Acyclovir is an antiviral currently used for the prevention and treatment of herpes simplex virus (HSV) and varicella-zoster virus (VZV) infections. This study aimed to characterize the pharmacokinetics (PK) of acyclovir and its oral prodrug valacyclovir to optimize dosing in children. Children receiving acyclovir or valacyclovir were included in this study. PK were described using nonlinear mixed-effect modeling. Dosing simulations were used to obtain trough concentrations above a 50% inhibitory concentration for HSV or VZV (0.
KEYWORDS: antiviral agents, children, population pharmacokinetics
ABSTRACT
Acyclovir is an antiviral currently used for the prevention and treatment of herpes simplex virus (HSV) and varicella-zoster virus (VZV) infections. This study aimed to characterize the pharmacokinetics (PK) of acyclovir and its oral prodrug valacyclovir to optimize dosing in children. Children receiving acyclovir or valacyclovir were included in this study. PK were described using nonlinear mixed-effect modeling. Dosing simulations were used to obtain trough concentrations above a 50% inhibitory concentration for HSV or VZV (0.56 mg/liter and 1.125 mg/liter, respectively) and maximal peak concentrations below 25 mg/liter. A total of 79 children (212 concentration-time observations) were included: 50 were taking intravenous (i.v.) acyclovir, 22 were taking oral acyclovir, and 7 were taking both i.v. and oral acyclovir, 57 for preventive and 22 for curative purposes. A one-compartment model with first-order elimination best described the data. An allometric model was used to describe body weight effect, and the estimated glomerular filtration rate (eGFR) was significantly associated with acyclovir elimination. To obtain target maximal and trough concentrations, the more suitable initial acyclovir i.v. dose was 10 mg/kg of body weight/6 h for children with normal renal function (eGFR ≤ 250 ml/min/1.73 m2) and 15 to 20 mg/kg/6 h for children with augmented renal clearance (ARC) (eGFR > 250 ml/min/1.73 m2). The 20-mg/kg/8 h dose for oral acyclovir and valacyclovir produced effective concentrations in more than 75% of children; however, a 15-mg/kg/6 h dose, if possible, is preferred. These doses should be prospectively confirmed, and therapeutic drug monitoring could be used to refine them individually. (This study has been registered at ClinicalTrials.gov under identifier NCT02539407.)
TEXT
Acyclovir {9-[(2-hydroxyethoxy)-methyl]guanine} is an antiviral used for curative or prophylactic treatment of herpes simplex virus (HSV) and varicella-zoster virus (VZV) infections and diseases. In some cases, it can also be used as a preventive treatment against cytomegalovirus (CMV) infections and diseases following solid organ or hematopoietic stem cell transplantation (1, 2), with the knowledge that immunocompromised patients are at risk of viral infections (3). The guanosine analogue structure of acyclovir shows a selective affinity for the HSV and VZV enzyme thymidine kinase, which allows inhibition of viral DNA replication (4, 5). Due to its low bioavailability (F), between 15% and 30% (6), acyclovir is used mostly in an intravenous (i.v.) form. Valacyclovir, a prodrug of acyclovir, is more lipophilic than acyclovir owing to the addition of an l-valyl ester and enables an increase in bioavailability up to 54%. Valacyclovir is more adapted to oral dosing because it is metabolized by intestinal and hepatic esterase to acyclovir (7, 8).
Acyclovir is poorly metabolized by the liver, and its metabolite, 9-carboxymethoxyguanine, has no antiviral activity. Up to 90% of acyclovir is directly eliminated via renal excretion, with an elimination half-life between 1.43 and 2.48 h in the pediatric population (9–11).
Depending on the indications, the usual recommended doses for the pediatric population range from 5 mg/kg to 20 mg/kg of body weight every 8 h with a 1-h infusion for the acyclovir i.v. dosing and from 20 mg/kg every 8 h to 2,000 mg every 12 h for the oral valacyclovir dosing. Renal impairment is taken into consideration, with dose adjustments when creatinine clearance is lower than 50 ml/min (12, 13). Augmented renal clearance (ARC) is present in 20 to 65% of critically ill patients and is characterized by increased creatinine clearance and elimination of renally eliminated medications. No recommendation is made for these patients.
Our study aimed to characterize the pharmacokinetics (PK) of i.v. acyclovir and oral valacyclovir with a population pharmacokinetic approach, defining relevant covariates that could explain interindividual variability (IIV). The final goal was to assess current dose recommendations for children and to suggest appropriate dosing for optimal exposure.
RESULTS
Plasma concentration data.
From the 79 patients, 212 acyclovir plasma concentrations were measured (1 to 10 samples per patient) (Fig. 1). Of the 79 children, 48 were from the pediatric immune-hematology unit (IHU), 19 were from the pediatric intensive care unit (PICU), 10 were from the department of pediatric surgery, and 2 were from the department of pediatrics of the Necker-Enfants Malades Hospital (Paris, France). Forty-six samples came from patients with oral dosing, and 166 samples came from patients with i.v. dosing. Table 1 summarizes patients’ characteristics (sex, age, body weight, height, body mass index, glomerular filtration rate [eGFR]), and indications for acyclovir treatment. Administration routes were i.v. for 50 patients, oral for 22 patients, and both i.v. and oral for 7 patients. Of the 29 patients who received the oral dose, 7 received acyclovir and 22 received valacyclovir tablets. The median (interquartile range [IQR]) administered doses were 51 (5 to 101) mg/kg/day and 22 (17 to 63) mg/kg/day for i.v. and oral administration, respectively.
