Abstract
Much of routine cancer care has been disrupted due to the perceived susceptibility to SARS-CoV-2 infection in cancer patients. Here, we systematically review the current evidence base pertaining to the prevalence, presentation and outcome of COVID-19 in cancer patients, in order to inform policy and practice going forwards. A keyword-structured systematic search was conducted on Pubmed, Cochrane, Embase and MedRxiv databases for studies reporting primary data on COVID-19 in cancer patients. Studies were critically appraised using the NIH National Heart, Lung and Blood Institute's quality assessment tool set. The pooled prevalence of cancer as a co-morbidity in patients with COVID-19 and pooled in-hospital mortality risk of COVID-19 in cancer patients were derived by random-effects meta-analyses. In total, 110 studies from 10 countries were included. The pooled prevalence of cancer as a co-morbidity in hospitalised patients with COVID-19 was 2.6% (95% confidence interval 1.8%, 3.5%, I2: 92.0%). Specifically, 1.7% (95% confidence interval 1.3%, 2.3%, I2: 57.6.%) in China and 5.6% (95% confidence interval 4.5%, 6.7%, I2: 82.3%) in Western countries. Patients most commonly presented with non-specific symptoms of fever, dyspnoea and chest tightness in addition to decreased arterial oxygen saturation, ground glass opacities on computer tomography and non-specific changes in inflammatory markers. The pooled in-hospital mortality risk among patients with COVID-19 and cancer was 14.1% (95% confidence interval 9.1%, 19.8%, I2: 52.3%). We identified impeding questions that need to be answered to provide the foundation for an iterative review of the developing evidence base, and inform policy and practice going forwards. Analyses of the available data corroborate an unfavourable outcome of hospitalised patients with COVID-19 and cancer. Our findings encourage future studies to report detailed social, demographic and clinical characteristics of cancer patients, including performance status, primary cancer type and stage, as well as a history of anti-cancer therapeutic interventions.
Key words: Cancer, COVID-19, mortality, prevalence, SARS-CoV-2, systematic review
Statement of Search Strategies Used and Sources of Information
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, the study protocol was prospectively registered on PROSPERO (CRD42020182103). Searches were conducted in PubMed, EMBASE (OVID) and MedRxiv for studies published between 1 December 2019 and 24 April 2020. Finally, the citation lists of all included studies were searched for studies not identified on systematic database searches.
Introduction
Populations at risk of severe outcomes of COVID-19 are gradually being identified [1] and thus far include people with diabetes, obesity and hypertension, as well as sociodemographic risk factors, such as male sex, ethnicity and smoking status [[2], [3], [4], [5]].
Patients with cancer represent a population of particular interest and are not only vulnerable to the direct impacts of COVID-19 infection, but also to the effects of healthcare reprioritisation, with subsequent delays in cancer diagnosis and treatment. Over the course of the pandemic, the UK's National Institute for Health and Care Excellence (NICE) advised clinicians to weigh up the risk of delaying in-hospital cancer care with the risk of nosocomial COVID-19 infection. However, there is still little robust data on the nature of the risks involved. Consequent post-pandemic surges in healthcare demands are expected to lead to decreased cancer survival rates [6,7].
Common immunosuppressive therapies are thought to increase vulnerability to severe outcomes of COVID-19 in cancer patients. Although recent studies of immunosuppressed patients indicate that outcomes may be less severe, larger studies of malignancy indicate an association with higher mortality rates [8,9]. These risks probably vary across specific tumour types [10,11] and therapeutic approaches [12]. Given the heterogenous nature of malignancies, determining the impact of cancer and its treatment on the presentation and prognosis of COVID-19 remains an unmet challenge.
Here we collated evidence pertaining to the manifestation of COVID-19 in patients with active malignancy. Specifically, we considered:
-
(i)
The prevalence of cancer in hospitalised patients with COVID-19;
-
(ii)
How COVID-19 presents in cancer patients;
-
(iii)
Whether patients with cancer and COVID-19 are at increased risk of mortality.
Cancer heterogeneity, including pathology, treatment and service-level determinants will probably influence COVID-19 outcomes. To meaningfully answer the questions above, we therefore considered demographic, social and healthcare factors, as well as primary cancer type and stage, and recent therapeutic management. Together, this study provides an overview of the evidence and quality of data currently available, to guide future reports of larger cohort studies with regards to the prevalence, presentation and outcome of patients with COVID-19 and cancer.
Materials and Methods
Protocol
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, the study protocol was prospectively registered on PROSPERO (CRD42020182103).
Inclusion and Exclusion Criteria
Patients of any age, sex, nationality and healthcare setting were included. Active malignancy was defined as current malignant disease or treatment for malignancy within the last 12 months. All study types reporting primary clinical data or original analyses of COVID-19 in patients with an existing diagnosis of active cancer in the English language were eligible for inclusion. To account for the rapid evolution of the evidence base and to minimise the effects of publication bias, preprint literature was included. Exclusion criteria were protocol-only publications, commentaries, opinion pieces and systematic reviews without original statistical analyses.
Sources and Search Strategy
We conducted searches in PubMed, EMBASE (OVID) and MedRxiv for studies published between 1 December 2019 and 24 April 2020 (see Appendix A). We subsequently manually searched the citation lists of all included studies for papers that were not identified on systematic database searches.
Study Selection
Two reviewers independently screened each title and abstract for eligibility, and an independent third reviewer resolved all disagreements. To ensure that cancer subgroups were not overlooked, abstracts without explicit mention of cancer patients were not discarded. Finally, we used the same approach to screen the full manuscripts of included studies.
