Phenotypic assay against Leishmania amazonensis in vitro and in vivo led to identification of an adamantyl-based phenyl sulfonyl acetamide (compound 1) as a promising antileishmanial agent. Compound 1 inhibited the growth of intracellular forms of L. amazonensis (50% inhibitory concentration [IC50] = 4 μM) and exhibited low toxicity to host cells, with a selectivity index (SI) of >125. However, in a cutaneous leishmaniasis (CL) mouse model, compound 1 did not reduce lesions and parasite load when administered as monotherapy or when given simultaneously with a suboptimal dose of miltefosine.
KEYWORDS: cutaneous leishmaniasis, adamantyl, phenyl sulfonyl acetamide, experimental chemotherapy
ABSTRACT
Phenotypic assay against Leishmania amazonensis in vitro and in vivo led to identification of an adamantyl-based phenyl sulfonyl acetamide (compound 1) as a promising antileishmanial agent. Compound 1 inhibited the growth of intracellular forms of L. amazonensis (50% inhibitory concentration [IC50] = 4 μM) and exhibited low toxicity to host cells, with a selectivity index (SI) of >125. However, in a cutaneous leishmaniasis (CL) mouse model, compound 1 did not reduce lesions and parasite load when administered as monotherapy or when given simultaneously with a suboptimal dose of miltefosine.
INTRODUCTION
Cutaneous leishmaniasis (CL) belongs to the leishmaniasis complex of diseases caused by over 20 different species of the kinetoplastid protozoan parasite Leishmania, which is transmitted through the bite of infected female sandflies. CL infections mostly lead to self-healing localized skin lesions (ulcers) but can also trigger disseminated ulcers and mucosa lesions (https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis). Approximately 1.2 million new cases of CL occur annually, leaving permanent scars, stigmas, and even serious disability, with huge social and public health impact (1). The current treatments for CL, namely pentavalent antimonials, amphotericin B, and miltefosine (Mt), have several drawbacks in terms of safety, drug resistance, cost, and efficacy, especially when used as monotherapies (2). CL is one of the neglected tropical diseases (NTDs), and its drug discovery and development pipeline remains sparse (3). There is a need for safer and more efficient oral and/or topical treatments for CL.
