Abstract
Fungal endocarditis, specifically from Candida species, is a rare but serious infection with a high mortality rate. Most cases occur in bioprosthetic or mechanical valves and are uncommon in native, structurally normal valves. When Candida endocarditis is detected and appropriate treatment is initiated earlier, there is an improvement in mortality. While the recommendation is usually to treat with a combination of surgery and antifungal medications, patient comorbidities may limit treatment options.
Keywords: clinical diagnostic tests, radiology (diagnostics), infections, tropical medicine (infectious disease), valvar diseases
Background
Candida endocarditis is a serious complication of candidemia. Fungi accounts for <10% of cases of infective endocarditis; however, the mortality is greater than 50%.1 Not accounting for culture negative endocarditis, Candida spp. are isolated in 53% of fungal endocarditis, with Candida albicans being the most common.2 3 Aspergillus spp are the second most common.4
Common clinical presentations of fungal endocarditis includes fever and a new or change in an existing heart murmur.3 Other manifestations include generalised body aches, dyspnoea, cough, lower extremity pains or clubbing.5 Fungal endocarditis has a preponderance for damaged heart valves, or hardware-like prosthetic valves (2%–10%), pacemakers (4.5%), implantable cardioverter defibrillators and ventricular assist devices (35%–39%).4 Native valve endocarditis is increasingly being recognised in the presence of concurrent risk factors like immunosuppression, intravenous drug use, prolonged antibiotic use, parenteral nutrition, chronic indwelling central venous catheters, neutropenia and diabetes mellitus.6 Since fungal endocarditis is more common in bioprosthetic or mechanical valves, a diagnosis of native valve fungal endocarditis may be missed as the clinical suspicion is usually low in such cases. Streptococcus species are the most common cause of native valve endocarditis (70%), followed by Staphylococcus (25%).1
Case presentation
An 84-year-old man with medical history significant for advanced Alzheimer’s dementia, urinary retention, malnutrition and chronic obstructive pulmonary disease (COPD) presented from a nursing home with productive cough and shortness of breath for 2 days. It was accompanied by dysphagia and vomiting. There was no history of cardiac disease. Two weeks prior to his presentation, he was admitted for pyelonephritis and sepsis from Escherichia coli (E. coli) and was treated with cefazolin for 10 days. He had COPD GOLD Stage 1, diagnosed 3 years prior, and only used albuterol inhaler as needed for shortness of breath. He had not been on steroids long term, or during the current admission. He had significant benign prostatic hyperplasia causing urinary retention and had been discharged with an indwelling urinary catheter. Physical examination findings were notable for a temperature of 39.5°C, heart rate 104 beats/min, respiratory rate 18 breaths/min, blood pressure 112/87 mm Hg, O2 saturation 94% and a new grade 2/6 systolic ejection murmur over the precordium.
Investigations
On admission, the total leucocyte count was 17.6×109/L (94.2% polymorphonuclear leucocytes, 1.7% lymphocytes and 3.8% monocytes). Chest radiography showed mild left perihilar and right infrahilar linear opacities suggestive of atelectasis. Electrocardiography showed sinus tachycardia at 104 beats/min and a first-degree heart block (P-R interval of 0.24 ms). Urinalysis showed mild leucocyte esterase, 10 white cell count and budding yeasts of Candida.
Bacterial and fungal blood cultures were drawn. Sepsis was diagnosed, and intravenous cefepime and vancomycin were empirically initiated.
Bacterial blood cultures showed no growth. Fungal cultures grew Candida albicans species on day 3 of admission (2/2 blood cultures). A diagnosis of candidemia was established and further workup pursued.
As the source of candidemia was not evident, a CT scan of chest, abdomen and pelvis was ordered.
The CT of the chest revealed a small right and trace left pleural effusion with associated atelectasis. No radiological signs of pneumonia were seen. Thickening of the distal oesophagus was seen (figure 1).
Figure 1.
