Occidiofungin is a nonribosomally synthesized cyclic lipopeptide that possesses broad-spectrum antifungal properties at submicromolar concentrations. This report explores multiple routes of administration and formulations of occidiofungin, as well as its toxicity in mice. Further, infection studies were performed in mice to assess the application of occidiofungin for treating systemic and intravaginal yeast infections. Formulations for intravenous and intravaginal administration of occidiofungin were prepared.
KEYWORDS: liposomal formulation, pharmacokinetics, Candida, antifungal agents
ABSTRACT
Occidiofungin is a nonribosomally synthesized cyclic lipopeptide that possesses broad-spectrum antifungal properties at submicromolar concentrations. This report explores multiple routes of administration and formulations of occidiofungin, as well as its toxicity in mice. Further, infection studies were performed in mice to assess the application of occidiofungin for treating systemic and intravaginal yeast infections. Formulations for intravenous and intravaginal administration of occidiofungin were prepared. Pharmacokinetic analyses were performed in a murine model, and a liquid chromatography-mass spectrometry (LC-MS) method was developed and used to quantify occidiofungin in mouse plasma samples. Toxicological and histopathological analyses of two repeat-dose studies using occidiofungin were performed. In these animal models, following intravenous administration, a liposomal formulation of occidiofungin improved the half-life and peak plasma drug concentration over that with a liposome-free formulation. Two long-term repeat-dosing toxicity studies of occidiofungin indicated the absence of toxicity in organ tissues. Murine models of a systemic yeast infection and a vulvovaginal yeast infection were performed. The findings of the systemic infection study revealed limitations in the use of occidiofungin that may be alleviated with the development of novel structural analogs or with further formulation studies. The gel formulation of occidiofungin demonstrated improved efficacy over that of the commercial product Monistat 3 in a vulvovaginal candidiasis study. This report outlines the optimal routes of administration of occidiofungin and demonstrates minimal toxicity following chronic exposure. Further, the results of these studies provide a clear indication for the use of occidiofungin for the treatment of recurrent vulvovaginal candidiasis (RVVC), which is a serious and clinically relevant issue.
INTRODUCTION
There is an urgent need to identify antifungal compounds with novel mechanisms of action and with low toxicity for the host organisms for the treatment of fungal infections that are resistant to currently available forms of treatment. According to some reports, systemic yeast infections have a mortality rate as high as 60% (1–4). The introduction of the echinocandin class of antifungals over a decade ago was the last introduction of an antifungal for the treatment of serious fungal infections. There are therapeutics for vulvovaginal candidiasis (VVC), but there is no clinically approved therapeutic for the treatment of recurrent VVC (RVVC) (5). The toxicity of antifungal compounds to humans is a major limiting factor in the clinical use and development of antifungal therapeutics. Furthermore, currently existing classes of antifungals are limited in their use, due to differences in their spectra of activity and antifungal resistance.
Occidiofungin is a cyclic, nonribosomally synthesized peptide (6). Occidiofungin has been reported to possess fungicidal activity against a wide spectrum of fungi that are pathogenic to plants, animals, and human beings (Trichophyton, Aspergillus, Fusarium, Mucor, Cryptococcus, and Candida species) (6, 7). Occidiofungin has a novel mechanism of action and a cellular target different from those of the currently available families of antifungals, which target primarily the cell wall and cell membrane stability (8). The cellular target of occidiofungin is actin. However, unlike other actin binding drugs, occidiofungin does not inhibit the polymerization or depolymerization of F-actin (7). Occidiofungin interferes with higher-order actin cable structures, thus leading to an apoptotic mechanism of cell death. This is an entirely novel mechanism of action for an antifungal and is encouraging in the face of extensive resistance to existing treatments (9). Previous research on toxicity in a murine model indicated that occidiofungin, when administered intraperitoneally (i.p.) or subcutaneously (s.c.), was well tolerated at a dose as high as 20 mg/kg of body weight (10).
In order to develop occidiofungin as a clinically viable drug, its bioavailability and toxicity following administration by different routes in animal models needed to be determined. In this study, different routes of administration and various formulations of occidiofungin were evaluated, and the parameters that indicate toxicity were monitored. The efficacy of occidiofungin for treating systemic and vaginal yeast infections was also evaluated.
RESULTS
Pharmacokinetics of occidiofungin following administration by different routes.
In order to determine the most efficient method of delivery of occidiofungin, four different routes of delivery were compared. A 2.5-mg/kg dose of occidiofungin formulated in phosphate-buffered saline (PBS) containing 1.5% hydroxypropyl-beta-cyclodextrin (β-CD) was delivered by the oral, subcutaneous (s.c.), intraperitoneal (i.p.), or intravenous (i.v.) route of administration (Fig. 1A). Comparison of the different routes of administration yielded important information regarding the absorption and retention of occidiofungin in blood. The oral and subcutaneous routes of administration yielded peak plasma occidiofungin concentrations near or below the limit of quantification (12.5 ng/ml), indicating that occidiofungin is not absorbed well from the gastrointestinal (GI) tract, nor is it readily absorbed from cutaneous tissue. Furthermore, the intraperitoneal route yielded a delayed peak plasma occidiofungin concentration of 196 ng/ml at 24 h postinjection (hpi), and occidiofungin was cleared by 48 hpi. The intravenous route of administration yielded a peak plasma occidiofungin concentration of 364 ng/ml at 1 hpi, and the compound was eliminated gradually from the bloodstream by 24 hpi, with an estimated half-life of approximately 3.2 h.
FIG 1.
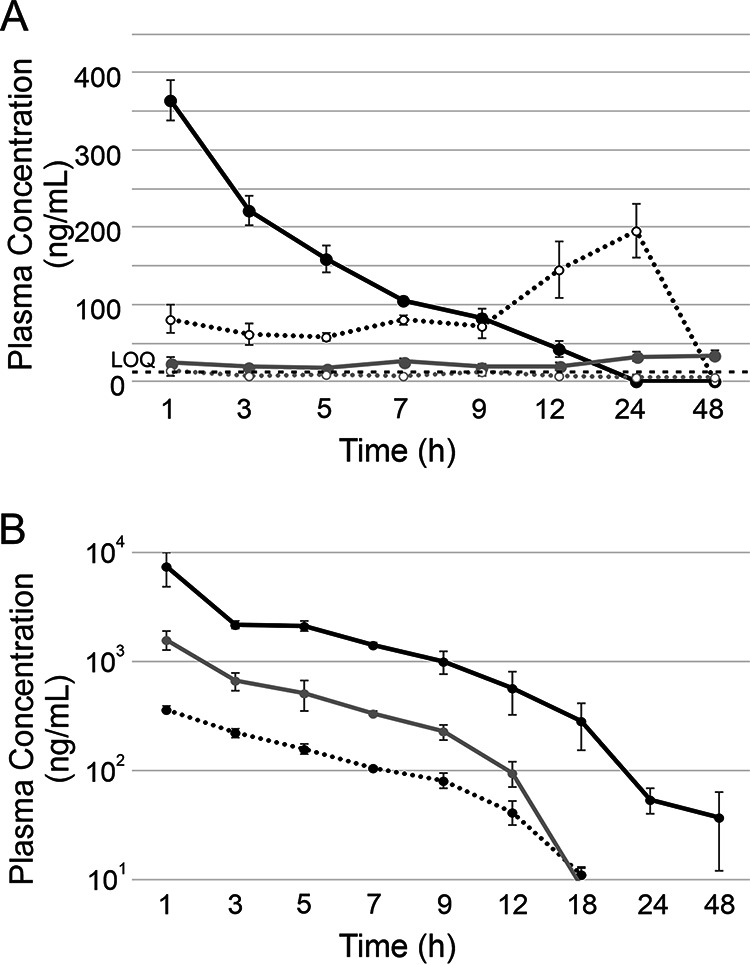
Pharmacokinetics of occidiofungin following a 2.5-mg/kg dose. (A) Comparison of concentrations of liposome-free occidiofungin in plasma following administration by the intravenous (solid black line), intraperitoneal (dotted black line), subcutaneous (solid gray line), and oral (dotted gray line) routes. Error bars indicate standard deviations. The limit of quantification (LOQ) was 12.5 ng/ml (indicated by a dashed black line). (B) Comparison of plasma occidiofungin concentrations following intravenous administration of liposome-free and liposomal occidiofungin. Dotted black line, liposome-free occidiofungin; solid gray line, DOPC vesicles; solid black line, DOPC:DPPG (9:1) vesicles.
