Carbapenem-resistant Enterobacterales (CRE) pose a significant threat to global public health. The most important mechanism for carbapenem resistance is the production of carbapenemases. Klebsiella pneumoniae carbapenemase (KPC) represents one of the main carbapenemases worldwide. Complex mechanisms of blaKPC dissemination have been reported in Colombia, a country with a high endemicity of carbapenem resistance. Here, we characterized the dynamics of dissemination of blaKPC gene among CRE infecting and colonizing patients in three hospitals localized in a highly endemic area of Colombia (2013 and 2015).
KEYWORDS: Klebsiella pneumoniae non-CG258, Enterobacterales, blaKPC-2, Colombia, whole-genome sequencing, IncN plasmid, outbreak
ABSTRACT
Carbapenem-resistant Enterobacterales (CRE) pose a significant threat to global public health. The most important mechanism for carbapenem resistance is the production of carbapenemases. Klebsiella pneumoniae carbapenemase (KPC) represents one of the main carbapenemases worldwide. Complex mechanisms of blaKPC dissemination have been reported in Colombia, a country with a high endemicity of carbapenem resistance. Here, we characterized the dynamics of dissemination of blaKPC gene among CRE infecting and colonizing patients in three hospitals localized in a highly endemic area of Colombia (2013 and 2015). We identified the genomic characteristics of KPC-producing Enterobacterales recovered from patients infected/colonized and reconstructed the dynamics of dissemination of blaKPC-2 using both short and long read sequencing. We found that spread of blaKPC-2 among Enterobacterales in the participating hospitals was due to intra- and interspecies horizontal gene transfer (HGT) mediated by promiscuous plasmids associated with transposable elements that was originated from a multispecies outbreak of KPC-producing Enterobacterales in a neonatal intensive care unit. The plasmids were detected in isolates recovered in other units within the same hospital and nearby hospitals. The gene “epidemic” was driven by IncN-pST15-type plasmids carrying a novel Tn4401b structure and non-Tn4401 elements (NTEKPC) in Klebsiella spp., Escherichia coli, Enterobacter spp., and Citrobacter spp. Of note, mcr-9 was found to coexist with blaKPC-2 in species of the Enterobacter cloacae complex. Our findings suggest that the main mechanism for dissemination of blaKPC-2 is HGT mediated by highly transferable plasmids among species of Enterobacterales in infected/colonized patients, presenting a major challenge for public health interventions in developing countries such as Colombia.
INTRODUCTION
Carbapenem-resistant Enterobacterales (CRE) currently pose a significant threat to global public health because the resulting infections are associated with high morbidity and mortality and very limited treatment options (1). In addition, the widespread transmission of carbapenem resistance via mobile genetic elements remains a main reason for concern (2). The most important mechanism for carbapenem resistance is the production of carbapenemases. Indeed, the Klebsiella pneumoniae carbapenemase (KPC) constitutes the most common class A beta-lactamase enzyme. KPC circulates worldwide and, along with New Delhi metallo-β-lactamase (NDM class B), Verona integron-encoded metallo-β-lactamase (VIM class B), imipenemase metallo-β-lactamases (IMP class B), and oxacilinase-48 (OXA-48 class D), is the most common carbapenemase identified worldwide (3, 4).
Since the first case of KPC identified in a K. pneumoniae clinical isolate from North Carolina in 1996 (5), international spread has occurred, and multiple outbreaks have been reported worldwide. In particular, a single clonal group (CG), designated CG258, has been responsible for the majority of infections, with two principal sequence types (STs) ST258 and ST512 (a single locus variant of ST258) representing the majority of blaKPC-containing K. pneumoniae. CG258 is responsible for >80% of outbreak isolates in the United States and about 90% of infections in Israel (6–9). The allelic variants blaKPC-2 and blaKPC-3 are the predominant genes associated with K. pneumoniae in Europe (Greece and Italy), Middle East (Israel), South America (Colombia and Argentina), and North America (especially the United States) (6–13). The blaKPC gene is often located on the mobile transposon Tn4401 and also on diverse plasmids, including broad-host-range plasmids of the incompatibility groups IncA/C, IncL/M, and IncN (14). Outbreaks of KPC-producing Enterobacterales caused by transferable plasmids have also been reported, specifically, by “promiscuous” plasmids of incompatibility group N (IncN) that harbor the blaKPC-2 gene (15).
In South America, the first detection of blaKPC on a conjugative plasmid was reported in Colombia (16), and since then, the country has become a region where strains producing carbapenemases are endemic (17). In Colombia, outbreaks of KPC-producing K. pneumoniae were initially attributed to strains belonging to clonal group CG258 (12, 13). Previous published data also suggested cocirculation of K. pneumoniae CG258 strains carrying blaKPC-3 and non-CG258 (from different STs) harboring blaKPC-2 (18, 19). However, a recent comprehensive genomic study indicated that the likely emergence of carbapenem resistance in Colombian hospitals was driven by the horizontal transfer of promiscuous plasmids harboring blaKPC-2 among Gram-negative bacteria instead of clonal dissemination of CG258 strains (19).
Here, we characterized the dynamics of transmission of blaKPC genes among CRE infecting and colonizing patients in an area of endemicity in Colombia around the city of Medellín, encompassing more than 2 million inhabitants. We provide evidence that the major mechanism for the spread of carbapenemase resistance (blaKPC-2) is the horizontal gene transfer (HGT) mediated by highly transferable plasmids, leading to a high endemicity of carbapenem resistance among Enterobacterales.
RESULTS
Infection/colonization by KPC-producing Enterobacterales in neonates and adults.
Between July 2013 and August 2015, 185 CRE clinical isolates of infected and colonized patients were collected from three hospitals serving the Medellin metropolitan area and adjacent communities (Central area of Colombia). Among the recovered organisms, 131 (70.8%) were positive for blaKPC (based on PCR), with 125 (95,4%) and 6 (4.6%) isolates carrying blaKPC-2, and blaKPC-3, respectively. These isolates were obtained from a group of 110 patients, encompassing 58 (53%) adults, 51 (46%) newborns, and one infant patient (1%) (see Data Set S1 in the supplemental material).
Among adult patients, 27 (47%) were colonized and 31 (43%) were considered infected. A total of 6 infected patients were previously colonized by KPC-producing Enterobacterales. More than half of the patients were males (56.9%, n = 33) and the majority were older (median age of 61 years; interquartile range [IQR] = 48 to 79 and 70 years; IQR = 56 to 81 years in colonized and infected patients, respectively). In the infected patients, KPC-producing Enterobacterales were most frequently isolated from urine and respiratory samples (32.3 and 25.8%, respectively). Overall, patients exhibited several underlying conditions, among which diabetes (34.5%), chronic obstructive pulmonary disease (20.7%), and chronic kidney disease (20.7%) were the most common. A total of 14 (24.1%) patients had previous antibiotic exposure. The most frequent antibiotics were cephalosporins (13.8%), beta-lactam/beta-lactamase inhibitor combination and carbapenems (10.3% for each) (see Table S1 in the supplemental material).
Among newborns, 46 (90.2%) were colonized, and 5 (9.8%) were infected by KPC-producing Enterobacterales. In colonized newborns, the mean length of hospital stay before sampling was 12 days (IQR = 5 to 30 days). A total of 26.1% (n = 12) were premature, and 36.9% (n = 17) had been previously exposed to antibiotics, mainly to aminoglycosides (26.1%), aminopenicillins (26.1%), and cephalosporins (23.9%). Several patients were colonized by up to 2 (n = 7) or 3 (n = 1) different species of Enterobacterales (see Table S2).
A total of 48 neonates (45 colonized and 3 infected) were involved in an outbreak that occurred during the study period in the neonatal intensive care unit (NICU) in hospital 1. Indeed, on 7 March 2014, 11 cases of CRE colonization in newborns were detected in the weekly surveillance cultures. Overall, 82 and 7 newborns, respectively, were colonized and infected by CRE and linked to the outbreak, which was declared over on 30 December 2014. Subsequently, five sporadic cases of colonization by carbapenem-resistant Enterobacter cloacae were detected between January and August 2015.
Non-CG258 K. pneumoniae in the outbreak setting shared an IncN plasmid.
Of the 57 isolates previously identified as carbapenem-resistant K. pneumoniae, 51 (89.5%) and 6 (10.5%) were positive for blaKPC-2 and blaKPC-3, respectively (see Data Set S1). Of these, 29 isolates were selected for WGS (n = 23/blaKPC-2, n = 6/blaKPC-3) based on initial characterization by rep-PCR/DiversiLab. The majority (n = 25) were identified as K. pneumoniae, 3 were identified as Klebsiella quasipneumoniae, and 1 was identified as Klebsiella variicola.