A series of natural product-inspired phenyl sulfonyl acetamides and acetates, as well as two pentacyclic triterpenoids (Fig. 1) previously investigated against Trypanosoma brucei, the causative agent of sleeping sickness (4, 5), were evaluated against L. amazonensis. Thus, the promising data on T. brucei prompted the present in vitro and in vivo investigation of compounds (numbered 1 to 23) on experimental models of Leishmania amazonensis infection (LTB0016 strain), one of the causative agents of CL. Statistical analysis was conducted in GraphPad Prism v.8.4.3 by ordinary one-way analysis of variance (ANOVA), Fisher’s least significant difference (LSD) test, and unpaired t test; significance was set at a P value of <0.05 (95% confidence interval).
FIG 1.
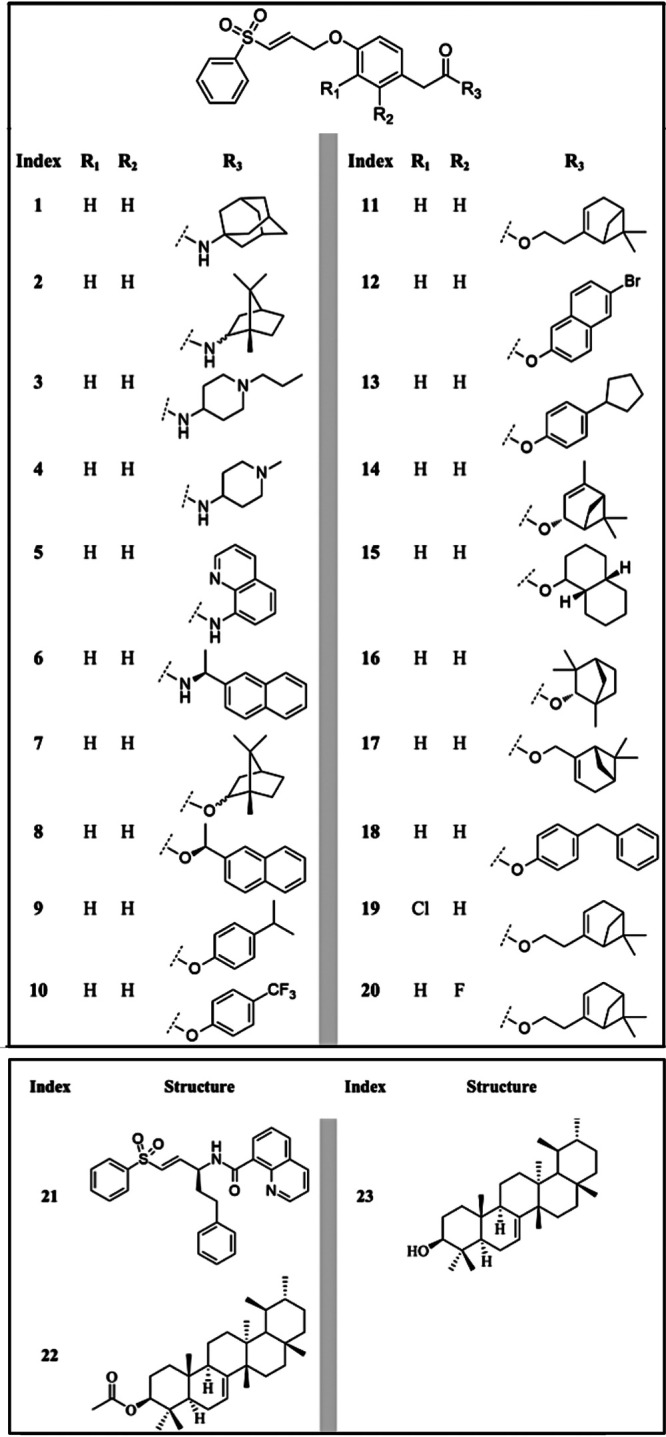
Chemical structures of tested compounds.
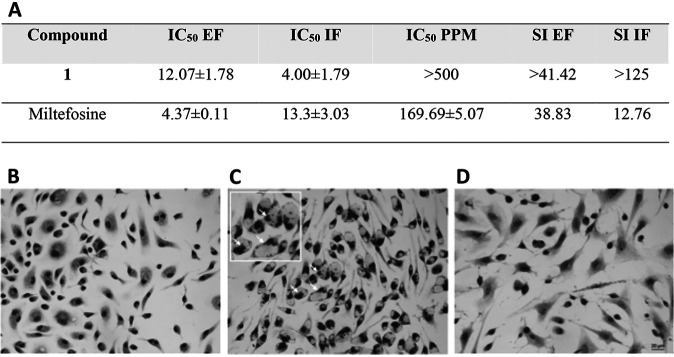
Initially, the compounds were screened using a colorimetric phenotypic assay (6) on extracellular amastigote forms (EF) of L. amazonensis. EF were purified from the skin foot paw lesions of male infected BALBc mice (7, 8). Compound 1 (Fig. 1) was active against EF (Fig. 2A). After 48 h of treatment, 5 μM compound 1 led to a 44% reduction in viable EF, and the 50% inhibitory concentration (IC50) was determined to be 12.07 ± 1.78 μM (Fig. 2A). Due to this promising profile, compound 1 was further evaluated by light microscopy against intracellular forms (IF) localized in primary cultures of peritoneal macrophages (PMM) (9). The intracellular forms are the relevant forms in mammalian infections. Compound 1 (IC50 = 4.00 ± 1.79 μM) was about 3-fold more potent (P = 0.0367) against IF than miltefosine (IC50 = 13.3 ± 3.03 μM), with no observable toxicity against PMM up to 500 μM and with a selectivity index (SI) of >125 (Fig. 2A). The relatively high SI of compound 1 makes it a good hit compound for CL (10). Moreover, analysis by light microscopy clearly demonstrate that compound 1 has a cidal rather than a static effect upon the intracellular forms, since it reduced not only the number of parasites per host cell but also the percentage of infected PMM, leading to cell culture sterilization (0% infection) at 10 μM (Fig. 2B and C).
FIG 2.
