Abstract
We report the case of a 70-year-old Japanese man who was referred from a local urologist because of acute urinary retention (detrusor underactivity revealed by a urodynamics examination). A neurogenic urinary retention workup failed to reveal the aetiology, but a spinal tap incidentally showed occult meningeal reaction with positive oligoclonal band. The patient had no headache, nausea/vomiting or fever. Considering his clinical laboratory findings, his neural lesions seemed to involve the meninges and spinal cord, suggestive of ‘form fruste’ meningitis-retention syndrome. When clinicians encounter patients with urinary retention of undetermined aetiology, a spinal tap should be considered.
Keywords: meningitis, neurology, spinal cord, urology, catheterisation / catheter care
Background
Urinary retention is a symptom of a urological emergency. Urinary retention in elderly men is common and is often attributed to prostate hypertrophy. In contrast, urinary retention with a normal prostate without any drug, systemic or neurological abnormality is extremely uncommon.1 2 We recently treated a patient in whom a spinal tap incidentally showed occult meningeal reaction, suggestive of ‘form fruste’ meningitis-retention syndrome (MRS).2–4
Case presentation
A 74-year-old Japanese man started to have difficult urination, which was soon followed by urinary retention. No prodromal infection/illness was noted. He was taking no drugs that might affect bladder function. A general urology doctor checked his problem, and at that time the patient provided questionnaires (overactive bladder symptom score (OABSS) of 7/15, international prostate symptom score (IPSS) of 23/35) showing difficult urination. His postvoid residual (PVR) was 300 mL as catheterised. He was prescribed 0.2 mg/day tamsulosin (adrenergic α1A blocker), but this did not ameliorate his symptom. Ultrasonography revealed a prostate size of 25 mL (normal <20 mL) with no protrusion of the inner gland. He showed an International Index of Erectile Dysfunction of 1/25, indicating erectile dysfunction. However, whether this problem occurred together with the urination symptom was unclear. He did not have constipation. Five years earlier, he had been diagnosed with hypertension and had been taking 5 mg/day amlodipine (a calcium channel antagonist) since then. He reported postural dizziness, but he did not remember whether this problem occurred together with the urination issue. He had no headache, nausea/vomiting or fever (though he reported a ‘subfever’ at 36.5°C rather than his usual temperature at 35.0°C–35.5°C). On arrival, his general condition and cognitive status were normal. No abnormality was found in the cranial nerves including eye movement and speech. His motor functions including extrapyramidal system, cerebellar system and pyramidal system were all normal. No sensory abnormality was found including the perineal area, and rectal tone was normal by digital examination.
Investigations
Urodynamics
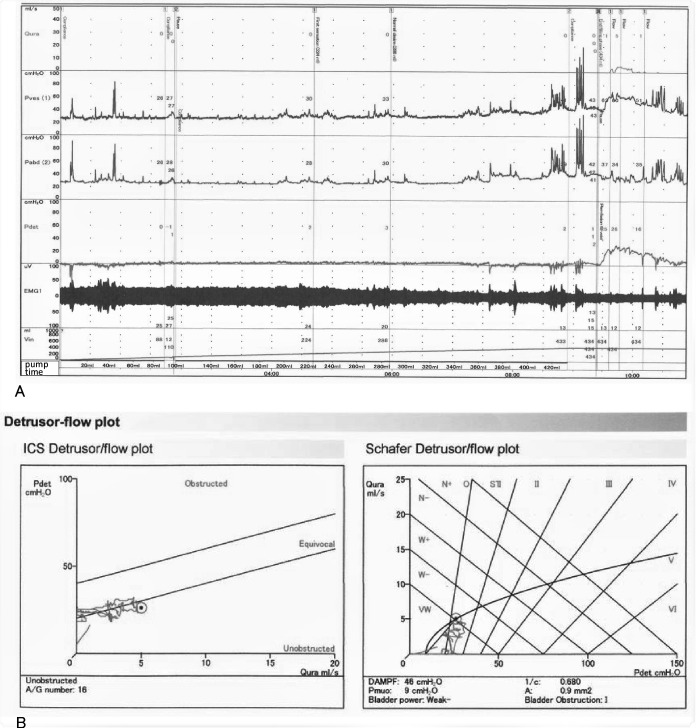
A urodynamics examination5 was performed at the 9th day after the patient’s symptom onset (ie, 2 days after he stopped taking tamsulosin). During the slow filling, he reported his first sensation at a volume of 224 mL and bladder capacity at 434 mL, and no detrusor overactivity was observed (figure 1). On voiding, electromyography sounds of the external sphincter muscles decreased. He micturated 70 mL, and a PVR volume of 364 mL remained (normal <30 mL). A pressure-flow analysis revealed no obstruction (Schafer obstruction grade 1) and a Schafer grade of ‘very weak’. Therefore, he was thought to have detrusor underactivity.
