Abstract
Objective
To describe the maternal clinical characteristics, maternal and perinatal outcomes in COVID-19-positive pregnant women.
Methods
Articles in all languages on the SARS-CoV-2 infection in pregnant women were sought from MEDLINE, EMBASE, Cochrane Library and LILACS; China National Knowledge Infrastructure Database (CNKI), Chinese Science and Technology Periodical Database (VIP) and Wan Fang Data between December 1, 2019 and April 27, 2020. Bulletins and national reports were also searched.
Results
From 12,168 retrieved articles, 143 were selected for full-text assessment; 33 for descriptive analyses, and 4 case-controls for meta-analysis. In 322 infected pregnant women, aged 20–45 years, the most frequent maternal comorbidity was obesity (24.2%). Forty-two (28.4%) were asymptomatic at admission. Cough (n = 148,59.7%) and fever (n = 147,59.3%) were the most prevalent symptoms. In the meta-analysis, fever (OR: 0.13,95% CI 0.05 to 0.36) and cough (0.26,95% CI 0.11 to 0.59) were lower in pregnant women with COVID-19 than non-pregnant women with COVID-19.195 (60.6%) delivered, and 125 (38.8%) remained pregnant during the study. Cesarean was reported in 99 (50.8%) women and vaginal delivery in 64 (32.8%). The main adverse obstetric outcome was premature birth (n = 37,18.9%). Thirty patients (10.3%) with COVID-19-related complications required intensive care, one (0.3%) died. SARS-CoV-2 was absent in breast milk, amniotic fluid, placenta or umbilical cord blood.
Conclusions
The maternal clinical characteristics of COVID-19-positive pregnant include frequently fever and cough; however significantly less frequently than non-pregnant women with COVID-19. Iatrogenic preterm birth is the main adverse obstetric outcome. Current data does not support vertical transmission in the third trimester.
Keywords: SARS-CoV-2, Novel coronavirus, COVID-19, Pregnancy, Coronavirus disease 2019, Vertical transmission, Perinatal outcome
1. Introduction
On February 11, 2020, the International Committee on Taxonomy of Virus officially named a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), as the causative agent of the new viral pandemic creating a global health crisis sickening over 51 million and killing about 1.2 million people hitherto [1,2]. The Coronavirus Disease 2019 (COVID-19) is highly transmissible, and the case fatality rate (CFR) is around 3.4%. However, this could vary depending on factors such as age, comorbidities and healthcare capacity [3]. Older people and those with pre-existing medical conditions—high blood pressure, heart problems and diabetes—are more vulnerable [4,5]. Moreover, owing to the major physiological changes pregnant women undergo—increased minute ventilation, diaphragm elevation and thorax deformation, edema of respiratory tract mucosa, altered cell immunity among others—pregnant women might be at a higher risk of infection and complications compared to non-pregnant women [6].
According to previous studies, 50% of pregnant women affected by Severe Acute Respiratory Syndrome (SARS), caused by a different coronavirus termed SARS-CoV, required intensive care; and 50% of them died [7]. Additionally, owing to the 79% shared similarity between SARS-CoV and SARS-CoV-2, COVID-19 could be seriously harmful for pregnant women [8].
Since despite the increasing cases COVID-19 in pregnant women, current clinical evidence is based on small series. We aim to review all cases from the current literature and provide up-to-date scientific evidence on the clinical characteristics, maternal outcomes, perinatal outcomes and the possibility of vertical transmission in the infected pregnant women.
2. Methods
2.1. Information sources, search strategy and eligibility criteria
The protocol for this systematic review was registered in the PROSPERO international prospective register of systematic reviews (Registration ID: CRD42020176534). This revision followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline [9]. Our wide search criterion included every article in any language on SARS-CoV-2 across MEDLINE, EMBASE, Cochrane Library and LILACS; and Chinese medical databases: China National Knowledge Infrastructure Database (CNKI), Chinese Science and Technology Periodical Database (VIP), and Wan Fang Data between December 01, 2019 and April 27, 2020. MeSH (medical subject heading) term, keywords or their variants were constructed. Moreover, bibliographic references of relevant articles were reviewed to search for additional reports (search formulas are available in Appendix A). Case-control studies, case series, case reports, letters and comments that reported SARS-CoV-2 in pregnant women, confirmed by a quantitative real-time polymerase chain reaction (qRT-PCR) on a nasopharyngeal sample, and a short-term outcome were included. The references of all identified articles for additional resources were examined. Official governmental e-sources with detailed description of cases such as Dutch Society Obstetrics & Gynecology were also included [10]. Articles were excluded if 1) they did not include pregnant women in their population, 2) their cases were reported in a previous article, 3) their full text was unavailable or 4) there was insufficient data or undefined outcome.
2.2. Study selection and data extraction
Three reviewers (R.N., W.Q. and P.Ll.) first independently screened articles by titles and abstracts. There was a consensus on the titles selected, and a fourth reviewer (W·V.) resolved the differences. Articles not meeting the selection criteria were excluded. The full-text was then reviewed in detail, and data about the characteristics of the study, the clinical characteristics of the infection and the maternal and perinatal outcomes was extracted, using Microsoft Excel (2013 version, Microsoft Corporation, Redmond, WA, USA). The inconsistencies were discussed, and a consensus was reached. Data duplication was avoided by choosing the most informative study from among cases published more than once. Only full-text articles were included and reviewed. The authors of articles were contacted as needed.
2.3. Assessment of methodological quality and risk of bias
The Newcastle Ottawa Scale (NOS) was employed to assess the risk of bias for case-control studies [11]. using which, a maximum of 9 points was allocated in four domains: 4 points for study group selection, 2 for group comparability and up to 3 points for exposure and outcome ascertainment. Additionally, a modified Newcastle Ottawa Scale (NOS) was used for case reports and case series to remove items relating to comparability and adjustment (irrelevant to non-comparative studies) and retaining items that focused on selection and were representatives of cases and outcome and exposure ascertainment. This modified NOS was tested in several systematic reviews with good interrater agreement [12]. We obtained five binary responses (yes/no) to questions, indicating whether the item suggests poor methodological quality or not. The quality of the report was deemed to be good when all five criteria were fulfilled, moderate when four were fulfilled and poor when three or less were fulfilled. The three reviewers assessed the methodological quality of the studies and discussed any disagreements.
2.4. Statistical approach
The distributions of absolute, relative and accumulated frequencies of the categorical variables were reported using descriptive analysis. For numerical variables, summary measures were calculated as averages and ranges. Observational studies reporting the proportion of symptoms, clinical and laboratory characteristics and perinatal outcomes were included for quantitative synthesis (meta-analysis). Since case reports lack a denominator for a given variable, they were excluded for the meta-analysis. Statistical analysis was performed using Stata Software (Stata Corp., College Station, TX, USA). The meta-analyses were performed using Review Manager 5.3 (RevMan version 5.3 Cochrane Collaboration). For the categorical variable, the Mantel-Haenszel M − H fixed effect model calculated the odds ratio (OR) and its 95% confidence interval (CI). Continuous data were analyzed using weighted mean difference (WMD). The degree of statistical heterogeneity was estimated using the I2 test. Funnel plots were used to investigate publication bias (Supplemental Fig. 1). The outcomes were compared in two subgroups [1]: pregnant women with COVID-19 versus pregnant women without COVID-19 and [2] pregnant women with COVID-19 versus non-pregnant women with COVID-19.
3. Results
3.1. Study selection
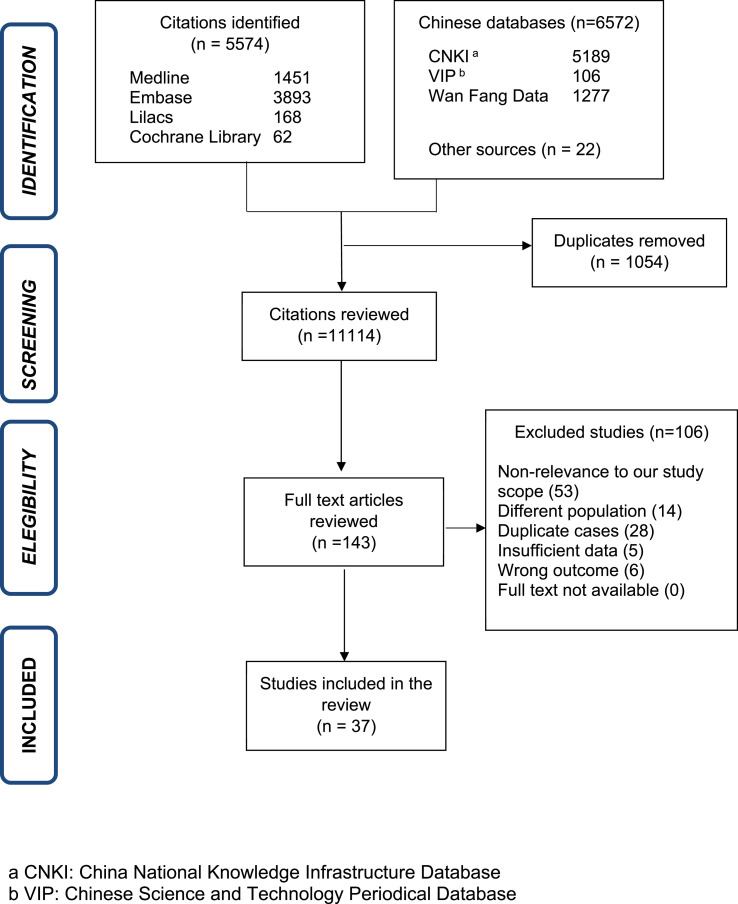
We identified 12,168 articles, removing 1054 duplicates and reviewing 11,114 as per titles and abstracts. Further, 10,971 without clinical information about pregnant women were removed. We evaluated 143 in full-text for eligibility. The references included in publications, websites of national reports and academic non-government organizations yielded 22 new articles fitting our criteria. We excluded 106 articles and, ultimately, included 37 articles in this review (Fig. 1 ): four case-control studies [[13], [14], [15], [16]], nine cases series [[17], [18], [19], [20], [21], [22], [23], [24], [25]], 23 case reports [[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]], and one national report [10].
