Abstract
Germ cell tumours (GCT) are the most common testicular neoplasms, seen mainly in young adults. Rarely they can affect extragonadal tissues, either as primary tumours or as metastases, most commonly to retroperitoneal lymph nodes. A ‘burned-out’ testicular tumour is a metastatic GCT with a relatively occult primary testicular tumour, which has histologically spontaneously regressed. We report a case of a 26-year-old man who presented with an acute history of lower back pain and leg swelling. CT demonstrated a large retroperitoneal soft tissue mass causing right-sided hydronephrosis with inferior vena cava and iliofemoral vein thrombosis. Although clinical examination of the testis was normal, ultrasound imaging of the scrotum identified a burned-out testicular primary. Orchiectomy confirmed the diagnosis and the patient responded well to chemotherapy, with no viable residual tumour on follow-up imaging. However, despite nephrostomy insertion, a dimercaptosuccinic acid (DMSA) scan demonstrated loss of function of the right kidney after treatment.
Keywords: urological cancer, pathology, radiology, urological surgery, surgical oncology
Background
The differential diagnosis for a solid retroperitoneal mass includes lymphoma, sarcoma, neurogenic tumours, retroperitoneal fibrosis and germ cell tumours (GCT).1 It is vital to consider GCT in the differential of a young man presenting with a retroperitoneal mass. Testicular clinical examination and ultrasound imaging is essential in the diagnosis and subsequent management. A ‘burned-out’ testicular primary tumour may be clinically occult and only identified with ultrasonography. It is crucial to make the diagnosis of metastatic GCT to initiate appropriate and timely treatment. Whereas a primary retroperitoneal GCT is treated with chemotherapy alone, metastatic disease secondary to a ‘burned-out’ primary testicular tumour requires additional orchiectomy, due to the high risk of premalignant lesions in the residual testicular parenchyma and residual tumour in case of incomplete regression.2
We report a case of a burned-out testicular tumour in a 26-year-old man presenting with back pain and leg swelling and no medical history. This case highlights the importance of early suspicion of a testicular primary and identification of relevant findings on imaging.
Case presentation
A previously fit and well 26-year-old man had presented with a 1 week history of acute lower back pain and left leg swelling. An ultrasound scan at his local hospital identified extensive left-sided iliofemoral deep vein thrombosis (DVT). He was then transferred to our institution for further investigation and specialist vascular input.
Investigations
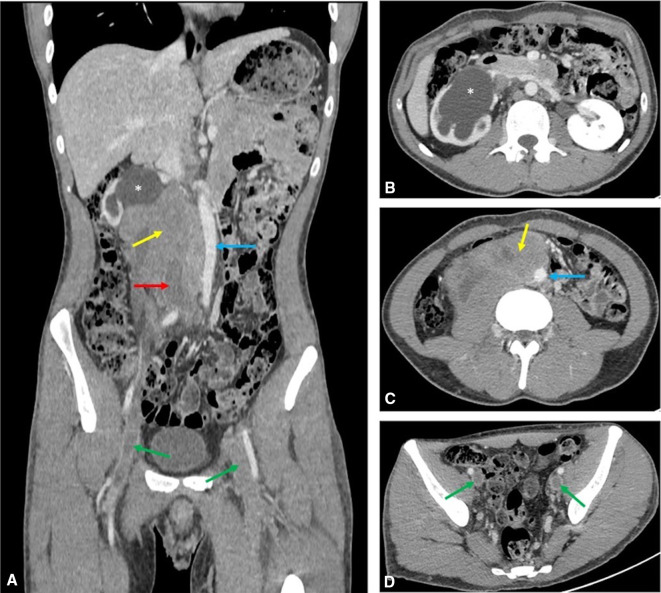
A CT venogram was requested on admission to characterise the extent of the DVT before vascular intervention. A large, predominantly right-sided retroperitoneal soft tissue mass was identified, encasing the upper inferior vena cava (IVC) and right ureter, with marked secondary right-sided hydronephrosis. There were thromboses of the lower IVC, iliac and common femoral veins and superficial femoral vein on the left (figure 1). A CT scan of the head, neck and chest for completion of staging revealed no further sites of disease.