Figure 1.
Urodynamic recording of the patient’s bladder on the ninth day after onset. (A) during slow filling (50 mL/min), when he reported his first sensation at a volume of 224 mL (100 mL <normal < 300 mL) and a maximum desire to void (bladder capacity) at 434 mL (200 mL <normal < 600 mL), we stopped Infusing saline into the bladder. In the meantime, he had no detrusor overactivity. On coughing he did not leak. During voiding, the sphincter electromyography sounds decreased in amplitude. He voided 70 mL and left a large PVR volume of 364 mL. (B, C:) pressure-flow analysis revealed no obstruction (Schafer obstruction grade 1; 0–6 grades: normal <1, 2=equivocal, 3–6=obstruction) and a Schafer grade of very weak (four grades: strong, normal, weak, very weak). Based on these results, he was considered to have detrusor underactivity. Flow, urinary flow; Pves, vesical (bladder) pressure; Pabd, abdominal (rectal) pressure; Pdiff, differential detrusor pressure=Pves – Pabd; PVR, postvoid residual; ICS, International Continence Society.
Neurophysiology
The results of a nerve conduction study of the four extremities, including F waves, were normal. The findings from an analysis of motor unit potentials in the external sphincter muscles were normal.6 The bulbocavernosus reflex was normal. Decelerating bursts and complex repetitive discharges, suggesting Fowler’s syndrome, were not observed.
Neuroimaging
The axial, sagittal and coronal plane images of 3T MRI with gadolinium enhancement in the lumbosacral spinal cord, dorsal root ganglia, cauda equina and vertebra/disc were normal. Brain MRI results were normal. 123I-metaiodo-benzylguanidine (MIBG) myocardial scintigraphy, a 123I-ioflupane (N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane) dopamine transporter (DAT) scan and 99mTc-L, L-ethylcysteinate dimer-single-photon emission CT were normal. At that time, we suspected that the patient may have premotor phase multiple system atrophy.7 8
Cerebrospinal fluid
A spinal tap was carried out 1 month after the onset of difficult urination to measure cerebrospinal fluid (CSF) alpha-synuclein, tau and other substances. Unexpectedly, the CSF showed mononuclear leucocytosis at 28/mm3 (normal <5), mildly increased protein content of 44 mg/dL (normal <40) and a normal glucose level at 56 mg/dL (59% of serum glucose). Bacterial smears and cultures were negative. The CSF enzyme immunoassay showed negative IgM and IgG antibodies of herpes simplex type-1 (HSV-1) and varicella-zoster viruses (VZV). CSF myelin-basic protein was negative (<31.3, normal). However, the CSF oligoclonal band was positive by immunoelectrophoresis (9 bands appeared, normal 0–1 band, IgG CSF vs serum=6.0:1510 (mg/dL)), suggesting an immune-mediated reaction. Serum aquaporin 4 antibody was negative. We did not measure myelin oligodendrocyte glycoprotein.