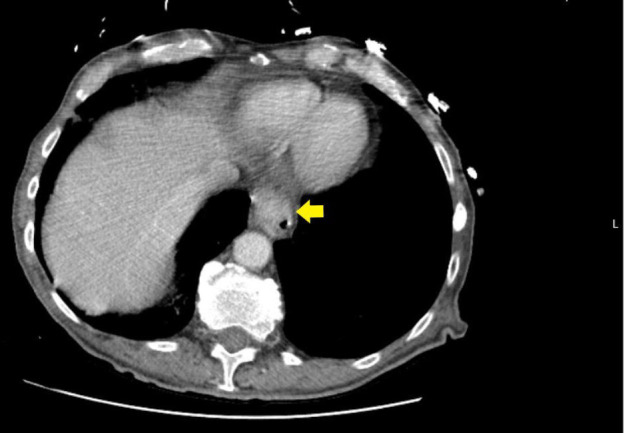
Distal oesophageal thickening (yellow arrow).
The CT scan of the abdomen and pelvis showed irregular circumferential urinary bladder wall thickening with small bilateral bladder diverticula (figure 2).
Figure 2.
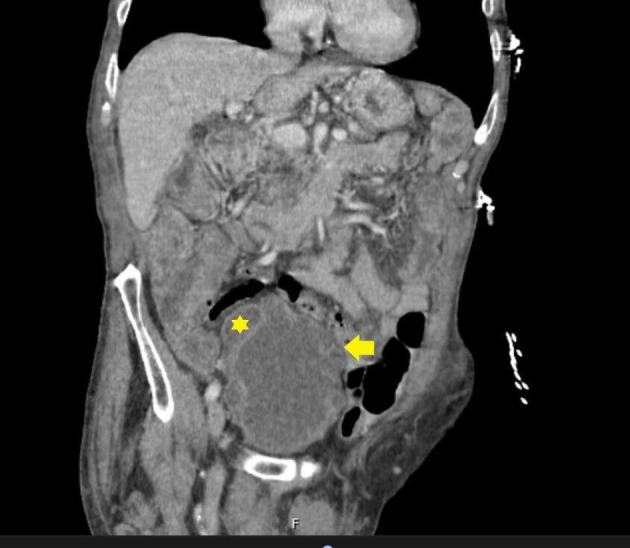
Urinary bladder wall thickening (yellow arrow) due to urinary retention. Also, note urinary bladder diverticula (yellow asterisk).
Given the oesophageal thickening and concomitant dysphagia, an oesophagogastroduodenoscopy (OGD) was performed which demonstrated oesophagitis in the lower one-third of the oesophagus with no evidence of neoplasia. Cultures and biopsy of the oesophagitis were obtained. No fungal growth was seen.
Ophthalmological examination revealed normal funduscopy bilaterally, and no evidence of Candida endophthalmitis.
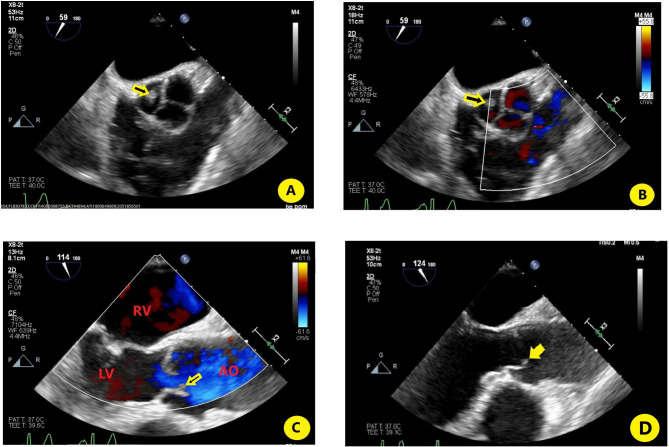
Infective endocarditis was subsequently entertained, and transoesophageal echocardiography (TOE) performed. TOE revealed left ventricular systolic dysfunction with an ejection fraction of 40%–45%. Aortic valve showed small echo dense structure attached primarily to the right coronary cusp (figure 3A–D). This abnormality was not present on a transthoracic echocardiogram (TTE) performed 3 months prior.
