Abstract
Coronavirus disease 2019 (COVID-19) is causing problems worldwide. Most people are susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but elderly populations are more susceptible. Elevated susceptibility and death rates in elderly COVID-19 patients, especially those with age-related complications, are challenges for pandemic prevention and control. In this paper, we review the clinical features of elderly patients with COVID-19 and explore the related molecular mechanisms that are essential for the exploration of preventive and therapeutic strategies in the current pandemic. Furthermore, we analyze the feasibility of currently recommended potential novel methods against COVID-19 among elderly populations.
Keywords: COVID-19, elderly, clinical feature, molecular mechanism, strategy
Coronavirus disease 2019 (COVID-19) is an emerging respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first identified in mid-December 2019 in Wuhan, China. Currently, it has spread globally and been declared a pandemic by the World Health Organization (WHO). Up to August 18,2020, more than 767,000 deaths and 21,500,000 confirmed positive cases have been reported around the world. Accumulating evidence shows that age and underlying conditions of virus contacts are crucial factors influencing personal fate toward different clinical severities of COVID-19, ranging from asymptomatic, mild, and moderate to death [1, 2]. COVID-19 shows a considerably elevated mortality rate in patients with advanced age and pre-existing comorbidities [3]. Under the situation of global aging, the COVID-19 pandemic creates challenges for not only widespread public health but also biomedical and clinical aging research [4].
Clinical features of COVID-19 in elderly patients
New coronavirus-exposed populations are generally susceptible, but elderly people, especially those with underlying conditions normally considered to be aging-associated diseases (diabetes, hypertension, and cardiovascular and cerebrovascular diseases), exhibit increased susceptibility [5]. Moreover, the spread of SARS-CoV-2 as well as severe acute respiratory syndrome coronavirus (SARS-CoV) in 2003 made it clear that these aging patients are more likely to progress to severe disease and die more easily from these infections than younger people [6-8]. After analyzing the data for 1099 COVID-19 patients from 552 hospitals in China, Nanshan Zhong et al. found that patients with severe disease were older than those with nonsevere disease and that coexisting illness was more common among patients in the severe group[9]. In the United States, 80% of deaths have occurred in patients over the age of 65 years, and patients aged 85 years and over have a high proportion of severe outcomes, mirroring the experience in China [10]. According to a meta-analysis, serious aging-related chronic diseases(associated with a pathological role of cellular senescence), including hypertension, diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular disease, and cerebrovascular disease, are independent risk factors associated with COVID-19 patients [11].
Similar to those in younger patients, fever, cough and sputum are the most common early symptoms in elderly patients. Elderly patients are more likely to have severe COVID-19 conditions, show lack of improvement, and die [12]. Analyzing a total of 56 COVID-19 patients, Liu et al. found that the pneumonia severity index (PSI) score of elderly patients was higher than that of young and middle-aged patients. The proportion of patients with PSI grades IV and V was significantly higher in elderly patients [13]. The incidence of the common fatal presentations of COVID-19 (including ARDS, shock, arrhythmia and acute cardiac injury) in elderly patients is higher than in young patients [12, 14, 15]. Wang et al. included patients with laboratory-confirmed COVID-19 and ascurtained the viral load by reverse transcriptase quantitative PCR (RT-qPCR). The results showed that older age was correlated with higher SARS-CoV-2 load [16]. Studies of SARS-CoV have shown that a high initial viral load is associated with death [17]. Accumulating evidence indicates that the SARS-CoV-2 viral load correlates with the risk of COVID-19 progression and poor prognosis [18, 19]. Thus, it is conceivable that the poor prognosis of elderly COVID-19 patients may be related to a higher viral load. However, the underlying mechanism requires further exploration.
Mechanism of COVID-19 in elderly patients
Senescent cells accumulate with age in vertebrates and promote aging mainly through the senescence-associated secretory phenotype (SASP) [20]. Data have shown that RNA viruses, such as influenza virus, display enhanced replication efficiency in senescent cells, which suggests that the accumulation of senescent cells in aging and age-related diseases may play a role in this phenomenon. However, the response to SARS-COV-2 that occurs in senescent cells is still poorly understood [8].
Angiotensin-converting enzyme 2 (ACE2) has been identified as areceptor for SARS-CoV-2, which directly interacts with COVID-19 spike(S) glycoproteins [21, 22]. During infection, the S protein is cleaved into S1 andS2 by the host transmembrane protease/serine subfamily member 2 (TMPRSS2)and protease furin [23].S1 directly binds to ACE2 and S2 plays a role in membrane fusion [24]. Zhou et al. analysed the expression of ACE2, together withTMPRSS2 and Furin by the method of single-cell RNA profiling combined with the protein information in different tissues. According to the rank list of candidate cells potentially vulnerable to SARS-CoV-2,the top targets were lung alveolar type 2 (AT2) cells and macrophages, followed by cardiomyocytes and adrenal gland stromal cells [25]. These findings were consistent with prominent lung symptoms, frequent heart damage and rare bowel symptoms. Cluster of differentiation 26 (CD26) is also recommended as a potential receptor for SARS-COV-2 [22, 26]. Intriguingly, both ACE2 and CD26 show associations with senescence and immunoregulation.
ACE2, which is widely distributed in the heart, kidneys, lungs, liver, intestine, brain and testes, is a known crucial component of the renin-angiotensin system (RAS)[27]. According to Khemais-Benkhiat et al., RAS is upregulated in endothelial cells with premature and replicative senescence [28]. It has been suggested that differential levels of ACE2 in human organs, especially the cardiac and pulmonary tissues of younger versus older adults, may be responsible for the disease virulence spectrum observed in COVID-19 patients [29]. Ziegler et al. discovered that ACE2 was a human interferon-stimulated gene in airway epithelial cells. SARS-CoV-2 could enhance infection through species-specific interferon-driven upregulation of ACE2 [30]. Smith et al. also identified ACE2 as an interferon-stimulated gene in lung cells. They found that chronic smoke exposure and inflammatory signaling could increase ACE2 expression levels in the respiratory tract. Their findings suggested that SARS-CoV-2 infections could create positive feedback loops that increase ACE2 expression and promote viral dissemination [31].
Interestingly, it has been previously established that ACE2 shows a protective effect in the lungs and that ACE2 expression in the lungs decreases during aging. The lower ACE2 level in the lungs may contribute to the poor prognosis of SARS in the aged group [32]. According to previous research, following SARS-COV entry, ACE2 expression is downregulated dramatically, which leads to a compensatory overproduction of angiotensin 2 (AngII), therefore increasing lung vascular permeability and aggravating lung failure [33]. A similar phenomenon is also observed in SARS-CoV-2-infected lung tissue [34]. According to Datta et al., SARS-CoV-2infects ACE2-expressing cells in the lung, inducing shedding of ACE2 from the cells and, thus, effectively reducing ACE2 expression levels [35]. These findings support the proposed role of ACE2 downregulation in both the pathogenesis and progression into ARDS in SARS and COVID-19 patients.
The contradictions between these research results indicate that SARS-CoV-2 regulation of ACE2 may be complex and dynamic. Whether the regulatory mechanism of SARS-CoV-2 toward ACE2 differs due to the patients’ age, underlying diseases, and stages of infection requires further exploration.
ACE2 is enriched in the heart and plays an essential role in the regulation of heart function [36]. Burrell et al. indicated that ACE2 expression is increased in myocardial infarction in humans and rats [37]. Chen et al. performed state-of-the-art single-cell atlas analysis of the adult human heart and revealed pericytes with high expression levels of ACE2 as target cells of SARS-CoV-2. Patients with coronary heart disease (CHD) showed increased ACE2expression at the mRNA and protein levels [38]. Patients with CHD and infected with SARS-CoV-2 are at an elevated risk of progressing into severe disease [11, 29]. Conversely, acute cardiac injury is reported as one of the most common complications in COVID-19 patients, which significantly exacerbates disease severity [39]. This evidence suggests that enrichment of ACE2 in the myocardium makes the heart one of the main target organs for SARS-CoV-2 infection. The expression level of ACE2 in cardiomyocytes is closely related to the poor prognosis of COVID-19 patients with heart diseases and the severity of COVID-19-induced heart injury.
