Dihydroartemisinin-piperaquine (DHA-PQ) provides highly effective therapy and chemoprevention for malaria in pregnant African women. PQ concentrations of >10.3 ng/ml have been associated with reduced maternal parasitemia, placental malaria, and improved birth outcomes. We characterized the population pharmacokinetics (PK) of PQ in a post hoc analysis of human immunodeficiency virus (HIV)-infected and -uninfected pregnant women receiving DHA-PQ as chemoprevention every 4 or 8 weeks.
KEYWORDS: pharmacokinetics, dihydroartemisinin-piperaquine, malaria prevention, pregnancy, drug-drug interactions, population pharmacokinetics
ABSTRACT
Dihydroartemisinin-piperaquine (DHA-PQ) provides highly effective therapy and chemoprevention for malaria in pregnant African women. PQ concentrations of >10.3 ng/ml have been associated with reduced maternal parasitemia, placental malaria, and improved birth outcomes. We characterized the population pharmacokinetics (PK) of PQ in a post hoc analysis of human immunodeficiency virus (HIV)-infected and -uninfected pregnant women receiving DHA-PQ as chemoprevention every 4 or 8 weeks. The effects of covariates such as pregnancy, nutritional status (body mass index [BMI]), and efavirenz (EFV)-based antiretroviral therapy were investigated. PQ concentrations from two chemoprevention trials were pooled to create a population PK database from 274 women and 2,218 PK observations. A three-compartment model with an absorption lag best fit the data. Consistent with our prior intensive PK evaluation, pregnancy and EFV use resulted in a 72% and 61% increased PQ clearance, compared to postpartum and HIV-uninfected pregnant women, respectively. Low BMI at 28 weeks of gestation was associated with increased clearance (2% increase per unit decrease in BMI). Low-BMI women given DHA-PQ every 8 weeks had a higher prevalence of parasitemia, malaria infection, and placental malaria compared to women with higher BMIs. The reduced piperaquine exposure in women with low BMI as well as during EFV coadministration, compared to pregnant women with higher BMIs and not taking EFV, suggests that these populations could benefit from weekly instead of monthly dosing for prevention of malaria parasitemia. Simulations indicated that because of the BMI-clearance relationship, weight-based regimens would not improve protection compared to a 2,880 mg fixed-dose regimen when provided monthly. (The clinical trials described in this paper have been registered at ClinicalTrials.gov under identifiers NCT02163447 and NCT02282293.)
INTRODUCTION
An estimated 40 to 50 million African women are at risk of malaria infection during pregnancy each year (1, 2). Without intervention, up to 41% of all pregnant African women living in malaria regions of endemicity are estimated to have placental malaria (3). Malaria during pregnancy can lead to an array of adverse outcomes for both the mother and developing fetus and is estimated to cause 900,000 low birth weight deliveries and 19.7% of all stillbirths in Africa annually (4–6).This situation is further complicated by common comorbidities such as malnutrition and human immunodeficiency virus (HIV) infection. Malnutrition is reported in up to 20% of African women of reproductive age (7–9). When combined with malaria infection, maternal malnutrition leads to a 17.8% increased risk of a low birth weight delivery compared to HIV-uninfected women without malnutrition (10). HIV-infected pregnant women are also at an increased risk for both contracting malaria and for worse birth outcomes compared to HIV-uninfected pregnant women (11–13). Given the geographic overlap of HIV infection and malnutrition in malaria regions of endemicity, there is a large pregnant population with comorbidities at risk for malaria (10, 14, 15).
The World Health Organization (WHO) recommends the use of long-lasting insecticide-treated bed nets (LLIN) and intermittent preventive treatment with sulfadoxine-pyrimethamine (IPTp-SP) during pregnancy in malaria regions of endemicity of Africa (16, 17). However, concerns regarding the efficacy of these prevention measures have arisen as a result of increased resistance of anopheline mosquitoes to pyrethroid insecticides used in LLINs and of malaria parasites to SP (18–20). In addition, HIV-infected women taking trimethoprim-sulfamethoxazole (SXT) as part of their HIV care to prevent opportunistic infections are not advised to use SP, as this might lead to increased risk of severe cutaneous reactions (13). A promising alternative for IPTp-SP is the artemisinin-based combination therapy (ACT) dihydroartemisinin-piperaquine (DHA-PQ) (21).
DHA-PQ is an appealing option for IPTp, as the DHA component rapidly kills circulating parasites and PQ has a slow clearance rate, maintaining protective concentrations against subsequent infections for about a month (22, 23). Previous studies have shown DHA-PQ to be safe and as effective as IPTp in both HIV-infected and -uninfected pregnant women, significantly lowering the malaria burden compared to IPTp-SP (24–26).
Few prevention studies have included a pharmacokinetic (PK) component to define the PK of PQ during pregnancy (27–30). We previously demonstrated in a focused intensive PK analysis that both pregnancy and efavirenz (EFV)-based antiretroviral therapy (ART) independently reduced PQ exposure at 28 weeks gestation (31). In a group of HIV-uninfected women, we evaluated the pharmacodynamics (PD) for DHA-PQ used as IPTp and established that 10.3 ng/ml PQ was 95% protective against parasitemia during pregnancy when parasitemia was measured with a highly sensitive molecular assay (27, 32). Other studies, including PK assessments with IPTp, were small and recorded PQ PK after only a single course of study drug (28, 29). To gain more comprehensive insights into sources of variability and optimal IPTp dosing regimens for women receiving DHA-PQ, we pooled data from two large clinical trials to perform a post hoc analysis. We included HIV-infected and -uninfected pregnant Ugandan women throughout the second and third trimesters, as well as postpartum women. Our goal was to provide a comprehensive understanding of PQ PK in both HIV-infected and -uninfected women during pregnancy.
RESULTS
A total of 274 (191 HIV-uninfected and 83 HIV-infected) pregnant women contributed 797 intensive and 1,001 monthly plasma samples used to build the population PK model (Fig. 1). Twenty-eight HIV-uninfected women given DHA-PQ and two given SP during pregnancy were reenrolled a minimum of 34 weeks postpartum and contributed an additional 420 intensive samples (Fig. 2A and B). The demographic characteristics of these participants are detailed in Table 1 and Table S2 in the supplemental material. At enrollment, 34 women had a BMI of less than 18.5 kg/m2, and at 28 weeks gestation, 70 women had a BMI less than or equal to 20.5 kg/m2 (Table 1 and Fig. S2).
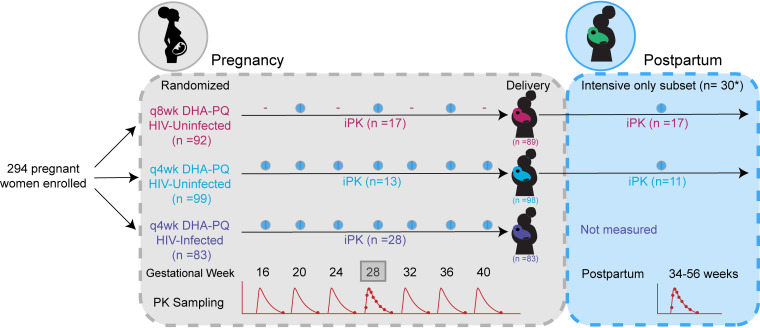
FIG 1.
Trial diagram. Women were enrolled at 16 to 28 weeks gestation. PK sampling began at 20 weeks gestation and continued until delivery. iPK (box) indicates intensive PK sampling at 28 weeks gestation. The asterisk indicates two of the women included in the postpartum sampling group received SP during pregnancy. The number of women enrolled and randomized reported here reflects only those who went on to initiate study drug. q4wk, doses given every 4 weeks; q8wk, doses given every 8 weeks.