(A) Compound 1 and miltefosine activity expressed as 50% inhibitory concentration (IC50) on L. amazonensis (LTB0016 strain) after 48 h of drug exposure against extracellular amastigote forms purified from animal lesion (EF) and intracellular forms (IF) within primary cultures of peritoneal macrophages (PMM), host cell toxicity, and respective selectivity indexes (SI). (B to D) Light microscopy images of Giemsa-stained uninfected (B) and infected PMM (C) subjected or not to 10 μM compound 1 (D), demonstrating parasite sterilization due to drug exposure. Arrows indicate intracellular parasites.
The leishmanicidal activity of compound 1 and with its relatively low IC90 (7.16 μM; IF) are desirable features to avoid parasitic relapses and drug resistance (11). According to the international target product profile recommended for novel drug candidates for leishmaniasis (10, 12), the sum of these phenotypic findings encouraged us to move compound 1 to LC mouse experimental models. In vivo assays were conducted using a CL mouse model by the left foot paw subcutaneous infection of BALBc male mice (18 to 20 g) with 2 × 105 amastigotes of L. amazonensis (BT0016 strain) as described by Feitosa and coworkers in 2019 (7) (full details are provided in the supplemental material). The drug treatment started at 15 days postinfection (dpi), on the onset of the lesions measuring 200 mm3, corresponding to a 3- to 4-mm diameter (3). Compound 1 at 10 mg/kg was given twice a day (b.i.d.), alone or in coadministration with a suboptimal dose of Milteforan (Mt) 4 mg/kg once a day (q.d.), for 14 days. As a positive control, Mt was administered at 40 mg/kg q.d. The animals were monitored daily, and the lesion size was regularly measured until the endpoint of 31 dpi. Thereafter, the animals were euthanized and their skin lesions collected for imprinting Giemsa analysis by light microscopy, as well as for parasite load quantification by quantitative PCR (qPCR), as reported previously (11, 13). All procedures were carried out in accordance with the guidelines established by the Fiocruz Committee of Ethics for the Use of Animals (CEUA L038/2017).
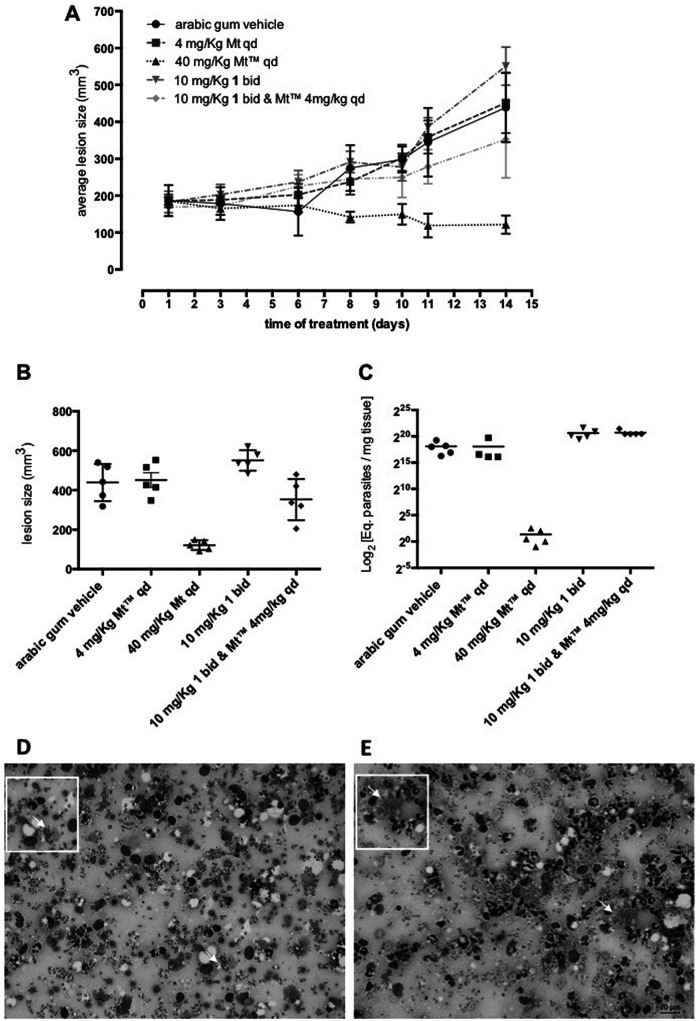
The in vivo infection led to a gradual increase in the size of the skin lesions of mice treated with the drug vehicle alone, which reached 438.8 ± 42.05 mm3 at the endpoint (Fig. 3A). Compound 1 given alone (10 mg/kg b.i.d. for 14 days) led to a mild increase (19%; P = 0.0328) in lesion sizes, with a mean value of 550.7 ± 23.15 mm3. When compound 1 was coadministered with a suboptimal dose of Mt (10 mg/kg of compound 1 plus 4 mg/kg of Mt, q.d. for 14 days), the lesions decreased by 20% (P = 0.0934). Mt alone at the optimal dose (40 mg/kg q.d. for 14 days) led to about a 72% significant reduction (P < 0.0001) in the size of the lesions, thereby reverting the clinical condition (Fig. 3A and B).
