In the phase 3 ASPECT-NP trial (NCT02070757), ceftolozane/tazobactam (C/T) was noninferior to meropenem for treatment of Gram-negative ventilated hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (vHABP/VABP). Here, we report outcomes in participants from ASPECT-NP with renal impairment (RI). Participants were categorized by their baseline renal function as follows: normal renal function (NRF; creatinine clearance [CLCR], ≥80 ml/min), mild RI (CLCR, >50 to <80 ml/min), moderate RI (CLCR, ≥30 to ≤50 ml/min), and severe RI (CLCR, ≥15 to <30 ml/min).
KEYWORDS: Pseudomonas aeruginosa, multidrug resistance, renal insufficiency, hospital-acquired pneumonia, ventilator-associated pneumonia
ABSTRACT
In the phase 3 ASPECT-NP trial (NCT02070757), ceftolozane/tazobactam (C/T) was noninferior to meropenem for treatment of Gram-negative ventilated hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (vHABP/VABP). Here, we report outcomes in participants from ASPECT-NP with renal impairment (RI). Participants were categorized by their baseline renal function as follows: normal renal function (NRF; creatinine clearance [CLCR], ≥80 ml/min), mild RI (CLCR, >50 to <80 ml/min), moderate RI (CLCR, ≥30 to ≤50 ml/min), and severe RI (CLCR, ≥15 to <30 ml/min). Dosing of both study drugs was adjusted based on renal function. The following C/T doses were administered every 8 h: NRF or mild RI, 3 g; moderate RI, 1.5 g; and severe RI, 0.75 g. The primary and key secondary endpoints were day 28 all-cause mortality (ACM) and clinical response at the test-of-cure visit in the intention-to-treat (ITT) population, respectively. In the ITT population, day 28 ACM rates for the C/T arm versus the meropenem arm were 17.6% versus 19.1% (NRF), 36.6% versus 28.6% (mild RI), 31.4% versus 38.5% (moderate RI), and 35.3% versus 61.9% (severe RI). Rates of clinical cure in the ITT population for the C/T arm versus the meropenem arm were 58.1% versus 58.5% (NRF), 54.9% versus 45.5% (mild RI), 37.1% versus 42.3% (moderate RI), and 41.2% versus 47.6% (severe RI). Small sample sizes in the RI groups resulted in large 95% confidence intervals (CIs), limiting conclusive interpretation of the analysis. Both drugs were well tolerated across all renal function groups. Overall, these results support the use of the study dosing regimens of C/T for treatment of vHABP/VABP in patients with RI. (This study has been registered at ClinicalTrials.gov under identifier NCT02070757.)
INTRODUCTION
Nosocomial pneumonia (NP), which includes hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP), is one of the most common hospital-associated infections (1, 2). Mortality rates are high in NP, ranging from approximately 10% to 20% for nonventilated HABP to 10% to 30% for VABP and 20% to 40% for ventilated HABP (vHABP) (3–5). Patients with HABP/VABP caused by antibacterial-resistant isolates have mortality rates 2.9-fold higher than those of patients infected with susceptible isolates (6).
Ceftolozane/tazobactam (C/T), a combination of the novel cephalosporin ceftolozane and the β-lactamase inhibitor tazobactam, has activity against many pathogens that cause HABP/VABP, including multidrug-resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase (ESBL)-producing Enterobacterales (7–10). The results from a clinical trial conducted to assess bronchopulmonary penetration of ceftolozane and tazobactam suggested that both agents penetrate the epithelial lining fluid of healthy participants and critically ill participants with confirmed or suspected pneumonia (11, 12). C/T was approved for the treatment of patients with HABP/VABP at a dose of 3 g (2 g ceftolozane and 1 g tazobactam) every 8 h based on the phase 3 ASPECT-NP trial, which demonstrated that C/T was noninferior to meropenem for the treatment of adults with vHABP or VABP (ClinicalTrials.gov registration NCT02070757) (13). The C/T dose approved for HABP/VABP is double that approved for the treatment of complicated intra-abdominal infections (cIAIs) and complicated urinary tract infections (cUTIs) (14).
Renal impairment (RI) is a common complication in critically ill patients and is associated with a substantial mortality risk, particularly among patients in the intensive care unit (15–17). C/T is primarily eliminated via the kidneys, and a previous clinical study in participants with RI, along with previous pharmacokinetic (PK) analyses, suggested that renal impairment was associated with reduced clearance of ceftolozane and tazobactam, leading to greater exposures of both agents; therefore, dosage reductions are recommended for patients with moderate and severe RI and are designed to match the exposures obtained with recommended C/T doses in patients with normal renal function (18–21).
As observed in clinical trials for other antibacterial agents, reduced clinical cure rates were reported for participants with RI in clinical trials of C/T for the treatment of cIAI and cUTI (22–25). ASPECT-NP included participants with severe RI on account of the critically ill nature of the HABP/VABP participant population, while previous studies of C/T for the treatment of cIAI/cUTI excluded these participants (13, 26, 27). Therefore, we performed a secondary analysis of ASPECT-NP data to assess the efficacy and safety of C/T for the treatment of vHABP/VABP in participants with RI, including participants with severe RI.
RESULTS
Participant demographics and baseline characteristics.
From January 2015 to April 2018, a total of 119 study sites enrolled and randomized 726 participants (intention-to-treat [ITT] population) to receive C/T (362 participants) or meropenem (364 participants). Among participants who were randomized to receive C/T and meropenem, 245 (67.7%) and 250 (68.7%), respectively, completed the study. Participant disposition is shown in Fig. S1 in the supplemental material.
For purposes of this analysis, participants were assigned to renal function groups based on their baseline creatinine clearance (CLCR) values. A total of 258 participants (C/T, n = 134; meropenem, n = 124) had RI at baseline; of these, 217 participants (84.1%; C/T, n = 115 [85.8%]; meropenem, n = 102 [82.3%]) continued to have RI throughout the study treatment period (Table S1). Of these 217 participants who remained renally impaired in both treatment arms, 171 (78.8%) remained in the same RI group, while 32 (14.7%) had improved renal function to a less severe category of RI (i.e., moderate to mild, or severe to moderate/mild) and 14 (6.5%) had worsening renal function to a more severe category. Among the 46 participants with RI whose renal function category changed, 43 (93.1%) had their study drug dosing adjusted according to the protocol, as described in Materials and Methods.
Demographics and baseline clinical characteristics were generally well balanced between treatment arms within renal function groups (Table 1) (28). Overall, participants with RI were older than those with normal renal function. Within both treatment arms, the proportion of participants with a diagnosis of vHABP increased with worsening RI. Participants with RI had greater severity of illness based on a higher proportion of participants with Acute Physiology and Chronic Health Evaluation (APACHE) II scores of >20 and Sequential Organ Failure Assessment (SOFA) scores of >7; mean APACHE II scores were highest among participants in the moderate and severe RI groups.
TABLE 1.