Figure 1.
Axial (B–D) and coronal (A) delayed phase (120 s) CT scan of the abdomen and pelvis demonstrating the right-sided retroperitoneal mass (yellow arrows) encasing the IVC (red arrow). The aorta is displaced to the right (blue arrow) and extensive thrombus is seen in the iliac veins (green arrows) and lower IVC. Severe right-sided hydronephrosis (asterisks) with associated cortical thinning also noted. IVC, inferior vena cava.
As a clinical examination of the testes was normal, the most likely diagnosis considered was of lymphoma and hence an ultrasound-guided biopsy of the retroperitoneal mass was performed for tissue diagnosis. Samples were sent for histopathological analysis and immunological staining at a specialist tertiary centre. Histological examination of the retroperitoneal tissue revealed intermediate size oval cells with pale eosinophilic cytoplasm and nuclei with fine chromatin and central nucleoli, set in a background of granulomatous reaction with areas of focal necrosis. Immunohistochemistry demonstrated positive staining for OCT3/4 and CD117 and was negative for AE1/AE3, CD30, AFP and PLAP. The overall appearances represented seminoma (figures 2–4).
Figure 2.
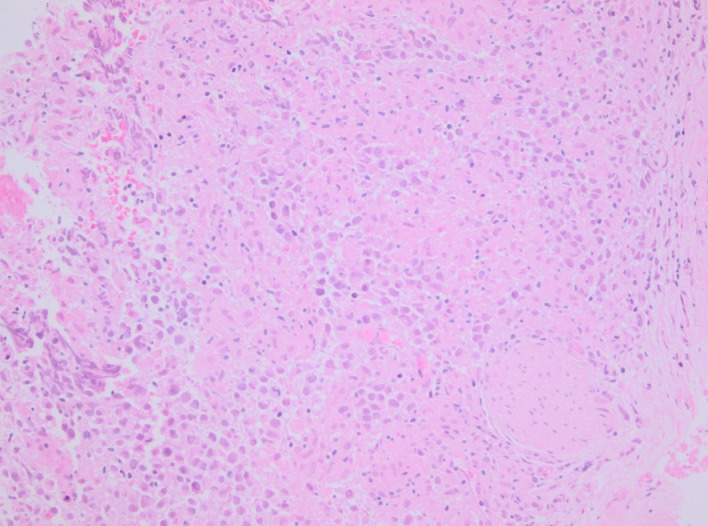
H&E stain. Retroperitoneal mass biopsy: the soft tissue is diffusely infiltrated by singly dispersed round cells with pale cytoplasm, oval to round nuclei and prominent nucleoli.
Figure 3.
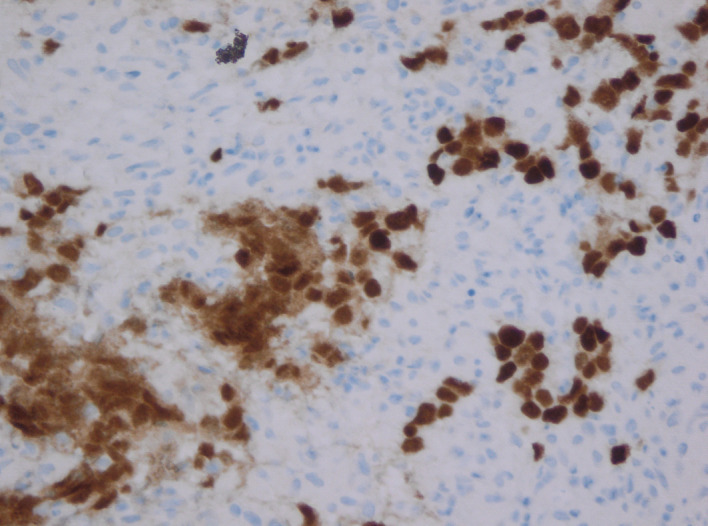
Cells show positive immunostaining with OCT3/4.