Data Collection and Quality Assessment
We developed a standardised data extraction tool and used the NIH National Heart, Lung and Blood Institute's Study Quality Assessment tools to quality assess included papers. Included papers underwent single-reviewer data extraction and quality assessment, and a 30% random sample was checked by a second reviewer. Using the NIH National Heart, Lung and Blood Institute's Study Quality Assessment tools, each study was quality appraised by two reviewers and converted into ‘high’, ‘moderate’ and ‘low’ risk of bias ratings through consensus between three reviewers.
Synthesis of Results
We reviewed all papers with respect to the primary questions and conducted qualitative evidence characterisation for each question. To account for variations in cancer prevalence, we categorised studies by geography into China and Western countries. We used results from the geographical subgroups to infer qualitative interpretations. We calculated (i) the pooled prevalence of co-morbid cancer in hospitalised COVID-19 patients and (ii) the pooled in-hospital mortality risk for patients with cancer and COVID-19. For the pooled analyses, we screened for studies that overlapped in terms of study site and recruitment window, in order to prevent duplication of data, i.e. double counting of the same patients within the quantitative synthesis. Where two or more studies were conducted at the same hospital with overlapping recruitment windows, the study with the longest recruitment window and/or the largest sample size of cancer patients was retained. Studies with inappropriate designs for the purposes of each pooled analysis were also excluded, i.e. studies deliberately selecting cancer patients or selecting on the basis of another co-morbidity/admission reason, for the pooled cancer prevalence analysis; studies with a short follow-up or not reporting death/discharge outcomes for the pooled mortality risk analysis; and case-control studies selecting patients on the basis of specific adverse outcomes such as death or intensive therapy unit admission for both analyses. Case series or reports, and studies including hospital staff or out-patients in their cohort were also excluded from both analyses. The pooled analyses were conducted using a random-effects model after the Freeman–Tukey Double Arcsine Transformation of variances in the Metaprop package in Stata 14 [13]. Here, exact or Clopper Pearson 95% confidence intervals were calculated for individual studies [14]. To address heterogeneity between studies, we used random-effects models that incorporate the assumption that studies are estimating different, however related, effects. Specifically, the I2 statistic was used to assess statistical heterogeneity.
Results
Study Selection
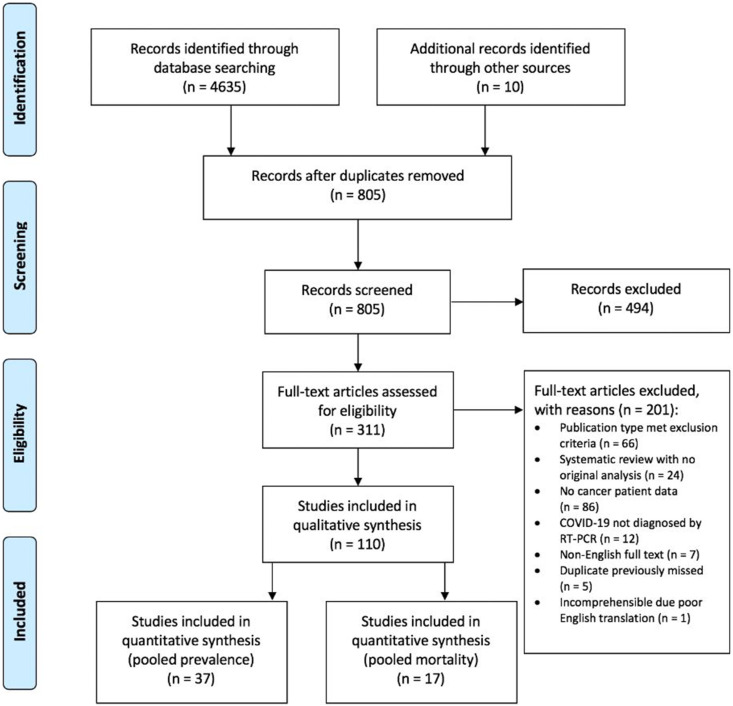
We retrieved 4635 titles through a systematic search and identified 10 further studies through manual searches of the reference lists. After removing duplicate records, 805 titles were retained for title and abstract screening, of which 311 articles were selected for full-text screening. In total, 110 records were subsequently included in the qualitative synthesis (see Appendix B). Of these, 37 studies were used to calculate the pooled prevalence of cancer in hospitalised patients with COVID-19, 30 studies were used to characterise the presenting features of COVID-19 and 17 studies were used to calculate the pooled mortality risk (see Appendix C).
Study Characteristics
Eighty cohort studies, six cross-sectional studies, two case-control studies, one interventional trial, 10 case series and 11 case reports were included (Figure 1 ). Sixty-seven (60.9%) of the included studies were preprint copies. Given the geographical emergence and spread of COVID-19, most studies were from China (n = 82), Italy (n = 10) and the US (n = 8). Two pairs of studies considered exactly the same cohorts [10,12,15,16], 31 studies had overlapping patient cohorts (study site and recruitment window) with possible duplication of included patients, two studies [10,17] described themselves as case series but were categorised as cohort studies, and one study described itself as a cross-sectional survey but was categorised as a case series [18]. Due to the inherent selection and reporting biases of this study design, all case series and reports (n = 21) were classified as high risk of bias. Eighty-one other papers were identified as having a high risk of bias, three a moderate risk of bias and only five a low risk of bias.
Fig 1.
Flowchart of included and excluded studies. Eighty cohort studies, six cross-sectional studies, two case-control studies, one interventional trial, 10 case series and 11 case reports were included.
The characteristics of the study populations are outlined in Table 1 . Most studies (n = 93) described a general cohort of COVID-19 patients with coincidental inclusion of co-morbid cancer. Socio-demographically, 23 studies reported age, 25 reported gender, six reported socioeconomic status, four reported smoking status and two reported occupational status. Thirty studies distinguished cancer types, nine identified tumour size and/or metastases and three provided cancer stage. Twenty-three studies reported recent cancer treatment (chemotherapy, radiotherapy, immunotherapy or surgery), six described the aim of treatment (palliative, radical, maintenance), 12 referenced the time since treatment (current or <12 months) and only one paper reported on immune competence.
