Abstract
Heart and brain development occur simultaneously during the embryogenesis, and both organ development and injuries are interconnected. Early neuronal and cardiac injury share mutual cellular events, such as angiogenesis and plasticity that could either delay disease progression or, in the long run, result in detrimental health effects. For this reason, the common mechanisms provide a new and previously undervalued window of opportunity for intervention.
Because angiogenesis, cardiogenesis and neurogenesis are essential for the development and regeneration of the heart and brain, we discuss therein the role of prokineticin as an angiogenic neuropeptide in heart-brain development and injuries. We focus on the role of prokineticin signaling and the effect of drugs targeting prokineticin receptors in neuroprotection and cardioprotection, with a special emphasis on heart failure, neurodegenerative Parkinson’s disease and ischemic heart and brain injuries. Indeed, prokineticin triggers common pro-survival signaling pathway in heart and brain.
Our review aims at stimulating researchers and clinicians in neurocardiology to focus on the role of prokineticin signaling in the reciprocal interaction between heart and brain. We hope to facilitate the discovery of new treatment strategies, acting in both heart and brain degenerative diseases.
Keywords: heart, brain, regeneration, prokineticin, neuropeptide, GPCR, heart failure, Parkinson, angiogenesis
Graphical Abstract
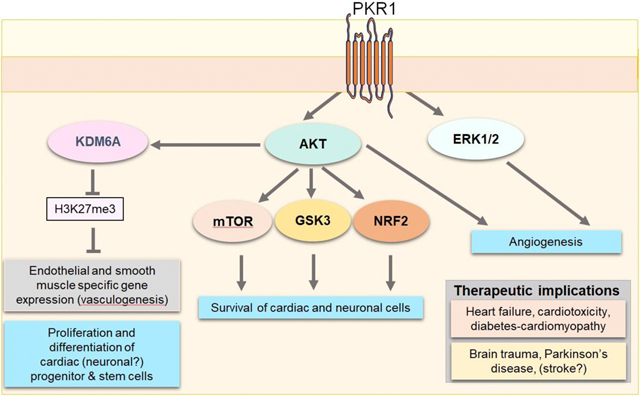
Prokineticin (PROK) cardio and neuroprotective signaling.
PROK2 via PKR1 receptor promotes AKT phosphorylation that demethylase the H3K27 via KDM6A, thereby unlocking endothelial and SMC gene expression in cardiac progenitor cells. PROK2 activates pro-survival pathways AKT and ERK1/2 in both heart and brain, regulates mTOR and GSK3 pathways and translocates NRF2 to reduce oxidative stress in the heart. PROK2 via PKR1 promotes angiogenesis via AKT/ERK kinase activation. Targeting PKR1 can protect against heart damages induced by myocardial infarction, anticancer drugs-mediated cardiotoxicity, diabetes-mediated cardiomyopathy, and brain damages induced by traumatic brain injury, probably stroke and Parkinson’s disease
1. Heart and brain development and injury
Heart and brain development occur concurrently, and error in cardiac development affect neuronal development during the embryogenesis. Moreover, congenital heart diseases are the most common birth defect often associated with impaired neuro developmental disorders [1]. Cardiac abnormalities can also alter the circulation, leading to flow disturbances affecting brain development. Cardiovascular and neurological diseases are also interconnected. For example, stress-related pathology and depression are associated with myocardial infarction and heart failure and vice versa [2].
Environmental risk factors such as psychosocial stress and diet-induced obesity can also induce cardiovascular and cerebral remodeling that result in the systolic and diastolic dysfunction and behavioral abnormalities (e.g., extreme anxiety and a diminished cognitive capacity) [3]. Moreover, ageing also triggers the remodeling of the heart and brain leading to degenerative diseases. A molecular mechanism is shared between angiogenesis, cardiogenesis and neurogenesis can be essential for functional recovery from development defects as well as heart and brain injuries.
Thus, identification of the common mediators of heart and brain development and damage will help to better understand the occurrences of these co-pathologies and develop new strategies to improve patient managements.
1.1. Cardiogenesis during development and injury
The first developing organ in the embryo is the heart that consists of three layers: the myocardium, the endocardium and the epicardium. Heart development is comprised of several rounds of epithelial-to-mesenchymal transition (EMT) and mesenchymal-to-epithelial transition (MET). The epicardium is not yet present at this tubular heart stage and originates from the proepicardium (PE). PE undergoes EMT and MET to form a type of epithelial layer called epicardium, and remains an intact layer [4]. Some of the epithelial cells will endure a second round of EMT and generate epicardium derived progenitor cells (EPDCs) that play a key role for proper myocardial development. EPDCs and epicardium play an important role on the transition of avascular hearts to heart with coronary circulatory system [5]. Indeed, most of the congenital heart diseases are resulted from errors in this EMT processes.
However, in the adult heart after myocardial infarction, EMT processes may exert deleterious effects on the heart and circulation [6]. EPDCs can differentiate into several cardiovascular cells in the mature heart [7]. Indeed, EPDCs also produces growth factors and cytokines that support myocardial growth and survival after myocardial infarction [8]. Myocardial regeneration requires the formation of the new vasculature that is essential for the survival of the cardiac tissue and to adapt to the changing environment after traumatic damage.
Therefore, activation of survival signaling in cardiomyocytes to protect the remaining cardiomyocytes, and activation of resident progenitor (e.g., EPDCs) to increase angiogenesis in failing hearts are crucial therapeutic approaches for treatment of heart failure [9].
1.2. Neurogenesis during development and injury
Neurogenesis mainly occurs during embryonic to early postnatal stages. However, in the adult mammalian brain, neurogenesis occurs in two neural niches in the hippocampus, more specifically in the subgranular layer (SGL) of the dentate gyrus (DG), and in the olfactory bulb (OB) throughout life and during the remodeling as a recovery process after stroke [10].
In the developing olfactory system, OB interneuron precursors are generated in the lateral ganglionic eminences, subventricular zone (SVZ), and septum of the embryonic forebrain, and migrate rostrally to the OB through a migration pathway known as the rostral migratory stream (RMS) [11]. The location of heterogeneous progenitor/stem cells has been shown within the adult SVZ to generate the types of OB interneurons [12, 13]. Indeed, after traumatic brain injury, the cells migrate from the SVZ via RMS to the injured cortex. The primary precursors in the SVZ have the characteristics of astrocytes and express glial fibrillary acidic protein (GFAP) [14]. GFAP+ cells can maintain a neurogenic potential and act as neural stem cells (NSCs) [15]. These cells can generate new neurons under normal conditions or after chemical removal.
Indeed, acute stroke induces neuronal regeneration and migration from a GFAP+ progenitor/stem cells [16] of the SVZ into a unique “neurovascular niche” in the peri-infarct cortex [17]. During functional recovery from brain injury, both angiogenesis and neurogenesis occur in this niche, indicating these events may provoke each other. In accord with this finding, systematic delivery of pro-angiogenic factors such as fibroblast growth factor 2 [18], stromal-derived factor-1 [19], and angiopoietin-1 [20] have been shown to enhance neurogenesis as well as angiogenesis in the peri-infarct cortex during functional recovery from brain injury [21]. Here we focus on prokineticin an angiogenic neuropeptide in cardiogenesis, angiogenesis and neurogenesis, during heart and brain development and injury.
2. Prokineticins (PROKs) and their receptors
Prokineticins (PROK) are considered as neuropeptides [22] or angiogenic hormones [23, 24]. They are comprised of 44% identical two groups: prokineticin-1 (PROK1, PK1; also called EG-VEGF,) and prokineticin-2 (PROK2, PK2; also called Bv8) with an N-terminal AVITGA sequence and ten cysteines forming five disulfide bridges [25]. The N-terminus is necessary for agonistic effect, and mutation in this region leads to antagonistic effect, whereas C-terminus is essential for its biological activity [26].
The PROK1 gene is located on human chromosomal regions of 1p13.1 and mouse chromosomal regions of 3. It does not have any splicing product. The PROK2 gene maps to regions of human chromosome 3p21 and mouse chromosome 6. Indeed, PROK2 has two splicing isoforms: a small form of PROK2 is composed of 3 exons, exon 1, 2, and 4. The long-form of PROK2 (PROK2L) has an additional 21 amino acids, composed of all four exons [27]. The PROK1 protein is composed of 86 amino acids, while its precursor has 105 amino acids. The PROK2 is composed of 81 amino acids, and the long-form PROK2L has 102 amino acids. The secreted PROK2L peptide can be cleaved by proteases to form PROK2β peptide with 47 amino acids that binds explicitly to prokineticin type 1 receptor [26].
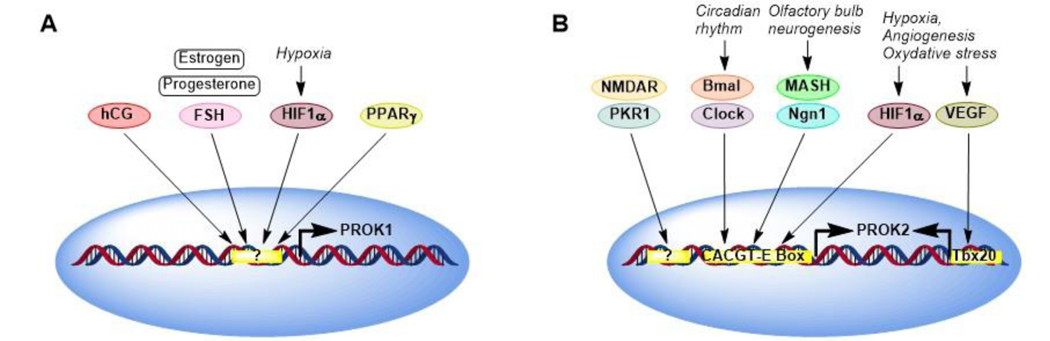
PROK1 and PROK2 are ubiquitously expressed in many tissues and regulated by different stimuli [28]. Expressions of PROK1 can be positively regulated by estrogen, progesterone, follicle stimulating hormone (FSH) and human chorionic gonadotropin (hCG), as well as hypoxia-inducible factor-1α (HIF-1α), peroxisome proliferator-activated receptor gamma (PPARγ )[29], and insulin [30]. Homeobox transcriptional factors (distal-less homeobox 1 and 2) can repress expression of PROK1. Expression of PROK2 is positively regulated by circadian rhythm genes, Bmal, and clock, olfactory neurogenesis basic helix-loop-helix factors, neurogenin1 (Ngn1) and Mash, [31], hypoxia and oxidative stress via CACGTG-E box elements on PROK2 promoters, [32] and angiogenesis via VEGF/Tbmx20 pathway [33] (Fig. 1). Granulocyte colony stimulating factor in human monocytes, lymphocytes and neurons also regulates PROK2 [34]. Both PROKs exert their biological activity by interacting with two highly related G protein-coupled receptors (GPCRs), namely prokineticin receptor 1 (PROKR1, PKR1) and 2 (PROKR2, PKR1) [35]. The PKRs are 85% identical in their amino acid sequences and consist of an extracellular N-terminus, seven transmembrane helices, three intracellular loops, three extracellular loops, and cytoplasmic C-terminal tails. PROK1 and PROK2 bind and activate both PRKR1 (393 amino acids) and PROKR2 (384 amino acids). However, PROK2 has a higher affinity to PKR1 than PKR2 [36].