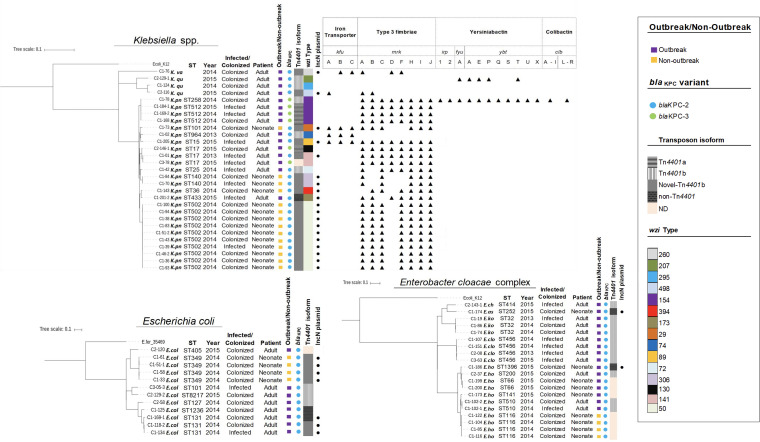
The KPC-2-producing K. pneumoniae isolates involved in the neonatal outbreak in hospital 1 (in 2014), exhibited a variety of genetic backgrounds that were not related to CG258. One main lineage was ST502 (n = 10) harboring capsular type wzi 50. Other isolates belonged to ST140 (n = 2), ST36 (n = 1), and ST101 (n = 1) exhibiting different capsular types (wzi 306, 394, and 29, respectively) and virulence factors. Despite the major differences in genetic backgrounds the isolates harbored a similar IncN plasmid replicon type and carried blaSHV, sul1, fosA, and aac(6′)-Ib-cr conferring, respectively, β-lactam, sulfonamide, fosfomycin, and aminoglycoside/quinolone resistance (Fig. 1 and Table 1). In addition, the blaKPC-2 gene was located on a novel Tn4401b transposon, which was flanked by a GATCT target site duplication and modified by the insertion of the Tn5403 element which was flanked by 34-bp inverted repeats into the ISKpn6 element (see Fig. S1 in the supplemental material).
FIG 1.
Phylogenetic tree showing the genetic relationships among isolates of Klebsiella spp. (n = 29), E. coli (n = 12), and Enterobacter cloacae complex (n = 20) harboring blaKPC. The results were generated by RAxML and visualized by the iTOL program. For species assignation, “K.va” indicates Klebsiella variicola, “K.qu” indicates Klebsiella quasipneumoniae, “K.pn” indicates Klebsiella pneumoniae, “E.col” indicates Escherichia coli, “E.ch” indicates Enterobacter chengduensis, “E.as” indicates Enterobacter asburiae, “E.ko” indicates Enterobacter kobei, “E.clo” indicates Enterobacter cloacae, and “E.ho” indicates Enterobacter hormaechei. Isolates were characterized by ST and year of isolation. We show whether the isolate was recovered from an infected/colonized patient, a neonate or an adult patient, and from a outbreak/nonoutbreak. We indicate the blaKPC variant, transposable element carrying blaKPC, capsular type (wzi typing), IncN replicon plasmid identified in the different species of Enterobacterales harboring blaKPC and virulence determinants identified in Klebsiella spp.
TABLE 1.
Molecular characteristics of 72 KPC-producing Enterobacterales isolates sequenced by Illumina platform
| Species | STa | Hospital | No. of isolates | Patient type | Variant of blaKPC-2 | Transposon isoformb | Additional resistance genes | Plasmid replicon typec |
|---|---|---|---|---|---|---|---|---|
| Klebsiella spp. | ||||||||
| K. pneumoniae | ST502 | 1 | 10 | Neonate | blaKPC-2 | Novel Tn4401b | blaSHV-62, sul1, aac(6′)-Ib-cr, fosA | IncN, IncR |
| ST140 | 1 | 2 | Neonate | blaKPC-2 | Novel Tn4401b | blaSHV-1, sul1, aac(6′)-Ib-cr, str(A,B), oqx(A,B), fosA | IncN, IncFIA, IncFII | |
| ST101 | 1 | 1 | Neonate | blaKPC-2 | Novel Tn4401b | blaSHV-1, sul1, aac(6′)-Ib-cr, aph(3″)-Ib, aph(6′)-Id, str(A,B), oqx(A,B), catA1, tet(D), fosA | IncN, IncFIB, IncFIB(K), IncFII, IncFII(K) | |
| ST36 | 1 | 1 | Neonate | blaKPC-2 | Novel Tn4401b | blaSHV-11, sul1, aac(6′)-Ib-cr, str(A,B), oqx(A,B) | IncN, IncR, IncFIB, IncFII | |
| ST17 | 1 | 1 | Adult | blaKPC-2 | Novel Tn4401b | blaSHV-11, sul1, aac(6′)-Ib-cr, oqx(A,B), tet(D), fosA | IncN, IncFIB(K), IncFII | |
| 2 | 1 | Adult | blaKPC-3 | Tn4401a | blaSHV-11, sul1, sul2, aadA2, oqx(A,B), erm(B), cmIA1, tet(B,D), fosA, dfrA15 | IncFIB(K), IncFIB(pQIL), IncFII, IncFII(K) | ||
| 3 | 1 | Adult | blaKPC-3 | ND | blaSHV-11, sul1, oqx(A,B), catA1, tetD, fosA | IncHI1B, IncFIB | ||
| ST15 | 1 | 1 | Adult | blaKPC-2 | Novel Tn4401b | sul1, aac(6′)-Ib-cr, aadA1, oqx(A,B), qnrB19, mph(A), tet(A), fos(A) | IncN, ColpVC, IncFIB(K), IncFII(K), IncI1 | |
| ST433 | 1 | 1 | Adult | blaKPC-2 | Non-Tn4401 | blaSHV-11, sul(1,2), aac(6′)-Ib-cr, aadA16, oqx(A,B), qnrB6, fosA5, arr3, dfrA27 | IncN, ColRNAI, IncFIB(K), IncFII(K), IncX3 | |
| ST964 | 1 | 1 | Adult | blaKPC-2 | Tn4401b | blaSHV-1, aac(3″)-IIa, aph(3″)-Ib, aph(6′)-Id, oqx(A,B), qnrB19, fosA | IncFIA, IncFIB(K), IncFII(K) | |
| ST25 | 1 | 1 | Adult | blaKPC-2 | Tn4401b | blaSHV-1, oqx(A,B), fosA | ND | |
| ST258 | 1 | 1 | Adult | blaKPC-3 | Tn4401b | blaSHV-28, sul(1,3), aac(3)-IVa, aac(6′)-Ib, aph(4)-Ia, aad(A2,A24), oqx(A,B), catA1, cmlA1, fosA, dfrA12 | ColRNAI, IncR, IncFIB(K), IncFII(K), Incl2 | |
| ST512 | 1 | 3 | Adult | blaKPC-3 | Tn4401a | blaSHV-11, sul1, aac(6′)-Ib, aph(3)-Ia, aadA2, oqx(A,B), catA1, fosA, dfrA12 | ColRNAI, IncFIB(K), IncFIB(pQIL), IncFII(K), IncX3 | |
| K. variicola | 1 | 1 | Adult | blaKPC-2 | Novel Tn4401b | blaLEN25, sul1, aac(6′)-Ib-cr, oqx(A,B). | IncN, IncFIB(K), IncFII(K) | |
| K. quasipneumoniae | 1 | 1 | Adult | blaKPC-2 | Tn4401b | blaOKP-A-5, sul1, oqx(A,B), fosA | IncR, IncFII(K) | |
| 2 | 1 | Adult | blaKPC-2 | Novel Tn4401b | blaOKP-A-5, sul1, aac(6′)-Ib-cr, oqx(A,B), fosA | IncN, IncR, IncFII(K) | ||
| 2 | 1 | Adult | blaKPC-2 | Tn4401b | blaOKP-B-3, sul2, rmtG, oqx(A,B), qnrB19, fosA | IncL/M(pMU407), IncHI1B, IncFIB, IncFII | ||
| E. coli | ST349 | 1 | 4 | Neonate | blaKPC-2 | Novel Tn4401b | blaOXA-1, sul1, aac(6′)-Ib-cr, aadA1, catA1, tet(B) | IncN, IncFIB |
| ST131 | 1 | 2 | Adult | blaKPC-2 | Novel Tn4401b | blaSHV-5, sul1, sul3, aac(3)-IV, aac(6′)-Ib-cr, aph(4)-Ia, aad(A1,A2), cmIA1 | IncN, IncHI2, IncHI2A, IncFIB, IncFII, IncI1 | |