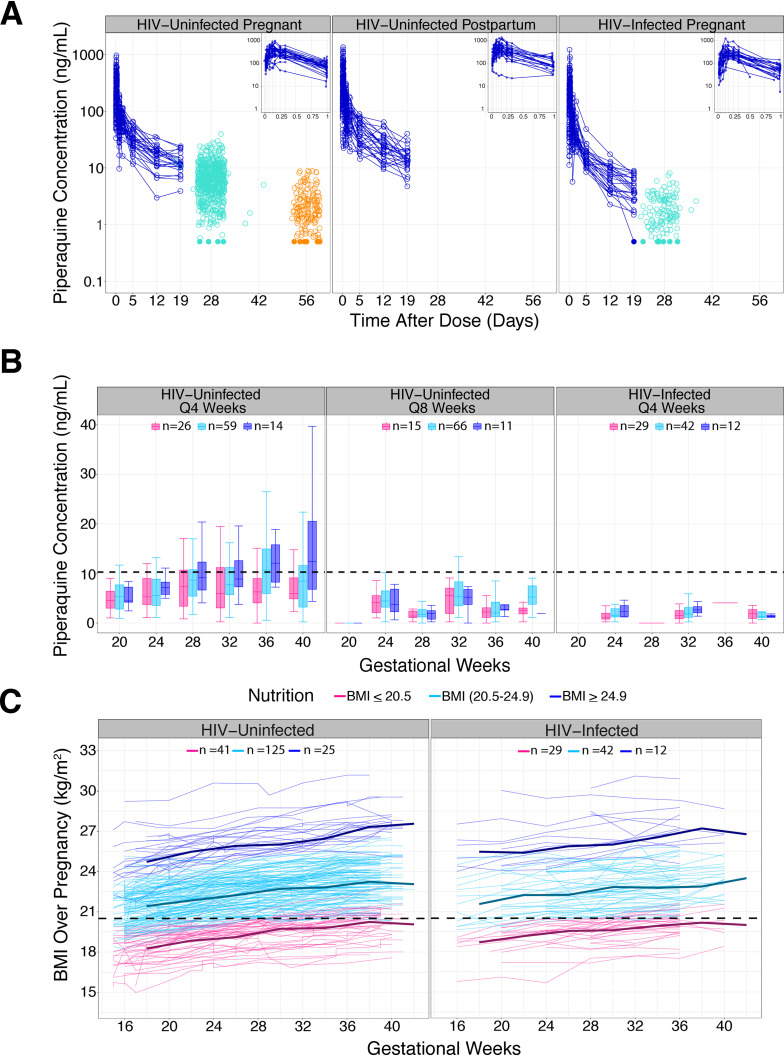
FIG 2.
Time profiles. (A) Piperaquine concentrations over time used to build the population PK model. The profiles in blue represent intensive PK sampling. Each line represents one individual. Monthly (28-day) concentrations are in green and 56-day trough concentrations are in orange. Insets in the upper right corner show the intensive PK profiles for the first day postdose. To avoid overlap of monthly points, random noise was added about the x axis to separate the data. (B) Piperaquine monthly concentrations stratified by treatment arm, HIV status, and BMI. Women were grouped based on gestational week-28 BMIs. The number of women in each group is displayed. The dashed line at 10.3 ng/ml marks the previously defined threshold for malaria protection in HIV-uninfected pregnant women. (C) BMI over time profile. Women were grouped based on week-28 BMIs. Each line represents one individual. The dashed line at 20.5 kg/m2 marks the plotting cutoff for defining a woman as malnourished during the third trimester (see the supplemental material).
TABLE 1.
Study participant characteristics
| Characteristice | HIV-uninfected pregnant |
HIV-infected pregnant |
HIV-uninfected postpartum |
|
|---|---|---|---|---|
| DHA-PQe every 8 wks (n = 92) | DHA-PQ every 4 wks (n = 99) | DHA-PQ every 4 wks (n = 83) | Single course DHA-PQ (n = 30)a ,e | |
| Age in yrs, (median [2.5–97.5% percentile]) | 21.5 (16.3–32.0) | 22.1 (17.1–32.0) | 30.3 (17.9–41.5) | 23.0 (19.6–29.9) |
| Intensive PK sample no.b | 237 | 182 | 378 | 420 |
| Monthly PK sample no. | 421 | 453 | 127 | NA |
| Gestational age (no. [%]) | ||||
| 16 wk | 63 (68.5) | 67 (67.7) | 19 (23.0) | NA |
| >16 to 20 wk | 29 (31.5) | 32 (32.3) | 25 (30.0) | NA |
| > 20 to 24 wk | 0 (0) | 0 (0) | 20 (24.0) | NA |
| >24 to 28 wk | 0 (0) | 0 (0) | 19 (23.0) | NA |
| Gravidity (no. [%]) | ||||
| 1 | 32 (34.8) | 36 (36.4) | 12 (14.5) | NA |
| 2 | 28 (30.4) | 28 (28.3) | 10 (12.0) | NA |
| ≥ 3 | 32 (34.8) | 35 (35.3) | 61 (73.5) | NA |
| Wt in kg (median [2.5–97.5% percentile]) | 56.4 (43.7–69.5) | 55.0 (46.3–70.8) | 56.2 (44.4–73.1) | 52.9 (41.1–66.8) |
| Ht in cm (median [2.5–97.5% percentile]) | 162 (150–177) | 162 (153–175) | 163 (150–173) | 162 (152–173) |
| BMI in kg/m2 (median [2.5–97.5% percentile]) | 21.1 (17.4–26.0) | 21.3 (17.0–26.6) | 21.4 (18.0–28.2) | 20.1 (16.1–23.5) |
| Low BMI at enrollment (no. [%])c | 11 (12.0) | 17 (17.0) | 6 (7.2) | 6 (20) |
| Low BMI at 28 wks gestation (no. [%])c | 15 (16.3) | 26 (26.3) | 29 (34.9) | NA |
| Wt gained in kg (median [2.5–97.5% percentile])d | 1.6 (−1.3–6.3) | 1.6 (−2.1–4.6) | 1.1 (−1.4–4.3) | NA |
Two of the women enrolled in the postpartum cohort received sulfadoxine-pyrimethamine in the parent trial.
30 HIV-uninfected women and 28 HIV-infected women contributed intensive PK samples.
Low BMI at enrollment was defined as a BMI of less than 18.5 kg/m2. At 28 weeks, low BMI was defined as a BMI of 20.5 kg/m2 or less to account for weight gained during pregnancy.
Weight gained was calculated from 28 gestational weeks through delivery in order to standardize the measurement.
DHA-PQ, dihydroartemisinin-piperaquine; PK, pharmacokinetic; BMI, body mass index; NA, not applicable.
A three-compartment disposition model with an absorption lag best fit the observed data (Fig. 3). Samples below the lower limit of quantification (LLOQ) made up only 4% (n = 94; 11 from HIV-infected women, 83 from HIV-uninfected women) of the data and were well captured when imputing the first sample to fall below the limit as half the LLOQ. Additionally, two samples with results that differed more than 10-fold from the patient’s previous and subsequent sample concentrations were deemed to be outliers and excluded from the analysis (see the supplemental material for more details). Residual error was well described by a combined error model. A linear relationship with a slope of 0.80 and an intercept estimated as 0.54 for HIV-uninfected women and intercept fixed to zero for HIV-infected women was used to describe the difference between venous and finger-stick PQ concentrations. Parameter estimates from the final model are listed in Table 2. Time profiles for intensive and monthly PQ concentrations are shown in Fig. 2A and B. Goodness of fit plots (Fig. S1) and visual predictive checks confirmed our model accurately fit and predicted the data in all populations (Fig. 4).
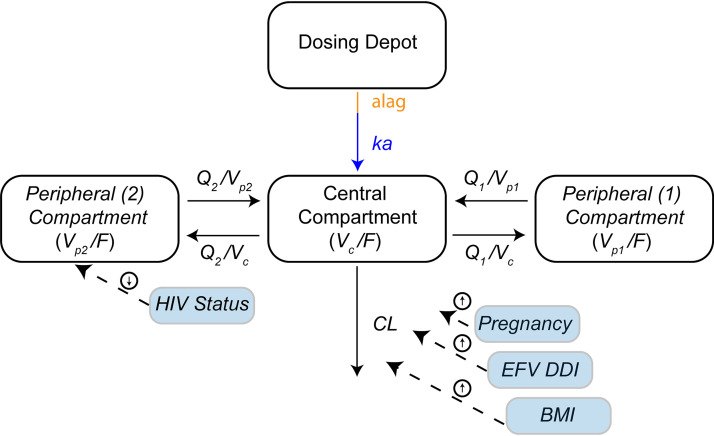
FIG 3.
Final piperaquine population pharmacokinetic model: a three-compartment model with an absorption lag. Four significant parameter-covariate relations were included in the final model. Covariates are shown in blue boxes, with dashed arrows indicating which parameter is influenced and the direction of the effect indicated by the arrows enclosed in circles. Clearance (CL) in this model is the oral clearance (CL/F).
TABLE 2.
