Abstract
Background
Mobile phone video call applications generally did not undergo testing in randomised controlled clinical trials prior to their implementation in patient care regarding the rate of successful patient visits and impact on the physician–patient relationship.
Methods
The National Center for Tumour Diseases (NCT) MOBILE trial was a monocentric open-label randomised controlled clinical trial of patients with solid tumours undergoing systemic cancer therapy with need of a follow-up visit with their consulting physician at outpatient clinics. 66 patients were 1:1 randomised to receive either a standard in-person follow-up visit at outpatient clinics or a video call via a mobile phone application. The primary outcome was feasibility defined as the proportion of patients successfully completing the first follow-up visit. Secondary outcomes included success rate of further video calls, time spent by patient and physician, patient satisfaction and quality of physician–patient relationship.
Findings
Success rate of the first follow-up visit in the intention-to-treat cohort was 87.9% (29 of 33) for in-person visits and 78.8% (26 of 33) for video calls (relative risk: RR 0.90, 95% CI 0.70 to 1.13, p=0.51). The most common reasons for failure were software incompatibility in the video call and no-show in the in-person visit arm. The success rate for further video visits was 91.7% (11 of 12). Standardised patient questionnaires showed significantly decreased total time spent and less direct costs for patients (Δmean −170.8 min, 95% CI −246 min to −95.5 min), p<0.0001; Δmean −€14.37, 95% CI −€23.9 to −€4.8, p<0.005) and comparable time spent for physicians in the video call arm (Δmean 0.5 min, 95% CI −5.4 min to 6.4 min, p=0.86). Physician–patient relationship quality mean scores assessed by a validated standardised questionnaire were higher in the video call arm (1.13-fold, p=0.02).
Interpretation
Follow-up visits with the tested mobile phone video call application were feasible but software compatibility should be critically evaluated.
Trial registration number
DRKS00015788.
Keywords: telemedicine, smartphone, physician-patient relationship, digital health, shared decision-making
Key questions.
What is already known about this subject?
In non-randomised clinical trials, video calls increased patient access and reduced costs in medical oncology care.
What does this study add?
We provide randomised controlled trial-level evidence of failure rate, cost and shared decision making in video calls for medical oncology care.
How might this impact on clinical practice?
If software compatibility can be warranted, video calls may help reduce cost while not affecting shared decision making in the clinical situation presented.
Background
Patients with solid tumours frequently undergo systemic cancer therapy for many months, especially in the metastatic setting. To monitor and treat adverse events and infections these patients often need to consult with their medical oncologist. Commutes to outpatient clinics of specialised comprehensive cancer centres can be long and strenuous for this fragile patient population. A retrospective analysis recently suggested that palliative systemic cancer therapy accounted for approximately 10% of the survival time awake remaining to pancreatic cancer patients with distant metastases.1
Telemedicine applications can facilitate patient access to specialised healthcare from remote and may therefore be ideally suited for the medical oncology setting. With the recent SARS-CoV-2 pandemic the need for remote healthcare access has risen substantially requiring thoroughly tested telemedicine applications.
Although there is evidence that these applications may reduce costs without negatively affecting clinical outcomes,2 3 few commercial applications underwent testing in randomised controlled trials prior to their clinical implementation.4 5 In vulnerable oncology patients undergoing systemic cancer therapy, it, therefore, remains unclear1 how robust these applications are with regards to their failure rate and2 how telemedicine applications affect communication strategies of physicians such as shared decision making and the resulting physician–patient relationship.
The primary objective of the National Center for Tumour Diseases (NCT) Assessment of Mobile Oncology Care by Interrogating Multifaceted Patient Experience (MOBILE) trial was to assess feasibility defined as the failure rate of video consultations as compared with in-person visits in patients with solid tumours undergoing systemic cancer therapy who required a follow-up appointment. By using standardised and validated questionnaires, we also assessed patient satisfaction, the economic burden and the effect on the physician–patient relationship.
Methods
Study design
The NCT MOBILE trial was a randomised controlled open-label clinical trial at the NCT in Heidelberg, Germany. Patients with solid tumours (International Classification of Diseases (ICD)-10 2016, C00-C97) undergoing systemic cancer therapy who required a follow-up visit in 2–14 days time were recruited by medical oncologists at NCT outpatient clinics. Patients were 1:1 randomised to receive their follow-up appointment at outpatient clinics (in-person) or via a dedicated smartphone application in German language ‘Minxli—Arzt via Video Chat’ (https://www.minxli.com/). The outcome of the appointment was documented by the treating physician in the case report form (CRF, 10.5281/zenodo.3902837) and by the patient in questionnaire ‘Q1’ (10.5281/zenodo.3902837). Patients in the video call group were eligible to schedule further video calls via the mobile phone application. After completion of oncological therapy (adjuvant/neoadjuvant patients) or 6 months after randomisation (palliative patients) patients in the video call group were asked to fill out questionnaire ‘Q2’. No changes to the study protocol were made after trial initiation. Patients were recruited from 29 November 2017 (first patient in) to 7 October 2019 (last patient in), follow-up period was 6 months for all patients with the last follow-up period ending on 7 April 2020. The trial is reported according to Consolidated Standards of Reporting Trials (CONSORT) Criteria (online supplemental file 2).