Figure 1. Regulation of expression of PROK1 (A) and PROK2 (B).
Expressions of PROK1 can be positively regulated by estrogen, progesterone, follicle stimulating hormone (FSH) and human chorionic gonadotropin (hCG), as well as hypoxia-inducible factor-1α (HIF-1α), peroxisome proliferator-activated receptor gamma (PPARγ), and insulin. Homeobox transcriptional factors (distal-less homeobox 1 and 2) can repress expression of PROK1. Expression of PROK2 is positively regulated by circadian rhythm genes (Bmal and clock), olfactory neurogenesis proneuronal basic helix-loop-helix factors (neurogenin1 (Ngn1), hypoxia and oxidative stress via CACGTG-E box elements on PROK2 promoters, and angiogenesis via vascular endothelial growth factor (VEGF)/Tbmx20, a T-box gene, pathway. Granulocyte colony stimulating factor in human monocytes, lymphocytes and neurons also regulates PROK2. PKR1 or NMDR also regulates expression of PROK2.
PROKs and their receptors (PKRs) are expressed in all mammalian tissues. Although PKR1 and PRKR2 are co-expressed in specific tissues [37–39], PKR1 is predominantly expressed in peripheral tissues, including the reproductive organs [40, 41], endocrine glands [25], the gastrointestinal tract [42, 43], spleen, pancreas, lungs, heart [44], and blood cells [45, 46]. On the other hand, PKR2 is mainly expressed in the central nervous system (CNS), including olfactory regions, cortex, thalamus and hypothalamus, septum and hippocampus, habenula, amygdala, nucleus tractus solitarius, and circumventricular organs such as subfornical organ (SFO), median eminence, area postrema, mammillary nuclei, periaqueductal gray (PAG), and dorsal raphe [47–51]. Because the pattern of PKR2 expression in the amygdala has a sexual dimorphism [52], PKR2 may have roles in a sexually dimorphic manner potentially on growth and development of CNS, the reproductive function and the motivated behaviors needs to be investigated. In contrast, PKR1 was found in fewer brain regions, such as the olfactory regions, dentate gyrus, zona incerta, and dorsal motor vagal nucleus [53].
2.1. PROK receptor (PKR) ligands and signaling
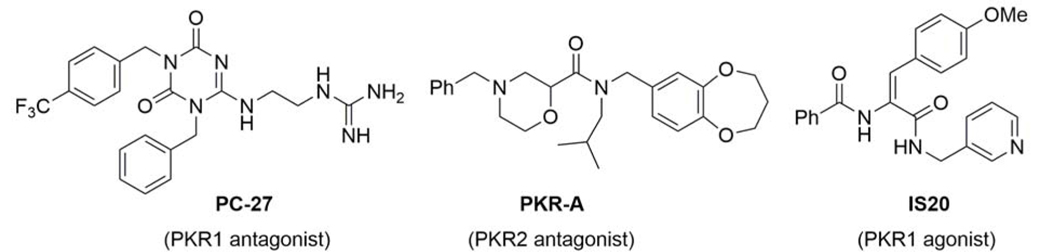
Several small molecules that modulate PKR1 and PKR2 have been identified (Fig. 2). At the current only two types of PKR antagonists have been reported: some triazinediones, such as PC-27, and PKR-A that are respectively selective for PKR1 and PKR2 [54, 55]. PC-27 was found to alleviate the Bv8-induced thermal hyperalgesia, and PKR-A to reduce infarct volume and central inflammation, while improving functional outcome in a mouse model of cerebral ischemic injury [56].
Figure 2.
Structure of small molecules that regulate PKR1 and PKR2 signaling
Using computational approaches and in vitro assays for drug screening, followed by validation of hits in ex vivo neonatal rat cardiomyocyte culture, a specific non-peptide PKR1 agonist (IS20) has been identified [57, 58]. Administration of IS20 in mice does not display any signs of toxicity.
PKR signaling includes the accumulation of inositol phosphate and mobilization of intracellular Ca2+, stimulation, or inhibition of cAMP [59], stimulation of mitogen-activated protein kinases (ERK), phospholipase Cβ and protein kinase B also called AKT [39]. PROK signaling has been implicated in several essential physiological functions, including gastrointestinal smooth muscle contraction, circadian rhythm regulation, neurogenesis, angiogenesis, pain perception, mood regulation, and reproduction [60]. PROK signaling is involved in several pathophysiologic events, including obesity, diabetes [61, 62], and heart failure [44]. Here, we discuss only neuroprotective and cardioprotective effects of the PROK system. Role of PROK axes in cardiac and neuronal cell functions is summarized in table 1.
Table 1.
Role of PROK in cardiac and neuronal cell functions
| Role in cells | Cell type | Function |
|---|---|---|
| Cell survival | Endothelial cells | Survival, proliferation, insulin uptake[38, 39, 58, 62, 72, 123] |
| Neuronal cells | Survival of GnRH neurons in the hypothalamus, activation of progenitor cells [76], dopaminergic neurons [110]. | |
| Haematopoietic cells | Survival [46] | |
| Cardiomyocytes | Antioxidant, antiapoptotic [38, 69, 96, 123, 124] | |
| Cell motility | Angiogenesis | Akt/ERK activation, vascular structure stability [33, 37, 39, 73] |
| Neurogenesis | Migration of GnRH neurons [74] and directed chemotaxis of astrocytes via PKR1 [117] | |
| Neural crest cells | Differentiation and proliferation [86, 87] | |
| Cardiogenesis | Asymmetric division, activation of EMT, cellular communications | |
| Cell differentiation | EPDCs and tcf+fibroblast progenitors | Epigenetic control of EPDC cell fate, differentiation into endothelial and smooth muscle cells [65] |
| Hematopoetic cells | Increase in total leukocyte, neutrophil, and monocyte counts [46] | |
| Preadipocytes, adipocyte precursors | Inhibition of adipogenesis [97] | |
| EPDCs and tcf+fibroblast progenitors | Inhibition of adipogenesis, and epicardial adipose tissue development after calorie overload [124] | |
| Cell excitability | Glutamate uptake in astrocytes | Promotion of glutamate uptake by upregulating GLAST [117] |
| Pain sensitization | Pain sensitization [28] | |
| Circadian rhythm | Regulation of sleep and circadian circle [22, 31, 47, 125] | |
| GABA-induced currents in suprachiasmatic nucleus (SCN) | Promotion of spontaneous firing rates in most the neurons from the dorsal SCN [49] |
3. Role of the PROK signaling in cardiac development and cardiac cell functions
PKR1 and PKR2 both are expressed in cardiovascular tissues, including cardiomyocytes, epicardial progenitor cells, endothelial cells, and fibroblasts [63]. PKR1 appears to be involved in heart development.
3.1. PKR1 signaling promotes EMT during heart development
Overexpression or activation of PKR1 by PROK2 in isolated embryonic EPDCs promotes EMT [64]. Genetic ablation of PKR1 in epicardium using cre-loxP technology (epicardial specific Wt1 and gata4 transgenic mice intercrossed by PKR1 loxP mice) induces abnormal heart development, leading to partial embryonic and postnatal lethality. These epicardial restricted PKR1 knockout mice in the adult stage displayed ischemic dilated cardiomyopathy and impaired cardiac functions. These genetic models of congenital cardiac dysfunction provide evidence that epicardial-PKR1 signaling is a key player in the coronary vascular network formation, and EPDC-cardiomyocytes interaction, regulating cardiomyocyte proliferation and rhythmicity.
Interestingly, hEPDCs derived from atrial appendage in culture spontaneously undergo EMT. They are not predetermined to differentiate into the vascular lineages. However, a new epigenetic pathway of PKR1 involves histone modifications by a demethylase (KDM6A) to overcome the intrinsic limitations of hEPDCs [65]. Thus, PROK2/PKR1 signaling inhibits EMT through activating a histone demethylase KDM6A and induces asymmetric division. This event promotes self-renewal and formation of vascular and epithelial/endothelial precursors. These precursors have angiogenic potential and are capable of differentiating into vascular smooth muscle and endothelial cells.
On the other hand, PROK2 via KDM6A inactivates histone H3K27me3-mediated repressive marks on the promoters of vascular genes that regulate the vascular lineage commitment and maturation. PROK2 via KDM6A stabilizes and accumulates β-catenin in the cytoplasm to repress peroxisome proliferator-activated receptor-γ (PPARγ) expression and activity, thereby inhibiting adipogenic signaling [65]. Thus, PKR1 is a promising target to manipulate hEPDCs-mediated cardiac repair/regeneration events in cardiovascular and metabolic diseases.
3.2. PKR1 controls fate of adult cardiac tcf21+ fibroblast progenitor cells originated from epicardium
Although cardiac fibroblasts and progenitors (CFPs) are derived from both epicardium and endothelium during embryogenesis, the majority of CFPs express the basic helix-loop-helix (bHLH) transcription factors tcf21, Wt1, and Tbx18 in the epicardium of developing heart [66]. Tcf21 continues to be expressed in the resting adult CPFs. Tcf+CFPs can be found in the epicardium, the perivascular and interstitial areas, depending on the injury type [67] and may be involved in the reparation of the heart. Indeed, a recent study has shown that adipogenesis and vasculogenesis share a common epicardial derived tcf21+CFPs, and PROK2 regulates a switch that controls CFP-vasculogenic- and CFP-adipocyte-transformation in the adult heart [68]. Under a high fat diet exposure, CFP-restricted inducible knockout mice (PKR1tcf−/−) exhibited a high level of fat deposition in the atrioventricular groove, perivascular area, and pericardium of adult mice. These mice had an impaired vascular network and cardiac dysfunction. Thus, targeting PKR1 could provide a novel therapeutic approach for the treatment of metabolic, cardiac diseases, and atherosclerosis.
3.3. PROK signaling in cardiomyocytes
Activation or overexpression of PKR1 in cardiomyocytes promotes survival pathway via AKT to protect cells against hypoxic insult [38, 69]. Transgenic mice overexpressing PKR1 in cardiomyocytes (TG-PKR1) displayed no spontaneous structural and functional abnormalities in the cardiomyocytes [70]. However, these mice showed an increased number of EPDCs, with an increase of capillary density and coronary arterioles. Indeed, cardiaomyocyte-PKR1 signaling upregulates its own ligand expression PROK2 that acts as a cardiocrine factor and promotes EPDC differentiation into endothelial and smooth muscle cells to generate new vessels. On the other hand, activation or overexpression of PKR2 in cardiomyocytes induces hypertrophy [71].
Transgenic mice overexpressing PKR2 in the cardiomyocytes (TG-PKR2) displayed eccentric hypertrophy and impaired endothelial integrity in a paracrine regulation. Thus, two PROK receptors have divergent roles in the cardiomyocytes [71].