Figure 4.
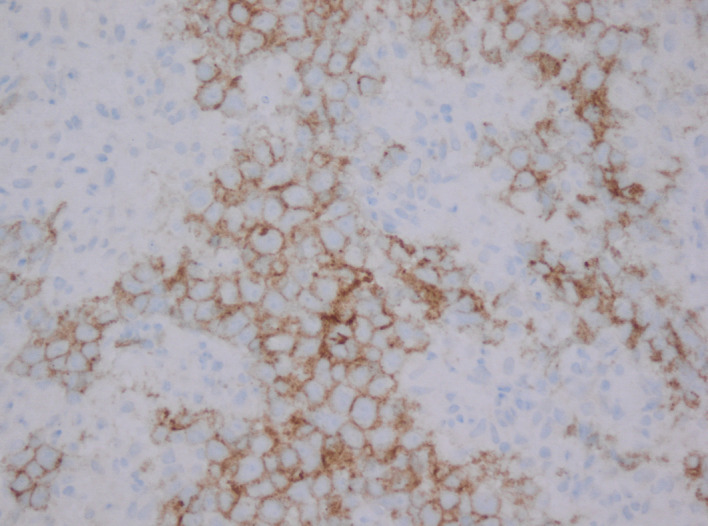
Cells show positive immunostaining with CD 117. Tumour cells were negative for AE1/3, CD30, AFP and PLAP.
A blood film showed mild microcytic hypochromic anaemia but no convincing lymphoid cells. Beta-human chorionic gonadotrophin (HCG) and lactate dehydrogenase (LDH) were mildly raised (23 IU/L and 329 IU/L, respectively). AFP, protein electrophoresis and IgG, IgA and IgM were all within the normal range.
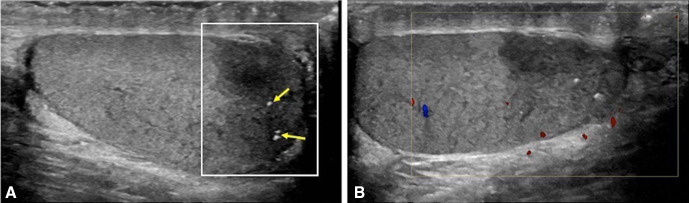
An ultrasound scan of the scrotum performed to exclude a testicular primary, demonstrated an ill-defined hypoechoic region of scarring in the lower pole of the right testicle with no internal vascularity, containing punctate calcifications (figure 5).
Figure 5.
Ill-defined hypoechoic area (A) within the lower pole of the right testis (white box) with scattered punctate calcifications (arrows) and no internal Doppler vascularity (B).
Differential diagnosis
The key differential diagnoses to be considered in a young adult man presenting with a retroperitoneal mass include lymphoma, sarcoma and GCT. The vital point is to identify that a retroperitoneal mass may be secondary to a testicular primary and to investigate this further with testicular clinical examination and ultrasonography with a high-frequency linear probe. Ultrasound should be performed even if clinical examination of the scrotum is normal, as a burned-out tumour will rarely be palpable.
The ultrasound findings for a burned-out primary tumour are non-specific and more subtle than an active testicular primary. They may include a combination of the following features: loss of testicular homogeneity, non-specific hypovascular hypoechoic areas within the parenchyma, focal areas of calcification, microlithiasis and testicular atrophy.2 In our case, scrotal sonography demonstrated an ill-defined hypoechoic region containing punctate calcifications within the lower pole of the right testicle.
In combination with the histological analysis of the retroperitoneal mass, the CT and ultrasound findings were consistent with a ‘burned-out’ primary seminomatous testicular tumour.