Final pharmacokinetic parameter estimates for piperaquine
| PK parametera | Population estimate (bootstrap 95% CI)a | CV (%) of BSV (bootstrap 95% CI)a |
|---|---|---|
| CL/F HIV-uninfected (liter/day) | 3,126 (2,712–3,397) | 21.8 (22–70) |
| Ka (day−1) | 20 (3.0–34) | |
| Vc/F (liter) | 3,155 (369–3,659) | 34.7 (5.4–91) |
| Vp1/F (liter) | 4,449 (3,772–7,952) | 36.3 (3.2–24)b |
| VP2/F (liter) | 3,1820 (23,707–39,216) | 36.3 (3.2–24)b |
| Q/F (liter/day) | 3,428 (2,418–5,874) | |
| Q2/F (liter/day) | 1,664 (974–39,216) | |
| Absorption lag time (day) | 0.026 (0.016–0.035) | |
| Proportional error (%) | 42 (36–44) | |
| Additive error HIV-infected (ng/ml) | 0.0001 (NA)d | |
| Additive error HIV-uninfected (ng/ml) | 0.29 (0.085–0.41) | |
| Intercept of venous/capillary ratio HIV-uninfected | 0.54 (0.075–1.4) | |
| Intercept of venous/capillary ratio HIV-infected | 0 (NA)d | |
| Ratio, venous/capillary | 0.80 (0.73–0.93) | |
| F (%) | 1 (NA)d | 34.7 (0.67–17) |
| θHIV; CL/F = (1+ θHIV) | 0.61 (0.48–0.85) | |
| θpostpartum; CL/F = (1+ θpostpartum) | −0.42 (−0.54 to −0.31) | |
| θBMI; CL/F = (1+ θBMI)b (θBMI-22.3)c | −0.020 (−0.032 to −0.0044) | |
| θHIV-infected; VP2/F = (1 + θHIV-infected)e | −0.49 (−0.59 to −0.33) |
CV, coefficient of variation; BSV, between subject variability; CI, confidence interval; CL, clearance; Ka, absorption rate constant; Vc, volume of the central compartment; Vp1, volume of the first peripheral compartment; Vp2, volume of the second peripheral compartment; F, bioavailability; Q and Q2, intercompartmental clearance.
The same term for variability was used for both peripheral volume compartments.
BMI effect on CL is the BMI at 28 weeks gestation.
Indicates term was fixed to reported value.
All HIV-infected women received efavirenz-based antiretroviral therapy. The HIV effect is thought to be a drug-drug interaction due to efavirenz.
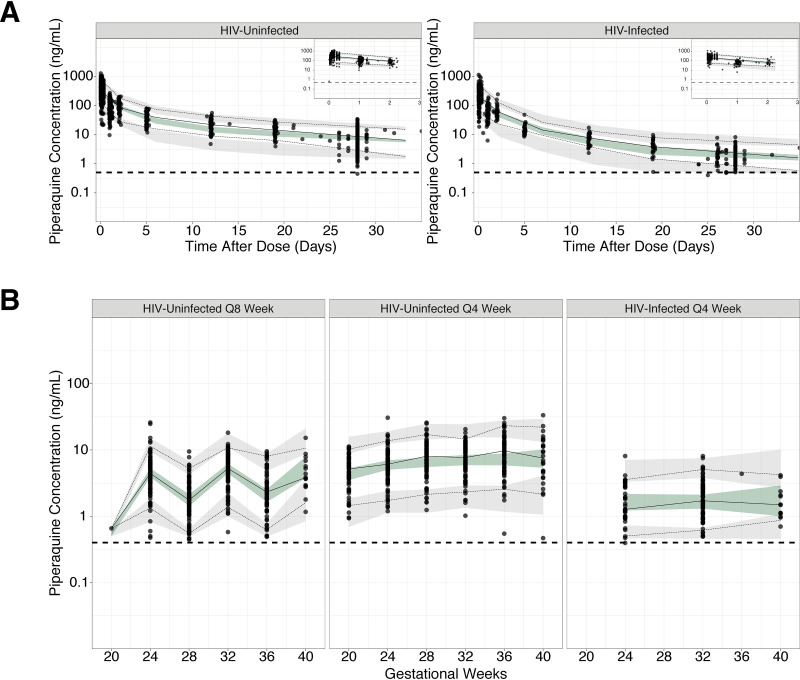
FIG 4.
Prediction-corrected visual predictive check of the final pharmacokinetic model. (A) Intensive profiles at 28 weeks gestation. Plot of intensive data, including day-28 levels stratified based on HIV status. Insets in the upper right corner show the intensive profiles for 3 days postdose. (B) Monthly concentrations plotted over pregnancy. The observed data for each subject are plotted as black circles. The solid and dashed lines are the observed median and 5th and 95th percentiles of the observed data. The shaded areas represent the 95% confidence intervals of the model simulated data. Q8, DHA-PQ dosing given every 8 weeks; Q4, DHA-PQ dosing given every 4 weeks.
Pregnancy, BMI, and EFV use in HIV-infected women were all found to independently increase PQ clearance (Table 2). Pregnancy was treated as a dichotomous variable and was found to increase PQ clearance by 72% compared to postpartum controls. Trimester was also explored and included during the forward covariate selection, indicating that PQ clearance increased during the third trimester, but this relationship was dropped during backward elimination (see the Materials and Methods). BMI at 28 weeks gestation was treated as a continuous variable and influenced PQ clearance in a linear fashion; low BMI pregnant women had higher elimination rates (2% increase for every unit drop in BMI) (Fig. S2). Similarly, HIV-infected women taking EFV had clearance increased by 61% in comparison to HIV-uninfected pregnant women. HIV-infected women also had a 51% smaller volume for the second peripheral compartment in comparison to HIV-uninfected women. No other covariate effects were identified.
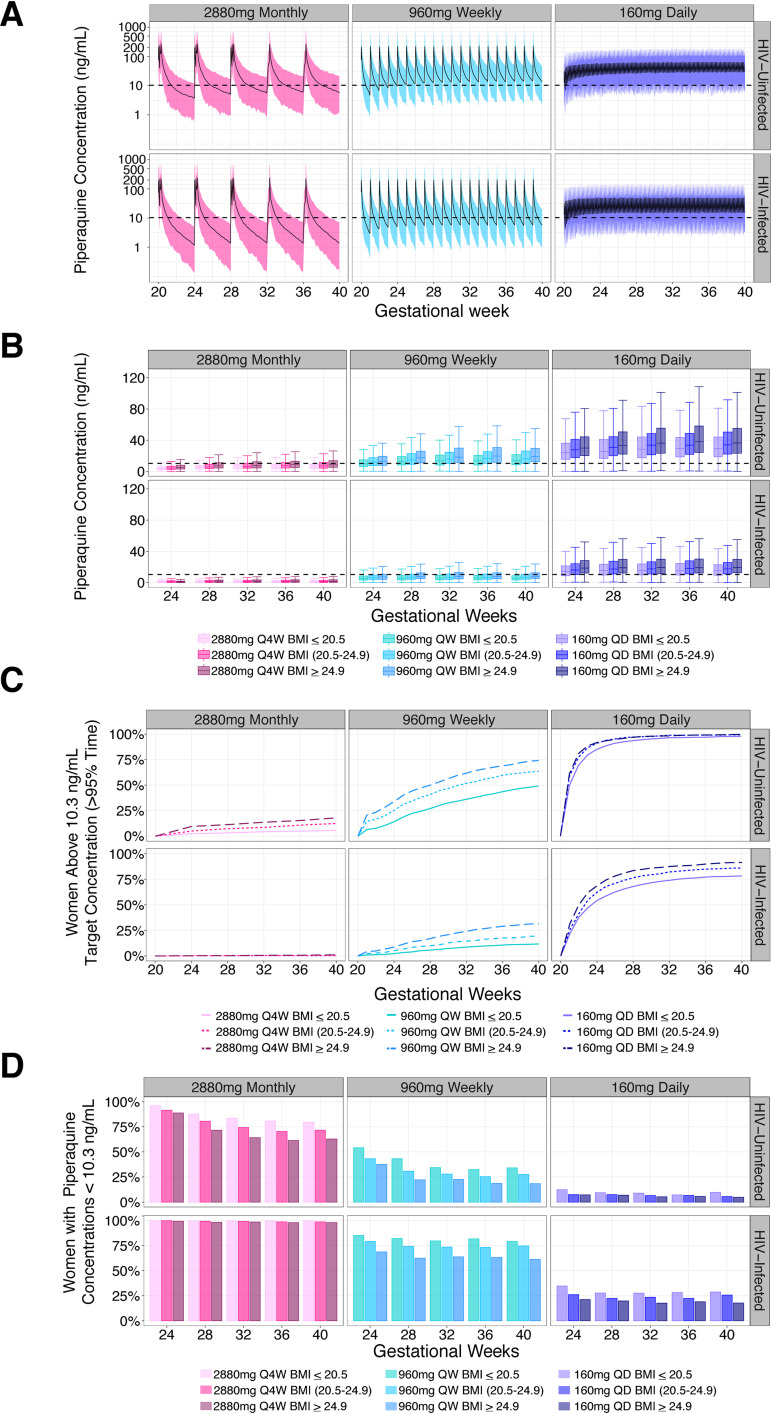
Simulations were performed to investigate whether alternative IPTp DHA-PQ regimens adjusting for dosage and frequency would provide higher PQ exposure and therefore improved protection against parasitemia (Fig. 5A to D and Fig. S5A to C). Monthly dosing of 2,880 mg (3 tabs × 3 days), regardless of HIV or nutritional status, provided inadequate protection against parasitemia throughout the second and third trimesters, as less than 20% of women stayed above the protective concentration (Fig. 5C and D). Weekly dosing of 960 mg (3 tabs × 1 day) resulted in protection for >45% (49 to 74.2%) and >10% (11.7 to 1.6%) of HIV-uninfected and -infected women, respectively. A daily dose of 160 mg (1 tab) provided the best protection for all women, with 75% protection reached before 24 and 32 weeks gestation in HIV-uninfected and -infected women, respectively. No regimen was predicted to result in QTc prolongation greater than 30 msec (Table S1). Monthly dosing resulted in the greatest prolongation and daily dosing resulted in the least, with HIV-infected women showing greater prolongation across all regimens compared to HIV-uninfected women. Low BMI and HIV-infected women consistently had the lowest protection regardless of dosing regimen and benefitted the most from increasing dosing frequency.