Fig. 1.
Selection of studies.
This review excluded 106 articles (Supplemental Table 1); 53 were excluded due to non-relevance to our scope (national reports, practice guidelines, etc.), 14 due to different population, five due to insufficient data, six due to wrong outcome (psychological evaluation of the pregnant woman), and 28 due to duplicate cases. The different characteristics of articles to consider duplication were evaluated: author, city, hospital of data source, number of pregnant women, period of admission or recruitment and specific characteristics attributed to patients (symptoms, laboratory results, birth weight). Twenty-six articles with Chinese pregnant women [[49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74]], were excluded as the patients were also included in a recent case series from Yan J. et al. [22] Two articles from USA [75,76], were also excluded since Breslin et al. [17] published a case series including the same population from the same hospitals (Appendix B) [17].
A descriptive analysis based on 33 articles and a meta-analysis based on 4 case-control studies was performed. For the descriptive analysis, we included a total of 322 pregnant women, 111 (34.5%) patients from Netherlands [10], 80(24.8%) from USA [17,19,21,40,42,46], 76(23.6%) from China [22,23,26,27,30,32,33,37,39,47], 43(13.3%) from Italy [18,29], 2(0.6%) from Canada [20], and one each from Honduras [28], South Korea [31], Sweden [34], Germany [35], Turkey [36], Iran [38], Australia [41], Spain [43], Peru [44], and India [45] (Supplemental Table 2). One case series(24) and one case report(48) from China were considered for analysis of vertical transmission. A different case series was consider to summarize immunoglobulin levels [25]. Of note, until April 27, around 400 cases were reported by different national reports; however, they were excluded because they were summary reports without descriptive information and some may have been included in previous research papers.
3.2. Risk of bias of included studies and quality assessment
Using NOS in the case-control studies, out of a maximum of nine stars, the highest score was eight; the lowest six. Every study had a proper case definition and was representative of the cases in hospitals; however, bias risk was considered in controls selection. Only two studies tried to control for some confounders by age-matching [14,16]. All studies had ascertainment of exposure with a standard method. Most studies had the same rate of non-response for both groups (Supplemental Table 3a). The quality of case series and case reports evaluated using the modified NOS were: three studies were deemed to be good [20,26,46], 23 were moderate [17,19,[22], [23], [24],[27], [28], [29], [30], [31],[34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44],47,48], and six were poor in methodological quality (Supplemental Table 3b) [18,21,25,32,33,45]. The Dutch Association for Obstetrics and Gynecology's official report was no assessed for quality [10].
3.3. Synthesis of results
3.3.1. - Maternal clinical characteristics
The main characteristics are summarized in Table 1 and detailed description in Supplemental Table 4. Maternal age between 20 and 45 years, and the gestational age ranged from 5 + 2 to 40 + 5 weeks (+days). Two twin pregnancies were reported. Data regarding time to diagnosis COVID-19 by trimester in 179 women were collected. Five (2.8%) were in the first trimester, nine (5.0%) in the second and 165 (92.2%) in the third. Forty-four (33.3%) patients reported exposure to a close area with active infection (i.e. Wuhan city). Forty-eight (36.4%) reported direct contact with an infected person. Maternal comorbidities were reported in 128 women, including obesity (n = 31,24.2%), asthma (n = 10,7.8%), gestational diabetes (n = 7,5.5%), type 2 diabetes (n = 4,3.1%), chronic hypertension (n = 3,2.3%) and hypothyroidism (n = 1,0.8%). Fourteen patients (10.9%) reported previous cesarean.
Table 1.
Maternal demographic, clinical characteristics and comorbidities.
| Demographic characteristics | Patient n (%) |
|---|---|
| Number of pregnant women | 322 |
| Maternal age in years | 20–45 |
| Single pregnancy | 177/179 (98.9) |
| Exposure to relevant environment a | 44/132 (33.3) |
| Contact with infected person | 48/132 (36.4) |
| Trimester of pregnancy at infection | |
| First trimester | 5/179 (2.8) |
| Second trimester | 9/179 (5.0) |
| Third trimester | 165/179 (92.2) |
| Maternal comorbidities | |
| Obesity | 31/128 (24.2) |
| Previous cesarean | 14/128 (10.9) |
| Asthma | 10/128 (7.8) |
| Gestational diabetes | 7/128 (5.5) |
| Type 2 diabetes | 4/128 (3.1) |
| Chronic hypertension | 3/128 (2.3) |
| Hypothyroidism | 1/128 (0.8) |
| Clinical characteristics | |
| Asymptomatic at admission | 42/169 (28.4) |
| Cough | 148/248 (59.7) |
| Fever at admission | 147/248 (59.3) |
| Tachypnea | 25/248 (10.1) |
| Myalgia | 23/248 (9.3) |
| Fatigue | 16/248 (6.5) |
| Dyspnea | 14/248 (5.6) |
| Sore throat | 13/248 (5.2) |
| Tachycardia | 13/248 (5.2) |
| Headache | 12/248 (4.8) |
| Chest pain | 8/248 (3.2) |
| Malaise | 4/248 (1.6) |
| Diarrhea | 4/248 (1.6) |
| Intra-partum fever | 2/248 (0.8) |
| Nasal congestion | 2/248 (0.8) |
| Post-partum fever | 1/248 (0.4) |
Exposure to a close area with active infection, i.e., Wuhan city.
Among 169 pregnant women, 42 (28.4%) were asymptomatic at admission but qRT-PCR tested SARS-CoV-2-positive. Among 248 women, the most common being cough (n = 148,59.7%) and fever (n = 147,59.3%). Less common symptoms were tachypnea (n = 25,10.1%), myalgia (n = 23,9.3%), fatigue (n = 16,6.5%), dyspnea (n = 14,5.6%), sore throat (n = 13,5.2%), tachycardia (n = 13,5.2%), headache (n = 12,4.8%), chest pain (n = 8,3.2%), malaise (n = 4,1.6%) and diarrhea (n = 4,1.6%). There were 125 ongoing pregnancies during the report. Among 111 of them, 92 (82.9%) were in home isolation with no or mild symptoms. (Detailed description in Supplemental Table 5).
Twenty articles including 88 patients reported the laboratory characteristics [19,20,22,28,30,31,[33], [34], [35], [36], [37], [38],40,[42], [43], [44],46,47]. Table 2 describes lymphopenia (n = 52,59.1%), elevated concentrations of C-reactive protein (n = 46, 52.3%) and leukopenia (n = 23,26.1%) as the most common findings. Leukocytosis (n = 9, 10.2%), high alanine transaminase ALT (n = 8,9.1%), high aspartic transaminase AST (n = 7,8.0%), high erythrocyte sedimentation rate (n = 6,6.8%), anemia (n = 6,6.8%) and thrombocytopenia (n = 5,5.7%) were also reported. All four patients from two studies had high IL-6 values(23, 35) and all 3 patients from one study had high IL-10 levels (100.0%) [23]. (Detailed description in Supplemental Table 6).
Table 2.
Maternal laboratory and imaging characteristics.
| Laboratory characteristics | Patient n (%) |
|---|---|
| Lymphopenia (<1.0 × 109/L) | 52/88 (59.1) |
| Elevated concentrations of CRP (>8.1 mg/L) | 46/88 (52.3) |
| Leukopenia (<4 × 109/L) | 23/88 (26.1) |
| Leukocytosis (>10 × 109/L) | 9/88 (10.2) |
| High ALT a (>33 U/L) | 8/88 (9.1) |
| High AST b (>33 U/L) | 7/88(8.0) |
| Erythrocyte sedimentation rate (>47 m/h) | 6/88 (6.8) |
| Anemia (<11 g/dL) | 6/88(6.8) |
| Trombocytopenia (<150 x 109/L) | 5/88 (5.7) |
| Serum ferritin (>117ng/mL) | 3/88 (3.4) |
| High creatine kinasa (>75 units/L) | 3/88 (3.4) |
| High creatinine | 1/88 (1.1) |
| High bilirubin (>1.1 mg/dL) | 0/88 (0.0) |
| Chest CT imaging characteristicsc | |
| CT evidence of pneumonia | 77/82 (93.9) |
| Ground-glass opacity | 65/82 (79.3) |
| Consolidation | 9/82 (11.0) |
| Pleural effusion | 5/82 (6.1) |
AST: Alanine aminotransferase.
ALT: Aspartate aminotransferase.
CT: Computed tomography.
Table 2 additionally shows 16 studies including 82 patients imaged by chest CT. with 77 (93.9%) diagnosed of pneumonia. The most characteristic findings on CT were ground-glass opacity (GGO) regions in 65 (79.3%), consolidation in nine (11.0%) and pleural effusion in five (6.1%). Data from 15 patients showed bilateral lesions in 13 (86.7%). Albeit other modalities were rarely reported, it is noteworthy that seven cases were reported with pneumonia by x-chest ray (77.8%) [19,20,37,38,40,42,43], and 2 reports found signs of pneumonia on ultrasound lung examination [36,40]. (Detailed description in Supplemental Table 6).