3.4. PROK signaling in endothelial cells
Activation or overexpression of PKR1 in coronary ECs promotes angiogenesis [39] as a consequence of the stimulation of EC proliferation, migration and branching [72]. Gα11 appears to regulate the activity of MAPK and AKT in angiogenic signaling of PKR1. The role of PKR1 in EC function in vivo has been demonstrated in endothelial-PKR1-deficient (PKR1ec−/−) mice [62] using Tie2-cre mice. These mice displayed severe lipid deposition and macrophage infiltration in their intima, leading to aortic aneurysms and impaired endothelium-dependent relaxation. Their blood pressure remained unaltered, probably due to altered cardiac output. More specifically, these mice displayed capillary rarefaction, apoptosis in their cardiomyocytes, interstitial fibrosis, leading to impaired diastolic function. Interestingly, these mice had abnormal insulin signaling in their hearts, leading to ectopic lipid deposition [62].
PKR2 signaling, unlike that of PKR1, does not induce angiogenesis [39, 73]. Activation or overexpression of PKR2 in ECs results in a fenestrated EC phenotype. PKR2 signaling causes loss of Zonula occludens protein-1 (ZO-1), a cell-cell adhesion molecule, at tight junctions, thereby inducing an abnormal organization of ECs. PKR1 and PKR2 can co-express in the cardiac capillary endothelial cells. PKR1 expression levels is dominant in the physiological condition, whereas PKR2 becomes dominant with the pathological stimuli [72]. Thus, the role of PKR2-specific antagonists on endothelial cell function remains to be investigated.
4. Role of PROK signaling in neuronal development and neuronal cell functions
4.1. Role of PROKs in neurogenesis
Indeed, PKR2 expression has been found in the SVZ, RMS, and the ependymal and subependymal layers of the olfactory ventricle (OV) [74] in the areas active in neurogenesis during adulthood. PROK2 acts as a chemoattractant for SVZ-derived neuronal progenitors. PROK2 is expressed in the granular and periglomerular layers of the OB. A recent study utilizing PROK2EGFP transgenic and PKR2LacZ/+ knock-in mice has demonstrated the presence of PROK2+ cells in the medial part of the RMS [75]. Moreover, in the OB, PROK2 is expressed in a subset of granule cells and tufted cells. Although co-expression of PROK2 and PKR2 in the OB cells were not found, PROK2 null mice (PROK2−/−) have reduced OB, along with the accumulation of neuronal progenitors in the RMS [76]. In accord with this data, PKR2 null mice (PKR2−/−) had hypoplasia and abnormal architecture of the OB [77] and no Gonadotropin-releasing hormone (GnRH) neurons was found in their hypothalamus. These findings indicate that PKR2 system may also be involved in the migration and survival of GnRH neurons in the hypothalamus [76]. Similarly, knockdown and knockout of PKR2 in zebrafish leads to abnormal development of GnRH3 fibers that was rescued by injection of PKR2 mRNA [78]. These phenotypes are similar to the clinical features of Kallmann syndrome [79], a human disease characterized by the association of hypogonadotropic hypogonadism and anosmia. Importantly, several PROK2 and PKR2 mutations are found in Kallmann syndrome [80].
Indeed, development and maturation of OB interneurons depend on the zinc finger homeodomain factor teashirt zinc finger family member 1 (TSHZ1) that binds and regulates expression of PKR2 [81]. Conditional deletion of Tshz1 in mice resulted in OB hypoplasia and severe olfactory deficits, similar to PKR2 deficient mice. The loss of intercalated cells (ITCs) in the mouse amygdala of Tshz1 mutant mice is associated with fear, depression, and social interaction phenotypes. The PKR2 gene was associated with not only a major depressive disorder [82] but also methamphetamine dependence [83]. The human patients with congenital aural atresia that were heterozygous for TSHZ1 loss-of-function mutations displayed hyposmia characterized by impaired odor discrimination and reduced olfactory sensitivity via negatively controlling the expression of PKR2 [81]. Indeed, PKR2 signaling modulates the electrical activity of neurons in the area postrema, subfornical organ, and paraventricular nucleus of the hypothalamus [84] and PROK2 suppresses GABA-activated current in sensory neurons[85].
Recently, it has been shown that severe tangential and radial migration defects of neuroblasts in the SVZ-RMS-OB result in loss of ~75% of GABAergic interneurons in the OB of PROK2 and PKR2 mutant mice. These interneurons are one of the few neuronal subpopulations that are continuously renewed throughout life from progenitors located in the adult SVZ [75]. These analyses demonstrate that PROK2/PROKR2 signaling is a key regulator of survival and migration of GnRH neurons and is crucial for the tangential and radial migration of OB interneurons rather than affecting OB neurogenesis [74].
4.2. Role of PROK signaling in enteric neural crest cells
PROK signaling cross-talks with glial cell line-derived neurotrophic factor (GDNF)/RET receptor tyrosine kinase (Ret) signaling in enteric neural crest cells (NCC) [86, 87]. These cells are multipotent progenitors, which give rise to neurons and glia of the enteric nervous system (ENS) during fetal development. Their survival, migration and differentiation are regulated by mainly (GDNF)/RET signaling. GDNF up-regulates PKR1 in enteric NCCs [88].
Furthermore, c-Ret deficient mice display a lower level of PKR1 in the enteric ganglions. Subsequently, functional analysis showed that GDNF potentiates the proliferative and differentiation effects of PROK1 via PKR1 in enteric NCCs. Moreover, PROK1 and GDNF signaling share some common downstream targets. Interestingly, in the c-Ret deficient NCCs, PROK1 signaling could induce the expression of both proliferation and differentiation markers, suggesting that the PROK1/PRKR1 pathway can be a compensatory/complementary pathway to GDNF signaling [89]. Indeed, PROK2 can compensate the loss of signaling from the GDNF/RET complex in Hirschsprung’s disease by controlling the differentiation and proliferation of neural crest cells [88]. These findings demonstrate that PROK1 cross-talks with GDNF/Ret signaling. PROK1 signaling also provides a compensatory mechanism to maintain proliferation and differentiation of enteric NCCs.
5. Targeting PROK receptors as therapeutic options in heart and brain injuries
5.1. PKR1 signaling protects heart against myocardial infarct-induced heart failure
Increased levels of PROK2 and their receptors were found after myocardial injury in mice model of heart failure [90]. However, the signaling pathways mediated by PKR1 and PKR2 act in diverse directions in the postnatal heart [44]. PROK system is associated with the pathologies of various cardiovascular and metabolic diseases, including heart failure [38], and abdominal aortic aneurysm [91].
Myocardial infarction and anticancer drugs [92] in humans cause cardiac injury, resulting in loss of myocardial cells, formation of fibrotic scar tissue, damaged progenitor cell plasticity and defective vessel networks. PKR1 gene transfer after coronary ligation in a mouse model of myocardial infarction (MI) increased the survival rate and improved the heart functions by promoting cardiac angiogenesis, cardiomyocyte survival, and the proliferation of EPDCs [70, 93]. The intraperitoneal administration of IS20, a PKR1 agonist, activated AKT survival pathway in the heart. Furthermore, IS20 decreased mortality and the size of the scar area, and improved heart function, similar to the effects of PKR1 gene transfer in mice with MI. Indeed, IS20 reduced apoptosis in cardiac cells, provoked EPDC proliferation and increased capillary formation [58].
5.2. PKR1 signaling inhibits dose and time-dependent doxorubicin-induced cardiovascular toxicity via myocardial and vascular protection
Heart failure occurrence during and following cancer treatments remains a subject of intense research and therapeutic interest. Doxorubicin (DOX) is one of the widely used anthracycline family of anticancer drugs and has a severe adverse effect on cardiovascular system [92]. Recently, Gasser and her colleagues have been shown that a low concentration of DOX (0.1 μM) inhibits angiogenesis and EPDC plasticity [94]. DOX at 1 μM induces apoptosis in all cardiac cells, including cardiomyocytes, endothelial cells and EPDCs. However, a high concentration (10–15 μM) of DOX promotes ROS accumulation in the cardiomyocytes. A PKR1 agonist, IS20, alleviates these effects of DOX in isolated cardiac cells. This effect of IS20 is due to the activation of PKR1 because genetic or pharmacological inactivation of PKR1 subdues these effects of IS20 in cardiac cells [94].
In a chronic mouse model of DOX-cardiotoxicity, IS20 has been shown to normalize an elevated serum marker of cardiotoxicity and vascular and EPDC deficits, attenuates apoptosis and fibrosis, and improves survival rate and cardiac function. Indeed, IS20 does not interfere with the cytotoxicity of DOX in breast cancer lines and in a mouse model of breast cancer. In addition, IS20 improves the decreases in LV diastolic volume induced by acute DOX treatments in mice [94]. This study identifies PKR1 as a promising target to protect the heart from the cardiac adverse effect of cancer treatments.
5.3. PROK/PKR1 pathway in diabetes-induced cardiomyopathy
Diabetic cardiomyopathy is the diabetes-associated cardiac complications manifested by structural, functional and metabolic changes, which often leads to heart failure. Recently studies in vitro and in vivo have shown that metformin, a widely used drug for the treatment of type 2 diabetes exerts a cardioprotective effect via PROK2/PKR1 signaling [95]. Reduced expression levels of PROK2, PKR1, and PKR2 and low p-AKT/AKT and p-GSK3β/GSK3β ratios have been found in diabetic mice. These anomalies were alleviated by Metformin treatment. Metformin or PROK2 [96] have cardioprotective effect against the high glucose-mediated injury in cardiomyocytes that was blocked by a PKR1 antagonist (PC7) or an AKT inhibitor [95]. This finding is in accord with a previous study showing that PROK2 and PKR1 inhibit the development of diabetes and obesity [97] and related cardiovascular disorders. Whether PKR1 agonists protect heart against diabetes-associated cardiac remodeling remain to be studied.
5.4. PROK/PKR1 pathway links obesity and cardiovascular diseases
The reciprocal interaction in the heart-brain axis can play key role in metabolic diseases such as obesity. Prokineticin-2 is an anorexic peptide that intracranial injection of PK2 in rats reduces food intake [98]. Contrarily, a neutralizing antibody for prokineticin-2 increases the food intake. Anorexic effect of PK2 was completely absent in the PKR1 deficient mice, indicating that the anorexic effects of PK2 are mediated by PKR1 in the hypothalamus. Interestingly, both PKR1 null mice and adipose tissue-restricted PKR1 knockout mice exhibit obesity. The peripheral effect of PKR1 signaling is due to suppression of proliferation and differentiation of preadipocytes into adipocytes[97]. Indeed, peripheral administration of PK2 act as an adipokine reduces food intake and body weight in diet-induced obesity models [99]. The polymorphism of PROK2 gene and the metabolic diseases (obesity and diabetes) have been correlated in human [100].
Therapeutic strategies targeting PKR1 could be important to treat obesity and obesity-associated insulin resistance and cardiovascular complications [101], because PKR1 signaling suppresses appetite, reduces adipocyte expansion, regulates visceral fat storage, and increases cardiovascular insulin sensitivity [102]. Thus improving the brain-heart axis function by PKR1 signaling can recover the risk of cerebral-cardiac disorders in obese individuals.