Baseline demographics and clinical characteristics by renal function group (ITT population)a
| Characteristic | Normal renal function (CLCR, ≥80 ml/min) |
Mild RI (CLCR, >50 to <80 ml/min) |
Moderate RI (CLCR, ≥30 to ≤50 ml/min) |
Severe RI (CLCR, ≥15 to <30 ml/min) |
||||
|---|---|---|---|---|---|---|---|---|
| C/T (n = 227) | MEM (n = 236) | C/T (n = 82) | MEM (n = 77) | C/T (n = 35) | MEM (n = 26) | C/T (n = 17) | MEM (n = 21) | |
| Primary diagnosis (n [%]) | ||||||||
| VABP | 183 (80.6) | 188 (79.7) | 56 (68.3) | 43 (55.8) | 18 (51.4) | 16 (61.5) | 5 (29.4) | 8 (38.1) |
| vHABP | 44 (19.4) | 48 (20.3) | 26 (31.7) | 34 (44.2) | 17 (48.6) | 10 (38.5) | 12 (70.6) | 13 (61.9) |
| Age, yrs | ||||||||
| <65 (n [%]) | 153 (67.4) | 170 (72.0) | 32 (39.0) | 22 (28.6) | 10 (28.6) | 5 (19.2) | 6 (35.3) | 5 (23.8) |
| ≥65 (n [%]) | 74 (32.6) | 66 (28.0) | 50 (61.0) | 55 (71.4) | 25 (71.4) | 21 (80.8) | 11 (64.7) | 16 (76.2) |
| Mean (SD) | 55.3 (16.7) | 53.7 (16.4) | 68.6 (12.7) | 70.0 (13.6) | 70.1 (10.7) | 71.2 (9.9) | 72.8 (12.2) | 72.9 (8.8) |
| Median (range) | 57.0 (18–90) | 56.0 (18–86) | 69.0 (24–89) | 71.0 (19–92) | 70.0 (44–89) | 72.0 (39–87) | 72.0 (53–98) | 73.0 (60–90) |
| Sex (n [%]) | ||||||||
| Male | 171 (75.3) | 173 (73.3) | 58 (70.7) | 47 (61.0) | 23 (65.7) | 17 (65.4) | 9 (52.9) | 16 (76.2) |
| Female | 56 (24.7) | 63 (26.7) | 24 (29.3) | 30 (39.0) | 12 (34.3) | 9 (34.6) | 8 (47.1) | 5 (23.8) |
| APACHE II score | ||||||||
| Mean (SD) | 16.9 (4.9) | 16.5 (5.4) | 17.9 (5.1) | 17.6 (5.0) | 20.2 (6.5) | 19.2 (6.3) | 18.9 (5.5) | 22.8 (7.2) |
| Median (range) | 17 (2–29) | 17 (2–30) | 18 (4–32) | 17 (7–34) | 21 (10–33) | 18 (10–38) | 17 (12–28) | 22 (12–39) |
| Failed prior antibacterial therapy for vHABP/VABP (n [%]) | 35 (15.4) | 26 (11.0) | 11 (13.4) | 9 (11.7) | 5 (14.3) | 3 (11.5) | 2 (11.8) | 2 (9.5) |
| Prior antibacterial therapy use (n [%] | 202 (89.0) | 211 (89.4) | 69 (84.1) | 69 (89.6) | 32 (91.4) | 24 (92.3) | 15 (88.2) | 18 (85.7) |
| Bacteremic at baseline (all Gram-negative respiratory pathogens) (n [%]) | 11 (4.8) | 14 (5.9) | 8 (9.8) | 3 (3.9) | 3 (8.6) | 1 (3.8) | 3 (17.6) | 1 (4.8) |
| Baseline Gram-negative adjunctive therapy (n [%]) | 66 (29.1) | 70 (29.7) | 19 (23.2) | 27 (35.1) | 12 (34.3) | 10 (38.5) | 6 (35.3) | 5 (23.8) |
| SOFA score at baseline (n [%]) | ||||||||
| ≤7 | 185 (81.5) | 162 (68.6) | 55 (67.1) | 57 (74.0) | 13 (37.1) | 13 (50.0) | 7 (41.2) | 5 (23.8) |
| >7 | 42 (18.5) | 74 (31.4) | 27 (32.9) | 20 (26.0) | 22 (62.9) | 13 (50.0) | 10 (58.8) | 16 (76.2) |
| CPIS at baseline (n [%]) | ||||||||
| ≤6 | 15 (6.6) | 21 (8.9) | 7 (8.5) | 8 (10.4) | 0 | 0 | 2 (11.8) | 2 (9.5) |
| 7 | 16 (7.0) | 28 (11.9) | 8 (9.8) | 3 (3.9) | 3 (8.6) | 1 (3.8) | 2 (11.8) | 2 (9.5) |
| 8 | 32 (14.1) | 30 (12.7) | 6 (7.3) | 6 (7.8) | 5 (14.3) | 4 (15.4) | 2 (11.8) | 1 (4.8) |
| >8 | 164 (72.2) | 157 (66.5) | 61 (74.4) | 60 (77.9) | 27 (77.1) | 21 (80.8) | 11 (64.7) | 16 (76.2) |
| Duration of mechanical ventilation before randomization | ||||||||
| <5 days (n [%]) | 94 (41.4) | 110 (46.6) | 47 (57.3) | 42 (54.5) | 24 (68.6) | 16 (61.5) | 13 (76.5) | 16 (76.2) |
| ≥5 days (n [%]) | 132 (58.1) | 125 (53.0) | 34 (41.5) | 35 (45.5) | 11 (31.4) | 10 (38.5) | 4 (23.5) | 5 (23.8) |
| Duration of hospitalization before randomization | ||||||||
| <5 days (n [%]) | 44 (19.4) | 55 (23.3) | 25 (30.5) | 14 (18.2) | 8 (22.9) | 6 (23.1) | 3 (17.6) | 6 (28.6) |
| ≥5 days (n [%]) | 181 (79.7) | 180 (76.3) | 57 (69.5) | 62 (80.5) | 26 (74.3) | 20 (76.9) | 14 (82.4) | 15 (71.4) |
| Randomized while in ICU (n [%]) | 211 (93.0) | 222 (94.1) | 79 (96.3) | 71 (92.2) | 28 (80.0) | 22 (84.6) | 16 (94.1) | 17 (81.0) |
Renal function groups were stratified by CLCR based on FDA guidance (28). BMI, body mass index; CPIS, Clinical Pulmonary Infection Score; CLCR, creatinine clearance; C/T, ceftolozane/tazobactam; ICU, intensive care unit; ITT, intention-to-treat; MEM, meropenem; SOFA, Sequential Organ Failure Assessment; VABP, ventilator-associated bacterial pneumonia; vHABP, ventilated hospital-acquired bacterial pneumonia.
Efficacy.