Cytokine storms, namely, hypercytokinemia, a frequent feature of severe SARS, MERS, H5N1 influenza and H7N9 influenza, are associated with disease severity and are a predictor of prognosis [40-42]. Accumulating data suggest that depletion of lymphocytes, activation of cytotoxic T-lymphocytes, neutrophils and neutrophil-mediated cytokine storms may play key roles in COVID-19 pathogenesis [43]. COVID-19 results in increased levels of plasma cytokines, including interleukin-6 (IL-6), interleukin-1β (IL-1β), interleukin-7 (IL-7), interleukin-8 (IL-8), interleukin-9 (IL-9), interleukin-10 (IL-10), granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), tumor necrosis factor (TNF-α) and vascular endothelial growth factor A (VEGFA). ICU patients with COVID-19 produced higher levels of IL-6 than those not requiring ICU care [44]. According to a meta-analysis, mean IL-6 serum levels are2.9 times higher in patients with severe disease than in those with nonsevere disease [45]. HighIL-6 levels are closely correlated with the serum SARS-CoV-2 viral load, which further affects vital signs and the occurrence of ARDS inCOVID-19 patients [46]. Moreover, as a mediator of SASP, IL-6 is the main functional marker of cell aging [5, 47], and the increase in IL-6 content may correspond to the age-dependent increase in mortality of COVID-19 patients [48].
ACE2 participates in the RAS and plays an important role in regulating IL-6-induced inflammatory injury through the NF-κB and STAT3 pathways [49]. Kubaet al. indicated that SARS-CoV infection could cause a reduction of ACE2 levels on cells, followed by increased serum AngII levels [50]. AngII is one of the key activators of NF-κB and STAT3, which stimulates the expression of IL-6[51]. In turn, we speculate that severe lung inflammation induced by SARS-CoV-2 infection may induce the dysregulation of the RAS followed by the development of ARDS.
CD26, also known as dipeptidyl peptidase 4 (DPP4), is a serine exopeptidase that is expressed ubiquitously in human tissues, including lung, kidney, liver, gut, and immune cell s(including T-cells, activated B-cells, activated natural killer cells and myeloid cells) [52, 53]. CD26 plays multiple roles in nutrition, metabolism, and the immune and endocrine systems. Using mass spectrometry analysis, Kim et al. identified CD26 as a surface protein that is expressed at significantly higher levels in senescent cells. CD26 activity is higher in older than in younger individuals [54]. Overly upregulated CD26 expression and activity are associated with diabetes, obesity and metabolic syndrome, which are reported to influence COVID-19 severity [22, 55].
Moreover, CD26 has been identified as the functional receptor of MERS-CoV [56, 57]. Research has indicated that MERS-CoV is associated with higher mortality among elderly populations with chronic debilitating diseases including diabetes, malignancy, pulmonary and renal diseases. MERS also appears with greater severity and higher mortality rates in elderly people, especially those with debilitated immune systemsorpre-existing comorbidities [58-60]. Therefore, we suggest that similar to the case for ACE2, DPP4 upregulation may be adeterminant of COVID-19 progression and prognosis.
Large numbers of studies have demonstrated the integral role of CD26 in T lymphocyte activation during immunesenescence and costimulation [61]. CD26 acts as a costimulatory agent that mediates T cell activation by binding to the ligand adenosine deaminase [62]. Additionally, CD26 enhances lymphocyte proliferation independent of ADA [63].
CD26 can exist in a soluble form that is considered to be released from the membrane into the plasma, which still retains its enzymatic activity[64]. Ikeda et al. found that soluble CD26 enhances the binding of transcription factors to the TNF-α and IL-6 promoters, thus increasing TNF-α and IL-6 mRNA and protein expression in THP-1 cells [65]. CD26 inhibitors decrease the concentration of proinflammatory factors, such as TNF-α and IL-6 [66]. Thus, some researchers have suggested that the activation of CD26 on T lymphocytes may partially contribute to the high expression of IL-6 in COVID-19 patients. However, the specific role of CD26 in COVID-19 still requires further exploration (Figure 1).
Figure1.
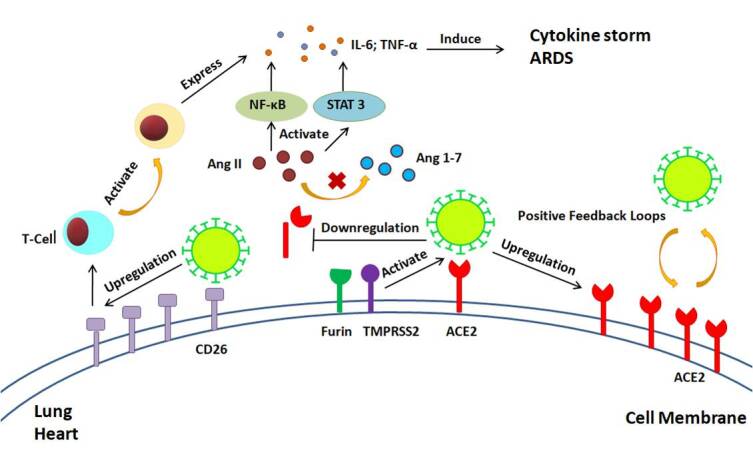
Interaction of SARS-CoV-2 with ACE2 and CD26. To enter the host cells, SARS-CoV-2 binds to membrane-bound ACE2 with the assistance of Furin and TMPRSS2. SARS-CoV-2 infections could create positive feedback loops that increase ACE2 expression and promote viral dissemination. On the other hand, SARS-CoV-2 infections may induce ACE2 shedding. ACE2 downregulation could lead to accumulation of Ang II, therefore inducing cytokine storm and ARDS. Activation of CD26 on T lymphocytes may partially contribute to the high expression of IL-6 in COVID-19 patients.
Proposed clinical applications in elderly covid-19 patients
Current evidence suggests that elderly patients with past comorbidities or dyspnea should be closely monitored for signs of disease progression, decompensation, and exacerbation of illness, especially 1-2 weeks after the onset of symptoms[10, 67]. Presently, there are no officially approved medicines available against SARS-CoV-2. Although the management of COVID-19 patients is primarily supportive, specific therapies are still under investigation. These therapies include current and potential antiviral drugs, anti-senescence drugs, immunosuppressive agents, steroids, mesenchymal stem cell (MSC) transplantation, and an artificial liver system (ALS), among others (Table 1). In the following paragraphs, we aim to highlight the proposed therapeutic strategies [68].
Table 1.