The liposomal formulations of occidiofungin were administered intravenously in order to determine whether liposomes would improve the pharmacokinetic parameters of occidiofungin. Drug encapsulation studies have shown previously that neutral or negatively charged liposomes improved the pharmacokinetics and pharmacodynamics of therapeutic drugs. Further, liposomal formulations are commonly used to deliver therapeutic drugs in the clinic (11). We used a liposomal formulation comprising 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DPPG). Intravenous administration of a 2.5-mg/kg dose of occidiofungin formulated with DOPC resulted in a peak concentration of occidiofungin in plasma higher than that without the liposome (Fig. 1B and Table 1). At 1 hpi, the concentration of occidiofungin in plasma was observed to be 1,588 ng/ml, and the compound was gradually cleared by 48 hpi. The rate of elimination of this formulation was similar to that of liposome-free occidiofungin formulated in PBS containing 1.5% β-CD; the half-life was determined to be 2.7 h. In comparison, with i.v. administration of a 2.5-mg/kg dose of occidiofungin formulated with DOPC:DPPG (9:1), the peak plasma occidiofungin concentration was observed to be about 7,377 ng/ml at 1 hpi (Fig. 1B). Interestingly, the half-life of occidiofungin for this formulation was calculated to be around 7.0 h. These results show that the half-life of occidiofungin can be modulated based on the formulation of the liposomes used to package and deliver occidiofungin.
TABLE 1.
Noncompartmental analysis of 2.5 mg/kg occidiofungin administered i.v.a
| Formulation | AUC0–t (μg·h/ml) | t1/2 (h) | Cmax (μg/ml) | MRT (h) |
|---|---|---|---|---|
| Liposome free | 2,176.54 | 3.16 | 363.95 | 4.85 |
| DOPC | 7,708.52 | 2.73 | 1,588.40 | 3.70 |
| DOPC:DPPG (9:1) | 37,267.68 | 6.99 | 7,377.37 | 5.63 |
Plasma occidiofungin concentration-time data were determined by PKSolver 2.0 (21). Parameters include the area under the concentration-time curve (AUC0–t), half-life (t1/2), maximum plasma occidiofungin concentration (Cmax), and mean residence time (MRT). All numbers were rounded up to two decimal places.
The peak plasma occidiofungin concentrations for the DOPC and DOPC:DPPG formulations were 4.4 and 20.3 times higher, respectively, than those with liposome-free occidiofungin. The DOPC formulation led to a peak plasma occidiofungin concentration 4-fold higher than that with the liposome-free formulation, while the DOPC:DPPG formulation led to a 20-fold-higher peak plasma occidiofungin concentration. The plasma occidiofungin concentration remained higher for the liposomal preparations than for liposome-free occidiofungin throughout the study. The half-lives of the DOPC formulation and the liposome-free preparations were not overtly different, with values of approximately 3.0 h. However, the half-life of the DOPC:DPPG formulation was >2-fold higher (estimated half-life, approximately 7.0 h).
The DOPC:DPPG formulation appeared to be cleared from the blood at a lower rate than the liposome-free and DOPC formulations (Fig. 1B and Table 1). Occidiofungin in the DOPC:DPPG formulation remained detectable in blood above the limit of quantification (12.5 ng/ml) for 24 h, whereas occidiofungin in the liposome-free formulation or the DOPC formulation was detectable above the limit of quantification for 12 h. The areas under the concentration-time curve (AUC0–t) of the DOPC- and DOPC:DPPG-formulated occidiofungin were 3.5- and 17.2-fold higher, respectively, than the AUC of liposome-free occidiofungin (Fig. 1B and Table 1). The AUC relative to the MIC (AUC/MIC ratio) against the pathogen is one of the most important factors linked to the effectiveness of an antibiotic for treating an infection, when the rate of inhibition of the pathogen is increased with increasing concentrations of the antibiotic above the MIC (12, 13). With both\DOPC- and the DOPC:DPPG-formulated occidiofungin, the AUCs were improved.
Toxicological and histopathological analyses of occidiofungin following intravenous administration.
The DOPC:DPPG liposomal formulation was evaluated for toxicity. Six female BALB/c mice received 2-mg/kg doses of DOPC:DPPG liposomal occidiofungin, while a control group of three female BALB/c mice received empty vesicles for the same duration. Following the first 2-mg/kg i.v. dose of DOPC:DPPG liposomal occidiofungin, mice lost approximately 5% of their initial body weight. Therefore, the dose was administered every 48 h for 28 days, and this regimen demonstrated that weight loss following administration plateaued (Fig. 2A). At 24 h after the last drug administration, mice were sacrificed, fixed in 10% neutral buffered formalin, and stored for histological examination. There were no behavioral signs of toxicity, and the histopathology report on these mice indicated no prolonged effects on the organ tissues (Fig. 2B). Sections of the lung, thyroid, trachea, small intestine, thymus, esophagus, stomach, brain, colon, adrenal gland, and heart were analyzed microscopically for abnormalities. They were found to be histologically within normal limits relative to the controls, suggesting that repeated dosing of mice with occidiofungin for a long duration did not have lasting effects on the organ tissues.
FIG 2.

Toxicological evaluation of DOPC:DPPG-formulated liposomal occidiofungin following repeated intravenous doses. (A) Comparison of the effects of a liposomal vehicle control (black line) and occidiofungin treatment (gray line) on body weight. Mice were treated with 2 mg/kg liposomal occidiofungin every 48 h for 28 days. Error bars indicate standard deviations. (B) Histopathological analysis of organs at the end of the 28-day repeated-dose toxicity study. All tissues were found to be normal. A representative image from a slide of each tissue is presented. Magnifications, ×10 for the brain and ×20 for all other tissues. (A) Lung; (B) trachea; (C) thyroid; (D) thymus; (E) esophagus; (F) kidney; (G) heart; (H) spleen; (I) liver; (J) stomach; (K) pancreas; (L) small intestine; (M) colon; (N) brain; (O) adrenal gland.