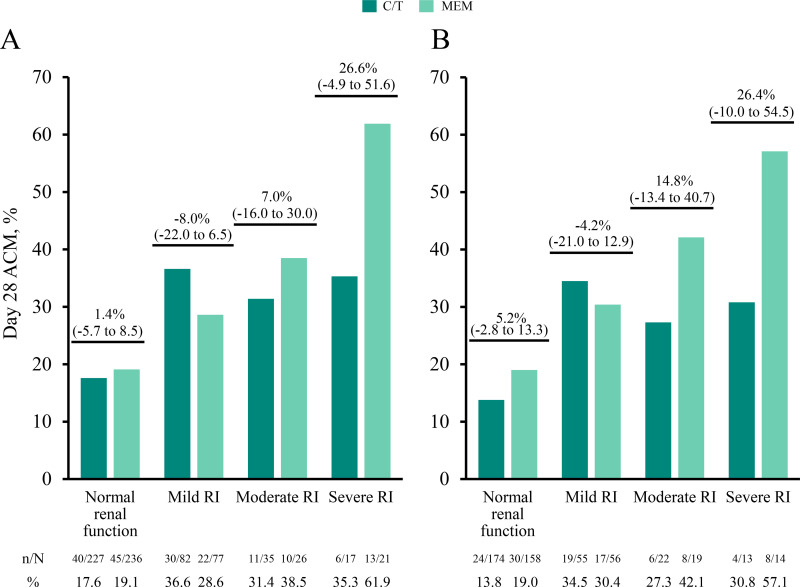
In both the ITT and microbiological ITT (mITT) groups, day 28 all-cause mortality (ACM) rates were numerically higher for all RI groups in both treatment arms compared with participants with normal renal function (Fig. 1A and B). Day 28 ACM rates for the C/T treatment arm were consistent across mild, moderate, and severe RI groups (31.4% to 36.6% [ITT]; 27.3% to 34.5% [mITT]), while rates for the meropenem arm increased as RI increased (mild RI, 28.6% [ITT] and 30.4% [mITT]; moderate RI, 38.5% [ITT] and 42.1% [mITT]; severe RI, 61.9% [ITT] and 57.1% [mITT]). Within renal function groups, day 28 ACM rates were generally comparable between treatment arms, as demonstrated by 95% confidence intervals (CIs) that included 0 (Fig. 1A and B).
FIG 1.
(A and B) Day 28 ACM by renal function group in the intention-to-treat (A) and microbiological intention-to-treat (B) populations. Participants were classified by renal function groups as follows: normal renal function (CLCR, ≥80 ml/min), mild RI (CLCR, >50 to <80 ml/min), moderate RI (CLCR, ≥30 to ≤50 ml/min), and severe RI (CLCR, ≥15 to <30 ml/min). Mortality rates (n/N, %) for each renal function group are provided below the graphs, and percentage differences between treatment groups (95% CI) are indicated above each renal function group. ACM, all-cause mortality; CI, confidence interval; CLCR, creatinine clearance; C/T, ceftolozane/tazobactam; MEM, meropenem; n, number of participants who died by day 28; N, number of participants in the treatment group; RI, renal impairment.
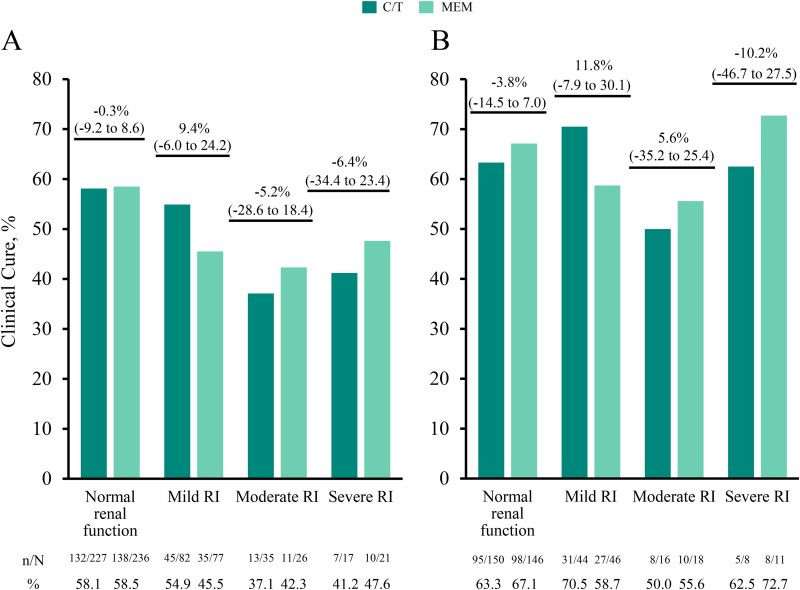
In the ITT population, clinical cure rates at the test-of-cure (TOC) visit were numerically lower in the moderate and severe RI groups across both treatment arms (C/T, 37.1% to 41.2%; meropenem, 42.3% to 47.6%) compared with participants with normal renal function (C/T, 58.1%; meropenem, 58.5%; Fig. 2A and Table S2). In the clinically evaluable (CE) population, numerical differences in clinical cure rates between the normal renal function group and the moderate and severe RI groups were smaller than in the ITT population and were generally similar across treatment arms (from 50.0% to 70.5% for the C/T arm and 55.6% to 72.7% for the meropenem arm; Fig. 2B).
FIG 2.
(A and B) Clinical cure rates at the test-of-cure visit by renal function group for the intention-to-treat (A) and clinically evaluable (B) populations. Participants were classified by renal function groups as follows: normal renal function (CLCR, ≥80 ml/min), mild RI (CLCR, >50 to <80 ml/min), moderate RI (CLCR, ≥30 to ≤50 ml/min), and severe RI (CLCR, ≥15 to <30 ml/min). Clinical cure rates (n/N, %) for each renal function group are provided below the graphs, and percentage differences between treatment groups (95% CI) are indicated above each renal function group. CI, confidence interval; CLCR, creatinine clearance; C/T, ceftolozane/tazobactam; MEM, meropenem; n, number of participants who experienced clinical cure; N, number of participants in the treatment group; RI, renal impairment.
The per-participant microbiological cure rates (cure or presumed cure) in the mITT population were robust across all renal function groups, ranging from 57.1% to 83.6% (Table 2). Cure rates were comparable between treatment arms, except for the mild RI group, where cure rates in the C/T group were higher than those in the meropenem group (83.6% versus 57.1%, respectively; difference, 26.5; 95% CI, 9.5 to 41.5). The per-pathogen clinical cure rates at the TOC visit in the mITT population are summarized in Table 3; overall, clinical cure rates for Gram-negative isolates were numerically higher in the normal renal function group (C/T, 66.5%; meropenem, 64.2%) than those in the RI groups (33.3% to 57.1%). Between the treatment arms, clinical cure rates were comparable within all renal function groups.
TABLE 2.
Per-participant microbiological cure at TOC visit by renal function group (mITT population)a
| Microbiological cure or presumed cureb | C/T (n/N [%]) | MEM (n/N [%]) | Between treatment arm difference (% [95% CI])c |
|---|---|---|---|
| Normal renal function (CLCR, ≥80 ml/min) | 125/174 (71.8) | 115/158 (72.8) | –0.9 (–10.5 to 8.7) |
| Mild RI (CLCR, >50 to <80 ml/min) | 46/55 (83.6) | 32/56 (57.1) | 26.5 (9.5 to 41.5) |
| Moderate RI (CLCR, ≥30 to ≤50 ml/min) | 13/22 (59.1) | 13/19 (68.4) | –9.3 (–35.4 to 19.2) |
| Severe RI (CLCR, ≥15 to <30 ml/min) | 9/13 (69.2) | 8/14 (57.1) | 12.1 (–22.3 to 42.6) |
CI, confidence interval; CLCR, creatinine clearance; C/T, ceftolozane/tazobactam; MEM, meropenem; mITT, microbiological intention to treat; RI, renal impairment; TOC, test of cure; n, number of participants who experienced microbiological cure; N, number of participants in the treatment group.
Participants who had a clinical outcome of cure but did not have follow-up cultures to document eradication were assessed as presumed cures.