esmoopen-2020-000912supp002.pdf (63.8KB, pdf)
Participants
Patients were eligible at age 18 years or older, performance status of Eastern Cooperative Oncology Group (ECOG) 0–2, owned a compatible smartphone with Android (Google, Mountain View, California, USA) or iOS (Apple, Cupertino, California, USA) operating system, were comfortable using it and agreed to the ‘Minxli—Arzt via Video Chat’ terms and conditions. Patients not proficient in the German language or patients with severe visual or auditory impairments were excluded from participation in the trial. All patients provided written informed consent.
Randomisation procedure
The allocation schedule and sequentially labelled sealed opaque envelopes containing the treatment allocations were prepared by the study’s statistician (JK) to conceal treatment allocation of the next patient from other investigators. The randomisation sequence was generated using a computer programme employing a random number generator with fixed seed. Block randomisation was used with a fixed block size of 6, and everybody was blinded to block length except the statistician. For each patient one envelope was opened by TW, JNK or LM or EG after the respective patient had provided informed written consent. Treatment allocation was conveyed to the patient and treating physician in an open-label design.
Mobile phone application
Patients were instructed to download and instal the smartphone application ‘Minxli—Arzt via Video Chat’ (V.1.3.1) from the Google Play Store (Google) or the application ‘Minxli—Arzt via Video Chat’ (V.1.2.8) from the Apple App Store (Apple). The application was provided in German language and compatible with the operating systems Android V.4.4 or higher (Google) and iOS V.10 or higher (Apple), respectively. Key features included scheduling encrypted video calls with verified physicians, a chat function with options to upload pictures, which was only available when a valid appointment had been scheduled by the patient and confirmed by the physician and a medication plan management function. Video calls scheduled by patients had to be confirmed and initialised by the treating physicians, thereby preventing patients from calling their physicians at unscheduled times. Video call data were transmitted using end-to-end encryption. All protected health information was encrypted in transit and stored on Amazon Web Services Simple Storage System (Amazon.com, Seattle, Washington, USA, servers in Frankfurt, Germany).
Outcomes
The primary outcome was feasibility defined as the proportion of patients successfully completing the first follow-up visit appointment of the type corresponding to the patient’s group assignment. A successful appointment was defined as a medical consultation between patient and physician that was unanimously finished and was not cancelled because of technical issues or other problems.
Secondary outcomes included patient satisfaction, content of the appointments, quality of the physician–patient relationship and cost-efficiency and time-efficiency during the first appointment as assessed in questionnaire ‘Q1’ which had to be filled out directly after the appointment to avoid recall bias. Further information about the time spent for the appointment by the treating physician and content of the appointment was assessed in the CRF (10.5281/zenodo.3902837).
Total time spent was calculated according to the following formula. Ttotal=2*Ttravel+Twait, with Ttotal: total time spent, Ttravel: time spent for one-way commute from home to NCT outpatient clinics, Twait: total waiting time spent at NCT outpatient clinics. Direct costs were reported by patients and indirect costs were calculated according to the following formula. costsindirect=Twork * S, with costsindirect: indirect costs, Twork: time absent from work due to the follow-up appointment as reported by the patient, S: average salary in the state of Baden-Württemberg, Germany of €24/hour (Statistisches Landesamt Baden-Württemberg, press release 158/2018, Stuttgart, Germany 12 July 2018, URI: http://www.statistikbw.de/Presse/Pressemitteilungen/2018158). Twork was set to 0 for all patients without employment, on sick-leave and retired patients.
After completion of the study, the general experience with the mobile phone application was assessed using questionnaire ‘Q2’. Age, gender, post code, oncological main diagnosis, Union for International Cancer Control (UICC) stage and time of initial oncological diagnosis were retrieved from NCT’s electronic medical documentation system. ECOG and therapy schema were retrieved from the CRF. Straight-line-distance from the supplied postal code to the hospital was calculated using Google Maps (Google).