| 1 | 1 | Adult | blaKPC-2 | Non-Tn4401 | sul(1,2), aac(3)-IId, aph(3″)-Ib, aph(3)-IId, aadA16, qnrB6, tet(A), arr3, dfrA12, dfrA27 | IncN, IncFIA, IncFIB, IncFII | ||
| ST1236 | 1 | 1 | Adult | blaKPC-2 | Non-Tn4401 | blaTEM-1A | ColRNAI, IncY | |
| ST405 | 2 | 1 | Adult | blaKPC-2 | ND | blaCMY-2, sul(1,2), aac(3)-IId, aadA5, aph(3″)-Ib, aph(6′)-Id, str(A,B), mph(A), tet(A), dfrA17 | IncFIB, IncFII, IncI1 | |
| ST8217 | 2 | 1 | Adult | blaKPC-2 | Tn4401 b | blaCTX-M-12, sul(1,2), aadA1, rmtG, qnrB19, mdf(A) | IncL/M(pMU407), IncHI1B, IncFIB, IncFII, IncI1 | |
| ST127 | 2 | 1 | Adult | blaKPC-2 | Tn4401b | aac(3″)-IIa, str(A,B), qnrB19 | IncN2, IncFII | |
| ST101 | 3 | 1 | Adult | blaKPC-2 | Tn4401b | sul(2,3), aad(A1,A2), str(A,B), qnrB19, cmIA1, floR, tet(A), tet(M), dfrA12 | IncL/M(pMU407), IncN2, IncFIB, IncX1 | |
| Enterobacter cloacae complex | ||||||||
| E. hormaechei | ST1396* | 1 | 1 | Neonate | blaKPC-2 | Non-Tn4401 | blaACT-7, sul1, aac(6′)-Ib-cr, aph(3″)-Ib, aadA16, qnrB6, fosA, arr-3, dfrA27 | IncN, IncFIB(K) |
| ST116 | 1 | 4 | Neonate | blaKPC-2 | ND | blaACT-7, sul2, aph(3″)-Ib, aph(6)-Id, str(A,B), tet(D), fos(A), dfrA14 | IncFIB, IncFII | |
| ST66 | 1 | 2 | Neonate | blaKPC-2 | ND | blaACT-7, blaACT-16, fosA, aph(6′)-Id | IncFIB, IncFII | |
| ST141 | 1 | 1 | Neonate | blaKPC-2 | ND | blaACT-7 | IncFIB(K), IncFII | |
| ST510 | 1 | 2 | Neonate | blaKPC-2 | Tn4401b | blaACT-7, sul(1,2), aac(3)-IId, aadA2, mph(A), tet(D), fosA, dfrA12 | IncFII, IncL/M, IncX5 | |
| ST200 | 2 | 1 | Adult | blaKPC-2 | Tn4401b | blaACT-16, blaCTX-M12, sul(1,2), aac(3)-IId, aac(6′)-Ian, aph(3″)-Ib, aph(6′)-Id, aadA2, qnrE1, mph(A), catA2, tet(D), fosA, dfrA12, mcr-9 | IncN2, IncHI2, IncHI2A, IncFIB, IncFII, IncX5 | |
| E. asburiae | ST252 | 1 | 1 | Neonate | blaKPC-2 | Non-Tn4401 | blaACT-3, blaACT-7, sul1, aac(6′)-Ib-cr, aadA16, qnrE1, qnrB6, fosA, arr3, dfrA27 | IncN, IncFIB(K) |
| E. cloacae | ST456 | 1 | 2 | Adult | blaKPC-2 | Tn4401b | blaCMH-3, sul1, aac(6′)-Ib-cr, aac(6′)-Ib3 aac(6′)-II, aadA1 aadA2, mphA, fosA, dfrA1, dfrA12 | ND |
| 2 | 1 | Adult | blaKPC-2 | Tn4401b | blaCMH-3, blaCTX-M-12, sul(1,2), aac(6′)-Ib-cr, aac(3)-IId, aac(6′)-Ib3, aac(6′)-II, aadA1, aadA2, rmtG, qnrB19, mph(A), tet(D), fosA, dfrA(1,12) | IncL/M | ||
| 3 | 1 | Adult | blaKPC-2 | Tn4401b | blaCHM-3, blaCTX-M-12, sul1, aac(3)-IId, aac(6′)-Ib3, aac(6′)-II, aad(A1,A2), aac(6′)-Ib-cr, mph(A), tet(D), fosA, dfr(A1,A12) | ND | ||
| E. kobei | ST32 | 1 | 2 | Adult | blaKPC-2 | Tn4401b | blaACT-9, aph(3″)-Ib aph(6)-Id, aacA4, fosA | IncN2 |
| 1 | 1 | Adult | blaKPC-2 | Tn4401b | blaACT-9, sul1, aac(3″)-IIa, aac(3)-VIa, aph(3″)-Ib aph(6)-Id, aadA1, qnrB19, tet(A), fosA, mcr-9 | IncN2 | ||
| E. chengduensis | ST414 | 2 | 1 | Adult | blaKPC-2 | Tn4401b | blaACT-6, aac(3″)-IIa, aph(3″)-Ib, aph(6′)-Id, qnrB19, fosA | IncN2 |
| Citrobacter spp. | ||||||||
| C. portucalensis | 1 | 1 | Adult | blaKPC-2 | Tn4401b | blaCMY-77, sul(1,2), aac(6′)-Ib-cr, aad(A2,A16), qnrB19, qnrB6, mph(A), tet(D), arr-3, dfrA12 dfrA27 | IncN, lncHI1A(CIT), IncHL1B(CIT), IncX5 | |
| C. freundii | ST215 | 1 | 1 | Adult | blaKPC-2 | Tn4401b | blaCMY-110, sul1, aac(6′)-Ib-cr, aadA16, qnrB6, arr-3, dfrA27 | IncN, RepA |
| ST169 | 2 | 1 | Adult | blaKPC-2 | Tn4401b | blaCMY-65, qnrB38 | Col440l, lncFlB(pHCM2), IncX5 | |
| ST504* | 2 | 1 | Adult | blaKPC-2 | Tn4401b | blaCMY-65, blaCTX-M15, sul1, aac(3″)-IIa, aac(6′)-Ib-cr, ant(2″)-Ia, aph(3″)-Ib, aph(6′)-Id, aadA1, qnrB60, qnrB38, mph(A), erm(B), catB3, cmlA1 | IncN2, IncFII(K), RepA | |
| ST22 | 2 | 1 | Adult | blaKPC-2 | Tn4401b | blaCMY-48, sul(1,2), aac(3″)-IIa, aac(6′)-Ian, aph(3″)-Ib, aph(3′)-Ia aph(6′)-Id, aad(A10,A1), qnrB19, tet(B), tet(D), dfrA1 | IncN2, RepA | |
| Serratia marcescens | 1 | 4 | Adult | blaKPC-2 | Tn4401 b | blaSRT-2, blaCTX-M-12, sul(1,2), aac(3″)-IIa, aac(6′)-Ic, qnrB19 aac(6′)-Ib-cr, tet(41) | IncA/C, IncA/C2, IncN2, IncR, IncHl1A(CIT), IncHL1B(CIT), IncP6 | |
| 3 | 1 | Neonate | blaKPC-2 | Tn4401b | sul(1,2), aac(3″)-IIa, aac(6′)-Ic, aac(6′)-Ian, aph(3″)-Ib, aph(3′)-Ia, aph(6′)-Id, aadA10, catA1, dfrA1 | IncA/C2, IncN2 | ||
| 3 | 1 | Adult | blaKPC-2 | Tn4401b | blaSRT-2, blaCTX-M-12, sul1, aac(6′)-II, aac(6′)-Ic, ant(2″)-Ia, aadA5 | IncL/M |
*, new sequence type (ST).
ND, not detected.
The IncN plasmid replicon type is indicated in boldface.
We also detected blaKPC-2 in non-CG258 K. pneumoniae (ST15, -17, -25, -433, and -964; Fig. 1) from colonized and infected adults in the participating hospitals (2013 to 2015). Some isolates that belonged to ST15 and ST17 (C1-205 and C1-01) carried blaKPC-2 on the novel Tn4401b. Of note, we found similar resistance genes and plasmid replicons (IncN) to that found in isolates that had been involved in the NICU outbreak (Fig. 1 and Table 1). In particular, a K. pneumoniae ST433 (C1-201-2) isolate carried blaKPC-2 on a non-Tn4401 genetic element (NTEKPC) of Tn3 elements-ΔblaTEM-blaKPC-2-ΔTn1721, previously described (20). This isolate carried five plasmid replicons, including IncN and genes conferring resistance to several antibiotics, including sulfonamides, rifampin, trimethoprim, aminoglycosides, and quinolones (Table 1).
