WCK 5222 (cefepime-zidebactam, 2 g + 1g, every 8 h [q8h]) is in clinical development for the treatment of infections caused by carbapenem-resistant and multidrug-resistant (MDR) Gram-negative bacilli. We determined the in vitro susceptibility of 1,385 clinical isolates of non-carbapenem-susceptible Enterobacterales, MDR Pseudomonas aeruginosa (also non-carbapenem susceptible), Stenotrophomonas maltophilia, and Burkholderia spp.
KEYWORDS: WCK 5222, cefepime, zidebactam, bicycloacyl hydrazide, PBP2, enhancer, β-lactamase inhibitors
ABSTRACT
WCK 5222 (cefepime-zidebactam, 2 g + 1g, every 8 h [q8h]) is in clinical development for the treatment of infections caused by carbapenem-resistant and multidrug-resistant (MDR) Gram-negative bacilli. We determined the in vitro susceptibility of 1,385 clinical isolates of non-carbapenem-susceptible Enterobacterales, MDR Pseudomonas aeruginosa (also non-carbapenem susceptible), Stenotrophomonas maltophilia, and Burkholderia spp. collected worldwide (49 countries) from 2014 to 2016 to cefepime-zidebactam (1:1 ratio), ceftazidime-avibactam, imipenem-relebactam, ceftolozane-tazobactam, and colistin using the CLSI broth microdilution method. Cefepime-zidebactam inhibited 98.5% of non-carbapenem-susceptible Enterobacterales (n = 1,018) at ≤8 μg/ml (provisional cefepime-zidebactam-susceptible MIC breakpoint). Against the subset of metallo-β-lactamase (MBL)-positive Enterobacterales (n = 214), cefepime-zidebactam inhibited 94.9% of isolates at ≤8 μg/ml. Further, it inhibited 99.6% of MDR P. aeruginosa (n = 262) isolates at ≤32 μg/ml (proposed cefepime-zidebactam-susceptible pharmacokinetic/pharmacodynamic MIC breakpoint), including all MBL-positive isolates (n = 94). Moreover, cefepime-zidebactam was active against the majority of isolates of Enterobacterales (≥95%) and P. aeruginosa (99%) that were not susceptible to ceftazidime-avibactam, ceftolozane-tazobactam, imipenem-relebactam, and colistin. Most isolates (99%) of S. maltophilia (n = 101; MIC50, 8 μg/ml; MIC90, 32 μg/ml) and Burkholderia spp. (n = 4; MIC range, 16 to 32 μg/ml) were also inhibited by cefepime-zidebactam at ≤32 μg/ml. The activity of cefepime-zidebactam against carbapenem-resistant Gram-negative bacteria is ascribed to its β-lactam enhancer mechanism of action (i.e., zidebactam binding to penicillin binding protein 2 [PBP2] and its universal stability to both serine β-lactamases and MBLs). The results from this study support the continued development of cefepime-zidebactam as a potential therapy for infections caused by Enterobacterales, P. aeruginosa, and other nonfermentative Gram-negative bacilli where resistance to marketed antimicrobial agents is a limiting factor.
INTRODUCTION
The prevalence of infections caused by carbapenem-resistant and multidrug-resistant (MDR) Enterobacterales, Pseudomonas aeruginosa, and other nonfermentative Gram-negative bacilli is increasing worldwide (1–3). These infections contribute significantly to increased patient morbidity and mortality, length of hospital stay, and medical costs; safe and effective treatment options for these infections may be limited for some patients (1, 4). The World Health Organization (WHO) recently recognized MDR Gram-negative bacilli as a global public health crisis and listed both carbapenem-resistant Enterobacterales and carbapenem-resistant P. aeruginosa as bacterial pathogens requiring critical priority for research and development of new antimicrobial agents (2).
In the recent past, development of new β-lactam/β-lactamase inhibitor combinations have shown success in overcoming resistance mediated by an evolving and expanding compendium of β-lactamases, including Ambler class A serine-based carbapenemases (e.g., Klebsiella pneumoniae carbapenemase [KPC]), acquired class C (AmpC) β-lactamases (e.g., CMY, DHA), and some class D (e.g., OXA-48-like) β-lactamases (5). However, these β-lactam/β-lactamase inhibitor combinations (ceftazidime-avibactam, ceftolozane-tazobactam, imipenem-relebactam, and meropenem-vaborbactam) do not provide inhibitory activity against isolates carrying Ambler class B metallo-β-lactamases (MBLs), a group of carbapenemases of increasing clinical importance worldwide (5). Further, these newer β-lactam/β-lactamase inhibitor combinations also lack comprehensive activity against MDR and extensively drug-resistant (XDR) P. aeruginosa, such as those expressing MBL and other nonenzymatic mechanisms of resistance concurrently. An additional emerging concern is the frequent reporting of Enterobacterales carrying mutations within serine carbapenemases (i.e., amino acid modifications within the Ω-loop) that demonstrate resistance to ceftazidime-avibactam, although many of these isolates regain susceptibility to carbapenems (5).
Compared to newer β-lactamase inhibitors (avibactam, relebactam, vaborbactam), zidebactam, a novel non-β-lactam bicycloacyl hydrazide and a component of WCK 5222, functions both as a β-lactamase inhibitor (inhibits Ambler class A [including KPCs and many extended-spectrum β-lactamase (ESBLs)] and class C serine β-lactamases) and a specific inhibitor of penicillin binding protein 2 (PBP2) (6, 7). Zidebactam is slated to enter phase 3 clinical development in combination with cefepime for the treatment of resistant Gram-negative infections using an anticipated clinical dose of 2 g cefepime/1 g zidebactam administered every 8 h (ClinicalTrials registration no. NCT02707107) (6).
Combining cefepime with zidebactam is rational for several reasons. Cefepime is a broad-spectrum cephem (i.e., fourth-generation cephalosporin) that binds primarily to PBP3 but also to PBP1a of Enterobacterales and possesses activity against aerobic/facultative Gram-positive and Gram-negative bacteria, including P. aeruginosa. AmpC has a low affinity for cefepime and, therefore, cefepime retains activity against AmpC derepressed species of Enterobacterales. Cefepime has multiple clinical indications in its current U.S. FDA product package insert that include the treatment of pneumonia (moderate to severe), empirical therapy for febrile neutropenic patients, uncomplicated and complicated urinary tract infections (including pyelonephritis), uncomplicated skin and skin structure infections, and complicated intra-abdominal infections (7). When cefepime is combined with zidebactam, the concomitant inactivation of multiple PBPs leads to pronounced improvement of antibacterial activity (β-lactam enhancer mechanism). Therefore, even though zidebactam does not inhibit MBLs and class D carbapenemases directly, the cefepime-zidebactam combination is active against isolates expressing these enzymes owing to its unhindered PBP2 binding, an outcome of its universal β-lactamase stability (both serine β-lactamases and MBLs) (8). Combining cefepime with zidebactam offers a potential treatment for infections with a current cefepime indication caused by isolates of Gram-negative bacilli resistant to cefepime alone, such as carbapenem-resistant (KPC and MBL-producing) isolates, and for many MDR isolates.