Potential strategies for the treatment of COVID-19.
| Treatment | Agent | Related Target/Pathways | Potential efficacy in COVID-19 |
|---|---|---|---|
| Antiviral drugs | Remdesivir LPV/RTV Favipiravir Arbidol |
Reduces the production of viral RNA Inhibits antiretroviral protease Targets RNA-dependent RNA polymerase Perturbs the virus membrane structure |
Shortens the recovery time in COVID-19 patients Shortens the viral shedding duration in patients Induces a shorter viral clearance time and greater improvement rate in chest imaging Shorter duration of positive RNA test compared to those treated with LPV/RTV |
| Antisenescence drugs | Azithromycin Chloroquine; hydroxychloroquine Rapamycin |
Targets and removes senescent cells; inhibits IL-6 and IL-1β expression; extends the lifespan of myofibroblasts Prevents the induction and accumulation of β-Gal; inhibits the replication of SARS-CoV in vitro Downregulates the IL-6 pathway; reduces the number of senescent T-cells through the mTOR-NLRP3-IL-1β axis |
Reduces airway inflammation; antifibrosis Reduces the viral load in COVID-19 patients Prevents and treats the severity of COVID-19 patients |
| ACE2-related therapy | ACE2 activator ACE2 inhibitor Human recombinant soluble ACE2 |
Avoids binding of S protein of SARS-CoV-2 to ACE2 Inhibits ACE2 expression Directly binds to SARS-CoV-2 in the circulation |
Requires scientific and clinical evidence Still under debate Blocks SARS-CoV-2 infection; prevents lung injury |
| CD26 inhibitor | Linagliptin | Attenuates DM-induced activation of NLRP3 inflammatory bodies | Decreases the concentration of cytokines, especially TNF-α and IL-6 |
| Immunosuppressive Therapy | Tocilizumab; sarilumab; siltuximab cyclosporine-cyclophilin A complex Corticosteroids |
Directly targets IL-6 receptors Halts the expression of TNF-α and IL-2; blocks the replication of coronaviruses Inhibits innate and adaptive immune responses as well as immune cells |
Improves clinical outcomes in severe cases Anti-inflammatory and antiviral properties in COVID-19 Improves clinical outcomes in COVID-19 patients with ARDS |
| MSC transplantation | / | Advantages in anti-inflammation, antifibrosis and injury repair | Improves pulmonary function and symptoms of patients |
| Artificial liver system | / | Attenuates the cytokine storm | Reduces the mortality of severe patients exhibiting rapid disease progression |
Abbreviations: COVID-19, coronavirus disease 2019; LPV/RTV, Lopinavir/ritonavir; IL-6, Interleukin-6; IL-1β, Interleukin-1β; β-Gal, beta-galactosidase; SARS-CoV, severe acute respiratory syndrome coronavirus; mTOR, mammalian target of rapamycin; NLRP3, nod-like receptor family pyrin domain-containing 3; ACE2, Angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CD26, cluster of differentiation 26; DM, diabetes mellitus; TNF-α, tumor necrosis factor-α; IL-2, Interleukin-2; MSC, mesenchymal stem cell.
Antiviral drugs
Timely antiviral therapy is highly recommended early the course of COVID-19 patients, especially elderly patients and patients with underlying conditions. Wu et al. retrospectively investigated the clinical data of 280 COVID-19 cases and recommended that timely antiviral treatment should be initiated to slow disease progression and improve prognosis in elderly patients [2]. Previously, oseltamivir, which could reduce the mortality of influenza patients, and ganciclovir, which is primarily used to treat cytomegalovirus [69], were widely used for SARS-CoV-2 patients. However, the efficiencies of neuraminidase inhibitors are currently being questioned, which makes them beyond recommendation [70, 71].
Remdesivir (GS-5734) is a monophosphoramidate prodrug of an adenosine analog that can affect viral RNA polymerase and reduce the production of viral RNA [72]. Remdesivir has shown a significant therapeutic effect in MERS-CoV mouse models [73]. It is effective in the EC50 range of 0.069 μM for SARS-CoV and 0.074 μM for MERS-CoV in tissue cultures as well as 0.77 μM in Vero E6 cells [72, 74]. Theoretically, remdesivir is currently the most promising drug for treating COVID-19. According to the result of a prospective, open-label study of remdesivir, Antinori et al. indicated that this drug could benefit hospitalized patients with SARS-CoV-2 pneumonia, with fewer adverse events observed [75]. The clinical trial conducted by Wang et al. in 237 patients at ten hospitals in Hubei Province showed that, although not statistically significant, the patients receiving remdesivir had a faster time to clinical improvement than those receiving placebo [76]. In the trial conducted by Beigel and colleagues, 1063 COVID-19 patients were randomly assigned to remdesivir or placebo. The results showed that remdesivir could obviously shorten the recovery time in hospitalized COVID-19 patients [77]. The authors also emphasized that earlier remdesivir treatment was probably more beneficial than later treatment [78]. Further explorations of the safety and efficiency of remdesivir for COVID-19 are still ongoing.
Lopinavir/ritonavir (LPV/RTV),which acts as antiretroviral protease inhibitor, is used as an anti-HIV drug [79]. In India, the Central Drugs Standard Control Organization approved the restricted public health use of the LPV/RTV combination in symptomatic COVID-19 patients [80]. However, the efficacy of LPV/RTV for COVID-19 is still controversial. A clinical trial in Hubei showed that early LPV/RTV treatment could shorten the viral shedding duration in COVID-19 patients, especially in those of an older age [81]. Cheng et al. obtained the exact opposite result among patients with mild pneumonia in Taiwan [82]. According to a randomized, controlled, open-label trial in 199 patients with severe COVID-19, mortality at 28 days and viral clearance time were similar in the LPV/RTV group and the standard-care group. The results suggested that no benefit was observed with LPV/RTV treatment [74]. Therefore, the WHO suggested larger trials with a greater variety of COVID-19 patients [83].
Favipiravir (FPV), a purine nucleic acid analog that targets RNA-dependent RNA polymerase (RdRP), is widely used as an oral anti-influenza drug [84]. Cai et al. conducted a clinical trial to evaluate the safety and efficacy of favipiravir in COVID-19 patients. A shorter viral clearance time as well as a significantly higher improvement rate in chest imaging was shown for the FPV group versus the LPV/r group. In addition, fewer adverse reactions were found in the FPV group than in the LPV/r group [85].
Arbidol, an important anti-viral drug candidate, also showed promising effects in COVID-19. Arbidol is a broad-spectrum antiviral molecule that inhibits both DNA as well as RNA viruses by altering the membrane structure of the virus [86]. According to Zhu et al., patients treated with arbidol show a shorter duration of positive RNA test compared to those treated with LPV/RTV, while nosignificant adverse effects are observed. The results indicated that arbidol monotherapy is superior to LPV/RTVin COVID-19 treatment [87]. Additionally, a retrospective cohort study showed that arbidol combined with LPV/RTV showed improved efficacy in COVID-19 patients [88]. Another retrospective, single-center study showed that the combination of Lianhuaqingwen and arbidol was effective for patients with mild symptoms within 5-7 days, and the cure rate was 98% [89]. Moreover, arbidol is considered to have a preventive effect. Yang et al. suggested that prophylactic oral arbidol was associated with a lower incidence of SARS-CoV-2 infection in medical staff [90]. Although accumulating evidence has demonstrated the potential clinical efficiency of arbidol, powered randomized control trials are still needed for further confirmation [91].
Antisenescence drugs
Azithromycin and the closely related drug roxithromycin are macrolide antibiotics that can act as senolytic drugs that target and remove senescent cells [92]. Additionally, azithromycin is known to exert an antifibrotic effect by significantly extending the lifespan of myofibroblast cells. Moreover, azithromycin has been proven to reduce airway inflammation by inhibiting IL-6 and IL-1β expression in mouse models [93, 94]. Therefore, azithromycin is recommended by a number of researchers as a potential COVID-19 treatment strategy. However, Gbinigie et al. conducted a rapid review of the current literature. The results indicated that other than in the case of bacterial super infection, there was no evidence supporting the use of azithromycin for the treatment of SARS-CoV-2 infection outside of the context of clinical trials [95]. According to a clinical trial conducted by Rosenberg et al., receipt of azithromycin alone could not significantly reduce in-hospital mortality of COVID-19 patients [96].