Outcome and follow-up
Thus, urinary retention with occult meningeal reaction and ‘form fruste’ MRS was the putative diagnosis. In MRS, corticosteroids might shorten the duration and severity of illness. However, since the patient’s illness seemed to improve spontaneously, we treated him without corticosteroids.2 Two weeks later, his PVR became <100 mL. One month later, a second CSF examination showed mononuclear leucocytosis at 19 /mm3, a protein content of 43 mg/dL and the glucose level 57 mg/dL (60% of serum glucose). At the same time, a second urodynamics examination was performed. During slow filling, his first sensation was 142 mL and the bladder capacity was 328 mL, and he had no detrusor overactivity or low compliance. A pressure-flow analysis indicated that his detrusor power represented by the Schafer grade had improved mildly from very weak to weak. We have been following the patient carefully for 6 months; his condition has remained unchanged.
Discussion
Based on the location of the lesion, isolated neurogenic urinary retention (of the urodynamic detrusor underactivity type) can be divided into the three subgroups of ‘upper neuron,’ ‘lower neuron,’ and ‘undetermined location’ as follows: (1) The upper neuron subgroup (possibly spinal cord; mostly autoimmune aetiologies; damaging micturition descending pathways as initial spinal shock; might be followed by detrusor overactivity) includes (MRS; fever, headache, stiff neck, minor upper motor neuron sign),2 9 10 multiple sclerosis spinal type, autoimmune myelitis11 and other conditions; (2) The lower neuron subgroup (eg, the sacral cord, cauda equina or peripheral nerves; various aetiologies; damaging the pelvic nerves) includes sacral herpes (dermatomal skin rush via sacral dorsal root ganglia),12 13 lumbar spondylosis (saddle pain/numbness with lower motor neuron sign), spina bifida occulta, diabetic polyneuropathy and other conditions and (3) The undetermined location subgroup includes urinary retention with occult meningeal reaction (table 1). Few such cases have been reported to date. The cases can be subdivided into infectious cases such as HSV type 214 and infectious mononucleosis (possibly Epstein-Barr virus)15 and non-infectious cases (autoimmune suspected). In the report by Vanneste et al,16 cases 1 and 3 had acute urinary retention and CSF abnormalities alone, but the neurological examinations were otherwise normal. Their case 3 showed no positive viral antibodies. In order to determine the responsible site for a patient’s urinary retention, a neurological examination as well as neurophysiology and neuroimaging seem to be necessary. However, in the previous reports, no extensive workup was done. Our patient was revealed to have an occult meningeal reaction, after the extensive consideration and exclusion of other aetiologies. Considering the complete lack of evidence of sacral or peripheral involvement and the positive oligoclonal band in his CSF, the neural lesions in our patient’s case seemed to involve the meninges and spinal cord, suggestive of ‘form fruste’ MRS.
Table 1.
Undetermined location cases in neurogenic urinary retention
| Year, author | patient | bladder-systemic-neurological symptoms and signs | Urodynamics | Other tests | Prognosis | |||||||||||||||||||
| Age | Sex | Retention | Fever | Head-ache | Stiff neck/kernig | Lower extermities | Sensation | Skin erruption | FS (mL) |
BC (mL) |
DO | DU | DSD | Neuro physiology | Neuro imaging | Cerebrospinal fluid (CSF) | CSF (weeks) |
Retention (weeks) |
||||||
| (Years) | (Systemic) | Power | Reflexes | Cell /mm3 (n<5) |
Protein mg/dL (n<40) |
Glucose mg/dL (n>50) |
OCB | MBP and virus titre |
||||||||||||||||
| Undetermined location cases: urinary retention with Some systemic sign No meningeal sign No upper neuron sign No lower neuron (sacral/peripheral) sign |
||||||||||||||||||||||||
| 1973 Sperber15 |
23 | F | + | + | + | np | N | np | N | – | np | +? | np | np | inc | inc | np | np | np | np | 1 | |||
| Sore throat, lymphadenopathy | (Exact number not mentioned) | |||||||||||||||||||||||