In the current study, we determined the in vitro activities of cefepime-zidebactam (in a fixed ratio of 1:1), ceftazidime-avibactam, imipenem-relebactam, ceftolozane-tazobactam, and colistin against a contemporary (2014 to 2016), global (Africa, Asia, Europe, Latin America, Middle East, North America, and South Pacific) collection of 1,385 clinical isolates of Gram-negative bacilli with non-carbapenem-susceptible and MDR phenotypes.
RESULTS
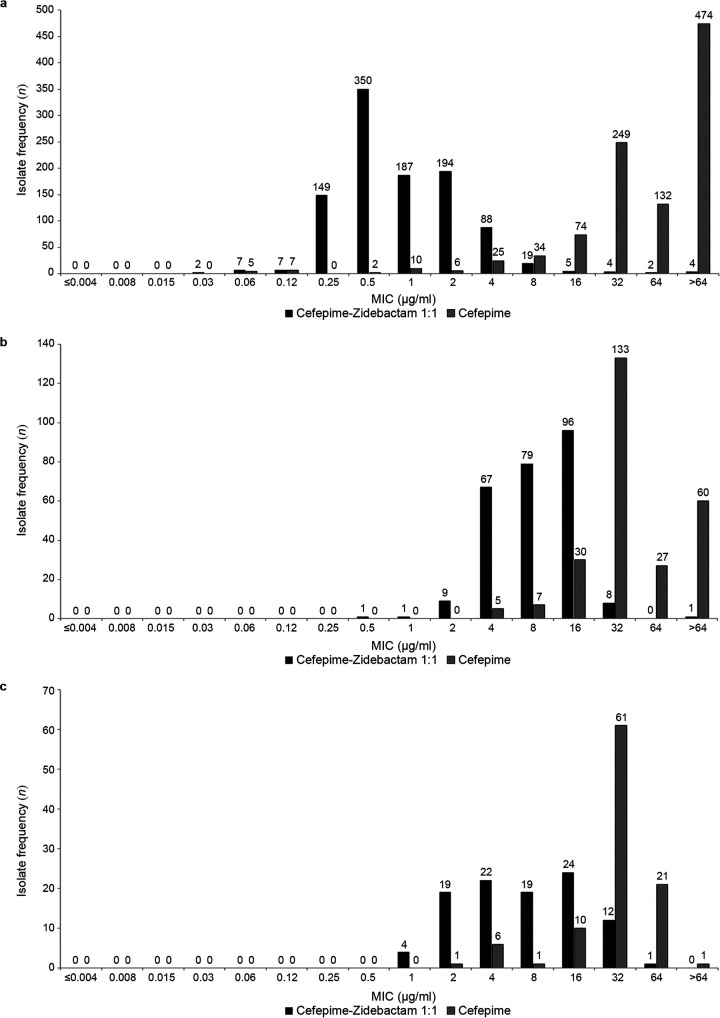
The in vitro activities of cefepime-zidebactam and comparator agents against 1,018 clinical isolates of Enterobacterales with non-carbapenem-susceptible phenotypes are summarized in Table 1. Individually, both cefepime and zidebactam exhibited limited activity (MIC90, >64 μg/ml) against these isolates. In combination, cefepime-zidebactam in a 1:1 ratio (MIC90, 4 μg/ml) demonstrated a ≥32-fold reduction in MIC90 compared to either cefepime or zidebactam alone. Figure 1 clearly demonstrates the shift to lower MICs for cefepime-zidebactam compared to cefepime alone. The combination cefepime-zidebactam inhibited 98.5% of isolates at the provisional cefepime-zidebactam-susceptible MIC breakpoint (≤8 μg/ml) based on the anticipated clinical dose of cefepime-zidebactam (2 g cefepime/1 g zidebactam administered every 8 h). In comparison, a clinical dose of cefepime alone of 2 g every 8 h supports the use of the cefepime susceptible-dose-dependent (SDD) category breakpoint for Enterobacterales (≤8 μg/ml) (9). The susceptibility of isolates of non-carbapenem-susceptible Enterobacterales to cefepime-zidebactam was greater than that to ceftazidime-avibactam (77.5%), imipenem-relebactam (64.1%), ceftolozane-tazobactam (2.6%), and colistin (78.1% intermediate susceptibility). Moreover, at ≤8 μg/ml, cefepime-zidebactam inhibited 94.8%, 98.5%, 96.4%, and 95.5% of Enterobacterales that were not susceptible to ceftazidime-avibactam, ceftolozane-tazobactam, or imipenem-relebactam or were colistin-resistant, respectively (Table 2).
TABLE 1.
In vitro activity of cefepime-zidebactam and comparator agents against 1,018 clinical isolates of non-carbapenem-susceptible Enterobacterales
| Antibacterial agent | MICs (μg/ml) |
MIC interpretationa,c |
|||||
|---|---|---|---|---|---|---|---|
| MIC range | MIC50 | MIC90 | S (%) | SDD (%) | I (%) | R (%) | |
| Cefepime-zidebactam 1:1b | ≤0.03 to >64 | 0.5 | 4 | 98.5 | NA | NA | 1.5 |
| Cefepime | ≤0.06 to >64 | 64 | >64 | 2.9 | 5.8 | NA | 91.3 |
| Zidebactam | ≤0.03 to >64 | 2 | >64 | NA | NA | NA | NA |
| Ceftazidime-avibactam | ≤0.06 to >64 | 1 | >64 | 77.5 | NA | NA | 22.5 |
| Ceftolozane-tazobactam | ≤0.06 to >64 | >64 | >64 | 2.6 | NA | 1.9 | 95.6 |
| Colistind | ≤0.25 to >8 | ≤0.25 | >8 | NA | NA | 78.1 | 21.9 |
| Imipenem-relebactam | ≤0.06 to >64 | 0.5 | 16 | 64.1 | NA | 8.7 | 27.1 |
| Meropeneme | ≤0.06 to >64 | 16 | >64 | 4.4 | NA | 5.2 | 90.4 |
S, susceptible; SDD, susceptible-dose dependent; I, intermediate; R, resistant.
Cefepime-zidebactam MICs were interpreted using provisional breakpoints of ≤8 μg/ml (susceptible) and ≥16 μg/ml (resistant) based on the anticipated clinical dose of cefepime-zidebactam (2 g cefepime and 1 g zidebactam administered every 8 h) despite PK/PD data supporting a cefepime-zidebactam susceptible MIC breakpoint of 64 μg/ml. A clinical dose of cefepime alone of 2 g every 8 h is published in Appendix E of the 2020 (M100, 30th edition) CLSI breakpoints to support use of the cefepime susceptible-dose dependent (SDD) category breakpoint for Enterobacterales (≤8 μg/ml).
NA, there are no MIC breakpoints available for this agent or there are no MIC breakpoint criteria for this interpretative category or the MIC breakpoint criteria are not applicable for a particular agent.