Toxicological and histopathological analyses of occidiofungin following intravaginal administration.
A long-term toxicity study was performed to evaluate the toxicity of the occidiofungin gel formulation that was used in the intravaginal yeast infection study presented below. Two groups of female BALB/c mice, receiving either a gel blank control (6 mice) or the gel formulation containing 5 mg of occidiofungin/ml (6 mice), were dosed intravaginally with 20 μl of gel. This treatment was given once daily for 28 days. The treated mice showed no signs of discomfort and no behaviors indicating toxicity. Further, the treated mice gained weight normally relative to the drug-free control group (data not shown). Vaginal and cervical tissues were examined histologically and evaluated for inflammation based on neutrophil infiltration and tissue damage. The scoring system used a scale of 0 to 3, as follows: 0, negative/normal; 1, 2, and 3, minimal, moderate, and severe damage, respectively. Luminal inflammation, wall inflammation, submucosal inflammation, and the amount of keratin in the vaginal and cervical tissues were scored (Fig. 3). For the most part, the level of inflammation present in all samples was negative or minimal. Moderate levels of submucosal inflammation were present in the cervix with the vehicle control and occidiofungin gel samples. Further, keratin was moderately elevated in some of the vaginal and cervical tissues with both the vehicle control and the occidiofungin gel samples. Keratin in the reproductive tract is related to the stage of the reproductive cycle; thus, vehicle control- and occidiofungin gel-treated mice had consistent 2+ cervical inflammation. Further, these elevations are likely attributable to the trauma of daily intravaginal administration. There were no statistical differences in the histological examination for inflammation between the vehicle control group and the occidiofungin-treated group (Fig. 3). Further, histological examination of the lung, heart, liver, kidney, and spleen showed no signs of organ-specific toxicity following the repeated-intravaginal-dose study (data not shown). These observations are not surprising, given that occidiofungin absorption into the bloodstream from the vaginal cavity was below the limit of quantification.
FIG 3.
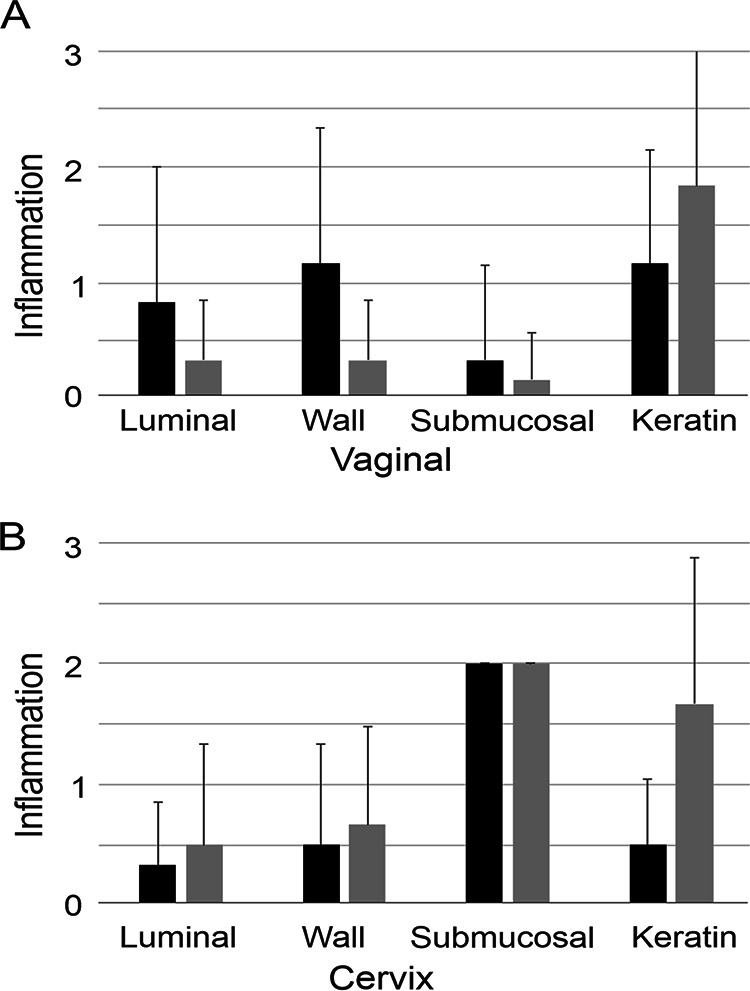
Toxicological evaluation of a gel formulation of occidiofungin following a repeated intravaginal daily dose for 28 days. The effects of treatment with the occidiofungin gel (5 mg/ml) (solid bars) and the gel vehicle control (shaded bars) on the inflammation of vaginal (A) and cervical (B) tissues were compared. Inflammation was scored on a scale of 0 to 3, as follows: 0, negative/normal; 1+, 2+, and 3+, minimal, moderate, and severe inflammation, respectively. There was no statistical difference between the occidiofungin gel and the vehicle control. Error bars indicate standard deviations.
Determination of the efficacy of occidiofungin in a murine model of systemic candidiasis.
Three groups of mice were evaluated in the systemic yeast infection model; they received either (i) a vehicle control containing DOPC:DPPG vesicles, (ii) a 2.5-mg/kg dose of DOPC:DPPG-formulated occidiofungin, or (iii) a 5-mg/kg dose of caspofungin. Immunocompromised mice were infected intravenously with 5 × 106 CFU of Candida glabrata ATCC 2001 (day 0 [D0]). A single dose of the vehicle control, occidiofungin, or caspofungin was administered 24 h (D1) following infection. The mice were sacrificed at 24 h post-drug treatment (D2), and the fungal loads in the kidneys were evaluated. The occidiofungin treatment group was not statistically significantly different from the vehicle control group, while the caspofungin treatment group was statistically significantly different (P < 0.05) from the vehicle control group (Fig. 4). The caspofungin treatment group had a 1-log reduction in CFU relative to levels in the occidiofungin and vehicle control groups. Based on the pharmacokinetic data described above, the DOPC:DPPG formulation should have been effective in reducing the fungal load in the systemic infection model. The plasma occidiofungin concentration was well above the measured MIC value of 0.5 μg/ml for Candida glabrata ATCC 2001 over a duration longer than that required to reduce the fungal load in our kill kinetic study, for which results are presented below.
FIG 4.
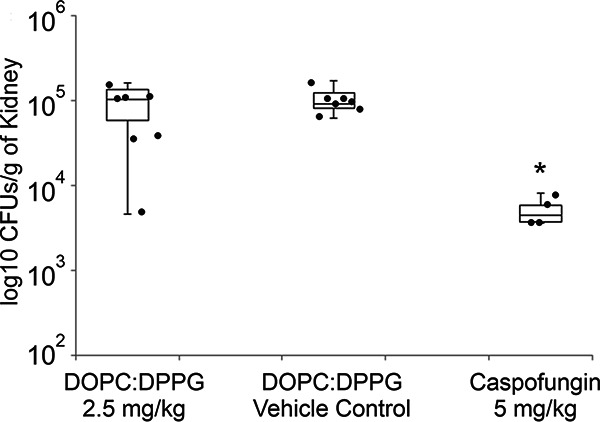
Murine model of systemic candidiasis. DOPC:DPPG-formulated occidiofungin (2.5 mg/kg) was compared to a vehicle control and caspofungin (5 mg/kg). No statistically significant reduction in fungal loads in kidneys (expressed in CFU per gram) was observed following liposomal occidiofungin treatment. The mice treated with 5 mg of caspofungin/kg demonstrated a log reduction in the fungal load from that with the vehicle control. Each black dot represents the value for an individual mouse. Error bars indicate standard deviations. The asterisk denotes statistical significance (P < 0.05).