Chloroquine (CQ) and its derivative hydroxychloroquine (HCQ) have the ability to induce alkalization, which functionally prevents the induction and accumulation of beta-galactosidase (β-Gal), known as a marker of senescence [97]. CQ and HCQ are widely used antimalarial and antiviral drugs and have recently received much attention as potential treatments for COVID-19 [98].CQ was recently demonstrated as an inhibitor ofSARS-CoV-2 in vitro, and the hydroxylated form, HCQ, has been proven to limit the replication of SARS-CoV-2 in vitro [99, 100]. Huang et al. found that CQ had a slight advantage over lopinavir/ritonavir in COVID-19 patients [101]. HCQ can also specifically inhibit the replication of SARS-CoV by interfering with the glycosylation of ACE2 [102]. Therefore, HCQ has been widely suggested as a potential treatment for patients with SARS-CoV-2 infection. A number of clinical trials have been conducted to explore the efficacies of CQ and HCQ. According to a small open-label nonrandomized clinical trial, HCQ treatment was significantly associated with viral load reduction in COVID-19 patients, and its efficacy could be reinforced by azithromycin [103]. However, according to a newly published comparative observational study among 181 COVID-19 patients with documented severe acute respiratory syndrome who required oxygen, HCQ did not show an obvious therapeutic effect. Additionally, eight patients in the HCQ group (10%) experienced electrocardiographic modifications that required discontinuation of treatment [104]. Despite the controversial results of these clinical trials, frequent side effects of CQ and HCQ, for example, worsening vision, nausea, digestive disorders and potential heart failure and even death, have hindered the wide application of these drugs [105]. Moreover, the results of a clinical trial conducted by Rosenberg et al., which involved 1438 COVID-19 patients, showed that compared with neither treatment, treatment with HCQ alone or combined with azithromycin was not significantly associated with differences in mortality [96].
Rapamycin is widely known as a key anti-aging drug and prevents the progression of senescence in human cell lines and animal models [106-108]. Rapamycin acts as an inhibitor of protein synthesis, inhibiting cytokine expression and viral replication [109]. In elderly patients, especially those with CHD or reduced T-cell counts, rapamycin can significantly reduce the expression of the serum senescence marker IL-6 [110]. As a candidate for potential use in COVID-19, rapamycin may prevent progression to severe forms of COVID-19 by downregulating the IL-6 pathway and reducing the number of senescent T-cells through the mTOR-NLRP3-IL-1β axis at the early stage of cytokine storms [111, 112]. Conversely, rapamycin is suggested as a novel intervention strategy beyond vaccines to prevent severe symptoms in COVID-19 [113]. Therefore, conducting clinical trials for rapamycin to prevent and treat the severity of COVID-19 patients, especially elderly patients, is strongly recommended.
ACE2-related therapies
Some researchers have suggested that SARS-CoV-2 induces initial damage effects by downregulating ACE2 expression and blocking ACE2-mediated activity and activating ACE2 may be much more efficacious. Accumulating evidence has illustrated that the activation of ACE2 could be a positive treatment method. ACE2 activators are purported to have two therapeutic effects: avoiding the binding of the S protein of SARS-CoV-2 to ACE2 and promoting the protective effects of different organs, preventing lung injury and fibrosis [34, 114, 115].ACE2 activators, such as diminazene aceturate, are recommended for application in COVID-19 patients [34].
However, due to the positive-feedback loop between virus infection and ACE2 expression, some researchers have suggested the use of ACE2 inhibitors to block SARS-CoV-2 infection. Considering the role of ACE2 in maintaining organ functioning, especially in the lungs, the clinical application of ACE2 inhibitors in COVID-19 is under question [116]. The findings regardingACE2 have sparked a debate regarding the potential use of angiotensin-converting enzyme inhibitors (ACEIs) and AngII receptor blockers (ARBs) among elderly COVID-19 patients in the context of the pandemic [117]. However, after reviewing published relevant animal, in vitro and clinical studies, Chung et al. announced that the results did not show a higher risk of infection with ACEI or ARB use [118]. Considering the contradictory hypotheses and lack of scientific evidence and clinical data worldwide, the European Society of Cardiology and the American College of Cardiology recommended the continuation of ACEIs or ARBs for COVID-19 patients who were already taking these medicines [119, 120].
Human recombinant soluble ACE2 (hrsACE2) is an FDA-approved treatment, with a 2017 phase II trial for ARDS [121]. Circulating soluble ACE2 can bind to SARS-CoV-2 but is unable to inhibit cell infection [122]. According to Monteil et al., clinical grade hrsACE2 reduces SARS-CoV-2 recovery from Vero cells by 1,000-5,000 times, demonstrating that hrsACE2 can not only block SARS-CoV-2 infection but also protect against lung injury, suggesting a possible therapeutic approach [123].
CD26 inhibitors
The clinical immune response to SARS-CoV-2 can be divided into 2 phases: an earlier phase of elimination by antiviral adaptive immunity and a later phase of damaged alveolar cells triggering innate inflammation [124]. According to Chen et al., the occurrence of ARDS may be associated with the later immune phase, and treatment to reduce inflammation during that phase may help reduce lung damage. CD26 inhibitors may potentially act to regulate an overactive immune reaction and prevent devastating lung injury [125]. In a mouse model of ARDS, which is the main cause of death in COVID-19 patients, CD26 inhibition by sitagliptin alleviated histological findings of lung injury by inhibiting the expression of IL-1β, TNF-α, and IL-6[126]. Birnbaum et al. reported that saxagliptin-mediated DPP4 inhibition could attenuate diabetes mellitus (DM) -induced activation of nod-like receptor family pyrin domain-containing 3 (NLRP3) inflammatory bodies, thus reducing serum CRP, TNF-α, IL-1β, IL-18 and IL-6 levels [127]. CD26 inhibitors, including sitagliptin, alogliptin, vildagliptin, saxagliptin, and linagliptin, are a class of drugs used effectively in the treatment of type 2 diabetes, which has demonstrated safety in elderly patients. These drugs have similar effects by binding to essentially the same catalytic site [128]. Research has shown that the administration of linagliptin can lead to a decrease in the concentration of proinflammatory factors, especially TNF-α and IL-6 [66]. Moreover, mathematical modeling has shown that the spread of MERS-CoV infection can be controlled by inhibiting the expression of CD26, with similar efficiency in SARS-CoV-2 infection [129]. Since the expression of CD26 is closely related to the pathogenesis and severity of COVID-19, CD26 inhibitors have recently been proposed as potential drugsagainst COVID-19 [130]. However, despite laboratory verification and theoretical feasibility, the actual efficacies of CD26 inhibitors in COVID-19 still require verification in further clinical trials.
Immunosuppressive agents
As we mentioned above, COVID-19 severity and outcomes are closely related to the characteristics of the immuneresponse and subsequent cytokine storm incited by pathogenic T cells and inflammatory monocytes with a high level of IL-6 secretion, which are more severe in elderly patients [131]. Some researchers have recommended monoclonal antibodies that target IL-6,TNF-α and other cytokine pathways as a potential strategy to attenuate the inflammatory storm [132].
Tocilizumab is a marketed humanized monoclonal antibody that directly targets IL-6 receptors. Accumulating clinical trials have confirmed its safety and effectiveness in rheumatoid arthritis treatment. In a clinical trial among 45 patients, tocilizumab exerted a rapidly beneficial effect on fever and inflammatory markers in COVID-19 patients [133]. According to Xu et al., within 5 days after additional application of tocilizumab, the percentage of lymphocytes in peripheral blood returned to normal in 52.6% of severe COVID-19 patients, and all patients were discharged after anaverage of 15.1 days. The results indicated that tocilizumab could improve the clinical outcomes in severe COVID-19 patients by suppressing the immune response [131]. Recently, tocilizumab was approved for patients with severe SARS-CoV-2 pulmonary complications by the National Health Commission of the People’s Republic of China. Other monoclonal antibodies specifically targeting IL-6 pathways, such as sarilumab and siltuximab, are also recommended for COVID-19 treatment. Clinical trials are still needed to evaluate their efficacy and safety in COVID-19 patients.