Applying 2020 (v 10.0) EUCAST breakpoints (≤2 μg/ml, susceptible; >2 μg/ml, resistant) to colistin MICs for Enterobacterales, 78.1% of isolates were colistin-susceptible and 21.9% of isolates were colistin-resistant.
Forty-five isolates that tested intermediate or resistant (not susceptible) to one or more carbapenems (ertapenem, imipenem, or meropenem) in previous studies tested susceptible to meropenem in the current study.
FIG 1.
MIC frequency distribution histograms comparing cefepime-zidebactam and cefepime alone for (a) 1,018 clinical isolates of non-carbapenem-susceptible Enterobacterales, (b) 262 clinical isolates of MDR (also not carbapenem susceptible) P. aeruginosa, and (c) 101 clinical isolates of S. maltophilia.
TABLE 2.
In vitro activity of cefepime-zidebactam against clinical isolates of Enterobacterales that were not carbapenem susceptible and not susceptible to comparator agents
| Category (n) | Data for cefepime-zidebactam 1:1a
|
||||
|---|---|---|---|---|---|
| MICs (μg/ml) |
MIC interpretationb
|
||||
| MIC range | MIC50 | MIC90 | S (%) | R (%) | |
| Ceftazidime-avibactam not susceptible (229) | 0.12 to >64 | 0.5 | 4 | 94.8 | 5.2 |
| Ceftolozane-tazobactam not susceptible (992) | 0.12 to >64 | 0.5 | 4 | 98.5 | 1.5 |
| Colistin-resistant (223) | 0.06 to >64 | 1 | 4 | 95.5 | 4.5 |
| Imipenem-relebactam not susceptible (365) | 0.06 to >64 | 1 | 4 | 96.4 | 3.6 |
Cefepime-zidebactam MICs were interpreted using provisional breakpoints of ≤8 μg/ml (susceptible) and ≥16 μg/ml (resistant) based on the anticipated clinical dose of cefepime-zidebactam (2 g cefepime and 1 g zidebactam administered every 8 h) despite PK/PD data supporting a cefepime-zidebactam susceptible MIC breakpoint of 64 μg/ml. A clinical dose of cefepime alone of 2 g every 8 h is published in Appendix E of the 2020 (M100, 30th edition) CLSI breakpoints to support use of the cefepime susceptible-dose dependent (SDD) category breakpoint for Enterobacterales (≤8 μg/ml).
SDD, susceptible-dose dependent; R, resistant.
The in vitro activities of cefepime-zidebactam and comparator agents against 262 clinical isolates of MDR P. aeruginosa (all MDR isolates were imipenem intermediate or resistant) are shown in Table 3. Cefepime (MIC90, >64 μg/ml) and zidebactam (MIC90, 32 μg/ml) were less potent individually than the combination of cefepime-zidebactam in a 1:1 ratio (MIC90, 16 μg/ml). Cefepime-zidebactam demonstrated at least a 2-fold reduction in the MIC90 value to 16 μg/ml compared to zidebactam alone and a >4-fold reduction compared to cefepime alone. Figure 1 compares the MIC frequency distributions for cefepime-zidebactam and cefepime alone against MDR P. aeruginosa and clearly demonstrates a shift to lower MICs for cefepime-zidebactam compared to cefepime alone. Cefepime-zidebactam inhibited 59.9% of isolates at ≤8 μg/ml (the cefepime-susceptible CLSI MIC breakpoint) (9) and 99.6% of isolates at the proposed cefepime-zidebactam-susceptible pharmacokinetic/pharmacodynamics (PK/PD) MIC breakpoint of ≤32 μg/ml (10–12). At the cefepime-susceptible CLSI MIC breakpoint and the PK/PD MIC breakpoint, the susceptibility of MDR P. aeruginosa to cefepime-zidebactam (59.9% and 99.6%, respectively) was greater than that of ceftazidime-avibactam (26.3%), ceftolozane-tazobactam (21.8%), and imipenem-relebactam (17.2%). Moreover, cefepime-zidebactam at ≤32 μg/ml inhibited >99% of ceftazidime-avibactam-resistant, non-ceftolozane-tazobactam-susceptible, and non-imipenem-relebactam-susceptible MDR P. aeruginosa.
TABLE 3.
In vitro activity of cefepime-zidebactam and comparator agents against 262 clinical isolates of MDR P. aeruginosa
| Antibacterial agent | MICs (μg/ml) |
MIC interpretation (%)a,c |
|||||
|---|---|---|---|---|---|---|---|
| MIC range | MIC50 | MIC90 | S PK/PD | S | I | R | |
| Cefepime-zidebactam 1:1b | 0.5 to >64 | 8 | 16 | 99.6 | 59.9 | 36.6 | 3.4 |
| Cefepime | 4 to >64 | 32 | >64 | NA | 4.6 | 11.5 | 84.0 |
| Zidebactam | 0.5 to >64 | 16 | 32 | NA | NA | NA | NA |
| Ceftazidime-avibactam | 0.5 to >64 | 32 | >64 | NA | 26.3 | NA | 73.7 |
| Ceftolozane-tazobactam | 0.5 to >64 | >64 | >64 | NA | 21.8 | 5.7 | 72.5 |
| Colistind | ≤0.25 to >8 | 1 | 1 | NA | NA | 99.6 | 0.4 |
| Imipenem-relebactam | 0.25 to >64 | 16 | >64 | NA | 17.2 | 22.1 | 60.7 |
| Meropeneme | 0.12 to >64 | 64 | >64 | NA | 1.9 | 1.2 | 97.0 |
S, susceptible; SDD, susceptible-dose dependent; I, intermediate; R, resistant.
Cefepime-zidebactam MICs for P. aeruginosa were interpreted using both the proposed PK/PD susceptible breakpoint of ≤32 μg/ml and using the 2020 (M100, 30th edition) CLSI breakpoints for cefepime tested against P. aeruginosa (≤8 μg/ml, susceptible; 16 μg/ml, intermediate; ≥32 μg/ml, resistant).
NA, there are no MIC breakpoints available for this agent or there are no MIC breakpoint criteria for this interpretative category.
Applying 2020 (v 10.0) EUCAST breakpoints (≤2 μg/ml, susceptible; >2 μg/ml, resistant) to colistin MICs for P. aeruginosa, 99.6% of isolates were colistin susceptible and 0.4% of isolates were colistin resistant.
Five isolates that tested intermediate or resistant to imipenem and/or meropenem in previous studies tested susceptible to meropenem in the current study.
The in vitro activities of cefepime-zidebactam and comparator agents against 101 clinical isolates of S. maltophilia are shown in Table 4. S. maltophilia is intrinsically resistant to carbapenems (9). The combination of cefepime-zidebactam (MIC50, 8 μg/ml; MIC90, 32 μg/ml) was 2- to 4-fold more potent than cefepime alone and 4- to >8-fold more potent than zidebactam alone. Figure 1 compares the MIC frequency distributions for cefepime-zidebactam and cefepime against the isolates of S. maltophilia tested and clearly demonstrates a shift to lower MICs for cefepime-zidebactam compared to cefepime alone. Colistin was the most active agent tested against S. maltophilia (MIC90, 4 μg/ml).