3.3.2. - Maternal outcomes
Maternal outcomes are listed on Table 3 and detailed description in Supplemental Table 7). Out of 322 pregnant women, 195 (60.6%) delivered and 125 (38.8%) were still pregnant during the study. Delivery occurred at term (≥37 weeks) in 127/195 (64.8%). Thirty-two (16.3%) did not reported gestational age. Adverse obstetrics outcomes included: preterm delivery in 37 out of 195 reporting gestational age (18.9%), premature labor treated with tocolysis in five cases (1.7%), intrauterine fetal distress (i.e. fetal hypoxia as stated in each article) in 17 (5.9%) and premature rupture of membranes in five (1.7%) women. There were 1(0.3%) stillbirth at 30 weeks of gestation that was delivered vaginally while the mother was on mechanical ventilation, who died subsequently because of severe COVID-19 complications [38]. One (0.3%) patient had a miscarriage presenting with fever and fatigue [22], and one (0.3%) opted for termination of the pregnancy at six-weeks’ gestation after receiving oxygen support and antiviral therapy [23]. Other adverse obstetrics outcomes were preeclampsia in three women (2.3%), gestational hypertension in 2 (1.6%) and cholestasis of pregnancy in 1 (0.8%). Cesarean was reported in 99 (50.8%) women and vaginal delivery in 64 (32.8%). There was no report on the delivery route in 32 (16.4%) cases. The main indication for cesarean was: COVID-19 (n = 31,31.3%). A total of 73 cases reported the median time between the onset of symptoms and delivery as five days (IQR 3–10 and range 1–38).
Table 3.
Maternal outcomes.
| Obstetric outcomes | Patient n (%) |
|---|---|
| Preterm delivery | 37/195 (18.9) |
| Miscarriage | 1/322 (0.3) |
| Interruption of pregnancy | 1/322 (0.3) |
| Intrauterine fetal distress | 17/290 (5.9) |
| Premature rupture of membrane | 5/290 (1.7) |
| Stillbirth | 1/290 (0.3) |
| Pre-eclampsia | 3/128 (2.3) |
| Gestational hypertension | 2/128 (1.6) |
| Cholestasis of pregnancy | 1/128 (0.8) |
| Delivery characteristics | |
| Cesarean | 99/195 (50.8) |
| COVID-19 infection | 31/99 (31.3) |
| Intrauterine fetal distress | 16/99 (16.2) |
| Labor dystocia | 10/99 (10.1) |
| Previous cesarean | 10/99 (10.1) |
| Preeclampsia | 3/99 (3.0) |
| Other obstetrics reason | 6/99 (6.1) |
| Not reported | 23/99 (23.2) |
| Vaginal | 64/195 (32.8) |
| Not reported | 32/195 (16.4) |
| Medical complications | |
| Severe pneumonia | 24/179 (13.4) |
| Acute respiratory distress syndrome | 19/179 (10.6) |
| Acute hepatic failure | 2/179 (1.1) |
| Acute renal failure | 2/179 (1.1) |
| Coagulopathy | 3/179 (1.7) |
| Cardiomyopathy | 2/179 (1.1) |
| Septic shock | 3/179 (1.7) |
| Intensive care unit admisaion | 30/290 (10.3) |
| Maternal mortality | 1/322 (0.3) |
| Treatment | |
| Nobtreatment | 129/183 (70.5) |
| Oxygen support | 52/290 (17.9) |
| Antivirals | 67/248 (27.0) |
| Antibiotics | 97/248 (39.1) |
| Corticosteroid | 41/248 (16.5) |
| Hydroxychloroquine | 13/248 (5.2) |
| Mechanical ventilation | 11/290a3.8) |
| Immune gamma globulin | 2/248 (0.8) |
| Extracorporeal Membrane Oxygenation | 1/290 (0.3) |
Table 3 shows 30 patients (10.3%) with COVID-19 complications requiring intensive care unit. Nine patients had data on the risk factors for admission to ICU being the most frequent obesity 3/9 (30%). All nine patients were admitted after 29 weeks of gestation, i.e. third trimester. Eight patients (8/9) underwent delivery by cesarean section. One patient had a preterm vaginal delivery spontaneously in the ICU.
The main complications were severe pneumonia occurred in 24 patients (13.4%) and acute respiratory distress syndrome in 19(10.6%). In six cases, the specific complication was unstated. One (0.3%) woman died of pneumonia by SARS-Cov-2 and multiorgan failure [38]. Table 3 also shows that 129 (70.5%) patients did not receive any medical treatment. Fifty-two (17.9%) patients reported the need for oxygen support; 67(27.0%) patients received some antiviral treatment such as oseltamivir, lopinavir, ritonavir and ganciclovir. Antibiotics were given to 97(39.1%) patients (moxifloxacin, cephalosporin, azithromycin, penicillin). Corticosteroids were given to 41(16.5%) patients. Other treatments reported were hydroxychloroquine (n = 13,5.2%), immune gamma globulin (n = 2,0.8%). Eleven (3.8%) patients needed mechanical ventilation and one needed extracorporeal membrane oxygenation ECMO (0.3%) (Detailed description in Supplemental Table 8).
3.3.3. - Perinatal outcomes
Neonatal outcomes are listed in Table 4 . Apgar scoring was higher than 7 at 1-min and 5-min in 84 (96.6%) and 85 (97.7%) newborns respectively. Breastfeeding restriction was reported in 28 (25.7%) infants. Low birth weight was reported in 9/62 newborns (14.5%). In this low birth weight group, we found seven neonates reporting some data. All seven patients had preterm delivery. One out of seven neonates were reported to have COVID-19 infection.
Table 4.
Neonatal outcomes.
| Neonatal outcomes | Patient n (%) a |
|---|---|
| APGAR >7 | |
| 1-minute | 84/87 (96.6) |
| 5-minute | 85/87 (97.7) |
| Low birth weight | 9/62 (14.5) |
| NICU admission for treatment b | 24/86 (27.9) |
| Respiratory distress syndrome | 2/36 (5.6) |
| Mechanical ventilation | 2/36 (5.6) |
| Asphyxia | 2/36 (5.6) |
| Pneumonia | 1/36 (2.8) |
| Sepsis | 0/36 (0.0) |
| Neonatal death | 1/118 (0.8) |
| No breastfeeding | 28/109 (25.7) |
| Discharge from hospital | 87/112 (77.7) |
196 neonates (including one pair of twin).
NICU: Neonatal intensive care unit.
Two infants had asphyxia (5.6%), two (5.6%) respiratory distress syndrome and one (2.8%) developed pneumonia. Twenty-four (27.9%) newborns were severely ill, requiring NICU admission, but only two (5.6%) required mechanical ventilation. We made a sub-analysis to identify risk factors and characteristics of patients for NICU admission. However, only seven neonates had some data. One out of seven had COVID-19 infection and five out of seven (71%) had preterm delivery. Yan J et al. [22] reported a newborn of 35 + 2 weeks delivered by cesarean with severe neonatal asphyxia treated with invasive ventilation died 2 h after birth. The mother developed severe pneumonia and septic shock. Eighty-seven (77.7%) infants had been discharged during the report (Detailed description in Supplemental Table 9).
Table 5 shows the data on vertical transmission. Every mother (322, 100%) included in this study was tested qRT-PCR positive for SARS-CoV-2 in a nasopharyngeal swab. Some patients were additionally tested in different type of samples. One out of 16 (6.25%) were vaginal swab-positive. SARS-CoV-2 was not found in breast milk (0/17), amniotic fluid (0/25), umbilical cord (0/16) or placenta (0/15). We found data of IgG and IgM antibodies on eight mothers and their neonates. Seven of them were qRT-PCR SARS-CoV-2 tested negative in nasopharyngeal swabs. Six infants had high IgG levels (i.e.>10AU/mL) and three had high IgM levels. Of note, the one qRT-PCR tested positive had no detection of IgG and IgM antibodies [44]. Among 138 neonates tested with nasopharyngeal swab, four (3.6%) were tested SARS-CoV-2 positive between 16 and 36 h after birth. Three of them had additionally a positive anal test. These four newborns were delivered by cesarean. Two infants had fever and two shortness of breath. Two of them required mechanical ventilation; however, none of these neonates died (Detailed description in Supplemental Table 10).
Table 5.
Results of SARS-CoV-2 testing on mothers and neonates.
| Type of test | Tested | Positive | (%) |
|---|---|---|---|
| Mother | |||
| Nasopharyngeal swabs | 322 | 322 | 100.00 |
| Breast milk | 17 | 0 | 0.00 |
| Vaginal swab | 16 | 1 | 6.25 |
| Cervical secretion | 1 | 0 | 0.00 |
| Sputum | 3 | 2 | 66.67 |
| Feces | 2 | 0 | 0.00 |
| Serum | 1 | 0 | 0.00 |
| IgM a | 8 | 6 | 75.00 |
| IgG a | 8 | 7 | 87.50 |
| Conception products | |||
| Amniotic fluid | 25 | 0 | 0.00 |
| Umbilical cord blood | 16 | 0 | 0.00 |
| Placenta | 15 | 0 | 0.00 |
| Neonate | |||
| Nasopharyngeal swab b | 138 | 4 | 3.60 |
| Serum | 4 | 0 | 0.00 |
| Anal b | 6 | 3 | 50.00 |
| Feces | 2 | 0 | 0.00 |
| Gastric juice | 1 | 0 | 0.00 |
| Urine | 1 | 0 | 0.00 |
| IgM a | 8 | 3 | 37.50 |
| IgG a | 8 | 6 | 75.00 |
We consider additional data of immune globulin levels from two articles: Zeng H et al.25 and Dong L et al.48.