| Atypical lymphocytosis | ||||||||||||||||||||||||
| (Infectious mononucleosis: common Epstein Barr (EB) virus infection) | ||||||||||||||||||||||||
| (Cases with EBV &both meningoencephalitis and peripheral neuropathy are cited) | ||||||||||||||||||||||||
| 1980 Vanneste16 |
20 | F | + | – | – | – | N | N | N | – | np | +? | np | np | 32 | 22 | 57 | np | np | np | 1 | |||
| case1 | Malaise, fatigue | |||||||||||||||||||||||
| (Viral infection suspected) | ||||||||||||||||||||||||
| 21 | F | + | – | – | – | N | N | N | – | np | +? | np | np | 161 | 70 | 54 | np | N | np | 1 | ||||
| case3 | (No malaise, fatigue) | |||||||||||||||||||||||
| 1991 Steinberg14 |
24 | M | + | – | – | – | N | N | N | – | 160 | 340 | – | + | np | BCR: N | Lumbar | 133 | 78 | 38 | – | HSV2 | 3 | 3 |
| (No malaise, fatigue; no leucocytosis) | (by CO2 cystometry) | MRI: N | ||||||||||||||||||||||
| (Central nervous system involvement by HSV is suspected) | ||||||||||||||||||||||||
| 2020 Present case |
74 | M | + | -+ | – | – | N | N | N | – | 224 | 434 | – | + | – | Sphincter | Lumbar | 28 | 44 | 56 | + | Normal | (3) | 5 |
| – | – | – | – | N | N | N | – | 142 | 328 | – | +- | – | EMG, BCR, | MRI, | 19 | 43 | 57 | np | np | |||||
| (No malaise, fatigue; no leucocytosis) | (Follow-up) | (Follow-up) | NCS incl. | brain MRI, | (Follow-up) | |||||||||||||||||||
| F wave | MIBG, DAT | |||||||||||||||||||||||
| : N | ECD-SPECT | |||||||||||||||||||||||
| : N | ||||||||||||||||||||||||
Hatched area indicates abnormality.
BC, bladder capacity (200 mL <N < 600 mL); BCR, bulbocavernosus reflex; DAT, 123I-ioflupane (N-ω-fluoropropyl-2-β-carbomethoxy-3β-(4-iodophenyl) nortropane) dopamine transporter scan; DO, detrusor overactivity; DSD, detrusor-sphincter dyssynergia; DU, detrusor underactivity; ECD, 99mTc-L, L-ethylcysteinate dimer-single-photon emission computed tomography (ECD-SPECT); FS, first sensation (100 mL <N < 300 mL); HSV, herpes simplex virus; MBP, myelin basic protein; MIBG, 123I-metaiodo-benzylguanidine (MIBG) myocardial scintigraphy; N, normal; np, not performed; OCB, oligoclonal band.
This case report has several limitations. We have followed our patient for only 6 months, and we performed imaging using only MRI, perfusion SPECT, DAT scan and MIBG myocardial scintigraphy. We measured only HSV1 and VZV antibody titres in the patient’s CSF. Therefore, other aetiologies were not completely excluded. In addition, the patient continues to have symptoms with an abnormal CSF on follow-up. This raises the possibility of chronic meningitis of unknown aetiology presenting as urinary retention rather than ‘meningeal reaction’ that is non-specific. CSF protein increase is known in Parkinson’s disease while CSF pleocytosis has not been clearly reported in neurodegenerative diseases. Therefore, the possibility that CSF abnormality in our case might reflect early stages of a neurodegenerative disease is not completely excluded. Nevertheless, diagnosing urinary retention with occult meningeal reaction, most probably ‘form fruste’ MRS, is important for the proper management and for the avoidance of unnecessary therapies, since MRS is a benign disease. It is important to emphasise that MRS is a diagnosis of exclusion after thorough evaluation/workup for other aetiologies. Further studies with a large number of patients are warranted. In addition, spinal tap is an option when clinicians encounter urinary retention of undetermined aetiology.
Learning points.
Urinary retention has not only urological but also neurological aetiology in men and women.
Neurological aetiology includes lumbar spondylosis, diabetic neuropathy and multiple system atrophy.
After excluding these aetiology, we should perform spinal tap to seek meningitis-retention syndrome (MRS).
MRS is regarded a mild form of acute disseminated encephalomyelopathy, often with positive oligoclonal band and myelin basic protein and with benign course.
It is not conclusive whether steroid might shorten the period of urinary retention in MRS.
