Abstract
Our previous work demonstrated that Piwil2 reactivated by the human papillomavirus oncoproteins E6 and E7 may reprogram somatic cells into tumor-initiating cells (TICs), which contribute to cervical neoplasia lesions. Maintaining the stemness of TICs is critical for the progression of cervical lesions. Here, we determined that canonical Wnt signaling was aberrantly activated in HaCaT cells transfected with lentivirus expressing Piwil2 and in cervical lesion specimens of low-grade squamous intraepithelial lesion, high-grade squamous intraepithelial lesion, and invasive carcinoma. Blocking the β-catenin and CREB binding protein interaction with ICG-001 significantly downregulated the reprogramming factors c-Myc, Nanog, Oct4, Sox2, and Klf4, thus leading to cell differentiation and preventing tumorigenicity in Piwil2-overexpressing HaCaT cells. Similarly, Piwil2 also critically regulated the canonical Wnt signaling pathway in cervical cancer. We further demonstrated that ICG-001 increased cisplatin sensitivity and significantly suppressed tumor growth of cervical cancer alone or in combination with cisplatin both in vitro and in vivo. The β-catenin/ CREB binding protein-mediated transcription activated by Piwil2 is essential for the maintenance of TICs, therefore contributing to the progression of cervical oncogenesis.
Keywords: Cervical cancer, Piwil2, Wnt signaling, Tumor initiating cell, CBP
Abbreviations: CBP, CREB binding protein; CSC, cancer stem cell; DICD, differentiation-induced cell death; FIGO, the international federation of gynecology and obstetrics; GSEA, gene set enrichment analysis; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; IHC, immunohistochemical; IPP, image-pro plus; LSIL, low-grade squamous intraepithelial lesion; pCSC, precancerous stem cell; PVDF, polyvinylidene fluoride; SCC, squamous carcinoma of the cervix; TCF, T-cell factor; TFs, transcription factors; TIC, tumor initiating cell
Introduction
Wnt signaling is an evolutionarily conserved pathway that plays essential roles during embryo development and in the maintenance of adult tissue homeostasis [1], [2], [3], [4], [5], [6]. Accumulating evidence also shows that the Wnt/β-catenin pathway regulates many key aspects of cancer development, including maintaining cancer stem cells and promoting metastasis, cell survival, and chemoresistance [1,[7], [8], [9], [10], [11], [12], [13]. Furthermore, the level of Wnt signaling correlates with tumor-initiating capacity in xenotransplantation assays [14], [15], [16]. Some studies show that differential coactivator usage in Wnt/β-catenin signaling appears to be a critical regulator of the maintenance of the stem state and the initiation of differentiation [11,[17], [18], [19], [20]. It has been demonstrated that the p300/β-catenin interaction drives the differentiation of adult stem/progenitor cells, while CREB binding protein (CBP)/β-catenin promotes progenitor maintenance/self-renewal [18,21].
Cervical cancer is intimately associated with high-risk human papillomavirus infection and progresses from its precursor stages of low-grade squamous intraepithelial lesion (LSIL) and high-grade squamous intraepithelial lesion (HSIL) to invasive carcinoma [22], [23], [24], [25], [26]. It is well known that the oncoproteins E6 and E7 of high-risk human papillomavirus are the primary viral factors responsible for the initiation and progression of cervical cancer [27], [28], [29], [30], [31]. A number of extensive studies have shown that some somatic cell reprogramming factors, such as c-Myc, Nanog, Oct4, Sox2, and Klf4, are abnormally modulated and functionally alter signaling pathways during cervical carcinogenesis [32], [33], [34], [35]. Our recent study further demonstrated that Piwil2, reactivated by E6 and E7, induces upregulation of these factors and subsequently initiates cell reprogramming, eventually leading to transformation of cervical epithelial cells into tumor initiating cells (TICs) [28]. However, the precise regulation of the maintenance of TIC stemness, which promotes cervical intraepithelial neoplasia to invasive cancer, and the treatment targeting these TICs is yet to be determined.
Here, we demonstrate that Piwil2, which initiated cell reprogramming, also synchronously activated Wnt3a signaling to enhance reprogramming and further maintain the stemness of TICs via the CBP/β-catenin interaction. Blocking the CBP/β-catenin interaction with ICG-001 can significantly downregulate the expression of cell reprogramming factors, thus inducing early cell differentiation and, to some extent, increasing cisplatin sensitivity in cervical cancer cells. Therefore, inhibiting the CBP/β-catenin interaction may be a potent therapeutic strategy for cervical precancerous lesions and cervical cancer when combined with cisplatin.
Materials and methods
Clinical samples
All specimens consisting of 8 normal cervixes, 10 LSIL, 8 HSIL, and 9 squamous carcinoma of the cervix (SCC) were collected at the Department of Pathology of China-Japan Friendship Hospital during the period of 2012 to 2018. The inclusion criteria included normal cervical tissues obtained from patients who underwent a hysterectomy for reasons other than neoplasia of the cervix and had no history of cervical lesions, and cervical neoplasm specimens obtained from patients diagnosed with LSIL, HSIL, or SCC and underwent surgery or cervical biopsy. The exclusion criteria were the patients who had received any tumor-specific therapy before the specimens were collected. The histological classifications and clinical staging were carried out in accordance with the International Federation of Gynecology and Obstetrics classification system published in 2018. The present study was approved by the Ethics Committee of China-Japan Friendship Hospital. All patients provided informed consent prior to specimen collection.
Immunohistochemistry
Cervical tissues from patients were fixed in 4% paraformaldehyde and subsequently embedded in paraffin. These paraffin blocks of specimens were cut into 5-µm slices. Then, the slices were deparaffinized, rehydrated, and antigen retrieval was performed using standard techniques. The slices were incubated overnight at 4 °C with primary antibodies used as follows: 1:500 for anti-Wnt3a (ab28472, Abcam, USA), 1:500 for anti-β-catenin (ab32572, Abcam), 1:1000 for anti-CBP (ab50702, Abcam), 1:1000 for anti-SOX2 (ab92494, Abcam), and 1:1000 for anti-involucrin (ab53112, Abcam). The binding of the primary antibodies was visualized using the ChemMate Detection Kit (Boster, China). The slices were lightly counterstained with Mayer's hematoxylin for 30 s.
Immunoreactivity was semiquantitatively evaluated. The immunoreactivity score was evaluated as the intensity score × proportion score. The intensity score was defined as follows: 0, negative; 1, weak; 2, moderate; or 3, strong. The proportion score was defined as follows: 0, negative; 1, <10%; 2, 11% to 50%; 3, 51% to 80%; or 4, >80% positive cells. The total score ranged from 0 to 12. Two different pathologists evaluated all the specimens in a blinded manner.