Questionnaire design
Our interdisciplinary research team developed the questionnaires ‘Q1’ (10.5281/zenodo.3902837) and ‘Q2’ (10.5281/zenodo.3902837) in German language based on a validated German instrument for the patient–physician interaction and self-developed questions adapted from prior assessments of telemedicine outcomes.6
Hence, Q1 consisted of two sections of self-developed questions and one validated instrument. Section 1 (13 items) assessed the general experience, time and money spent for the first video call or in-person appointment, section 2 (7 items) reported the content of appointment (physical examinations etc) using five-point Likert scale and some open questions to different statements. Section 3 (13 items) assessed the physician-patient relationship using the validated questionnaire on quality of physician–patient interaction (QQPPI) in German language.7–9 To calculate the QQPPI total score, Likert levels of all items were summed per patient with a high score indicating high and a low score indicating low satisfaction with the interaction with the physician, respectively. QQPPI total scores were only calculated for patients who completed all 13 questions because the QQPPI score lacks validation for incomplete questionnaires.
Q2 assessed the desire to repeat the appointment, technical difficulties and the number of appointments in total as remembered by the patient at the end of oncological therapy (neoadjuvant/adjuvant patients) or 6 months after treatment initiation (palliative patients). Q2 consisted out of 10 self-developed items, of which 5 were open questions to be answered in free text and 5 were five level Likert scale items.
All questionnaires were sent back to the study lead in a pseudonymised format. All original questionnaires as well as their translations are provided under a Creative Commons Attribution V.4.0 International Licence (http://doi.org/10.5281/zenodo.3902837).
Statistical analysis
The primary outcome parameter was compared between patients in the video call and in-person visit group using Fisher’s exact test (two sided) in the intention-to-treat cohort. For secondary outcomes, patients who obviously evaluated the wrong appointment in questionnaire Q1 were excluded from analysis (n=3, eg, video call patients indicating journey from home). Likert scale scores were compared using Mann-Whitney U tests (two sided), spending in € and time spent were compared using unpaired t-tests (two sided). Multiple comparisons were accounted for using the Benjamini-Hochberg method with a q<0.05 considered as statistically significant and indicated with an asterisk. No statistical sample size estimation was performed for this trial. P values and CIs were calculated using GraphPad Prism V.8.4.2 (GraphPad Software) for Windows 10 (Microsoft, Redmond, Washington, USA) or the ‘blakerci’ function from the ‘PropCI’ package V.0.3.0 from the Comprehensive R Archive Network for independent proportions. Cronbach’s alpha was calculated using the ‘alpha’ function of the ‘psych’ package V.2.0.8 and the Benjamini-Hochberg correction was calculated using the ‘p.adjust’ function in the R base package. All packages were executed in R Studio V.1.2.5003.
Results
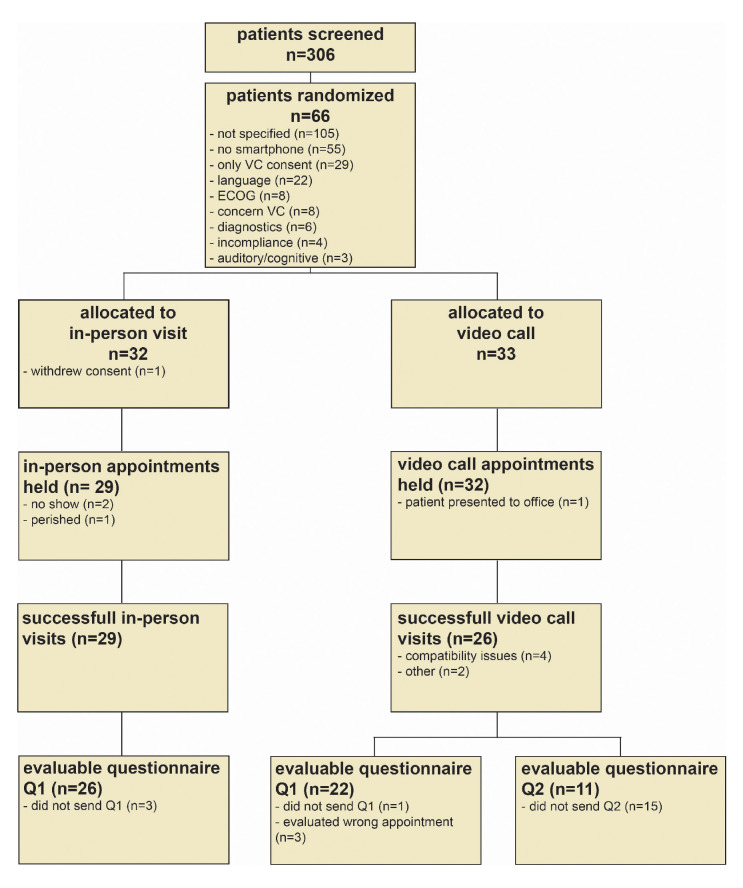
Between 29 November 2017 and 10 July 2019 we screened 306 patients as potentially eligible (figure 1). Of these, 43 patients (14%) were deemed ineligible prior to randomisation due to insufficient proficiency in the German language (n=22), ECOG 3 or 4 (n=8), the need of in-house diagnostics (n=6), incompliance (n=4) or severe auditory or cognitive impairments (n=3). Of the remaining patients, 29 (9%) objected to randomisation because they solely preferred video calls and 105 (34%) were unwilling to participate due to unspecified reasons. Another 55 patients (18%) were not adept at using or not owning a smartphone a priori compatible with the application (iOS V.10 or higher, Android V.4.4 or higher) and eight patients (3%) had concerns regarding the safety of the video call.