The 95% CIs are unstratified.
TABLE 3.
Per-pathogen clinical cure at TOC visit by renal function group (mITT population)a
| Baseline pathogen, n/N1 (%) | Normal renal function (CLCR, ≥80 ml/min) |
Mild RI (CLCR, >50 to <80 ml/min) |
Moderate RI (CLCR, ≥30 to ≤50 ml/min) |
Severe RI (CLCR, ≥15 to <30 ml/min) |
||||
|---|---|---|---|---|---|---|---|---|
| C/T (N = 227) | MEM (N = 235) | C/T (N = 82) | MEM (N = 77) | C/T (N = 35) | MEM (N = 26) | C/T (N = 17) | MEM (N = 21) | |
| Gram-negative | 113/170 (66.5) | 97/151 (64.2) | 30/53 (56.6) | 24/56 (42.9) | 7/21 (33.3) | 6/17 (35.3) | 6/12 (50.0) | 8/14 (57.1) |
| Pseudomonas aeruginosa | 25/38 (65.8) | 27/41 (65.9) | 9/16 (56.3) | 11/19 (57.9) | 2/5 (40.0) | 1/2 (50.0) | 0/3 | 0/3 |
| Enterobacterales | 87/127 (68.5) | 73/115 (63.5) | 21/40 (52.5) | 18/41 (43.9) | 5/15 (33.3) | 6/17 (35.3) | 6/10 (60.0) | 6/10 (60.0) |
| Escherichia coli | 23/33 (69.7) | 19/26 (73.1) | 7/14 (50.0) | 4/11 (36.4) | 1/3 (33.3) | 1/2 (50.0) | 1/1 (100.0) | 2/3 (66.7) |
| Klebsiella pneumoniae | 38/53 (71.7) | 42/58 (72.4) | 10/21 (47.6) | 11/22 (50.0) | 1/5 (20.0) | 2/5 (40.0) | 3/6 (50.0) | 3/6 (50.0) |
| ESBL-producing Enterobacterales | 37/57 (64.9) | 31/45 (68.9) | 9/15 (60.0) | 8/17 (47.1) | 1/7 (14.3) | 2/4 (50.0) | 1/4 (25.0) | 4/7 (57.1) |
Cure was defined as eradication or presumed eradication. Eradication was defined as a ≥1-log reduction in bacterial burden from the baseline lower respiratory tract specimen and a per-pathogen count of ≤104 CFU/ml for endotracheal aspiration or sputum specimens, ≤103 CFU/ml for bronchoalveolar lavage fluid specimens, or ≤102 CFU/ml for protected brush specimens. Presumed eradication was defined as the absence of material to culture in a participant deemed a clinical cure. CLCR, creatinine clearance; C/T, ceftolozane/tazobactam; ESBL, extended-spectrum β-lactamase; MEM, meropenem; mITT, microbiological intention-to-treat; n, number of participants who experienced clinical cure; N, number of participants within the subgroup; N1, number of participants in the subgroup with the specified pathogen; RI, renal impairment; TOC, test of cure.
Pharmacokinetic analysis by renal function group.
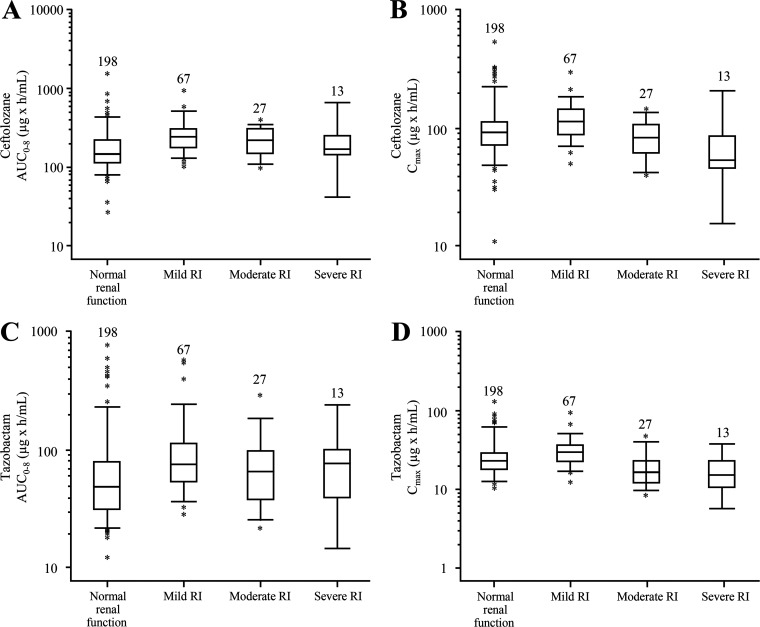
A total of 305 participants receiving C/T in ASPECT-NP had evaluable PK exposure data. Summaries of plasma exposures by renal function group are shown in Fig. 3. For both ceftolozane and tazobactam, the median area under the concentration-time curve for the first 8 h after dosing and the maximum plasma concentration for each RI group were within the 5th to 95th percentile range of the normal renal function group, and the converse was within the same range, indicating that exposures resulting from the proposed HABP/VABP dosing regimens were generally comparable. Additionally, the plasma probability of target attainment (PTA) for ceftolozane at up to a 2-log kill PK/pharmacodynamic (PD) target (at up to 50% of the time that the free drug concentration exceeded the MIC [fT>MIC] = 4 μg/ml), as well as the PTA for tazobactam at the PK/PD target associated with restoring ceftolozane antibacterial activity to 1-log kill (35% fT > threshold concentration [fT>CT] = 1 μg/ml), was >98% in all RI groups.
FIG 3.
Ceftolozane and tazobactam steady-state exposures by renal function group. (A to D) The ceftolozane AUC0–8 (A) and Cmax (B) and tazobactam AUC0–8 (C) and Cmax (D) are shown. Participants were classified by renal function groups as follows: normal renal function (CLCR, ≥80 ml/min), mild RI (CLCR, >50 to <80 ml/min), moderate RI (CLCR, ≥30 to ≤50 ml/min), and severe RI (CLCR, ≥15 to <30 ml/min). Boxes are the 25th, 50th, and 75th percentiles; whiskers are the 5th to 95th percentiles. Asterisks show data points outside this range. Participant numbers are indicated above each box. AUC0–8, area under the concentration-time curve for the first 8 h after dosing; CLCR, creatinine clearance; Cmax, maximum plasma concentration; RI, renal impairment.
Safety results.
Table 4 contains rates of treatment-emergent adverse events (AEs) by renal function group and treatment arm. Overall, AE rates were similar between treatment arms within renal function groups, with rates generally increasing with increasing severity of RI. Rates of serious AEs were observed to be higher in participants with moderate and severe RI (50.0% to 71.4%) compared with participants with normal renal function or mild RI (30.2% to 51.2%). Rates of treatment-related AEs and discontinuations due to treatment-related AEs were low (1.3% to 15.4% and 0% to 5.9%, respectively), and these were similar between treatment arms within renal function groups; the incidence of treatment-related AEs was generally similar between participants with normal renal function and those with mild, moderate, and severe RI. No treatment-related deaths were reported in the trial. Rates of specific AEs were comparable between renal function groups and treatment arms (Table S3).
TABLE 4.