Table 1.
Study characteristics
| Study characteristics | No. of studies n (%) |
|---|---|
| Publication status | |
| Peer-reviewed | 43 (39.1) |
| Non-peer-reviewed (preprint) | 67 (60.9) |
| Country | |
| China | 82 (74.5) |
| Italy | 10 (9.1) |
| USA | 8 (7.3) |
| UK | 2 (1.8) |
| South Korea | 1 (0.9) |
| France | 1 (0.9) |
| Spain | 1 (0.9) |
| Netherlands | 1 (0.9) |
| Denmark | 1 (0.9) |
| Brazil | 1 (0.9) |
| Multiple | 2 (1.8) |
| Study design | |
| Interventional trial | 1 (0.9) |
| Prospective cohort | 6 (5.5) |
| Retrospective cohort | 74 (67.3) |
| Case-control | 2 (1.8) |
| Cross-sectional | 6 (5.5) |
| Case series (≥2 cases) | 10 (9.1) |
| Case report (1 case) | 11 (10.0) |
| Study setting | |
| Community (out-patient) | 5 (4.5) |
| Hospital (in-patient) | 95 (86.4) |
| Community and hospital | 10 (9.1) |
| Population | |
| Patients with COVID-19, including some with cancer | 93 (84.5) |
| Patients with cancer, including some with COVID-19 | 4 (3.6) |
| Patients with cancer and COVID-19 only | 13 (11.8) |
| Reporting of cancer cohort features | |
| Age (median) | 23 (20.9) |
| Gender (male:female) | 25 (22.7) |
| Other co-morbidities than cancer | 13 (11.8) |
| Lifestyle factors | 3 (2.7) |
| Cancer type | 32 (29.1) |
| Cancer stage | 15 (13.6) |
| Time since last treatment | 21 (19.1) |
| Treatment type | 21 (19.1) |
| Treatment objective (palliative, radical, maintenance) | 8 (7.3) |
| Study duration (days) | |
| <7 | 5 (4.5) |
| 7–13 | 9 (8.1) |
| 14–29 | 19 (17.3) |
| ≥30 days | 8 (7.3) |
| Not reported | 69 (62.7) |
| Reported outcomes for patients with cancer and COVID-19 | |
| Disease severity | 63 (57.3) |
| Mortality | 52 (47.3) |
| Not reported | 40 (36.4) |
| Risk of bias | |
| Low (good quality) | 5 (4.5) |
| Moderate (fair quality) | 3 (2.7) |
| High (poor quality) | 102 (92.7) |
Prevalence of Cancer in Hospitalised COVID-19 Patients
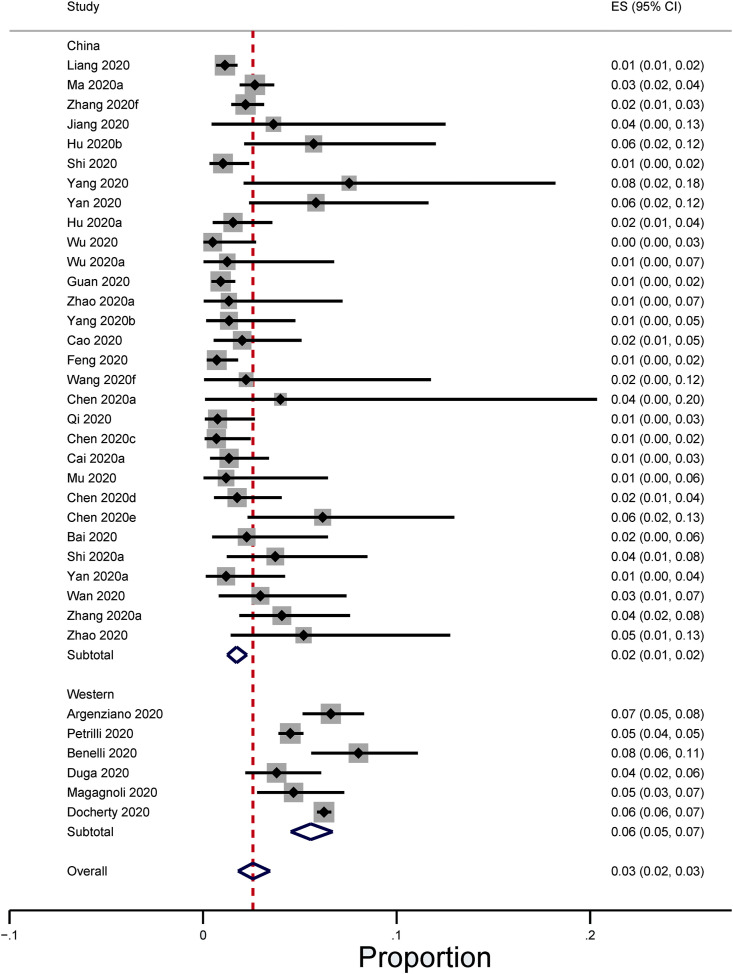
The pooled prevalence of active cancer in hospitalised patients with COVID-19 across 37 cohort studies was 2.6% (95% confidence interval 1.8%, 3.5%) (Fig 2, Fig 3 ) [10,12,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]]. The prevalence in China and Western countries was 1.7% (95% confidence interval 1.3%, 2.3%) [10,12,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]] and 5.6% (95% confidence interval 4.5%, 6.7%), respectively [[46], [47], [48], [49], [50], [51]]. There was significant heterogeneity in the estimates for each group (P < 0.001), with I2 = 92.0% across all 10 countries, and specifically, 57.6% for China and 82.3% for Western countries.
Fig 2.