FIG 5.
Alternative IPTp regimen simulations. (A) Full PK profiles. Simulated PQ concentrations over pregnancy stratified based on HIV status for three different dosing regimens. The dashed line at 10.3 ng/ml marks the previously defined threshold for malaria protection in HIV-uninfected pregnant women. (B) Day-28 concentrations. Simulated PQ day-28 concentrations over pregnancy stratified based on HIV status and week-28 BMI. The dashed line at 10.3 ng/ml marks the previously defined threshold for malaria protection in pregnant women. (C) Percentage of women protected. Percentage of women achieving protection based on HIV status and week-28 BMI for different prevention regimens over pregnancy. Protection was defined as sustaining a PQ concentration of 10.3 ng/ml or greater for 95% of their pregnancy. (D) Percentage of women with day-28 concentrations below 10.3 ng/ml. Based on simulated PQ concentrations, the percentage of women not protected at the end of the month is stratified based on HIV status and week-28 BMI. Q4W, doses given every 4 weeks; QW, doses given every week; QD, doses given daily.
The prevalence of malaria parasitemia, placental malaria, and number of women with ≥1 episode of symptomatic malaria is reported in Table 3. HIV-uninfected women with low BMI who received DHA-PQ every 8 weeks had a higher percentage of outcomes for all measures (22.1% parasitemia; 40% placental malaria; 26.7% symptomatic malaria) compared to women in the highest BMI group receiving 8-week dosing (17% parasitemia; 20% placental malaria; 10% symptomatic malaria), as well as those in the highest BMI group receiving monthly dosing (6% parasitemia; 28.6% placental malaria; 0% symptomatic malaria). No difference in outcomes was detected between BMI groups in the monthly dosing group. HIV-infected women receiving concomitant indoor residual spraying of insecticides (IRS) had the lowest number of outcomes among the dosing groups.
TABLE 3.
Malaria and parasitological outcomes in HIV-infected and -uninfected pregnant women
| Malaria and parasitological outcomec | HIV-uninfected pregnant (no. of positive samples/total samples [%]) |
HIV-infected pregnantb
(no. of positive samples/total samples [%]) |
|
|---|---|---|---|
| DHA-PQ every 8 wks (n = 88)a ,c | DHA-PQ every 4 wks (n = 96)a | DHA-PQ every 4 wks (n = 81)a | |
| Parasite prevalence by monthly LAMP detection | |||
| BMI at 28 wks gestation <20.5 kg/m2 | 17/77 (22.1) | 6/129 (4.7) | 0/113 (0) |
| BMI at 28 wks gestation >20.5 and <24.9 kg/m2 | 49/321 (15.3) | 16/300 (5.3) | 5/227 (2.2) |
| BMI at 28 wks gestation >24.9 kg/m2 | 8/47 (17.0) | 4/67 (6.0) | 0/59 (0) |
| Prevalence of placental malaria by histopathological assessment | |||
| BMI at 28 wks gestation <20.5 kg/m2 | 6/15 (40.0) | 4/25 (16) | 3/28 (10.7) |
| BMI at 28 wks gestation >20.5 and <24.9 kg/m2 | 22/63 (35.0) | 18/57 (31.6) | 2/41 (4.9) |
| BMI at 28 wks gestation >24.9 kg/m2 | 2/10 (20.0) | 4/14 (28.6) | 0/12 (0) |
| Women with at least one episode of malaria on chemoprevention | |||
| BMI at 28 wks gestation <20.5 kg/m2 | 4/15 (26.7) | 0/25 (0) | 0/28 (0) |
| BMI at 28 wks gestation >20.5 and <24.9 kg/m2 | 7/63 (11.1) | 0/57 (0) | 0/41 (0) |
| BMI at 28 wks gestation >24.9 kg/m2 | 1/10 (10.0) | 0/14 (0) | 0/12 (0) |
Two HIV-infected women, one woman in the every 8 week and 2 women in the every 4 week DHA-PQ arm were excluded from this analysis because they did not have a placental sample collected for histopathological assessment.
These women also received indoor residual spraying of insecticides.
DHA-PQ; dihydroartemisinin-piperaquine; LAMP; loop-mediated isothermal amplification; BMI, body mass index.
DISCUSSION
We evaluated the population PK of PQ in a cohort of 274 pregnant women receiving DHA-PQ for malaria prevention. We employed a population approach to identify and quantify the effects of important covariates which might affect drug exposure in a post hoc analysis. A three-compartment model with an absorption lag time best described our data, using pregnancy, 28-week gestational BMI, and EFV use as significant covariates. Pregnancy increased clearance by 72% compared to postpartum controls. Interestingly, we identified a trend in which for every 1 unit decrease in 28 -week gestational BMI, there was a 2% increase in clearance, revealing that low-BMI pregnant women have lower PQ exposures. HIV-infected women who were receiving EFV based-ART had a 61% increased clearance and a 51% smaller volume for the second peripheral compartment compared to HIV-uninfected pregnant women. Simulations suggested that increasing PQ dosing frequency may improve efficacy, with low daily dosing of DHA-PQ resulting in the highest number of women maintaining protective concentrations. Furthermore, due to the association between low BMI with higher clearance, weight-based dosing was associated with an increased disparity between PQ levels (Fig. S4). These findings suggest that weight-based dosing for pregnant women may not be needed, as heavier women are able to achieve adequate exposure when given fixed-dose (non-weight-based) regimens. Given the pragmatic benefits of fixed-dose regimens, we recommend this option. By building a PK model which simultaneously fits three different populations, we have created a novel integrated model which is also a tool that others can use when designing future clinical trials and evaluating PQ levels.
Piperaquine metabolism is primarily hepatic and mediated by cytochrome P450 (CYP) 3A4 enzymes (33). The physiological changes that occur during pregnancy are known to alter CYP activity, including that of CYP3A4, likely leading to the increased clearance compared to nonpregnant adults that was noted in this study (34, 35). By including longitudinal samples throughout the second and third trimester, our model was able to confirm our previous findings (from just the intensive cohort) that pregnancy increases PQ clearance. Additionally, we explored whether this pregnancy effect changed over trimesters, as some studies have shown the effect of pregnancy is greater or is only clinically relevant during the third trimester (36–38). Our analysis indicates that pregnancy’s effect is consistent over the second and third trimesters (31). Previous trials investigating DHA-PQ for malaria treatment and prevention in Thai and Papua New Guinean women found similar increases of 42 to 45% in PQ clearance compared to nonpregnant control women (28, 39). In contrast, a treatment trial in Sudanese women did not find a significant pregnancy effect, possibly due to a small trial size, although we cannot exclude impacts of ethnicity or genetics on PQ PK (40).
An inverse trend was identified between PQ clearance and maternal 28-week gestational BMI in which low-BMI women displayed increased clearance after controlling for the effects of HIV and pregnancy (Fig. S2). When comparing the clearance values for women with the lowest and highest recorded BMIs at 28 weeks gestation (17.1 and 30.5 kg/m2, respectively), low-BMI women had a 24.1% higher clearance. We predict that following fixed monthly dosing of 2,880 mg PQ, women with a 28-week BMI of ≤20.5 kg/m2 will have 3- and 6-fold less time above protective concentrations compared to HIV-uninfected and -infected women with a BMI of ≥25 kg/m2, respectively (Fig. 5C). Our findings suggest the use of weight-bands for PQ dosing, as per current World Health Organization guidelines, may not provide the intended benefit over fixed-dose regimens (21). For example, when using the current weight-based treatment guidelines, women with a BMI of ≥25 kg/m2 (average weight: 70.2 kg) will receive higher (160/1,280 mg DHA/PQ total dose) DHA-PQ doses compared to women with a BMI of ≤20.5 kg/m2 at 28 weeks (average weight: 52 kg; 120/960 mg DHA/PQ total dose) resulting in 4- and 8-fold less time protected for HIV-uninfected and -infected women of lower BMIs, respectively (Fig. S4). Our findings indicate that malnourished HIV-infected pregnant women are consistently the least protected population. This finding is concerning, as previous studies have reported up to 14.6% of HIV-infected pregnant women lose weight during pregnancy and no study to date has directly investigated DHA-PQ dosing in this population (15).