Cell culture, transfection, and treatment
Cervical cancer cell lines (HeLa, SiHa) and HaCaT cells were purchased from the American Type Culture Collection and cultured according to their specifications at 37 °C in a humidified 5% CO2 incubator.
HeLa, SiHa and HaCaT cells were cultured in 96-well cell culture dishes and transfected with the lentiviral vector pLenti-CMV-Piwil2-SV40-EGFP for Piwil2 overexpression. To knock down Piwil2 expression, HeLa and SiHa cells were transfected with the lentiviral vector pLenti-shPiwil2-Ub-EGFP-IRES-puro (Table S1). Cells stably expressing the shRNA were selected with puromycin (9620, Sigma-Aldrich, USA) for 2 wk. Cells transfected with lentiviral empty vector were used as the control.
ICG-001 (20 µM, S2662, Selleckchem, USA), a specific inhibitor of the β-catenin/CBP interaction, IQ-1 (20 µM, S8248, Selleckchem), indirectly blocking the p300/β-catenin interaction by binding to the PR72/130 subunit of the serine/threonine phosphatase PP2A, or Dimethyl Sulfoxide (DMSO) (vehicle control) was added to media from the time of cell plating through completion of the experiment. Cervical cancer cell lines were treated with cisplatin (3, 5, 10, 15 µg/mL, P4394, Sigma-Aldrich) alone or in combination with ICG-001 or IQ-1. The control cells were treated with vehicle alone.
Cell viability and colony formation
Cells were seeded in 96-well plates at a concentration of 1 × 103 cells/well and treated as described above. Cell viability was evaluated using the WST-8 Cell Counting kit (CCK-8, Dojindo, Japan) per the manufacturer's instructions at 24, 48, 72, and 96 h. The LD50 values were calculated by nonlinear regression analysis using GraphPad Prism software (San Diego, USA). All experiments were performed independently in triplicate. To test colony formation, 2 × 103 cells were harvested and cultured in 6-well plates after treatment with ICG-001 or IQ-1 for 48 h. The medium was changed every 3 d. Approximately 2 wk later, the clones were imaged and quantified with Image-Pro Plus 6.0 (IPP6.0, Media Cybernetics, Inc.) after staining with 0.05% crystal violet.
Real-time RT-PCR
Total RNA was isolated and reverse transcribed by using Superscript III reverse transcriptase (2680, TaKaRa, Japan). Real-time RT-PCR was performed using the iQ SYBR Green Supermix kit (170-8880, Bio-Rad, USA) on a CFX96 Touch real-time polymerase chain reaction (PCR) instrument (Bio-Rad). The primer sets are presented in Table S2.
Immunoblotting
Antibodies specific to Piwil2 (ab85084, 1:1000), Wnt3a (ab28472, 1:1000), β-catenin (ab32572, 1:5000), T-cell factor (TCF; ab76151, 1:2000), P300 (ab14984, 1:500), CBP (ab50702, 1:100), c-Myc (ab32072, 1:500), Nanog (ab80892, 1:500), Oct4 (ab181557, 1:1000), Sox2 (ab137385, 1:500), Klf4 (ab72543, 1:1000), loricrin (ab137533, 1:500), and involucrin (ab53112, 1:500) were obtained from Abcam. Antibody specific to histone H3 (AH433, 1:1000) was purchased from Beyotime Biotechnology. Antibody specific to GAPDH (#2118, 1:2000) was purchased from Cell Signaling Technology. Protein was prepared from the cells and tissues according to the kit manual (89900, Thermo, USA) except for the addition of an acid extraction step for histones. After electrophoresis, the proteins were transferred to polyvinylidene fluoride (IPFL00010, Merck Millipore, Germany) membranes and probed with the indicated primary antibodies. Incubation with species-specific secondary antibodies (Cell Signaling) was performed at room temperature for 1 h. The blots were developed with chemiluminescent substrate (34080, Thermo), and autoradiography was performed with X-OMAT film (Kodak, Rochester, NY, USA).
Coimmunoprecipitation
For coimmunoprecipitation of β-catenin with CBP or p300, HaCaT-Piwil2 cells were treated with 20 µM ICG-001 or 20 µM IQ-1 for 24 h, after which they were washed and lysed. The nuclear fraction was isolated and precleared with an antibody against β-catenin (ab32572, 1:50). The immunoprecipitated proteins were then subjected to Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and detected by using an antibody against CBP (ab50702, 1:100) or p300 (ab14984, 1:500).
Flow cytometry analysis
An Annexin V-633/PI Apoptosis Detection Kit (AD11, Dojindo) was used to evaluate cell apoptosis. After treatment with 20 µM ICG-001 or 20 µM IQ-1 for 24 h, the cells were collected and labeled with 5 µL Annexin V-633 and 5 µl PI for 15 min in the dark at room temperature. For analysis of the expression of stem cell markers, the cells were labeled with anti-ALDH1A1 (ab52492, 1:20) and subsequently stained with the following cocktail of antibodies: anti-rabbit IgG-Alexa Fluor Plus 647, anti-CD49f-eFluor 450, anti-338-PE, and anti-Oct4-eFluor 660 (A32733, 48-0495-82, 12-8888-42, 50-5841-82, Invitrogen, USA). Cells were then washed, resuspended in 1 × PBS with 2% FBS, and analyzed by flow cytometry (FACSCalibur, BD).
Immunofluorescence staining
Cells were cultured on adhesive slides and treated with 20 µM ICG-001, 20 µM IQ-1, or vehicle alone for 24 h, fixed with prechilled methanol at −20 °C for 5 min and subsequently permeabilized with 0.2% Triton X-100 in 1 × PBS for 10 min. Immunostaining was carried out by using standard protocols. Primary antibodies were used at the following dilutions: anti-CK 17 (ab53707, 1:500) and anti-CK14 (sc-53253, Santa Cruz Biotechnology, 1:100). The secondary antibody was TRITC goat anti-mouse IgG (H+L) (ZF-0313, ZSGB-Bio, 1:100). After costaining with DAPI, cells were imaged by using an Olympus IX-71 microscope.
Microarray and gene set enrichment analysis
Three HaCaT-Lenti cells and three HaCaT-Piwil2 cells were used for the microarrays. Genome-wide mRNA microarray analysis was performed as described previously [28]. Gene expression data were analyzed with Gene Set Enrichment Analysis (GSEA) software (http://software.Broadinstitute.org/gsea/index.jsp) [36].