FIG 1.
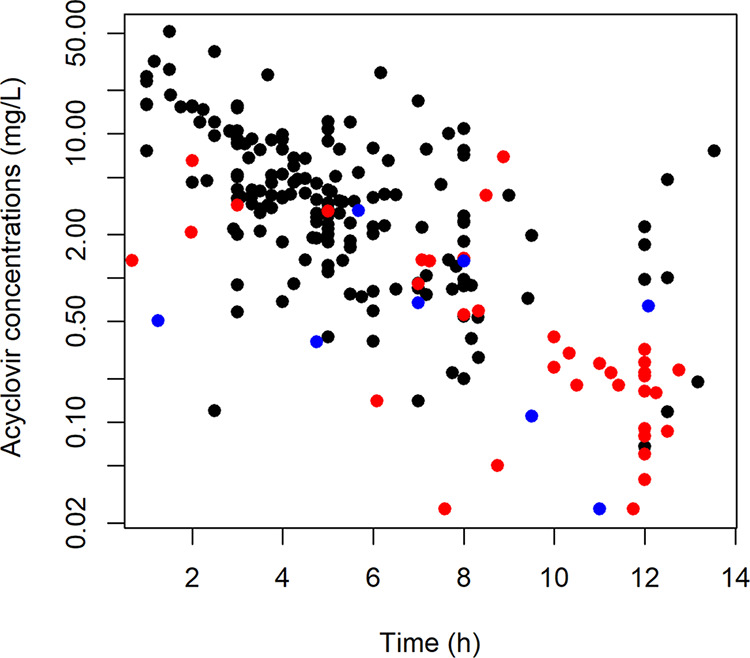
Acyclovir concentrations as a function of time after dose of acyclovir i.v. (black circles), oral acyclovir (blue circles), and oral valacyclovir (red circles).
TABLE 1.
Characteristics of the population
| Characteristic | Median (range) |
|---|---|
| No. of patients (no. of samples) | 79 (212) |
| Administration route (oral/i.v./i.v. and oral) (no. of patients) | 22/50/7 |
| Sex (no. of males/females) | 48/31 |
| Age (yr) | 4.1 (0.02–18) |
| Age at inclusion (no.): 0–1 yr/1–12 yrs/12–18 yrs | 20/41/18 |
| Wt (kg) | 15 (3.1–66) |
| Ht (cm) | 108 (49–169) |
| Body mass index (kg/m2) | 16.9 (11.5–50.0) |
| Plasmatic creatinine (μmol liter−1) | 29 (10–172) |
| eGFR (ml/min/1.73 m2) | 164 (56–399) |
| No. using acyclovir as a curative treatment | 22 |
| Against HSV-1/2 | 17 |
| Against VZV | 5 |
| No. using acyclovir as a preventive treatment | 57 |
| After hepatic transplantation | 8 |
| After pulmonary transplantation | 1 |
| After hematopoietic stem-cell transplantation (medullar) | 6 |
| After peripheral stem cell transplantation | 4 |
| After bone marrow transplantation | 36 |
| Others | 2 |
Population pharmacokinetics.
(i) Structural model building. A one-compartment model with first-order absorption and elimination best described the data (Fig. 2). The parameters of the model were bioavailability (F), absorption rate constant (ka), volume of distribution (V), and clearance (CL). Only three acyclovir concentrations were lower than the limit of quantification (LOQ), accounting for 1.4% of the data; thus, only the method of half of the LOQ was tested. The available data were not sufficient to estimate IIV for bioavailability and ka, and fixing the variance of these random effects to zero had no influence on the objective function value (OFV). The residual variability was best described by a proportional error model. An allometric model was added to the structural model, with a decrease in the OFV of 60 U and decreases from 0.73 to 0.434 for IIV in CL and from 0.738 to 0.496 for IIV in V. eGRF was added to CL because it decreased the OFV by 51 U (and the IIV from 0.46 to 0.389), whereas the addition of the plasma creatinine decreased the OFV by 29 U and the addition of both plasma creatinine and height decreased the OFV by 48 U. The effect of other covariates was not statistically significant. Final PK parameter estimates are summarized in Table 2.
FIG 2.