Figure 3.
(A) Transoesophageal echocardiography (TOE) (cross section) focusing on the aortic valve showing the vegetation on the right coronary cusp of the aortic valve (yellow arrow); (B) TOE showing the same as (A), with colour Doppler; (C) parasternal long axis of right ventricle showing small vegetation on aortic valve present during ventricular systole; (D) TOE (longitudinal axis) focusing on the aortic valve showing the vegetation on the right coronary cusp of the aortic valve (yellow arrow). AO, aorta; LV, left ventricle; RV, right ventricle; Vegetation, yellow arrow.
Treatment
Non-invasive bilevel positive airway pressure ventilation and intravenous diuretics were initiated. Cardiothoracic surgery and infectious diseases were consulted. Cefepime and vancomycin were discontinued and antifungal therapy with intravenous fluconazole 400 mg daily started.
Besides medical factors (advanced Alzheimer’s dementia, advanced age, size of the vegetation, haemodynamic stability), social aspects were an impediment to surgical candidacy (lack of capacity secondary to dementia, no family/surrogate decision maker, lack of social support, living in nursing home, impaired activity of daily living). After assessment of the risk–benefit ratio, a joint decision to treat medically was taken.
Medical management was continued. After 7 days of intravenous fluconazole, oral fluconazole 400 mg daily was initiated for a total of 6 weeks. It was decided to continue lifelong antifungal suppression thereafter with fluconazole 200 mg daily.
Since the presumptive source of candidemia was the urinary tract, as evidenced by candiduria at presentation, the chronic indwelling Foley catheter was changed to intermittent supervised catherisation.
Outcome and follow-up
The patient was transferred in stable condition to a skilled nursing facility.
The patient was reassessed after 6 weeks of antifungal therapy. He was clinically stable with no fevers, chills, new or recurrent systemic, or cardiac symptoms. A TTE showed a structurally intact aortic valve with no evidence of vegetation or masses.
Discussion
Candida spp are normal flora in the gastrointestinal system and in the lower genitourinary system.7 The pathophysiology of Candida endocarditis starts with blood stream infection/colonisation by the yeast. The blastospores must then convert to a filamentous form, a phenotypical switch, in order to adhere to and invade the endocardium. Adherence is mediated by cell surface proteins in the fungal wall, specifically the Int1p and Septin complex protein in Candida spp, as well as leucocyte-induced adhesion factors. After adherence, the filamentous Candida proliferate and destroy cardiac tissue.8
Given the higher associated mortality, often greater than 50%, early detection and treatment is crucial1 and can reduce mortality from fungal endocarditis.4 Patients usually have a predisposing factor for developing candidemia. In our patient, chronic urinary retention from benign prostatic hyperplasia led to urinary bladder diverticula (figure 2), subsequent urinary stasis and was likely the predisposing factor for candidemia. In addition, the presence of a chronic indwelling urinary catheter further contributed to colonisation of the urinary bladder with Candida.
While in this case 2/2 blood cultures were positive for Candida albicans, blood cultures may not always be reliable in fungal endocarditis. They often have poor yield and are only positive in less than 50% of the cases.1 Fungal endocarditis is an important cause of culture-negative endocarditis. Cardiac imaging, most often with TOE, is valuable in the diagnosis of endocarditis. Lesions are seen more often on the left side of the heart although they can be bilateral in immunocompromised patients.8 Candida endocarditis is much more likely to affect prosthetic valves.9
Due to the paucity of clinical trial data, defining the optimal treatment regimen for fungal endocarditis is challenging. Treatment should be individualised based on overall health, haemodynamic stability, presence or absence of valvular destruction, and overall life expectancy. A combination of antifungal pharmacotherapy and surgical treatment is often required.3
Management also involves removal of any affected cardiac devices for source control.6 Candida spp should initially be treated with either a lipid formulation of amphotericin B with or without flucytosine or a high-dose echinocandin including caspofungin, micafungin or anidulafungin. Use of amphotericin B-containing products is associated with infusion-related rigours, hypotension and bronchospasm. Delayed adverse effects include nephrotoxicity and a cation wasting. Blood cultures should be drawn until negative at which time treatment can be scaled back to oral fluconazole, voriconazole or posaconazole depending on specific susceptibilities of the organism.10 In this patient, treatment with fluconazole was chosen to avoid the adverse effects associated with more toxic drugs and the availability of an oral formulation.