Due to lower than expected parasitological outcomes observed in these studies as a result of concurrent IRS, we were not able to investigate associations between PK covariates and malaria outcomes. However, we observed in the raw data that low-BMI women who received DHA-PQ every 8 weeks had the highest prevalence of parasitemia, the highest percentage of women with a malaria infection, and the highest prevalence of placental malaria (Table 3) compared to women with higher BMIs and those given DHA-PQ monthly. HIV-uninfected women given DHA-PQ monthly had fewer outcomes, likely indicating the benefit of more frequent dosing. It is likely that malnourished HIV-infected women would have even higher outcome rates; however, these women were also protected by IRS. Given IRS’s efficacy, only 5 women had parasitemia and placental malaria detected.
Previous studies have reported increased phenylbutazone clearance and shorter antipyrine half-life in malnourished men compared to well-nourished men (41, 42). In a similar cohort of HIV-infected Ugandan pregnant women, food insecurity was found to significantly reduce the bioavailability of different ART combinations compared to healthy nourished controls (43). While BMI did not appear to have any significant effects on bioavailability in our model, it is possible that decreased protein binding or an array of other physiological changes induced by malnutrition led to the increased clearance of PQ (42, 44–46). Multiple studies have shown malnutrition alone or in combination with other diseases such as HIV and malaria is associated with adverse birth outcomes (10, 15). Given that malnutrition is a modifiable, albeit difficult, risk factor, prevention regimens which include nutritional supplementation could potentially lead to improved maternal and birth outcomes and warrant investigation. However, by increasing the dosing frequency, optimal chemoprevention can readily be achieved in this special population.
It is possible that this inverse BMI-clearance relationship is a result of physiological changes due to maternal malnutrition, given that recent studies report inadequate weight gain in up to 62% of Ugandan women during pregnancy (15, 47). Indeed, in the present study, 21 women (16 HIV-infected; 5 HIV-uninfected) lost weight during pregnancy. We have investigated both weight and BMI as potential covariates for clearance as often the two are correlated (in our case r2 = 0.65). We found that BMI was superior and the only significant predictor of clearance (P = 0.027 versus P = 0.32). Furthermore, it is difficult to classify a pregnant woman as malnourished, given that no guidelines using weight-based measures exist. Instead, measures of maternal nutrition are defined by weight gained during pregnancy, and only criteria regarding prepregnancy BMI are used to classify a woman as malnourished (48). While weight gained during pregnancy was tested as a covariate, this measure was highly variable, and was potentially confounded by gains due to the growing fetus, possibly explaining a lack of relationship. Additionally, prepregnancy BMI and weight gained during pregnancy are not clinically useful measures for determining dosage guidelines, as many women do not know their prepregnancy BMI, and weight gained during pregnancy can only be determined retrospectively. In contrast, BMI at 28 weeks of gestation could be used clinically to guide dosing recommendations.
The HIV-infected women in this trial received concomitant EFV-based ART; EFV is a known CYP3A4 inducer (49, 50). After controlling for the effect of pregnancy, there was an additional 61% increase in PQ clearance in women receiving EFV, which we attributed to EFV-mediated induction of CYP3A4. This extends our previous work by confirming the effects of EFV and indicating this effect lasts throughout the second and third trimesters (31). In the only other study that investigated administration of EFV and PQ in HIV-positive nonpregnant adults, the PQ area under the curve from 0 to 28 days (AUC0–28days) was 43% lower than that in patients not receiving EFV, in agreement with our findings (51). Our model identified a difference in peripheral volume, whereby HIV-infected pregnant women had a 51% reduction in the second peripheral compartment. This finding is likely an artifact due to differences in terminal PK sampling between trials, where some HIV-uninfected women had PK samples obtained up to 56 days postdose. There is evidence to suggest that if sampling does not sufficiently capture the elimination phase for drugs with long terminal half-lives, such as PQ, the true terminal phase will not be not defined, and models will under predict the volume and/or compartment number (52).
Pregnancy, low BMI, and EFV use all decreased PQ exposure, potentially reducing the efficacy of DHA-PQ for IPTp. Given that both pregnant women and HIV-infected individuals are at an increased risk for contracting malaria, it is essential to optimize prevention measures to protect these high risk groups (11, 12). When administered monthly, less than 20% of HIV-uninfected women and less than 2% of HIV-infected women were predicted to maintain PQ exposure above the protective level (Fig. 5 and Fig. S5). Regardless of HIV status, malnourished women had the smallest amount of time above the protective concentration. Simulations showed that increasing the frequency of dosing improved protection, with low daily dosing achieving the best protective coverage of >75% of both HIV-infected and malnourished women protected. Animal toxicity studies have documented that prolonged exposure to artemisinins can cause neurological and auditory toxicity (53–55). Unfortunately, limited clinical data exist (56, 57). Clinical trials which explore more frequent dosing, including daily, will need to include neurological and auditory toxicity assessments to ensure these regimens are, in fact, safe. Regimens with more frequent lower doses showed less QTc prolongation, indicating they are less cardiotoxic (Table S1).
As alternative dosing regimens are explored, clinical trials which employ fixed dosing should be conducted. The trials which provided data for this analysis used a fixed dose of 3 tablets per dosing day. The results indicate that heavier women did not disproportionately contract malaria or parasitemia and therefore may not need a higher dose (Table 3). Instead, low-BMI women given DHA-PQ every 8 weeks had the highest prevalence of parasitological outcomes compared to all other groups. Additionally, fixed dosing is more pragmatic, especially in resource-limited settings.
This study had some limitations. Information regarding prepregnancy weight, mid-upper-arm circumference, plasma protein, free drug, and nutrient levels was unavailable. To decrease bias associated with variable enrollment times, we used weight and BMI measures at 28 weeks gestation as a baseline measurement. It is possible that by using BMI at 28 weeks gestation we underestimated the effects of malnutrition on clearance. The studies that enrolled our subjects did not record food intake; thus, we cannot account for food effects on drug absorption. Further, only the first of three daily DHA-PQ doses each month was directly observed, and so limited adherence to prevention may have affected results. Lastly, the parasite prevalence and malaria outcomes among these women were low (Table 3) due to DHA-PQ’s efficacy and the effects of other prevention measures such as IRS. As a result, we were unable to fully explore the effects of malnutrition as well as HIV/EFV use on parasitological outcomes. Given that we could not establish a PK/PD relationship for these two groups, the protective concentration based on HIV-uninfected women was used instead.
DHA-PQ is a safe and effective regimen which shows promise as an alternative for IPTp-SP. In order to best protect all women from malaria and parasitemia, it is important to carefully consider dosing strategies in vulnerable populations. Our findings indicate that pregnant women, especially those who are low BMI and/or receiving concomitant CYP3A4 inducers such as EFV, have reduced PQ exposure, increasing their risk for malaria. It is these malnourished/HIV-infected pregnant women who may benefit from weekly or low daily dosing using fixed-dose regimens. Trials exploring alternative DHA-PQ regimens in high-risk populations, such as malnourished women, are needed to confirm our recommendations for IPTp.
MATERIALS AND METHODS
Study population.
Data were pooled from two clinical trials conducted in Tororo, Uganda between December 2014 and March 2016 investigating the efficacy of DHA-PQ given as an IPTp (25, 26). For the first parent study, HIV-uninfected pregnant women were randomized to receive either standard treatment doses of SP given every 8 weeks or DHA-PQ given every 4 or every 8 weeks during the 2nd and 3rd trimesters of pregnancy (note that “pregnancy” in this report refers to the second and third trimesters). Additionally, a subset of the HIV-uninfected pregnant women underwent intensive PK sampling postpartum, providing nonpregnant control samples. In the second parent study, HIV-infected pregnant women receiving EFV-based ART were randomized to receive either monthly DHA-PQ in combination with daily SXT (standard of care for HIV-infected populations to prevent opportunistic infections) or SXT alone. Eligible participants were pregnant women between 12 and 28 weeks gestation confirmed by ultrasound, ≥16 years of age, living within 30 km of the study clinic, and having known HIV status. Only women randomized to DHA-PQ were included in our PK analyses.
Written informed consent was obtained from all study participants. Study protocols were approved by the ethics committees at Makerere University, the Ugandan National Council of Science and Technology, and the University of California, San Francisco. The clinical trial registration numbers are NCT02163447 and NCT02282293.
Study design.
At enrollment, each subject was given a long-lasting insecticide-treated net and underwent a routine medical examination, including height and weight measurements and a blood smear to detect parasitemia. Women received all their medical care at the study clinic and were encouraged to come to the clinic any time they felt ill. Routine visits occurred every month, at which placebo or study drug was administered and finger-stick or venous blood was taken for blood, a PK sample collection, and detection of submicroscopic parasitemia by loop-mediated isothermal amplification (LAMP). Symptomatic malaria was diagnosed when a woman presented to the clinic with a fever or history of fever (tympanic temperature ≥38°C) and a positive blood smear. At delivery, presence of placental malaria was detected by histopathology (32, 58).