Proportion of hospitalised COVID-19 patients with cancer. The pooled prevalence of active cancer in hospitalised patients with COVID-19 across 37 cohort studies was 2.6% (95% confidence interval 1.8%, 3.5%). In China and Western countries, the prevalence figures were 1.7% (95% confidence interval 1.3%, 2.3%) and 5.6% (95% confidence interval 4.5%, 6.7%), respectively.
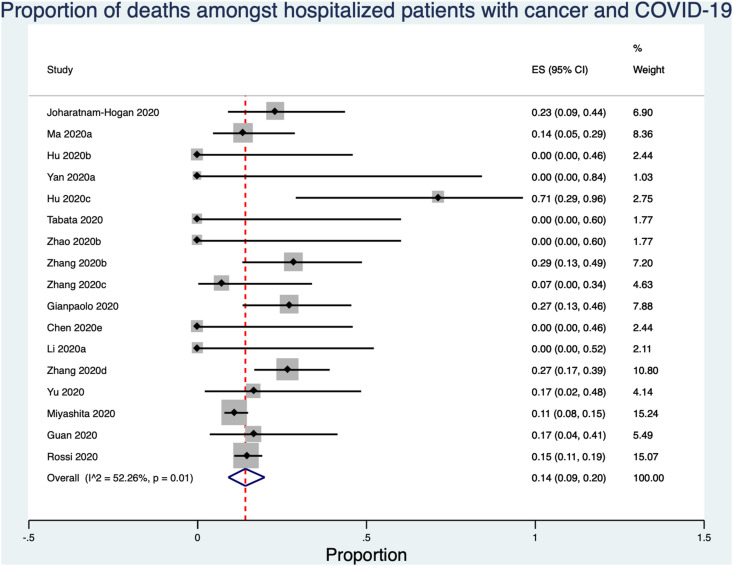
Fig 3.
Proportion of deaths among hospitalised patients with cancer and COVID-19. The pooled in-hospital mortality risk among patients with COVID-19 and cancer was 14.1% (95% confidence interval 9.1%, 19.8%).
Presenting Features of COVID-19 in Cancer Patients
The clinical presentation of COVID-19 in hospitalised cancer patients across 30 studies is detailed in Table 2 . Here, 16 (53.3%) were observational cohort studies, five were case series and nine were case reports. Patients most commonly presented with non-specific symptoms of fever, cough, dyspnoea, fatigue, myalgia, chest tightness and headache. Where computed tomography was carried out, abnormal changes (including ground glass opacities) were seen up to 6 days in advance of the clinical presentation. Laboratory findings included non-specific changes in inflammatory markers and decreased arterial oxygen saturations.
Table 2.
Clinical presentation of COVID-19 in cancer patients
| Features | 4 observational and cohort studies |
14 case reports and case series |
|||
|---|---|---|---|---|---|
| Ma et al., 2020 (n = 37) | Hrusak et al., 2020 (n = 9) | Zhang et al., 2020 (n = 67) | Yang et al., 2020 (n = 3) | n = 23 | |
| Fever | 75.7% | 77.8% | 79.1% | 100% | n = 14 |
| Cough | 56.8% | ND | 74.6% | 33.3% | n = 8 |
| Dyspnoea | 32.4% | ND | 65.7% | ND | n = 12 |
| Hypoxia/reduced SpO2 | ND | ND | ND | 33.3% | n = 1 |
| WBC | ↑ neutrophil | ↓ neutrophil and lymphocyte | ND | ↑ in 33.3% | ↑ lymphocyte: n = 2 ↑ neutrophil: n = 1 |
| CRP | ND | ND | ↑ | ↑ | 9 |
| Other inflammatory markers | ↑ IL-6 and LDH | ND | ↑ LDH | 66.6% ↑ D-dimer | ↑ LDH: n = 2 ↑ D-dimer: n = 7 |
| Imaging modality | ND | CT | CT | ND | CT: n = 18 X-ray: n = 2 |
| Other symptoms | Headache, myalgia, fatigue, diarrhoea | Diarrhoea | Fatigue, diarrhoea, nausea, myalgia, and vomiting | ND | Myalgia: n = 3 Diarrhoea: n = 3 Nausea and vomiting: n = 2 Fatigue: n = 2 Asymptomatic cases: n = 3 |
CRP, C-reactive protein; CT, computed tomography; IL-6, interleukin-6; LDH, lactate dehydrogenase; ND, no data available; WBC, white blood cells.
In-hospital Mortality Risk for Cancer Patients with COVID-19
Across 17 retrospective cohort studies, clinical outcomes were identified in 904 hospitalised patients with cancer and COVID-19 [11,16,22,28,30,39,43,44,47,[52], [53], [54], [55], [56], [57], [58]]. The pooled in-hospital mortality risk was 14.1% (95% confidence interval 9.1%, 19.8%; Figure 3). There was significant heterogeneity in the estimate (P = 0.01) with I2 = 55.9%. Only six of 17 studies reported the outcome for all cancer patients at the end of the study observation window [11,22,43,44,56,59]. The median length of in-hospital observation was 7–31 days [39,43,44,[53], [54], [55], [56],59] and 0–75% of the cancer patients remained hospitalised at the end of the study [11,22,43,44,56,59]. Furthermore, only eight studies specified the cancer type and severity and/or treatment during admission or within the past 12 months [9,11,22,30,43,55,56,[58], [59], [60]]. Of these, only two studies reported both cancer type and treatment for all included patients [11,43]. Together, cancer types were reported for 276 patients and genitourinary, gastrointestinal and respiratory system cancers accounted for 91.6% of all reported cancers [1,9,11,22,26,30,43,55,56,58,59,61]. Oncological treatments were reported for 68 patients, and more than a third were treated with chemotherapy [11,30,43,55,56,59].