TABLE 4.
In vitro activity of cefepime-zidebactam and comparator agents against 101 clinical isolates of S. maltophilia
| Antibacterial agent | MICs (μg/ml) |
||
|---|---|---|---|
| MIC range | MIC50 | MIC90 | |
| Cefepime-zidebactam 1:1 | 1 to 64 | 8 | 32 |
| Cefepime | 2 to >64 | 32 | 64 |
| Zidebactam | >64 | >64 | >64 |
| Ceftazidime-avibactam | 0.5 to >64 | 16 | 64 |
| Ceftolozane-tazobactam | ≤0.25 to >64 | 16 | >64 |
| Colistin | ≤0.25 to >8 | 0.5 | 4 |
| Imipenem-relebactam | 1 to >64 | >64 | >64 |
| Meropenem | 1 to >64 | >64 | >64 |
Zidebactam alone and cefepime alone both exhibited limited activity against the four isolates of Burkholderia spp. tested, with MIC ranges of 16 to 32 and 64 to >64 μg/ml, respectively. Cefepime-zidebactam in combination demonstrated MICs in the range of 16 to 32 μg/ml. All four Burkholderia spp. isolates were susceptible to meropenem (MIC, ≤4 μg/ml) and ceftazidime-avibactam (ceftazidime MIC, ≤8 μg/ml) (9).
Table 5 summarizes the carbapenemases present in 994 clinical isolates of Enterobacterales and compares MICs for cefepime-zidebactam, ceftazidime-avibactam, imipenem-relebactam, and ceftolozane-tazobactam tested against MBL-positive isolates and MBL-negative, serine carbapenemase-positive isolates. The 214 isolates of Enterobacterales expressing MBL (96 Enterobacter cloacae, 73 Klebsiella pneumoniae, 14 Citrobacter freundii, 12 Escherichia coli, 9 Serratia marcescens, 8 Klebsiella oxytoca, 1 Enterobacter asburiae, and 1 Enterobacter kobei) comprised 115 isolates harboring NDM, 92 isolates harboring VIM, and 7 isolates harboring IMP. The majority of these isolates (79.0%, 169/214) also carried one or more ESBLs, AmpC β-lactamases, and/or serine carbapenemases (data not shown). Cefepime-zidebactam at ≤8 μg/ml inhibited 94.9% of 214 isolates of MBL-positive Enterobacterales (inclusive of 19 isolates coexpressing MBL and OXA-48-like and 4 isolates coexpressing MBL and KPC). Expectedly, ceftazidime-avibactam, imipenem-relebactam, and ceftolozane-tazobactam were inactive against isolates harboring an MBL. Cefepime-zidebactam and ceftazidime-avibactam both inhibited all or the majority of isolates carrying KPC and/or OXA-48-like or GES serine carbapenemases; imipenem-relebactam was poorly active or inactive against isolates with OXA-48-like (18.9% susceptible) or GES carbapenemases (50.0% susceptible), and ceftolozane-tazobactam was inactive against all serine carbapenemases.
TABLE 5.
In vitro activity of cefepime-zidebactam and comparator agents against isolates of non-carbapenem-susceptible Enterobacterales carrying carbapenemase genes
| Group (n)a | Results for FPZb,c |
Results for CZAd |
Results for IMRe
|
Results for C/Tf
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MICs (μg/ml) |
S (%) | MICs (μg/ml) |
S (%) | MICs (μg/ml) |
S (%) | MICs (μg/ml) |
S (%) | |||||
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |||||
| MBL-positiveg | ||||||||||||
| MBL (191) | 0.5 | 4 | 94.8 | >64 | >64 | 1.0 | 16 | 64 | 1.6 | >64 | >64 | 0.5 |
| MBL + OXA-48-like (19) | 1 | 8 | 94.7 | >64 | >64 | 26.3 | 64 | >64 | 0 | >64 | 0 | |
| MBL + KPC (4)h | 0.5–4 | 100 | >64 | 0 | 1-64 | 25.0 | >64 | 0 | ||||
| MBL-negative, serine carbapenemase-positive | ||||||||||||
| KPC (561) | 0.5 | 2 | 100 | 1 | 4 | 97.9 | 0.25 | 1 | 94.3 | 64 | >64 | 0.9 |
| KPC + OXA-48-like (2)h | 0.5–1 | 100 | 0.5–2 | 100 | 0.25–1 | 100 | >64 | 0 | ||||
| OXA-48-like (111) | 0.5 | 2 | 100 | 0.5 | 2 | 97.3 | 2 | 8 | 18.9 | >64 | >64 | 0.9 |
| GES carbapenemase (2)h ,i | 1–2 | 100 | 4 | 100 | 1–2 | 50.0 | >64 | 0 | ||||
| MBL-negative, serine carbapenemase-negative (104)j | 4 | 8 | 96.4 | 1 | 4 | 97.3 | 0.5 | 4 | 77.3 | >64 | >64 | 14.5 |
Isolates not susceptible to imipenem were screened for β-lactamase genes; 45 isolates that tested intermediate or resistant to imipenem and/or meropenem and/or ertapenem in previous studies tested susceptible to meropenem in the current study; 994 of 1,018 non-carbapenem-susceptible Enterobacterales were molecularly characterized. Most isolates (93.1%, 925/994) cocarried extended-spectrum β-lactamases (ESBLs), original-spectrum β-lactamases (e.g., TEM-1, SHV-1, SHV-11), and/or chromosomal- and plasmid-mediated AmpC cephalosporinases which were not included in the analysis because they do not affect the activity of cefepime-zidebactam.
FPZ, cefepime-zidebactam.
Cefepime-zidebactam MICs were interpreted using provisional breakpoints of ≤8 μg/ml (susceptible) and ≥16 μg/ml (resistant) based on the anticipated clinical dose of cefepime-zidebactam (2 g cefepime and 1 g zidebactam administered every 8 h) despite PK/PD data supporting a cefepime-zidebactam susceptible MIC breakpoint of 64 μg/ml. A clinical dose of cefepime alone of 2 g every 8 h is published in Appendix E of the 2020 (M100, 30th edition) CLSI breakpoints to support use of the cefepime susceptible-dose dependent (SDD) category breakpoint for Enterobacterales (≤8 μg/ml).
CZA, ceftazidime-avibactam.
IMR, imipenem-relebactam.
C/T, ceftolozane-tazobactam.
MBLs included NDM (115 isolates), VIM (92 isolates), and IMP (7 isolates); the ESBLs included SHV, CTX-M, VEB, and the endogenous ESBL common to Klebsiella oxytoca.
No MIC50 or MIC90 is provided if <10 isolates were present in a group; in those instances, an MIC range is provided in the MIC50 column.
The GES carbapenemase-positive isolates both carried GES-20.
A total of 15 of 104 isolates did not have an acquired β-lactamase (i.e., ESBL, plasmid-mediated AmpC) detected.