Furthermore, K. variicola (C1-76) and K. quasipneumoniae (C2-116) isolates recovered from colonized adults at hospital 1 and hospital 2, also carried the blaKPC-2 on the novel Tn4401b. We also identified the IncN plasmid replicon and resistance determinants similar to those isolates from the neonatal outbreak (Fig. 1 and Table 1). Overall, our results suggest that the spread of blaKPC-2 across different clones of K. pneumoniae non-CG258, K. variicola, and K. quasipneumoniae strains was due to the same IncN type conjugative plasmid that carried the gene blaKPC-2 into the modified Tn4401b via insertion of a Tn5403 element. This plasmid seems to have the ability to broadly disseminate between hospitals.
Simultaneous circulation of K. pneumoniae CG258 harboring blaKPC-3 favors dissemination of carbapenem resistance.
Phylogenetic analyses showed four CG258 K. pneumoniae isolates that harbored blaKPC-3. They belonged to ST512 (C1-184-1, C1-169-2, and C1-168) and ST258 (C1-78). All of them contained the wzi 154 capsular type and were recovered from infected and colonized adult patients from a single hospital (Fig. 1). In the ST258 isolate, blaKPC-3 was located on a Tn4401b transposon, and we were able to identify virulence factors, such as type 3 fimbriae, yersiniabactin, and colibactin, as well as five plasmid replicons, including IncI2 (Fig. 1 and Table 1). A BLAST analysis indicated high similarity to an Incl2 plasmid previously described in US isolates and to a K. pneumoniae isolate from Colombia (COL-Kpn48) (19, 21) (see Fig. S2). In contrast, the blaKPC-3 of ST512 isolates was located on a Tn4401a transposon and contained only the type 3 fimbriae (Fig. 1). We also identified a set of four plasmid replicons that were shared, including the IncFIB(pQIL) plasmid replicon (two isolates, C1-168 and C1-169-2) (Table 1). Of note, the IncFIB(pQIL) plasmid is similar to a ST512 strain previously associated with an outbreak in the city of Medellin (19). Thus, our findings suggest that carbapenem-resistant K. pneumoniae CG258 isolates are also circulating concomitantly with non-CG258 K. pneumoniae, favoring the dissemination of carbapenem resistance.
Interspecies transmission of IncN plasmids carrying blaKPC-2 carbapenemase.
During the sampling period, we collected 74 isolates from species other than K. pneumoniae carrying blaKPC-2 in colonized/infected neonates and adults (see Data Set S1). In total, 43 of these isolates were selected for WGS based on initial characterization by rep-PCR/DiversiLab. The organisms identified were Escherichia coli (n = 12, 7 STs), Enterobacter cloacae complex (including Enterobacter hormaechei [n = 11, 5 STs, 1 new ST], Enterobacter cloacae [n = 4, 1 ST], Enterobacter kobei [n = 3, 1ST], Enterobacter asburiae [n = 1, 1 ST], and a recently described species of Enterobacter chengduensis [n = 1, 1 ST] [22]) (Fig. 1), Citrobacter freundii (n = 4, 3 STs, 1 new ST), Citrobacter portucalensis (n = 1), and Serratia marcescens (n = 6) (see Fig. S3).
Most isolates carried genes conferring resistance to several antibiotics, including amynoglycosides, sulfonamides, tetracyclines, trimethroprim, quinolones, phenicols, fosfomycin, and β-lactams (Table 1). Notably, two isolates of Enterobacter cloacae complex (E. kobei ST32 and E. hormaechei ST200) harbored the mcr-9 gene, related to resistance to polymyxins (23) (Table 1). Of note, the genetic environment of mcr-9 was similar to the one found in the chromosome of E. hormaechei strain S5 (accession number CP031571.1) recovered in the United States in 2015 (24) (see Fig. S4).
The blaKPC-2 gene was located on the Tn4401b isoform in 25/43 isolates sequenced. The remaining isolates carried the novel Tn4401b, including six E. coli isolates (ST349 [n = 4] and ST131 [n = 2]) (Fig. 1). The non-Tn4401 element (NTEKPC) found in K. pneumoniae ST433 (see above) was also detected in two E. coli isolates (ST131 and ST1236). In E. hormaechei ST1396 and E. asburiae ST252, NTEKPC was identified with a insertion of Tn5403 into the tnpA(Tn3). Of note, these isolates shared the IncN plasmid replicon (Table 1). In E. coli ST405 (n = 1) and E. hormaechei ST116 (n = 4), ST66 (n = 2), and ST141 (n = 1), blaKPC-2 was not detected in the genome, suggesting the loss of the plasmid or blaKPC gene during storage or subculture processing prior to sequencing.
Taken together, our results support interspecies dissemination of blaKPC-2 among Enterobacterales driven by promiscuous plasmids, predominantly of the incompatibility N group (IncN) carrying a novel Tn4401b structure and non-Tn4401 elements (NTEKPC).
Characterization of IncN plasmids involved in dissemination of blaKPC-2.
We evaluated the transferability of blaKPC-2 by mating assays, selecting one K. pneumoniae ST15 (C1-205) isolate carrying the blaKPC-2 on the novel Tn4401b and IncN plasmid replicon type as donor. We readily transferred blaKPC-2 into E. coli J53 with a conjugation efficiency of 1 × 10−2 per donor cells. We selected the transconjugant (E. coli Tc_C1-205) and other Enterobacterales (C1-70, C1-83, C1-93, C1-94, C1-143, C1-134, C1-205, and C2-116) to characterize the genetic location of blaKPC-2. Our results indicated that all isolates had blaKPC2 located on an ∼56-Kb plasmid (see Fig. S5).
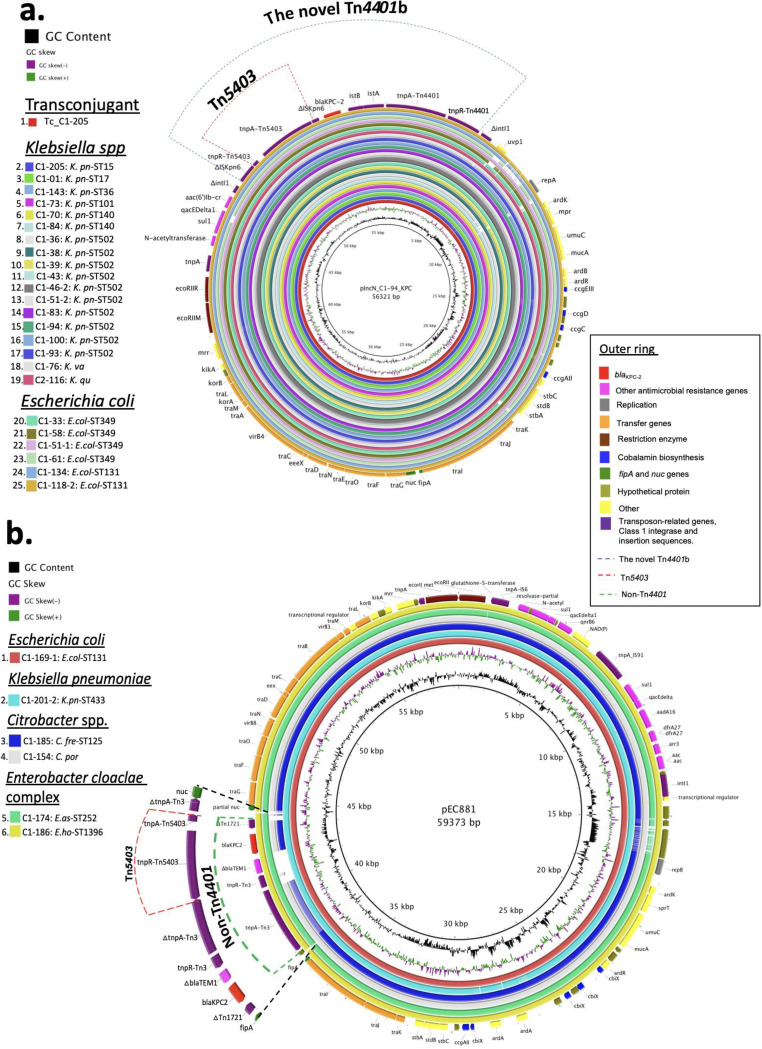
To reconstruct the complete plasmid sequence, we selected one K. pneumoniae ST502 isolate (C1-94) and sequenced the genome using the MinION platform (long-read sequencing). The 56,321-bp plasmid was designated pIncN_C1-94_KPC and determined to be ST15 at the pMLST (Fig. 2a). We confirmed that the plasmid carried the novel Tn4401b associated with a class 1 integron and other resistance genes [sul1, qacEDelta1, aac(6′)Ib-cr]. Subsequently, we used this plasmid sequence to determine its presence in each isolate using the Illumina data (short read). We identified the plasmid in the transconjugant strain (E. coli Tc_C1-205) and 24 Enterobacterales isolates (Fig. 2a). Our findings support the notion that pIncN_C1-94-KPC mediated the initial neonatal outbreak, spreading to other units within the same hospital, and was circulating simultaneously in other regional hospitals into different Enterobacterales.