FIG 3.
Activity of compound 1 in the L. amazonensis-BALBc model of CL. The graphics show average lesion size during treatment (A) and average lesion size (B) and parasite load by quantitative PCR (qPCR) at 31 days postinfection (C), according to each experimental group. Light microscopy of lesion imprints of infected mice treated with vehicle (D) and after oral (p.o.) administration of compound 1 at 10 mg/kg b.i.d. (E). Arrows indicates intracellular parasites.
For qPCR, standard curves were constructed using DNA samples extracted from mouse skin fragments spiked with 106 amastigotes of L. amazonensis. Parasite load expressed as equivalents of parasite DNA/mg tissue (eq par/mg tissue) showed a 93.2% efficiency for the target 18S rRNA gene in Leishmania, with a linearity coefficient of 0.98 (Fig. S1A). For the mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) target, an efficiency of 91.4% was observed, with a linearity coefficient of 0.99 (Fig. S1B), confirming the sensitivity and accuracy of parasite detection and quantification (13). The molecular readout showed that mice treated with compound 1 alone (160,496 ± 45,419 eq par/mg tissue) or in coadministration with Mt (170,706 ± 28,584 eq par/mg tissue) led to an increase in parasite load (6- to 7-fold; P = 0.0018 and 0.0010, respectively) compared to that in the vehicle-treated group (27,753 ± 9,539 eq par/mg tissue) at 31 dpi (Fig. 3C). As expected, Mt given at 40 mg/kg suppressed parasite load (0.256 ± 0.102 eq par/mg tissue, 99.99%; P = 0.00196) in infected mice (8).
Analysis of lesion imprints on Giemsa-stained slides using light microscopy (Fig. 3D and E) showed that vehicle-treated mice (Fig. 3D) have numerous intracellular parasites (arrow) in macrophages besides the inflammatory diffuse infiltrates. Compound 1 lesions (Fig. 3E) also revealed a similar pattern, exhibiting high parasitemia, while the Mt group displayed only a few nonviable parasites. Our findings revealed that compound 1 has leishmanicidal activity in vitro but lacks in vivo efficacy under the treatment conditions used in this study. The lack of efficacy is potentially tied to compound 1’s lipophilicity. Its relatively high lipophilicity (cLogP of 4.3) actually impaired its use at a higher dose such as the one used for the reference drug Mt (40 mg/kg). Drugs in clinical use for leishmaniasis generally have high aqueous solubility. Thus, ongoing work on analogues of compound 1 is aimed at improving aqueous solubility and using absorption, distribution, metabolism, and excretion (ADME) experiments to reduce obvious metabolic liabilities. Analogues of compound 1 with improved bioavailability could potentially contribute to the drug discovery and development pipeline of leishmaniasis.
Finally, although it did not reach successfully in vivo outcomes, our present phenotypic study brings novel knowledge to the field of drug discovery for LC, which aims to achieve a future delivery of new therapies for patients suffering from this disregarded neglected tropical disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Roberson Donola Girão, Ana Lia Mazzetti, and Cristiane França da Silva (Laboratory of Cellular Biology/IOC/Fiocruz) for their excellent technical contribution.
We thank the Fortalecimento dos Programas de Gestão Estratégica de Pesquisa da Fiocruz Rede de Plataformas Fiocruz (grant VPPLR-001-Fio-14) and the Programa de Excelência Acadêmica (PROEX) from CAPES. The present study was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional Desenvolvimento científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação Oswaldo Cruz, PAEF/CNPq/Fiocruz. The work at Jackson State University (H.Z. and I.V.O.) was supported by the U.S. National Institutes of Health (grants SC3GM122629 and G12MD007581).