Table 6 shows β-lactamases present in 229 clinical isolates of P. aeruginosa and compares MICs for cefepime-zidebactam, ceftazidime-avibactam, imipenem-relebactam, and ceftolozane-tazobactam tested against MBL-positive isolates and against MBL-negative isolates with specific types of acquired serine β-lactamases. The 94 isolates with an MBL were composed of 89 isolates with VIM, 3 isolates with NDM, and 2 isolates with IMP. The majority of isolates with an MBL did not carry an ESBL and/or serine carbapenemase (94.6%, 89/94). At the proposed PK/PD susceptible breakpoint of ≤32 μg/ml, cefepime-zidebactam inhibited 100% of isolates carrying an MBL, 100% of MBL-negative isolates carrying an acquired serine β-lactamase, and 98.9% of non-carbapenem-susceptible isolates where no β-lactamase genes were detected. Ceftazidime-avibactam, imipenem-relebactam, and ceftolozane-tazobactam were inactive against MBL-harboring P. aeruginosa. Even MBL-negative, serine carbapenemase-positive isolates of P. aeruginosa exhibited limited susceptibility to these agents. It is likely that the P. aeruginosa tested in this study possessed other non-β-lactamase-mediated mechanisms of β-lactam resistance (e.g., efflux, porin loss, PBP mutations).
TABLE 6.
In vitro activity of cefepime-zidebactam and comparative agents against isolates of non-carbapenem-susceptible P. aeruginosa carrying β-lactamase genes
| Group (n)a,b | Results for FPZd,e
|
Results for CZAf |
Results for IMRg |
Results for C/Th |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MICs (μg/ml) |
S PK/PD (%) | MICs (μg/ml) |
S (%) | MICs (μg/ml) |
S (%) | MICs (μg/ml) |
S (%) | |||||
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |||||
| MBL-positive (94)i | 8 | 16 | 100 | 32 | >64 | 1.1 | >64 | >64 | 0 | >64 | >64 | 0 |
| MBL-negative, acquired serine β-lactamase-positive | ||||||||||||
| All isolates (43) | 8 | 16 | 100 | 32 | >64 | 23.8 | 8 | 32 | 7.1 | >64 | >64 | 0 |
| GES carbapenemase (14)j | 4 | 16 | 100 | 4 | 64 | 64.3 | 16 | 64 | 0 | 16 | >64 | 0 |
| GES ESBL (5)c,k | 4–8 | 100 | 16–32 | 0 | 4–8 | 0 | 64–>64 | 0 | ||||
| VEB (16) | 8 | 16 | 100 | 64 | >64 | 0 | 4 | 8 | 12.5 | >64 | >64 | 0 |
| PER (7)c | 16–32 | 100 | 16–64 | 0 | 4–8 | 0 | 64–>64 | 0 | ||||
| KPC (1)c | 4 | 100 | 2 | 100 | 1 | 100 | >64 | 0 | ||||
| No acquired β-lactamase detected (93) | 8 | 16 | 98.9 | 8 | >64 | 53.8 | 4 | 8 | 38.7 | 4 | 64 | 54.8 |
Isolates not susceptible to imipenem were screened for β-lactamase genes; 5 isolates that tested intermediate or resistant to imipenem and/or meropenem in previous studies tested susceptible to meropenem in the current study; 229 of 262 non-carbapenem-susceptible P. aeruginosa were molecularly characterized.
All isolates, including those where no acquired β-lactamase was detected, are presumed to contain the chromosomal ampC gene (PDC) common to P. aeruginosa.
No MIC50 or MIC90 is provided if <10 isolates were present in a group; in those instances, an individual MIC or an MIC range is provided in the MIC50 column.
FPZ, cefepime-zidebactam.
Cefepime-zidebactam MICs for P. aeruginosa were interpreted based on the proposed PK/PD susceptible breakpoint of ≥32 μg/ml.
CZA, ceftazidime-avibactam.
IMR, imipenem-relebactam.
C/T, ceftolozane-tazobactam.
The MBLs included VIM (89 isolates), NDM (3 isolates), and IMP (2 isolates). Five isolates carried an ESBL in addition to an MBL; the five ESBL-positive isolates comprised 3 isolates with VEB, and 1 isolate each with GES-1 and SHV. The MBL present in all isolates with an ESBL was VIM.
GES enzymes with carbapenemase activity were GES-2 (2 isolates), GES-5 (5 isolates), GES-6 (3 isolates), and GES-19-20 (4 isolates).
GES enzymes with ESBL activity were GES-1 (5 isolates).
DISCUSSION
The spread of carbapenem-resistant and MDR Gram-negative pathogens frequently involves successful high-risk clones with enhanced abilities to develop and/or acquire antimicrobial resistance determinants and to cause nosocomial outbreaks (1, 2, 13–15). Carbapenemase genes carried by mobile genetic elements on plasmids facilitate horizontal spread within and between species and promote the success of epidemic clones (13, 14). The development of new antimicrobial agents active against carbapenem-resistant, particularly MBL-producing, and MDR Gram-negative bacilli is critical to address current and projected increases in infections caused by these pathogens (2). The in vitro and in vivo PK/PD studies conducted in the past have shown potent activity for cefepime-zidebactam against MDR Enterobacterales and P. aeruginosa (8, 10–12, 16–18) and synergistic, rapid cidality against isolates of Gram-negative bacilli carrying both serine-based β-lactamases and MBLs (10, 19–21).
In the current in vitro study, we observed that cefepime-zidebactam (1:1 ratio) inhibited 98.5% of Enterobacterales at the provisional cefepime-zidebactam-susceptible MIC breakpoint (≤8 μg/ml) (9). In a previous study, >99% of 2,560 unselected isolates of Enterobacterales (E. coli, K. pneumoniae, Enterobacter spp.) prospectively collected over a 3-month period in 2017 from seven medical centers in New York City had cefepime-zidebactam MICs (tested at a ratio of 1:1) of ≤2 μg/ml (22). Moreover, 93 additional (selected) isolates of blaKPC-positive K. pneumoniae were tested for which cefepime-zidebactam exhibited an MIC90 of 2 μg/ml. In another study of prospectively collected isolates (global surveillance program in 2013 and 2015), Sader et al. (16) reported that 99.3% of carbapenem-resistant Enterobacterales (n = 153) had cefepime-zidebactam (1:1 ratio) MICs of ≤8 μg/ml, similar to the observations we made in the current study. The observations noted in a second study by Sader et al., showing cefepime-zidebactam MICs of ≤2 μg/ml against KPC-positive isolates of Enterobacterales (17), are also in line with our current study.
In the current study, cefepime-zidebactam inhibited 94.9% of MBL-positive isolates of Enterobacterales at ≤8 μg/ml (the provisional cefepime-zidebactam-susceptible MIC breakpoint) (9). Sader et al., previously tested 20 isolates of MBL-positive Enterobacterales and reported a similar result (MIC50, 0.5 μg/ml; MIC90, 8 μg/ml) (17). Livermore et al. reported that 31 of 35 isolates of Enterobacterales with MBLs had MICs of ≤2 μg/ml for cefepime-zidebactam (tested at a ratio of 1:1) (8). Lutgring et al. tested 275 contemporary NDM-producing Enterobacterales collected from 30 U.S. states through the Centers for Disease Control and Prevention’s Antibiotic Resistance Laboratory Network and reported an MIC50 of 0.25 μg/ml and an MIC90 of 4 μg/ml for cefepime-zidebactam (23).