Animal studies
In vivo experiments were performed in accordance with the institutional guidelines for the use of laboratory animals. Four-wk-old female nu/nu nude mice (Beijing Vital River Laboratory Animal Technology Co., Ltd, China) were fed in a pathogen-free animal facility for at least 1 wk before use. Cells (5 × 106) in the exponential growth phase were harvested and injected subcutaneously into the dorsum of each mouse. Once palpable tumors were established, to observe tumor-suppressing effects, the mice were randomly assigned into six groups based on the treatment received: ICG-001 (300 mg/kg), IQ-1 (300 mg/kg), and DDP (2 mg/kg) treatment alone; ICG-001 and DDP in combination; and IQ-1 and DDP in combination. Moreover, to evaluate the tumorigenesis of HaCaT-Piwil2 cells, the mice were administered ICG-001 (300 mg/kg) or IQ-1 (300 mg/kg). The mice received intraperitoneal injections twice weekly. The control group received the vehicle alone following the same schedule. Tumors were measured with calipers twice weekly, and the volume was calculated as V=(length × width2)/2. At the end of the experiment, the mice were sacrificed, and the tumors were collected and weighed.
Statistical analyses
The data are presented as the mean ± SD. To assess the statistical significance of the differences, one-way analysis of variance or unpaired Student's t test (SPSS software, version 19.0, SPSS Inc., USA) was performed. A P value < 0.05 was considered significant.
Results
Piwil2 initiates the gene expression signatures of stem cells and activates Wnt3a/β-catenin signaling
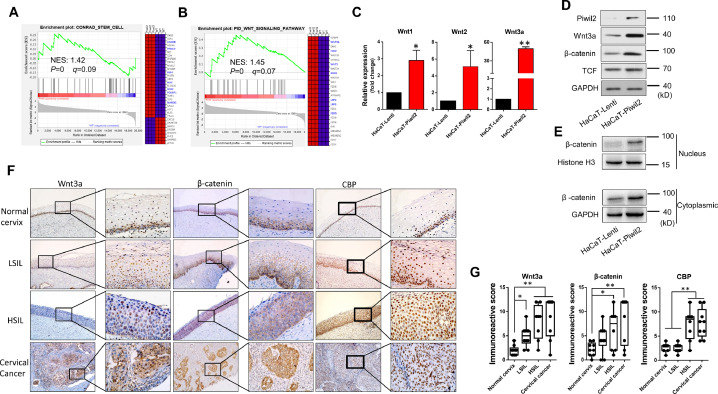
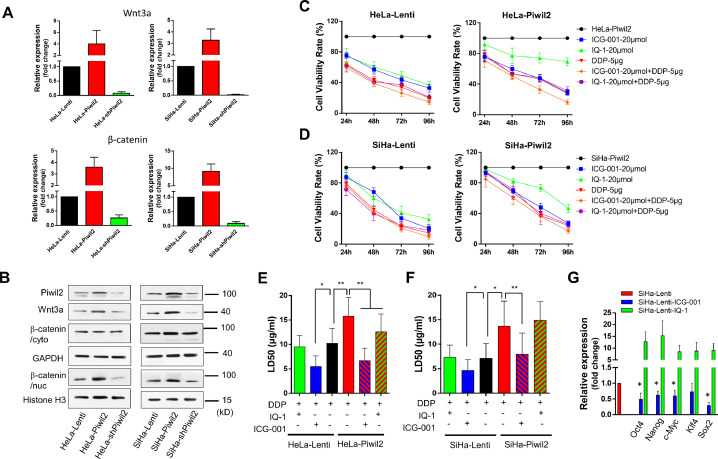
Microarray assay and gene set enrichment analysis (GSEA) performed on HaCaT cells with or without Piwil2 overexpression revealed a positive enrichment of gene signatures related to stem cells (MSigDB v5.0, C2) in HaCaT-Piwil2 cells (Figure 1A and Table S3). In particular, the essential genes Lin28B, Klf4, Myc, Sox2, Pou5f1 (Oct4), and Nanog were differentially upregulated in HaCaT-Piwil2 vs HaCaT-Lenti cells (Figure 1A). Moreover, the level of the gene set for the Wnt signaling pathway (MSigDB v5.0, C2) was also elevated in HaCaT-Piwil2 cells (Figure 1B and Table S3), which may play a key role in maintaining cancer stem cells and oncogenesis. We further confirmed that Piwil2 overexpression induced a significant upregulation of Wnt3a and activated Wnt/β-catenin signaling (Figure 1C–E). As shown in Figure 1E, β-catenin accumulated in the cytoplasm, and nuclear and TCFs were also upregulated in HaCaT-Piwil2 cells, which is the main hallmark of Wnt activation [37]. Together, these observations indicated that Piwil2 led to the transformation of somatic cells to TICs and the accompanying activation of the canonical Wnt signaling pathway, subsequently eliciting Wnt target gene expression via TCF.
Figure 1.
Wnt/β-catenin signaling activation in Piwil2 reprograming HaCaT cell and in cervical lesions. (A) GSEA plot showing a significant enrichment of the stemness gene set and heat map showing the gene lists related to cell reprogramming in HaCaT cells overexpressing Piwil2. (B) GSEA demonstrating that Piwil2 expression positively correlated with Wnt/β-catenin signaling pathway activation. (C) Real-time PCR analysis of Wnt1, Wnt2, and Wnt3a mRNA expression in HaCaT and HaCaT-Piwil2 cells. (D and E) Immunoblotting of Wnt3a, TCF, and β-catenin in the cytoplasm and nucleus. (F) Representative sections showing immunohistochemical staining of Wnt3a, β-catenin, and CBP in normal cervix, LSIL, HSIL, and cervical cancer specimens. (G) The immunoreactivity score for Wnt3a, β-catenin, and CBP. The data are presented as the mean±SD. *P < 0.05 and **P < 0.01 by Student's t test.
Further we explored the histological patterns of the Wnt signal transduction cascade throughout cervical cancer development. We measured Wnt3a and β-catenin expression by immunohistochemical (IHC) analysis of specimens derived from cervical lesions (Figure 1F and G). The IHC showed that in the normal cervix, the reserve cells in the basal layer, a potential stem cell population of the uterine cervix, were consistently positive for Wnt3a, β-catenin and CBP. In preneoplastic lesions, LSIL showed 25% to 50% of positive cells in the intermediate compartment, while HSIL showed immunostaining with a greater percentage of 75% to 100%. In cervical cancer, the Wnt pathway components were all positively stained with strong immunoreactivity intensity. In addition, the expression pattern of Sox2 was similar to that of its upstream Wnt/β-catenin in cervical lesions, while involucrin, an early differentiation marker of the epidermis, was negatively correlated with the degree of cervical lesion (Figure S1).