FIG 2.
(a) BLAST Ring Image Generator (BRIG 0.95 and BLASTN v2.2.29) alignment of 24 sequenced blaKPC-2-carrying Enterobacterales and the Tc_C1-205 transconjugant harboring the annotated pIncN_C1-94_KPC (accession number “contig_241_1” within WGS genome SAMN07291374; inside ring). Each isolate and the Tc_C1-205 transconjugant are indicated by a ring of different color (left panel). The outer ring shows resistance genes, as well as the structural genes of pIncN_C1-94_KPC indicated by different colors (right panel). The novel transposon Tn4401 (blue dotted line) harboring an insertion of Tn5403 (red dotted line) into ISkpn6 is also shown. (b) BLAST Ring Image Generator (BRIG 0.95 and BLASTN v2.2.29) of six sequenced blaKPC-2-carrying Enterobacterales isolates using plasmid pEC881 as reference (accession number CP019026.1) from an E. coli recovered in Cali, Colombia, 2013 (inside ring). Each isolate is indicated by a ring of different color (left panel). The outer ring shows the resistance and structural genes indicated by different colors (right panel). The NTEKPC element (green dotted line) harboring an insertion of Tn5403 (red dotted line) into tnpA(Tn3) in C1-174 and C1-186 isolates is also shown.
We also detected a second IncN plasmid in some isolates that carried the blaKPC-2 on non-Tn4401 elements (NTEKPC) (C1-169-1, C1-201-2, C1-186, and C1-174). This plasmid was similar to a completely sequenced pEC881_KPC plasmid (accession number CP019026.1) from an E. coli strain recovered in Cali, Colombia, in 2013 (20) (Fig. 2b). Likewise, we identified the backbone of this plasmid in Citrobacter portucalensis (C1-154) and C. freundii ST215 (C1-185) isolates recovered from adults at hospital 1 (Fig. 2b).
DISCUSSION
In this study, we characterized the dynamics of dissemination of blaKPC-2 in different genera and species of Enterobacterales in hospitals serving an area of endemicity in Colombia with more than 2 million inhabitants. We show that broad dissemination of blaKPC is facilitated by intra- and interspecies HGT mediated by promiscuous plasmids associated with transposable elements. This mechanism of spread led initially to a multispecies outbreak of KPC-2-producing Enterobacterales in a neonatal care unit. Subsequently, the blaKPC-2-carrying plasmids were detected in Enterobacterales recovered from adult units in the same hospital and nearby hospitals over 3 years. The gene “epidemic” was driven by IncN-pST15-type plasmids carrying a novel Tn4401b structure and non-Tn4401 elements (NTEKPC).
Previous studies (18, 19) had suggested that dissemination of blaKPC-2 among K. pneumoniae non-CG258 in Colombia was mainly due to HGT, as well as its dissemination in other Enterobacterales (25, 26). Our study, using and extended and comprehensive evaluation of Enterobacterales recovered from colonized and infected patients in a high-endemicity area of Colombia, supports that concept and shows the efficient and expanding spread of carbapenem resistance, occurring mainly from hospitalized patients with the possibility of “spillover” to the community maintaining high circulation of these genes. Consistent with previous observations, K. pneumoniae isolates belonging to CG258 and carrying blaKPC-3 also circulated simultaneously with other genetic backgrounds (mostly harboring blaKPC-2). This phenomenon has created a “perfect storm” for dissemination of carbapenem resistance in Colombia (19).
Interestingly, multiple species of KPC-2-producing Enterobacterales (n = 24) were recovered in the NICU outbreak and adult units from hospital 1 and, subsequently, in nearby hospitals. The isolates exhibited heterogeneous genetic backgrounds but had in common the location of blaKPC-2 gene within a novel Tn4401b element which harbors an insertion of Tn5403 transposon into the ISKpn6 (Fig. 1 and see Fig. S1). The Tn5403 element was previously identified as a “helper” element that participated in the transfer of nonconjugative plasmids, found in isolates of K. pneumoniae recovered from polluted aquatic environments (27). It was also found in isolates of Raoultella ornithinolytica, a microorganism widely distributed in aquatic environments, insects, and fish (28). Of note, Tn5403 only contains genes related to transpositions function and is known to transpose by replicative transposition. The element seems to play as “disruptive” or “reorganizing” force via transposition within plasmids from clinical isolates (29). Thus, it is tempting to speculate that this novel Tn4401b structure could have originated through the introduction of Tn5403 element mediated by homologous recombination between environmental and human-adapted hospital isolates.
Our analysis of short- and long-read whole-genome sequencing revealed that the novel Tn4401b element was harbored within the pIncN_C1-94_KPC plasmid, which was identified in the 24 Enterobacterales isolates described above (Fig. 2a), showing a high conjugation efficiency, as has been previously described in IncN-type plasmids (30–32). An additional non-Tn4401 element “carrier” of blaKPC-2 (NTEKPC) was also identified in different species of Enterobacterales from neonates and adults (Table 1), harbored in a plasmid similar to the IncN pEC881_KPC reported previously (20) (Fig. 2b). Thus, these two IncN plasmids played a key role in the transmission interspecies of blaKPC-2 in some of the participating hospitals. Presumably, transposition of Tn5403 occurred and facilitated plasmid rearrangements (29).
Our findings are in agreement with previous studies (15, 32–37) by showing that the interspecies spread of blaKPC-2 through plasmids adds an additional layer of complexity to the molecular investigation of multispecies outbreaks and strongly suggest conjugation capacity and high plasticity of IncN ST15 plasmids in the gastrointestinal environment within a patient. Thus, the data in the study indicated that dissemination of the blaKPC-2 gene started in July 2013 in a K. pneumoniae ST17 isolate from an infected adult patient localized in non-ICU in hospital 1 that carried the blaKPC2 within the novel Tn4401b into pIncN_C1-94_KPC plasmid. This plasmid seems to be the source of the subsequent outbreaks in the neonatal unit.
Figure 3 attempts to reconstruct the events of dissemination of blaKPC2 of the outbreak. Our data suggest that, in the beginning of the outbreak (March 2014), the clonal dissemination of blaKPC2 by non-CG258 lineage of K. pneumoniae ST502 occurred, followed by emergence of KPC-2-producing E. coli ST349 by acquisition of the pIncN_C1-94_KPC plasmid (Fig. 2a) and subsequent spread to other species of Enterobacterales (March to December 2014). In parallel, an E. coli ST131 recovered from an adult patient in non-ICU setting carried the blaKPC-2 on NTEKPC within a plasmid highly similar to pECC881_KPC (Fig. 2b). After the outbreak was controlled (2015), we detected sporadic cases in neonates colonized by E. asburiae ST252 (C1-174) and E. hormaechei ST1396 (C1-186) carrying blaKPC-2 in the same plasmid pEC881_KPC, with some variations in the region containing the blaKPC2. Also, in the adult unit C. freundii ST215 (C1-185) and C. portucalensis (C1-154) were identified carrying the backbone of pEC881_KPC, albeit carrying a Tn4401b (Fig. 2b), suggesting gene rearrangement likely through transposition and homologous recombination. Likewise, one isolate of K. quasipneumoniae (C2-116) recovered from an adult patient in hospital 2 (January 2015), harbored the pIncN_C1-94_KPC plasmid.
FIG 3.
Timeline of the outbreak and dissemination of blaKPC2 among different Enterobacterales in hospitals located in Medellin and surrounding areas (2013 to 2015). The neonates are in orange, adult from hospital 1 in brown and adult from hospital 2 in dark blue. The asterisk indicates the place of stay in ICU (red) and non-ICU (light blue) at the time of sampling. The type of plasmid identified in the isolates are indicated with black and green ring. Those that exhibited variations within mobile elements are indicated with a green arrow pointing to the right.