Women randomized to DHA-PQ every 8 weeks received the study drug at 20, 28, and 36 weeks gestation, while those randomized to DHA-PQ every 4 weeks received the study drug beginning at enrollment (16 to 28 weeks gestation) (Fig. 1). A standard dose of 3 tablets (40 mg DHA/320 mg PQ; Duo-Cotecxin, Holley-Cotec) was given once a day for 3 consecutive days, with the first dose observed in the clinic and the remaining two taken at home. A subset of 30 HIV-uninfected women (28 enrolled from the DHA-PQ arms, 2 enrolled from the SP arm) were reenrolled at 34 to 54 weeks postpartum to provide nonpregnant control data. Twenty seven of these 30 women were those who contributed intensive sampling at 28 weeks of gestation. HIV-infected women received efavirenz/tenofovir/lamivudine, which was initiated at least 4 weeks prior to PK sampling; they were instructed to take it every morning.
Estimation of nutritional status.
The weight of each woman was recorded at monthly visits during pregnancy and postpartum (for those providing control data). Weight was used to calculate body mass index (BMI), the rate of weight gain during pregnancy, as well as to group women into weight and BMI tertiles. For plotting purposes only, a week-28 BMI of 20.5 kg/m2 or less was used to classify pregnant women as malnourished. This value was derived using the enrollment weight from a woman in our trial with a BMI of 18.3 kg/m2 (a value considered to define a woman as malnourished prepregnancy) and weight gain guidelines during the second and third trimesters from the Institute of Medicine (see the supplemental material for further explanation of this calculation) (48). This threshold was used as there are no weight-based guidelines for nutritional status during pregnancy.
Initially, BMI at enrollment was identified as a significant covariate on PQ clearance in the covariate search. However, women were enrolled at various points throughout the second trimester. Women who were enrolled later would have had more time over which to gain weight, potentially biasing the results. In order to standardize this measure, BMI as a continuous variable at 28 weeks gestation (the earliest time point at which all women were enrolled) was tested and found to be significant.
Pharmacokinetic sample collection and analysis.
All 191 HIV-uninfected women provided monthly samples. Venous samples were collected at 20, 28, and 36 weeks and finger-stick samples at 24, 32, and 40 gestational weeks. A subset of 30 women (n = 17 every 8 weeks; n = 13 every 4 weeks) were enrolled in an intensive PK substudy: between 27 and 28 weeks gestation, these women had venous plasma samples taken before and after their last dose at times predose, 0.5, 1, 2, 3, 4, 6, 8, and 24 h post-last-dose. Finger-stick samples were collected at 24 h post-last-dose, and days 4, 7, 14, and 21 postdose. This intensive sampling schedule was also followed during the postpartum visit. Venous and finger-stick samples (24 h time point) were collected simultaneously in order to establish a relationship between these two sample types, allowing for simultaneous fitting of all data. Identical intensive sampling procedures were followed for 28 HIV-infected women. Monthly samples were quantified in a convenience sample of 83 HIV-infected women.
Plasma PQ concentrations were determined using high-performance liquid chromatography tandem mass spectrometry, as previously described (31, 59). Two different methods were used and the calibration ranges were 10 to 1,000 ng/ml and 0.5 to 50 ng/ml, with 0.5 ng/ml as the lower limit of quantification (LLOQ). The inter- and intrarun coefficient of variation (CV) was below 10% for all quality control samples for both assays.
Population modeling.
Piperaquine PK data were analyzed using nonlinear mixed-effects modeling in the software NONMEM VII (Icon Development Solutions, Ellicott City, MD). All parameters were estimated using the first order conditional estimation with interaction algorithm. Both exclusion and inclusion of samples below the LLOQ were tested (60). One-, two-, and three-compartment models with first order absorption were explored. An absorption lag time and transit compartments were also tested. Venous and finger-stick samples were modeled simultaneously using a linear relationship to describe any concentration differences. Between-subject variability was evaluated on structural model parameters assuming a log-normal distribution. A combined error model with both additive and proportional terms was used to describe the residual unexplained variability.
A stepwise covariate (SCM) search was performed to identify characteristics that influenced PQ PK. Characteristics tested were pregnancy status, gravidity, gestational weeks, trimester, weight, weight tertile, weight gained, rate of weight gain during pregnancy, BMI, BMI tertile, age, HIV status, and treatment arm. Gravidity, weight gained, age, HIV status, and treatment arm were treated as time independent. All other characteristics were tested as time-dependent variables and as time independent using the respective enrollment values. Linear and nonlinear relationships between parameters and covariates were investigated, including allometric scaling. Covariate-parameter relationships were sequentially tested with a significance cutoff of P < 0.05 for forward inclusion, followed by backward elimination with a cutoff of P < 0.01, in order to account for multiple hypothesis testing.
Model development and selection was guided by goodness of fit plots, the objective function value, parameter estimates, and their respective relative standard error values. Simulation-based diagnostics such as visual predictive checks (n = 500) and a nonparametric bootstrap (n =1000) were also performed to determine the model’s predictive power and the precision of parameter estimates.
Optimal dosing assessment.
The final PK model was used to perform simulations, adjusting for the dose frequency and amount. Monthly (2,880 mg once per month), weekly (960 mg every 7 days), and low daily (160 mg) doses were evaluated. PQ PK was simulated over 1,000 times for pregnant HIV-uninfected and -infected women with week-28 BMIs ranging from 16 to 27 kg/m2. Weight-based dosing simulations were performed by simulating the 274 women from our database over 50 times. To assess the relationship between PK and PQ’s known QTc prolongation, we utilized two previously developed PK-QTc models (one for HIV-uninfected and one for HIV-infected women) which described the linear relationship between PQ concentration and change in QT interval (27, 30). The maximum PQ concentrations predicted from each regimen were input into the QTc models to assess if any clinically significant prolongation (>60 msec) was predicted to occur.
Each dosing schedule was evaluated based on the number of women who maintained 10.3 ng/ml PQ, how quickly this threshold was achieved, and if the maximum concentrations were predicted to result in QT prolongation greater than 60 msec. Adequate protection for this analysis was considered as maintaining 10.3 ng/ml PQ for 95% of the time on prevention. These criteria were based upon a prior study that concluded that maintaining 10.3 ng/ml PQ provided 95% protection against parasitemia in HIV-uninfected pregnant women, and the FDA's safety guidelines regarding QT prolongation (27, 61).
Data availability.
The data used in this study come from two clinical trials conducted in Tororo, Uganda (25, 26). Both the raw data and NONMEM formatted data are available upon request. Please contact Grant Dorsey (grant.dorsey@ucsf.edu) to obtain the raw data and Rada Savic for the NONMEM formatted set and/or model code (rada.savic@ucsf.edu).
Supplementary Material
ACKNOWLEDGMENTS
The investigators thank Ali Mohamed, Maria Garcia-Cremades, and the rest of the Savic lab for their insight and analysis suggestions. Additionally, we thank Florence Marzan and David Gingrich of the Aweeka laboratory for their excellent analytical work providing drug levels and the staff of the Infectious Disease Research Collaboration, including Catherine Tugaineyo and Bridget Nzarubara. Last, we thank the women in Tororo who participated in all study procedures.