The cyclosporine-cyclophilin A complex, including cyclophilin and tacrolimus, is considered to suppress organ rejection by halting the expression of TNF-α and IL-2 [134]. Moreover, according to Pfefferle et al., cyclophilin can block the replication of coronaviruses, including SARS-CoV, human CoV-229E and CoV-NL-63, feline CoV, among others. These results suggest that cyclophilin is a broad-spectrum coronavirus inhibitor and might be used for therapy in emerging coronavirus infections [135]. Therefore, the anti-inflammatory and antiviral properties of the cyclosporine-cyclophilin A complex make it a potential clinical application in severe COVID-19. However, considering the nephrotoxicity and hepatotoxicity of cyclophilin and tacrolimus, safety issues must be explored in COVID-19 patients, especially elderly individuals with liver and kidney involvement.
Corticosteroids show inhibitory effects on both innate and adaptive immune responses, as well as multiple types of immune cells [136]. Corticosteroids were among the first therapies tested in trials for preventing ARDS [137]. Meduri et al. constructed a clinical trial to investigate the efficacy of long-term corticosteroid treatment in patients with ARDS. The results showed that prolonged corticosteroid application could accelerate the resolution of ARDS and decrease hospital mortality [138]. Fadel et al. conducted a single pretest, single posttest quasi-experiment among 213 COVID-19 patients. The results indicated that an early short course of methylprednisolone application could reduce escalation of care and improve clinical outcomes in patients with moderate-to-severe COVID-19 [139]. However, clinical management of severe SARS and MERS patients revealed that corticosteroid therapy did not decrease mortality; in contrast, it caused delayed viral shedding [140]. In addition, Zha et al. announced that after analyzing the data of 31 COVID-19 patients, they found no association between corticosteroid therapy and outcomes in patients without ARDS [141]. The efficacy of corticosteroids in COVID-19 patients remains controversial. Thus, the Guideline for the Diagnosis and Treatment of COVID-19 (7th version) recommended low-to-moderate doses and short courses of corticosteroid application in selected COVID-19 cases that represent excessive immune response activation or rapid progression of imaging changes
MSC transplantation
MSCs are nonhematopoietic stem cells derived from the mesoderm, which can be isolated from various tissues, such as bone marrow, adipose tissue, umbilical cord blood, placenta, menstrual blood, dental pulp, and amniotic fluid [142]. Accumulating evidence from preclinical and clinical trials has demonstrated that MSCs have great advantages in anti-inflammation, anti-fibrosis and injury repair [143-145]. Shao et al. reported that MSCs could strongly suppress IL-6 production, thereby disrupting the development of cytokine storms [146]. Previously, it was demonstrated that MSC transplantation could significantly reduce the mortality of patients with H7N9-induced ARDS (17.6% died in the MSC group, whereas54.5% died in the control group). Moreover, no significant adverse effects were observed in these patients [147]. According to Leng et al., MSC transplantation could significantly improve pulmonary function and symptoms of COVID-19 patients without obvious adverse effects. Moreover, decreased TNF-α and increased IL-10 levels were detected in the MSC treatment group compared with the control group [148]. These results indicated that MSC-based therapy could be a potential alternative for managing patients with severe symptoms of COVID-19.
Artificial liver system
The artificial liver system (ALS) is widely used as an effective support therapy in severe liver failure patients [149]. As mentioned above, the cytokine storm is the main motivator of COVID-19 progression and a poor prognosis, and its severity is closely associated with advanced age. Previously, clinical trials have demonstrated that the plasma exchange module of ALS exhibited high efficacy in attenuating the cytokine storm of H7N9 infection [150]. A single-center study showed that artificial liver plasma exchange could significantly reduce inflammatory cytokine levels, thus reducing mortality in critically ill patients with COVID-19 [151]. Our colleagues found that the application of ALS showed excellent prognosis in the treatment of severeCOVID-19 patients presenting a cytokine storm [152]. According to the Diagnosis and Treatment of COVID-19 (7th version) published in China, ALS is recommended in patients who exhibit rapid disease progression confirmed by lung imaging and a cytokine storm.
Nutritional support in elderly people
To date, considerable evidence has demonstrated that food and nutrients could affect immune system function. Poor nutritional status is widely considered one of the significant risk factors for severe COVID-19 [153]. It has been directly highlighted that nutritional support may play an important role in determining COVID-19 outcomes [154]. Generally, there are nutritional deficiencies in calcium, vitamin C, vitamin D, folate, and zinc among the elderly population, especially among in-hospital patients [155]. According to a study in Wuhan, the prevalence of malnutrition is elevated in elderly patients with COVID-19 [156]. The researchers suggested that nutritional support should be strengthened during treatment. Malnutrition can exacerbate the immune system deficiency in elderly individuals, making them susceptible to SARS-CoV-2 infection [157]. A healthy, balanced diet can provide elderly individuals with necessary macro- and micronutrients, prebiotics, probiotics, and symbiotics to restore and maintain immune cell function, thus reducing the incidence of SARS-CoV-2 infection [153].
Problems faced by the progress and application of the vaccine
The development of a safe and effective vaccine against SARS-CoV-2 for nonimmune individuals is an urgent and critical task for controlling the ongoing pandemic [68]. The developing coronavirus vaccines and drugs mainly target the spike glycoprotein or S protein, the major inducers of neutralizing antibodies. To date, Wei Chen et al. conducted a dose-escalation, single-center, open-label, nonrandomized, first-in-human trial of a recombinant adenovirus type5-vectored COVID-19 vaccine in Wuhan, China. The vaccine was tolerable and immunogenic 28 days after vaccination. The humoral responses against SARS-CoV-2 peaked at day 28 after vaccination in healthy adults, and rapid specific T-cell responses were noted from day 14 after vaccination [158].
However, even if a successful vaccine for SARS-CoV-2 becomes available, an individual’s immune response must be sufficiently strong to respond to the vaccine, and once exposure occurs, the reaction can later confer protection against the pathogen. Thus, vaccines cannot provide complete protection in elderly populations due to age-related declines in immune function and the accumulation of various diseases. According to Montoet al., influenza outbreaks still occur in elderly nursing homes even when vaccination rates reach 80-98% utilization [159]. Thus, the deficiency of the elderly group could become a gap in herd immunity that relies on vaccines. Some researchers recommended remodeling of the senescent immune system of elderly persons by allo-priming as a method to restore cellular immune function. Heterologous immunity can enhance the panviral protection upon each viral exposure, thereby providing long-term protection. The alloantigen priming strategy has been proposed in conjunction with viral-specific vaccines in the elderly population [160]. However, the efficiency requires further exploration.
Moreover, accumulating reports worldwide of some COVID-19 patients testing positive again after initially testing negative indicate the potential of re-infections and short-lasting immunity against COVID-19 [161]. To et al. firstly reported the case of COVID-19 re-infection by a phylogenetically distinct SARS-corona-virus-2 strain [162]. According to Ibarrondo et al., antibody loss in COVID-19 patients is quicker than that reported for SARS-CoV [163]. It means that humoral immunity against SARS-CoV-2 may not be long lasting in human bodies. Consequently, people doubt whether the vaccine could produce long-term immunity in the population.
Conclusions
The COVID-19 pandemic suggests that we are facing a historic challenge to our capacity to protect the health of our elderly population. Protecting aging populations is now a central question in maintaining global health and biosecurity [4]. Lloyd-Sherlock et al. announced that due to great barriers in access to health services and support, older people in low-and middle-income countries are bearing the brunt of COVID-19 [164]. An open letter suggested that the WHO should act immediately to redress its neglect of older people, and member states must prioritize the needs of the elderly population in national responses and support for low- and middle-income countries [165]. The current results of biomedical, clinical and public health studies also highlight the need for therapy and prevention strategies for aging COVID-19 patients. We hope that this review can provide insight regarding the prevention and treatment of COVID-19 in elderly populations.
Acknowledgement
This study was supported by the National Key Research and Development Program of China (2016YFC 1101304/3) and Zhejiang Province Key Research and Development Plan Emergency Project (No. 2020C03123-1).