Treatment
The patient was treated with anticoagulants (dalteparin 10 000 units subcutaneously, two times per day followed by rivaroxaban 20 mg orally one time per day) and referred to urology for a right orchiectomy. The histological analysis identified a dense fibrous scar corresponding to the location of the sonographic findings, with no viable tumour (figure 6). The adjacent testicular tissue showed atrophy of most of the seminiferous tubules with only sertoli cells and thickening of the basement membrane (figure 7). There was no evidence of intratubular germ cell neoplasia.
Figure 6.
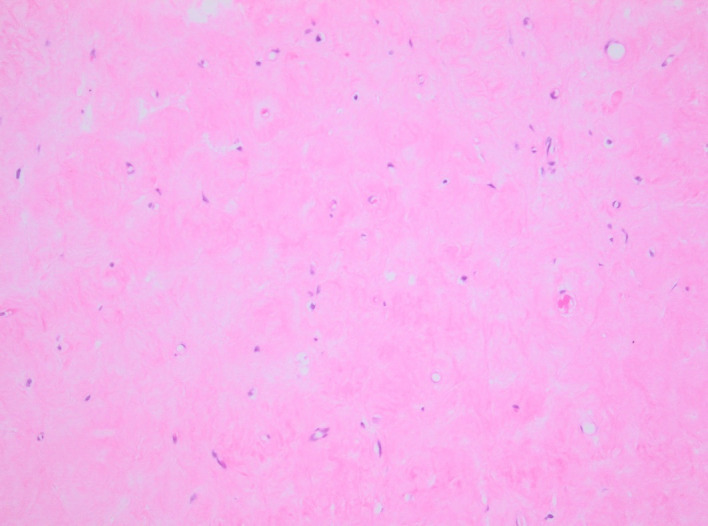
H&E stain. Testis showing scar tissue.
Figure 7.
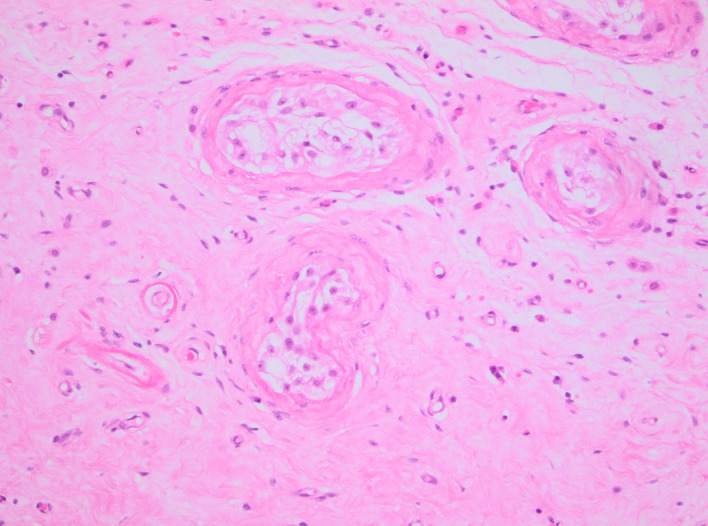
H&E stain. Testis showing seminiferous tubules (upper right and centre) as well as scar tissue.
Due to the extensive compressive effect of the retroperitoneal mass on the right ureter causing significant hydronephrosis, attempts at both antegrade and retrograde ureteric stent insertion were not successful and a nephrostomy was placed instead.
The patient was then treated initially with etoposide 300 mg/m2 and cisplatin 60 mg/m2, followed by three cycles of BEP chemotherapy—bleomycin 30 000 IU day 1, 8 and 15, etoposide 500 mg/m2 and cisplatin 100 mg/m2.