We consider additional data of newborns with qRT-PCR positive for SARS-CoV-2 from the article of Zeng L et al.24.
3.4. Meta-analysis
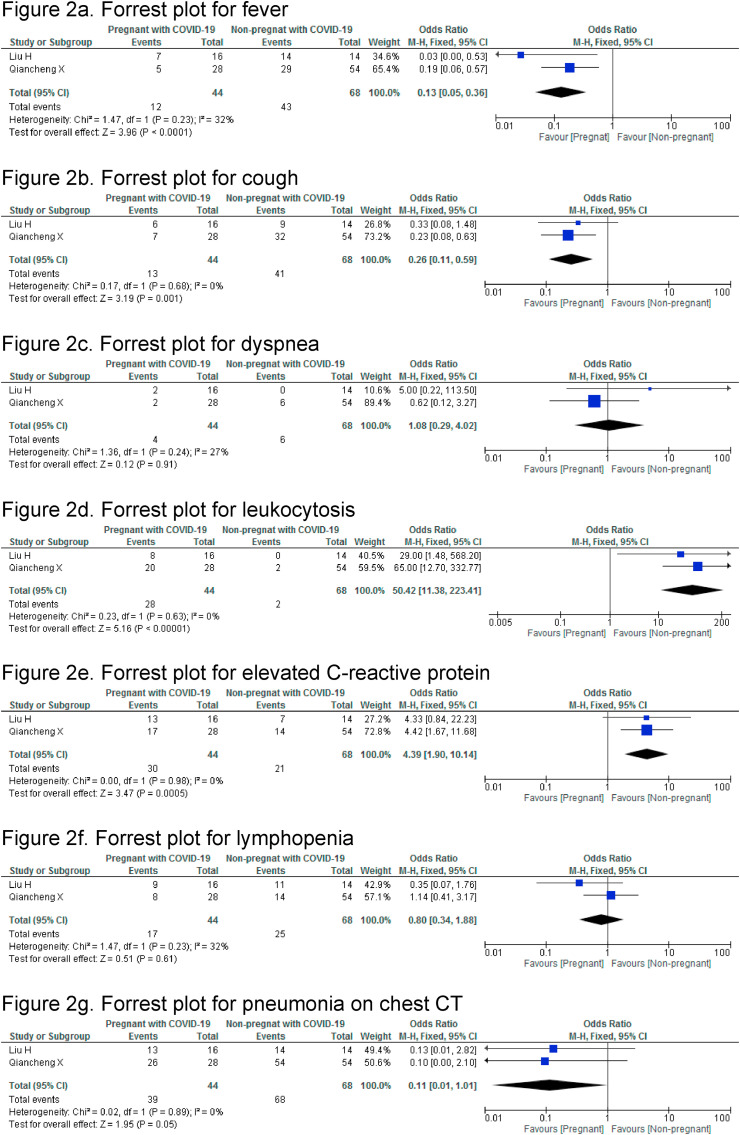
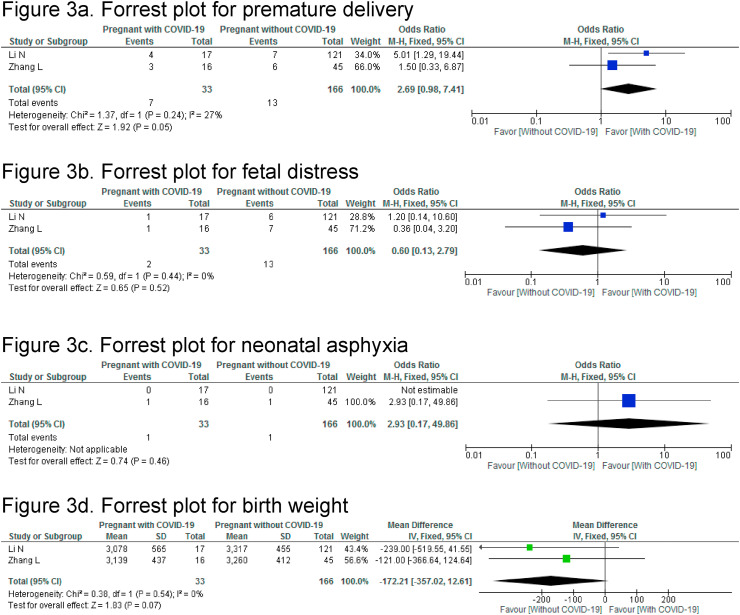
Two case-control studies compared clinical characteristics, laboratory and chest CT findings between pregnant women with COVID-19 and non-pregnant women with COVID-19 (Fig. 2 ) [15,16]. Among the clinical characteristics, fever (OR:0.13,95%CI 0.05–0.36) and cough (0.26,95%CI 0.11–0.59) were significantly lower in pregnant women with COVID-19 than non-pregnant women with COVID-19. There was no significant difference in dyspnea (OR:1.08, 95% CI 0.29–4.02). Leukocytosis (OR: 50.42, 95% CI 11.38–233.41) and high levels of C-reactive protein (OR:4.39,95%CI 1.9–10.14) were significantly higher in pregnant women with COVID-19 than non-pregnant COVID-19-infected women. The rates of lymphopenia between the two groups was comparable, OR:0.80 (95% CI 0.34–1.88). Similarly, signs of pneumonia on chest CT were not significantly different, OR: 0.11, (95% CI, 0.01–1.01). Two different case-controls studies compared pregnant women with and without COVID-19 (Fig. 3 ) [13,14]. The ORs for premature delivery (2.69, 95%CI 0.98–7.41, p = 0.05), fetal distress (0.6, 95%CI 0.13–2.79, p = 0.52) and neonatal asphyxia (2.93, 95%CI 0.17–49.86, p = 0.46) were not significantly different. The pooled estimate showed a comparable birth weight in newborns of women with and without COVID-19 (mean difference MD (IV, fixed-effect): 172 g, (95%CI-357 to 12.61).
Fig. 2.
Meta-analysis: Pregnant women with COVID-19 vs. non-pregnant women with COVID-19
2a Forrest plot for fever
2b. Forrest plot for cough
2c. Forrest plot for dyspnea
2d. Forrest plot for leukocytosis
2e. Forrest plot for elevated C
-reactive protein
2f. Forrest plot for lymphopenia
2g. Forrest plot for pneumonia on chest CT.
Fig. 3.
Meta-analysis: Pregnant women with COVID-19 vs pregnant women without COVID-19
3a. Forrest plot for premature delivery
3b. Forrest plot for fetal distress
3c. Forrest plot for neonatal asphyxia
3d. Forrest plot for birth weight.
4. Discussion
4.1. Main findings
This systematic review summarizes information about maternal clinical characteristics; and maternal and perinatal outcomes among 322 pregnant women with COVID-19.
We report that maternal mortality is rare. However, but there is a considerable proportion of women that required ICU due to complication of COVID-19 infection. Additionally, we report that maternal clinical characteristics are slightly different in pregnant women compared with the general population. The common symptom reported is cough and fever if they are symptomatic, but these are frequently less than the general population. There is a considerable proportion of asymptomatic pregnant women presenting at hospital for obstetric reasons. The main adverse obstetric outcome found is iatrogenic preterm delivery (i.e. cesarean or induction delivery due to worsening of the medical or obstetric condition). Additionally, there was a considerable proportion of low birth weight infants and neonates requiring admission to the NICU. The data collected hitherto does not support vertical transmission.
COVID-19 has posed a daunting challenge to the public health worldwide leading to researchers to focus their efforts in shedding light on our understanding of the disease. Consequently, we are facing an unprecedent number of articles being published, making it difficult to endeavor an updated systematic review. Yet, as there are several published large case series of COVID-19 in adults, there are still few published case series focusing on pregnant women. Furthermore, several articles include cases that had been previously included in different articles, even with different authors. This has raised serious concern since it represents a lapse in ethical standards of scientific reporting and potentially misleads our understanding of the disease and responding to it with ineffective public health decision [77]. In order to adhere to our strict protocol, we had to exclude many articles with every reason stated in Supplemental Table 1.
4.2. Comparison with existing literature
4.2.1. - Clinical characteristics
Among pregnant women with COVID-19 presented at hospital for obstetric reasons, three hospital-based studies recently reported the proportion of asymptomatic pregnant women at presentation in the range of 66%–88% when a universal testing was performed in areas with high prevalence of the disease [17,21,78]. A lower proportion of asymptomatic pregnant women, of about 28%, was found here, since we included most cases from the patients being admitted to hospitals, likely reflecting a bias selection for more complicated cases. Furthermore, we should consider that some of the women were asymptomatic at admission, but they might have developed symptoms later, i.e. they were presymptomatic rather than asymptomatic, which is unstated in the three previous reports. An epidemiological study reported the proportion of asymptomatic in the general population being 50% at first testing; and after data modelling, they could estimate that only 18% would be truly asymptomatic [79]. In the pediatric population, the disease is known to be mild, with mortality being extremely low; even among them, the proportion of truly asymptomatic children is 4%, and 50% of them will have very mild symptoms [80]. Thus, the proportion of asymptomatic in pregnant women could be less than what is stated by the three studies. Nevertheless, people in the asymptomatic or presymptomatic period can be transmit the virus, and as health-workers, we should bear in mind the potential risk of managing pregnant women and, where possible, include a universal screening protocol according to the capabilities of our health system [81]. We found fever and cough as the most frequent symptoms reported among pregnant women with COVID-19. However, cough was present in less proportion than in the general population as confirmed in the meta-analysis when compared with non-pregnant women with COVID-19 [5]. Similarly, fever can be found in 89% of the general population and here we reported a lower frequency of fever, highlighted in the meta-analysis when compared with non-pregnant women with COVID-19 [5,82,83].