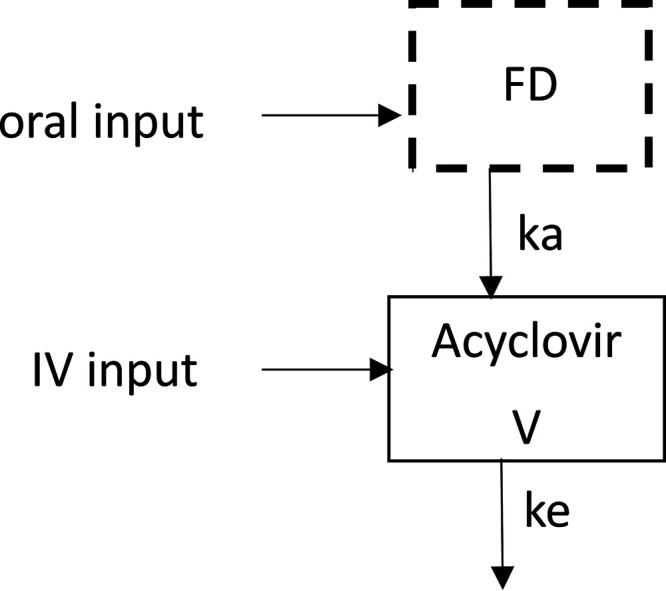
Pharmacokinetic compartmental model for acyclovir plasma concentration after oral or i.v. dose D. F, bioavailability of acyclovir; ka, first-order absorption rate constant; V, acyclovir distribution volume; ke, acyclovir elimination rate constant.
TABLE 2.
Pharmacokinetic parameters for a body weight of 15 kg, with a CLCR of 164 ml/min/1.73 m2a
| Population parameter | Estimated value | RSEb (%) |
|---|---|---|
| Fixed population effects | ||
| Absorption rate constant (h−1) | 0.376 | 11 |
| Clearance (liters/h) | 5.15 | 8 |
| Vol of distribution (liters) | 16.2 | 13 |
| Bioavailability | 1c | |
| Oral valacyclovir on bioavailability | 0.768 | 17 |
| Oral acyclovir on bioavailability | 0.397 | 24 |
| eGFR effect on clearance | 0.658 | 11 |
| Interindividual variability | ||
| For clearance | 0.389 | 14 |
| For vol of distribution | 0.434 | 28 |
| Residual variability | ||
| Residual proportional error | 0.48 | 6 |
RSE, relative standard error.
Bioavailability is fixed to 1 for i.v. administration.
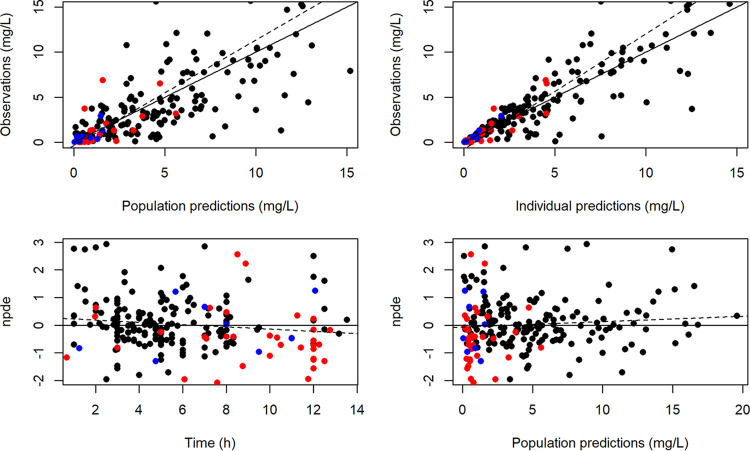
(ii) Model assessment. Diagnostic plots from the final model are shown in Fig. 3. Very high concentrations were not correctly fitted by the model. Prediction-corrected visual predictive check (pc-VPC) of the final population PK model showed the comparison between the 5th, 95th, and 50th predicted percentiles for the 1,000 simulations and the observed concentrations of acyclovir. This evaluation method provided good proof of the model adequacy (Fig. 4).
FIG 3.
(Upper graphs) Observation data versus population predictions (upper left) and versus individual predictions (upper right) in the final model, after doses of acyclovir i.v. (black circles), oral acyclovir (blue circles), and oral valacyclovir (red circles). The solid black line is the identity line. (Lower graphs) Normalized prediction distribution error (npde) versus time (lower left) and predictions (lower right), after doses of acyclovir i.v. (black circles), oral acyclovir (blue circles), and oral valacyclovir (red circles). The solid horizontal line is the theoretical mean (0), and the dashed line corresponds to regression.
FIG 4.
Prediction-corrected visual predictive check for acyclovir concentrations, following an i.v. acyclovir dose in linear scale (left) and in log-linear scale (middle) and an oral valacyclovir dose in linear scale (right). Colored areas represent 95% confidence intervals (CIs) of 5th, 95th (dark grey), and 50th (light grey) simulated percentiles. Lines indicate empirical (observed) 5th, 50th, and 95th percentiles. Dots represent observed data.
(iii) Subpopulation definition. An eGFR limit of 250 ml/min/1.73 m2 was chosen to identify children with normal and augmented renal clearance. This limit was high compared to the 130 ml/min/1.73 m2 value classically used in adults. Children considered to have ARC in this study (eGFR ≥ 250 ml/min/1.73 m2) represented 16% of the children; only 24% of children were from the PICU.