Figure 1.
CONSORT flow chart. Reasons for exclusion/failure are highlighted with bullet points. CONSORT, Consolidated Standards of Reporting Trials; ECOG, Eastern Cooperative Oncology Group; Q1, questionnaire Q1; Q2, questionnaire; VC, video call.
This resulted in a total of 66 patients who were 1:1 randomised to the video call (n=33) and in-person visit (n=33) arm (figure 1). The cohort included patients with a range of different tumour types and therapies including cytotoxic chemotherapy, targeted and immunotherapy in either palliative or (neo)adjuvant intention (table 1). Patients in the video call and in-person visit cohorts showed representative age distribution for medical oncology patients (table 1). ECOG performance status and distance to the hospital were similar in both groups (table 1). However, we observed a higher number of female patients with breast cancer in the video call (n=10) as compared with the in-person visit group (n=2), resulting in a higher number of UICC stage 1 patients (n=6 vs n=1) in the video call arm (table 1).
Table 1.
Patient baseline characteristics
| General | In-person (n=32) | Video call (n=33) |
| Patient no | 32 | 33 |
| Age—yr | ||
| Median | 59.5 | 54 |
| Range | 29–72 | 22–74 |
| Sex—no (%) | ||
| Female | 13 (40.6) | 17 (51.5) |
| Male | 19 (59.3) | 16 (48.4) |
| ECOG performance status score—no (%) | ||
| 0 | 14 (43.7) | 17 (51.5) |
| 1 | 15 (46.8) | 14 (42.4) |
| 2 | 3 (9.3) | 2 (6.0) |
| UICC stage—no (%) | ||
| 1 | 1 (3.1) | 6 (18.1) |
| 2 | 2 (6.2) | 3 (9.0) |
| 3 | 2 (6.2) | 6 (18.1) |
| 4 | 27 (84.3) | 18 (54.5) |
| Tumour type—no (%) | CRC 9 (28.1) PDAC: 7 (21.8) PCa: 3 (9.3) BRCA: 2 (6.2) HNSCC: 2 (6.2) CUP: 1 (3.1) ESCA: 1 (3.1) RCC: 1 (3.1) NET: 1 (3.1) OV: 1 (3.1) SARC: 1 (3.1) GC: 1 (3.1) UCEC: 1 (3.1) VUL: 1 (3.1) |
BRCA: 10 (30.3) CRC: 5 (15.1) PDAC: 4 (12.1) PCa: 3 (9.0) GC: 3 (9.0) BLCA: 2 (6.0) NET: 2 (6.0) CESC: 1 (3.0) ESCA: 1 (3.0) HNSCC: 1 (3.0) HCC: 1 (3.0) |
| Therapy scheme—no (%) | Platinum triplet (±mab): 8 (25.0) FOLFOX/FLO/CapOx (±mab):5(15.6) anti-PD-1/PD-L1: 4 (12.5) FOLFIRI (±mab): 4 (12.5) Platin +Taxane(±mab): 4 (12.5) CisTopo (±mab): 2 (6.25) Docetaxel: 2 (6.25) Gem +nabPac: 1 (3.125) EC/DC: 1 (3.125) INN-doxorubicin: 1 (3.125) |
EC/DC: 8 (24.2) Platinum triplet (±mab): 6 (18.1) FOLFOX/FLO/CapOx (±mab):5(15.1) anti-PD-1/PD-L1: 4 (12.1) FOLFIRI (±mab): 4 (12.1) Docetaxel: 2 (6.0) CisBev: 1 (3.0) Epirubicin: 1 (3.0) Platin+taxane(±mab): 1 (3.0) Sunitinib/lanreotid: 1 (3.0) |
| Linear distance to hospital—km (SD) | 34.1 (38.7) | 28.4 (20.5) |
Table indicating patient characteristics at baseline.