A number of infections in pregnancy are presenting with little symptoms or even they are asymptomatic. For instance, less than 5% of cytomegalovirus infection are reported to be symptomatic in pregnancy [84]. Similarly, in toxoplasmosis infection around 10% of pregnant women become symptomatic [85]. Similar to other infections, in COVID-19 fever is the commonest symptom was found pregnant women. Influenza is another common infection during pregnancy and it usually presents with fever, cough, sore through, rhinorrhea, accompanied of unspecific symptoms such as fever, headache, fatigue, body aches among others. The symptoms are enhanced in the third trimester due to the physiological changes that are more evident as pregnancy progresses. Similarly, here we found more symptomatic pregnant women in the third trimester [86].
Although anosmia and dysgeusia were reported as common signs in some reports including non-pregnant adult population, the number of articles reporting these symptoms in our systematic search were scarce.
Among the laboratory findings, lymphopenia and elevated CRP was also commonly found in pregnant women [5,83]. Among pregnant women, data from Liu H et al. and Qiancheg X. et al. shows that leukocytosis is significantly more frequently observed in pregnant COVID-19-afected women compared with COVID-19-afected non-pregnant women, though an analysis of the data reported by Liu H et al. shows that the number of leukocytes is nearly in the high normal range of values for pregnant women, where it is far known that the total number of leukocytes in blood increases especially in the third trimester [15,16]. Among women reporting contact with a possible infected patient or living in an infected area, a third reported positively. However, most articles did not report on this and it is likely that coming articles will not report this epidemiological characteristic as COVID-19 is currently everywhere. It is important to highlight that obesity was found in 24% of those reporting comorbidities which is a major risk factor for contracting COVID-19 [4,5].
Similar to adult population, findings of pneumonia were found in about 94% of pregnant women with COVID-19 undergoing chest CT [5,87]. When compared with non-pregnant women with COVID-19, there is no significant difference, but there is a tendency in the diagnosis of pneumonia to be more present in pregnant women. Lung ultrasound may help diagnose pneumonia as was shown in the general population [88]. Inchingolo R. et al. have recently reported pneumonia diagnosis using lung ultrasound in symptomatic pregnant women with COVID-19 [89]. Thus, further research should clarify the use of chest CT or lung ultrasound in pregnant women with COVID-19 showing no or mild symptoms.
Albeit about 70% women were not treated, some women received oxygen support and a variety of antiviral and antibiotics as the most frequent treatments as observed in adult population since there is no evidence-based treatment for COVID-19 thus far. Since there are no clear recommendations on the use of antiviral in pregnancy, all patients reported here had delivered before using antivirals. Data is still scarce on effective medical treatment for COVID-19 in pregnant women as they are excluded in any known trial exploring treatment options for COVID-19. Corticosteroids were given in 16% patients, mostly after delivery, i.e. as medical management of COVID-19. We were unable to determine the number of pregnant women receiving corticosteroid prenatally as per protocol for lung maturation. Nonetheless, current guidelines recommend proceeding with caution and uniquely weighing the maternal risks and fetal benefits when considering its use, particularly in early preterm delivery [90].
4.2.2. - Maternal outcomes
Two recent reports from New York suggest that the overall clinical outcomes in pregnant women are similar to those in non-pregnant women [17,21]. A rationale to believe that COVID-19 may have a deleterious effect on pregnancy is the similarity of SARS-CoV-2 with the SARS-causing virus, which is more aggressive than COVID-19 in pregnant women, leading to high admission at ICU and high mortality [7]. A rationale to believe that COVID-19 might have a deleterious effect on pregnancy is the similarity of SARS-CoV-2 with SARS-Cov-1 and MERS-CoV, which are more aggressive than COVID-19 in pregnant women [7], leading to higher rates of admission to ICU (MERS-CoV: 44.6%, SARS-CoV-1: 53.3% and SARS-CoV-2: 9.3%), need for mechanical ventilation (MERS: 40.9%, SARS-COv-1: 40% and SARS-CoV-2: 5.4%) and higher rate of mortality (MERS: 28.6%, SARS-COv-1: 25.8% and SARS-CoV-2: 0%) as reported in a recent systematic review [91].
Here, we found that about 10% pregnant women required intensive care because of respiratory complications. Similarly, Blitz MJ et al. described in a series of women with COVID-19 that the reason of admission to ICU due to worsening respiratory symptoms is 10% in pregnant and non-pregnant women [92]. Recently, the Public Health Agency of Sweden released a report on 13 pregnant and postpartum women with a substantial higher risk (RR: 5.4%, 95CI 2.89–10.08) of being admitted to ICU and, additionally, a higher risk of requiring mechanical ventilation support (RR: 4.0,95% CI 1.75–9.14%) raising concern about the real impact of COVID-19 in pregnant women [93]. However, further prospective studies will clarify whether pregnancy per se is a factor to become severe ill and require ICU admission or instead the comorbidities are the risk factor for clinical deterioration. The burden of COVID-19 on maternal mortality remains unclear as there may be a delay in reporting maternal death. Here, we report a case of maternal death due to COVID-19 in Iran [38]. However, following the closing date of this review, there have been a series of eight maternal deaths among women critically ill with COVID-19, surprisingly, from Iran again [82], and few reports from other low-income countries [94]. Though it is difficult to determine an exact case fatality rate (CFR), some researchers have suggested it to be around 0.4% among those between 10 and 49 years, regardless of the gender, which is the range where pregnant women are [95]. Thus, considering women have less mortality rate, pregnant women without comorbidity could have a CFR less than 0.4% [96].
It is still unclear whether pregnancy is an immunological contributor to severe and controlled COVID-19 infection.
In normal pregnancy, anti-inflammatory cytokines are secreted to enrich the intrauterine milieu. Similarly, in patients with COVID-19 infection there are increased secretion of T-helper-2 (Th2) cytokines that suppresses inflammation [83]. This suggests that in pregnancy is preserved the anti-inflammatory environment, suppressing the severity of the COVID-19 infection. It is unknown whether COVID-19 infection has any effect on placental hormone production [97].
4.2.3. - Perinatal outcomes
We report one miscarriage in a very symptomatic woman and one intrauterine death at 30 weeks in a woman with subsequent death due to COVID-19 [38]. Similarly, only one neonatal death was reported in a woman with severe disease. Data on the effects of the virus in the first and second trimester are sparse. Further prospective studies will clarify if the prolonged hypoxemia in those severe cases of the infected women could harm placentation and cause miscarriage, intrauterine death or fetal growth restriction. Regarding the route of delivery, at least half of the COVID-19-infected women underwent cesarean; in 30% of them, the main indication was stated as COVID-19-complication.
Respiratory viruses such as H1N1 possess women at higher risk for severe complications leading to iatrogenic preterm birth [98]. Therefore, there is some concern regarding COVID-19 and the risk of preterm birth. Here, we have found a risk of preterm birth of 19%, higher than that commonly reported in pregnant women (about 10%) [99]. Interestingly, most of the preterm births reported had a medical indication, supporting the thesis that COVID-19-complications lead to iatrogenic preterm birth rather than causing spontaneous preterm birth. Low birth weight was found slightly increased in neonates born to infected mothers and could be explained by the increased number of preterm births. Data on fetal growth restriction as a diagnosis were not collected. In our review, most of the neonates scored Apgar greater than 7 at first and 5 min, suggesting no acute intrauterine hypoxic environment at delivery in the infected women. A very high proportion (28%) of neonates being admitted to NICU was found. However, it is likely that a bias is present as we cannot exclude those admitted due to most common indications such as preterm birth and those admitted as a cautious measure to avoid any possible transmission or following a local protocol recommending temporally separating infants born to mothers with COVID-19. Data collected on the clinical manifestation among neonates is insufficient to draw any conclusion from this systematic review, though data from other studies suggest a mild clinical course with relatively quick recovery and no major concern about the morbidity of infants born to mother with COVID-19 [54,82].
4.2.4. - Vertical transmission
Mother-to-child transmission of SARS-CoV-2 has been intensively researched, as it could potentially occur prenatally (in utero), perinatally (labor and partum) or postnatally when breastfeeding [100,101].