Outcome and follow-up
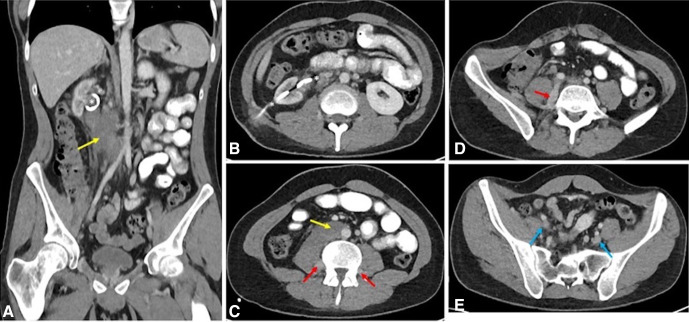
Chemotherapy was well-tolerated, and there has been an excellent response, with a significant reduction in the bulk of the retroperitoneal mass (figure 8). We reasoned that the chemotherapy-induced tumour regression would allow a stent placement and thus render the patient nephrostomy free, without the need for an external drainage device. However, a further attempt at an antegrade ureteric stent insertion was still unsuccessful.
Figure 8.
Axial (B–E) and coronal (A) CT images post-treatment demonstrating significant reduction in the size of the retroperitoneal mass (yellow arrows). The IVC and right ureter remain encased, but there is resolution of the extensive venous thrombus. Partial recanalisation of the iliac veins (blue arrow) is seen with drainage through the ascending lumbar veins (red arrows). Right nephrostomy noted in situ (asterisks). IVC, inferior vena cava.
A subsequent renal DMSA scan showed a non-functioning right kidney, contributing only 2.5% of split function (figure 9). The daily urine output from the nephrostomy immediately following its insertion was approximately 100–200 mL; however, the output had decreased within 4 months to 20–50 mL. There was significant right renal cortical atrophy with a delayed enhancement of the parenchyma on the initial CT which was again demonstrated on follow-up imaging postnephrostomy insertion, despite the reduction in the bulk of the retroperitoneal tumour. The minimal cortical tracer uptake seen on the DMSA which was performed 4 months post follow-up CT correlates with the clinical picture of that time. Furthermore, the interval administration of chemotherapy likely contributed to further deterioration of his renal function, as has been observed in other studies as well.3 4 Progressive renal atrophy on follow-up CT imaging, as seen in our patient has also been noted previously in patients treated with these chemotherapy agents.4
Figure 9.
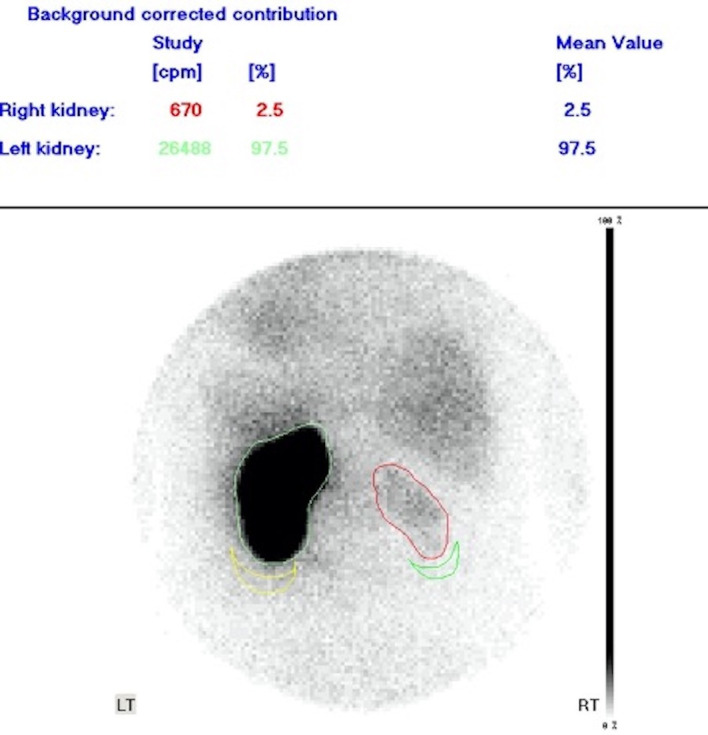
DMSA scan demonstrates an atrophic non-functioning right kidney with minimal cortical tracer uptake.