Footnotes
Contributors: All authors contributed significantly and are in agreement with the content of the manuscript. RS: study concept and design, data acquisition and analysis, and manuscript writing, FT, YA and DS: data acquisition.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Panicker JN, Sakakibara R. Lower urinary tract and bowel dysfunction in neurologic disease. Continuum 2020;26:178–99. 10.1212/CON.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 2.Sakakibara R. Neurogenic lower urinary tract dysfunction in multiple sclerosis, neuromyelitis optica, and related disorders. Clin Auton Res 2019;29:313–20. 10.1007/s10286-018-0551-x [DOI] [PubMed] [Google Scholar]
- 3.Basoulis D, Mylona M, Toskas P, et al. . Meningitis-Retention syndrome. Int Neurourol J 2015;19:207–9. 10.5213/inj.2015.19.3.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregušová A, Klézl P, Mašková V, et al. . Acute urinary retention in aseptic meningitis: Meningitis-retention syndrome. Neuro Endocrinol Lett 2019;40:166–8. [PubMed] [Google Scholar]
- 5.Abrams P, Cardozo L, Fall M, et al. . The standardisation of terminology of lower urinary tract function: report from the standardisation Sub-committee of the International continence Society. Neurourol Urodyn 2002;21:167–78. 10.1002/nau.10052 [DOI] [PubMed] [Google Scholar]
- 6.Sakakibara R, Uchiyama T, Yamanishi T, et al. . Sphincter EMG as a diagnostic tool in autonomic disorders. Clin Auton Res 2009;19:20–31. 10.1007/s10286-008-0489-5 [DOI] [PubMed] [Google Scholar]
- 7.Panicker JN, Simeoni S, Miki Y, et al. . Early presentation of urinary retention in multiple system atrophy: can the disease begin in the sacral spinal cord? J Neurol 2020;267:659–64. 10.1007/s00415-019-09597-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakakibara R, Panicker JN, Aiba Y, et al. . Possible "Premotor" Multiple System Atrophy-Cerebellar Form. Eur Neurol 2020;83:80–6. 10.1159/000506983 [DOI] [PubMed] [Google Scholar]
- 9.de Groat WC, Yoshimura N. Anatomy and physiology of the lower urinary tract. Handb Clin Neurol 2015;130:61–108. 10.1016/B978-0-444-63247-0.00005-5 [DOI] [PubMed] [Google Scholar]
- 10.Hiraga A, Sakakibara R, Mori M, et al. . Bilateral lesion in the lateral columns and complete urinary retention: association with the spinal cord descending pathway for micturition. Neurourol Urodyn 2005;24:398–9. 10.1002/nau.20129 [DOI] [PubMed] [Google Scholar]
- 11.Hiraga A, Sakakibara R, Mori M, et al. . Urinary retention can be the sole initial manifestation of acute myelitis. J Neurol Sci 2006;251:110–2. 10.1016/j.jns.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 12.Yamanishi T, Yasuda K, Sakakibara R, et al. . Urinary retention due to herpes virus infections. Neurourol Urodyn 1998;17:613–9. [DOI] [PubMed] [Google Scholar]
- 13.He H, Tang C, Yi X, et al. . Herpes zoster-induced acute urinary retention: two cases and literature review. Niger J Clin Pract 2018;21:534–7. 10.4103/njcp.njcp_244_16 [DOI] [PubMed] [Google Scholar]
- 14.Steinberg J, Rukstalis DB, Vickers MA. Acute urinary retention secondary to herpes simplex meningitis. J Urol 1991;145:359–60. 10.1016/S0022-5347(17)38340-4 [DOI] [PubMed] [Google Scholar]
- 15.Sperber A, Tessler AN, Berczeller P. Infectious mononucleosis with acute urinary retention. Urology 1973;2:456–7. 10.1016/0090-4295(73)90027-7 [DOI] [PubMed] [Google Scholar]
- 16.Vanneste JA, Karthaus PP, Davies G. Acute urinary retention due to sacral myeloradiculitis. J Neurol Neurosurg Psychiatry 1980;43:954–6. 10.1136/jnnp.43.10.954 [DOI] [PMC free article] [PubMed] [Google Scholar]