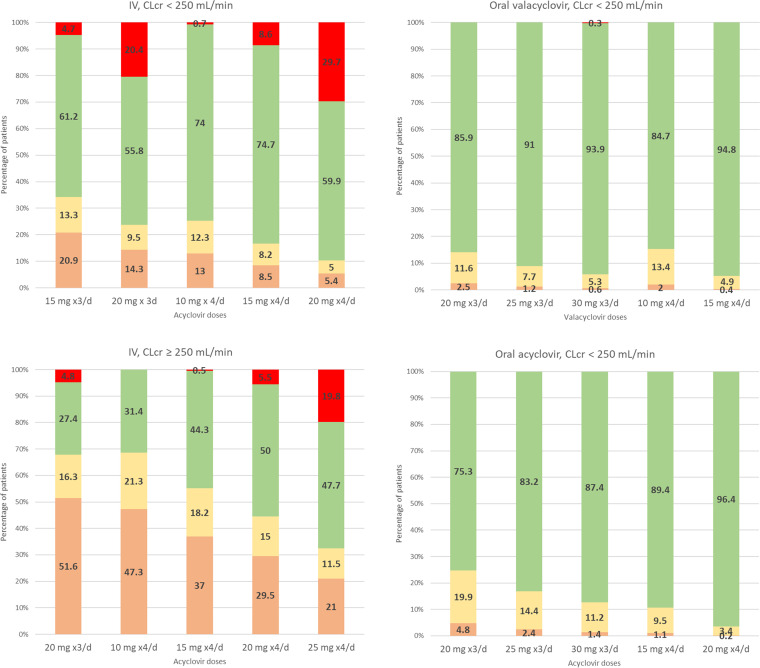
(iv) Plasma concentration simulation. Several i.v. and oral dosing regimens were tested, i.e., from 10 to 30 mg/kg every 8 h and from 10 to 25 mg/kg every 6 h. The percentages of patients with (i) a trough concentration below 0.56 mg/liter, (ii) a trough concentration between 0.56 and 1.125 mg/liter, or (iii) a trough concentration above 1.125 mg/liter and with (iv) a peak concentration below or above 25 mg/liter are reported in Fig. 5. For all tested oral valacyclovir and oral acyclovir doses, <0.1% of children had a peak greater than 25 mg/liter. The 20-mg/kg/8 h oral acyclovir and valacyclovir administrations allowed 97.5% and 95.2% of children, respectively, to be above the target trough concentration of 0.56 mg/liter and 85.9% and 75.3%, respectively, to be above 1.125 mg/liter. The same total daily dose but given as 15 mg/kg/6 h increased these percentages. For i.v. administration, the percentage of patients with a high peak concentration is more important than for oral administration, due to bioavailabilities of 77% for valacyclovir and 40% for oral acyclovir. A 10-mg/kg/6 h i.v. dose in children with normal renal function allowed a higher percentage of children to have a trough concentration above 0.56 mg/liter, with only 0.7% of children with a peak above 25 mg/liter. A 15- to 20-mg/kg/6 h dose for children with ARC would lead to 29.5% to 37% of the children to be underdosed and 0.5 to 5.5% to be overdosed, with no child having a maximal concentration above the neurotoxic limit of 50 mg/liter. These doses are proposals for the initiation of the treatment, but therapeutic drug monitoring could help to adjust these doses individually. Proposed initial doses are reported in Table 3.
FIG 5.
Simulations of trough (Ctrough) and peak (Cmax) concentrations according to the route of administration (i.v. or oral) and renal function (eGFR of <250 or >250 ml/min/1.73 m2). Color code: red, Cmax of >25 mg/liter; green, Ctrough of >1.125 mg/liter and Cmax of <25 mg/liter; yellow, Ctrough of <1.125 mg/liter and Cmax of <25 mg/liter; orange, Ctrough of <0.56 mg/liter and Cmax of <25 mg/liter.
TABLE 3.
Proposed initial doses according to the route of administration (i.v. or oral) and renal function (eGFR of <250 or >250 ml/min/1.73 m2)
| eGFR (ml/min/1.73 m2) | i.v. acyclovir doses | Oral acyclovir doses | Valacyclovir doses (oral) |
|---|---|---|---|
| <250 | 10 mg/kg/6 h | 15 to 20 mg/kg/6 h | 10 to 15 mg/kg/6 h |
| ≥250 | 15 to 20 mg/kg/6 h |
DISCUSSION
The concentrations of acyclovir and valacyclovir were satisfactorily described by a one-compartment model with first-order absorption to represent valacyclovir administration and first-order elimination. A one- or a two-compartment model has already been used to describe acyclovir PK. PK parameters were comparable to those previously reported in immunocompromised children; clearance was 0.35 liters/h/kg in our study of children with a median body weight of 15.6 kg, compared to 0.2 liters/h/kg reported by Zeng et al. (9) and 0.5 liters/h/kg reported by Eksborg et al. (11) and Nadal et al. (10) in children with a mean body weight of 20 kg. The volume of distribution was 1.2 liters/kg in our study, compared to 0.4 liters/kg in the study by Zeng et al. and 1.2 to 1.3 liters/kg in the studies by Eksborg et al. and Nadal et al. An allometric model was also used, and the effect of eGFR was demonstrated on elimination clearance by Zeng et al., using the Counahan-Barratt formula [eGFR calculated as (0.43 × height in cm)/serum creatinine concentration]. All patients were cotreated with various drugs according to their conditions, but none of those drugs are known to interact significantly with acyclovir (14).