Previous studies show no evidence of vertical SARS transmission [102]. Here, we report SARS-CoV-2 was not found in placenta, amniotic fluid, umbilical cord or breast milk. There are two reasons to believe that the SARS-CoV-2 could enter bloodstream and may be able to reach the placenta. First, it has been detected in blood samples, particularly from those severe cases [103,104]; and second, it utilizes the membrane-bound angiotensin-converting enzyme 2 (ACE2) to gain access to its target cell, known to be expressed in the human placenta, though in low levels [105]. Thus, in some severe cases, SARS-CoV-2 could be found in the placenta. Thus not surprisingly, Algarroba GN et al. [106] and Hosier et al. [107] recently reported a visualization of SARS-CoV-2 virions invading syncytiothrophoblasts using electron microscopy and positive immunohistochemical staining for SARS-CoV-2 spike protein, demonstrating viral localization in syncytiothrophoblasts cells. Their findings are based on infected women in their second trimester and in severe cases with high viremia. Though SARS-CoV2 was found in nasopharyngeal of four neonates, it does not prove vertical transmission since these neonates could have been contaminated in the hospital by professional health workers. Some other authors claim that the presence of SARS-CoV-2 IgM antibody found in blood of newborns could represent an evidence of vertical transmission [25,48]. Since IgM antibodies are too large to cross the placenta, it's reasonable to believe they could be produced by the fetus. However, the two reports could be false positive since they failed to demonstrate the presence of virus in newborns, along with the unknown performance characteristics of the blood antibodies test for SARS-CoV2 [108].
4.3. Strengths and limitations
This is a systematic review based on case series and case reports, focusing on four small case-control studies with an overall small number of cases. No prospective longitudinal studies were identified; thus, the data is mainly descriptive. Moreover, the summarized findings are largely from hospitalized COVID-19 patients; therefore, the analysis is likely biased toward severe cases. This study was also performed over a four-month period, and since most of the included pregnant women were in their third trimester, the impact of COVID-19 in their first and second trimester remains unknown, including any effect on fetus development and long-term outcomes. Currently, with articles generated at an unprecedented rate, this systematic review warrants to be updated with the publication of new data published. An important strength of this systematic review is that we have included only patients confirmed by qRT-PCR testing for SARS-CoV-2 thus limiting population bias. Moreover, no previous systematic review on COVID-19 and pregnancy has included a thorough search including Chinese databases. Large and multinational prospective studies focusing on pregnant women from the first trimester and a complete follow-up are necessary to better estimate the impact of COVID-19 on pregnant women [109].
5. Conclusions
Maternal clinical characteristics in pregnant women with COVID-19 are slightly different than the general population. The usual symptom at presentation is cough and fever, but these two symptoms are significantly less frequent in pregnant women with COVID-19 compared with non-pregnant women with COVID-19. Iatrogenic preterm birth is the main adverse obstetric outcome. Maternal severe morbidity and mortality seems to be similar to those reported for non-pregnant women. However, impaired clinical course of COVID-19 could have some impact on morbidity and cause adverse obstetric and perinatal outcomes. No convincing evidence of vertical transmission exists, at least during the third trimester of pregnancy.
Author contribution
RHN and WV formulated the research questions, designed the study, developed the preliminary search strategy, and drafted the manuscript. WQ, PL, KUQ, EGR refined the search strategy by conducting iterative database queries and incorporating new search terms. RHN, WQ, PLL, KUQ, and EGR searched and collected the articles. RHN and WQ conducted the quality assessment. All authors critically reviewed the manuscript for relevant intellectual content. All authors have read and approved the final version of the manuscript.
Funding source
No funding.
Disclosures
The authors have no conflict of interests.
Ethical approval
Approval was not required.
Declaration of competing interest
All authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2020.101919.
Appendix A. Full search formula
MEDLINE ((((((((((("Novel coronavirus"[All Fields] OR "Novel coronavirus 2019"[All Fields]) OR "2019 nCoV"[All Fields]) OR "COVID-19"[All Fields]) OR "COVID-2019"[All Fields]) OR "COVID"[All Fields]) OR "Wuhan coronavirus"[All Fields]) OR "Wuhan pneumonia"[All Fields]) OR "SARS-CoV-2"[All Fields]) OR "2019nCoV"[All Fields]) OR "2019 novel coronavirus"[All Fields]) OR (("COVID-19"[Supplementary Concept] OR "COVID-19"[All Fields]) OR "covid19"[All Fields])) OR "new coronavirus"[All Fields]) OR ("Wuhan"[Title/Abstract] AND (("coronavirus"[MeSH Terms] OR "coronavirus"[All Fields]) OR "coronaviruses"[All Fields])); EMBASE ('coronaviridae infection'/exp OR 'coronaviridae infection' OR 'novel coronavirus':ti,ab, kw OR 'novel coronavirus 2019':ti,ab, kw OR '2019 ncov':ti,ab, kw OR 'covid 19':ti,ab, kw OR 'covid-2019':ti,ab, kw OR covid:ti,ab, kw OR 'wuhan coronavirus':ti,ab, kw OR 'wuhan pneumonia':ti,ab, kw OR 'sars-cov-2':ti,ab, kw OR '2019ncov':ti,ab, kw OR '2019 novel coronavirus':ti,ab, kw OR covid19:ti,ab, kw OR 'new coronavirus':ti,ab, kw OR (wuhan:ti,ab, kw AND coronavirus:ti,ab,kw)); Cochrane library (("Novel coronavirus"):ti,ab, kw OR ("Novel coronavirus 2019"):ti,ab, kw OR ("2019 nCoV"):ti,ab, kw OR ("COVID-19"):ti,ab, kw OR ("COVID-2019"):ti,ab, kw OR (COVID):ti,ab, kw OR ("Wuhan coronavirus"):ti,ab, kw OR ("Wuhan pneumonia"):ti,ab, kw OR ("SARS-CoV-2"):ti,ab, kw OR ("2019nCoV"):ti,ab, kw OR ("2019 novel coronavirus"):ti,ab, kw OR (COVID19):ti,ab, kw OR ("new coronavirus"):ti,ab, kw OR ((Wuhan AND coronavirus)):ti,ab,kw); LILACS ((( ( ( "CORONAVIRUS" or "COVID-19") or "SARS-COV/") or "SARS-RELATED CORONAVIRUS") or "SARS-RELATED CORONAVIRUS/") or "2019NCOV") or ("WUHAN" AND "CORONAVIRUS") [Palavras]. The search terms used in Chinese medical databases were: “SARS-CoV-2”, “新冠肺炎”,”2019年冠狀病毒”, “武漢市肺炎”, “新型冠状病毒肺炎”, “新型冠状病毒”, “COVID-19”. Limited for human studies. Subsequently, the search was restricted to pregnant women with COVID-19.
Appendix B. Detailed articles excluded because of duplicate cases
- Articles from China
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology
Nan Yu of Tongji Hospital has three publications 51-53 with similar period of recruitment and probably some cases are the same. Furthermore, these cases are included in the report of Yan J et al. 22 Wang S et al. 50 reported a case of neonate infected with COVID-19, but this case has the same characteristics than reported by Nan Yu in two articles 51, 52. Liu Wei et al. 54 and Liu W et al. 49 articles were excluded because probably the cases are the same of previous report in Tongji Hospital.52
Renmin Hospital of Wuhan University
Chen R et al.,55 Fan et al.57 and the two articles of Khan Suliman et al.56, 58 were excluded because these cases probably overlap each other. They have similar period of recruitment and we consider that probably most of them are included in the report of Yan J et al.22
Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology, Wuhan
Chen Siyu et al.,59 Yang H et al.60 and Wu C et al.61 have the same time period of recruitment and probably these cases are the same and they are included in the series of Yan J et al.22
Union Hospital, Tongji Medical College, Huazhong University of Science and Technology
The patients reported by Chen S et al. 62 and Chen Y et al.63 probably there were included in the article of Liu D et al.64 and these cases are included in the series of Yan J et al.22
Zhongnan Hospital of Wuhan University
Chen H et al.65 and Yang P et al.66 probably have the same cases and we excluded them because of Yan J et al.22 published a larger series including the same cases.
The First Affiliated Hospital, College of Medicine, Zhejiang University
Huang J-w et al.,67, Li Y et al.68 and Kang et al.69 were excluded since they reported the same case. This case was published in the case series of Chen X et al.23 that was included in our systematic review.
Central Hospital of Wuhan, Tongji Medical College
Wu X et al.70 has the same period and probably these cases are included in the series of Yan J et al.22
Wuhan Red Cross Hospital
Xia et al.71 was excluded because the case was included in Yan J et al.22
Multicenter
Liu Y et al.73 and Zhu et al.74 was excluded because Yan J et al.22 published a larger series including the same cases. Chen L et al.72 reported 118 patients of all 50 designated hospitals in Wuhan city and probably these cases overlap with as those reported by Yan J et al.22. We excluded Chen L et al.72 because the data published by Yan J et al.22 provides with more detailed description.
- Articles from USA
New York–Presbyterian Allen Hospital and Columbia University Irving Medical Center
Breslin et al.75 was excluded because these 7 cases were included in the expanded series of 43 cases of Breslin et al.17 Sutton et al.76 was excluded because some of their cases were reported probably by Breslin et al.17
Appendix C. Supplementary data
The following are the Supplementary data to this article:
Assessment of reporting biases
1a. Funnel plot of comparison between pregnant women with COVID-19 and those without COVID-19. Outcome: Premature delivery. 1.b. Funnel plot of comparison between pregnant and non-pregnant women with COVID-19. Outcome: Cough.