Inhibition of the β-catenin/CBP interaction induces HaCaT-Piwil2 cell differentiation
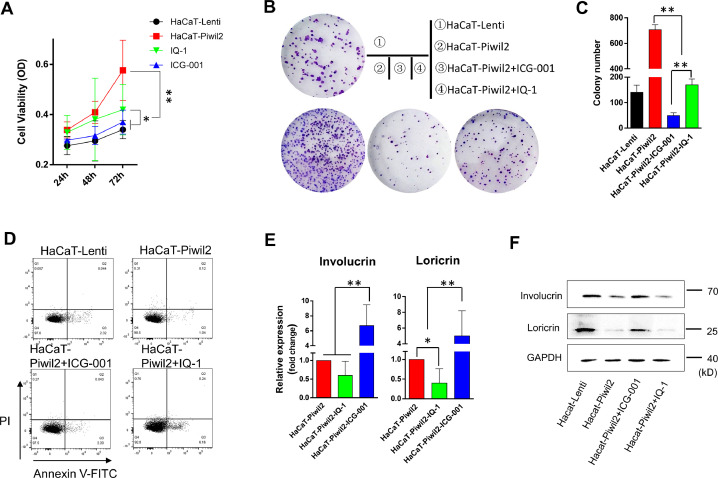
Differential recruitment of CBP and p300 accounts for the dichotomous behavior of Wnt/β-catenin-dependent signaling in controlling stem cell function [18]. Blocking the β-catenin/CBP interaction with 20 µM ICG-001 led to a significant inhibition of cell proliferation and a remarkable decrease in colony formation in HaCaT-Piwil2 cells (Figure 2A–C). IQ-1, which also specifically inhibits the β-catenin/p300 interaction, also caused an evident decrease in both cell proliferation and colony formation, but the efficiency was inferior to that of ICG-001 (Figure 2A–C). Cell apoptosis was detected after HaCaT-Piwil2 cells were exposed to 20 µM ICG-001 or IQ-1 for 24 h. The data showed that both treatments caused no significant increase in cell apoptosis (Figure 2D). As shown in Figure 2E and F, Piwil2 overexpression significantly downregulated the expression of markers of epidermal differentiation loricrin and involucrin. However, ICG-001 treatment for 24 h led to a remarkable increase in loricrin and involucrin, while 24-h exposure to IQ-1, caused a decrease in these markers to some extent (Figure 2E and F). These findings suggest that β-catenin/CBP-mediated transcription is critical for maintaining the stemness phenotype and proliferation, whereas blocking the β-catenin/p300 interaction can prevent cell differentiation.
Figure 2.
Blocking the β-catenin/CBP interaction induces HaCaT-Piwil2 cell differentiation. (A) HaCaT cells were transfected with lentivirus containing Piwil2 and treated with ICG-001 or IQ-1, and cell viability was evaluated daily. (B and C) Colony formation numbers were measured in HaCaT-Piwil2 cells after treatment with 20 µM ICG-001 or IQ-1. (D) Cell apoptosis in HaCaT-Piwil2 cells after treatment with 20 µM ICG-001 or IQ-1. (E and F) The expression of the markers of epidermal differentiation loricrin and involucrin was determined by real-time PCR and immunoblotting in HaCaT-Piwil2 cells treated with 20 µM ICG-001 or IQ-1 for 24 h. The data are presented as the mean ± SD. *P < 0.05 and **P < 0.01 by Student's t test.
ICG-001 suppresses tumorigenesis of HaCaT-Piwil2 cells in vivo
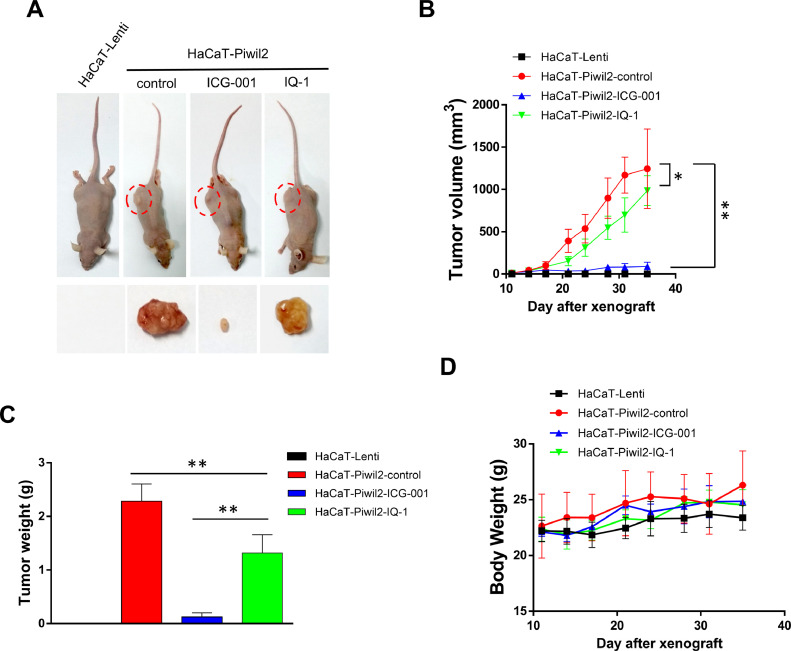
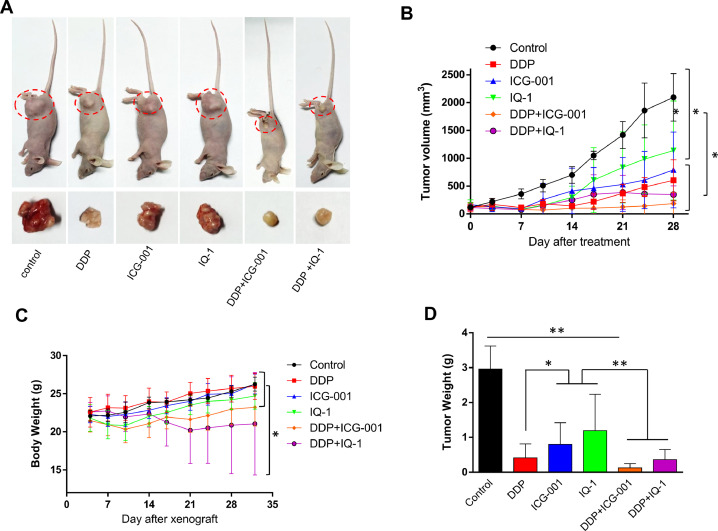
To assess the tumorigenicity of HaCaT cells reprogrammed by Piwil2, 5 × 106 HaCaT-Piwil2 cells subcutaneously transplanted into nude mice formed tumors with a mean volume of 1243.97 ± 469.43 mm3 at the end of 4 wk, whereas no tumor formation was observed in the mice with subcutaneous transplantation of HaCaT-Lenti cells (Figure 3A and B). To further identify the effect of ICG-001 and IQ-1 on tumor xenograft growth, the mice received intraperitoneal injections of 300 mg/kg ICG-001 or IQ-1 twice weekly for 4 wk as the tumors were established. After 4 wk of treatment, the tumors were significantly suppressed by ICG-001 (89.04 ± 51.68 mm3) but to a lesser extent suppressed by IQ-1 (986.19 ± 175.67 mm3; Figure 3A and B). Consistent with the tumor volume data, the mean tumor weight of the control group was 2.28 ± 0.32 g, while the tumor weights of the ICG-001 and IQ-1 groups were 0.12 ± 0.08 g and 1.32 ± 0.34 g, respectively (Figure 3C). Both ICG-001 and IQ-1 showed no overt toxicity in mice, as no weight loss was observed after treatment (Figure 3D).