Renal excretion is an important elimination pathway for acyclovir, and thus the influence of renal function on acyclovir CL was expected. eGFR was calculated according to the Schwartz formula. However, use of the Schwartz formula, based on serum creatinine, to evaluate renal function may have limitations, especially for critically ill children. Factors such as age, gender, muscle mass, nutritional and/or hydration status, and liver dysfunction interfere with serum creatinine concentrations. Serum creatinine concentrations may therefore have low sensitivity in evaluating renal function in this specific population (15). Endogenous biomarkers such as cystatin C or exogenous markers such as renal inulin or radiolabeled agent clearance might have made a better alternative (16, 17) but were not recorded in our study and are not commonly used. The most appropriate method to estimate the GFR is creatinine clearance (CLCR), calculated with both urine (UCR) and plasmatic (SCR) creatinine concentrations. Unfortunately, missing data on UCR and urine output does not allow calculation of the CLCR (18). In our model, two groups of patients could be distinguished according to their renal function, those with eGFRs of <250 ml/min/1.73 m2 and those with eGFRs of >250 ml/min/1.73 m2, corresponding to ARC. With an incidence between 16% and 80%, ARC (usually defined as >130 ml/min/1.73 m2) appears to be a common phenomenon found in critically ill patients (16, 19). Elevated renal clearances were also reported in a pediatric liver transplant population before surgery and within 3 months posttransplantation (20). Several potential mechanisms may contribute to the occurrence of ARC, including endogenous responses to increased metabolism and solute production, alterations in neurohormonal balance, and therapeutic maneuvers such as fluid resuscitation; the precise mechanism of ARC is not completely resolved. But, for many drugs, especially antibiotics such as vancomycin or β-lactam, underexposure had been pointed out in cases of ARC, increasing the risk of treatment failure (21). Any renally cleared drugs are potentially vulnerable to this phenomenon. Acyclovir is almost exclusively eliminated by renal excretion and seems to not be an exception. Increased acyclovir clearance had also been reported during infant maturation (22).
Target values were chosen for peak and trough acyclovir concentrations. Goals were to maintain trough concentrations above the recommended 50% inhibitory concentrations for HSV and VZV (0.56 mg/liter and 1.125 mg/liter, respectively) and to maintain peak concentrations below 25 mg/liter, the limit considered to result in moderate or severe adverse side effects such as nausea, abdominal pain, vomiting, renal failure, and neutropenia (23). A peak higher than 50 mg/liter has been proposed for an increase in neurotoxicity (24).
Dosing adaptations are currently prescribed only for renal impairment below 50 ml/min. In contrast, no recommendations exist for patients with enhanced renal function, and thus our work has an important impact, providing dosing recommendations for this population. Furthermore, simulated data flagged the potential inefficiency of current doses for the ARC group. Considering all our results, the oral form is less likely to produce toxic peak concentrations even with a dosing regimen of 30 mg/kg/8 h. Since the absorption phase is absent from the i.v. route, maximal concentrations must be excessively high in order to maintain the plasma concentration above the maximum efficacy threshold of 1.125 mg/liter or 0.56 mg/liter throughout treatment for patients with elevated GFR. We suggest that the dosing regimens be adapted depending on the renal function of the patient.
The more suitable initial doses to ensure a trough concentration of >1.125 mg/liter and a peak concentration of <25 mg/liter were 10 mg/kg/6 h of i.v. acyclovir for children with normal renal function (eGFR ≤ 250 ml/min/1.73 m2) and 15 to 20 mg/kg/6 h of i.v. acyclovir for children with augmented renal clearance (ARC) (eGFR > 250 ml/min/1.73 m2). The 20-mg/kg/8 h dose oral for oral acyclovir and valacyclovir produced effective concentrations in more than 75% of children; however, a 15-mg/kg/6 h dose, if possible, is preferred to increase this percentage. A higher dose of acyclovir than valacyclovir was needed to result in the same percentage of children in the target (Table 3).
A limitation of this study is that most of the children received valacyclovir as crushed tablets, which probably produce approximate doses and modify the absorption of the drug, and we did not have this information. Furthermore, we were not able to suggest dose adaptation for impaired renal function due to the lack of cases in our population. All our proposals aim to obtain the maximal efficacy/safety ratio against HSV and VZV disease and infections or as a preventive treatment. Our dosing regimen proposals need to be confirmed prospectively. Therapeutic drug monitoring, combining drug measurement with Bayesian estimation from a population model, is then a useful tool to adapt the dose individually. Indeed, in critically ill children, renal function changes quickly, and the use of these methods is helpful for individual dosing optimization.
Regarding other indications, even if it seems to provide a clinically and economically valuable alternative to valganciclovir for CMV prophylaxis in young people with solid organ and bone marrow transplantation (2, 25, 26), valacyclovir requires a much higher dose for this indication (27). Indeed, with a 50% inhibitory concentration (IC50) of 47.1 mg/liter (28) against CMV, acyclovir would not be able to obtain efficient and nontoxic concentrations for this indication.
Conclusions.
This study provided optimal dosing stratification of acyclovir and valacyclovir PK in children with various body weights and renal clearances. Simulations from modeling suggested that dose or administration frequency be adjusted depending on eGFR. The use of our suggested dosing adaptations combined with therapeutic drug monitoring should improve clinical outcomes.
MATERIALS AND METHODS
Patients.