References
- 1.He F., Deng Y., Li W. Coronavirus Disease 2019: what we know? J Med Virol. 2020;92:719–725. doi: 10.1002/jmv.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John Hopkins University COVID-19 dashboard by the center for systems science and engineering (CSSE) at john hopkins university (JHU) https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 3.Rajgor D.D., Lee M.H., Archuleta S., Bagdasarian N., Quek S.C. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20:776–777. doi: 10.1016/S1473-3099(20)30244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H., Wang L.L., Zhao S.J., Kwak-Kim J., Mor G., Liao A.H. Why are pregnant women susceptible to viral infection: an immunological viewpoint? J Reprod Immunol. 2020;139:103122. doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Wang Y., Zeng Y., Song T., Pan X., Jia M. Critically ill pregnant patient with COVID‐19 and neonatal death within two hours of birth. Int J Gynaecol Obstet. 2020;150:126–128. doi: 10.1002/ijgo.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nederlandse Vereniging Voor Obstetrie. Gynaecologie Dutch Association for Obstetrics and Gynecology. Update registration COVID-19 positive pregnant women in NethOSS 2020. https://www.nvog.nl/actueel/registratie-van-covid-19-positieve-zwangeren-in-nethoss/
- 11.Wells G., Shea B., O'Connell D., Peterson J., Welch V., Losos M. 2009. The NewcastleOttawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [cited 2020 May 15] [Google Scholar]
- 12.Bazerbachi F., Haffar S., Szarka L.A., Wang Z., Prokop L.J., Murad M.H. Secretory diarrhea and hypokalemia associated with colonic pseudo‐obstruction: a case study and systematic analysis of the literature. Neuro Gastroenterol Motil. 2017;29 doi: 10.1111/nmo.13120. 10.1111/nmo.13120. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Jiang Y., Wei M., Cheng B.H., Zhou X.C., Li J. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province] Zhonghua Fu Chan Ke Za Zhi. 2020;55:166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. Chinese. [DOI] [PubMed] [Google Scholar]
- 14.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa352. ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H., Liu F., Li J., Zhang T., Wang D., Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80:7–13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiancheng X., Jian S., Lingling P., Lei H., Xiaogan J., Weihua L. Coronavirus disease 2019 in pregnancy. Int J Infect Dis. 2020;95:376–383. doi: 10.1016/j.ijid.2020.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrazzi E.M., Frigerio L., Cetin I., Vergani P., Spinillo A., Prefumo F. COVID‐19 Obstetrics Task Force, Lombardy, Italy: executive management summary and short report of outcome. Int J Gynaecol Obstet. 2020;149:377–378. doi: 10.1002/ijgo.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juusela A., Nazir M., Gimovsky M. Two cases of coronavirus 2019–related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM. 2020;2:100113. doi: 10.1016/j.ajogmf.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlachodimitropoulou Koumoutsea E., Vivanti A.J., Shehata N., Benachi A., Le Gouez A., Desconclois C. COVID19 and acute coagulopathy in pregnancy. J Thromb Haemostasis. 2020;18:1648–1652. doi: 10.1111/jth.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vintzileos W.S., Muscat J., Hoffmann E., John N., Vertichio R., Vintzileos A.M. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease. Am J Obstet Gynecol. 2020;223:284–286. doi: 10.1016/j.ajog.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan J., Guo J., Fan C., Juan J., Yu X., Li J. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1–111.e14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Chen, Yang L.I., Wang Jinxi, Cai Hongliu, Cao Hongcui, Sheng Jifang. [Pregnant women complicated with COVID-19: a clinical analysis of 3 cases] J Zhejiang Univ. 2020;49:240–244. doi: 10.3785/j.issn.1008-9292.2020.03.08. Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in wuhan, China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W. Antibodies in infants born to mothers with COVID-19 pneumonia. J Am Med Assoc. 2020;323:1848‐9. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 Novel Coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020;71:844–846. doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen R., Sun Y., Xing Q.S. A patient with SARS-CoV-2 infection during pregnancy in Qingdao, China. J Microbiol Immunol Infect. 2020;53:499–500. doi: 10.1016/j.jmii.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zambrano L.I., Fuentes-Barahona I.C., Bejarano-Torres D.A., Bustillo C., Gonzales G., Vallecillo-Chinchilla G. A pregnant woman with COVID-19 in Central America. Trav Med Infect Dis. 2020;36:101639. doi: 10.1016/j.tmaid.2020.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Indraccolo U. A pregnant woman and the SARS-CoV-2 infection: how are barriers easily crossed? Recenti Prog Med. 2020;111:259–260. doi: 10.1701/3347.33190. [DOI] [PubMed] [Google Scholar]
- 30.Liao X., Yang H., Kong J., Yang H. Chest CT findings in a pregnant patient with 2019 novel coronavirus disease. Balkan Med J. 2020;37:226–228. doi: 10.4274/balkanmedj.galenos.2020.2020.3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee D.H., Lee J., Kim E., Woo K., Park H.Y., An J. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) confirmed patient. Korean J Anesthesiol. 2020;73:347–351. doi: 10.4097/kja.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu Zhongliang, Duan Zhao, Liu Ming, Wang Huiping, Wang Min, Zhang Hong. [Experience of infection protection during cesarean section of pregnant women with new coronavirus pneumonia] China Family Planning and Obstetrics and Gynecology. 2020;12:93–95. doi: 10.3969/j.issn.1674-4020.2020.02.25. Chinese. [DOI] [Google Scholar]
- 33.Huang Liqun, Wang Junping, Xiong Chumei, Sun Dianxing. [Successful treatment of the first pregnancy with severe new coronavirus pneumonia] Med & Pharm J Chin PLA. 2020:1–4. doi: 10.3969/j.issn.2095-140X.2020.04.001. Chinese. [DOI] [Google Scholar]
- 34.Gidlöf S., Savchenko J., Brune T., Josefsson H. COVID-19 in pregnancy with comorbidities: more liberal testing strategy is needed. Acta Obstet Gynecol Scand. 2020;99:948–949. doi: 10.1111/aogs.13862. [DOI] [PubMed] [Google Scholar]
- 35.Kleinwechter H., Laubner K. [Coronavirus disease 2019 (COVID-19) and pregnancy. Overview and report of the first German case with COVID-19 and gestational diabetes] Diabetologe. 2020:1–4. doi: 10.1007/s11428-020-00611-0. German. [DOI] [Google Scholar]
- 36.Kalafat E., Yaprak E., Cinar G., Varli B., Ozisik S., Uzun C. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol. 2020;55:835–837. doi: 10.1002/uog.22034. [DOI] [PubMed] [Google Scholar]
- 37.Xiong X., Wei H., Zhang Z., Chang J., Ma X., Gao X. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25857. 10.1002/jmv.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karami P., Naghavi M., Feyzi A., Aghamohammadi M., Novin M.S., Mobaien A. Mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Trav Med Infect Dis. 2020:101665. doi: 10.1016/j.tmaid.2020.101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng Z., Wang J., Mo Y., Duan W., Xiang G., Yi M. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health. 2020;13:818–820. doi: 10.1016/j.jiph.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnettler W.T., Al Ahwel Y., Suhag A. Severe ARDS in COVID-19-infected pregnancy: obstetric and intensive care considerations. Am J Obstet Gynecol MFM. 2020:100120. doi: 10.1016/j.ajogmf.2020.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowe B., Bopp B. COVID-19 vaginal delivery – a case report. Aust N Z J Obstet Gynaecol. 2020;60:465–466. doi: 10.1111/ajo.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iqbal S.N., Overcash R., Mokhtari N., Saeed H., Gold S., Auguste T. An uncomplicated delivery in a patient with covid-19 in the United States. N Engl J Med. 2020;382:e34. doi: 10.1056/NEJMc2007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González Romero D., Ocampo Pérez J., González Bautista L., Santana-Cabrera L. [Pregnancy and perinatal outcome of a woman with COVID-19 infection] Rev Clin Esp. 2020;220:533–534. doi: 10.1016/j.rce.2020.04.006. Spanish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission: a case report. Am J Perinatol. 2020;37:861–865. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma K.A., Kumari R., Kachhawa G., Chhabra A., Agarwal R., Sharma A. Management of the first patient with confirmed COVID-19 in pregnancy in India: from guidelines to frontlines. Int J Gynaecol Obstet. 2020;150:116–118. doi: 10.1002/ijgo.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Browne P.C., Linfert J.B., Perez-Jorge E. Successful treatment of preterm labor in association with acute COVID-19 infection. Am J Perinatol. 2020;37:866–868. doi: 10.1055/s-0040-1709993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu D., Sang L., Du S., Li T., Chang Y., Yang X.-A. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020 doi: 10.1002/jmv.25927. 10.1002/jmv.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong L., Tian J., He S., Zhu C., Wang J., Liu C. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. J Am Med Assoc. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W., Wang Q., Zhang Q., Chen L., Chen J., Zhang B. Coronavirus disease 2019 (COVID-19) during pregnancy: a case series. https://www.preprints.org/manuscript/202002.0373/v1 Preprints 2020: 2020020373. [cited 2020 May 15]
- 50.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20(5):559‐564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nan Yu, Fang Zixuan, Wu Jianli, Zeng Wanjiang, Deng Dongrui, Chen Suhua. [Perinatal outcome of new coronary pneumonia in late pregnancy] Prog Obstet Gynecol. 2020:167–169. doi: 10.13283/j.cnki.xdfckjz.2020.03.004. 03. Chinese. [DOI] [Google Scholar]