5.5. Controversy functions of PROK2 in ischemic models of cerebral injury
In a murine model of stroke (occlusion of middle cerebral artery), PROK2 expression was increased in the ischemic cortex and striatum. Intracranial administration of PROK2 (10 pmol) after the stroke increased the infarct volume and CD68+ inflammatory cells in mice brain [103]. However, the administration of a PROK2 receptor antagonist reduced infarct volume and central inflammation.
On the contrary, at higher concentrations (10–100nM), PROK2 exerts a protective role in an in vitro cerebral ischemia model, using oxygen-glucose deprivation (OGD) in the primary murine cortical cell cultures [104]. Neuroprotective effect of PROK2 is mediated by an activation of ERK1 and AKT survival pathway. When the ischemic tolerance had been experimentally induced by exposing hippocampal slices to subtoxic pharmacological stimulus NMDA, before the exposure to OGD, the development of OGD tolerance was associated with an increase in the expression of PROK2, PKR1 and PKR2 mRNA and proteins [104]. PROK2 at nM concentration attenuated the injury that was reversed by the PKR antagonist PC7. This study also showed that the PROK system is up-regulated during the cerebral injury [104]. PROK2 activates the ERK1/2 and AKT in the neuroprotective pathway.
These discrepancies have been explained by several mechanisms: A) At low doses PROK2 may use stress activated protein kinase (SAPK)/JNK, transducers of death signals, to induce the neuronal damage [103, 105], however, at the high doses PROK2 uses ERK/AKT survival pathway to prevent from cerebral injury [104, 106] as observed with nM scale of PROK2 for cardioprotection [44]. B) An altered balance of the expression levels PKR2 versus PKR1 may be detrimental during the stroke as observed in the heart [44]. The involvement PKR1 and PKR2 in ischemic stroke events and the mechanisms still needs to be fully elucidated. C) Stroke levels or type of the brain injury models could be also important controlling the expression of PROK2 and its receptors PKR1 and PK2 (Fig. 3) and off-target effects of PK antagonist needs to be clarified.
Figure 3. The role of PROK2 via its receptors PKR1 and PKR2 in brain and heart injuries.
Overall PROK2 (nM concentration) promotes a survival pathway to protect the neuron and cardiac cells against injury. However low dose (pM) of PROK2 may induces proapoptotic signaling that is detrimental for neuronal injury. Whether these contrary roles of PROK2 in CNS are depending on the different concentration, injury type or receptors have not been clarified yet. PKR1 signaling is beneficial, whereas PKR2 signaling detrimental in heart injuries.
5.6. Neuroprotective role of PROK2 in the model of traumatic brain Injury
In a traumatic brain injury (TBI) mice model by physical trauma of the cortex, the migration of SVT cells to the olfactory bulb was not impaired. Indeed, mobilization of proliferating cells from the SVZ neurogenic niche to injured cortex and lack of alteration in the RMS migration depends on the extent and type of the injury. The high expression of PROK2 was found only after the traumatic cortical injury in the mice cortical microglia of the cortex, but not in the GFAP positive astrocytes, doublecortin (DCX) positive immature neurons and CD45 positive leucocytes [107]. Note that the intact brain does not express PROK2. However, high levels of PROK have been found in the astrocytes and neurons in the glutamate- [105] and amyloid-beta-induced toxicity models, where a PKR antagonist has beneficial effects [108, 109].
Contrarily, administration of a PKR antagonist is detrimental in TBI model, because it inhibits the SVT cell migration [107]. When the neurospheres [107] or SVZ explants [74] were cocultured with the PROK2-expressing cells, the migration of cells from the neurospheres or SVZ demonstrated that PROK2 is essential in recruiting SVZ cells to injured areas.
The activation of PROK2 to promote the migration of SVZ cells to injured areas during brain injuries and neurodegenerative diseases is an important finding. However, which receptor is involved in the beneficial effects of PROK2 in the traumatic brain injury needs to be clarified (Fig. 3).
5.7. Protective role of PROK signaling in Parkinson’s disease
The nigral dopaminergic neurons are the other neuronal targets of the PROK system. Although PROK2 expression has been found at a low level in the nigral system, its receptors PROKR1 and PROKR2 are constitutively expressed on nigrostriatal neurons. PROK expression is increased in surviving nigral dopaminergic neurons of brains of patients with Parkinson’s disease [110]. Recently, an elevated PROK2 expression has also been found in nigral dopaminergic neurons during the early stages of neurodegeneration in mouse models of Parkinson’s disease such as toxin-induced PROK2 reporter mice and MitoPark transgenic mice [110]. Indeed, elevated PROK2 levels have also been found in an experimental model of Parkinson’s disease induced by the dopaminergic cell death following in vitro tumor necrosis factor α (TNFα) treatment [111].
PROK2 overexpression or PROK2 treatment protects dopaminergic neurons against oxidative stress, mitochondrial dysfunction, and neurodegeneration induced by the parkinsonian neurotoxicant (MPTP) [112]. Indeed, PROK2 activates ERK and AKT survival signaling pathways and promotes mitochondrial biogenesis, thereby exerting potent neuroprotection [110]. In accord with these findings, PROK overexpression plays a protective role, whereas antagonist of PKRs exacerbates dopaminergic degeneration in experimental Parkinson’s disease. Altogether, these data identify PROK2 as a novel player secreted from dopaminergic neurons during degeneration, acting as a compensatory neuroprotective cytokine in nigral dopaminergic neurons.
5.8. Protective effect of PROK2 in astrocytes against neurotoxicity induced damage
Astrocytes regulate the migration of postnatal neuroblasts and promote functional coordination of the brain [113]. The dysfunction of astrocytes may contribute to either neuronal death or the process of neural disturbances [114, 115]. Neuroinflammation and ischemia can elicit two different types of reactive astrocytes, termed pro-inflammatory A1s and anti-inflammatory A2s that reduces excitotoxicity and promotes long-term neuronal survival [116]. Astrocytes also express a high level of PKRs. Overexpression of PROK2 in cultured astrocytes or in mouse brain promotes the A2 type of astrocyte phenotype confirmed with the expression of A2 type markers such as Arginase-1, Nrf2, PTX3, SPHK1, and TM4SF1 [117]. PROK2 also elevates astrocyte A2 phenotype, increases mitochondrial energy metabolism and antioxidant pathway, while decreases inflammatory factors [117]. Furthermore, PROK2, through its receptor PKR1 on astrocytes, promotes directed chemotaxis.
A small-molecule PKR1 agonist, IS20, mimics the neuroprotective effect of PROK2 in primary astrocyte cultures [117]. It also increases astrocyte migration and glutamate uptake by upregulating the glutamate aspartate transporter (GLAST). PROKR1 signaling appears to favor the A2 astrocyte phenotype versus A1 in neurodegenerative diseases such as Parkinson’s disease. Notably, IS20 blocks MPTP-induced reductions in the A2 phenotype. It also mitigates the elevation of the A1 phenotype in this in vitro Parkinson’s disease model [117]. PROK2 via PKR1 regulates a novel neuron-astrocyte signaling mechanism in Parkinson’s disease. Thus, these findings could be exploited for the development of novel therapeutic strategies targeting PKR1 for Parkinson’s disease and other related chronic neurodegenerative diseases.
Although astrocytes develop stem cell properties during brain ischemic events, the multipotency of astrocytes is reliant on growth factors as observed in in vitro settings [118]. In vitro assays have shown that some of the reactive astrocytes in the traumatic or ischemic brain acquire neurosphere (NS)-forming ability, multipotency, and long-term self-renewal, while others remain within their astrocyte lineage in vivo [118]. Whether PROK/PKRs promote NS-forming ability remains unknown.
6. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Therapeutic angiogenesis is a key component to combat neurodegenerative diseases and heart failure as it plays a critical role in supplying the high metabolic demand of neuronal activity and myocardial contractility. Angiogenesis can be a compensatory mechanism triggered by ischemic injuries, associated with improved functional outcomes after ischemic stroke. In contrast, defective capillary network compromises tissue oxygen availability and is associated with neuronal loss in the animal models of heart failure and neurodegenerative disorders [119]. However, the role of angiogenesis in acute and chronic neurodegenerative disorders is not fully characterized, which may contribute to the clinical trial failures of some neuroprotective agents. Current available data supporting the role of angiogenesis in neuronal damage are mainly derived from experimental animal models. However, therapeutic angiogenesis and vasculogenesis can be a promising tool to improve the treatment of cerebral ischemia and neuronal damage in clinical settings.
Similarly, therapeutic angiogenesis and vasculogenesis are essential for the regeneration of the heart. Promoting PROK signaling is a promising approach to provide angiogenesis cardiac and neuronal protection in an autocrine/paracrine fashion in cardiac and neuronal cells. A further interrogation into the PROK signaling on functions of stem progenitor cells in CNS and cardiovascular system will be very important to further determine pro-survival role of PROK. The ability of PROK to form neurospheres or cardiospheres, promoting transformation into specific neuronal or cardiac cells remains to be investigated.
Another question concerns the efficacy neuroprotective effect of PROK signaling in multiple models of CNS injuries in different species. Altered expression of the PROK axes in the preclinical models and human neuronal and cardiovascular diseases are listed in table 2 and table 3. Whether two different PKRs involved in PROK2 mediated beneficial and detrimental effects in the stroke models needs to be clarified. PKR agonists and antagonists have been identified as powerful tools to investigate the functions of PKRs. However, additional studies will be required to characterize their off-target effects.
Table 2.
Role of PROK in preclinical models of cardiac and neuronal diseases
| System | Models | Functions |
|---|---|---|
| Cardiovascular (CV) system | ||
| Preclinical CV disease models | MI Infarction | PKR1 agonist IS20 has cardiovascular (CV) protective effect, increases capillary network Preclinical CV development, increases EPDCs number and disease models improves CV functions [58]. |
| Doxorubicin (DOX)-mediated cardiotoxicity | IS20 protects cardiomyocytes endothelial cells and EPDCs, provides vascular stability, by increasing AKt survival and antioxidant pathways and improves CV functions [123]. | |
| Breast cancer mice models | No alteration on antitumor efficacy of DOX by PKR1 agonist [123]. | |
| Global PKR1 knockout –PKR1-KO) | Dilated cardiomyopathy, kidney atrophy urine retention [63]. | |
| Obesity, diabetes [97]. | ||
| Knockout mice models | Adipose restricted PKR1-KO | Obesity [97]. |
| Endothelial restricted PKR1-KO | Lipodyslipidemia, insulin insensitivity, CV and renal disorders [62]. | |
| Epicardium restricted PKR1-KO prenatal | Embryonic lethality due to error in epicardial cardiomyocyte communication and lack of EMT | |
| Epicardial originated fibroblast progenitor restricted PKR1-KO postnatal | Development of epicardial adipose tissue (EAT) perivascular adipose tissue (PAT), loss of vascular network, impaired cardiac function, arrhythmia [64]. | |
| Epicardin positive renal progenitor restricted PKR1-KO | Abnormal glomerular structure, atrophic kidney, urine retention [93]. | |
| Overexpression of PKR1 cardiomyocytes of transgenic mice hearts | No abnormalities in cardiomyocytes, but increased vascularization and activation of adult EPDCs proliferation and differentiation into vascular cell type [70]. | |
| Transgenic overexpression mice models | Overexpression of PKR2 in cardiomyocytes of transgenic mice hearts | Hypertrophic cardiomyopathy and endotheliopathy [71] |
| CNS | ||
| Cerebral disease models | Cerebral ischemia | PROK2 at high dose plays neuroprotective role models via activating ERK/Akt signaling [104], while at low dose increases the infarct volume [103]. |
| Neurodegenerative models | Parkinson’s diseases | PROK2 via PKR1 protects dopaminergic neurons and prevents neurodegeneration [110]. PKR1 agonist, IS20 controls neuron-astrocyte A2 protective signaling, leads to an increase in antioxidant and anti-inflammatory proteins [117]. |
| OB defects, Kalmann syndrome like phenotype [80]. | ||
| Knockout mice models | PROK2 null mice | Low circadian rhythmicity [126] affecting physiological and behavioral parameters, including sleep/wake cycle [125], locomotor activity, and anxiolytic and antidepressant-like effects [127], food intake and thermoregulation [128]. |
| Partial deletion of PROK2 in the MOB promotes insulin resistance, and increases food intake in vivo [100] | ||
| PKR1 null mice | Feeding [98] [99] | |
| PKR2 deficient mice | OB developmental abnormalities [77], circadian rhythm dysregulation [129], Kalmann syndrome like phenotype, abnormal pain sensation [130]. | |
| Transgenic overexpressing models | PK2 overexpressing transgenic mice and zebrafish | Augmentation of sleep in light and suppression of sleep in dark [131] via PKR2[132] |
Table 3.