Across 14 case reports and series, 24 cancer patients with COVID-19 were identified [18,[60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72]]. Nine died, three remained in hospital and 12 were discharged by the end of the study period. Of these, 23 had active cancer [18,[60], [61], [62], [63], [64], [65], [66], [67], [68],[70], [71], [72]] and one was in remission [69]. The average in-patient stay was 18 days. Cancer types included 20 solid organ cancers (lung, ovarian, cervical, endometrial, breast) [60,61,[63], [64], [65], [66],[68], [69], [70],72] and four haematological cancers [18,62,67,71] with different stages of severity. Although cancer treatments were non-homogenous, all patients received oxygen therapy, antibiotics, intensive care admission and mechanical ventilation for treatment of COVID-19 [18,[60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72]].
Discussion
We narratively synthesised results from 110 studies across 10 countries. Included studies were predominantly from China (n = 82), Italy (n = 10) and the USA (n = 8), in line with the evolution of the pandemic.
Prevalence of Co-morbid Cancer
From 37 studies, the overall prevalence of active cancer as a co-morbidity among hospitalised patients with COVID-19 was estimated to be 2–4%, with a higher prevalence seen in studies from Western settings (5–7%) compared with studies conducted in China (1–2%). These findings are unsurprising considering a recent study that reported a lower incidence of total cancer in China compared with Western countries such as the USA and UK [73].
Clinical Presentation
Cancer patients with COVID-19 seem to most commonly present with non-specific symptoms of fever, dyspnoea and chest tightness, in addition to decreased arterial oxygen saturation, ground glass opacities on computed tomography and non-specific changes in inflammatory markers. One study [55] reported that cancer patients more commonly presented with confusion. Consistent with the presentation of COVID-19 in non-cancer patients [74], laboratory findings in cancer patients showed non-specific changes in inflammatory markers, decreased arterial oxygen saturations and abnormal computed tomography scans. Where patients presented with lymphopenia, non-localising symptoms and disseminated infection, underlying immunosuppression must be considered.
Mortality
The estimated in-hospital mortality risk for patients with COVID-19 and cancer is between 9 and 20% across all settings. The pooled mortality risk estimate of 14.1% among hospitalised patients with COVID-19 and cancer is at least five times higher than the reported mortality risk of COVID-19 in non-elderly patients without underlying predisposing conditions across Europe and America [75]. These results corroborate a recently published systematic review and meta-analysis [76] that reported that malignancy, among age and other co-morbidities, is associated with a greater risk of death from COVID-19 infection.
The findings in this review are in line with findings from four of the included studies that independently reported increased mortality risks in their respective cohorts. Specifically, two studies reported a higher risk of mortality in cancer patients with COVID-19 when adjusting for age and sex (hazard ratio 1.4; 95% confidence interval 1.0–2.0) [43,77] and another reported a significantly higher relative risk of mortality in cancer patients under the age of 50 years compared with non-cancer patients in the same age group (relative risk 5.01; 95% confidence interval 1.55–16.2) [58]. Finally, cancer patients were found to have a higher composite risk of admission to an intensive care unit, invasive ventilation or death when adjusting for both age and smoking status (hazard ratio 3.501; 95% confidence interval 1.604–7.643) [16].
Methodological Considerations
There are several important limitations inherent in the available evidence that restrict the inferences that can be drawn from these findings. Here, we first review the limitations of the available data and then address specific measures that should be undertaken to ensure that studies examining prevalence, risk and mortality differences are epidemiologically robust from the outset.
All included studies accurately reported the prevalence of cancer in hospitalised patients with a confirmed diagnosis of COVID-19. Although this provides insight into the proportion of co-morbid cancer, it is not a measure of the risk of COVID-19 acquisition among people with cancer. Notably, the need for hospitalisation may be inflated due to extensive clinical monitoring, a group coached to present early with infection or may have been an incidental finding [58]. Together, behavioural and healthcare factors such as self-surveillance, health literacy, access to healthcare and thresholds for testing or admission may lead to earlier presentation and over-representation of cancer patients within the COVID-19 cohort. Cancer patients with COVID-19 were largely identified coincidentally in cohort studies, where the primary objective was not to investigate the prevalence, clinical presentation or course of disease. Consequently, relevant confounders, including demographic, social and clinical characteristics, were not considered or reported. Most studies did not report the specific cancer type or treatment history for included cancer patients, nor did they outline the primary reason for hospital admission. This means that for certain patients with cancers requiring closer monitoring or more frequent contact with healthcare services, there may be a bias with regards to the detection of COVID-19 symptoms and consequent diagnosis. There may also be a bias towards detection of COVID-19 in patients whose disease or therapy causes symptoms similar to COVID-19, prompting higher rates of testing and, consequently, detection. For this review, only studies examining data from hospital in-patients were considered. However, none of the included studies specified the original reason for admission. Therefore, it remains uncertain whether included patients were originally admitted for reasons specific to their cancer diagnosis, such as elective admission for cancer treatment or emergency admission for cancer-related complications, or whether they were admitted due to illness caused primarily by COVID-19, which poses a significant challenge when interpreting the available data with regards to risk of infection. Furthermore, differences in study design, study population and risk of bias inevitably lead to heterogeneity between studies, and this is particularly present in combined pooled estimates of prevalence in the West and China. To address these heterogeneities, we used a random-effects model that incorporates an assumption that studies are estimating different, yet related, effects. We also examined these regions separately and heterogeneity was lower when we did this, particularly for China, which shows that some of the heterogeneity is explained by between-country differences. Heterogeneity is often high in meta-analyses of proportions [[78], [79], [80], [81]] and care must be taken if the results from the analysis are used for clinical decision making.