In the current study, cefepime-zidebactam inhibited 99.6% of MDR P. aeruginosa at the proposed PK/PD susceptible MIC breakpoint (≤32 μg/ml) (10–12). Sader et al. previously reported that 99.5% of prospectively collected (unselected) isolates (n = 1,291) of P. aeruginosa collected by a global surveillance program in 2013 and 2015 had cefepime-zidebactam (tested at a ratio of 1:1) MICs of ≤8 μg/ml (16). Similarly, Khan et al. reported that 98.5% of 271 isolates of P. aeruginosa prospectively collected in a 3-month period in 2017 from seven medical centers in New York City had cefepime-zidebactam MICs of ≤8 μg/ml (tested at a ratio of 1:1) (22). Khan et al. also reported that 77.8% of carbapenem-resistant P. aeruginosa (n = 126) isolates had cefepime-zidebactam MICs of ≤8 μg/ml (22). Another study of P. aeruginosa causing pneumonia in U.S. hospitals in 2018 reported that cefepime-zidebactam at a concentration of ≤16 μg/ml inhibited 99.5% of MDR (n = 186) and non-meropenem-susceptible (n = 194) isolates, 99.2% of XDR (n = 119) isolates, and 97.2% of non-ceftazidime-avibactam-susceptible (n = 36) isolates; all resistant isolates of P. aeruginosa were inhibited by cefepime-zidebactam at a concentration of ≤64 μg/ml (24).
We observed that at ≤32 μg/ml (PK/PD breakpoint), cefepime-zidebactam inhibited most P. aeruginosa isolates carrying an MBL (100%), most MBL-negative isolates carrying an acquired serine β-lactamase (100%), and most non-carbapenem-susceptible isolates where no β-lactamase genes were detected (98.9%). Sader et al. previously tested 12 isolates of MBL-positive P. aeruginosa and reported that 91.7% of isolates had cefepime-zidebactam MICs of ≤8 μg/ml (MIC50, 4 μg/ml; MIC90, 8 μg/ml) and tested 21 isolates of P. aeruginosa that overexpressed AmpC and reported that 90.5% of isolates had cefepime-zidebactam MICs of ≤8 μg/ml (MIC50, 4 μg/ml; MIC90, 8 μg/ml) (17). Livermore et al. reported that 9 of 10 isolates with derepressed AmpC (PDC), 8 of 10 with MBLs, and 8 of 10 with upregulated efflux were susceptible to cefepime-zidebactam at 8 μg/ml (tested at a ratio of 1:1) (8).
In the current study, the combination of cefepime-zidebactam (MIC50, 8 μg/ml; MIC90, 32 μg/ml) was 2- to 4-fold more potent than cefepime alone and 4- to >8-fold more potent than zidebactam alone against S. maltophilia (Table 4). Livermore et al. previously reported that zidebactam potentiated the in vitro activity of cefepime against most isolates of S. maltophilia, reflecting either an enhancer effect or, more probably, inhibition of the L-2 cephalosporinase, which is known to result in resistance to cefepime (8).
The activity of cefepime-zidebactam against non-carbapenem-susceptible isolates is credited to its novel β-lactam enhancer mechanism of action (i.e., the ability of zidebactam to bind to PBP2 and its universal β-lactamase stability, including both serine β-lactamases and MBLs). A recent study showed that cefepime was able to rapidly and efficiently bind with its target PBP3 in P. aeruginosa amid MBL expression and that the addition of PBP2 binding by zidebactam resulted in synergistic antibacterial action (25). Despite zidebactam lacking direct inhibitory activity against MBLs, it is able to enhance the activity of cefepime through unhindered binding to PBP2 (owing to β-lactamase stability), thus obviating the need for β-lactamase inhibition, a feature distinct from combinations such as ceftazidime-avibactam and imipenem-relebactam. Recently, Monogue et al. and Kidd et al. demonstrated pronounced bactericidal effects (1 to 2 log10 bacterial killing) of a human-simulated regimen of cefepime-zidebactam against MDR/XDR P. aeruginosa (including MBL producers; cefepime-zidebactam MICs up to 32 μg/ml) in neutropenic mouse thigh and lung infection models, respectively. (10, 12). Lepak et al. showed in vivo efficacy of cefepime-zidebactam against MBL-producing Enterobacterales in a neutropenic mouse lung infection model (21).
In conclusion, we studied a recent worldwide collection of non-carbapenem-susceptible Gram-negative bacilli and observed that cefepime-zidebactam demonstrated potent in vitro activity against Enterobacterales (MIC90, 4 μg/ml) and P. aeruginosa (MIC90, 16 μg/ml) producing the most common and important β-lactamases currently circulating, including ESBLs, AmpCs, OXA-48-like, KPCs, and MBLs for which treatment options are currently limited. The current study challenged cefepime-zidebactam with Gram-negative bacilli isolates producing multiple β-lactamases of the same class or different classes and extends data presented in previous studies (8, 10, 16–22). Results from the current study support further clinical development of cefepime-zidebactam, which demonstrates the potential to provide a therapeutic option for the treatment of infections caused by carbapenem-resistant and MDR Gram-negative bacilli.
MATERIALS AND METHODS
Isolate collection.
The 1,385 isolates of Gram-negative bacilli included in this study were selected from frozen stocked isolates maintained by IHMA (Schaumburg, IL, USA) based upon their predetermined resistance phenotypes. Isolates of Enterobacterales (n = 1,018) were chosen because they were not susceptible to carbapenems (imipenem or meropenem; MIC, ≥2 μg/ml) (9). Isolates of P. aeruginosa (n = 262) were selected based on possession of an MDR phenotype not susceptible to imipenem (MIC, ≥4 μg/ml) and resistance to both amikacin and a fluoroquinolone (9). Clinical isolates of S. maltophilia (n = 101) and Burkholderia spp. (n = 4) were selected irrespective of a previously known resistance phenotype. Species distributions of the 1,018 isolates of Enterobacterales and 367 isolates of non-Enterobacterales are provided in Table S1 in the supplemental material. All isolates included in the current study were collected during IHMA global surveillance studies from 2014 to 2016. The isolates were obtained from 204 clinical laboratories distributed across 49 countries. The geographical origins of the isolates are summarized in Table S2. All isolates were cultured from specimens collected from patients with intra-abdominal, urinary tract, skin and soft tissue, lower respiratory tract, or bloodstream infections. The identities of all of the isolates were previously confirmed by IHMA using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Billerica, MA, USA).
Isolates of Enterobacterales and P. aeruginosa were screened for the presence of genes encoding β-lactamases using published multiplex PCR assays, followed by full-gene DNA sequencing as described previously (26, 27). Specifically, isolates were screened for genes encoding metallo-β-lactamases (IMP, VIM, NDM, GIM, and SPM), serine carbapenemases (KPC, GES, and OXA-48-like [Enterobacterales] or OXA-24-like [P. aeruginosa]), ESBLs (SHV, TEM, CTX-M, VEB, PER, and GES), acquired AmpC β-lactamases (ACC, ACT, CMY, DHA, FOX, MIR, and MOX), and the chromosomal AmpC intrinsic to P. aeruginosa (PDC).