Another important finding in our study is the identification of a group of closely related E. cloacae ST456 isolates (n = 4) carrying blaKPC-2 on Tn4401b (Fig. 1), a finding only previously reported in Norway (38). Of note, E. kobei ST32 and E. hormaechei ST200 isolates recovered from infected and colonized adult patients carried the mcr-9 gene within a genetic environment similar to a previous isolate described in USA (Fig. S2). The mcr-9 was described initially in Salmonella enterica and E. coli (23, 39), and later in Enterobacter spp. and Klebsiella pneumoniae and has been associated with carbapenemase-encoding genes (blaNDM-1, blaVIM-1, blaVIM-4, and blaOXA-48) in the United States, China, and European countries (24, 40, 41). In South America, it has recently been described in K. quasipneumoniae associated with blaNDM-1 in Argentina (42). To our knowledge, this is the first report of Enterobacter spp. harboring mcr-9 and blaKPC-2. However, at the time of isolation, the organisms were susceptible to colistin therefore it is likely that the mcr-9 gene was not expressed, a finding consistent with previous reports (43). Of note, mcr-9 had not been previously reported in Colombia.
Some limitations of the study include the limited number of hospitals in the region and that we were not able to resolve all plasmid sequences by long-read sequencing. Seven isolates of E. hormaechei involved in the NICU outbreak could have lost the blaKPC gene or the plasmid that contained it during storage or subculture processing prior to sequencing. Therefore, it was not possible to define the genetic environment of blaKPC gene in those isolates. However, we used the analysis of those genomes in the study to describe aspects related to the outbreak in NICU from hospital 1.
In conclusion, the KPC carbapenemase epidemic in an area of high endemicity in Colombia is driven by the horizontal transfer of promiscuous plasmids harboring blaKPC-2 among members of the Enterobacterales. This phenomenon has resulted in the occurrence of multispecies outbreak and high-level of genetic diversity of KPC-producing Enterobacterales. Our findings provided evidence of an epidemic of plasmid carrying blaKPC rather than clonal expansion of successful genetic lineage. Interrupting plasmid transmission is a challenge for public health interventions in developing countries.
MATERIALS AND METHODS
Strain selection and clinical and epidemiological characterization.
We conducted a prospective surveillance study of CRE in three tertiary-care hospitals in Colombia between 2013 and 2015. Hospital 1 is located in Medellín and, hospitals 2 and 3 are located in surrounding areas (202, 143, and 146 beds, respectively). All hospitals have functional intensive care units (ICU) for adults and two of them have neonatal intensive care units (NICU).
We isolated Enterobacterales from infected or colonized patients. Colonization was defined as a CRE recovered from a surveillance rectal culture (only in two hospitals) or a clinical sample without associated symptoms or disease. Infection was defined by a compatible clinical syndrome with confirmation by the infectious disease services and/or infection control committee, retrospectively. Rectal swabs were cultured on a selective chromogenic medium (chromID CARBA; bioMérieux). The collected Enterobacterales exhibited nonsusceptibility to at least one carbapenem (imipenem, meropenem or ertapenem). Multiple CRE isolates from an individual patient, in the same or different specimens, were also included.
Surveillance rectal swabs were collected in the following patients: (i) individuals with previous stay in the ICU, (ii) patients who received broad-spectrum antibiotic treatment (piperacillin-tazobactam, cefepime, quinolones, or carbapenems) within 90 days before admission, (iii) immunosuppressed patients due to hematologic disease or cancer, (iv) patients who underwent kidney transplantation, (v) use of a long-term urinary catheter or surgical wound drainage, (vi) suspected infection at a different institution, and (vii) hemodialysis and peritoneal dialysis. Clinical and demographic data from colonized and infected patients (i.e., age, sex, colonization or infection by more than one CRE, ICU admission, antibiotic use, use of invasive medical devices, underlying diseases, and comorbidities) were collected from electronically medical records and recorded in a Microsoft Access Database. This study was approved by the Institutional Review Board (IRB) of each participating hospital.
Bacterial identification, antibiotic susceptibility testing, and detection of the blaKPC gene.
Identification and antimicrobial susceptibility of Enterobacterales were performed by using the automated Vitek-2 system (bioMérieux Marcy-l’Étoile, France). The antimicrobial agents tested included ertapenem, imipenem, meropenem, cefotaxime, ceftazidime, ceftriaxone, cefepime, gentamicin, amikacin, and ciprofloxacin. MICs results were interpreted following the Clinical and Laboratory Standards Institute (CLSI) breakpoints (44).
All isolates classified as carbapenem-resistant were tested by PCR for the presence of the blaKPC gene using primers and assays previously described (5). Klebsiella pneumoniae ATCC BAA-1705 and Klebsiella pneumoniae ATCC BAA-1706 were used as positive and negative controls for the PCR assay, respectively. DNA sequencing was performed on PCR amplification products and the results were compared and aligned with reference sequence using the online BLAST database for detection of variants of the blaKPC gene.
Whole-genome sequencing.
A total of 72 isolates of CRE carrying blaKPC were selected for whole-genome sequencing (WGS) based on initial characterization by rep-PCR/DiversiLab (bioMérieux Marcy-l’Étoile, France) and choosing representatives strains of major clonotypes. Genomic DNA was extracted with the GeneJET Genomic DNA purification kit (Thermo Fisher Scientific, Waltham, MA). DNA libraries were prepared using a NexteraXT DNA sample preparation kit and multiplexed with a NexteraXT index primer kit on the Illumina platform (Illumina, San Diego, CA). Genomic libraries were sequenced on a MiSeq sequencer to obtain 250-bp paired-end reads using kit v2 and v3 (Illumina). The readings were processed to eliminate low quality bases and contamination with sequences of adapters and later assembled by de novo assembly. Cleaning and assembly were carried out using CLC Genomics Workbench assembler, version 8.5. The genomes were annotated using the RAST server (http://rast.nmpdr.org).
In order to reconstruct a fully closed plasmid sequence from a Klebsiella pneumoniae isolate carrying the blaKPC-2 and isolated from hospital 1, we used the MinION (Oxford Nanopore Technologies, Oxford, UK) platform to obtain long-read sequences. Genomic DNA was extracted using the DNeasy blood and tissue kit (Qiagen), and MinION libraries were prepared using the SQK-LSK208 kit according to the manufacturer´s protocol. We used both Illumina and MinION reads to fully generate the plasmid sequence. The BLAST Ring Image Generator (BRIG) (45) was used to align the assembled reads of the sequenced clinical isolates and the transconjugant Tc_C1-205 strain to the blaKPC-2 carrying K. pneumoniae C1-94 plasmid (pIncN_C1-94_KPC) (accession number “contig_241_1” within WGS genome SAMN07291374). In addition, we aligned the assembled reads of some isolates sequenced to the reference plasmids carrying blaKPC included pEC881_KPC (accession number CP019026.1) and pBK15692 (accession number NC_022520).
In silico MLST, genome annotation, virulence factors, and capsular typing.
The assemblies were typed on the web server of the Center for Genomic Epidemiology using the multilocus sequence typing (MLST; MLST 2.0 server; https://cge.cbs.dtu.dk/services/MLST/) (46). The determination of resistance elements, the plasmid replicon, and plasmid multilocus sequence type (pMLST) were identified using Resfinder 2.1 (https://cge.cbs.dtu.dk/services/ResFinder/), Plasmidfinder 2.1 (https://cge.cbs.dtu.dk/services/PlasmidFinder/), and pMLST 2.0, (https://cge.cbs.dtu.dk/services/pMLST/), respectively (47, 48), using an identity percentage higher than 95% and a coverage cutoff greater than 90%. The Tn4401 isoforms were determined by BLASTn comparing the region surrounding each blaKPC gene to the sequences of the Tn4401 isoforms as described previously (49).
In addition, we identified virulence genes and capsular typing (wzi sequencing) of isolates of K. pneumoniae using the Institut Pasteur’s Klebsiella BigsDB site (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
Phylogenetic analyses.
The phylogenetic reconstruction of the isolates was carried out by detecting single nucleotide polymorphisms (SNP) against reference genomes specific to each family of Enterobacterales: Klebsiella spp (accession number NZ_LAKK01000012.1), Escherichia coli (accession number CP010876.1), Citrobacter freundii (accession number NZ_CP011612.1), Enterobacter complex (accession number NRIS01000001.1), and Serratia marcescens (accession number NZ_HG326223.1). We also included the following reference genomes as an external group: E. coli K-12 MG1655 (accession number NC_000913), Escherichia fergusonii ATCC 35469 (accession number NC_011740.1), and Salmonella enterica CT18 (accession number NC_003198.1). An SNP matrix was constructed and used to reconstruct the phylogeny of the strains with RAxML (50). We used the general time reversal model with a GAMMA distribution and the Lewis correction for the parameters to determine the best phylogenetic reconstruction by maximum likelihood because the SNP matrix constitutes a matrix without invariant characters. We performed 20 runs and chose the one with the best score. In addition, 100 bootstraps were made to support the reconstructions. The trees obtained were visualized by iTOL (51).