Outcomes for 75% [82] of patients in cohort studies remain unknown due to insufficient follow-up periods and the specific causes of mortality are not specified. Without cause of death information, it is not possible to distinguish between mortality caused by cancer or its associated treatment complications and mortality as a direct result of COVID-19; in cases where COVID-19 causes decompensation of underlying disease, even cause of death data may be unreliable. Excess all-cause mortality in cancer patients diagnosed with COVID-19 would provide the only robust metric of the risk posed by COVID-19 and requires routine setting-specific data and large sample sizes to ascertain.
Development of Future Studies
Included studies were largely (92.7%) assessed as having a high potential of bias, and we uncovered significant limitations in the existing literature base, which must be addressed (Figure 4 ). It is recommended that future studies include data on cancer type and treatment regimens of all cancer patients, as well as primary admission reason for all patients, with details on the causes of death if mortality data are presented. To estimate the relative risk of SARS-CoV-2 infection in cancer patients, the incidence of COVID-19 should be measured through longitudinal follow-up of cohorts of individuals with and without cancer. Additionally, sub-cohorts of specific cancer types and stages should be considered; ideally, without prior or current SARS-CoV-2 infection at baseline. Cohorts must be selected through probabilistic sampling methods to ensure they are balanced and representative, particularly with respect to other demographic and clinical variables. Follow-up duration should adequately allow for declining incidence, and inconsistency in follow-up duration should be addressed using person–time metrics. Studies must define, collect and present data on all potential confounding and interacting variables, such as those discussed under ‘Methodological considerations’, and adjust for these at the point of design or analysis. We further encourage future studies to report comprehensive social, demographic and clinic characteristics of cancer patients. Clinical characteristics of interests pertain specifically to performance status, co-morbidities, cancer type, cancer stage, treatment type, history and plan.
Fig 4.
Recommendations for future studies of COVID-19 in cancer patients.
Future studies will need to reflect the changing epidemiology of COVID-19 with regards to their design and outcomes of interest. In light of the current resurgence, or ‘second wave’, with high community transmission nationally, community-based follow-up of known cancer patients should also be undertaken; specifically considering household and occupational exposures, as well as healthcare exposures, and linking this with hospital admission and clinical outcomes data. Aggregated findings from outbreak analyses based on household or institutional clusters may reveal insights regarding transmission risk for cancer patients compared with non-cancer contacts of COVID-19 cases. In order to examine mortality risks, it is essential to capture both in-hospital and out-of-hospital deaths. Routine mortality data, such as cause of death certification, should be utilised, which can be linked with both national COVID-19 surveillance data and healthcare datasets. When community transmission is effectively controlled, researchers should continue to monitor large cohorts of cancer patients over the course of treatment, with a focus on nosocomial transmission.
Going forward, ceilings of care for those receiving palliative versus curative therapy should be considered, and the effects of immunosuppressive chemotherapy versus targeted immunotherapy, radiotherapy, surgery or other types of treatment should be reported.
Conclusions
The results from this review show that cancer constitutes a co-morbidity in 1–2% of hospitalised COVID-19 patients in China and 5–7% in Western countries. They present similarly clinically to non-cancer patients, and the preliminary evidence suggests there is an increased in-hospital mortality risk in patients with cancer and COVID-19. However, a comprehensive assessment of the qualities of the included studies show the need for longitudinal follow-up studies, where cohorts are selected through probabilistic sampling methods, and follow-up duration is extended to encompass clinical progression and outcome. In order to improve generalisability, granularity and understanding of heterogeneity, we further encourage future studies to report detailed social, demographic and clinic characteristics of cancer patients, including but not limited to, performance status, other co-morbidities, cancer type and stage, and treatment type, history and plan. We recommend that future studies include primary admission reason for all patients, with details on the causes of death if mortality data are presented. Finally, in light of the current ‘second wave’ of COVID-19 with high community transmission, it is essential to report both in-hospital and out-of-hospital deaths, and community-based follow-up should be implemented.
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Acknowledgements
The authors would like to thank Akhila Kadgathur Jayaram and Polygeia, the youth-led global think tank based in the UK, USA and Hong Kong.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clon.2020.11.006.
Appendices. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 3.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 4.Jin J.-M., Bai P., He W., Wu F., Liu X.-F., Han D.-M. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan D., Sze S., Minhas J.S., Bangash M.N., Pareek N., Divall P. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Lancet Oncology COVID-19: global consequences for oncology. Lancet Oncol. 2020;21:467. doi: 10.1016/S1470-2045(20)30175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai A.G., Pasea L., Banerjee A., Denaxas S., Katsoulis M., Chang W.H. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. medRxiv. 2020 2020.2005.2027.20083287. [Google Scholar]