Summary of the number of participants with AEs by renal function group (safety population)a
| AE category, n (%) | Normal renal function (CLCR, ≥80 ml/min) |
Mild RI (CLCR, >50 to <80 ml/min) |
Moderate RI (CLCR, ≥30 to ≤50 ml/min) |
Severe RI (CLCR, ≥15 to <30 ml/min) |
||||
|---|---|---|---|---|---|---|---|---|
| C/T (n = 227) | MEM (n = 235) | C/T (n = 82) | MEM (n = 77) | C/T (n = 35) | MEM (n = 26) | C/T (n = 17) | MEM (n = 21) | |
| Any AEs | 189 (83.3) | 193 (82.1) | 72 (87.8) | 62 (80.5) | 33 (94.3) | 24 (92.3) | 16 (94.1) | 20 (95.2) |
| AEs by severity | ||||||||
| Mild | 48 (21.1) | 44 (18.7) | 15 (18.3) | 10 (13.0) | 3 (8.6) | 3 (11.5) | 3 (17.6) | 1 (4.8) |
| Moderate | 65 (28.6) | 74 (31.5) | 19 (23.2) | 22 (28.6) | 11 (31.4) | 6 (23.1) | 3 (17.6) | 3 (14.3) |
| Severe | 76 (33.5) | 75 (31.9) | 38 (46.3) | 30 (39.0) | 19 (54.3) | 15 (57.7) | 10 (58.8) | 16 (76.2) |
| Any treatment-related AEsb | 23 (10.1) | 19 (8.1) | 11 (13.4) | 1 (1.3) | 2 (5.7) | 4 (15.4) | 2 (11.8) | 3 (14.3) |
| Any serious AEs | 79 (34.8) | 71 (30.2) | 42 (51.2) | 30 (39.0) | 21 (60.0) | 13 (50.0) | 10 (58.8) | 15 (71.4) |
| Any treatment-related serious AEsb | 7 (3.1) | 2 (0.9) | 1 (1.2) | 0 | 0 | 0 | 0 | 0 |
| Any AEs that led to discontinuation of study drug | 16 (7.0) | 23 (9.8) | 10 (12.2) | 10 (13.0) | 7 (20.0) | 3 (11.5) | 4 (23.5) | 6 (28.6) |
| Any treatment-related AEs that led to discontinuation of study drugb | 3 (1.3) | 3 (1.3) | 0 | 1 (1.3) | 0 | 1 (3.8) | 1 (5.9) | 0 |
| Any AEs that resulted in death | 51 (22.5) | 50 (21.3) | 33 (40.2) | 27 (35.1) | 13 (37.1) | 11 (42.3) | 8 (47.1) | 13 (61.9) |
| Any treatment-related AEs that resulted in deathb | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AE, adverse event; CLCR, creatinine clearance; C/T, ceftolozane/tazobactam; MEM, meropenem; RI, renal impairment.
In participants with multiple events for which at least one was treatment related, the AE was counted as related to study drug.
DISCUSSION
The ASPECT-NP trial demonstrated the noninferiority of C/T to meropenem for day 28 ACM and clinical response for the treatment of vHABP/VABP, a common, difficult-to-treat infection with a high morbidity and mortality rate (1, 29–31). In this analysis of participants from ASPECT-NP with RI at baseline, day 28 ACM rates and clinical cure rates were comparable between the C/T and meropenem arms within all RI groups, with 95% CIs for treatment differences that included 0, supporting the efficacy of C/T in participants with RI. For both treatment arms, day 28 ACM rates were higher in participants with RI than in participants with normal renal function. Given that both acute and chronic RI are associated with higher mortality rates in patients in the intensive care unit, this finding is expected (32, 33). In addition, participants with RI in this study were older (approximately 70% were ≥65 years of age), and those with moderate or severe RI had higher SOFA scores than participants with normal renal function; thus, advanced age and increased severity of illness could account for some of the mortality differences between the moderate and severe RI groups compared with participants with normal renal function. Finally, a primary diagnosis of vHABP was more common in participants with RI, particularly those with severe RI, than in participants with normal renal function (20% in participants with normal renal function, 43% in all participants with RI, and 66% in participants with severe RI), which may also account for part of the mortality difference, as vHABP is associated with higher mortality than VABP (4). The lower observed clinical cure rates in participants with RI in both treatment arms were more pronounced in the ITT population than in the CE population. This difference may be explained in part by the higher mortality rates observed in the RI groups, since participants in the ITT population who died from a cause other than vHABP/VABP before the TOC visit were categorized as having an indeterminate clinical response but were assessed as noncures (i.e., clinical failures) in the ITT clinical response analyses (Table S2). This group of participants was excluded from the CE population, where clinical cure rates were similar between the RI and normal renal function groups. These results support the efficacy of the C/T dosing regimens used in this study in participants with RI.
Per-participant microbiological cure rates were similar between treatment arms within all renal function groups, with high rates of cure or presumed cure (≥50%) observed across all groups. Per-pathogen cure rates were also similar across treatment arms, although a trend was observed in decreasing Gram-negative cure rates with increasing RI (from 67% and 64% cure rates in participants with normal renal function who received C/T and meropenem, respectively, to 33% and 35% cure rates in participants with moderate RI who received C/T and meropenem, respectively). Because of the small sample sizes in the RI groups, we cannot draw meaningful conclusions regarding this apparent decrease in per-pathogen cure rates.
Our simulations using individual PK parameter estimates from the final PK models suggested that steady-state exposures (measured by the area under the concentration-time curve and maximum plasma concentration during the 8-h dosing interval) were generally comparable between participants with normal renal function and those with RI (12). Ceftolozane and tazobactam lung epithelial lining fluid exposures, driven by plasma exposures, are therefore expected to be similar between participants with normal renal function and those with RI, as was previously demonstrated. Additionally, the plasma PTA for both ceftolozane and tazobactam at up to the 2-log kill PK/PD target for ceftolozane (up to 50% fT>MIC = 4 μg/ml) and the PK/PD target associated with restoring ceftolozane antibacterial activity to 1-log kill for tazobactam (35% fT>CT = 1 μg/ml) was >98% for both agents in all RI groups. As previously reported, %fT>MIC and %fT>CT are the PK/PD indices that drive efficacy for ceftolozane and tazobactam, respectively (34, 35). Caro and colleagues also reported that C/T achieved 50% to 60% lung penetration in critically ill participants and that concentrations in plasma achieved ƒT>MIC = 4 μg/ml for ceftolozane and ƒT>CT = 1 μg/ml for tazobactam for approximately 100% and 80% of the dosing interval, respectively (12, 36). The efficacy and exposure results across renal function groups support the use of the recommended C/T doses of 3 g (CLCR, >50 ml/min), 1.5 g (CLCR, 30 to 50 ml/min), and 0.75 g (CLCR, 15 to 29 ml/min) for treatment of vHABP/VABP.
Both study drugs were well tolerated across renal function groups, with similar rates of AEs in both treatment arms in all renal function groups; rates generally increased with increasing RI severity, which was expected in this critically ill population. However, rates of treatment-related AEs were low, with less than 16% of participants in any group or treatment arm experiencing treatment-related AEs. Rates of study drug discontinuation due to AEs were also generally low, with higher rates of up to 28.6% in those with severe RI; however, less than 6% of participants in any RI group in either treatment arm discontinued due to a treatment-related AE. No participants died due to treatment-related AEs in the trial. These data suggest that C/T is well tolerated in participants across the renal function spectrum when administered at dosages that account for reduced CLCR.