Altered expression of the prokineticin axes in neuronal and cardiovascular diseases
| Diseases | Mutated/Altered Expression |
|---|---|
| Kallmann syndrome, idiopathic hypogonadotropic hypogonadism | PKR2, PROK2 [133] |
| Hirschsprung disease | PROK1, PROK2, PKR1, PKR2 [88] |
| Neuroblastoma progression | PROK1, PROK2, PKR1, PKR2 [134] |
| Parkinson’s disease | PROK2 [111] |
| Circadian Rhythm / sleep and Mood disorders | PROK2, PKR2 [135] [82, 125] |
| Autoimmune demyelination/Multiple sclerosis | PROK2[135] |
| Heart failure | PKR1, PROK2 [38] |
| Abdominal aortic aneurysm | PROK2 [91] |
| Metabolic diseases (Obesity and diabetes) | PROK2 [100] |
A new discipline, “neurocardiology”, examines the reciprocal interaction between heart and brain [120] For example; cardiac arrhythmias and congestive heart failure can lead to cerebral accidents or ischemic attacks. Cerebrovascular dysfunction can cause electrocardiographic disorders or cardiac rhythm disturbances. Since the hippocampal formation particularly DG plays a pivotal role in the regulation of the brain-heart axis [3], whether PROK system contributes to heart-brain interaction remains to be confirmed.
Recent studies have been shown that GPCRs are delivered by exosomes, smallest extracellular vesicles [121], which are alternative biomarker sources, drug delivery carriers and therapeutic approaches for protection of organs in several diseases [122]. It is possible that PKR1 carrying exosomes can play a key role in the reciprocal interaction between heart and brain, and can be potential therapeutic approaches for the heart-brain axis-related diseases.
Nevertheless, PKR signaling drives neurogenesis and cardiogenesis during development, and promotes survival signaling against heart injuries (e.g., myocardial infarction, anti-cancer drug and diabetes induced injuries) and brain injuries (e.g., Parkinson’s disease and traumatic brain injury) (Fig. 4).
Figure 4. Role of prokineticin (PROK) signaling in development and injury of brain and heart.
. Prokineticin receptors (mostly PKR1) signaling drives neurogenesis and cardiogenesis during development, and promotes survival signaling against heart and brain injuries. Prokineticin signaling may represent a common mechanism in neuroprotection and cardioprotection. Application of integrative medicine in neuro-cardiology will provide future therapeutic approach to treat chronic neurodegenerative and cardiovascular disorders.
Highlights.
Angiogenesis and vasculogenesis are essential for the regeneration of the heart and brain.
prokineticin 2 (PROK2) as an angiogenic neuropeptide has a key role on cardiac and neuronal development.
The activation of neuropeptide PROK2 promotes the migration of SVZ cells to injured areas during brain injuries and neurodegenerative diseases.
PROK receptor agonists may have potential therapeutic implications for mitigating dopaminergic neuron loss in Parkinson diseases.
The prokineticin signaling pathways involved in the heart development specifically proliferation and differentiation of epicardial derived progenitor cells and the communication between epicardial cells and cardiomyocytes that regulates cardiomyocyte proliferation and rhythmicity.
PKR1 agonist is a promising target to protect the heart from the cardiac adverse effect of cancer treatments, myocardial infarction mediated ischemic damage and diabetes-induced cardiomyopathy.
Some antagonists of PKR1 and PKR2 have been discovered.
Some biased PKR1 agonist display neuro-protectant activities in Parkinson’s diseases.
Advances in medicinal chemistry are determining the future clinical development of PKR1 agonist.
A new discipline “neurocardiology”, may focus on role of PROK signaling on the reciprocal interaction between heart and brain.
Acknowledgements
We thank to Prof. Emel Songu-Mize for her critical reading and editing. We also thank Dr. Huajun Jin for assistance in preparing this manuscript. The drawings in the Figures were produced and adapted, using ChemDraw Professional 16.0.
Funding
This work was supported in part by grants of CGN and LD from the ANR-16-ECVD-000, ERA-CVD (JTC 2016) “Transnational Research Projects on Cardiovascular Diseases,” “Centre National de la Recherche Scientifique (CNRS),” and “Université de Strasbourg.” National Institutes of Health grants NS08820, NS100090 and ES026892 the W. E. Lloyd Endowed Chair and Eminent Scholar to AGK are also acknowledged.
Glossary
- Prokineticin
A small peptide hormone/cytokine/neuropeptide
- GPCR
G protein coupled seven transmembrane domain receptors involved in transmission of signal from extracellular space by binding to the ligands (Neuropeptides, cytokines etc.) to exert biological response. They are target of approximately 40% of clinically used drugs
- Enteric neural crest cells (NCC)
neural crest cells form the entire enteric nervous system, including neurons and glia of the gastrointestinal tract
- Glial fibrillary acidic protein (GFAP)
It is an intermediate filament protein that is expresses in astrocytes
- Hyposmia
reduced ability to smell and to detect odors
- Kallmann syndrome
genetic disorder causes incompletion of puberty due to hypogonadotropic hypogonadism
- Olfactory bulb (OB)
neural structure composed of neuronal cell bodies, astrocytes and oligodendrocytes synapse and capillaries in the forebrain responsible for the sense of smell
- Subgranular layer (SGL)
hippocampus brain region where adult neurogenesis occurs
- Dentate gyrus (DG)
hippocampal formation part in the temporal lobe
- Subventricular zone (SVZ)
proliferative zone containing neural progenitor cells responsible of the neurogenesis during embryogenesis and repair in adult brain
- Rostral migratory stream (RMS)
migratory pathway of neuronal progenitor and precursors derived from SVZ located between SVZ and OB
- Myocardial infarction
Heart attack due to mainly sudden blockage in a coronary artery leading to ischemia and lack of blood supply and eventually heart failure
- Cardiotoxicity
damage in the heart muscle due to anticancer drugs
- Vasculogenesis
the de novo formation of a primitive vascular network via differentiation of angioblast or endothelial precursors into endothelial cells
- Angiogenesis
formation of new capillaries from pre-existing vessel throughout life in both health and disease
- Parkinson’s disease
A neurodegenerative disease due to the cell death in the substantia nigra and midbrain, affecting motor system
Footnotes
Conflict of Interest
AGK has an equity interest in PK Biosciences Corporation located in Ames, IA USA. The terms of this arrangement have been reviewed and approved by Iowa State University in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].White BR, Rogers LS, Kirschen MP, Recent advances in our understanding of neurodevelopmental outcomes in congenital heart disease, Curr Opin Pediatr, 31 (2019) 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zou R, Shi W, Tao J, Li H, Lin X, Yang S, Hua P, Neurocardiology: Cardiovascular Changes and Specific Brain Region Infarcts, Biomed Res Int, 2017 (2017) 5646348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agrimi J, Spalletti C, Baroni C, Keceli G, Zhu G, Caragnano A, Matteucci M, Chelko S, Ramirez-Correa GA, Bedja D, Casieri V, Di Lascio N, Scalco A, Beltrami AP, Paolocci N, Caleo M, Lionetti V, Obese mice exposed to psychosocial stress display cardiac and hippocampal dysfunction associated with local brain-derived neurotrophic factor depletion, EBioMedicine, 47 (2019) 384–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tan CMJ, Lewandowski AJ, The Transitional Heart: From Early Embryonic and Fetal Development to Neonatal Life, Fetal Diagn Ther, (2019) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kapuria S, Yoshida T, Lien CL, Coronary Vasculature in Cardiac Development and Regeneration, J Cardiovasc Dev Dis, 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Callender T, Woodward M, Roth G, Farzadfar F, Lemarie JC, Gicquel S, Atherton J, Rahimzadeh S, Ghaziani M, Shaikh M, Bennett D, Patel A, Lam CS, Sliwa K, Barretto A, Siswanto BB, Diaz A, Herpin D, Krum H, Eliasz T, Forbes A, Kiszely A, Khosla R, Petrinic T, Praveen D, Shrivastava R, Xin D, MacMahon S, McMurray J, Rahimi K, Heart failure care in low- and middle-income countries: a systematic review and meta-analysis, PLoS Med, 11 (2014) e1001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nebigil CG, Desaubry L, The role of GPCR signaling in cardiac Epithelial to Mesenchymal Transformation (EMT), Trends Cardiovasc Med, 29 (2019) 200–204. [DOI] [PubMed] [Google Scholar]
- [8].Duenas A, Aranega AE, Franco D, More than Just a Simple Cardiac Envelope; Cellular Contributions of the Epicardium, Front Cell Dev Biol, 5 (2017) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Samak M, Hinkel R, Stem Cells in Cardiovascular Medicine: Historical Overview and Future Prospects, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bond AM, Ming GL, Song H, Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later, Cell Stem Cell, 17 (2015) 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A, In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain, Development, 128 (2001) 3759–3771. [DOI] [PubMed] [Google Scholar]
- [12].Azim K, Zweifel S, Klaus F, Yoshikawa K, Amrein I, Raineteau O, Early decline in progenitor diversity in the marmoset lateral ventricle, Cereb Cortex, 23 (2013) 922–931. [DOI] [PubMed] [Google Scholar]
- [13].Merkle FT, Mirzadeh Z, Alvarez-Buylla A, Mosaic organization of neural stem cells in the adult brain, Science, 317 (2007) 381–384. [DOI] [PubMed] [Google Scholar]
- [14].Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA, Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain, Proc Natl Acad Sci U S A, 97 (2000) 13883–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Magnusson JP, Frisen J, Stars from the darkest night: unlocking the neurogenic potential of astrocytes in different brain regions, Development, 143 (2016) 1075–1086. [DOI] [PubMed] [Google Scholar]