BLCA, bladder urothelial carcinoma; BRCA, breast cancer; CapOx, capecitabine+oxaliplatin; CESC, cervical squamous cell carcinoma; CisBev, cisplatin+bevacizumab; CRC, colorectal adenocarcinoma; CUP, carcinoma of unknown primary; DC, doxorubicin+cyclophosphamide; EC, epirubicin+cyclophosphamide; ECOG, Eastern Cooperative Oncology Group; ESCA, oesophageal carcinoma; FLO, floururacil+oxaliplatin; FOLFIRI, floururacil+irinotecan; FOLFOX, floururacil+oxaliplatin; GC, gastric adenocarcinoma; Gem+nabPac, gemcitabine +nanosomal albumin bound paclitaxel; HCC, liver hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; INN, pegylated liposomal; mab, monoclonal antibody; NET, neuroendocrine tumour; OV, ovarian adenocarcinoma; PCA, prostate adenocarcinoma; PDAC, pancreatic adenocarcinoma; platin+taxane, carboplatin +paclitaxel; RCC, renal cell carcinoma; SARC, soft tissue sarcoma; TCbHP, docetaxel+carboplatin+trastuzumab +pertuzumab; UCEC, uterine corpus endometrium cancer; UICC, Union for International Cancer Control; VUL, vulvar squamous cell carcinoma; yr, years.
The first appointment took place as scheduled in 29 (91%) of patients of the in-person visit and 32 (97%) of patients in the video call arm (figure 1). In the in-person visit arm, one patient withdrew consent after randomisation, two patients did not present to outpatient clinics and could not be contacted by any means and one patient died before the scheduled appointment. In the video call arm one patient erroneously presented to outpatient clinics.
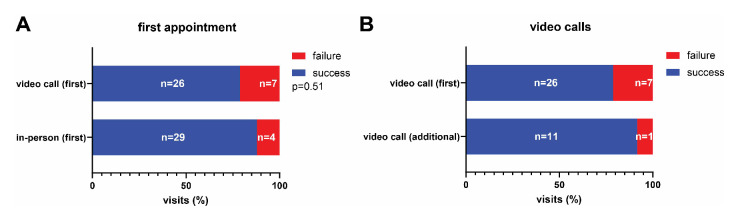
All of the remaining 29 patients in the in-person visit arm completed the appointment successfully. In the video call arm, 26 (81.3%) patients completed their appointments via mobile phone application successfully. Six (18.8%) patients experienced technical difficulties resulting in premature termination of the video call appointment. These patients could be contacted by phone and did not have to present to outpatient clinics. The most common reasons for failure were software compatibility issues (n=4), followed by unstable internet connection (n=1) and problems with the appointment scheduling function of the mobile phone application (n=1). In summary, this resulted in a success rate of 29 (87.9%) of 33 patients (95% CI 71% to 96%) for in-person visits and 26 (78.8%) of 33 patients (95% CI 62% to 0.90%) for video calls in the intention-to-treat cohort, which was not significantly different (RR 0.90, 95% CI 0.70 to 1.13); p=0.51) (figure 2A). The success rate for further video calls after the first appointment was 11 (91.7%) of 12 patients (95% CI 63% to 100%) (figure 2B). Technical difficulties were not associated with age (failed video calls: median=53 years, all video calls: median=54 years) but we detected a non-significant difference in gender with more males experiencing technical difficulties (failed video calls: 4 (80%) of 5 male, successful video calls : 11 (42%) of 26 male, p=0.17).
Figure 2.
Success rate of video call and in-person visits. Stacked bar graphs indicating success rates of patients in the in-person visit and video call arms. (A) Success rates at first scheduled appointment in the in-person and video call arms (prespecified). (B) Success rates for video call appointments at the first scheduled and at any additional appointments (exploratory). P value was calculated using Fisher’s exact test.
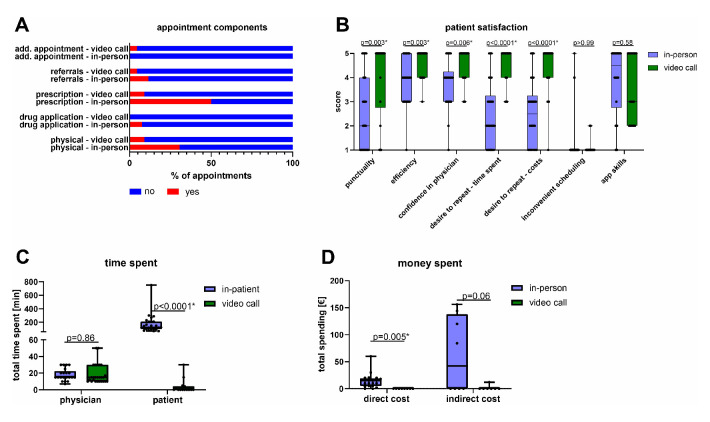
Patients who successfully completed in-person and video visits were asked to fill out a questionnaire (Q1) directly after the appointment to avoid recall bias. We received 26 evaluable Q1 questionnaires in the in-person and 22 in the video call group (figure 1). We assessed the content of the video visits including physical and instrumental examinations, administered treatments, prescriptions, referrals and scheduling of additional follow-up appointments. These contents differed from in-person appointments although these differences did not reach statistical significance (figure 3A). Physical examinations were performed in 8 (31%) of 26 in-person as compared with 2 (9%) of 22 video calls. Physicians filled out prescriptions in 13 (50%) of 26 in-person as compared with 2 (9%) of 22 video calls. Moreover, medication was directly administered in 2 (8%) of 26 in-person appointments. Additional appointments had to be scheduled in 1 (5%) of 22 video calls but not in the in-person group. Physicians referred patients to other healthcare professionals in 3 (12%) of 26 in-person but only in 1 (5%) of 22 video call appointments.