Transferability of the blaKPC gene and plasmid analysis.
We selected one isolate of K. pneumoniae (C1-205) from hospital 1 carrying the blaKPC gene (according to short-read WGS-base analysis) and used it as donor in a conjugation experiment, with sodium azide-resistant E. coli strain J53 as the recipient. The experiments were performed as previously described (37). Briefly, donor and recipient were grown overnight with agitation at 37°C in tryptic soy broth (TSB). Cultures were transferred into fresh TSB (ratio 1:100) and incubated with agitation for approximately 5 h at 37°C, until the culture reached exponential growth (i.e., an optical density at 600 nm of 0.5 to 0.6). Donor and recipient cultures were mixed at a 1:10 ratio in fresh TSB and incubated at 37°C without agitation for 20 h. The conjugation mixture was diluted in phosphate-buffered saline, and dilutions were plated on tryptic soy agar (TSA) containing imipenem (4 μg/ml) and azide (150 μg/ml) for selection of transconjugants. The conjugation efficiency was calculated by dividing the number of transconjugant by donor cells. Antimicrobial susceptibility testing and PCR amplification of blaKPC gene were subsequently performed to confirm the transconjugant Tc_C1-205.
To confirm the localization of blaKPC-2 gene on a plasmid, we selected eight isolates of different Enterobacterales of hospitals 1 and 2 carrying blaKPC genes (C1-70, C1-83, C1-93, C1-94, C1-143, C1-134, C1-205, and C2-116) based on short-read WGS analysis and the E. coli transconjugant (Tc_C1-205). S1 digestion and pulsed-field gel electrophoresis was performed using the previously described protocol (52, 53), followed by Southern hybridization using a digoxigenin (DIG) DNA-labeled blaKPC probe (DIG-High Prime DNA labeling and detection starter kit II; Roche, Germany), following the manufacturer’s instructions. A transconjugant (Tc_C1-205) was sequenced on the Illumina MiSeq platform (Illumina), and the reads were assembled de novo using CLC Genomics Workbench assembler (v8.5) with default parameters, and the contigs were mapped against Escherichia coli J53 reference genome (accession number AICK01). Identification of plasmids associated with transfer of the blaKPC gene was performed with BLASTn, using the unmapped contig harboring blaKPC in E. coli Tc_C1-205 (accession number SAMN12815781).
Data availability.
The sequence data for the isolates included in this study were submitted to the NCBI GenBank database under BioProject number PRJNA391501.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Hospital San Juan de Dios, the Fundación Clínica del Norte, and the Clínica Universitaria Bolivariana for providing clinical and microbiological information and sending isolates.
This study was supported by funding (grant 1115-5693-3375) from the Colombian Agency for Science, Technology, and Innovation (COLCIENCIAS) (to E.R. and N.O.); Institución Universitaria Colegio Mayor de Antioquia grant FCS46-2016 (to A.M.R.); NIH/National Institutes of Allergy and Infectious Diseases (NIAID) grants K24-AI121296 (C.A.A.), R01AI093749 (C.A.A.), and R01AI134637 (to C.A.A.); a University of Texas System Faculty Science and Technology Acquisition and Retention (STAR) Award (to C.A.A.); and a University of Texas Health Science Center at Houston Presidential Award (to C.A.A.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. C.A.A. has received grant support from Merck, Inc.; Entasis Pharmaceuticals; and MeMed. M.V.V. has received grant support from Merck, Inc.; Pfizer; and West Química. All other authors declare no conflicts of interest.
Significant contributions to laboratory processing were made by E.D.C., N.O., D.P., and A.M.R. Collection and analysis of clinical data were done by C.A., C.C., and C.P. Short-read (Illumina) sequencing was performed by R.R., L.D., A.Q.D., and A.M.R. Long-read (MinION) sequencing was performed by B.M.H. Sequence data processing and analysis were performed by R.R., L.D., and A.M.R. A.M.R. and C.A.A. wrote the manuscript, which was reviewed by A.C., M.V.V., and E.R.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Logan LK, Weinstein RA. 2017. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meletis G. 2016. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis 3:15–21. doi: 10.1177/2049936115621709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel G, Bonomo RA. 2013. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol 4:48. doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordmann P, Poirel L. 2014. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect 20:821–830. doi: 10.1111/1469-0691.12719. [DOI] [PubMed] [Google Scholar]
- 5.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. 2001. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navon-Venezia S, Leavitt A, Schwaber MJ, Rasheed JK, Srinivasan A, Patel JB, Carmeli Y, Israeli KPC Kpn Study Group. 2009. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 53:818–820. doi: 10.1128/AAC.00987-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitchel B, Rasheed JK, Patel JB, Srinivasan A, Navon-Venezia S, Carmeli Y, Brolund A, Giske CG. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother 53:3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 9.Woodford N, Tierno PM Jr, Young K, Tysall L, Palepou MF, Ward E, Painter RE, Suber DF, Shungu D, Silver LL, Inglima K, Kornblum J, Livermore DM. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 48:4793–4799. doi: 10.1128/AAC.48.12.4793-4799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pournaras S, Protonotariou E, Voulgari E, Kristo I, Dimitroulia E, Vitti D, Tsalidou M, Maniatis AN, Tsakris A, Sofianou D. 2009. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J Antimicrob Chemother 64:348–352. doi: 10.1093/jac/dkp207. [DOI] [PubMed] [Google Scholar]
- 11.Mammina C, Palma DM, Bonura C, Anna Plano MR, Monastero R, Sodano C, Calà C, Tetamo R. 2010. Outbreak of infection with Klebsiella pneumoniae sequence type 258 producing Klebsiella pneumoniae carbapenemase 3 in an intensive care unit in Italy. J Clin Microbiol 48:1506–1507. doi: 10.1128/JCM.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ocampo AM, Vargas CA, Sierra P, Cienfuegos AV, Jiménez JN. 2015. Molecular characterization of an outbreak of carbapenem resistant Klebsiella pneumonia in a high complexity hospital in Medellín, Colombia. Biomedica 35:496–504. doi: 10.7705/biomedica.v35i4.2610. [DOI] [PubMed] [Google Scholar]
- 13.Lopez JA, Correa A, Navon-Venezia S, Correa AL, Torres JA, Briceño DF, Montealegre MC, Quinn JP, Carmeli Y, Villegas MV. 2011. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 17:52–56. doi: 10.1111/j.1469-0691.2010.03209.x. [DOI] [PubMed] [Google Scholar]
- 14.Mathers AJ, Peirano G, Pitout JD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweizer C, Bischoff P, Bender J, Kola A, Gastmeier P, Hummel M, Klefisch FR, Schoenrath F, Frühauf A, Pfeifer Y. 2019. Plasmid-mediated transmission of KPC-2 carbapenemase in Enterobacteriaceae in critically III patients. Front Microbiol 10:276. doi: 10.3389/fmicb.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villegas MV, Lolans K, Correa A, Suarez CJ, Lopez JA, Vallejo M, Quinn JP, Colombian Nosocomial Resistance Study Group. 2006. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother 50:2880–2882. doi: 10.1128/AAC.00186-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rada AM, Hernández-Gómez C, Restrepo E, Villegas MV. 2019. Distribution and molecular characterization of beta-lactamases in Gram-negative bacteria in Colombia, 2001–2016. Biomedica 39:199–220. doi: 10.7705/biomedica.v39i3.4351. [DOI] [PubMed] [Google Scholar]