Determination of the efficacy of occidiofungin in a murine model of vulvovaginal yeast infection.
In a murine vulvovaginal yeast infection model, 6- to 8-week-old BALB/c mice were infected intravaginally with Candida albicans (D0). Four groups were treated intravaginally with 0.5, 0.25, 0.1, or 0.0% gel-formulated occidiofungin. The fifth group was treated with the commercial antifungal vaginal cream Monistat 3, which contains 4% miconazole. Mice were dosed once per day with the formulated occidiofungin gel or with Monistat 3 for 3 days (D1, D2, and D3). The occidiofungin- and miconazole-treated groups were compared to a vehicle control group. The group treated with 0.5% occidiofungin showed a 2-log reduction in fungal loads (Fig. 5), while the 0.25% occidiofungin and Monistat 3 groups each showed a 1-log reduction in fungal loads. The 0.5 and 0.25% occidiofungin and Monistat 3 treatment groups were statistically significantly different (P < 0.05) from the vehicle control. During the course of the study, the mice were examined for outward signs of distress or irritation. No behavioral changes, including sluggishness, stretching, or reluctance to consume food, were observed. Furthermore, no vaginal bleeding or swelling was observed following treatment.
FIG 5.
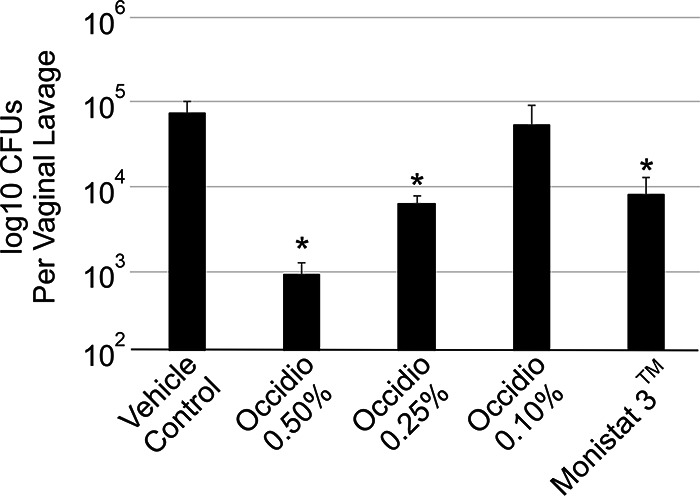
Murine model of vulvovaginal candidiasis. Gel-formulated occidiofungin (0.1%, 0.25%, or 0.5%) was compared to a vehicle control and Monistat 3 (4% miconazole). Mice treated with 0.25 and 0.5% occidiofungin demonstrated 1-log and 2-log reductions, respectively, in fungal loads from those with the vehicle control. The group treated with 0.25% occidiofungin showed an effect similar to that for the Monistat 3-treated group. Fungal loads for mice treated with occidiofungin at 0.1% were not statistically significantly different from those for mice given the vehicle control. Error bars indicate standard deviations; asterisks indicate statistical significance (P < 0.05).
In vitro bioactivities of free and liposomal occidiofungin.
Given the inability to demonstrate efficacy in the systemic candidiasis infection study, the inhibitory activity of the DOPC:DPPG-formulated occidiofungin was tested in comparison to that of liposome-free occidiofungin in order to determine whether the formulation reduced the inhibitory activity of occidiofungin. The MICs of DOPC:DPPG-formulated occidiofungin and liposome-free occidiofungin in yeast extract-peptone-dextrose (YPD) medium were both 0.5 μg/ml (Table 2). Further, in vitro kill kinetics analysis of purified DOPC:DPPG-formulated occidiofungin indicated no major differences from liposome-free occidiofungin in the rate of killing of C. glabrata ATCC 2001 at 2× MIC (Fig. 6). There was a moderately lower rate of killing for DOPC:DPPG-formulated occidiofungin than for liposome-free occidiofungin. However, no viable cells were observed in either sample by 8 h. The liposome formulation of occidiofungin does not appear to be the reason for the lack of efficacy observed in the systemic candidiasis study. When determined in the presence of 50% mouse serum, the activity of occidiofungin against C. glabrata ATCC 2001 was reduced by >32-fold from its activity in YPD medium (Table 2). To determine whether the loss in activity was specific to mouse serum, sera and whole blood from other animals (rat, porcine, and human sera; whole blood from hamsters, guinea pigs, beagles, rhesus monkeys, and humans) were evaluated. The MICs of liposome-free and DOPC:DPPG-formulated occidiofungin in the presence of rat and porcine sera were also >16 μg/ml. Further, when the mouse serum was heat inactivated or when esterase inhibitors were added, the MICs of liposome-free and DOPC:DPPG-formulated occidiofungin were still >16 μg/ml. When the assay was carried out using commercial human serum, the MICs for the liposome-free and DOPC:DPPG-formulated occidiofungin were 8 and 4 μg/ml, respectively. Inhibitory activity also increased in the presence of human serum, but the inhibitory activity was lower compared to other serum tested. The activity of DOPC:DPPG-formulated occidiofungin was improved 2-fold over that of liposome-free occidiofungin. The same 2-fold improvement in activity was observed in minimum lethal concentrations (MLCs) when the assay was carried out using 50% human whole blood instead of serum. When the activities of liposome-free and DOPC:DPPG-formulated occidiofungin were tested against hamster and guinea pig blood, the MLCs were observed to be >16 μg/ml. However, DOPC:DPPG-formulated occidiofungin had an MLC of 16 μg/ml in beagle and rhesus monkey blood, whereas the MLC of liposome-free occidiofungin was >16 μg/ml. An increase in the inhibitory activity of DOPC:DPPG-formulated occidiofungin was observed in human blood, with an MLC of 8 μg/ml, whereas the liposome-free occidiofungin had an MLC of 16 μg/ml.
TABLE 2.
Activities of free and DOPC:DPPG liposomal occidiofungin in 50% serum and 50% blood
| Medium | MIC (μg/ml) |
MLC (μg/ml) |
||
|---|---|---|---|---|
| Liposome-free occidiofungin | Liposomal occidiofungin | Liposome-free occidiofungin | Liposomal occidiofungin | |
| YPD | 0.5 | 0.5 | NDa | ND |
| Serum | ||||
| Mouse | >16 | >16 | ND | ND |
| Mouse (heat inactivated) | >16 | >16 | ND | ND |
| Mouse (with esterase inhibitors) | >16 | >16 | ND | ND |
| Rat | >16 | >16 | ND | ND |
| Human | 8 | 4 | ND | ND |
| Porcine | >16 | 16 | ND | ND |
| Whole blood | ||||
| Hamster | ND | ND | >16 | >16 |
| Guinea pig | ND | ND | >16 | >16 |
| Beagle | ND | ND | >16 | 16 |
| Rhesus monkey | ND | ND | >16 | 16 |
| Human | ND | ND | 16 | 8 |
ND, not determined.