Antimicrobial susceptibility testing.
Broth microdilution panels were prepared with cation-adjusted Mueller-Hinton broth (BBL, Becton, Dickinson, Sparks, MD) following standardized CLSI methodology (9, 28). Panels were frozen at –80°C and thawed to room temperature prior to use. Doubling dilutions for cefepime-zidebactam were prepared at a ratio of the two components of 1:1 (10). A 1:1 ratio of cefepime and zidebactam was used because both agents are active antibacterials and the use of a 1:1 ratio for MIC determination eliminates activity bias due to either of the components (29, 30). Testing cefepime-zidebactam at a 1:1 ratio was accepted by the CLSI in 2017 and first published in 2018 in the 28th edition of the CLSI M100 Performance Standards for Antimicrobial Susceptibility Testing document. The CLSI decision to support the use of a 1:1 cefepime-zidebactam ratio was based on an M23 study which involved replicate MIC determinations in eight U.S. laboratories. The M23 study showed that cefepime-zidebactam MICs determined at a 1:1 ratio were highly reproducible, and quality control ranges were successfully established (29). Ceftazidime-avibactam, ceftolozane-tazobactam, and imipenem-relebactam were tested at fixed concentrations of avibactam (4 μg/ml), tazobactam (4 μg/ml), and relebactam (4 μg/ml), respectively (9). MICs were determined following the CLSI standard method for broth microdilution (9, 28). MIC endpoints were read following panel incubation at 35°C for 20 h in ambient air. Quality control testing was performed each day of testing using Escherichia coli ATCC 25922, E. coli ATCC 35218, P. aeruginosa ATCC 27853, K. pneumoniae ATCC 700603, and K. pneumoniae ATCC BAA-1705.
MICs were interpreted as susceptible, SDD (cefepime), intermediate, or resistant using CLSI breakpoints (9) for all agents tested against isolates of Enterobacterales and P. aeruginosa, with the following exceptions. Cefepime-zidebactam MICs were interpreted using provisional breakpoints of ≤8 μg/ml (susceptible) and ≥16 μg/ml (resistant) based on the anticipated clinical dose of cefepime-zidebactam (2 g cefepime-1 g zidebactam administered every 8 h) despite PK/PD data supporting a cefepime-zidebactam-susceptible MIC breakpoint of 64 μg/ml (31, 32). A clinical dose of cefepime alone of 2 g every 8 h is published in Appendix E of the 2020 (M100, 30th edition) CLSI breakpoints to support use of the cefepime SDD category breakpoint for Enterobacterales (≤8 μg/ml) (9). For P. aeruginosa, a PK/PD susceptible breakpoint of ≤32 μg/ml (based on in vivo PK/PD studies) (10–12) was employed for determining susceptibility to cefepime-zidebactam. Zidebactam MICs were not interpreted, as no breakpoints exist for it as a standalone agent. In regard to colistin tested against Enterobacterales and P. aeruginosa, EUCAST MIC interpretative breakpoints (susceptible, ≤2 μg/ml; resistant, >2 μg/ml) (33) were applied in addition to those of the CLSI (9) because of the interpretative differences that exist between these two sets of breakpoints. Imipenem-relebactam MICs were interpreted using FDA breakpoints for Enterobacterales (susceptible, ≤1 μg/ml; intermediate, 2 μg/ml; resistant, ≥4 μg/ml) and P. aeruginosa (susceptible, ≤2 μg/ml; intermediate, 4 μg/ml; resistant, ≥8 μg/ml) (34).
Regarding PK/PD MIC breakpoints for cefepime-zidebactam, the PK/PD breakpoint for cefepime-zidebactam was identified based on pharmacodynamic targets derived from a neutropenic mouse infection model and probability of attainment (PTA) targets identified (for cefepime-zidebactam, 2 g + 1 g, 1 h infusion, every 8 h [q8h]) employing a population PK (popPK) model built using phase 1 PK data (31, 32). These studies/analyses established a >98% PTA for MICs up to 64 μg/ml, thus identifying the PK/PD breakpoint for both Enterobacterales and nonfermenters. For Enterobacterales, in light of low cefepime-zidebactam MICs obtained in multiple surveillance studies, the breakpoint of ≤64 μg/ml which is supported by PK/PD target attainment analyses is several doubling dilutions higher than the MIC90 (generally 0.12 μg/ml) (16). Moreover, surveillance studies (16) show that few isolates exist at cefepime-zidebactam MICs of 16, 32, or 64 μg/ml. However, for CRE and MDR subpopulations of Enterobacterales, cefepime-zidebactam MIC frequency distributions shift to the right (higher MICs) compared to the whole population with a MIC98.5 of ≤8 μg/ml (Table 1) (16). For a drug expected to tackle contemporary CRE and MDR pathogens, the putative breakpoint should comprehensively cover such resistant isolates, provided PTA-supported breakpoints are high enough. Therefore, taking into account the above-described analyses, for Enterobacterales, a conservative susceptibility breakpoint of 8 μg/ml was employed in the current study. Likewise, for P. aeruginosa, the cefepime-zidebactam MIC frequency distribution for MDR isolates in the current study led to the identification of a susceptibility breakpoint of ≤32 μg/ml.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by Wockhardt Bio AG, Switzerland. J.A.K. is an employee of Shared Health Manitoba and the University of Manitoba and is a consultant to IHMA, Inc. M.A.H., S.K.B., and D.F.S. are employees of IHMA, which received funding from Wockhardt Bio AG to perform this study and write the manuscript. J.A.K. and the IHMA authors do not have personal financial interests in the sponsorship of the manuscript (Wockhardt Bio AG).
All authors participated in data analysis and have read and approved the final version of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. 2018. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis 66:1290–1297. doi: 10.1093/cid/cix893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 3.Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, Benito N, Grau S. 2019. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev 32:e00031-19. doi: 10.1128/CMR.00031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca-Doñate R, Moliner-Lahoz J. 2017. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis 65:644–652. doi: 10.1093/cid/cix411. [DOI] [PubMed] [Google Scholar]
- 5.Bush K, Bradford PA. 2019. Interplay between β-lactamases and new β-lactamases inhibitors. Nat Rev Microbiol 17:295–306. doi: 10.1038/s41579-019-0159-8. [DOI] [PubMed] [Google Scholar]
- 6.NIH U.S. National Library of Medicine. Clinicaltrials.gov. https://clinicaltrials.gov. Accessed 6 January 2020.
- 7.Baxter Healthcare Corporation. Cefepime package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050817s007lbl.pdf.