Currently, he feels well in himself. His renal function is stable with a serum creatinine of 135 µmol/L and an Estimated glomerular filtration rate (eGFR) of 65. He is awaiting a right nephrectomy and continues to be under periodic oncological review.
Discussion
Testicular cancer is one of the most common malignancies in men aged 15–40, with peak incidences in the third and fourth decades.5 In total, 90%–95% of all primary testicular tumours develop from germ cells. GCT include seminomatous and non-seminomatous types (embryonic carcinoma, yolk sac tumour, teratoma, choriocarcinoma) and mixed forms. Five per cent of all GCT are extragonadal, originating from migrated primordial germ cells, with the anterior mediastinum, retroperitoneum and central nervous system being the most common sites. Retroperitoneal GCT, however, are more commonly secondary, with an occult burned-out testicular tumour in about 10% of cases of presumed primary retroperitoneal disease.6
First described in 1927, a ‘burned out’ tumour typically refers to a testicular GCT in which the primary lesion has spontaneously regressed, often with only a residual parenchymal scar.7 Metastatic disease may or may not be present. It is a rare entity with poorly understood pathophysiology. Unconfirmed mechanisms for this phenomenon have been proposed, including the neoplasm outgrowing its blood supply or an immune-mediated response by the host.8 9
Since the regressed primary testicular tumour is rarely palpable or symptomatic, patients usually present late with symptoms from metastatic disease, most commonly abdominal and back pain, palpable mass and weight loss. Infrequently, testicular pain or a palpable abnormality will be present.10
Although retroperitoneal disease is the most commonly documented site of metastasis, mediastinal, supraclavicular, cervical or axillary lymph node involvement has also been reported, as well as metastases to the liver, lung, brain and other organs.11–13 Paraneoplastic syndromes have also been documented.14
It is always essential to consider GCT when confronted with a retroperitoneal mass of unknown origin and to obtain a testicular ultrasound even if no mass is evident on clinical examination. Physical examination is not sufficient to eliminate a primary testicular tumour, whereas ultrasound sensitivity for diagnosing GCT is close to 100%.3
Despite the presence of metastatic disease, there is often no viable tumour remaining within the testicle, with only a residual scar seen on imaging and histopathology. Germ cell neoplasia in situ is found in 50% of cases on histology, and residual GCT may be present if regression is incomplete.8 Therefore, orchiectomy is essential not only for a definitive diagnosis but also for treatment, combined with systemic chemotherapy.
Current guidelines from the European Association of Urology state that for metastatic seminomatous tumours, chemotherapy is the mainstay of treatment, with evidence in favour of a cisplatin-based regime.15 16 Standard therapy in cases such as this patient is with BEP or EP if bleomycin is contraindicated.17
At present, no data are available comparing prognosis of burned-out versus non-regressed gonadal and extragonadal GCT. Therefore, longitudinal studies on a larger cohort may show the long-term outcomes of such cases with regards to the quality of life, fertility outcomes and treatment-induced second malignancies.
Learning points.
Consider testicular primary germ cell tumour in a young adult with a retroperitoneal mass.
Examine the testes and proceed to scrotal ultrasonography even if no palpable abnormality is present.
A burned-out tumour is rare, and a high index of suspicion for timely diagnosis is necessary, as it requires orchiectomy before systemic chemotherapy for metastatic disease.
Acknowledgments
The authors gratefully acknowledge Dr Anand Sharma (Consultant Medical Oncologist) for his help in the management of the patient and Dr Nidhi Prasad (Consultant Histopathologist) for providing histopathology inputs.