References
- [1].Gasmi A, Noor S, Tippairote T, Dadar M, Menzel A, Bjorklund G (2020). Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol, 215:108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wu J, Li W, Shi X, Chen Z, Jiang B, Liu J, et al. (2020). Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med. [DOI] [PubMed] [Google Scholar]
- [3].Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. (2020). Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ, 368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Koff WC, Williams MA (2020). Covid-19 and Immunity in Aging Populations - A New Research Agenda. N Engl J Med, in press. [DOI] [PubMed] [Google Scholar]
- [5].Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chan-Yeung M, Xu RH (2003). SARS: epidemiology. Respirology, 8 Suppl:S9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kobayashi T, Jung SM, Linton NM, Kinoshita R, Hayashi K, Miyama T, et al. (2020). Communicating the Risk of Death from Novel Coronavirus Disease (COVID-19). J Clin Med, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Malavolta M, Giacconi R, Brunetti D, Provinciali M, Maggi F (2020). Exploring the Relevance of Senotherapeutics for the Current SARS-CoV-2 Emergency and Similar Future Global Health Threats. Cells, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. (2020). Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med, 382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ (2020). SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience, 42:505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang B, Li R, Lu Z, Huang Y (2020). Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY), 12:6049-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, et al. (2020). Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect, 26:767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu K, Chen Y, Lin R, Han K (2020). Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect, 80:e14-e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lei S, Jiang F, Su W, Chen C, Chen J, Mei W, et al. (2020). Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine, 21:100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. (2020). Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. (2020). Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis, 20:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chu CM, Poon LL, Cheng VC, Chan KS, Hung IF, Wong MM, et al. (2004). Initial viral load and the outcomes of SARS. CMAJ, 171:1349-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yu X, Sun S, Shi Y, Wang H, Zhao R, Sheng J (2020). SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit Care, 24:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. (2020). Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ, 369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, et al. (2020). Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell, 19:e13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579:270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bassendine MF, Bridge SH, McCaughan GW, Gorrell MD (2020). COVID-19 and comorbidities: A role for dipeptidyl peptidase 4 (DPP4) in disease severity? J Diabetes. [DOI] [PubMed] [Google Scholar]
- [23].Sternberg A, Naujokat C (2020). Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life Sci, 257:118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ragia G, Manolopoulos VG (2020). Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: a promising approach for uncovering early COVID-19 drug therapies. Eur J Clin Pharmacol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou L, Niu Z, Jiang X, Zhang Z, Zheng Y, Wang Z, et al. (2020). Systemic analysis of tissue cells potentially vulnerable to SARS-CoV-2 infection by the protein-proofed single-cell RNA profiling of ACE2, TMPRSS2 and Furin proteases. bioRxiv. [Google Scholar]
- [26].Vankadari N, Wilce JA (2020). Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect, 9:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Patel S, Rauf A, Khan H, Abu-Izneid T (2017). Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed Pharmacother, 94:317-325. [DOI] [PubMed] [Google Scholar]
- [28].Khemais-Benkhiat S, Idris-Khodja N, Ribeiro TP, Silva GC, Abbas M, Kheloufi M, et al. (2016). The Redox-sensitive Induction of the Local Angiotensin System Promotes Both Premature and Replicative Endothelial Senescence: Preventive Effect of a Standardized Crataegus Extract. J Gerontol A Biol Sci Med Sci, 71:1581-1590. [DOI] [PubMed] [Google Scholar]
- [29].Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. (2020). Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. (2020). SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell, 181:1016-1035 e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Smith JC, Sausville EL, Girish V, Yuan ML, Vasudevan A, John KM, et al. (2020). Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell, 53:514-529 e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xie X, Chen J, Wang X, Zhang F, Liu Y (2006). Age- and gender-related difference of ACE2 expression in rat lung. Life Sci, 78:2166-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. (2005). Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature, 436:112-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rodriguez-Puertas R (2020). ACE2 Activators for the Treatment of Covid 19 Patients. J Med Virol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Datta PK, Liu F, Fischer T, Rappaport J, Qin X (2020). SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics, 10:7448-7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. (2002). Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature, 417:822-828. [DOI] [PubMed] [Google Scholar]
- [37].Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, et al. (2005). Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J, 26:369-375; discussion 322-364. [DOI] [PubMed] [Google Scholar]
- [38].Chen L, Li X, Chen M, Feng Y, Xiong C (2020). The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res, 116:1097-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guo J, Huang F, Liu J, Chen Y, Wang W, Cao B, et al. (2015). The Serum Profile of Hypercytokinemia Factors Identified in H7N9-Infected Patients can Predict Fatal Outcomes. Sci Rep, 5:10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, et al. (2006). Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med, 12:1203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA (2018). MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine, 104:8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kuppalli K, Rasmussen AL (2020). A glimpse into the eye of the COVID-19 cytokine storm. EBioMedicine, 55:102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rothan HA, Byrareddy SN (2020). The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun, 109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Aziz M, Fatima R, Assaly R (2020). Elevated Interleukin-6 and Severe COVID-19: A Meta-Analysis. J Med Virol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. (2020). Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ershler WB (1993). Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc, 41:176-181. [DOI] [PubMed] [Google Scholar]
- [48].Hirano T, Murakami M (2020). COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity, 52:731-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].de Wit E, van Doremalen N, Falzarano D, Munster VJ (2016). SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol, 14:523-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. (2005). A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med, 11:875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li C, Wang Y, Qiu Q, Shi T, Wu Y, Han J, et al. (2014). Qishenyiqi protects ligation-induced left ventricular remodeling by attenuating inflammation and fibrosis via STAT3 and NF-kappaB signaling pathway. PLoS One, 9:e104255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Strollo R, Pozzilli P (2020). DPP4 inhibition: preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab Res Rev, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Abbott CA, Baker E, Sutherland GR, McCaughan GW (1994). Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics, 40:331-338. [DOI] [PubMed] [Google Scholar]
- [54].Kim KM, Noh JH, Bodogai M, Martindale JL, Yang X, Indig FE, et al. (2017). Identification of senescent cell surface targetable protein DPP4. Genes Dev, 31:1529-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wu Z, McGoogan JM (2020). Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA, in press. [DOI] [PubMed] [Google Scholar]
- [56].Mubarak A, Alturaiki W, Hemida MG (2019). Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development. J Immunol Res, 2019:6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, et al. (2013). Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature, 495:251-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nassar MS, Bakhrebah MA, Meo SA, Alsuabeyl MS, Zaher WA (2018). Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: epidemiology, pathogenesis and clinical characteristics. Eur Rev Med Pharmacol Sci, 22:4956-4961. [DOI] [PubMed] [Google Scholar]
- [59].Gralinski LE, Baric RS (2015). Molecular pathology of emerging coronavirus infections. J Pathol, 235:185-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ahmed AE (2017). The predictors of 3- and 30-day mortality in 660 MERS-CoV patients. BMC Infect Dis, 17:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Klemann C, Wagner L, Stephan M, von Horsten S (2016). Cut to the chase: a review of CD26/dipeptidyl peptidase-4's (DPP4) entanglement in the immune system. Clin Exp Immunol, 185:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Abbott CA, McCaughan GW, Levy MT, Church WB, Gorrell MD (1999). Binding to human dipeptidyl peptidase IV by adenosine deaminase and antibodies that inhibit ligand binding involves overlapping, discontinuous sites on a predicted beta propeller domain. Eur J Biochem, 266:798-810. [DOI] [PubMed] [Google Scholar]