M.N.C.S. is a CNPq researcher fellow and CNE researcher. O.C.M. is a research fellow of CNPq and a JCNE researcher.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:1–12. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drugs for Neglected Diseases initiative. 2018. Making medical history to meet the needs of neglected patients. Drugs for Neglected Diseases initiative, Geneva, Switzerland: https://www.dndi.org/wp-content/uploads/2019/07/DNDi_2018_AnnualReport.pdf. [Google Scholar]
- 3.Van Bocxlaer K, Caridha D, Black C, Vesely B, Leed S, Sciotti RJ, Wijnant GJ, Yardley V, Braillard S, Mowbray CE, Ioset JR, Croft SL. 2019. Novel benzoxaborole, nitroimidazole and aminopyrazoles with activity against experimental cutaneous leishmaniasis. Int J Parasitol Drugs Drug Resist 11:129–138. doi: 10.1016/j.ijpddr.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Collins J, Nyamwihura R, Ware S, Kaiser M, Ogungbe IV. 2018. Discovery of a quinoline-based phenyl sulfone derivative as an antitrypanosomal agent. Bioorg Med Chem Lett 28:1647–1651. doi: 10.1016/j.bmcl.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carothers S, Nyamwihura R, Collins J, Zhang H, Park H, Setzer WN, Ogungbe IV. 2018. Bauerenol acetate, the pentacyclic triterpenoid from Tabernaemontana longipes, is an antitrypanosomal agent. Molecules 23:355. doi: 10.3390/molecules23020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikus J, Steverding D. 2000. A simple colorimetric to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol Int 48:265–269. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 7.Feitosa LM, da Silva ER, Hoelz LVB, Souza DL, Come JAASS, Cardoso-Santos C, Batista MM, Soeiro M. d N C, Boechat N, Pinheiro LCS. 2019. New pyrazolopyrimidine derivatives as Leishmania amazonensis arginase inhibitors. Bioorg Med Chem 27:3061–3069. doi: 10.1016/j.bmc.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Godinho JL, Simas-Rodrigues C, Silva R, Ürmenyi TP, de Souza W, Rodrigues JC. 2012. Efficacy of miltefosine treatment in Leishmania amazonensis-infected BALB/c mice. Int J Antimicrob Agents 39:326–331. doi: 10.1016/j.ijantimicag.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Santos CC, Lionel JR, Peres RB, Batista MM, da Silva PB, de Oliveira GM, da Silva CF, Batista D, Souza S, Andrade CH, Neves BJ, Braga RC, Patrick DA, Bakunova SM, Tidwell RR, Soeiro M. 2017. In vitro, in silico, and in vivo analyses of novel aromatic amidines against Trypanosoma cruzi. Antimicrob Agents Chemother 62:e02205-17. doi: 10.1128/AAC.02205-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsuno K, Burrows JN, Duncan K, Hooft van Huijsduijnen R, Kaneko T, Kita K, Mowbray CE, Schmatz D, Warner P, Slingsby BT. 2015. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov 14:751–758. doi: 10.1038/nrd4683. [DOI] [PubMed] [Google Scholar]
- 11.Cal M, Ioset J-R, Fügi MA, Mäser P, Kaiser M. 2016. Assessing anti-T. cruzi candidates in vitro for sterile cidality. Int J Parasitol Drugs Drug Resist 6:165–170. doi: 10.1016/j.ijpddr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caridha D, Vesely B, van Bocxlaer K, Arana B, Mowbray CE, Rafati S, Uliana S, Reguera R, Kreishman-Deitrick M, Sciotti R, Buffet P, Croft SL. 2019. Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis. Int J Parasitol Drugs Drug Resist 11:106–117. doi: 10.1016/j.ijpddr.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro-Romão RP, Saavedra AF, Da-Cruz AM, Pinto EF, Moreira OC. 2016. Development of real-time PCR assays for evaluation of immune response and parasite load in golden hamster (Mesocricetus auratus) infected by Leishmania (Viannia) braziliensis. Parasit Vectors 9:361. doi: 10.1186/s13071-016-1647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.