- [16].Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV, GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain, Nat Neurosci, 7 (2004) 1233–1241. [DOI] [PubMed] [Google Scholar]
- [17].Ohab JJ, Fleming S, Blesch A, Carmichael ST, A neurovascular niche for neurogenesis after stroke, J Neurosci, 26 (2006) 13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Woodbury ME, Ikezu T, Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration, J Neuroimmune Pharmacol, 9 (2014) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cui L, Qu H, Xiao T, Zhao M, Jolkkonen J, Zhao C, Stromal cell-derived factor-1 and its receptor CXCR4 in adult neurogenesis after cerebral ischemia, Restor Neurol Neurosci, 31 (2013) 239–251. [DOI] [PubMed] [Google Scholar]
- [20].Rosa AI, Goncalves J, Cortes L, Bernardino L, Malva JO, Agasse F, The angiogenic factor angiopoietin-1 is a proneurogenic peptide on subventricular zone stem/progenitor cells, J Neurosci, 30 (2010) 4573–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang L, Zhang Z, Wang Y, Zhang R, Chopp M, Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats, Stroke, 35 (2004) 1732–1737. [DOI] [PubMed] [Google Scholar]
- [22].Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY, Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus, Nature, 417 (2002) 405–410. [DOI] [PubMed] [Google Scholar]
- [23].Shojaei F, Singh M, Thompson JD, Ferrara N, Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression, Proc Natl Acad Sci U S A, 105 (2008) 2640–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferrara N, LeCouter J, Lin R, Endocrine gland vascular endothelial growth factor (EG-VEGF) and the hypothesis of tissue-specific regulation of angiogenesis, Endocr Res, 28 (2002) 763–764. [DOI] [PubMed] [Google Scholar]
- [25].LeCouter J, Ferrara N, EG-VEGF and Bv8. a novel family of tissue-selective mediators of angiogenesis, endothelial phenotype, and function, Trends Cardiovasc Med, 13 (2003) 276–282. [DOI] [PubMed] [Google Scholar]
- [26].Chen J, Kuei C, Sutton S, Wilson S, Yu J, Kamme F, Mazur C, Lovenberg T, Liu C, Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1, Mol Pharmacol, 67 (2005) 2070–2076. [DOI] [PubMed] [Google Scholar]
- [27].Marsango S, di Patti MC, Barra D, Miele R, The Bv8 gene from Bombina orientalis: molecular cloning, genomic organization and functional characterization of the promoter, Peptides, 30 (2009) 2182–2190. [DOI] [PubMed] [Google Scholar]
- [28].Negri L, Maftei D, Targeting the Prokineticin System to Control Chronic Pain and Inflammation, Curr Med Chem, 25 (2018) 3883–3894. [DOI] [PubMed] [Google Scholar]
- [29].Traboulsi W, Brouillet S, Sergent F, Boufettal H, Samouh N, Aboussaouira T, Hoffmann P, Feige JJ, Benharouga M, Alfaidy N, Prokineticins in central and peripheral control of human reproduction, Horm Mol Biol Clin Investig, 24 (2015) 73–81. [DOI] [PubMed] [Google Scholar]
- [30].Ujvari D, Jakson I, Oldmark C, Attarha S, Alkasalias T, Salamon D, Gidlof S, Hirschberg AL, Prokineticin 1 is up-regulated by insulin in decidualizing human endometrial stromal cells, J Cell Mol Med, 22 (2018) 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cheng MY, Bittman EL, Hattar S, Zhou QY, Regulation of prokineticin 2 expression by light and the circadian clock, BMC Neurosci, 6 (2005) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Watthanasurorot A, Saelee N, Phongdara A, Roytrakul S, Jiravanichpaisal P, Soderhall K, Soderhall I, Astakine 2--the dark knight linking melatonin to circadian regulation in crustaceans, PLoS Genet, 9 (2013) e1003361. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [33].Meng S, Gu Q, Yang X, Lv J, Owusu I, Matrone G, Chen K, Cooke JP, Fang L, TBX20 Regulates Angiogenesis Through the Prokineticin 2-Prokineticin Receptor 1 Pathway, Circulation, 138 (2018) 913–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Abou-Hamdan H, Desaubry L, Scalable 9-Step Synthesis of the Splicing Modulator NVS-SM2, J Org Chem, 83 (2018) 2954–2958. [DOI] [PubMed] [Google Scholar]
- [35].Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, Yamada T, Hinuma S, Inatomi N, Ohtaki T, Onda H, Fujino M, Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors, Biochem Biophys Res Commun, 293 (2002) 396–402. [DOI] [PubMed] [Google Scholar]
- [36].Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K, Molecular cloning and characterization of prokineticin receptors, Biochim Biophys Acta, 1579 (2002) 173–179. [DOI] [PubMed] [Google Scholar]
- [37].LeCouter J, Lin R, Tejada M, Frantz G, Peale F, Hillan KJ, Ferrara N, The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells, Proc Natl Acad Sci U S A, 100 (2003) 2685–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Urayama K, Guilini C, Messaddeq N, Hu K, Steenman M, Kurose H, Ert G, Nebigil CG, The prokineticin receptor-1 (GPR73) promotes cardiomyocyte survival and angiogenesis, FASEB J, 21 (2007) 2980–2993. [DOI] [PubMed] [Google Scholar]
- [39].Guilini C, Urayama K, Turkeri G, Dedeoglu DB, Kurose H, Messaddeq N, Nebigil CG, Divergent roles of prokineticin receptors in the endothelial cells: angiogenesis and fenestration, Am J Physiol Heart Circ Physiol, 298 (2010) H844–852. [DOI] [PubMed] [Google Scholar]
- [40].Kisliouk T, Levy N, Hurwitz A, Meidan R, Presence and regulation of endocrine gland vascular endothelial growth factor/prokineticin-1 and its receptors in ovarian cells, J Clin Endocrinol Metab, 88 (2003) 3700–3707. [DOI] [PubMed] [Google Scholar]
- [41].Gorowiec MR, Catalano RD, Norman JE, Denison FC, Jabbour HN, Prokineticin 1 induces inflammatory response in human myometrium: a potential role in initiating term and preterm parturition, Am J Pathol, 179 2709–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bassil AK, Dass NB, Murray CD, Muir A, Sanger GJ, Prokineticin-2, motilin, ghrelin and metoclopramide: prokinetic utility in mouse stomach and colon, Eur J Pharmacol, 524 (2005) 138–144. [DOI] [PubMed] [Google Scholar]
- [43].Wade PR, Palmer JM, Mabus J, Saunders PR, Prouty S, Chevalier K, Gareau MG, McKenney S, Hornby PJ, Prokineticin-1 evokes secretory and contractile activity in rat small intestine, Neurogastroenterol Motil, 22 e152–161. [DOI] [PubMed] [Google Scholar]
- [44].Nebigil CG, Prokineticin receptors in cardiovascular function: foe or friend?, Trends Cardiovasc Med, 19 (2009) 55–60. [DOI] [PubMed] [Google Scholar]
- [45].Dorsch M, Qiu Y, Soler D, Frank N, Duong T, Goodearl A, O’Neil S, Lora J, Fraser CC, PK1/EG-VEGF induces monocyte differentiation and activation, J Leukoc Biol, 78 (2005) 426–434. [DOI] [PubMed] [Google Scholar]
- [46].LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N, Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization, Proc Natl Acad Sci U S A, 101 (2004) 16813–16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ingves MV, Ferguson AV, Prokineticin 2 modulates the excitability of area postrema neurons in vitro in the rat, Am J Physiol Regul Integr Comp Physiol, 298 R617–626. [DOI] [PubMed] [Google Scholar]
- [48].Qiu CY, Liu YQ, Qiu F, Wu J, Zhou QY, Hu WP, Prokineticin 2 potentiates acid-sensing ion channel activity in rat dorsal root ganglion neurons, J Neuroinflammation, 9 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ren P, Zhang H, Qiu F, Liu YQ, Gu H, O’Dowd DK, Zhou QY, Hu WP, Prokineticin 2 regulates the electrical activity of rat suprachiasmatic nuclei neurons, PLoS One, 6 e20263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Xiong YC, Li XM, Wang XJ, Liu YQ, Qiu F, Wu D, Gan YB, Wang BH, Hu WP, Prokineticin 2 suppresses GABA-activated current in rat primary sensory neurons, Neuropharmacology, 59 589–594. [DOI] [PubMed] [Google Scholar]
- [51].Cottrell GT, Zhou QY, Ferguson AV, Prokineticin 2 modulates the excitability of subfornical organ neurons, J Neurosci, 24 (2004) 2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mohsen Z, Sim H, Garcia-Galiano D, Han X, Bellefontaine N, Saunders TL, Elias CF, Sexually dimorphic distribution of Prokr2 neurons revealed by the Prokr2-Cre mouse model, Brain Struct Funct, 222 (2017) 4111–4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cheng MY, Leslie FM, Zhou QY, Expression of prokineticins and their receptors in the adult mouse brain, J Comp Neurol, 498 (2006) 796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Congiu C, Onnis V, Deplano A, Salvadori S, Marconi V, Maftei D, Negri L, Lattanzi R, Balboni G, A new convenient synthetic method and preliminary pharmacological characterization of triazinediones as prokineticin receptor antagonists, European Journal of Medicinal Chemistry, 81 (2014) 334–340. [DOI] [PubMed] [Google Scholar]
- [55].Thompson WJ, Melamed JY, Preparation of morpholinecarboxamides as prokineticin 2 receptor antagonists, Merck & Co., Inc., USA: 2007, pp. 100 pp. [Google Scholar]
- [56].Cheng MY, Lee AG, Culbertson C, Sun G, Talati RK, Manley NC, Li X, Zhao H, Lyons DM, Zhou Q-Y, Steinberg GK, Sapolsky RM, Prokineticin 2 is an endangering mediator of cerebral ischemic injury, Proceedings of the National Academy of Sciences, 109 (2012) 5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Brogi S, Tafi A, Desaubry L, Nebigil CG, Discovery of GPCR ligands for probing signal transduction pathways, Front Pharmacol, 5 (2014) 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gasser A, Brogi S, Urayama K, Nishi T, Kurose H, Tafi A, Ribeiro N, Desaubry L, Nebigil CG, Discovery and cardioprotective effects of the first non-Peptide agonists of the G protein-coupled prokineticin receptor-1, PLoS One, 10 (2015) e0121027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ngan ES, Tam PK, Prokineticin-signaling pathway, Int J Biochem Cell Biol, 40 (2008) 1679–1684. [DOI] [PubMed] [Google Scholar]
- [60].Zhao Y WJ, Wang X, Jia H, Chen DN, Li JD, Prokineticins and their G protein-coupled receptors in health and disease., Prog Mol Biol Transl Sci, 161 (2019) 149–179. [DOI] [PubMed] [Google Scholar]