- 53.Yu N., Li W., Kang Q., Zeng W., Feng L., Wu J. No SARS-CoV-2 detected in amniotic fluid in mid-pregnancy. Lancet Infect Dis. 2020;S1473–3099(20) doi: 10.1016/S1473-3099(20)30320-0. 30320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W., Wang J., Li W., Zhou Z., Liu S., Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020;14(2):193–198. doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen R., Zhang Y., Huang L., Cheng B.H., Xia Z.Y., Meng Q.T. [Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients] Journal canadien d'anesthésie. 2020;67:655–663. doi: 10.1007/s12630-020-01630-7. French. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khan S., Peng L., Siddique R., Nabi G., Nawsherwan, Xue M. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol. 2020;41:748–750. doi: 10.1017/ice.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. 2020;41:741–750. doi: 10.1093/cid/ciaa226. [DOI] [Google Scholar]
- 58.Khan S., Jun L., Siddique R., Li Y., Han G., Xue M. Association of COVID-19 infection with pregnancy outcomes in healthcare workers and general women. Clin Microbiol Infect. 2020;26:788–790. doi: 10.1016/j.cmi.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25789. 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H., Sun G., Tang F., Peng M., Gao Y., Peng J. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J Infect. 2020;81:e40–e44. doi: 10.1016/j.jinf.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu C., Yang W., Wu X., Zhang T., Zhao Y., Ren W. Clinical manifestation and laboratory characteristics of SARS-CoV-2 infection in pregnant women. Virol Sin. 2020;35:305–310. doi: 10.1007/s12250-020-00227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S., Huang B., Luo D., Li X., Yang F., Zhao Y. [Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases] Zhonghua Bing Li Xue Za Zhi. 2020;49:E005. doi: 10.3760/cma.j.cn112151-20200225-00138. Chinese. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020;215:127–132. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 65.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang P., Wang X., Liu P., Wei C., He B., Zheng J. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020;127:104356. doi: 10.1016/j.jcv.2020.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J.W., Zhou X.Y., Lu S.J., Xu Y., Hu J.B., Huang Ml. [Dialectical behavior therapy-based psychological intervention for woman in late pregnancy and early postpartum suffering from COVID-19: a case report] J Zhejiang Univ - Sci B. 2020;21:394–399. doi: 10.1631/jzus.B2010012. Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26:1335–1336. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang Xianhui, Zhang Rong, He Huiliang, Yao Yongxing, Zheng Yueying, Wen Xiaohong. [Anesthesia management in cesarean section for patient with COVID-19: a case report] J Zhejiang Univ. 2020;49 doi: 10.3785/j.issn.1008-9292.2020.03.04. 249-2. Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu X., Sun R., Chen J., Xie Y., Zhang S., Wang X. Radiological findings and clinical characteristics of pregnant women with COVID‐19 pneumonia. Int J Gynaecol Obstet. 2020;150:58–63. doi: 10.1002/ijgo.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia H., Zhao S., Wu Z., Luo H., Zhou C., Chen X. Emergency Caesarean delivery in a patient with confirmed coronavirus disease 2019 under spinal anaesthesia. Br J Anaesth. 2020;124:e216–e218. doi: 10.1016/j.bja.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen L., Li Q., Zheng D., Jiang H., Wei Y., Zou L. Clinical characteristics of pregnant women with covid-19 in wuhan, China. N Engl J Med. 2020;382 doi: 10.1056/NEJMc2009226. e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020;S0163–4453(20) doi: 10.1016/j.jinf.2020.02.028. 30109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breslin N., Baptiste C., Miller R., Fuchs K., Goffman D., Gyamfi-Bannerman C. COVID-19 in pregnancy: early lessons. Am J Obstet Gynecol MFM. 2020;2(2):100111. doi: 10.1016/j.ajogmf.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sutton D., Fuchs K., D'Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bauchner H., Golub R.M., Zylke J. Editorial concern-possible reporting of the same patients with COVID-19 in different reports. J Am Med Assoc. 2020;323:1256. doi: 10.1001/jama.2020.3980. [DOI] [PubMed] [Google Scholar]
- 78.Khalil A, Hill R, Ladhani S, Pattisson K, O'Brien P. SARS-CoV-2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet Gynecol;223:296-297. 10.1016/j.ajog.2020.05.005. [DOI] [PMC free article] [PubMed]
- 79.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. e20200702. [DOI] [PubMed] [Google Scholar]
- 81.Kim S.E., Jeong H.S., Yu Y., Shin S.U., Kim S., Oh T.H. Viral kinetics of SARS-CoV-2 in asymptomatic carriers and presymptomatic patients. Int J Infect Dis. 2020;95:441–443. doi: 10.1016/j.ijid.2020.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., Seferovic M.D., Aski S.K., Arian S.E. Maternal death due to COVID-19 disease. Am J Obstet Gynecol. 2020;223:109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonalumi S., Trapanese A., Santamaria A., D'Emidio L., Mobili L. Cytomegalovirus infection in pregnancy: review of the literature. J Prenat Med. 2011;5:1–8. [PMC free article] [PubMed] [Google Scholar]
- 85.Wong S.-Y., Remington J.S. Toxoplasmosis in pregnancy. Clin Infect Dis. 1994;18:853–861. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]
- 86.Carlson A., Thung S.F., Norwitz E.R. H1N1 influenza in pregnancy: what all obstetric care providers ought to know. Rev Obstet Gynecol. 2009;2:139–145. doi: 10.3909/riog0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Govind A., Essien S., Karthikeyan A., Fakokunde A., Janga D., Yoong W. Re: novel Coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol. 2020;251:272–274. doi: 10.1016/j.ejogrb.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zamaniyan M., Ebadi A., Aghajanpoor Mir S., Rahmani Z., Haghshenas M., Azizi S. Preterm delivery in pregnant woman with critical COVID‐19 pneumonia and vertical transmission. Prenat Diagn. 2020 doi: 10.1002/pd.5713. 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vibert F., Kretz M., Thuet V., Barthel F., De Marcillac F., Deruelle P. Prone positioning and high-flow oxygen improved respiratory function in a 25-week pregnant woman with COVID-19. Eur J Obstet Gynecol Reprod Biol. 2020;250:257–258. doi: 10.1016/j.ejogrb.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sichitiu J., Fakhouri F., Desseauve D. Antenatal corticosteroid therapy and COVID-19: pathophysiological considerations. Acta Obstet Gynecol Scand. 2020;99:252. doi: 10.1111/aogs.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M. Outcome of Coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blitz M.J., Grünebaum A., Tekbali A., Bornstein E., Rochelson B., Nimaroff M. Intensive care unit admissions for pregnant and non-pregnant women with COVID-19. Am J Obstet Gynecol. 2020;223:290–291. doi: 10.1016/j.ajog.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Collin J., Bystrom E., Carnahan A., Ahrne M. Pregnant and postpartum women with SARS-CoV-2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99:819–822. doi: 10.1111/aogs.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amorim M.M.R., Soligo Takemoto M.L., Fonseca E.B. Maternal Deaths with Covid19: a different outcome from mid to low resource countries? Am J Obstet Gynecol. 2020;223:298–299. doi: 10.1016/j.ajog.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 96.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanna N., Hanna M., Sharma S. Is pregnancy an immunological contributor to severe or controlled COVID‐19 disease? Am J Reprod Immunol. 2020;84:e13317. doi: 10.1111/aji.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mosby L.G., Rasmussen S.A., Jamieson D.J. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205:10–18. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 99.Beck S., Wojdyla D., Say L., Betran A.P., Merialdi M., Requejo J.H. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sisman J., Jaleel M.A., Moreno W., Rajaram V., Collins R.R., Savani R.C. Intrauterine transmission of SARS-COV-2 infection in a preterm infant. Pediatr Infect Dis J. 2020;39:e265–e267. doi: 10.1097/INF.0000000000002815. [DOI] [PubMed] [Google Scholar]
- 101.Siberry G.K., Reddy U.M., Mofenson L.M. SARS-COV-2 maternal–child transmission: can it occur before delivery and how do we prove it? Pediatr Infect Dis J. 2020;39:e263–e264. doi: 10.1097/INF.0000000000002820. [DOI] [PubMed] [Google Scholar]
- 102.Shek C.C., Ng P.C., Fung G.P., Cheng F.W., Chan P.K., Peiris M.J. Infants born to mothers with severe acute respiratory syndrome. Pediatrics. 2003;112:e254. doi: 10.1542/peds.112.4.e254. [DOI] [PubMed] [Google Scholar]
- 103.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. J Am Med Assoc. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang W., Du R.H., Li B., Zheng X.S., Yang X.L., Hu B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microb Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valdes G., Neves L.A., Anton L., Corthorn J., Chacon C., Germain A.M. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 106.Algarroba G.N., Rekawek P., Vahanian S.A., Khullar P., Palaia T., Peltier M.R. Visualization of SARS-CoV-2 virus invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hosier H., Farhadian S., Morotti R., Deshmukh U., Lu-Culligan A., Campbell K. SARS-CoV-2 infection of the placenta. MedRxiv. 2020 doi: 10.1101/2020.04.30.20083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kimberlin D.W., Stagno S. Can SARS-CoV-2 infection Be acquired in utero?: more definitive evidence is needed. J Am Med Assoc. 2020;323:1788–1789. doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- 109.Panchaud A., Favre G., Pomar L., Vouga M., Aebi-Popp K., Baud D. An international registry for emergent pathogens and pregnancy. Lancet. 2020;395:1483–1484. doi: 10.1016/S0140-6736(20)30981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of reporting biases
1a. Funnel plot of comparison between pregnant women with COVID-19 and those without COVID-19. Outcome: Premature delivery. 1.b. Funnel plot of comparison between pregnant and non-pregnant women with COVID-19. Outcome: Cough.