- 18.Ocampo AM, Chen L, Cienfuegos AV, Roncancio G, Chavda KD, Kreiswirth BN, Jiménez JN. 2016. A two-year surveillance in five Colombian tertiary care hospitals reveals high frequency of non-CG258 clones of carbapenem-resistant Klebsiella pneumoniae with distinct clinical characteristics. Antimicrob Agents Chemother 60:332–342. doi: 10.1128/AAC.01775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rojas LJ, Weinstock GM, De La Cadena E, Diaz L, Rios R, Hanson BM, Brown JS, Vats P, Phillips DS, Nguyen H, Hujer KM, Correa A, Adams MD, Perez F, Sodergren E, Narechania A, Planet PJ, Villegas MV, Bonomo RA, Arias CA. 2018. An analysis of the epidemic of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: convergence of two evolutionary mechanisms creates the “perfect storm.” J Infect Dis 217:82–92. doi: 10.1093/infdis/jix524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoesser N, Sheppard AE, Peirano G, Anson LW, Pankhurst L, Sebra R, Phan HTT, Kasarskis A, Mathers AJ, Peto TEA, Bradford P, Motyl MR, Walker AS, Crook DW, Pitout JD. 2017. Genomic epidemiology of global Klebsiella pneumoniae carbapenemase (KPC)-producing Escherichia coli. Sci Rep 7:5917. doi: 10.1038/s41598-017-06256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Chavda KD, Al Laham N, Melano RG, Jacobs MR, Bonomo RA, Kreiswirth BN. 2013. Complete nucleotide sequence of a blaKPC-harboring IncI2 plasmid and its dissemination in New Jersey and New York hospitals. Antimicrob Agents Chemother 57:5019–5025. doi: 10.1128/AAC.01397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu W, Feng Y, Zong Z. 2019. Characterization of a strain representing a new Enterobacter species, Enterobacter chengduensis sp. nov. Antonie Van Leeuwenhoek 112:491–500. doi: 10.1007/s10482-018-1180-z. [DOI] [PubMed] [Google Scholar]
- 23.Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, Wiedmann M. 2019. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium isolate. mBio 10:e00853-19. doi: 10.1128/mBio.00853-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan Y, Li Y, Wang G, Li C, Xiang L, She J, Yang Y, Zhong F, Zhang L. 2019. Coproduction of MCR-9 and NDM-1 by colistin-resistant Enterobacter hormaechei isolated from bloodstream infection. Infect Drug Resist 12:2979–2985. doi: 10.2147/IDR.S217168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuzon G, Naas T, Correa A, Quinn JP, Villegas MV, Nordmann P. 2013. Dissemination of the KPC-2 carbapenemase in non-Klebsiella pneumoniae enterobacterial isolates from Colombia. Int J Antimicrob Agents 42:59–62. doi: 10.1016/j.ijantimicag.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Mojica MF, Correa A, Vargas DA, Maya JJ, Montealegre MC, Rojas LJ, Ruiz SJ, Quinn JP, Villegas MV, Colombian Nosocomial Bacterial Resistance Study Group. 2012. Molecular correlates of the spread of KPC-producing Enterobacteriaceae in Colombia. Int J Antimicrob Agents 40:277–279. doi: 10.1016/j.ijantimicag.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Rinkel M, Hubert J, Roux B, Lett M. 1994. Identification of a new transposon Tn5403 in a Klebsiella pneumoniae strain isolated from a polluted aquatic environment. Curr Microbiol 29:249–254. doi: 10.1007/BF01577436. [DOI] [PubMed] [Google Scholar]
- 28.Sun F, Yin Z, Feng J, Qiu Y, Zhang D, Luo W, Yang H, Yang W, Wang J, Chen W, Xia P, Zhou D. 2015. Production of plasmid-encoding NDM-1 in clinical Raoultella ornithinolytica and Leclercia adecarboxylata from China. Front Microbiol 6:458. doi: 10.3389/fmicb.2015.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S, Chandler M, Varani AM, Hickman AB, Dekker JP, Dyda F. 2016. Mechanisms of evolution in high-consequence drug resistance plasmids. mBio 7:e01987-16. doi: 10.1128/mBio.01987-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jørgensen NH, Stenderup A. 1982. An IncN plasmid exhibiting a high frequency of transfer in broth media. Acta Pathol Microbiol Immunol Scand B 90:323–324. doi: 10.1111/j.1699-0463.1982.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 31.Li J-J, Lee C-S, Sheng J-F, Doi Y. 2014. Complete sequence of a conjugative IncN plasmid harboring blaKPC-2, blaSHV-12, and qnrS1 from an Escherichia coli sequence type 648 strain. Antimicrob Agents Chemother 58:6974–6977. doi: 10.1128/AAC.03632-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler A, Khabra E, Paikin S, Carmeli Y. 2016. Dissemination of the blaKPC gene by clonal spread and horizontal gene transfer: comparative study of incidence and molecular mechanisms. J Antimicrob Chemother 71:2143–2146. doi: 10.1093/jac/dkw106. [DOI] [PubMed] [Google Scholar]
- 33.Mathers AJ, Cox HL, Kitchel B, Bonatti H, Brassinga AK, Carroll J, Scheld WM, Hazen KC, Sifri CD. 2011. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. mBio 2:e00204-11. doi: 10.1128/mBio.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kukla R, Chudejova K, Papagiannitsis CC, Medvecky M, Habalova K, Hobzova L, Bolehovska R, Pliskova L, Hrabak J, Zemlickova H. 2018. Characterization of KPC-encoding plasmids from Enterobacteriaceae isolated in a Czech hospital. Antimicrob Agents Chemother 62:e02152-17. doi: 10.1128/AAC.02152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoesser N, Phan HTT, Seale AC, Aiken Z, Thomas S, Smith M, Wyllie D, George R, Sebra R, Mathers AJ, Vaughan A, Peto TEA, Ellington MJ, Hopkins KL, Crook DW, Orlek A, Welfare W, Cawthorne J, Lenney C, Dodgson A, Woodford N, Walker AS, TRACE Investigators’ Group. 2020. Genomic epidemiology of complex, multispecies, plasmid-borne blaKPC carbapenemase in Enterobacterales in the United Kingdom from 2009 to 2014. Antimicrob Agents Chemother 21:e02244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chmelnitsky I, Shklyar M, Leavitt A, Sadovsky E, Navon-Venezia S, Ben Dalak M, Edgar R, Carmeli Y. 2014. Mix and match of KPC-2 encoding plasmids in Enterobacteriaceae-comparative genomics. Diagn Microbiol Infect Dis 79:255–260. doi: 10.1016/j.diagmicrobio.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Hardiman CA, Weingarten RA, Conlan S, Khil P, Dekker JP, Mathers AJ, Sheppard AE, Segre JA, Frank KM. 2016. Horizontal transfer of carbapenemase-encoding plasmids and comparison with hospital epidemiology data. Antimicrob Agents Chemother 60:4910–4919. doi: 10.1128/AAC.00014-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuelsen Ø, Overballe-Petersen S, Bjørnholt JV, Brisse S, Doumith M, Woodford N, Hopkins KL, Aasnæs B, Haldorsen B, Sundsfjord A, Norwegian Study Group on CPE. 2017. Molecular and epidemiological characterization of carbapenemase-producing Enterobacteriaceae in Norway, 2007 to 2014. PLoS One 12:e0187832. doi: 10.1371/journal.pone.0187832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kieffer N, Royer G, Decousser JW, Bourrel AS, Palmieri M, Ortiz De La Rosa JM, Jacquier H, Denamur E, Nordmann P, Poirel L. 2019. mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and its origin. Antimicrob Agents Chemother 63:e00965-19. doi: 10.1128/AAC.00965-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavda KD, Westblade LF, Satlin MJ, Hemmert AC, Castanheira M, Jenkins SG, Chen L, Kreiswirth BN. 2019. First report of blaVIM-4- and mcr-9-coharboring Enterobacter species isolated from a pediatric patient. mSphere 4:e00629-19. doi: 10.1128/mSphere.00629-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Liu F, Hu Y, Zhang G, Zhu B, Gao GF. 2020. Detection of mobile colistin resistance gene mcr-9 in carbapenem-resistant Klebsiella pneumoniae strains of human origin in Europe. J Infect 80:578–606. doi: 10.1016/j.jinf.2019.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Faccone D, Martino F, Albornoz E, Gomez S, Corso A, Petroni A. 2020. Plasmid carrying mcr-9 from an extensively drug-resistant NDM-1-producing Klebsiella quasipneumoniae subsp. quasipneumoniae clinical isolate. Infect Genet Evol 81:104273. doi: 10.1016/j.meegid.2020.104273. [DOI] [PubMed] [Google Scholar]
- 43.Tyson GH, Li C, Hsu CH, Ayers S, Borenstein S, Mukherjee S, Tran TT, McDermott PF, Zhao S. 2020. The mcr-9 gene of Salmonella and Escherichia coli is not associated with colistin resistance in the United States. Antimicrob Agents Chemother 64:e00573-20. doi: 10.1128/AAC.00573-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing: 27th informational supplement–M100-S27. CLSI, Wayne, PA. [Google Scholar]
- 45.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST ring image generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Pontén T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naas T, Cuzon G, Truong HV, Nordmann P. 2012. Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob Agents Chemother 56:4753–4759. doi: 10.1128/AAC.00334-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Letunic I, Bork P. 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 53.Liu SL, Hessel A, Sanderson KE. 1993. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc Natl Acad Sci U S A 90:6874–6878. doi: 10.1073/pnas.90.14.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence data for the isolates included in this study were submitted to the NCBI GenBank database under BioProject number PRJNA391501.