FIG 6.
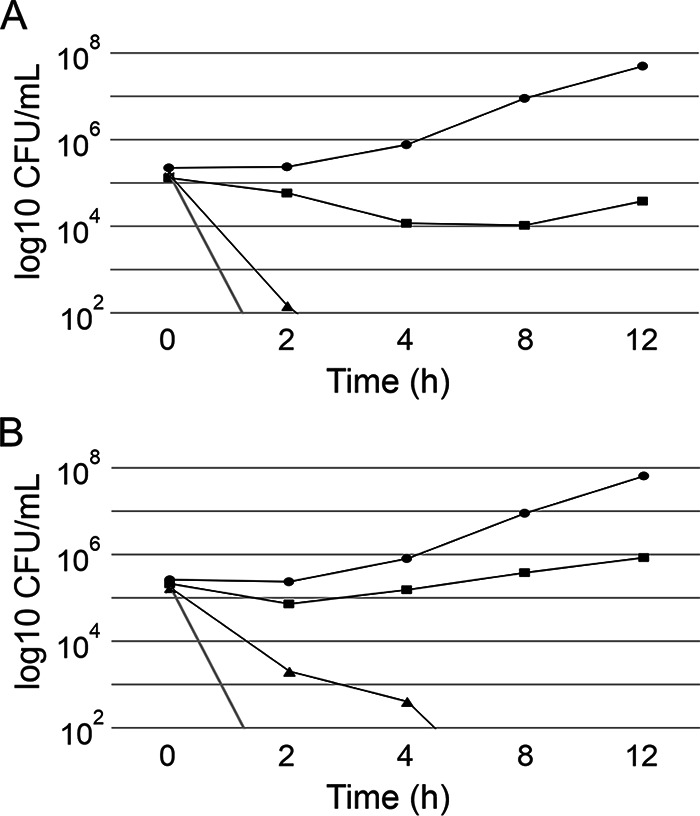
Comparison of the kill kinetics of liposome-free occidiofungin and DOPC:DPPG-formulated occidiofungin against Candida glabrata ATCC 2001. (A) Liposome-free occidiofungin. Black line and circles, vehicle control; black line and squares, 0.5× MIC; black line and triangles, 1× MIC; gray line, 2× MIC. (B) Liposomal occidiofungin. Lines and symbols are as described for panel A.
DISCUSSION
Previous research on toxicity in murine models indicated that occidiofungin, when administered intraperitoneally or subcutaneously, was well tolerated at a dose of 20 mg/kg. Histopathology revealed no major organ tissue toxicity (10). Repeated dosing at 2 mg/kg administered intraperitoneally for 5 days indicated as much as a 12% loss in body weight, which was recovered when treatment was stopped. Blood chemistry and microscopic tissue analyses indicated no severe effects on organ function and health (10). Further, toxicological evaluations following an intravenous dose (5 mg/kg) of occidiofungin showed a transient allergic response, indicated by an increase in neutrophils (14). The body weight changes observed were within normal limits, and tubular necrosis seen in the kidney tissue was found to be transient (14). Following these earlier studies, questions regarding the formulation and other possible routes of administration of occidiofungin remain. The findings from this study include (i) determination of the optimal route of occidiofungin administration, (ii) a liposomal formulation of occidiofungin with enhanced pharmacokinetic properties, (iii) a toxicological profile of novel formulations of occidiofungin for use against a systemic yeast infection and a murine VVC infection, and (iv) a demonstrated superiority in antifungal activity of an occidiofungin gel over the commercial drug Monistat 3 in a murine model of VVC.
We evaluated four different routes of administration of occidiofungin formulated in 1.5% hydroxypropyl-beta-cyclodextrin and showed that occidiofungin has low absorption properties by the oral, subcutaneous, and intraperitoneal routes of administration. Two liposomal formulations of occidiofungin for i.v. administration were evaluated to determine their effects on occidiofungin pharmacokinetics. A marked increase in the peak plasma occidiofungin concentration following intravenous administration was seen for both the DOPC and DOPC:DPPG liposomal formulations. The DOPC:DPPG combination yielded a peak plasma occidiofungin concentration and AUC0-t superior to those of the liposome-free and DOPC formulations. Kill curve assays conducted with liposome-free and DOPC:DPPG liposomal occidiofungin demonstrated that the rates of killing were not overtly different for the two formulations. Therefore, by formulating occidiofungin with liposomes, we were able to improve its bioactivity and pharmacokinetics. The DOPC:DPPG (9:1) liposomal formulation of occidiofungin was used for further studies to evaluate toxicity following a 28-day repeated-dosing study.
Toxicity analysis of the DOPC:DPPG liposomal formulation containing 2 mg of occidiofungin/kg was performed by i.v. administration every 48 h for 28 days. The study showed that fluctuations in body weight occurred when each dose was administered but that body weight was rapidly recovered, by the next day. No behavioral changes could be seen following administration. Histopathology assays done on multiple organs at the end of 28 days suggested that no significant organ toxicity could be observed following repeated dosing. In addition to having improved inhibitory activity and pharmacokinetic properties over those of liposome-free occidiofungin, the liposomal formulation of occidiofungin was well tolerated by the mice.
An efficacy study conducted in a murine model of systemic candidiasis using 2.5 mg of liposomal occidiofungin/kg in comparison with an empty-vesicle control indicated that there was no significant difference in fungal loads in the kidneys between the treated and control groups. A positive-control group of mice that received 5 mg/kg caspofungin had a CFU count >1 log lower than that of the negative control. This finding indicated that even though there was sufficient occidiofungin in the plasma to cause a reduction in the fungal load following administration, efficacy with regard to reduction in fungal loads could not be observed. This suggests that occidiofungin is not bioavailable in vivo, possibly due to binding to serum proteins. This was confirmed by bioactivity assays carried out using 50% serum and blood from different animals that could be used as model systems. Inhibitory activity could be observed in porcine, beagle, primate, and human blood or serum, but activity was not observed in mouse, rat, hamster, or guinea pig blood or serum. Interestingly, the inhibitory activity of occidiofungin was superior in human blood and serum with both liposomal and liposome-free occidiofungin. This is an interesting observation, suggesting that the murine model may not be the optimal animal model for a systemic antifungal study of occidiofungin. Liposomal occidiofungin was consistently more active than free occidiofungin in the presence of sera and blood from different animals. Possibly, chemical analogs of occidiofungin or the development of an alternative formulation will improve bioactivity in the presence of serum proteins.