A key limitation of this analysis is the small sample sizes of the RI groups, particularly of the moderate and severe RI groups. These small samples, resulting in large 95% CIs, limit conclusive interpretation of the data.
Consistent with the primary and key secondary endpoints of the ASPECT-NP study, day 28 ACM and clinical cure rates were similar between treatment arms across the spectrum of renal function. The PK results suggest that adjusted C/T doses provide adequate exposure in participants with RI. Both drugs were well tolerated, and no new safety signals were identified. The results of this analysis demonstrate that the use of dose-adjusted C/T is appropriate in patients with vHABP/VABP and RI.
MATERIALS AND METHODS
Study design and participants.
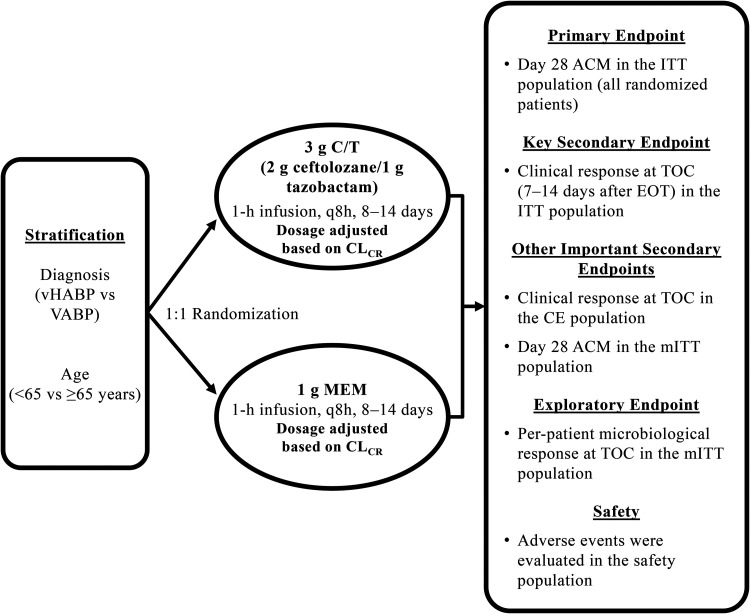
ASPECT-NP (protocol MK-7625A-008) was a phase 3, randomized, controlled, double-blind, multicenter noninferiority study that compared the safety and efficacy of C/T with meropenem for the treatment of vHABP/VABP. The methods have been previously described (13). The study design is shown in Fig. 4. ASPECT-NP was conducted at 263 hospitals in 34 countries. The study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. All participants (or legally acceptable representatives) provided informed consent.
FIG 4.
ASPECT-NP study design. ACM, all-cause mortality; CE, clinically evaluable; C/T, ceftolozane-tazobactam; CLCR, creatinine clearance; EOT, end of treatment; ITT, intention-to-treat; MEM, meropenem; mITT, microbiological intention-to-treat; q8h, every 8 h; TOC, test of cure; VABP, ventilator-associated bacterial pneumonia; vHABP, ventilated hospital-acquired bacterial pneumonia.
Eligible participants were ≥18 years of age, were intubated and mechanically ventilated, and had confirmed vHABP or VABP (13). Pneumonia was diagnosed based on clinical and radiological evidence. Diagnosis of vHABP required the presence of symptoms within 24 h before intubation or within 48 h after intubation in participants who had been hospitalized at least 48 h. Diagnosis of VABP required participants to have received mechanical ventilation for at least 48 h before symptom onset. Participants were also required to have chest radiographs indicating infiltrate suggestive of bacterial pneumonia, plus purulent tracheal secretions with ≥1 additional clinical criterion (e.g., fever [body temperature ≥38°C], hypothermia [body temperature ≤35°C], elevated/decreased white blood cell counts [≥10,000 cells/mm3 or ≤4,500 cells/mm3], or ≥15% immature neutrophils) within 24 h before the first dose of study drug. Collection of a baseline lower respiratory tract (LRT) specimen (for Gram stain and quantitative culture) was required within 36 h before the first dose of study drug. Key exclusion criteria included end-stage renal disease (defined as CLCR < 15 ml/min), requirement for peritoneal dialysis/hemodialysis/hemofiltration, or decreased urine output (<20 ml/h over a 24-h period).
Procedures.
Participants were stratified according to diagnosis (vHABP versus VABP) and age (<65 versus ≥65 years) and were randomized 1:1 to receive C/T or meropenem as a 1-h infusion every 8 h. Dosing by renal function group is shown in Table 5. Per the protocol, dose adjustments were required within 24 h if the renal function category changed during the treatment period; all dose adjustments were implemented by an unblinded pharmacist. Participants with CLCR of ≤50 ml/min received dummy dosing of intravenous saline to maintain blinding. The duration of study drug treatment was 8 to 14 days (24 to 42 doses). While initial dosing was determined by baseline CLCR (calculated using the Cockcroft-Gault formula), dosage was adjusted during the study period if a participant’s CLCR changed to levels within a different dosing category (37). Administration of the study drug was discontinued in participants who developed end-stage renal disease or required initiation of renal replacement therapy. A baseline LRT specimen was obtained for Gram stain and quantitative culture within 36 h before the first dose of study drug. Pathogen identification and susceptibility were performed at the sites’ local laboratories and confirmed at a central laboratory. Empiric adjunctive Gram-positive therapy (600 mg linezolid every 12 h or standard-of-care alternative) was administered to all participants at baseline until culture results confirmed the absence of Staphylococcus aureus. Empiric Gram-negative adjunctive therapy (intravenous amikacin, 15 mg/kg of body weight, once daily or alternative standard-of-care aminoglycoside) was permitted for up to 72 h after the first dose of study drug at sites where the local prevalence of meropenem-resistant P. aeruginosa was at least 15% (based on local antibiograms).
TABLE 5.
Dose by renal function groupa
| Renal function category | C/T |
MEM |
||
|---|---|---|---|---|
| Dose | IV infusion frequency | Dose | IV infusion frequency | |
| CLCR, ≥50 ml/min | 3 g (2 g ceftolozane and 1 g tazobactam) | q8h | 1 g | q8h |
| CLCR, 30–50 ml/min | 1.5 g (1 g ceftolozane and 0.5 g tazobactam) | q8h | 1 g | q12h |
| CLCR, 26–29 ml/min | 0.75 g (0.5 g ceftolozane and 0.25 g tazobactam) | q8h | 1 g | q12h |
| CLCR, 15–25 ml/min | 0.75 g (0.5 g ceftolozane and 0.25 g tazobactam) | q8h | 0.5 g | q12h |
| CLCR, <15 ml/min or initiation of renal replacement therapy | Discontinue study drug | Discontinue study drug | ||
CLCR, creatinine clearance; C/T, ceftolozane/tazobactam; IV, intravenous; MEM, meropenem; q8h, every 8 h; q12h, every 12 h.
Study visits and analysis populations.