- [63].Yu DM, Slaitini L, Gysbers V, Riekhoff AG, Kahne T, Knott HM, et al. (2011). Soluble CD26 / dipeptidyl peptidase IV enhances human lymphocyte proliferation in vitro independent of dipeptidyl peptidase enzyme activity and adenosine deaminase binding. Scand J Immunol, 73:102-111. [DOI] [PubMed] [Google Scholar]
- [64].Cordero OJ, Salgado FJ, Nogueira M (2009). On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother, 58:1723-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ikeda T, Kumagai E, Iwata S, Yamakawa A (2013). Soluble CD26/Dipeptidyl Peptidase IV Enhances the Transcription of IL-6 and TNF-alpha in THP-1 Cells and Monocytes. PLoS One, 8:e66520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wicinski M, Gorski K, Walczak M, Wodkiewicz E, Slupski M, Pawlak-Osinska K, et al. (2019). Neuroprotective Properties of Linagliptin: Focus on Biochemical Mechanisms in Cerebral Ischemia, Vascular Dysfunction and Certain Neurodegenerative Diseases. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Goh KJ, Choong MC, Cheong EH, Kalimuddin S, Duu Wen S, Phua GC, et al. (2020). Rapid Progression to Acute Respiratory Distress Syndrome: Review of Current Understanding of Critical Illness from COVID-19 Infection. Ann Acad Med Singapore, 49:108-118. [PubMed] [Google Scholar]
- [68].Yi Y, Lagniton PNP, Ye S, Li E, Xu RH (2020). COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci, 16:1753-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 395:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yousefi B, Valizadeh S, Ghaffari H, Vahedi A, Karbalaei M, Eslami M (2020). A global treatments for coronaviruses including COVID-19. J Cell Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Li G, De Clercq E (2020). Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov, 19:149-150. [DOI] [PubMed] [Google Scholar]
- [72].Brown AJ, Won JJ, Graham RL, Dinnon KH 3rd, Sims AC, Feng JY, et al. (2019). Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res, 169:104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sheahan TP, Sims AC, Leist SR, Schafer A, Won J, Brown AJ, et al. (2020). Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun, 11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. (2020). A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med, 382:1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Antinori S, Cossu MV, Ridolfo AL, Rech R, Bonazzetti C, Pagani G, et al. (2020). Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: Clinical outcome and differences in post-treatment hospitalisation status. Pharmacol Res, 158:104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. (2020). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet, 395:1569-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. (2020). Remdesivir for the Treatment of Covid-19 - Preliminary Report.N Engl J Med, in press. [DOI] [PubMed] [Google Scholar]
- [78].Beigel JH, Tomashek KM, Dodd LE (2020). Remdesivir for the Treatment of Covid-19 - Preliminary Report. Reply. N Engl J Med, 383. [DOI] [PubMed] [Google Scholar]
- [79].Venkatasubbaiah M, Dwarakanadha Reddy P, Satyanarayana SV (2020). Literature-based review of the drugs used for the treatment of COVID-19. Curr Med Res Pract, 10:100-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bhatnagar T, Murhekar MV, Soneja M, Gupta N, Giri S, Wig N, et al. (2020). Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: Protocol for restricted public health emergency use. Indian J Med Res, 151:184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yan D, Liu XY, Zhu YN, Huang L, Dan BT, Zhang GJ, et al. (2020). Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur Respir J, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Cheng CY, Lee YL, Chen CP, Lin YC, Liu CE, Liao CH, et al. (2020). Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J Microbiol Immunol Infect, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Costanzo M, De Giglio MAR, Roviello GN (2020). SARS-CoV-2: Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and Other Drugs for the Treatment of the New Coronavirus. Curr Med Chem. [DOI] [PubMed] [Google Scholar]
- [84].Du YX, Chen XP (2020). Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection. Clin Pharmacol Ther, in press. [DOI] [PubMed] [Google Scholar]
- [85].Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. (2020). Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing), in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Galiano V, Villalain J (2016). The Location of the Protonated and Unprotonated Forms of Arbidol in the Membrane: A Molecular Dynamics Study. J Membr Biol, 249:381-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, et al. (2020). Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect, 81:e21-e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, et al. (2020). Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect, 81:e1-e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Khan S, Ali A, Shi H, Siddique R, Shabana, Nabi G, et al. (2020). COVID-19: Clinical aspects and therapeutics responses. Saudi Pharm J, 28:1004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yang C, Ke C, Yue D, Li W, Hu Z, Liu W, et al. (2020). Effectiveness of Arbidol for COVID-19 Prevention in Health Professionals. Front Public Health, 8:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jomah S, Asdaq SMB, Al-Yamani MJ (2020). Clinical efficacy of antivirals against novel coronavirus (COVID-19): A review. J Infect Public Health, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ozsvari B, Nuttall JR, Sotgia F, Lisanti MP (2018). Azithromycin and Roxithromycin define a new family of "senolytic" drugs that target senescent human fibroblasts. Aging (Albany NY), 10:3294-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mosquera RA, De Jesus-Rojas W, Stark JM, Yadav A, Jon CK, Atkins CL, et al. (2018). Role of prophylactic azithromycin to reduce airway inflammation and mortality in a RSV mouse infection model. Pediatr Pulmonol, 53:567-574. [DOI] [PubMed] [Google Scholar]
- [94].Tang F, Li R, Xue J, Lan J, Xu H, Liu Y, et al. (2017). Azithromycin attenuates acute radiation-induced lung injury in mice. Oncol Lett, 14:5211-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gbinigie K, Frie K (2020). Should azithromycin be used to treat COVID-19? A rapid review. BJGP Open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. (2020). Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000). Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci, 113(Pt 20):3613-3622. [DOI] [PubMed] [Google Scholar]
- [98].Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. (2020). Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Devaux CA, Rolain JM, Colson P, Raoult D (2020). New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents, 55:105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res, 30:269-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Huang M, Tang T, Pang P, Li M, Ma R, Lu J, et al. (2020). Treating COVID-19 with Chloroquine. J Mol Cell Biol, 12:322-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. (2005). Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J, 2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents, 105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [104].Mahevas M, Tran VT, Roumier M, Chabrol A, Paule R, Guillaud C, et al. (2020). Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ, 369:m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Abd El-Aziz TM, Stockand JD (2020). Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2) - an update on the status. Infect Genet Evol, 83:104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV (2009). Rapamycin decelerates cellular senescence. Cell Cycle, 8:1888-1895. [DOI] [PubMed] [Google Scholar]
- [107].Bielas J, Herbst A, Widjaja K, Hui J, Aiken JM, McKenzie D, et al. (2018). Long term rapamycin treatment improves mitochondrial DNA quality in aging mice. Exp Gerontol, 106:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Arriola Apelo SI, Lamming DW (2016). Rapamycin: An InhibiTOR of Aging Emerges From the Soil of Easter Island. J Gerontol A Biol Sci Med Sci, 71:841-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sargiacomo C, Sotgia F, Lisanti MP (2020). COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging (Albany NY), 12:6511-6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Singh M, Jensen MD, Lerman A, Kushwaha S, Rihal CS, Gersh BJ, et al. (2016). Effect of Low-Dose Rapamycin on Senescence Markers and Physical Functioning in Older Adults with Coronary Artery Disease: Results of a Pilot Study. J Frailty Aging, 5:204-207. [DOI] [PubMed] [Google Scholar]
- [111].Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F (2020). Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2.Cell Discov, 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Omarjee L, Janin A, Perrot F, Laviolle B, Meilhac O, Mahe G (2020). Targeting T-cell senescence and cytokine storm with rapamycin to prevent severe progression in COVID-19. Clin Immunol, 216:108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zheng Y, Li R, Liu S (2020). Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: A novel intervention strategy beyond vaccines and specific antiviral medicines. J Med Virol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Dhawale VS, Amara VR, Karpe PA, Malek V, Patel D, Tikoo K (2016). Activation of angiotensin-converting enzyme 2 (ACE2) attenuates allergic airway inflammation in rat asthma model. Toxicol Appl Pharmacol, 306:17-26. [DOI] [PubMed] [Google Scholar]