This prospective study was part of the Optimome study, which was approved by the Ethics Committee of the Necker-Enfants Malades Hospital and is registered at https://www.clinicaltrials.gov (ClinicalTrials registration no. NCT02539407). All children aged less than or equal to 18 years old, weighing more than 2.5 kg, and receiving oral or intravenous acyclovir, oral acyclovir, or oral valacyclovir were included. The study was conducted in the pediatric intensive care unit (PICU), the pediatric immune-hematology unit (IHU), and the department of pediatric surgery and department of pediatrics of Necker-Enfants Malades Hospital (Paris, France). Prior to inclusion, oral consent was obtained from the patient’s legal representative after oral and written information. Patients with hemofiltration or extracorporeal membrane oxygenation assistance were excluded.
Baseline patient characteristics were recorded, including sex, age, body weight (BW), height (HT), body mass index, creatinine level, and reason for acyclovir administration. The estimated glomerular filtration rate (eGFR, ml/min/1.73 m2) was derived from the Schwartz formula (29), as follows:
where is a coefficient equal to 0.45 for patients aged <2 years and with a BW of ≥2.5 kg, 0.55 for patients aged ≥2 and <13 years and for female patients aged ≥13 years, or 0.7 for males aged ≥13 years.
Drug administration regimens and blood sampling.
The prescribed doses refer to a child’s medical history (preventive/curative treatment), the disease, the local protocol, and the route of administration. Intravenous acyclovir was diluted in 0.9% saline solution. Oral acyclovir can be given, using acyclovir (Zovirax) or valacyclovir (Zelitrex) tablets, crushed if needed. Acyclovir was mostly administered three times daily but could also be given every 6, 12, or 24 h. Median (range) daily doses were 59 (5 to 101) mg/kg/day for i.v. acyclovir, 35 (20 to 54) mg/kg/day for oral acyclovir, and 21 (17 to 63) mg/kg/day for oral valacyclovir. The median delay between drug intake and sample collection was 5.1 h, and the interquartile range was 3.5 to 8 h.
Assay.
Acyclovir concentrations in plasma samples were measured using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. Samples were centrifuged (4,000 × g, 5 min) to yield plasma and then stored at –20°C before analysis. The analysis was performed using TSQ Quantum discovery max chromatographic system (Fisher Scientific, Les Ulis, France). A volume of 100 μl of each plasma sample was precipitated with 500 μl of acetonitrile. The supernatants were evaporated to dryness under a +40°C nitrogen flow. The residues were then reconstituted in 500 μl of water, and a volume of 10 μl was injected into the chromatographic system. Chromatographic separation was carried out on an Atlantis T3 C18 column (Waters, Saint-Quentin, France) using a mobile phase composed of water (0.05% [vol/vol] formic acid) and methanol (0.05% [vol/vol] formic acid). The method was fully validated according to FDA guidelines for validation of bioanalytical assays. The calibration of acyclovir was linear over the range of 0.05 to 16 μg/ml with a limit of quantification (LOQ) of 0.05 μg/ml.
Population pharmacokinetics.
Data were analyzed using the nonlinear mixed-effect modeling software MONOLIX (version 2018R1), along with the SAEM algorithm. To model acyclovir and valacyclovir administration simultaneously, doses and concentrations were converted to molarity, dividing mass concentrations by the molarity of each compound. Analytical solutions were used to code i.v. and oral routes simultaneously. Numerical and graphical outputs were also obtained with MONOLIX software. Simulations were performed with NONMEM 7.4, using the final parameters obtained in MONOLIX. Maximal trough concentrations and the percentages of patients in each interval were calculated using R software (version 3.6.1) and represented with Excel.
(i) Structural model building. One- and two-compartment models were tested to describe the data. Acyclovir concentrations below the lower limit of quantification (LOQ) were set to half of the LOQ. Proportional, additive, and combined models were investigated to describe residual variability. IIV was defined by an exponential model.
Data for acyclovir and valacyclovir were then fitted jointly. Only significant interindividual variabilities of the PK parameters were kept, e.g., a decrease in the objective function value (OFV) of ≥3.84 U.
The continuous covariates considered were body weight, age, height, and eGFR by using the Schwarz formula (29).
Continuous covariates were integrated as follows:
where is the typical value of clearance or volume of distribution for a patient with the median covariate value, Covi is the covariate value for individual i, and β is the estimated influential factor for the continuous covariate estimated by the modeling software. For body weight, according to the allometric rule, β was fixed at 0.75 for the clearance parameter and 1 for the volume of distribution parameter (30), and median body weight was 15.6 kg. Categorical covariates such as sex were tested as follows:
where Covi equals 0 or 1.
A covariate was retained in the model if its effect was biologically plausible, if it produced a reduction in the variability of the PK parameter IIV, and if the OFV was decreased by at least 3.84 (equal to chi squared with 1 degree of freedom equivalent to a P value of 0.05) in the forward inclusion phase and was increased by more than 6 (equal to chi squared with 1 degree of freedom equivalent to a P value of 0.01) in the backward phase.
(ii) Model evaluation. For evaluation of the goodness of fit, the following graphs were plotted: observed concentrations versus population predictions and versus individual predictions, normalized prediction distribution error metrics (npde) versus time and versus predictions.