This work was funded by the National Institutes of Health (grant numbers R01 AI117001-02 [to P.J.R. and F.A.] and P01 HD059454 [to G.D.]).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. 2010. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 7:e1000221. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2019. World Malaria Report 2019. World Health Organization, Geneva, Switzerland: https://www.who.int/publications/i/item/9789241565721. [Google Scholar]
- 3.Walker PG, ter Kuile FO, Garske T, Menendez C, Ghani AC. 2014. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health 2:e460–e467. doi: 10.1016/S2214-109X(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 4.Akinyemi RO, Izzeldin IM, Dotchin C, Gray WK, Adeniji O, Seidi OA, Mwakisambwe JJ, Mhina CJ, Mutesi F, Msechu HZ, Mteta KA, Ahmed MA, Hamid SH, Abuelgasim NA, Mohamed SA, Mohamed AY, Adesina F, Hamzat M, Olunuga T, Maro VP, Walker R. 2014. Contribution of noncommunicable diseases to medical admissions of elderly adults in Africa: a prospective, cross-sectional study in Nigeria, Sudan, and Tanzania. J Am Geriatr Soc 62:1460–1466. doi: 10.1111/jgs.12940. [DOI] [PubMed] [Google Scholar]
- 5.Moore KA, Fowkes FJI, Wiladphaingern J, Wai NS, Paw MK, Pimanpanarak M, Carrara VI, Raksuansak J, Simpson JA, White NJ, Nosten F, McGready R. 2017. Mediation of the effect of malaria in pregnancy on stillbirth and neonatal death in an area of low transmission: observational data analysis. BMC Med 15:98. doi: 10.1186/s12916-017-0863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KA, Simpson JA, Scoullar MJL, McGready R, Fowkes FJI. 2017. Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. Lancet Glob Health 5:e1101–e1112. doi: 10.1016/S2214-109X(17)30340-6. [DOI] [PubMed] [Google Scholar]
- 7.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R, Maternal and Child Nutrition Study Group. 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 8.Ververs MT, Antierens A, Sackl A, Staderini N, Captier V. 2013. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr 5. doi: 10.1371/currents.dis.54a8b618c1bc031ea140e3f2934599c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lartey A. 2008. Maternal and child nutrition in Sub-Saharan Africa: challenges and interventions. Proc Nutr Soc 67:105–108. doi: 10.1017/S0029665108006083. [DOI] [PubMed] [Google Scholar]
- 10.Cates JE, Unger HW, Briand V, Fievet N, Valea I, Tinto H, D’Alessandro U, Landis SH, Adu-Afarwuah S, Dewey KG, ter Kuile FO, Desai M, Dellicour S, Ouma P, Gutman J, Oneko M, Slutsker L, Terlouw DJ, Kariuki S, Ayisi J, Madanitsa M, Mwapasa V, Ashorn P, Maleta K, Mueller I, Stanisic D, Schmiegelow C, Lusingu JPA, van Eijk AM, Bauserman M, Adair L, Cole SR, Westreich D, Meshnick S, Rogerson S. 2017. Malaria, malnutrition, and birthweight: a meta-analysis using individual participant data. PLoS Med 14:e1002373. doi: 10.1371/journal.pmed.1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaponda EB, Chandramohan D, Michelo C, Mharakurwa S, Chipeta J, Chico RM. 2015. High burden of malaria infection in pregnant women in a rural district of Zambia: a cross-sectional study. Malar J 14:380. doi: 10.1186/s12936-015-0866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steketee RW, Wirima JJ, Bloland PB, Chilima B, Mermin JH, Chitsulo L, Breman JG. 1996. Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg 55:42–49. doi: 10.4269/ajtmh.1996.55.42. [DOI] [PubMed] [Google Scholar]
- 13.ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, van Eijk AM, Rogerson SJ, Steketee RW. 2004. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg 71:41–54. doi: 10.4269/ajtmh.2004.71.41. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. 2004. Malaria and HIV interactions and their implications for public health policy. World Health Organization, Geneva, Switzerland: https://www.who.int/hiv/pub/prev_care/malaria/en/. [Google Scholar]
- 15.Young S, Murray K, Mwesigwa J, Natureeba P, Osterbauer B, Achan J, Arinaitwe E, Clark T, Ades V, Plenty A, Charlebois E, Ruel T, Kamya M, Havlir D, Cohan D. 2012. Maternal nutritional status predicts adverse birth outcomes among HIV-infected rural Ugandan women receiving combination antiretroviral therapy. PLoS One 7:e41934. doi: 10.1371/journal.pone.0041934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. 2018. World Malaria Report 2018. World Health Organization, Geneva, Switzerland: https://www.who.int/malaria/publications/world-malaria-report-2018/en/. [Google Scholar]
- 17.World Health Organization. 2014. WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). World Health Organization, Geneva, Switzerland: https://www.who.int/malaria/publications/atoz/policy_brief_iptp_sp_policy_recommendation/en/. [Google Scholar]
- 18.Walker PG, Floyd J, Ter Kuile F, Cairns M. 2017. Estimated impact on birth weight of scaling up intermittent preventive treatment of malaria in pregnancy given sulphadoxine-pyrimethamine resistance in Africa: a mathematical model. PLoS Med 14:e1002243. doi: 10.1371/journal.pmed.1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigira V, Kapisi J, Clark TD, Kinara S, Mwangwa F, Muhindo MK, Osterbauer B, Aweeka FT, Huang L, Achan J, Havlir DV, Rosenthal PJ, Kamya MR, Dorsey G. 2014. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 11:e1001689. doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naidoo I, Roper C. 2011. Drug resistance maps to guide intermittent preventive treatment of malaria in African infants. Parasitology 138:1469–1479. doi: 10.1017/S0031182011000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. 2015. Guidelines for the treatment of malaria, 3rd ed World Health Organization, Geneva, Switzerland: https://www.who.int/malaria/publications/atoz/9789241549127/en/. [Google Scholar]
- 22.Hoglund RM, Workman L, Edstein MD, Thanh NX, Quang NN, Zongo I, Ouedraogo JB, Borrmann S, Mwai L, Nsanzabana C, Price RN, Dahal P, Sambol NC, Parikh S, Nosten F, Ashley EA, Phyo AP, Lwin KM, McGready R, Day NP, Guerin PJ, White NJ, Barnes KI, Tarning J. 2017. Population pharmacokinetic properties of piperaquine in Falciparum malaria: an individual participant data meta-analysis. PLoS Med 14:e1002212. doi: 10.1371/journal.pmed.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarning J, Ashley EA, Lindegardh N, Stepniewska K, Phaiphun L, Day NP, McGready R, Ashton M, Nosten F, White NJ. 2008. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob Agents Chemother 52:1052–1061. doi: 10.1128/AAC.00955-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajubi R, Ochieng T, Kakuru A, Jagannathan P, Nakalembe M, Ruel T, Opira B, Ochokoru H, Ategeka J, Nayebare P, Clark TD, Havlir DV, Kamya MR, Dorsey G. 2019. Monthly sulfadoxine-pyrimethamine versus dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnancy: a double-blind, randomised, controlled, superiority trial. Lancet 393:1428–1439. doi: 10.1016/S0140-6736(18)32224-4. [DOI] [PubMed] [Google Scholar]
- 25.Kakuru A, Jagannathan P, Muhindo MK, Natureeba P, Awori P, Nakalembe M, Opira B, Olwoch P, Ategeka J, Nayebare P, Clark TD, Feeney ME, Charlebois ED, Rizzuto G, Muehlenbachs A, Havlir DV, Kamya MR, Dorsey G. 2016. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med 374:928–939. doi: 10.1056/NEJMoa1509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natureeba P, Kakuru A, Muhindo M, Ochieng T, Ategeka J, Koss CA, Plenty A, Charlebois ED, Clark TD, Nzarubara B, Nakalembe M, Cohan D, Rizzuto G, Muehlenbachs A, Ruel T, Jagannathan P, Havlir DV, Kamya MR, Dorsey G. 2017. Intermittent preventive treatment with dihydroartemisinin-piperaquine for the prevention of malaria among HIV-infected pregnant women. J Infect Dis 216:29–35. doi: 10.1093/infdis/jix110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savic RM, Jagannathan P, Kajubi R, Huang L, Zhang N, Were M, Kakuru A, Muhindo MK, Mwebaza N, Wallender E, Clark TD, Opira B, Kamya M, Havlir DV, Rosenthal PJ, Dorsey G, Aweeka FT. 2018. Intermittent preventive treatment for malaria in pregnancy: optimization of target concentrations of dihydroartemisinin-piperaquine. Clin Infect Dis 67:1079–1088. doi: 10.1093/cid/ciy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamin JM, Moore BR, Salman S, Page-Sharp M, Tawat S, Yadi G, Lorry L, Siba PM, Batty KT, Robinson LJ, Mueller I, Davis TM. 2015. Population pharmacokinetics, tolerability, and safety of dihydroartemisinin-piperaquine and sulfadoxine-pyrimethamine-piperaquine in pregnant and nonpregnant Papua New Guinean women. Antimicrob Agents Chemother 59:4260–4271. doi: 10.1128/AAC.00326-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore BR, Benjamin JM, Auyeung SO, Salman S, Yadi G, Griffin S, Page-Sharp M, Batty KT, Siba PM, Mueller I, Rogerson SJ, Davis TM. 2016. Safety, tolerability and pharmacokinetic properties of coadministered azithromycin and piperaquine in pregnant Papua New Guinean women. Br J Clin Pharmacol 82:199–212. doi: 10.1111/bcp.