Footnotes
Twitter: @DeepakBatura
Contributors: PdS, CWS, DB, WG and EV involved in conceptualisation, designing, data acquisition and interpretation. PdS, CWS, DB, WG and EV involved in drafting and reviewing the article. PdS, CWS, DB, WG and EV contributed to the final approval of the article. PdS, DB and EV accountability regards accuracy and integrity.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rajiah P, Sinha R, Cuevas C, et al. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949–76. 10.1148/rg.314095132 [DOI] [PubMed] [Google Scholar]
- 2.Astigueta JC, Abad-Licham MA, Agreda FM, et al. Spontaneous testicular tumor regression: case report and historical review. Ecancermedicalscience 2018;12:888. 10.3332/ecancer.2018.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann JT, Kollmannsberger C, Kanz L, et al. Platinum organ toxicity and possible prevention in patients with testicular cancer. Int J Cancer 1999;83:866–9. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda A, Kawai K, Ando S, et al. Management of ureteral obstruction in advanced testicular tumor with lymph node metastasis. Jpn J Clin Oncol 2012;42:748–52. 10.1093/jjco/hys094 [DOI] [PubMed] [Google Scholar]
- 5.Chia VM, Quraishi SM, Devesa SS, et al. Cancer Epidemiol biomarkers Prev 2010;19:1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kühn MW, Weissbach L. Localization, incidence, diagnosis and treatment of extratesticular germ cell tumors. Urol Int 1985;40:166–72. 10.1159/000281074 [DOI] [PubMed] [Google Scholar]
- 7.Prym P. Spontanheilung eines bösartigen, wahrscheinlich chorionepitheliomatösen Gewächses Im Hoden. Virchows Arch. path Anat. 1927;265:239–58. 10.1007/BF01894164 [DOI] [Google Scholar]
- 8.Johnson K, Brunet B. Brain Metastases as Presenting Feature in 'Burned Out' Testicular Germ Cell Tumor. Cureus 2016;8:e551. 10.7759/cureus.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosillo C, Scagnoli S, Pomati G, et al. Burned-Out testicular cancer: really a different history? Case Rep Oncol 2017;10:846–50. 10.1159/000480493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balzer BL, Ulbright TM. Spontaneous regression of testicular germ cell tumors: an analysis of 42 cases. Am J Surg Pathol 2006;30:858–65. 10.1097/01.pas.0000209831.24230.56 [DOI] [PubMed] [Google Scholar]
- 11.Mesa H, Rawal A, Rezcallah A, et al. "Burned out" testicular seminoma presenting as a primary gastric malignancy. Int J Clin Oncol 2009;14:74–7. 10.1007/s10147-008-0804-0 [DOI] [PubMed] [Google Scholar]
- 12.Nakazaki H, Tokuyasu H, Takemoto Y, et al. Pulmonary metastatic choriocarcinoma from a Burned-out testicular tumor. Intern Med 2016;55:1481–5. 10.2169/internalmedicine.55.5679 [DOI] [PubMed] [Google Scholar]
- 13.Nishisho T, Sakaki M, Miyagi R, et al. Burned-out seminoma revealed by solitary rib bone metastasis. Skeletal Radiol 2017;46:1415–20. 10.1007/s00256-017-2701-y [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa H, Kawada N, Taniguchi A, et al. Paraneoplastic neurological syndrome due to burned-out testicular tumor showing hot cross-bun sign. Acta Neurol Scand 2016;133:398–402. 10.1111/ane.12469 [DOI] [PubMed] [Google Scholar]
- 15.Laguna MP, Albers P, Algaba F, et al. EAU guidelines:Testicular cancer. Edn. presented at the EAU Annual Congress Amsterdam. Arnhem, The Netherlands: EAU Guidelines Office, 2020: 978–94. https://uroweb.org/guideline/testicular-cancer/ [Google Scholar]
- 16.Bokemeyer C, Kollmannsberger C, Stenning S, et al. Metastatic seminoma treated with either single agent carboplatin or cisplatin-based combination chemotherapy: a pooled analysis of two randomised trials. Br J Cancer 2004;91:683–7. 10.1038/sj.bjc.6602020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Wit R. Refining the optimal chemotherapy regimen in good prognosis germ cell cancer: interpretation of the current body of knowledge. J Clin Oncol 2007;25:4346–9. 10.1200/JCO.2007.13.3777 [DOI] [PubMed] [Google Scholar]