From the final model, Monte Carlo simulations were performed to compute the prediction-corrected visual predictive check (pc-VPC). The observed concentration data were overlaid on the 5th, 50th, and 95th percentiles of the simulated concentrations at each time, and a visual inspection was performed.
(iii) Subpopulation definition. Patients were split into two groups according to their eGFRs: children with normal renal clearance and children with augmented renal clearance. This eGFR limit was found by choosing the lowest P value for the Wilcoxon tests, comparing trough concentrations between the two eGFR groups.
(iv) Simulations. One thousand Monte Carlo simulations were performed, from the final population PK model, to simulate acyclovir trough and peak concentrations. According to previously published reports, curative antiviral efficiency is reached with acyclovir plasma concentrations above the in vitro 50% inhibitory concentration (IC50, 0.56 mg/liter and 1.125 mg/liter for HSV and VZV, respectively) for more than 12 h (9, 31). Although acyclovir has a large therapeutic window, moderate and severe side effects from the drug seem to appear when peak concentrations exceed 25 mg/liter (23). Several dosing regimens were tested, from 10 mg/kg to 30 mg/kg every 8 h and from 10 mg/kg to 25 mg/kg every 6 h, for both oral valacyclovir and i.v. acyclovir. Trough concentrations were calculated using the equation of i.v. or oral concentration as a function of time at 8 h after drug intake for a thrice daily regimen, at 12 h for a twice daily regimen, and at 24 h for a once daily dose. The percentages of patients with trough concentrations below 0.56 mg/liter, between 0.56 and 1.125 mg/liter, and above 1.125 mg/liter and with a maximal or peak concentration below or above 25 mg/liter were reported.
ACKNOWLEDGMENTS
We have no funding to declare.
We have no conflicts of interest to declare.
REFERENCES
- 1.Fila M, Dechartes A, Maisin A, Dossier C, Zhao W, Deschênes G, Baudouin V. 2015. Comparison between valganciclovir and aciclovir/valaciclovir for CMV prophylaxis in pediatric renal transplantation. Saudi J Kidney Dis Transpl 26:453–459. doi: 10.4103/1319-2442.157306. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Gandul C, Stampf S, Héquet D, Mueller NJ, Cusini A, van Delden C, Khanna N, Boggian K, Hirzel C, Soccal P, Hirsch HH, Pascual M, Meylan P, Manuel O, Swiss Transplant Cohort Study (STCS). 2017. Preventive strategies against cytomegalovirus and incidence of α-herpesvirus infections in solid organ transplant recipients: a nationwide cohort study. Am J Transplant 17:1813–1822. doi: 10.1111/ajt.14192. [DOI] [PubMed] [Google Scholar]
- 3.Levin MJ, Weinberg A, Schmid DS. 2016. Herpes simplex virus and varicella-zoster virus. Microbiol Spectr 4:DMIH2-0017-2015. doi: 10.1128/microbiolspec.DMIH2-0017-2015. [DOI] [PubMed] [Google Scholar]
- 4.King DH. 1988. History, pharmacokinetics, and pharmacology of acyclovir. J Am Acad Dermatol 18:176–179. doi: 10.1016/s0190-9622(88)70022-5. [DOI] [PubMed] [Google Scholar]
- 5.Gnann JW, Barton NH, Whitley RJ. 1983. Acyclovir: mechanism of action, pharmacokinetics, safety and clinical applications. Pharmacotherapy 3:275–283. doi: 10.1002/j.1875-9114.1983.tb03274.x. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher C, Bean B. 1985. Evaluation of oral acyclovir therapy. Drug Intell Clin Pharm 19:518–524. doi: 10.1177/106002808501900703. [DOI] [PubMed] [Google Scholar]
- 7.Granero GE, Amidon GL. 2006. Stability of valacyclovir: implications for its oral bioavailability. Int J Pharm 317:14–18. doi: 10.1016/j.ijpharm.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 8.MacDougall C, Guglielmo BJ. 2004. Pharmacokinetics of valaciclovir. J Antimicrob Chemother 53:899–901. doi: 10.1093/jac/dkh244. [DOI] [PubMed] [Google Scholar]
- 9.Zeng L, Nath CE, Blair EYL, Shaw PJ, Stephen K, Earl JW, Coakley JC, McLachlan AJ. 2009. Population pharmacokinetics of acyclovir in children and young people with malignancy after administration of intravenous acyclovir or oral valacyclovir. Antimicrob Agents Chemother 53:2918–2927. doi: 10.1128/AAC.01138-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadal D, Leverger G, Sokal EM, Floret D, Perel Y, Leibundgut K, Weller S. 2002. An investigation of the steady-state pharmacokinetics of oral valacyclovir in immunocompromised children. J Infect Dis 186(Suppl 1):S123–S130. doi: 10.1086/342968. [DOI] [PubMed] [Google Scholar]