Occidiofungin was as effective as the control drug (Monistat 3) when administered intravaginally to mice infected with C. albicans. It is worth noting that in this study, occidiofungin was found to be an effective antifungal agent at concentrations 16-fold lower than that of the control drug. These results augur well for the effectiveness of occidiofungin in the treatment of vulvovaginal candidiasis. Vulvovaginal candidiasis has been estimated to affect approximately 75% of all women, and 5 to 10% of all women will develop recurrent VVC (5, 15, 16). Several factors have been implicated in the onset and recurrence of this disease, such as personal hygiene, sexual partners, contraceptives, diabetes, antibiotic use, and asymptomatic vaginal colonization by Candida species. Approximately 90% of VVC cases in the United States are caused by C. albicans, while the remaining 10% are caused by C. glabrata, Candida tropicalis, Candida parapsilosis, or Candida krusei. These non-albicans VVC-causing Candida species are generally resistant to azole treatments. There have been no new therapeutic developments for recurrent VVC in decades. The fact that there is no established medical treatment method for VVC has led to the development of several ineffective treatment methods (5, 17, 18). These generally include the use of a rigorous dosing regimen of antifungals followed by a long period of prophylactic dosing. Among the different approaches in use by physicians, none have been particularly effective in the treatment of RVVC (5, 18). This often forces patients to try nonconventional approaches, such as probiotics, oils, and readily available herbal and acidifying agents. These options are often less effective than the physician-prescribed antifungal treatment methods and can lead to adverse effects that compound the problem with allergic responses and vaginal irritation (3, 18). There is an absolute need for an alternative antifungal treatment option for vaginal fungal infections. Hence, the development of a new class of antifungals with a different mechanism of action and spectrum of activity is desperately needed. Occidiofungin is a unique natural product with demonstrated attributes that can potentially fill this need.
Occidiofungin has a wide spectrum of activity against several types of fungi and yeasts that are resistant to the commonly used classes of antifungals (6–8, 19). The advantages of occidiofungin over currently used antifungals arise from its fungicidal activity and novel mechanism of action (7, 8, 20) in conjunction with the minimal toxicity observed in animal studies (10). In the current study, limitations in the use of occidiofungin for the treatment of a systemic yeast infection have been identified. The studies point to the need for an alternative animal model for efficacy studies and possibly the synthesis of novel chemical analogs. Occidiofungin was more effective than the positive-control drug (Monistat 3)in the VVC study. It is worth noting that in this study, occidiofungin was found to be an effective antifungal agent at concentrations 16-fold lower than the concentration of miconazole present in the positive-control drug. These results support the continued development of occidiofungin for the treatment of vulvovaginal candidiasis.
MATERIALS AND METHODS
Formulation of occidiofungin.
Occidiofungin was isolated as described previously (6) and was formulated in phosphate-buffered saline (PBS) containing 1.5% β-CD for evaluating the subcutaneous (s.c.), oral, intraperitoneal (i.p.), and initial intravenous (i.v.) routes of administration. Further, the formulation and testing of occidiofungin in vesicles for i.v. administration were performed using DOPC and DPPG. Vesicles were prepared per manufacturer specifications (Avanti Polar Lipids). Two types of liposomal formulations were tested: the first type of formulation using 100% DOPC and the second type using a DOPC:DPPG mixture at a 9:1 ratio. In each case, the appropriate amount of lipid for achieving a final amount of 20 mg was dissolved in 1 ml of chloroform before being added to the bottom of a 50-ml glass beaker. The lipids were vacuum dried to create a lipid cake. The lipids were then rehydrated using 1 ml of a 0.5-mg/ml PBS solution of occidiofungin in 1.5% (wt/vol) β-CD. The initial suspension was transferred to a 1.8-ml centrifuge tube. Sonication was carried out using a probe sonicator (Branson SLPe) with 30-s on-off cycles while the tube was placed on ice to minimize thermal effects. Sonication was done until the solution became translucent. The quantity of occidiofungin in the vesicles was estimated using a gel filtration column by collecting the vesicles in the void volume. The column was prepared using a bed volume of 15 ml of Sephadex G-10 beads (GE Healthcare Life Sciences). The void volume was estimated using blue dextran as the marker. Occidiofungin from the vesicles was extracted using 50% methanol and was purified by reversed-phase high-performance liquid chromatography (RP-HPLC) using a 4.6- by 250-mm C18 column (catalog no. 201TP54; Grace-Vydac) on a Bio-Rad BioLogic F10 DuoFlow system with a QuadTec UV-Vis detector. The solvents used were water-trifluoroacetic acid (TFA) (99.9%:0.1%) and acetonitrile-TFA (99.9%:0.1%). The amount of occidiofungin extracted from the vesicles was compared to a standard sample of occidiofungin. Approximately 50 to 60% of occidiofungin was determined to be encapsulated or bound to both the DOPC and DOPC:DPPG (9:1) vesicle preparations. Isolated vesicles were used in the MIC and kill kinetic studies described below, while the liposomal formulation of occidiofungin containing encapsulated and nonencapsulated occidiofungin was used in the in vivo animal efficacy and pharmacokinetic studies.
Formulation and testing of occidiofungin in a gel for intravaginal administration were performed using a 0.1, 0.25, or 0.5% (wt/vol) concentration. The occidiofungin gel formulation comprised 4% glycerol, 5% polysorbate 80, 0.1% sorbic acid, and 2.5% hydroxyethyl cellulose, 3,400 cP (HEC), in 25 mM acetate buffer. Glycerol was added to 25 mM acetate buffer and vortexed until it was uniformly dispersed. Polysorbate 80 was used to solubilize occidiofungin and was added to the mixture of glycerol and the acetate buffer. The mixture was heated at 45°C for 10 min with gentle mixing until the occidiofungin was completely dissolved. The pH of the solution was adjusted to 6.8 using 6 N sodium hydroxide before the gelling agent HEC was added. After gelation was achieved, the pH was adjusted to 5.5 using 6 N hydrochloric acid. The material was stored at room temperature until use.
Pharmacokinetic analysis of occidiofungin in a murine model.
Six- to 8-week-old female BALB/c mice were used for all in vivo studies. Four different routes of administration (oral, s.c., i.p., and i.v.) were evaluated in the study. Occidiofungin in 1.5% (wt/vol) β-CD suspended in PBS was administered to the mice at a dose of 2.5 mg/kg. Additional i.v. routes of administration used the DOPC- or DOPC:DPPG (9:1)-formulated occidiofungin at a dose of 2.5 mg/kg. Four groups of nine mice each were used, each group corresponding to a different route of administration. Two groups of nine mice each were used to evaluate the liposomal preparations of occidiofungin. The mice were weighed prior to occidiofungin administration, and changes in body weight were monitored every 24 h. The nine mice in each group were separated into three groups of three for blood draws. Following occidiofungin administration by the intravenous route, blood was drawn from the lateral saphenous vein of the mouse for the initial two time points and from the tail vein for the remaining time points at 1, 3, 5, 7, 9, 12, 18, 24, and 48 hpi. For each time point, 50 μl of blood was drawn from each of the three mice, and the blood was pooled. Sodium citrate (0.6% [wt/vol]) was used as an anticoagulant. The samples were then spun at ∼17,500 × g for 10 min, and the supernatant was removed and stored at –20°C. Plasma samples were prepared for liquid chromatography-tandem mass spectrometry (LC–MS-MS) analysis as described below.
Quantification of occidiofungin in plasma.