- 8.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1385. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. M100 CLSI, Wayne, PA. [Google Scholar]
- 10.Kidd JM, Abdelraouf K, Nicolau DP. 2020. Efficacy of human-simulated bronchopulmonary exposures of cefepime, zidebactam and the combination (WCK 5222) against MDR Pseudomonas aeruginosa in a neutropenic murine pneumonia model. J Antimicrob Chemother 75:149–155. doi: 10.1093/jac/dkz414. [DOI] [PubMed] [Google Scholar]
- 11.Bhagwat SS, Takalkar SS, Chavan RP, Friedland HD, Patel MV. 2017. WCK 5222 [Cefepime (FEP)-WCK 5107 (Zidebactam, ZID)]: unravelling sub-MIC pharmacodynamic (PD) effects employing in vivo dose fractionation studies and translating into MIC-based PK/PD target for MBL-expressing P aeruginosa (PA), abstr P283. ASM Microbe, 1 to 5 June 2017, New Orleans, LA, USA. [Google Scholar]
- 12.Monogue ML, Tabor-Rennie J, Abdelraouf K, Nicolau DP. 2019. In vivo efficacy of WCK 5222 (cefepime-zidebactam) against multidrug-resistant Pseudomonas aeruginosa in the neutropenic murine thigh infection model. Antimicrob Agents Chemother 63:e00233-19. doi: 10.1128/AAC.00233-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerqueira GC, Earl AM, Ernst CM, Grad YH, Dekker JP, Feldgarden M, Chapman SB, Reis-Cunha JL, Shea TP, Young S, Zeng Q, Delaney ML, Kim D, Peterson EM, O’Brien TF, Ferraro MJ, Hooper DC, Huang SS, Kirby JE, Onderdonk AB, Birren BW, Hung DT, Cosimi LA, Wortman JR, Murphy CI, Hanage WP. 2017. Multi-institute analysis of carbapenem resistance reveals remarkable diversity, unexplained mechanisms, and limited clonal outbreaks. Proc Natl Acad Sci U S A 114:1135–1140. doi: 10.1073/pnas.1616248114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitout JDD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 16.Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61:e00072-17. doi: 10.1128/AAC.00072-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M. 2017. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother 72:1696–1703. doi: 10.1093/jac/dkx050. [DOI] [PubMed] [Google Scholar]
- 18.Thomson KS, AbdelGhani S, Snyder JW, Thomson GK. 2019. Activity of cefepime-zidebactam against multidrug-resistant (MDR) Gram-negative pathogens. Antibiotics 8:32. doi: 10.3390/antibiotics8010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 2017. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. doi: 10.1128/AAC.02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moya B, Barcelo IM, Cabot G, Torrens G, Palwe S, Joshi P, Umarkar K, Takalkar S, Periasamy H, Bhagwat S, Patel M, Bou G, Oliver A. 2019. In vitro and in vivo activities of β-lactams in combination with the novel β-lactam enhancers zidebactam and WCK 5153 against multidrug-resistant metallo-β-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 63:e00128-19. doi: 10.1128/AAC.00128-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepak AJ, Zhao M, Andes DR. 2019. WCK 5222 (cefepime-zidebactam) pharmacodynamic target analysis against metallo-β-lactamase-producing Enterobacteriaceae in the neutropenic mouse pneumonia model. Antimicrob Agents Chemother 63:e01648-19. doi: 10.1128/AAC.01648-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan Z, Iregui A, Landman D, Quale J. 2019. Activity of cefepime/zidebactam (WCK 5222) against Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter baumannii endemic to New York City medical centres. J Antimicrob Chemother 74:2938–2942. doi: 10.1093/jac/dkz294. [DOI] [PubMed] [Google Scholar]
- 23.Lutgring JD, Balbuena R, Reese N, Gilbert SE, Ansari U, Bhatnagar A, Boyd S, Campbell D, Cochran J, Haynie J, Ilutsik J, Longo C, Swint S, Rasheed JK, Brown AC, Karlsson M. 2020. Antibiotic susceptibility of NDM-producing Enterobacterales collected in the United States in 2017 and 2018. Antimicrob Agents Chemother 64:e00499-20. doi: 10.1128/AAC.00499-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sader HS, Carvalhaes CG, Mendes RE, Castanheira M, Flamm RK. 2019. Comparison of cefepime-zidebactam (WCK 5222), ceftazidime-avibactam, and ceftolozane-tazobactam tested against Gram-negative organisms causing pneumonia in United States hospitals in 2018, poster no. 2214. IDWeek 2019, Washington, DC. [Google Scholar]
- 25.Moya B, Bhagwat S, Cabot G, Bou G, Patel M, Oliver A. 2020. Effective inhibition of PBPs by cefepime and zidebactam in the presence of VIM-1 drives potent bactericidal activity against MBL-expressing Pseudomonas aeruginosa. J Antimicrob Chemother 75:1474–1478. doi: 10.1093/jac/dkaa036. [DOI] [PubMed] [Google Scholar]
- 26.Lob SH, Biedenbach DJ, Badal RE, Kazmierczak KM, Sahm DF. 2015. Antimicrobial resistance and resistance mechanisms of Enterobacteriaceae in ICU and non-ICU wards in Europe and North America: SMART 2011–2013. J Glob Antimicrob Resist 3:190–197. doi: 10.1016/j.jgar.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Nichols WW, de Jonge BL, Kazmierczak KM, Karlowsky JA, Sahm DF. 2016. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother 60:4743–4749. doi: 10.1128/AAC.00220-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 11th ed. M07-A11 CLSI, Wayne, PA, USA. [Google Scholar]
- 29.Traczewski MM, Bhagwat SS. 2017. Cefepime-zidebactam (FEP-ZID) or WCK 5222 tier 2 broth microdilution MIC quality control versus E. coli ATCC 25922, K. pneumoniae ATCC 700603, P. aeruginosa ATCC 27853, A. baumannii NCTC 13304 and E. coli NCTC 13353, poster no. Saturday 284. ASM Microbe, 1 to 5 June 2017, New Orleans, LA, USA. [Google Scholar]
- 30.Bhagwat SS, Periasamy H, Takalkar SS, Palwe SR, Khande HN, Patel MV. 2019. The novel β-lactam enhancer zidebactam augments the in vivo pharmacodynamic activity of cefepime in a neutropenic mouse lung Acinetobacter baumannii infection model. Antimicrob Agents Chemother 63:e02146-18. doi: 10.1128/AAC.02146-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller A, Bhagwat S, Patel M, Mouton J. 2017. Population pharmacokinetics, Monte Carlo simulations and dosing recommendations of cefepime using 90 minutes infusion, including renal impairment. Poster EP0943. ECCMID 2017, Vienna, Austria. [Google Scholar]
- 32.Muller A, Mouton J. 2017. Population pharmacokinetics of zidebactam (WCK 5107), a novel beta-lactam enhancer antibiotic, in individuals with various renal functions. Poster P1954. ECCMID 2019, Amsterdam, Netherlands. [Google Scholar]
- 33.European Committee on Antimicrobial Susceptibility Testing. 2020. Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0, 2020 http://www.eucast.org. Accessed 5 January 2020.
- 34.U.S. Food and Drug Administration. Antibacterial susceptibility test interpretative criteria. https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria. Content current as of 1 January 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.