- 11.Eksborg S, Pal N, Kalin M, Palm C, Söderhäll S. 2002. Pharmacokinetics of acyclovir in immunocompromized children with leukopenia and mucositis after chemotherapy: can intravenous acyclovir be substituted by oral valacyclovir? Med Pediatr Oncol 38:240–246. doi: 10.1002/mpo.1317. [DOI] [PubMed] [Google Scholar]
- 12.DailyMed. 2019. Acyclovir sodium—acyclovir sodium injection, solution. National Library of Medicine, Bethesda, MD: Accessed 7 June 2020. [Google Scholar]
- 13.DailyMed. 2020. Valacyclovir—valacyclovir hydrochloride tablet. National Library of Medicine, Bethesda, MD: Accessed 7 June 2020. [Google Scholar]
- 14.Roche. 2020. Rovalcyte prescribing information. Roche, Boulogne-Billancourt, France. [Google Scholar]
- 15.Hoste EAJ, Damen J, Vanholder RC, Lameire NH, Delanghe JR, Van den Hauwe K, Colardyn FA. 2005. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Transplant 20:747–753. doi: 10.1093/ndt/gfh707. [DOI] [PubMed] [Google Scholar]
- 16.Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA. 2020. Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol 35:25–39. doi: 10.1007/s00467-018-4120-2. [DOI] [PubMed] [Google Scholar]
- 17.Selimoğlu MA, Varol İ, Karabiber H, Tabel Y, Keçeli M, Yılmaz S. 2016. Evaluation of renal functions in pediatric liver transplantation. Pediatr Transplant 20:83–88. doi: 10.1111/petr.12642. [DOI] [PubMed] [Google Scholar]
- 18.Lubowitz H, Slatopolsky E, Shankel S, Rieselbach RE, Bricker NS. 1967. Glomerular filtration rate. Determination in patients with chronic renal disease. JAMA 199:252–256. doi: 10.1001/jama.199.4.252. [DOI] [PubMed] [Google Scholar]
- 19.Baptista JP, Roberts JA, Udy AA. 2019. Augmented renal clearance: a real phenomenon with an uncertain cause. Anaesth Crit Care Pain Med 38:335–336. doi: 10.1016/j.accpm.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Wiesmayr S, Jungraithmayr TC, Ellemunter H, Stelzmüller I, Bonatti H, Margreiter R, Zimmerhackl LB. 2005. Long-term glomerular filtration rate following pediatric liver transplantation. Pediatr Transplant 9:604–611. doi: 10.1111/j.1399-3046.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 21.Cook AM, Hatton-Kolpek J. 2019. Augmented renal clearance. Pharmacotherapy 39:346–354. doi: 10.1002/phar.2231. [DOI] [PubMed] [Google Scholar]
- 22.Sampson MR, Bloom BT, Lenfestey RW, Harper B, Kashuba AD, Anand R, Benjamin DK, Capparelli E, Cohen-Wolkowiez M, Smith PB, Best Pharmaceuticals for Children Act—Pediatric Trials Network. 2014. Population pharmacokinetics of intravenous acyclovir in preterm and term infants. Pediatr Infect Dis J 33:42–49. doi: 10.1097/01.inf.0000435509.75114.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bean B, Aeppli D. 1985. Adverse effects of high-dose intravenous acyclovir in ambulatory patients with acute herpes zoster. J Infect Dis 151:362–365. doi: 10.1093/infdis/151.2.362. [DOI] [PubMed] [Google Scholar]
- 24.Haefeli WE, Schoenenberger RA, Weiss P, Ritz RF. 1993. Acyclovir-induced neurotoxicity: concentration-side effect relationship in acyclovir overdose. Am J Med 94:212–215. doi: 10.1016/0002-9343(93)90186-s. [DOI] [PubMed] [Google Scholar]
- 25.Ljungman P, de La Camara R, Milpied N, Volin L, Russell CA, Crisp A, Webster A, Valacyclovir International Bone Marrow Transplant Study Group. 2002. Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood 99:3050–3056. doi: 10.1182/blood.V99.8.3050. [DOI] [PubMed] [Google Scholar]
- 26.Squifflet J-P, Legendre C. 2002. The economic value of valacyclovir prophylaxis in transplantation. J Infect Dis 186(Suppl 1):S116–S122. doi: 10.1086/342961. [DOI] [PubMed] [Google Scholar]
- 27.Ong S-Y, Truong H-T-T, Diong CP, Linn Y-C, Ho AY-L, Goh Y-T, Hwang WY-K. 2015. Use of valacyclovir for the treatment of cytomegalovirus antigenemia after hematopoietic stem cell transplantation. BMC Hematol 15:8. doi: 10.1186/s12878-015-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winston DJ, Yeager AM, Chandrasekar PH, Snydman DR, Petersen FB, Territo MC, Valacyclovir Cytomegalovirus Study Group. 2003. Randomized comparison of oral valacyclovir and intravenous ganciclovir for prevention of cytomegalovirus disease after allogeneic bone marrow transplantation. Clin Infect Dis 36:749–758. doi: 10.1086/367836. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A. 1976. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263. [PubMed] [Google Scholar]
- 30.Anderson BJ, Holford NHG. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 31.Tod M, Lokiec F, Bidault R, De Bony F, Petitjean O, Aujard Y. 2001. Pharmacokinetics of oral acyclovir in neonates and in infants: a population analysis. Antimicrob Agents Chemother 45:150–157. doi: 10.1128/AAC.45.1.150-157.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]