- 8.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anil I., Arnold R., Benkwitz-Beford S., Branford S., Campton N., Cazier J.-B. The UK Coronavirus Cancer Monitoring Project: protecting patients with cancer in the era of COVID-19. Lancet Oncol. 2020;21:622–624. doi: 10.1016/S1470-2045(20)30230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clopper C.J., Pearson E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 15.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D.-Y., Guo J.-M., Yang Z.-Z., You Y., Chen Z.-C., Chen S.-M. The first report of the prevalence of COVID-19 in chronic myelogenous leukemia patients in the core epidemic area of China: multicentre, cross-sectional survey. medRxiv. 2020 2020.2003.2012.20034876. [Google Scholar]
- 19.Bai X., Fang C., Zhou Y., Bai S., Liu Z., Chen Q. Predicting COVID-19 malignant progression with AI techniques. medRxiv. 2020:2020. 2003.2020.20037325. [Google Scholar]
- 20.Cai Q., Huang D., Ou P., Yu H., Zhu Z., Xia Z. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 21.Cao M., Zhang D., Wang Y., Lu Y., Zhu X., Li Y. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv. 2020 2020.2003.2004.20030395. [Google Scholar]
- 22.Chen M., Tu C., Tan C., Zheng X., Wang X., Wu J. Key to successful treatment of COVID-19: accurate identification of severe risks and early intervention of disease progression. medRxiv. 2020 2020.2004.2006.20054890. [Google Scholar]
- 23.Chen X., Ling J., Mo P., Zhang Y., Jiang Q., Ma Z. Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients. medRxiv. 2020 2020.2003.2003.20030437. [Google Scholar]
- 24.Chen X., Zhang Y., Zhu B., Zeng J., Hong W., He X. Associations of clinical characteristics and antiviral drugs with viral RNA clearance in patients with COVID-19 in Guangzhou, China: a retrospective cohort study. medRxiv. 2020 2020.2004.2009.20058941. [Google Scholar]
- 25.Chen X., Zheng F., Qing Y., Ding S., Yang D., Lei C. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. medRxiv. 2020 2020.2003.2003.20030353. [Google Scholar]
- 26.Feng Z., Li J., Yao S., Yu Q., Zhou W., Mao X. The use of adjuvant therapy in preventing progression to severe pneumonia in patients with coronavirus disease 2019: a multicenter data analysis. medRxiv. 2020 2020.2004.2008.20057539. [Google Scholar]
- 27.Guan W-j, Ni Z-y, Hu Y., Liang W-h, Ou C-q, He J-x. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu H., Yao N., Qiu Y. Comparing rapid scoring systems in mortality prediction of critically ill patients with novel coronavirus disease. Acad Emerg Med. 2020;27:461–468. doi: 10.1111/acem.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu L., Chen S., Fu Y., Gao Z., Long H., Ren H-w. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. medRxiv. 2020 doi: 10.1093/cid/ciaa539. 2020.2003.2025.20037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma J., Yin J., Qian Y., Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's retrospective study. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma K.-L., Liu Z.-H., Cao C-f, Liu M.-K., Liao J., Zou J.-B. COVID-19 myocarditis and severity factors an adult cohort study. medRxiv. 2020 2020.2003.2019.20034124. [Google Scholar]
- 32.Qi D., Yan X., Tang X., Peng J., Yu Q., Feng L. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple-center study. medRxiv. 2020 2020.2003.2001.20029397. [Google Scholar]
- 33.Shi P., Ren G., Yang J., Li Z., Deng S., Li M. Clinical characteristics of imported and second-generation COVID-19 cases outside Wuhan, China: a multicenter retrospective study. medRxiv. 2020 doi: 10.1017/S0950268820002332. 2020.2004.2019.20071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C., Deng R., Gou L., Fu Z., Zhang X., Shao F. Preliminary study to identify severe from moderate cases of COVID-19 using NLR&RDW-SD combination parameter. medRxiv. 2020 doi: 10.21037/atm-20-3391. 2020.2004.2009.20058594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020;71:706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan D., Liu X-y, Zhu Y-n, Huang L., Dan B-t, Zhang G-j. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in patients with SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1183/13993003.00799-2020. 2020.2003.2022.20040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan S., Song X., Lin F., Zhu H., Wang X., Li M. Clinical characteristics of coronavirus disease 2019 in Hainan, China. medRxiv. 2020 2020.2003.2019.20038539. [Google Scholar]
- 40.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y., Shen C., Li J., Yuan J., Yang M., Wang F. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020 2020.2003.2002.20029975. [Google Scholar]
- 42.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1016/j.jcv.2020.104364. 2020.2003.2002.20030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao W., Yu S., Zha X., Wang N., Pang Q., Li T. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: a retrospective cohort study. medRxiv. 2020 2020.2003.2013.20035436. [Google Scholar]
- 45.Zhao Z., Xie J., Yin M., Yang Y., He H., Jin T. Clinical and laboratory profiles of 75 hospitalized patients with novel coronavirus disease 2019 in Hefei, China. medRxiv. 2020 2020.2003.2001.20029785. [Google Scholar]
- 46.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benelli G., Buscarini E., Canetta C., La Piana G., Merli G., Scartabellati A. SARS-COV-2 comorbidity network and outcome in hospitalized patients in Crema, Italy. medRxiv. 2020 doi: 10.1371/journal.pone.0248498. 2020.2004.2014.20053090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duga S., Asselta R., Lazzeri M., Guazzoni G., Azzolini E., Buffi N. Impact of anti-androgenic therapies on COVID-19: an observational study in male population from a COVID-19 regional centre of Lombardy (Italy) medRxiv. 2020 2020.2004.2020.20068056. [Google Scholar]
- 50.Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin J.W., Sutton S.S. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020 doi: 10.1016/j.medj.2020.06.001. 2020.2004.2016.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li K., Chen D., Chen S., Feng Y., Chang C., Wang Z. Radiographic findings and other predictors in adults with covid-19. medRxiv. 2020 doi: 10.1186/s12931-020-01411-2. 2020.2003.2023.20041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giorgi Rossi P., Marino M., Formisano D., Venturelli F., Vicentini M., Grilli R. Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the Province of Reggio Emilia. Italy medRxiv. 2020 doi: 10.1371/journal.pone.0238281. 2020.2004.2013.20063545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabata S., Imai K., Kawano S., Ikeda M., Kodama T., Miyoshi K. The clinical characteristics of COVID-19: a retrospective analysis of 104 patients from the outbreak on board the Diamond Princess cruise ship in Japan. medRxiv. 2020 doi: 10.1016/S1473-3099(20)30482-5. 2020.2003.2018.20038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joharatnam-Hogan N., Hochhauser D., Shiu K.-K., Rush H., Crolley V., Butcher E. Outcomes of the 2019 novel coronavirus in patients with or without a history of cancer – a multi-centre North London experience. medRxiv. 2020 doi: 10.1177/1758835920956803. 2020.2004.2016.20061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu K., Zhao Y., Wang M., Zeng Q., Wang X., Wang M. Identification of a super-spreading chain of transmission associated with COVID-19. medRxiv. 2020 2020.2003.2019.20026245. [Google Scholar]