Figure 3.
ICG-001 suppresses tumorigenesis of HaCaT-Piwil2 cells in vivo. A total of 5 × 106 HaCaT-Piwil2 or HaCaT-Lenti cells were injected subcutaneously into nude mice. After the tumors were palpable, the mice received an intraperitoneal injection of 300 mg/kg of ICG-001 or IQ-1 twice weekly for 4 wk. Five mice per group were used. (A) Representative image of mice carrying tumors and excised tumors after 4 wk of treatment. (B) Tumor volume was monitored by caliper measurements twice a week. (C) Tumor weight was measured after sacrifice at the end of the experiment. (D) The body weight of the mice was measured twice a week. The data are presented as the mean ± SD. *P < 0.05 and **P < 0.01 by Student's t test.
CBP/β-catenin promotes the maintenance of stem cell reprogramming by Piwil2
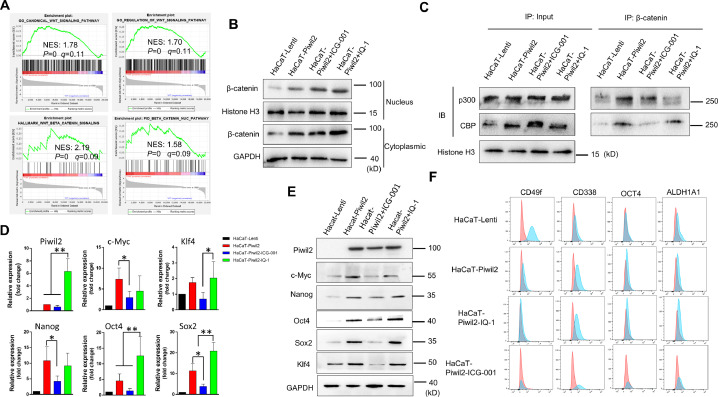
We analyzed the biological role of Piwil2 in the activation of canonical Wnt signaling in HaCaT-Piwil2 cells by GSEA. The GSEA results showed that overexpression of Piwil2 was positively associated with Wnt/β-catenin signaling pathway activation and translocation of β-catenin into the nucleus (Figure 4A and Table S3). We determined that significant accumulation of β-catenin in the cytoplasm coincided with more translocation of β-catenin into the nucleus and clear upregulation of TCF in HaCaT-Piwil2 cells compared to HaCaT-Lenti cells, while both ICG-001 and IQ-1 showed no inhibition of it (Figures 1D and 4B). We further demonstrated that β-catenin in the nucleus interacted with the coactivators CBP and p300, and the balance could be interrupted if the interaction was inhibited with specific inhibitors. The results of coimmunoprecipitation showed that ICG-001 caused a dramatic increase in the relative amount of p300, while IQ-1 led to an evident increase in CBP (Figure 4C). We further investigated β-catenin/CBP- and β-catenin/p300-mediated gene expression. As our previous report described, overexpression of Piwil2 induced a significant upregulation of c-Myc, Nanog, Oct4, Sox2, and Klf4, and these pluripotency transcription factors (TFs) are closely related to cell reprogramming and stemness maintenance [28,32,33,35]. Blocking the β-catenin/CBP interaction with ICG-001 led to a significant decrease in these TFs, while IQ-1 inhibition of the β-catenin/p300 interaction enhanced the expression of these TFs compared with ICG-001 treatment (Figure 4D and E). Moreover, IQ-1 treatment also upregulated Piwil2 expression significantly while ICG-001, to some extent, led to downregulation of Piwil2 (Figure 4D and E). Therefore, ICG-001 induced cellular differentiation, and IQ-1 contributed to the maintenance of stemness dependent on β-catenin/p300 switching to β-catenin/CBP (Figure 4F and Figure S2).
Figure 4.
CBP/β-catenin promotes the maintenance of stem cell reprogramming by Piwil2. (A) GSEA plot showing significant enrichment of the Wnt/β-catenin signaling activation modules in HaCaT-Piwil2 cells. (B) Immunoblotting of β-catenin translocated into the nucleus in HaCaT-Piwil2 cells after treatment with 20 µM ICG-001 or IQ-1 for 24 h. (C) Nuclear lysates from HaCaT-Piwil2 cells treated with 20 µM ICG-001, 20 µM IQ-1, or DMSO were coimmunoprecipitated with antisera to β-catenin and immunoblotted for CBP and p300. (D and E) The expression of Piwil2 and the “reprogramming” factors c-Myc, Nanog, Oct4, Sox2, and Klf4 was determined by real-time PCR and immunoblotting in HaCaT-Piwil2 cells treated with 20 µM ICG-001, 20 µM IQ-1, or DMSO for 24 h. (F) The proportion of CD49f-, CD338-, OCT4-, and ALDHA1-positive cells, determined by flow cytometry in HaCaT-Piwil2 cells treated with 20 µM ICG-001, 20 µM IQ-1, or DMSO for 24 h. The data are presented as the mean ± SD. *P < 0.05 and **P < 0.01 by Student's t test.