Figure 3.
Appointment characteristics and patient satisfaction. Components of the appointment, patient satisfaction, time and cost were assessed for the first scheduled appointment. (A) Stacked bars indicating characteristics of the first appointment in the in-person and video call arms. (B) Box plots indicating different dimensions of patient satisfaction and the desire to repeat the appointment in the in-person (n=26) or video call (n=22) group. P values were calculated using Mann-Whitney U tests (two sided). (A, B) Indicated are descriptive titles for the items. (C) Box plots indicating total time spent for physicians (n=47) and patients (n=39). P values were calculated using unpaired t-tests (two sided). (D) Box plots indicating total direct (n=29) and indirect costs (n=15) for patients in the in-person and video call arm. P values were calculated using unpaired t-tests (two sided). (B–D) Multiple comparisons were accounted for using the Benjamini-Hochberg method within each panel (A–D). Statistically significant exploratory comparisons are indicated with an asterisk (q<0.05). Boxes indicate IQR, bars indicate median and whiskers range.
Patients indicated higher overall satisfaction in the video call group (figure 3B). Patients ranked confidence in their physician (p=0.006), efficiency (p=0.003) and punctuality (p=0.003) higher in the video call group as compared with the in-person appointment (figure 3B). Accordingly, patients in the video call group preferred the video call setting for future visits with respect to saving time (p<0.0001) and cost (p<0.0001) (figure 3B). Indeed, patients in the video call group saved 170 min (Δmean −170.8 min, 95% CI −246 min to −95.5 min)) and €14.37 in direct costs (Δmean −€14.37, 95% CI −€23.9 to −€4.8€) on average as compared with the in-person visit group (figure 3C, D). This positive assessment of the video consultations and desire to repeat them was maintained throughout the study period as assessed by the end of study questionnaire Q2 (online supplemental table S1). Patients in the video call group saved an average of €58 (Δmean −€58.3, 95% CI −€120.6 to €4.0, p=0.064) in indirect costs due to reduced absence from work although this difference was not statistically significant (figure 3D). Time spent was comparable for physicians in the video call and in-person visit arm (figure 3C) (Δmean 0.5 min, 95% CI −5.4 min to −6.4 min, p=0.86).
esmoopen-2020-000912supp001.pdf (36.1KB, pdf)
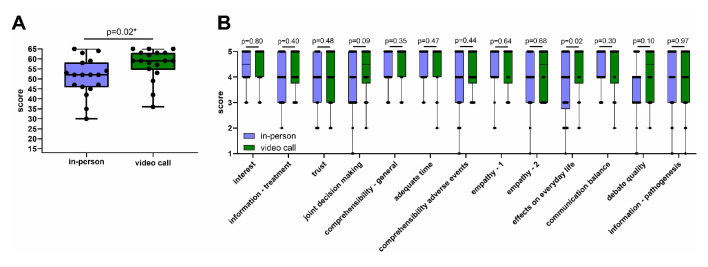
We assessed physician–patient relationship quality using the validated standardised QQPPI which is characterised by high reliability and unidimensionality (this study: Cronbach’s α = 0.93) and has been successfully established and applied to randomised clinical trials before (Cronbach’s α initial report = 0.97).7 8 We found that QQPPI scores were higher in patients of the video call as compared with the in-person visit group (figure 4A). This effect was consistent across the different items of the questionnaire (figure 4B) suggesting that the physician–patient relationship is not negatively affected but may be positively affected by video calls.
Figure 4.
Physician–patient relationship assessment using the questionnaire on quality of physician–patient interaction. Physician–patient relationship after the first appointment was assessed using the Questionnaire on Quality of Physician–Patient Interaction (QQPPI) Questionnaire. (A) Box plots indicating QQPPI total score in the in-person (n=18) and video call groups (n=18). (B) Box plots indicating patient agreement with individual items of the QQPPI questionnaire (n=48). Indicated are descriptive titles for the items. (A, B) P values were calculated using Mann-Whitney-U tests. Multiple comparisons were accounted for using the Benjamini-Hochberg method. Asterisks indicate significant exploratory comparisons (q<0.05). Boxes indicate IQR, bars indicate median and whiskers range.