Baseline screening assessments were performed within 24 h before randomization and the initiation of study drug administration. The TOC visit was conducted within 7 to 14 days after the end-of-treatment visit. The ITT population included all randomized participants, regardless of whether they received study drug. The mITT population, which was a subset of the ITT population, included all participants who received any amount of study drug and had at least one bacterial respiratory pathogen isolated from the baseline LRT culture that was susceptible to at least one study drug. The CE population, which was a subset of the ITT population, included all participants who received study drug, adhered to the study protocol through the TOC visit, and had an evaluable clinical outcome (cure or failure) at the TOC visit. The safety population, which was a subset of the ITT population, included all participants who received any amount of study drug.
Endpoints and clinical assessments.
The primary efficacy endpoint of ASPECT-NP was day 28 ACM in the ITT population, and the key secondary endpoint was clinical response at the TOC visit in the ITT population (13). Additional efficacy endpoints included day 28 ACM in the mITT population and clinical response at the TOC visit in the CE population. This secondary analysis assessed these efficacy responses by renal function group. Clinical cure of vHABP/VABP was defined as a participant who was alive, with complete resolution of all or most of the clinical signs and symptoms of pneumonia, with no new signs or symptoms attributable to vHABP/VABP and no additional antibacterial therapy administered for vHABP/VABP other than approved adjunctive therapy. Clinical failure was defined as a participant who died from vHABP/VABP, who had progression, relapse, or recurrence of new symptoms or lack of resolution, or who had a need for alternative or prolonged antibacterial therapy for vHABP/VABP. Microbiological eradication was defined as a ≥1-log reduction in bacterial burden from the baseline LRT pathogen and a per-pathogen count of ≤104 CFU/ml for endotracheal aspirate or sputum specimens, ≤103 CFU/ml for bronchoalveolar lavage fluid specimens, and ≤102 CFU/ml for protected brush specimens from a follow-up LRT culture. Persistence was defined as the continued presence of the baseline LRT pathogen at or after the end of treatment (<1-log reduction or >104 CFU/ml for endotracheal aspirate or sputum specimens, >103 CFU/ml for bronchoalveolar lavage fluid specimens, and >102 CFU/ml for protected brush specimens). Microbiological assessments were performed on a per-participant and per-pathogen basis. The incidence of AEs, serious AEs, and early termination of study medication or participation due to an AE was also determined for each participant.
Pharmacokinetic analysis.
To facilitate assessment of ceftolozane and tazobactam PK, plasma samples were collected from participants enrolled in ASPECT-NP and receiving C/T. Population PK modeling and simulation for C/T in adult participants with vHABP/VABP was performed, wherein the previously developed plasma population PK models for ceftolozane and tazobactam were refined using PK data from participants with vHABP/VABP from ASPECT-NP (20). In the updated model, CLCR was identified as a significant covariate on clearance (38). Stochastic simulations were performed using the refined population PK model to estimate steady-state plasma ceftolozane and tazobactam exposures in participants who received adjusted dosing regimens based on RI. The PTA for each drug and each renal function group was also assessed using Monte Carlo simulations (N = 1,000 for each RI group). The PTA for ceftolozane was defined as the percentage of participants who achieved the PK/PD target at MIC up to 64 μg/ml. The ceftolozane PK/PD target was to maintain the ceftolozane concentration above the MIC based on free drug concentrations (%ƒT>MIC) for up to 50% of the dosing interval. The PTA for tazobactam was defined as the percentage of participants who achieved the PK/PD target at a CT of 1 μg/ml. The tazobactam PK/PD target was to maintain a tazobactam concentration above the CT based on free drug concentrations (%ƒT>CT) for up to 35% of the dosing interval.
Statistical analysis.
The 2-sided 95% CIs for the treatment difference between C/T and meropenem were calculated as unstratified Newcombe CIs. For negative outcomes (e.g., day 28 ACM), the difference between groups was calculated as meropenem minus C/T. For positive outcomes (e.g., clinical cure), the difference was calculated as C/T minus meropenem. This subgroup analysis was not powered for noninferiority testing. Safety data were analyzed descriptively. All statistical analyses were performed using SAS versions 9.3 and 9.4 (SAS Institute, Inc., Cary, NC).
Data availability.
The data sharing policy of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Supplementary Material
ACKNOWLEDGMENTS
We thank the participants and their families and caregivers for participating in this study, along with all investigators and site personnel.
All authors are responsible for the work described in this paper. All authors were involved in at least one of the following: conception, design of work or acquisition, analysis, interpretation of data, and drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). The authors are employees of MSD who may own stock and/or hold stock options in Merck & Co., Inc., Kenilworth, NJ, USA. Medical writing and/or editorial assistance was provided by Rebecca Brady, PhD, and Todd Waldron, PhD, CMPP, of The Lockwood Group, Stamford, CT, USA. This assistance was funded by MSD.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, Ray SM, Beldavs Z, Gross C, Bamberg W, Sievers M, Concannon C, Buhr N, Warnke L, Maloney M, Ocampo V, Brooks J, Oyewumi T, Sharmin S, Richards K, Rainbow J, Samper M, Hancock EB, Leaptrot D, Scalise E, Badrun F, Phelps R, Edwards JR, Emerging Infections Program Hospital Prevalence Survey Team. 2018. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 379:1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suetens C, Latour K, Karki T, Ricchizzi E, Kinross P, Moro ML, Jans B, Hopkins S, Hansen S, Lyytikainen O, Reilly J, Deptula A, Zingg W, Plachouras D, Monnet DL, the Healthcare-Associated Infections Prevalence Study Group. 2018. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill 23:1800516 https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2018.23.46.1800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. 2005. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 4.Talbot GH, Das A, Cush S, Dane A, Wible M, Echols R, Torres A, Cammarata S, Rex JH, Powers JH, Fleming T, Loutit J, Hoffmann S, Foundation for the National Institutes of Health Biomarkers Consortium HABP VABP Project Team. 2019. Evidence-based study design for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. J Infect Dis 219:1536–1544. doi: 10.1093/infdis/jiy578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melsen WG, Rovers MM, Groenwold RHH, Bergmans DCJJ, Camus C, Bauer TT, Hanisch EW, Klarin B, Koeman M, Krueger WA, Lacherade J-C, Lorente L, Memish ZA, Morrow LE, Nardi G, van Nieuwenhoven CA, O’Keefe GE, Nakos G, Scannapieco FA, Seguin P, Staudinger T, Topeli A, Ferrer M, Bonten MJM. 2013. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis 13:665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Loeches I, Torres A, Rinaudo M, Terraneo S, de Rosa F, Ramirez P, Diaz E, Fernandez-Barat L, Li Bassi GL, Ferrer M. 2015. Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J Infect 70:213–222. doi: 10.1016/j.jinf.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Juan C, Zamorano L, Pérez JL, Ge Y, Oliver A, Spanish Group for the Study of Pseudomonas, Spanish Network for Research in Infectious Diseases. 2010. Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem-resistant and multidrug-resistant Pseudomonas aeruginosa clinical strains. Antimicrob Agents Chemother 54:846–851. doi: 10.1128/AAC.00834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sader HS, Rhomberg PR, Farrell DJ, Jones RN. 2011. Antimicrobial activity of CXA-101, a novel cephalosporin tested in combination with tazobactam against Enterobacteriaceae, Pseudomonas aeruginosa, and Bacteroides fragilis strains having various resistance phenotypes. Antimicrob Agents Chemother 55:2390–2394. doi: 10.1128/AAC.01737-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, Lagace-Wiens PR, Rubinstein E, Gin AS, Walkty A, Hoban DJ, Lynch JP 3rd, Karlowsky JA. 2014. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 74:31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 10.Carvalhaes CG, Castanheira M, Sader HS, Flamm RK, Shortridge D. 2019. Antimicrobial activity of ceftolozane-tazobactam tested against Gram-negative contemporary (2015–2017) isolates from hospitalized patients with pneumonia in US medical centers. Diagn Microbiol Infect Dis 94:93–102. doi: 10.1016/j.diagmicrobio.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Chandorkar G, Huntington JA, Gotfried MH, Rodvold KA, Umeh O. 2012. Intrapulmonary penetration of ceftolozane/tazobactam and piperacillin/tazobactam in healthy adult subjects. J Antimicrob Chemother 67:2463–2469. doi: 10.1093/jac/dks246. [DOI] [PubMed] [Google Scholar]
- 12.Caro L, Nicolau DP, De Waele J, Kuti JL, Larson KB, Gadzicki E, Yu B, Zeng Z, Adedoyin A, Rhee EG. 2020. Lung penetration, bronchopulmonary pharmacokinetic/pharmacodynamic profile and safety of 3 g ceftolozane/tazobactam administered to ventilated, critically ill patients with pneumonia. J Antimicrob Chemother 75:1546–1553. doi: 10.1093/jac/dkaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kollef MH, Nováček M, Kivistik Ü, Réa-Neto Á, Shime N, Martin-Loeches I, Timsit J-F, Wunderink RG, Bruno CJ, Huntington JA, Lin G, Yu B, Butterton JR, Rhee EG. 2019. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 19:1299–1311. doi: 10.1016/S1473-3099(19)30403-7. [DOI] [PubMed] [Google Scholar]
- 14.Merck Sharp & Dohme Corp. 2020. ZERBAXA (ceftolozane and tazobactam) for injection, for intravenous use. Prescribing information. Merck Sharp & Dohme Corp, Whitehouse Station, NJ. [Google Scholar]
- 15.Barrantes F, Tian J, Vazquez R, Amoateng-Adjepong Y, Manthous CA. 2008. Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med 36:1397–1403. 10.1097/CCM.0b013e318168fbe0. doi: 10.1097/CCM.0b013e318168fbe0. [DOI] [PubMed] [Google Scholar]
- 16.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. 2005. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 17.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honore PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. 2015. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 18.Wooley M, Miller B, Krishna G, Hershberger E, Chandorkar G. 2014. Impact of renal function on the pharmacokinetics and safety of ceftolozane-tazobactam. Antimicrob Agents Chemother 58:2249–2255. doi: 10.1128/AAC.02151-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. 2012. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother 56:3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandorkar G, Xiao A, Mouksassi MS, Hershberger E, Krishna G. 2015. Population pharmacokinetics of ceftolozane/tazobactam in healthy volunteers, subjects with varying degrees of renal function and patients with bacterial infections. J Clin Pharmacol 55:230–239. doi: 10.1002/jcph.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao AJ, Miller BW, Huntington JA, Nicolau DP. 2016. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived dose justification for phase 3 studies in patients with nosocomial pneumonia. J Clin Pharmacol 56:56–66. doi: 10.1002/jcph.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kullar R, Wagenlehner FM, Popejoy MW, Long J, Yu B, Goldstein EJ. 2017. Does moderate renal impairment affect clinical outcomes in complicated intra-abdominal and complicated urinary tract infections? Analysis of two randomized controlled trials with ceftolozane/tazobactam. J Antimicrob Chemother 72:900–905. doi: 10.1093/jac/dkw486. [DOI] [PubMed] [Google Scholar]
- 23.Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J. 2016. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 62:1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagenlehner FME, Cloutier DJ, Komirenko AS, Cebrik DS, Krause KM, Keepers TR, Connolly LE, Miller LG, Friedland I, Dwyer JP, Epic Study Group. 2019. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med 380:729–740. doi: 10.1056/NEJMoa1801467. [DOI] [PubMed] [Google Scholar]
- 25.Mathers A, Hope WW, Kaye KS, Loutit J, Alexander E, Dudley M, Vazquez JA. 2017. Meropenem-vaborbactam (VABOMERE): outcomes in subjects with renal impairment in phase 3 studies TANGO I and TANGO II. Abstr IDWeek, October 4–8, 2017, San Diego, CA. [Google Scholar]
- 26.Solomkin J, Hershberger E, Miller B, Popejoy M, Friedland I, Steenbergen J, Yoon M, Collins S, Yuan G, Barie PS, Eckmann C. 2015. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis 60:1462–1471. doi: 10.1093/cid/civ097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. 2015. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet 385:1949–1956. doi: 10.1016/S0140-6736(14)62220-0. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. 1998. Guidance for industry: pharmacokinetics in patients with impaired renal function: study design, data analysis, and impact on dosing and labeling. Center for Drug Evaluation and Research, Silver Spring, MD: https://www.fda.gov/media/71334/download. [Google Scholar]
- 29.Giuliano KK, Baker D, Quinn B. 2018. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control 46:322–327. doi: 10.1016/j.ajic.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Micek ST, Wunderink RG, Kollef MH, Chen C, Rello J, Chastre J, Antonelli M, Welte T, Clair B, Ostermann H, Calbo E, Torres A, Menichetti F, Schramm GE, Menon V. 2015. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care 19:219. doi: 10.1186/s13054-015-0926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Razazi K, Mekontso Dessap A, Carteaux G, Jansen C, Decousser JW, de Prost N, Brun-Buisson C. 2017. Frequency, associated factors and outcome of multi-drug-resistant intensive care unit-acquired pneumonia among patients colonized with extended-spectrum β-lactamase-producing Enterobacteriaceae. Ann Intensive Care 7:61. doi: 10.1186/s13613-017-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimes-Stigare C, Frumento P, Bottai M, Martensson J, Martling CR, Walther SM, Karlstrom G, Bell M. 2015. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care 19:221. doi: 10.1186/s13054-015-0920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. 2002. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int 62:986–996. doi: 10.1046/j.1523-1755.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 34.Craig WA, Andes DR. 2013. In vivo activities of ceftolozane, a new cephalosporin, with and without tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae, including strains with extended-spectrum beta-lactamases, in the thighs of neutropenic mice. Antimicrob Agents Chemother 57:1577–1582. doi: 10.1128/AAC.01590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melchers MJ, Mavridou E, van Mil AC, Lagarde C, Mouton JW. 2016. Pharmacodynamics of ceftolozane combined with tazobactam against Enterobacteriaceae in a neutropenic mouse thigh model. Antimicrob Agents Chemother 60:7272–7279. 10.1128/AAC.01580-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodvold KA, Hope WW, Boyd SE. 2017. Considerations for effect site pharmacokinetics to estimate drug exposure: concentrations of antibiotics in the lung. Curr Opin Pharmacol 36:114–123. doi: 10.1016/j.coph.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Patel YT, Fiedler-Kelly J, Feng HP, Bruno CJ, Gao W. 2020. Population pharmacokinetic analysis for plasma and epithelial lining fluid ceftolozane/tazobactam concentrations in patients with ventilated nosocomial pneumonia. J Clin Pharmacol doi: 10.1002/jcph.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.