- 57.Zhang J., Wang X., Jia X., Li J., Hu K., Chen G. Risk factors for disease severity, unimprovement, and mortality of COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26:767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyashita H., Mikami T., Chopra N., Yamada T., Chernyavsky S., Rizk D. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31:1088–1089. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H.-Y., Wang L.-W., Chen Y.-Y., Shen X.-K., Wang Q., Yan Y.-Q. A multicentre study of 2019 novel coronavirus disease outcomes of cancer patients in Wuhan, China. medRxiv. 2020 2020.2003.2021.20037127. [Google Scholar]
- 60.Yang S., Zhang Y., Cai J., Wang Z. Clinical characteristics of COVID-19 after gynecologic oncology surgery in three women: a retrospective review of medical records. Oncologist. 2020;25:982–985. doi: 10.1634/theoncologist.2020-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suppli M.H., Riisgaard de Blanck S., Elgaard T., Josipovic M., Pohl M. Early appearance of coronavirus disease 2019 associated pulmonary infiltrates during daily radiotherapy imaging for lung cancer. J Thorac Oncol. 2020;15:1081–1084. doi: 10.1016/j.jtho.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H., Huang Y., Xie C. The treatment and outcome of a lung cancer patient infected with SARS-CoV-2. J Thorac Oncol. 2020;15:63–64. doi: 10.1016/j.jtho.2020.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouyang W., Yu J., Zhang J., Xie C. Alert to potential contagiousness: a case of lung cancer with asymptomatic SARS-CoV-2 infection. J Thorac Oncol. 2020;15:82–83. doi: 10.1016/j.jtho.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cai Y., Hao Z., Gao Y., Ping W., Wang Q., Peng S. COVID-19 in the perioperative period of lung resection: a brief report from a single thoracic surgery department in Wuhan, China. J Thorac Oncol. 2020;15:1065–1072. doi: 10.1016/j.jtho.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J., Lin H., Wu Y., Fang Y., Kumar R., Chen G. COVID-19 in posttransplant patients-report of 2 cases. Am J Transplant. 2020;20:1879–1881. doi: 10.1111/ajt.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qu J., Yang R., Song L., Kamel I.R. Atypical lung feature on chest CT in a lung adenocarcinoma cancer patient infected with COVID-19. Ann Oncol. 2020;31:825–826. doi: 10.1016/j.annonc.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonomi L., Ghilardi L., Arnoldi E., Tondini C.A., Bettini A.C. A rapid fatal evolution of Coronavirus Disease-19 (COVID-19) in an advanced lung cancer patient with a long time response to nivolumab. J Thorac Oncol. 2020;15:83–85. doi: 10.1016/j.jtho.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin J., Wang H., Qin X., Zhang P., Zhu L., Cai J. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology. 2020;72:1491–1493. doi: 10.1002/hep.31257. [DOI] [PubMed] [Google Scholar]
- 71.Jin X.-H., Zheng K.I., Pan K.-H., Xie Y.-P., Zheng M.-H. COVID-19 in a patient with chronic lymphocytic leukaemia. Lancet Haematol. 2020;7:e351–e352. doi: 10.1016/S2352-3026(20)30074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spezzani V., Piunno A., Iselin H.U. Benign COVID-19 in an immunocompromised cancer patient - the case of a married couple. Swiss Med Wkly. 2020;150:w20246. doi: 10.4414/smw.2020.20246. [DOI] [PubMed] [Google Scholar]
- 73.Feng R.M., Zong Y.N., Cao S.M., Xu R.H. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., Villamizar-Pena R., Holguin-Rivera Y., Escalera-Antezana J.P. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ioannidis J.P.A., Axfors C., Contopoulos-Ioannidis D.G. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. medRxiv. 2020 doi: 10.1016/j.envres.2020.109890. 2020.2004.2005.20054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parohan M., Yaghoubi S., Seraji A., Javanbakht M.H., Sarraf P., Djalali M. Risk factors for mortality in patients with coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. medRxiv. 2020 doi: 10.1080/13685538.2020.1774748. 2020.2004.2009.20056291. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y., Cui Y., Shen M., Zhang J., Liu B., Dai M. Comorbid diabetes mellitus was associated with poorer prognosis in patients with COVID-19: a retrospective cohort study. medRxiv. 2020 2020.2003.2024.20042358. [Google Scholar]
- 78.Chao D.T., Lim J.K., Ayoub W.S., Nguyen L.H., Nguyen M.H. Systematic review with meta-analysis: the proportion of chronic hepatitis B patients with normal alanine transaminase </= 40 IU/L and significant hepatic fibrosis. Aliment Pharmacol Ther. 2014;39:349–358. doi: 10.1111/apt.12590. [DOI] [PubMed] [Google Scholar]
- 79.Sonda T., Kumburu H., van Zwetselaar M., Alifrangis M., Lund O., Kibiki G. Meta-analysis of proportion estimates of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob Resist Infect Control. 2016;5:18. doi: 10.1186/s13756-016-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agarwal R., Aggarwal A.N., Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care. 2010;55:1653–1660. [PubMed] [Google Scholar]
- 81.Price J., Lee J., Willcox M., Harnden A. Place of death, care-seeking and care pathway progression in the final illnesses of children under five years of age in sub-Saharan Africa: a systematic review. J Glob Health. 2019;9 doi: 10.7189/jogh.09.020422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu R., Ming X., Xu O., Zhou J., Peng H., Xiang N. Association of cardiovascular manifestations with in-hospital outcomes in patients with COVID-19: a hospital staff data. medRxiv. 2020 2020.2002.2029.20029348. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.