Discussion
Here, we present evidence that video call applications are feasible in medical oncology patients with no significant differences in success rate in the intention-to-treat analysis. However, this trial was not planned with a non-inferiority design prohibiting strong conclusions about comparability of failure rates. Despite low patient numbers, our cohort covered a broad range of tumour types and was representative for medical oncology patients regarding age and administered therapies.
Screening failures revealed non-availability of a smartphone or non-adeptness in its use as the most frequent reason for non-participation in the trial. Along these lines, poor availability and infrequent use of information and communication technology have been linked to other inequalities in non-digital healthcare access highlighting the risk that telemedicine may reinforce these social inequalities.10 11 However, the net effect of telemedicine on social inequalities will ultimately depend on current non-digital healthcare access for underprivileged groups and their strength of digital illiteracy.
We observed different reasons for failure in the video call and in-person visit group. In the video call group, we observed a relevant number of patients experiencing compatibility issues which could not be resolved by technical support. None of the video call patients who could not be contacted by the smartphone application were at evident risk, were contacted by phone and did not have to present to outpatient clinics. However, it is conceivable that video call failures may lead to unscheduled visits in rare cases or alternatively may affect the physician’s ability to correctly assess a condition of a patient which also relies on visual inspection. Hence, compatibility of mobile phone applications should be critically evaluated prior to their implementation.
In the in-person group, two patients did not present to outpatient-clinics and one patient withdrew consent after randomisation suggesting that patients may skip appointments which they deem unnecessary due to the effort of presenting to outpatient clinics. Along these lines, 9% (n=29) of all screened patients refused participation in the trial because they were only interested in the video call group highlighting the strong interest of patients to avoid supposedly dispensable commutes. In contrast, to the video call arm where all patients could be contacted, two no-show patients in the in-person visit arm could not be contacted by any means at the time of their scheduled appointment. In high-risk situations difficult to understand for the patient, such as profound neutropenia, ensuring compliance may be critical. In our cohort, compliance was better in the video call arm. Thus, our data suggest that compliance may be positively affected by use of the tested video call application.
Given the failure rates and reasons for failure of the appointment observed in our video call and in-person visit groups, a nuanced evaluation of the setting used for an appointment may be adequate for clinical practice. For situations with digitally less-literate patients or risk factors for technical difficulties such as old operating systems and poor internet connection an in-person appointment should be scheduled. For digitally literate patients and a risk for non-compliance or no-show a video call seems more adequate based on the data presented. In situations where visiting outpatient clinics may present a considerable health hazard for patients such as during the recent SARS-CoV-2 pandemic, video calls may become a necessity for oncology patients. Along these lines, during the SARS-CoV-2 pandemic, cancer screenings and non-emergency in-person visits of new patients were frequently declined for the sake of epidemiological infectious disease control.12
Similar to previous studies in other medical specialties, our patients were highly satisfied with their video call experience.3 6 13 Reasons for high patient satisfaction may be the observed cost and times savings, which were similar to previous reports.6 13 For physicians, the application did not result in increased time expenditure. The application also prevented unscheduled consultations and enabled physicians to remain in full control of initialising the appointments. This may be an important factor to ensure work–life balance and increase acceptance of these applications in physicians.
In our study, the patients’ perception of the physician–patient relationship was more positive in the video call group as compared with the in-person visit arm. This outcome was unexpected given frequent concerns about the importance of physical interactions voiced by healthcare professionals.14 Importantly, the physician–patient relationship is bidirectional, and we only assessed the patients’ perception. It is possible that the decreased distress from the need to commute to outpatient clinics positively influenced the patients’ perception of the physician–patient relationship in our study. We hypothesise that the direct link created between physician and patient via the application may give patients a feeling of exclusivity and privacy which cannot be guaranteed to the same degree in high volume outpatient clinics. Because all patients in this study had an in-person appointment at our centre prior to the video call, participants benefited from an established physician–patient relationship in most cases. Effects of video calls on physician–patient relationships may be different when using video calls at the first consultation. Moreover, patients in our trial consulted their physician regarding treatment-related follow-ups via video call. Thus, most questions concerned symptoms and side effects and not psychologically more challenging questions such as limiting treatment at the end of life.15 In these situations, in-person interactions may have a more positive impact on the physician–patient relationship.
Our study highlights the potential of video call applications to reduce factors of socioeconomic distress in patients with solid tumours undergoing systemic cancer therapy while maintaining the physician–patient relationship. Compatibility of smartphone applications should be critically assessed prior to their implementation. Larger studies and longer follow-ups are required to judge whether differences in physical examinations, laboratory diagnostics and other factors influenced by video call consultations affect patient hospitalisations and survival.
Acknowledgments
We thank all patients for participation in the trial and reviewers for critical assessment of the manuscript.