The optimal solvent for the extraction of occidiofungin from plasma was 50% methanol with 0.2% formic acid (data not shown). LC was carried out using a Waters XBridge BEH C18 column (length, 100 mm; inside diameter [i.d.], 2.1 mm; particle size, 5 μm) to quantify occidiofungin in the plasma samples. A mobile phase of water with 0.1% acetic acid (mobile phase A) and methanol with 0.1% acetic acid (mobile phase B) was used to elute occidiofungin. The gradient conditions were as follows: a hold at 95% A and 5% B for 0.5 min, a linear increase of B to 95% over 12 min, a hold at 95% B for 2 min, a decrease of B to 5% over 4 min, and reequilibration under the initial conditions for 2 min. The overall run time was 20 min, and the flow rate was 0.2 ml/min. MS-MS was performed using a Thermo Scientific TSQ Access Max mass spectrometer. Occidiofungin (parent mass, 1,200 m/z) was fragmented, yielding a daughter ion of 1,068 m/z (collision energy, 37 eV; T lens, 185; capillary temperature, 270°C). The 1,068 m/z corresponds to an occidiofungin fragment lacking the xylose sugar, and this mass was used for the quantification of occidiofungin in plasma. A calibration curve of occidiofungin was obtained by fortifying commercially available plasma from BALB/c mice (Innovative Research, Novi, MI) with variable amounts of occidiofungin and a fixed amount of the internal standard (azithromycin, 100 ng/ml). An initial concentration of 1,000 ng of occidiofungin/ml was set up, and serial dilutions were made in plasma down to a concentration of 12.5 ng/ml. Therefore, the lower limit of quantification was 12.5 ng/ml. Plasma concentration-time data were determined by PKSolver 2.0 (21).
Toxicological evaluation of a repeated dose of occidiofungin.
The DOPC:DPPG liposomal formulation for i.v. administration and the gel formulation for intravaginal administration of occidiofungin were evaluated for toxicity. In the i.v. administration study, 6- to 8-week-old female BALB/c mice received 2 mg of DOPC:DPPG liposomal occidiofungin/kg every 48 h for 28 days, in order to study the effects of repeated dosing of occidiofungin. A control group of three mice received empty vesicles for the same duration. After 28 days, mice were sacrificed and fixed in 10% neutral buffered formalin. Each organ was examined histologically by embedding tissues in paraffin and staining with hematoxylin and eosin (H&E). The mice were monitored for clinical signs of discomfort and stereotypical stretching behavior: ruffled fur, loss of nesting behavior, or locomotor dysfunction. Body weight was measured immediately before each treatment and was monitored every 24 h. In the intravaginal administration study, two groups (6 mice each) of 6- to 8-week-old female BALB/c mice were treated intravaginally with 20 μl of the gel formulation of occidiofungin (5 mg/ml, or 0.5%) every day for 28 days. A gel was prepared without occidiofungin and was administered to the control group. Blood was drawn from the tail vein at the end of the study, and the plasma was used to determine occidiofungin absorption from the vaginal cavity as described above. At the end of 28 days, mice were sacrificed and fixed in 10% neutral buffered formalin. Each organ was examined histologically by embedding tissue in paraffin and staining with H&E at Mississippi State University’s Animal Health Center, which is accredited by the American Association of Veterinary Laboratory Diagnosticians (AAVLD). Duplicate tissue sections were scored in a blind fashion by Timothy Morgan, who is certified by the American Board of Veterinary Practitioners (ABVP).
Determination of the efficacy of occidiofungin in a murine model of systemic candidiasis.
The DOPC:DPPG liposomal formulation of occidiofungin was evaluated in a systemic candidiasis infection model using 18 female 6-week-old BALB/c mice. Liposomal occidiofungin was prepared using a 9:1 ratio of DOPC:DPPG as described above. Neutropenia was induced in the mice by administering 150 mg of cyclophosphamide/kg in sterile PBS via the intraperitoneal route. After 72 h, the mice were infected intravenously with 5 × 106 CFU of Candida glabrata ATCC 2001 in 100 μl of sterile PBS (day 0 [D0]). A single dose of drug was administered 24 h (D1) following infection. The first group, consisting of seven mice, was treated intravenously with 2.5 mg of liposomal occidiofungin/kg; the second group (seven mice) was treated intravenously with an equal volume of empty vesicles; and the last group (four mice) was treated intraperitoneally with caspofungin in PBS at a dose (5 mg/kg) that has been widely reported (22–24). The mice were returned to the cages, and their behavior and body weight changes were monitored. The mice were sacrificed at 24 h post-drug treatment (D2), and the right and left kidneys were removed, weighed, macerated in YPD, and plated on YPD plates for the determination of fungal loads. Statistical analyses (t tests) were performed to compare the control group with the treated groups and to compare differences between treated groups. All the analyses were two sided, and a P value of <0.05 was considered statistically significant.
Determination of the efficacy of occidiofungin in a murine model of vulvovaginal yeast infection.
The murine model of vulvovaginal candidiasis used in this study has been reported previously (25, 26). A variation of this method was used. Five groups of six mice each were used to evaluate four concentrations of occidiofungin (0.5, 0.25, 0.1, and 0.0% [vehicle control]) and Monistat 3 as a positive control. Briefly, 6- to 8-week-old BALB/c mice were treated subcutaneously with 200 ng of β-estradiol 17-valerate 3 days prior to inoculation with C. albicans ATCC MYA-2876 (D−3). A subcutaneous dose of estradiol was administered every 3 days (D0 and D3) until the end of the experiment to induce pseudo-estrus. Intravaginal inoculations of approximately 20 μl of C. albicans at 2.5 × 106 CFU/ml were performed on D0 of the VVC study. On the same day as inoculation (D0), mice received another subcutaneous injection of estradiol. A 20-μl dose of a prepared gel containing 0.5, 0.25, 0.1, or 0.0% occidiofungin or Monistat 3 cream was administered intravaginally. Drug treatments were performed on D2, D3, and D4 of the study. On D5, the vaginal lumen was lavaged with 100 μl of sterile PBS with a 200-μl pipette tip. Serial dilutions and total CFU per vaginal lavage fluid specimen were determined by plating on YPD plates containing 50 μg/ml of chloramphenicol. Body weights, signs of vaginal irritation such as swelling or bleeding, and clinical signs of discomfort (stereotypical stretching behavior) were monitored. Statistical analyses (t tests) were performed to compare the control group to the treated groups and to compare differences between treated groups. All the analyses were two sided, and a P value of <0.05 was considered statistically significant.
Bioactivities of free and liposomal occidiofungin.
MICs were determined using a modified CLSI M27-A3 method (27). The strain used to carry out the bioactivity assays was Candida glabrata ATCC 2001. A time course analysis (kill kinetic study) of yeast cell death in YPD medium using free and liposomal occidiofungin was performed as reported previously (20). Further, the bioactivities of the liposome-free and DOPC:DPPG-formulated occidiofungin were tested in the presence of 50% serum and whole blood from different animals (rat, mouse, hamster, horse, beagle, rhesus monkey, and human). Occidiofungin was diluted in 100% serum or blood to achieve a starting concentration of 16 μg/ml. The activities of liposome-free and DOPC:DPPG-formulated occidiofungin were also tested using heat-inactivated mouse serum. The mouse serum was heat inactivated by placement in a water bath at 56°C for 15 min. Additionally, the assay was repeated using a protease and esterase inhibitor cocktail (Millipore Sigma, Calbiochem). The cocktail was added to mouse serum at a 1× concentration according to the manufacturer’s instructions. The MIC was read at 24 h postinoculation. MLCs for assays carried out using whole blood were determined by plating 100 μl from each well onto YPD plates and incubating at 35°C for 24 h. The lowest concentration at which no colonies were visible on the plates was determined to be the MLC.