Blocking the β-catenin/CBP interaction reduces cell viability, drug resistance, and pluripotency transcription factors in cervical cancer cells
To further confirm that Piwil2 activates the canonical Wnt pathway, we evaluated the expression of Wnt3a and β-catenin in cervical cancer cell lines as Piwil2 was overexpressed or silenced. Our data showed that the overexpression of Piwil2 caused significant upregulation of Wnt3a and accumulation of β-catenin in the nucleus, while Piwil2 knockdown led to the opposite results (Figure 5A and B). Thus, to some extent, treatment with 20 µM ICG-001 or IQ-1 inhibited the growth of HeLa-Lenti and SiHa-Lenti cells and resulted in a significantly higher inhibition rate in combination with 5 µg/mL cisplatin. However, IQ-1 was less effective than ICG-001 when used alone in HeLa and SiHa cells overexpressing Piwil2. The combination of ICG-001 and cisplatin exerted a more significant effect on growth inhibition than either ICG-001, IQ-1 or cisplatin alone or IQ-1 in combination with cisplatin (Figure 5C and D). Moreover, ICG-001 treatment led to significant downregulation of c-Myc, Nanog, Oct4, and Sox2 but inconspicuous downregulation of Klf4 (Figure 5G), which was accompanied by a significant LD50 decrease in cisplatin in HeLa and SiHa cells with or without Piwil2 overexpression (Figure 5E and F). There was no notable effect of IQ-1 on modulating the sensitivity to cisplatin even though IQ-1 treatment caused a significant upregulation of c-Myc, Nanog, Oct4, Sox2, and Klf4 (Figure 5G).
Figure 5.
Blocking the β-catenin/CBP interaction reduces cell viability, drug resistance, and the expression of pluripotency transcription factors in cervical cancer cells. (A) The expression of Wnt3a and β-catenin was measured by real-time PCR in cervical cancer cell lines with Piwil2 overexpression or knockdown. (B) Immunoblotting for Wnt3a and β-catenin in the cytoplasm and nucleus, respectively, in cervical cancer cell lines with Piwil2 overexpression or knockdown. (C and D) Cervical cancer cell lines with or without Piwil2 overexpression were treated with 20 µM ICG-001, 20 µM IQ-1, or 5 µg/mL cisplatin alone, 20 µM ICG-001 and 5 µg/mL cisplatin in combination, or 20 µM IQ-1 and 5 µg/mL cisplatin in combination, and cell viability was evaluated daily. (E and F) The LD50 dose of cisplatin in the presence of ICG-001 or IQ-1 in cervical cancer cell lines with or without Piwil2 overexpression. G The expression of pluripotency transcription factors c-Myc, Nanog, Oct4, Sox2, and Klf4 was determined by real-time PCR in SiHa cells treated with 20 µM ICG-001 or 20 µM IQ-1 for 24 h. The data are presented as the mean ± SD. *P < 0.05 and **P < 0.01 by Student's t test.
Blocking Wnt/β-catenin confers tumor-suppressing effects in cervical cancer xenografts
Experiments testing the therapeutic efficacy of ICG-001 and IQ-1 were extended to an in vivo model of cervical cancer. As shown in Figure 6, ICG-001 was more effective in suppressing tumor xenograft growth than IQ-1 but inferior to cisplatin. Notably, cotreatment with ICG-001 and cisplatin strongly inhibited tumor growth. In addition, chronic administration of ICG-001 and cisplatin, at the indicated dose and schedule, was well tolerated with no significant weight loss observed in the animals, while treatment with the combination of IQ-1 and cisplatin led to obvious weight loss from 3 wk after administration (Figure 6C). Together, these data highlight the therapeutic efficacy of ICG-001 and cisplatin in inhibiting tumor progression.
Figure 6.
Blocking Wnt/β-catenin suppressed the xenograft growth of cervical cancer in vivo. Female nude mice were subcutaneously injected with 5 × 106 HeLa cells. After the tumors were palpable, the mice received intraperitoneal injections of ICG-001 (300 mg/kg), IQ-1 (300 mg/kg), or DDP (2 mg/kg) alone, ICG-001, and DDP in combination or IQ-1 and DDP in combination twice weekly for 4 wk. (A) Representative image of mice carrying tumors and excised tumors after 4 wk of treatment. (B) Tumor volume was monitored by caliper measurements twice a week. (C) The body weight of the mice was measured twice a week. (D) Tumor weight was measured after sacrifice at the end of the experiment. The data are presented as the mean ± SD. *P < 0.05 and **P < 0.01 by Student's t test.
Discussion
We have demonstrated that Piwil2, which is reactivated by oncoproteins E6 and E7 in high-risk HPV-infected cervical epithelium, reprogrammed somatic cells into TICs, thereby leading to oncogenesis of the cervix [28]. Maintaining the stem cell potential of TICs will constantly promote the transition of cervical neoplasia into invasive cancer, while inducing the differentiation of TICs and differentiation-induced cell death may lead to spontaneous regression of cervical lesions [38], [39], [40]. It is now widely accepted that Wnt/β-catenin signaling may preferentially influence progenitor cell expansion in development and in cancer [7,[41], [42], [43], and aberrant Wnt signaling is essential for the tumorigenesis and maintenance of cancer stem cells in many types of cancer [1,[44], [45], [46], [47]. In the present study, gene set enrichment analysis revealed significant upregulation of the canonical_Wnt_signaling and β-catenin_nuc_pathway gene sets in HaCaT-Piwil2 cells, which was further verified by Q-PCR and Western blot analysis. Wnt3a notably increased, and more β-catenin translocated into the nucleus, accompanied by upregulation of TCF (Figure 1). In the specimens of cervical lesions, the IHC results also showed that Wnt3a and β-catenin were positive in LSIL, HSIL, and cervical cancer and well correlated with pathological grading (Figure 1F and Figure S1). These data indicate that Wnt/β-catenin signaling plays an essential role in maintaining the stemness of TICs reprogrammed by Piwil2, which inevitably contributes to cervical neoplasia progression.
In the canonical Wnt signaling cascade, β-catenin partners with members of the TCF/LEF family of transcription factors and recruits coactivator CBP or p300 to transcribe Wnt target genes [10,21,48]. Thus, Wnt/β-catenin signaling may play a dichotomous role in stem cell biology in terms of self-renewal, cell proliferation and differentiation [10,18,49]. Consistent with previous studies [19,49], blocking the CBP/β-catenin interaction with 20 µM ICG-001 significantly promoted cell differentiation in HaCaT-Piwil2 cells, while blocking the p300/β-catenin interaction with 20 µM IQ-1 inhibited cell differentiation and maintained the undifferentiated state of HaCaT-Piwil2 cells by upregulating the TFs c-Myc, Nanog, Oct4, Sox2, and Klf4 (Figure 4). Thus, diminishing the β-catenin/p300 interaction and promoting β-catenin coactivator switching from p300 to CBP is critical for maintenance of TICs reprogrammed by Piwil2. Our data showed that Piwil2 expression also be affected by these two coactivators, supposedly maybe attribute to the stemness of TICs. However, the exact mechanism merit further investigation. As IQ-1 was not the direct specific p300/β-catenin antagonist, IQ-1 also inhibited cell growth and colony formation in vitro and suppressed tumorigenesis in vivo, even though ICG-001 had superior efficacy to IQ-1 (Figures 2 and 3). This likely implies that IQ-1 may have other effects independent of blocking the p300/β-catenin interaction via binding to the PR72/130, which need to be further investigated in TICs.