- [115].Prata LO, Rodrigues CR, Martins JM, Vasconcelos PC, Oliveira FM, Ferreira AJ, et al. (2017). Original Research: ACE2 activator associated with physical exercise potentiates the reduction of pulmonary fibrosis. Exp Biol Med (Maywood), 242:8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cheng H, Wang Y, Wang GQ (2020).Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD (2020). Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med, 382:1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chung MK, Karnik S, Saef J, Bergmann C, Barnard J, Lederman MM, et al. (2020). SARS-CoV-2 and ACE2: The biology and clinical data settling the ARB and ACEI controversy. EBioMedicine, 58:102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rico-Mesa JS, White A, Anderson AS (2020). Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB.Curr Cardiol Rep, 22:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sienko J, Kotowski M, Bogacz A, Lechowicz K, Drozdzal S, Rosik J, et al. (2020). COVID-19: The Influence of ACE Genotype and ACE-I and ARBs on the Course of SARS-CoV-2 Infection in Elderly Patients. Clin Interv Aging, 15:1231-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, et al. (2017). A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care, 21:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Alexandre J, Cracowski JL, Richard V, Bouhanick B, Drugs C-wgotFSoPT (2020).Renin-angiotensin-aldosterone system and COVID-19 infection. Ann Endocrinol (Paris). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, et al. (2020). Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell, 181:905-913 e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. (2020). COVID-19 infection: the perspectives on immune responses. Cell Death Differ, 27:1451-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chen CF, Chien CH, Yang YP, Chou SJ, Wang ML, Huo TI, et al. (2020). Role of Dipeptidyl Peptidase 4 Inhibitors in Diabetic Patients with Coronavirus-19 Infection. J Chin Med Assoc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kawasaki T, Chen W, Htwe YM, Tatsumi K, Dudek SM (2018). DPP4 inhibition by sitagliptin attenuates LPS-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol, 315:L834-L845. [DOI] [PubMed] [Google Scholar]
- [127].Birnbaum Y, Bajaj M, Qian J, Ye Y (2016). Dipeptidyl peptidase-4 inhibition by Saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ Open Diabetes Res Care, 4:e000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Berger JP, SinhaRoy R, Pocai A, Kelly TM, Scapin G, Gao YD, et al. (2018). A comparative study of the binding properties, dipeptidyl peptidase-4 (DPP-4) inhibitory activity and glucose-lowering efficacy of the DPP-4 inhibitors alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin in mice. Endocrinol Diabetes Metab, 1:e00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Tang S, Ma W, Bai P (2017). A Novel Dynamic Model Describing the Spread of the MERS-CoV and the Expression of Dipeptidyl Peptidase 4. Comput Math Methods Med, 2017:5285810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Iacobellis G (2020). COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res Clin Pract, 162:108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. (2020). Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A, 117:10970-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Buonaguro FM, Puzanov I, Ascierto PA (2020).Anti-IL6R role in treatment of COVID-19-related ARDS. J Transl Med, 18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Morena V, Milazzo L, Oreni L, Bestetti G, Fossali T, Bassoli C, et al. (2020). Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O'Callaghan A, Pardos-Gea J, Quintana A, et al. (2020). Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun Rev, 102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Pfefferle S, Schopf J, Kogl M, Friedel CC, Muller MA, Carbajo-Lozoya J, et al. (2011). The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog, 7:e1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Vandewalle J, Luypaert A, De Bosscher K, Libert C (2018). Therapeutic Mechanisms of Glucocorticoids.Trends Endocrinol Metab, 29:42-54. [DOI] [PubMed] [Google Scholar]
- [137].Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF (1988). Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock.Am Rev Respir Dis, 138:62-68. [DOI] [PubMed] [Google Scholar]
- [138].Meduri GU, Schwingshackl A, Hermans G (2016). Prolonged Glucocorticoid Treatment in ARDS: Impact on Intensive Care Unit-Acquired Weakness. Front Pediatr, 4:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, et al. (2020). Early Short Course Corticosteroids in Hospitalized Patients with COVID-19. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Stockman LJ, Bellamy R, Garner P (2006). SARS: systematic review of treatment effects. PLoS Med, 3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zha L, Li S, Pan L, Tefsen B, Li Y, French N, et al. (2020). Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med J Aust, 212:416-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Klingemann H, Matzilevich D, Marchand J (2008).Mesenchymal Stem Cells - Sources and Clinical Applications. Transfus Med Hemother, 35:272-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Han KH, Ro H, Hong JH, Lee EM, Cho B, Yeom HJ, et al. (2011). Immunosuppressive mechanisms of embryonic stem cells and mesenchymal stem cells in alloimmune response. Transpl Immunol, 25:7-15. [DOI] [PubMed] [Google Scholar]
- [144].Zanoni M, Cortesi M, Zamagni A, Tesei A (2019). The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis.Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Takeda K, Ning F, Domenico J, Okamoto M, Ashino S, Kim SH, et al. (2018). Activation of p70S6 Kinase-1 in Mesenchymal Stem Cells Is Essential to Lung Tissue Repair. Stem Cells Transl Med, 7:551-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, et al. (2020). Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther, 11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, et al. (2020). Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering (Beijing). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. (2020). Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis, 11:216-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Rademacher S, Oppert M, Jorres A (2011).Artificial extracorporeal liver support therapy in patients with severe liver failure. Expert Rev Gastroenterol Hepatol, 5:591-599. [DOI] [PubMed] [Google Scholar]
- [150].Liu X, Zhang Y, Xu X, Du W, Su K, Zhu C, et al. (2015). Evaluation of plasma exchange and continuous veno-venous hemofiltration for the treatment of severe avian influenza A (H7N9): a cohort study. Ther Apher Dial, 19:178-184. [DOI] [PubMed] [Google Scholar]
- [151].Liu J, Dong YQ, Yin J, He G, Wu X, Li J, et al. (2020). Critically ill patients with COVID-19 with ECMO and artificial liver plasma exchange: A retrospective study. Medicine (Baltimore), 99:e21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhang Y, Yu L, Tang L, Zhu M, Jin Y, Wang Z, et al. (2020). A Promising Anti-Cytokine-Storm Targeted Therapy for COVID-19: The Artificial-Liver Blood-Purification System. Engineering (Beijing), in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Zabetakis I, Lordan R, Norton C, Tsoupras A (2020). COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Laviano A, Koverech A, Zanetti M (2020).Nutrition support in the time of SARS-CoV-2 (COVID-19). Nutrition, 74:110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Power SE, Jeffery IB, Ross RP, Stanton C, O'Toole PW, O'Connor EM, et al. (2014). Food and nutrient intake of Irish community-dwelling elderly subjects: who is at nutritional risk? J Nutr Health Aging, 18:561-572. [DOI] [PubMed] [Google Scholar]
- [156].Li T, Zhang Y, Gong C, Wang J, Liu B, Shi L, et al. (2020). Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Haase H, Rink L (2009).The immune system and the impact of zinc during aging. Immun Ageing, 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, et al. (2020). Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Monto AS, Rotthoff J, Teich E, Herlocher ML, Truscon R, Yen HL, et al. (2004). Detection and control of influenza outbreaks in well-vaccinated nursing home populations. Clin Infect Dis, 39:459-464. [DOI] [PubMed] [Google Scholar]
- [160].Ventura MT, Casciaro M, Gangemi S, Buquicchio R (2017). Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy, 15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Sheikh A, Sheikh A, Sheikh Z, Dhami S, Sridhar D (2020). What's the way out? Potential exit strategies from the COVID-19 lockdown. J Glob Health, 10:010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR, et al. (2020). COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin Infect Dis, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. (2020). Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Lloyd-Sherlock P, Ebrahim S, Geffen L, McKee M (2020).Bearing the brunt of covid-19: older people in low and middle income countries. BMJ, 368:m1052. [DOI] [PubMed] [Google Scholar]
- [165].Lloyd-Sherlock PG, Kalache A, McKee M, Derbyshire J, Geffen L, Casas FG (2020). WHO must prioritise the needs of older people in its response to the covid-19 pandemic. BMJ, 368:m1164. [DOI] [PubMed] [Google Scholar]