- [61].Von Hunolstein JJ, Nebigil CG, Can prokineticin prevent obesity and insulin resistance?, Curr Opin Endocrinol Diabetes Obes, 22 (2015) 367–373. [DOI] [PubMed] [Google Scholar]
- [62].Dormishian M, Turkeri G, Urayama K, Nguyen TL, Boulberdaa M, Messaddeq N, Renault G, Henrion D, Nebigil CG, Prokineticin receptor-1 is a new regulator of endothelial insulin uptake and capillary formation to control insulin sensitivity and cardiovascular and kidney functions, J Am Heart Assoc, 2 (2013) e000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Boulberdaa M, Urayama K, Nebigil CG, Prokineticin receptor 1 (PKR1) signalling in cardiovascular and kidney functions, Cardiovasc Res, 92 (2011) 191–198. [DOI] [PubMed] [Google Scholar]
- [64].Arora H, Boulberdaa M, Qureshi R, Bitirim V, Gasser A, Messaddeq N, Dolle P, Nebigil CG, Prokineticin receptor-1 signaling promotes Epicardial to Mesenchymal Transition during heart development, Sci Rep, 6 (2016) 25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Qureshi R, Kindo M, Boulberdaa M, von Hunolstein JJ, Steenman M, Nebigil CG, A prokineticin-driven epigenetic switch regulates human epicardial cell stemness and fate, Stem Cells, 36 (2018) 1589–1602. [DOI] [PubMed] [Google Scholar]
- [66].Ivey MJ, Tallquist MD, Defining the Cardiac Fibroblast, Circ J, 80 (2016) 2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD, The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors, Development, 139 (2012) 2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Qureshi R, Kindo M, Arora H, Boulberdaa M, Steenman M, Nebigil CG, Prokineticin receptor-1-dependent paracrine and autocrine pathways control cardiac tcf21(+) fibroblast progenitor cell transformation into adipocytes and vascular cells, Sci Rep-Uk, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Su G, Sun G, Liu H, Shu L, Zhang W, Liang Z, Prokineticin 2 relieves hypoxia/reoxygenation-induced injury through activation of Akt/mTOR pathway in H9c2 cardiomyocytes, Artif Cells Nanomed Biotechnol, 48 (2020) 345–352. [DOI] [PubMed] [Google Scholar]
- [70].Urayama K, Guilini C, Turkeri G, Takir S, Kurose H, Messaddeq N, Dierich A, Nebigil CG, Prokineticin receptor-1 induces neovascularization and epicardial-derived progenitor cell differentiation, Arterioscler Thromb Vasc Biol, 28 (2008) 841–849. [DOI] [PubMed] [Google Scholar]
- [71].Urayama K, Dedeoglu DB, Guilini C, Frantz S, Ertl G, Messaddeq N, Nebigil CG, Transgenic myocardial overexpression of prokineticin receptor-2 (GPR73b) induces hypertrophy and capillary vessel leakage, Cardiovasc Res, 81 (2009) 28–37. [DOI] [PubMed] [Google Scholar]
- [72].Nebigil CG, Updates on Endothelial Functions of Proangiogenic Prokineticin, Hypertension, 68 (2016) 1091–1097. [DOI] [PubMed] [Google Scholar]
- [73].Brouillet S, Hoffmann P, Benharouga M, Salomon A, Schaal JP, Feige JJ, Alfaidy N, Molecular characterization of EG-VEGF-mediated angiogenesis: differential effects on microvascular and macrovascular endothelial cells, Mol Biol Cell, 21 2832–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY, Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling, Science, 308 (2005) 1923–1927. [DOI] [PubMed] [Google Scholar]
- [75].Wen Y, Zhang Z, Li Z, Liu G, Tao G, Song X, Xu Z, Shang Z, Guo T, Su Z, Chen H, You Y, Li J, Yang Z, The PROK2/PROKR2 signaling pathway is required for the migration of most olfactory bulb interneurons, J Comp Neurol, (2019). [DOI] [PubMed] [Google Scholar]
- [76].Prosser HM, Bradley A, Caldwell MA, Olfactory bulb hypoplasia in Prokr2 null mice stems from defective neuronal progenitor migration and differentiation, Eur J Neurosci, 26 (2007) 3339–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y, Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2, Proc Natl Acad Sci U S A, 103 (2006) 4140–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bassi I, Luzzani F, Marelli F, Vezzoli V, Cotellessa L, Prober DA, Persani L, Gothilf Y, Bonomi M, Knocking-down of the Prokineticin receptor 2 affects reveals its complex role in the regulation of the hypothalamus-pituitary-gonadal axis in the zebrafish model, Sci Rep, 10 (2020) 7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, Morgan G, Murat A, Toublanc JE, Wolczynski S, Delpech M, Petit C, Young J, Hardelin JP, Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2, PLoS Genet, 2 (2006) e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Dode C, Rondard P, PROK2/PROKR2 Signaling and Kallmann Syndrome, Front Endocrinol (Lausanne), 4 (2013) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kuerbitz J, Arnett M, Ehrman S, Williams MT, Vorhees CV, Fisher SE, Garratt AN, Muglia LJ, Waclaw RR, Campbell K, Loss of Intercalated Cells (ITCs) in the Mouse Amygdala of Tshz1 Mutants Correlates with Fear, Depression, and Social Interaction Phenotypes, J Neurosci, 38 (2018) 1160–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kishi T, Kitajima T, Tsunoka T, Okumura T, Ikeda M, Okochi T, Kinoshita Y, Kawashima K, Yamanouchi Y, Ozaki N, Iwata N, Possible association of prokineticin 2 receptor gene (PROKR2) with mood disorders in the Japanese population, Neuromolecular Med, 11 (2009) 114–122. [DOI] [PubMed] [Google Scholar]
- [83].Kishi T, Kitajima T, Tsunoka T, Okumura T, Okochi T, Kawashima K, Inada T, Ujike H, Yamada M, Uchimura N, Sora I, Iyo M, Ozaki N, Iwata N, PROKR2 is associated with methamphetamine dependence in the Japanese population, Prog Neuropsychopharmacol Biol Psychiatry, 34 (2010) 1033–1036. [DOI] [PubMed] [Google Scholar]
- [84].Ingves MV, Ferguson AV, Prokineticin 2 modulates the excitability of area postrema neurons in vitro in the rat, Am J Physiol Regul Integr Comp Physiol, 298 (2010) R617–626. [DOI] [PubMed] [Google Scholar]
- [85].Xiong YC, Li XM, Wang XJ, Liu YQ, Qiu F, Wu D, Gan YB, Wang BH, Hu WP, Prokineticin 2 suppresses GABA-activated current in rat primary sensory neurons, Neuropharmacology, 59 (2010) 589–594. [DOI] [PubMed] [Google Scholar]
- [86].Ngan ES, Shum CK, Poon HC, Sham MH, Garcia-Barcelo MM, Lui VC, Tam PK, Prokineticin-1 (Prok-1) works coordinately with glial cell line-derived neurotrophic factor (GDNF) to mediate proliferation and differentiation of enteric neural crest cells, Biochim Biophys Acta, 1783 (2008) 467–478. [DOI] [PubMed] [Google Scholar]
- [87].Ngan ES, Lee KY, Sit FY, Poon HC, Chan JK, Sham MH, Lui VC, Tam PK, Prokineticin-1 modulates proliferation and differentiation of enteric neural crest cells, Biochim Biophys Acta, 1773 (2007) 536–545. [DOI] [PubMed] [Google Scholar]
- [88].Ruiz-Ferrer M, Torroglosa A, Nunez-Torres R, de Agustin JC, Antinolo G, Borrego S, Expression of PROKR1 and PROKR2 in human enteric neural precursor cells and identification of sequence variants suggest a role in HSCR, PLoS One, 6 (2011) e23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ngan ES, Sit FY, Lee K, Miao X, Yuan Z, Wang W, Nicholls JM, Wong KK, Garcia-Barcelo M, Lui VC, Tam PK, Implications of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 signaling in human neuroblastoma progression, Clin Cancer Res, 13 (2007) 868–875. [DOI] [PubMed] [Google Scholar]
- [90].Nguyen T, Gasser A, Nebigil CG,, Role of Prokineticin Receptor-1 in Epicardial Progenitor Cells, J. Dev. Biol, 1 (2013) 20–31. [DOI] [PubMed] [Google Scholar]
- [91].Choke E, Cockerill GW, Laing K, Dawson J, Wilson WR, Loftus IM, Thompson MM, Whole genome-expression profiling reveals a role for immune and inflammatory response in abdominal aortic aneurysm rupture, Eur J Vasc Endovasc Surg, 37 (2009) 305–310. [DOI] [PubMed] [Google Scholar]
- [92].Nebigil CG, Desaubry L, Updates in Anthracycline-Mediated Cardiotoxicity, Front Pharmacol, 9 (2018) 1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Arora H, Boulberdaa M, Qureshi R, Bitirim V, Messadeq N, Dolle P, Nebigil CG, Prokineticin receptor 1 is required for mesenchymal-epithelial transition in kidney development, FASEB J, 30 (2016) 2733–2740. [DOI] [PubMed] [Google Scholar]
- [94].Gasser A CY, Audebrand A, Daglayan A, Charavin M, Escoubet B, Karpov P, Tetko I, Chan MWY, Cardinale D, Désaubry L and Nebigil CG, Prokineticin Receptor-1 Signaling Inhibits Dose- and Time-Dependent Anthracycline-Induced Cardiovascular Toxicity Via Myocardial and Vascular Protection, JACC Cardiooncology, 1 (2019) 84–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yang Z, Wang M, Zhang Y, Cai F, Jiang B, Zha W, Yu W, Metformin Ameliorates Diabetic Cardiomyopathy by Activating the PK2/PKR Pathway, Front Physiol, 11 (2020) 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yang Z, Wu Y, Wang L, Qiu P, Zha W, Yu W, Prokineticin 2 (PK2) Rescues Cardiomyocytes from High Glucose/High Palmitic Acid-Induced Damage by Regulating the AKT/GSK3beta Pathway In Vitro, Oxid Med Cell Longev, 2020 (2020) 3163629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Szatkowski C, Vallet J, Dormishian M, Messaddeq N, Valet P, Boulberdaa M, Metzger D, Chambon P, Nebigil CG, Prokineticin receptor 1 as a novel suppressor of preadipocyte proliferation and differentiation to control obesity, PLoS One, 8 (2013) e81175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gardiner JV, Bataveljic A, Patel NA, Bewick GA, Roy D, Campbell D, Greenwood HC, Murphy KG, Hameed S, Jethwa PH, Ebling FJP, Vickers SP, Cheetham S, Ghatei MA, Bloom SR, Dhillo WS, Prokineticin 2 Is a Hypothalamic Neuropeptide That Potently Inhibits Food Intake, Diabetes, 59 (2010) 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Beale K, Gardiner J, Bewick G, Hostomska K, Patel N, Hussain S, Jayasena C, Ebling F, Jethwa P, Prosser H, Lattanzi R, Negri L, Ghatei M, Bloom S, Dhillo W, Peripheral administration of prokineticin 2 potently reduces food intake and body weight in mice via the brainstem, British Journal of Pharmacology, 168 (2013) 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mortreux M, Foppen E, Denis RG, Montaner M, Kassis N, Denom J, Vincent M, Fumeron F, Kujawski-Lafourcade M, Andreelli F, Balkau B, Marre M, Roussel R, Magnan C, Gurden H, Migrenne-Li S, New roles for prokineticin 2 in feeding behavior, insulin resistance and type 2 diabetes: Studies in mice and humans, Mol Metab, 29 (2019) 182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Von Hunolstein J-J, Nebigil CG, Can prokineticin prevent obesity and insulin resistance?, Current Opinion in Endocrinology, Diabetes and Obesity, 22 (2015) 367–373. [DOI] [PubMed] [Google Scholar]