Piwil2 is thought to play an important role in tumor initiation because it is predominantly expressed in precancerous stem cells and cancer stem cells [38,[50], [51], [52]. Here, we verified that Wnt3a can be induced by Piwil2 in HaCaT cells and was positively correlated with the pathological grading of cervical lesions. Knocking down Piwil2 led to significant downregulation of Wnt3a in cervical cancer cell lines (Figure 5). Furthermore, blocking the CBP/β-catenin interaction with ICG-001 remarkably increased cisplatin sensitivity and thereafter ameliorated toxicity when combined with cisplatin in the treatment of cervical cancer both in vitro and in vivo (Figures 5 and 6). All of these findings indicate that the Wnt/β-catenin pathway regulates many key aspects of cervical neoplasia development, such as maintaining TICs, cancer cell survival, and chemoresistance.
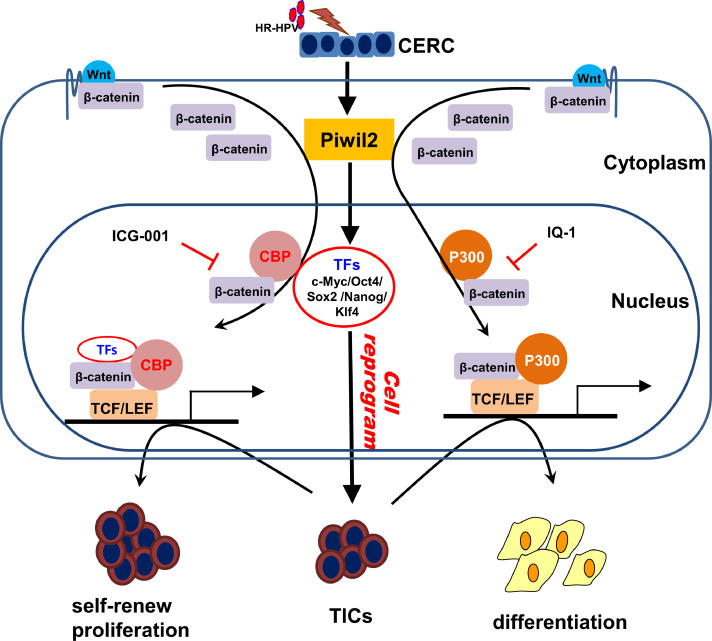
Some reports suggest that Wnt signaling is also involved in stem cell maintenance by modulating the levels of intrinsic pluripotency factors, such as Oct4, Nanog, and Sox2 [21,32,33,49]. Moreover, Wnt/β-catenin signaling has also been highlighted to stimulate nuclear reprogramming [49,53,54]. It has been reported that Wnt signaling turned on a “reprogramming” factor in the nuclei of pluripotent cells, which allowed more effective conversion of differentiated cell nuclei to a pluripotent program [55], [56], [57]. We previously verified that Piwil2 may initiate cell reprogramming via evident upregulation of the “reprogramming” factors c-Myc, Nanog, Oct4, Sox2, and Klf4, subsequently leading to tumorigenesis [28]. Blocking the CBP/β-catenin interaction significantly downregulated these “reprogramming” factors, thus leading to cell differentiation, whereas IQ-1 treatment resulted in diminished p300/β-catenin interaction and a concomitant increase in these “reprogramming” factors (Figure 4), which was likely attributed to IQ-1 preventing β-catenin from switching coactivator usage from CBP to p300 [19]. Therefore, it is worth noting that IQ-1 may enhance cell reprogramming and the sequential progression of neoplasia even though it, to some extent, prohibits differentiated cell growth. This indicates that β-catenin mainly recruits and partners with coactivator CBP and some of these “reprogramming” factors work together to maintain the undifferentiated state of TICs in cervical neoplasia lesions (Figure 7).
Figure 7.
The proposed model of Wnt/β-catenin signaling for the maintenance of TICs reprogrammed by Piwil2 in cervical oncogenesis. Piwil2 reprogrammed cervical epithelium into TICs by upregulation of “reprogramming” factors and, at the same time, activated Wnt/β-catenin signaling. The β-catenin/CBP-mediated transcription of the “reprogramming” factors was critical for the maintenance of TICs and chemoresistance and promoted cervical oncogenesis.
In summary, the current study demonstrated that Wnt/β-catenin signaling was activated by Piwil2 in cervical neoplasia lesions. The β-catenin/CBP-mediated transcription of c-Myc, Nanog, Oct4, Sox2, and Klf4 was critical for the maintenance of TICs reprogrammed by Piwil2 and for chemoresistance, which contributed to the progression of preneoplastic lesions to invasive cervical cancer (Figure 7). Therefore, it is likely that targeting the β-catenin/CBP interaction in the Wnt pathway could make a great difference to cure cervical neoplasia and invasive cancer.
Author contributions
All authors contributed to the design, investigation and project administration of the work, the interpretation of results, the drafting and editing of the manuscript. They give their final approval and agree to be accountable for the work herein.
Acknowledgments
We would like to thank Dr. Jie Lin and Dr. Aiping Song (Department of Pathology, China-Japan Friendship Hospital) for their histological work. This work was supported by the National Key R&D Program of China (2018YFC1003900), the National Natural Science Foundation of China (81372777, 81372779), and the Fundamental Research Funds for the Central Universities and Research projects on biomedical transformation of China-Japan Friendship Hospital (No. PYBZ1827).
Footnotes
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This work was supported by the National Key R&D Program of China (2018YFC1003900), the National Natural Science Foundation of China (81372777, 81372779), and the Fundamental Research Funds for the Central Universities and Research projects on biomedical transformation of China-Japan Friendship Hospital (No. PYBZ1827).
Conflict of interest: The authors declare that they have no conflicts to disclose.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2020.10.013.