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallender E, Vucicevic K, Jagannathan P, Huang L, Natureeba P, Kakuru A, Muhindo M, Nakalembe M, Havlir D, Kamya M, Aweeka F, Dorsey G, Rosenthal PJ, Savic RM. 2018. Predicting optimal dihydroartemisinin-piperaquine regimens to prevent malaria during pregnancy for human immunodeficiency virus-infected women receiving efavirenz. J Infect Dis 217:964–972. doi: 10.1093/infdis/jix660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kajubi R, Huang L, Jagannathan P, Chamankhah N, Were M, Ruel T, Koss CA, Kakuru A, Mwebaza N, Kamya M, Havlir D, Dorsey G, Rosenthal PJ, Aweeka FT. 2017. Antiretroviral therapy with efavirenz accentuates pregnancy-associated reduction of dihydroartemisinin-piperaquine exposure during malaria chemoprevention. Clin Pharmacol Ther 102:520–528. doi: 10.1002/cpt.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopkins H, Gonzalez IJ, Polley SD, Angutoko P, Ategeka J, Asiimwe C, Agaba B, Kyabayinze DJ, Sutherland CJ, Perkins MD, Bell D. 2013. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis 208:645–652. doi: 10.1093/infdis/jit184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee TM, Huang L, Johnson MK, Lizak P, Kroetz D, Aweeka F, Parikh S. 2012. In vitro metabolism of piperaquine is primarily mediated by CYP3A4. Xenobiotica 42:1088–1095. doi: 10.3109/00498254.2012.693972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isoherranen N, Thummel KE. 2013. Drug metabolism and transport during pregnancy: how does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab Dispos 41:256–262. doi: 10.1124/dmd.112.050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson GD. 2005. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 36.Gardner MJ, Schatz M, Cousins L, Zeiger R, Middleton E, Jusko WJ. 1987. Longitudinal effects of pregnancy on the pharmacokinetics of theophylline. Eur J Clin Pharmacol 32:289–295. doi: 10.1007/BF00607577. [DOI] [PubMed] [Google Scholar]
- 37.Tran AH, Best BM, Stek A, Wang J, Capparelli EV, Burchett SK, Kreitchmann R, Rungruengthanakit K, George K, Cressey TR, Chakhtoura N, Smith E, Shapiro DE, Mirochnick M, Team IPP. 2016. Pharmacokinetics of rilpivirine in HIV-infected pregnant women. J Acquir Immune Defic Syndr 72:289–296. doi: 10.1097/QAI.0000000000000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu T, Campbell SC, Stockmann C, Tak C, Schoen K, Clark EA, Varner MW, Spigarelli MG, Sherwin CM. 2016. Pregnancy-induced changes in the pharmacokinetics of caffeine and its metabolites. J Clin Pharmacol 56:590–596. doi: 10.1002/jcph.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarning J, Rijken MJ, McGready R, Phyo AP, Hanpithakpong W, Day NP, White NJ, Nosten F, Lindegardh N. 2012. Population pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and nonpregnant women with uncomplicated malaria. Antimicrob Agents Chemother 56:1997–2007. doi: 10.1128/AAC.05756-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoglund RM, Adam I, Hanpithakpong W, Ashton M, Lindegardh N, Day NP, White NJ, Nosten F, Tarning J. 2012. A population pharmacokinetic model of piperaquine in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum malaria in Sudan. Malar J 11:398. doi: 10.1186/1475-2875-11-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnaswamy K, Ushasri V, Naidu NA. 1981. The effect of malnutrition on the pharmacokinetics of phenylbutazone. Clin Pharmacokinet 6:152–159. doi: 10.2165/00003088-198106020-00005. [DOI] [PubMed] [Google Scholar]
- 42.Krishnaswamy K, Naidu AN. 1977. Microsomal enzymes in malnutrition as determined by plasma half life of antipyrine. Br Med J 1:538–540. doi: 10.1136/bmj.1.6060.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartelink IH, Savic RM, Mwesigwa J, Achan J, Clark T, Plenty A, Charlebois E, Kamya M, Young SL, Gandhi M, Havlir D, Cohan D, Aweeka F. 2014. Pharmacokinetics of lopinavir/ritonavir and efavirenz in food insecure HIV-infected pregnant and breastfeeding women in Tororo, Uganda. J Clin Pharmacol 54:121–132. doi: 10.1002/jcph.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ducharme J, Farinotti R. 1996. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet 31:257–274. doi: 10.2165/00003088-199631040-00003. [DOI] [PubMed] [Google Scholar]
- 45.Krishnaswamy K. 1989. Drug metabolism and pharmacokinetics in malnourished children. Clin Pharmacokinet 17(Suppl 1):68–88. doi: 10.2165/00003088-198900171-00006. [DOI] [PubMed] [Google Scholar]
- 46.Krishnaswamy K. 1978. Drug metabolism and pharmacokinetics in malutrition. Clin Pharmacokinet 3:216–240. doi: 10.2165/00003088-197803030-00003. [DOI] [PubMed] [Google Scholar]
- 47.Wanyama R, Obai G, Odongo P, Kagawa MN, Baingana RK. 2018. Are women in Uganda gaining adequate gestational weight? A prospective study in low income urban Kampala. Reprod Health 15:160. doi: 10.1186/s12978-018-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasmussen KM, Yaktine AL (ed). 2009. Weight gain during pregnancy: reexamining the guidelines. National Academies Press, Washington (DC). [PubMed] [Google Scholar]
- 49.Michaud V, Ogburn E, Thong N, Aregbe AO, Quigg TC, Flockhart DA, Desta Z. 2012. Induction of CYP2C19 and CYP3A activity following repeated administration of efavirenz in healthy volunteers. Clin Pharmacol Ther 91:475–482. doi: 10.1038/clpt.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. 2004. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol 44:1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- 51.Banda CG, Dzinjalamala F, Mukaka M, Mallewa J, Maiden V, Terlouw DJ, Lalloo DG, Khoo SH, Mwapasa V. 2018. Pharmacokinetics of piperaquine and safety profile of dihydroartemisinin-piperaquine coadministered with antiretroviral therapy in malaria-uninfected HIV-positive Malawian adults. Antimicrob Agents Chemother 62:e00634-18. doi: 10.1128/AAC.00634-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarning J, Lindegardh N, Annerberg A, Singtoroj T, Day NP, Ashton M, White NJ. 2005. Pitfalls in estimating piperaquine elimination. Antimicrob Agents Chemother 49:5127–5128. doi: 10.1128/AAC.49.12.5127-5128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brewer TG, Peggins JO, Grate SJ, Petras JM, Levine BS, Weina PJ, Swearengen J, Heiffer MH, Schuster BG. 1994. Neurotoxicity in animals due to arteether and artemether. Trans R Soc Trop Med Hyg 88(Suppl 1):33–36. doi: 10.1016/0035-9203(94)90469-3. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Xu Z, Yuan Y, Ru L, Yuan Z, Zhang S, Wang Q, Song J, Xu Q. 2019. Sub-acute toxicological study of artemisinin-piperaquine tablets in rhesus monkeys. Regul Toxicol Pharmacol 109:104486. doi: 10.1016/j.yrtph.2019.104486. [DOI] [PubMed] [Google Scholar]
- 55.Brewer TG, Grate SJ, Peggins JO, Weina PJ, Petras JM, Levine BS, Heiffer MH, Schuster BG. 1994. Fatal neurotoxicity of arteether and artemether. Am J Trop Med Hyg 51:251–259. doi: 10.4269/ajtmh.1994.51.251. [DOI] [PubMed] [Google Scholar]
- 56.Toovey S, Jamieson A. 2004. Audiometric changes associated with the treatment of uncomplicated falciparum malaria with co-artemether. Trans R Soc Trop Med Hyg 98:261–269. doi: 10.1016/j.trstmh.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Efferth T, Kaina B. 2010. Toxicity of the antimalarial artemisinin and its dervatives. Crit Rev Toxicol 40:405–421. doi: 10.3109/10408441003610571. [DOI] [PubMed] [Google Scholar]
- 58.Natureeba P, Ades V, Luwedde F, Mwesigwa J, Plenty A, Okong P, Charlebois ED, Clark TD, Nzarubara B, Havlir DV, Achan J, Kamya MR, Cohan D, Dorsey G. 2014. Lopinavir/ritonavir-based antiretroviral treatment (ART) versus efavirenz-based ART for the prevention of malaria among HIV-infected pregnant women. J Infect Dis 210:1938–1945. doi: 10.1093/infdis/jiu346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kjellin LL, Dorsey G, Rosenthal PJ, Aweeka F, Huang L. 2014. Determination of the antimalarial drug piperaquine in small volume pediatric plasma samples by LC-MS/MS. Bioanalysis 6:3081–3089. doi: 10.4155/bio.14.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahn JE, Karlsson MO, Dunne A, Ludden TM. 2008. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn 35:401–421. doi: 10.1007/s10928-008-9094-4. [DOI] [PubMed] [Google Scholar]
- 61.United States Food and Drug Administration. 2005. E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e14-clinical-evaluation-qtqtc-interval-prolongation-and-proarrhythmic-potential-non-antiarrhythmic-0. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study come from two clinical trials conducted in Tororo, Uganda (25, 26). Both the raw data and NONMEM formatted data are available upon request. Please contact Grant Dorsey (grant.dorsey@ucsf.edu) to obtain the raw data and Rada Savic for the NONMEM formatted set and/or model code (rada.savic@ucsf.edu).