- [102].Nebigil CG, Prokineticin Is a New Linker between Obesity and Cardiovascular Diseases, Frontiers in Cardiovascular Medicine, 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Cheng MY, Lee AG, Culbertson C, Sun G, Talati RK, Manley NC, Li X, Zhao H, Lyons DM, Zhou QY, Steinberg GK, Sapolsky RM, Prokineticin 2 is an endangering mediator of cerebral ischemic injury, Proc Natl Acad Sci U S A, 109 (2012) 5475–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Landucci E, Lattanzi R, Gerace E, Scartabelli T, Balboni G, Negri L, Pellegrini-Giampietro DE, Prokineticins are neuroprotective in models of cerebral ischemia and ischemic tolerance in vitro, Neuropharmacology, 108 (2016) 39–48. [DOI] [PubMed] [Google Scholar]
- [105].Severini C, Lattanzi R, Maftei D, Marconi V, Ciotti MT, Petrocchi Passeri P, Florenzano F, Del Duca E, Caioli S, Zona C, Balboni G, Salvadori S, Nistico R, Negri L, Bv8/prokineticin 2 is involved in Abeta-induced neurotoxicity, Sci Rep, 5 (2015) 15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Melchiorri D, Bruno V, Besong G, Ngomba RT, Cuomo L, De Blasi A, Copani A, Moschella C, Storto M, Nicoletti F, Lepperdinger G, Passarelli F, The mammalian homologue of the novel peptide Bv8 is expressed in the central nervous system and supports neuronal survival by activating the MAP kinase/PI-3-kinase pathways, Eur J Neurosci, 13 (2001) 1694–1702. [DOI] [PubMed] [Google Scholar]
- [107].Mundim MV, Zamproni LN, Pinto AAS, Galindo LT, Xavier AM, Glezer I, Porcionatto M, A new function for Prokineticin 2: Recruitment of SVZ-derived neuroblasts to the injured cortex in a mouse model of traumatic brain injury, Mol Cell Neurosci, 94 (2019) 1–10. [DOI] [PubMed] [Google Scholar]
- [108].Caioli S, Severini C, Ciotti T, Florenzano F, Pimpinella D, Petrocchi Passeri P, Balboni G, Polisca P, Lattanzi R, Nistico R, Negri L, Zona C, Prokineticin system modulation as a new target to counteract the amyloid beta toxicity induced by glutamatergic alterations in an in vitro model of Alzheimer’s disease, Neuropharmacology, 116 (2017) 82–97. [DOI] [PubMed] [Google Scholar]
- [109].Maftei D, Ratano P, Fusco I, Marconi V, Squillace S, Negri L, Severini C, Balboni G, Steardo L, Bronzuoli MR, Scuderi C, Campolongo P, Lattanzi R, The prokineticin receptor antagonist PC1 rescues memory impairment induced by beta amyloid administration through the modulation of prokineticin system, Neuropharmacology, 158 (2019) 107739. [DOI] [PubMed] [Google Scholar]
- [110].Gordon R, Neal ML, Luo J, Langley MR, Harischandra DS, Panicker N, Charli A, Jin H, Anantharam V, Woodruff TM, Zhou QY, Kanthasamy AG, Kanthasamy A, Prokineticin-2 upregulation during neuronal injury mediates a compensatory protective response against dopaminergic neuronal degeneration, Nat Commun, 7 (2016) 12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gordon R, Anantharam V, Kanthasamy AG, Kanthasamy A, Proteolytic activation of proapoptotic kinase protein kinase Cdelta by tumor necrosis factor alpha death receptor signaling in dopaminergic neurons during neuroinflammation, J Neuroinflammation, 9 (2012) 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Luo J, Padhi P, Jin H, Anantharam V, Zenitsky G, Wang Q, Willette AA, Kanthasamy A, Kanthasamy AG, Utilization of the CRISPR-Cas9 Gene Editing System to Dissect Neuroinflammatory and Neuropharmacological Mechanisms in Parkinson’s Disease, J Neuroimmune Pharmacol, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gengatharan A, Bammann RR, Saghatelyan A, The Role of Astrocytes in the Generation, Migration, and Integration of New Neurons in the Adult Olfactory Bulb, Front Neurosci, 10 (2016) 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].di Val Cervo P. Rivetti, Romanov RA, Spigolon G, Masini D, Martin-Montanez E, Toledo EM, La Manno G, Feyder M, Pifl C, Ng YH, Sanchez SP, Linnarsson S, Wernig M, Harkany T, Fisone G, Arenas E, Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model, Nat Biotechnol, 35 (2017) 444–452. [DOI] [PubMed] [Google Scholar]
- [115].Liddelow SA, Barres BA, Reactive Astrocytes: Production, Function, and Therapeutic Potential, Immunity, 46 (2017) 957–967. [DOI] [PubMed] [Google Scholar]
- [116].Becerra-Calixto A, Cardona-Gomez GP, The Role of Astrocytes in Neuroprotection after Brain Stroke: Potential in Cell Therapy, Front Mol Neurosci, 10 (2017) 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Neal M, Luo J, Harischandra DS, Gordon R, Sarkar S, Jin H, Anantharam V, Desaubry L, Kanthasamy A, Kanthasamy A, Prokineticin-2 promotes chemotaxis and alternative A2 reactivity of astrocytes, Glia, 66 (2018) 2137–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gotz M, Sirko S, Beckers J, Irmler M, Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis, Glia, 63 (2015) 1452–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Erdener SE, Dalkara T, Small Vessels Are a Big Problem in Neurodegeneration and Neuroprotection, Front Neurol, 10 (2019) 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Manea MM, Comsa M, Minca A, Dragos D, Popa C, Brain-heart axis--Review Article, J Med Life, 8 (2015) 266–271. [PMC free article] [PubMed] [Google Scholar]
- [121].Medapati MR, Singh A, Korupally RR, Henderson D, Klonisch T, Manda SV, Chelikani P, Characterization of GPCRs in extracellular vesicle (EV), Methods Cell Biol, 142 (2017) 119–132. [DOI] [PubMed] [Google Scholar]
- [122].Chong SY, Lee CK, Huang C, Ou YH, Charles CJ, Richards AM, Neupane YR, Pavon MV, Zharkova O, Pastorin G, Wang JW, Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers, Int J Mol Sci, 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Gasser A, Chen Y-W, Audebrand A, Daglayan A, Charavin M, Escoubet B, Karpov P, Tetko I, Chan MWY, Cardinale D, Désaubry L, Nebigil CG, Prokineticin Receptor-1 Signaling Inhibits Dose- and Time-Dependent Anthracycline-Induced Cardiovascular Toxicity Via Myocardial and Vascular Protection, JACC: CardioOncology, 1 (2019) 84–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Qureshi R, Kindo M, Arora H, Boulberdaa M, Steenman M, Nebigil CG, Prokineticin receptor-1-dependent paracrine and autocrine pathways control cardiac tcf21(+) fibroblast progenitor cell transformation into adipocytes and vascular cells, Sci Rep, 7 (2017) 12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hu WP, Li JD, Zhang C, Boehmer L, Siegel JM, Zhou QY, Altered circadian and homeostatic sleep regulation in prokineticin 2-deficient mice, Sleep, 30 (2007) 247–256. [PMC free article] [PubMed] [Google Scholar]
- [126].Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY, Attenuated circadian rhythms in mice lacking the prokineticin 2 gene, J Neurosci, 26 (2006) 11615–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Li JD, Hu WP, Zhou QY, Disruption of the circadian output molecule prokineticin 2 results in anxiolytic and antidepressant-like effects in mice, Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 34 (2009) 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhou WB, Li JD, Hu WP, Cheng MY, Zhou QY, Prokineticin 2 is involved in the thermoregulation and energy expenditure, Regul Peptides, 179 (2012) 84–90. [DOI] [PubMed] [Google Scholar]
- [129].Prosser HM, Bradley A, Chesham JE, Ebling FJ, Hastings MH, Maywood ES, Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei, Proc Natl Acad Sci U S A, 104 (2007) 648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Maftei D, Vellani V, Artico M, Giacomoni C, Severini C, Lattanzi R, Abnormal Pain Sensation in Mice Lacking the Prokineticin Receptor PKR2: Interaction of PKR2 with Transient Receptor Potential TRPV1 and TRPA1, Neuroscience, 427 (2020) 16–28. [DOI] [PubMed] [Google Scholar]
- [131].Li X, Zhang C, Zhou QY, Overexpression of Prokineticin 2 in Transgenic Mice Leads to Reduced Circadian Behavioral Rhythmicity and Altered Molecular Rhythms in the Suprachiasmatic Clock, Journal of circadian rhythms, 16 (2018) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Chen S, Reichert S, Singh C, Oikonomou G, Rihel J, Prober DA, Light-Dependent Regulation of Sleep and Wake States by Prokineticin 2 in Zebrafish, Neuron, 95 (2017) 153–168.e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sarfati J, Guiochon-Mantel A, Rondard P, Arnulf I, Garcia-Pinero A, Wolczynski S, Brailly-Tabard S, Bidet M, Ramos-Arroyo M, Mathieu M, Lienhardt-Roussie A, Morgan G, Turki Z, Bremont C, Lespinasse J, Du Boullay H, Chabbert-Buffet N, Jacquemont S, Reach G, De Talence N, Tonella P, Conrad B, Despert F, Delobel B, Brue T, Bouvattier C, Cabrol S, Pugeat M, Murat A, Bouchard P, Hardelin JP, Dode C, Young J, A comparative phenotypic study of kallmann syndrome patients carrying monoallelic and biallelic mutations in the prokineticin 2 or prokineticin receptor 2 genes, J Clin Endocrinol Metab, 95 659–669. [DOI] [PubMed] [Google Scholar]
- [134].Lau ST, Hansford LM, Chan WK, Chan GC, Wan TS, Wong KK, Kaplan DR, Tam PK, Ngan ES, Prokineticin signaling is required for the maintenance of a de novo population of c-KIT(+) cells to sustain neuroblastoma progression, Oncogene, 34 (2015) 1019–1034. [DOI] [PubMed] [Google Scholar]
- [135].Abou-Hamdan M, Costanza M, Fontana E, Di Dario M, Musio S, Congiu C, Onnis V, Lattanzi R, Radaelli M, Martinelli V, Salvadori S, Negri L, Poliani PL, Farina C, Balboni G, Steinman L, Pedotti R, Critical role for prokineticin 2 in CNS autoimmunity, Neurol Neuroimmunol Neuroinflamm, 2 (2015) e95. [DOI] [PMC free article] [PubMed] [Google Scholar]