A meta-analysis11 by Steinbach et al demonstrated that antifungal monotherapy, although not preferred, is a reasonable alternative for those who cannot undergo surgery. Lifelong antifungal prophylaxis should be considered given the high rate of fungal endocarditis recurrence.1 This was the approach adopted in our patient. This approach may also be considered for patients in whom the infected cardiac device cannot be removed, such as a left ventricular assist device.10
Learning points.
Fungal endocarditis can also occur in immunocompetent individuals.
Fungal endocarditis does not necessarily require damaged heart valves or foreign objects to establish infection.
Fungal endocarditis can be treated successfully with medical therapy if surgical intervention is prohibitive.
If treated with medical therapy alone, addition of chronic antifungal prophylaxis may be necessary.
Footnotes
Contributors: TS and JCT were involved in the conception and design of the work. TS, SRZ, JCT and MTA were involved in manuscript writing and reviewing and revising of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pierrotti LC, Baddour LM. Fungal endocarditis, 1995-2000. Chest 2002;122:302–10. 10.1378/chest.122.1.302 [DOI] [PubMed] [Google Scholar]
- 2.Negi N, Ahmad A. Current updates on fungal endocarditis. Fungal Biol Rev 2018;32:1–9. 10.1016/j.fbr.2017.11.001 [DOI] [Google Scholar]
- 3.Yuan S-M. Fungal endocarditis. Braz J Cardiovasc Surg 2016;31:252–5. 10.5935/1678-9741.20160026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badiee P, Amirghofran AA, Ghazi Nour M, et al. Incidence and outcome of documented fungal endocarditis. Int Cardiovasc Res J 2014;8:152–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Rubinstein E, Lang R. Fungal endocarditis. Eur Heart J 1995;16(Suppl B):84–9. 10.1093/eurheartj/16.suppl_B.84 [DOI] [PubMed] [Google Scholar]
- 6.Castillo JC, Anguita MP, Ruiz M, et al. [Changing epidemiology of native valve infective endocarditis]. Rev Esp Cardiol 2011;64:594–8. 10.1016/j.recesp.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 7.Mnge P, Okeleye BI, Vasaikar SD, et al. Species distribution and antifungal susceptibility patterns of Candida isolates from a public tertiary teaching hospital in the eastern Cape Province, South Africa. Braz J Med Biol Res 2017;50:5797. 10.1590/1414-431x20175797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spellberg B, Edwards JE. The pathophysiology and treatment of Candida sepsis. Curr Infect Dis Rep 2002;4:387–99. 10.1007/s11908-002-0005-3 [DOI] [PubMed] [Google Scholar]
- 9.Benjamin DK, Mirò JM, Hoen B, et al. Candida endocarditis: contemporary cases from the International collaboration of infectious endocarditis merged database (ICE-mD). Scand J Infect Dis 2004;36:453–5. 10.1080/00365540410020703 [DOI] [PubMed] [Google Scholar]
- 10.Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases Society of America. Clin Infect Dis 2016;62:409–17. 10.1093/cid/civ1194 [DOI] [PubMed] [Google Scholar]
- 11.Steinbach WJ, Perfect JR, Cabell CH, et al. A meta-analysis of medical versus surgical therapy for Candida endocarditis. J Infect 2005;51:230–47. 10.1016/j.jinf.2004.10.016 [DOI] [PubMed] [Google Scholar]