Footnotes
Twitter: @science_wallet, @FpkMd
JNK and ECW contributed equally.
Contributors: Conceptualisation: TW, ECW, JNK and JK. Questionnaire design: JNK, ECW; Methodology: TW, JNK, ECW and JK. Data analysis: TW, EE and LM. Writing: TW; Review and editing: TW, ECW, JNK, EE, HMS, LM and JK. Patient recruitment: TW, EE, LM, HMS, EG, BG, HH, AI, BK, FK, AMa, AMo, CP, DS, HS, NS and LW; Supervision of patient care: ECW, DJ, MD and AS. Visualisation: TW. Funding Acquisition: JNK, ECW and TW. Supervision: ECW and JNK.
Funding: Funding provided by Minxli, München, Germany for salary of LM and in form of the mobile phone application.
Competing interests: LM is an employee of Minxli, München, Germany.
Patient consent for publication: Not required.
Ethics approval: Ethical approval was granted by the ethics commission of the medical faculty at Heidelberg University (S-090/2017).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All requests for data and materials should be made to the corresponding author, following verification of any intellectual property or confidentiality obligations.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Bange EM, Doucette A, Gabriel PE, et al. . Opportunity costs of receiving palliative chemotherapy for metastatic pancreatic ductal adenocarcinoma. JCO Oncol Pract 2020;16:e678–87. 10.1200/JOP.19.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruse CS, Krowski N, Rodriguez B, et al. . Telehealth and patient satisfaction: a systematic review and narrative analysis. BMJ Open 2017;7:e016242. 10.1136/bmjopen-2017-016242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flodgren G, Rachas A, Farmer AJ, et al. . Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2015;9:CD002098. 10.1002/14651858.CD002098.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitamura C, Zurawel-Balaura L, Wong RKS. How effective is video consultation in clinical oncology? A systematic review. Curr Oncol 2010;17:17–27. 10.3747/co.v17i3.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sirintrapun SJ, Lopez AM. Telemedicine in cancer care. Am Soc Clin Oncol Educ Book 2018;38:540–5. 10.1200/EDBK_200141 [DOI] [PubMed] [Google Scholar]
- 6.Viers BR, Lightner DJ, Rivera ME, et al. . Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol 2015;68:729–35. 10.1016/j.eururo.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 7.Bieber C, Nicolai J, Mueller K, et al. . Der fragebogen zur Arzt-patient-interaktion (FAPI) – validierung und psychometrische optimierung anhand einer stichprobe chronischer schmerzpatienten. Klinische Diagnostik und Evaluation 2011;4:78–93. [Google Scholar]
- 8.Bieber C, Müller KG, Blumenstiel K, et al. . A shared decision-making communication training program for physicians treating fibromyalgia patients: effects of a randomized controlled trial. J Psychosom Res 2008;64:13–20. 10.1016/j.jpsychores.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 9.Bieber C, Müller KG, Nicolai J, et al. . How does your doctor talk with you? preliminary validation of a brief patient self-report questionnaire on the quality of physician-patient interaction. J Clin Psychol Med Settings 2010;17:125–36. 10.1007/s10880-010-9189-0 [DOI] [PubMed] [Google Scholar]
- 10.Fang ML, Canham SL, Battersby L, et al. . Exploring privilege in the digital divide: implications for theory, policy, and practice. Gerontologist 2018;59:e1–15. 10.1093/geront/gny037 [DOI] [PubMed] [Google Scholar]
- 11.Hazin R, Qaddoumi I. Teleoncology: current and future applications for improving cancer care globally. Lancet Oncol 2010;11:204–10. 10.1016/S1470-2045(09)70288-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harky A, Chiu CM, Yau THL, et al. . Cancer patient care during COVID-19. Cancer Cell 2020;37:749–50. 10.1016/j.ccell.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller KI, Alstadhaug KB, Bekkelund SI, Acceptability BSI. Acceptability, feasibility, and cost of telemedicine for nonacute headaches: a randomized study comparing video and traditional consultations. J Med Internet Res 2016;18:e140. 10.2196/jmir.5221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel H, Sulmasy LS, Health and Public Policy Committee of the American College of Physicians . Policy recommendations to guide the use of telemedicine in primary care settings: an American College of physicians position paper. Ann Intern Med 2015;163:787–9. 10.7326/M15-0498 [DOI] [PubMed] [Google Scholar]
- 15.Winkler EC, Heußner P. [Advance care planning and decisions to limit treatment at the end of life - the view from medical ethics and psychooncology]. Dtsch Med Wochenschr 2016;141:394–8. 10.1055/s-0041-110421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000912supp002.pdf (63.8KB, pdf)
esmoopen-2020-000912supp001.pdf (36.1KB, pdf)