ACKNOWLEDGMENTS
We acknowledge Lawrence J. Dangott for expertise and graduate student training on mass spectrometry techniques and equipment (Protein Chemistry Lab, Texas A&M). We also thank Timothy Morgan (veterinary pathologist at the College of Veterinary Medicine at Mississippi State University) for assistance with the histological examination of mice.
The research was funded by National Institutes of Health grants R41AI131792-01 and 2R42AI131792-02A1.
L.S., F.A., and S.-E.L. are board members of Sano Chemicals, Inc. Sano Chemicals is actively developing occidiofungin for the treatment of serious fungal infections.
REFERENCES
- 1.Charlier C, Hart E, Lefort A, Ribaud P, Dromer F, Denning DW, Lortholary O. 2006. Fluconazole for the management of invasive candidiasis: where do we stand after 15 years? J Antimicrob Chemother 57:384–410. doi: 10.1093/jac/dki473. [DOI] [PubMed] [Google Scholar]
- 2.Hibberd PL, Rubin RH. 1994. Clinical aspects of fungal infection in organ transplant recipients. Clin Infect Dis 19(Suppl 1):S33–S40. doi: 10.1093/clinids/19.supplement_1.s33. [DOI] [PubMed] [Google Scholar]
- 3.Nyirjesy P, Weitz MV, Grody MH, Lorber B. 1997. Over-the-counter and alternative medicines in the treatment of chronic vaginal symptoms. Obstet Gynecol 90:50–53. doi: 10.1016/S0029-7844(97)00242-1. [DOI] [PubMed] [Google Scholar]
- 4.Schaenman JM, Rosso F, Austin JM, Baron EJ, Gamberg P, Miller J, Oyer PE, Robbins RC, Montoya JG. 2009. Trends in invasive disease due to Candida species following heart and lung transplantation. Transplant Infect Dis 11:112–121. doi: 10.1111/j.1399-3062.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 5.Matheson A, Mazza D. 2017. Recurrent vulvovaginal candidiasis: a review of guideline recommendations. Aust N Z J Obstet Gynaecol 57:139–145. doi: 10.1111/ajo.12592. [DOI] [PubMed] [Google Scholar]
- 6.Lu S-E, Novak J, Austin FW, Gu G, Ellis D, Kirk M, Wilson-Stanford S, Tonelli M, Smith L. 2009. Occidiofungin, a unique antifungal glycopeptide produced by a strain of Burkholderia contaminans. Biochemistry 48:8312–8321. doi: 10.1021/bi900814c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravichandran A, Geng M, Hull KG, Li J, Romo D, Lu S-E, Albee A, Nutter C, Gordon DM, Ghannoum MA, Lockless SW, Smith L. 2018. A novel actin binding drug with in vivo efficacy. Antimicrob Agents Chemother 63:e01585-18. doi: 10.1128/AAC.01585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emrick D, Ravichandran A, Gosai J, Lu S, Gordon DM, Smith L. 2013. The antifungal occidiofungin triggers an apoptotic mechanism of cell death in yeast. J Nat Prod 76:829–838. doi: 10.1021/np300678e. [DOI] [PubMed] [Google Scholar]
- 9.Ghannoum MA, Rice LB. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517. doi: 10.1128/CMR.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei T, Cooley J, Austin F, Lu S, Smith L, Pruett S. 2012. Pre-clinical toxicological evaluation of occidiofungin, a unique glyco-lipopeptide antifungal. Int J Toxicol 31:326–336. doi: 10.1177/1091581812445185. [DOI] [PubMed] [Google Scholar]
- 11.Bulbake U, Doppalapudi S, Kommineni N, Khan W. 2017. Liposomal formulations in clinical use: an updated review. Pharmaceutics 9:12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andes D, Craig WA. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob Agents Chemother 46:1665–1670. doi: 10.1128/aac.46.6.1665-1670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig WA, Redington J, Ebert SC. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J Antimicrob Chemother 27(Suppl C):29–40. doi: 10.1093/jac/27.suppl_c.29. [DOI] [PubMed] [Google Scholar]
- 14.Lai-Hing S, Ravichandran A, Escano J, Cooley J, Austin FW, Lu S, Pruett S, Smith L. 2014. Toxicological evaluation of occidiofungin against mice and human cancer cell lines. Pharmacol Pharm 5:1085–1093. doi: 10.4236/pp.2014.511118. [DOI] [Google Scholar]
- 15.Sheary B, Dayan L. 2005. Recurrent vulvovaginal candidiasis. Aust Fam Physician 34:147–150. [PubMed] [Google Scholar]
- 16.Sobel JD. 1992. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis 14(Suppl 1):S148–S153. doi: 10.1093/clinids/14.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 17.Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. 2016. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol 42:905–927. doi: 10.3109/1040841X.2015.1091805. [DOI] [PubMed] [Google Scholar]
- 18.Watson C, Calabretto H. 2007. Comprehensive review of conventional and non-conventional methods of management of recurrent vulvovaginal candidiasis. Aust N Z J Obstet Gynaecol 47:262–272. doi: 10.1111/j.1479-828X.2007.00736.x. [DOI] [PubMed] [Google Scholar]
- 19.Ravichandran A, Gu G, Escano J, Lu S-E, Smith L. 2013. The presence of two cyclase thioesterases expands the conformational freedom of the cyclic peptide occidiofungin. J Nat Prod 76:150–156. doi: 10.1021/np3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis D, Gosai J, Emrick C, Heintz R, Romans L, Gordon D, Lu S, Austin F, Smith L. 2012. Occidiofungin’s chemical stability and in vitro potency against Candida species. Antimicrob Agents Chemother 56:765–769. doi: 10.1128/AAC.05231-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Huo M, Zhou J, Xie S. 2010. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Olson JA, Adler-Moore JP, Smith PJ, Proffitt RT. 2005. Treatment of Candida glabrata infection in immunosuprressed mice by using a combination of liposomal amphotericin B with caspofungin or micafungin. Antimicrob Agents Chemother 49:4895–4902. doi: 10.1128/AAC.49.12.4895-4902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozcan SK, Budak F, Willke A, Filiz S, Costur P, Dalcik H. 2006. Efficacies of caspofungin and a combination of caspofungin and meropenem in the treatment of murine disseminated candidiasis. APMIS 114:829–836. doi: 10.1111/j.1600-0463.2006.apm_450.x. [DOI] [PubMed] [Google Scholar]
- 24.Singh G, Imai J, Clemons KV, Stevens DA. 2005. Efficacy of caspofungin against central nervous system Aspergillus fumigatus infection in mice determined by TaqMan PCR and CFU methods. Antimicrob Agents Chemother 49:1369–1376. doi: 10.1128/AAC.49.4.1369-1376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anonymous. Standard operating procedure (SOP): Candida albicans murine vulvovaginal candidiasis (VVC) model. NIH/NIAID task order A13. NIH/NIAID contract no. HHSN272201000038I. NIAID, Bethesda, MD. [Google Scholar]
- 26.Yano J, Fidel PL Jr, 2011. Protocols for vaginal inoculation and sample collection in the experimental mouse model of Candida vaginitis. J Vis Exp 2011:3382. doi: 10.3791/3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]