Appendix. Supplementary materials
References
- 1.Klaus A., Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 2.Banziger C., Soldini D., Schutt C., Zipperlen P., Hausmann G., Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 3.ten Berge D., Koole W., Fuerer C., Fish M., Eroglu E., Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang G., Zhou J., Teng Y., Xie J., Lin J., Guo X., Gao Y., He M., Yang X., Wang S. Mesenchymal TGF-beta signaling orchestrates dental epithelial stem cell homeostasis through Wnt signaling. Stem Cells. 2014;32:2939–2948. doi: 10.1002/stem.1772. [DOI] [PubMed] [Google Scholar]
- 5.Scheibner K., Bakhti M., Bastidas-Ponce A., Lickert H. Wnt signaling: implications in endoderm development and pancreas organogenesis. Curr Opin Cell Biol. 2019;61:48–55. doi: 10.1016/j.ceb.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 6.R.J. Garriock, R.B. Chalamalasetty, J. Zhu, M.W. Kennedy, A. Kumar, S. Mackem, T.P. Yamaguchi, A dorsal-ventral gradient of Wnt3a/beta-catenin signals controls mouse hindgut extension and colon formation, Development, 147 (2020). [DOI] [PMC free article] [PubMed]
- 7.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 8.Chen H.Y., Lang Y.D., Lin H.N., Liu Y.R., Liao C.C., Nana A.W., Yen Y., Chen R.H. miR-103/107 prolong Wnt/beta-catenin signaling and colorectal cancer stemness by targeting Axin2. Sci Rep. 2019;9:9687. doi: 10.1038/s41598-019-41053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie S.L., Fan S., Zhang S.Y., Chen W.X., Li Q.X., Pan G.K., Zhang H.Q., Wang W.W., Weng B., Zhang Z. SOX8 regulates cancer stem-like properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/beta-catenin pathway. Int J Cancer. 2018;142:1252–1265. doi: 10.1002/ijc.31134. [DOI] [PubMed] [Google Scholar]
- 10.Zhan T., Rindtorff N., Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atlasi Y., Looijenga L., Fodde R. Cancer stem cells, pluripotency, and cellular heterogeneity: a WNTer perspective. Curr Top Dev Biol. 2014;107:373–404. doi: 10.1016/B978-0-12-416022-4.00013-5. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Li J., Han R., Deng X., Shi J., Huang H., Hamad N., McCaughley A., Liu J., Wang C. Deletion of tetraspanin CD151 alters the Wnt oncogene-induced mammary tumorigenesis: a cell type-linked function and signaling. Neoplasia. 2019;21:1151–1163. doi: 10.1016/j.neo.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghasemi F., Shafiee M., Banikazemi Z., Pourhanifeh M.H., Khanbabaei H., Shamshirian A., Amiri Moghadam S., ArefNezhad R., Sahebkar A., Avan A. Curcumin inhibits NF-kB and Wnt/beta-catenin pathways in cervical cancer cells. Pathol Res Pract. 2019;215 doi: 10.1016/j.prp.2019.152556. [DOI] [PubMed] [Google Scholar]
- 14.Batlle E., Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 15.Shenoy A.K., Fisher R.C., Butterworth E.A., Pi L., Chang L.J., Appelman H.D., Chang M., Scott E.W., Huang E.H. Transition from colitis to cancer: high Wnt activity sustains the tumor-initiating potential of colon cancer stem cell precursors. Cancer Res. 2012;72:5091–5100. doi: 10.1158/0008-5472.CAN-12-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai K.K.Y., Kweon S.M., Chi F., Hwang E., Kabe Y., Higashiyama R., Qin L., Yan R., Wu R.P., Lai K. Stearoyl-CoA desaturase promotes liver fibrosis and tumor development in mice via a Wnt positive-signaling loop by stabilization of low-density lipoprotein-receptor-related proteins 5 and 6. Gastroenterology. 2017;152:1477–1491. doi: 10.1053/j.gastro.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manegold P., Lai K.K.Y., Wu Y., Teo J.L., Lenz H.J., Genyk Y.S., Pandol S.J., Wu K., Lin D.P., Chen Y. Differentiation therapy targeting the beta-catenin/CBP interaction in pancreatic cancer. Cancers (Basel) 2018;10:95. doi: 10.3390/cancers10040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieger M.E., Zhou B., Solomon N., Sunohara M., Li C., Nguyen C., Liu Y., Pan J.H., Minoo P., Crandall E.D. p300/beta-catenin interactions regulate adult progenitor cell differentiation downstream of WNT5a/protein kinase C (PKC) J Biol Chem. 2016;291:6569–6582. doi: 10.1074/jbc.M115.706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyabayashi T., Teo J.L., Yamamoto M., McMillan M., Nguyen C., Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;104:5668–5673. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espada J., Calvo M.B., Diaz-Prado S., Medina V. Wnt signalling and cancer stem cells. Clin Transl Oncol. 2009;11:411–427. doi: 10.1007/s12094-009-0380-4. [DOI] [PubMed] [Google Scholar]
- 21.Kahn M. Symmetric division versus asymmetric division: a tale of two coactivators. Future Med Chem. 2011;3:1745–1763. doi: 10.4155/fmc.11.126. [DOI] [PubMed] [Google Scholar]
- 22.Sultana F., Winch K., Saville M., Brotherton J.M.L. Is the positive predictive value of high-grade cytology in predicting high-grade cervical disease falling due to HPV vaccination? Int J Cancer. 2019;144:2964–2971. doi: 10.1002/ijc.32050. [DOI] [PubMed] [Google Scholar]
- 23.Schlecht N.F., Platt R.W., Duarte-Franco E., Costa M.C., Sobrinho J.P., Prado J.C., Ferenczy A., Rohan T.E., Villa L.L., Franco E.L. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003;95:1336–1343. doi: 10.1093/jnci/djg037. [DOI] [PubMed] [Google Scholar]
- 24.Malagon T., Kulasingam S., Mayrand M.H., Ogilvie G., Smith L., Bouchard C., Gotlieb W., Franco E.L. Age at last screening and remaining lifetime risk of cervical cancer in older, unvaccinated, HPV-negative women: a modelling study. Lancet Oncol. 2018;19:1569–1578. doi: 10.1016/S1470-2045(18)30536-9. [DOI] [PubMed] [Google Scholar]
- 25.Sadri Nahand J., Moghoofei M., Salmaninejad A., Bahmanpour Z., Karimzadeh M., Nasiri M., Mirzaei H.R., Pourhanifeh M.H., Bokharaei-Salim F., Mirzaei H. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: a review. Int J Cancer. 2020;146:305–320. doi: 10.1002/ijc.32688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nahand J.S., Taghizadeh-Boroujeni S., Karimzadeh M., Borran S., Pourhanifeh M.H., Moghoofei M., Bokharaei-Salim F., Karampoor S., Jafari A. microRNAs: new prognostic, diagnostic, and therapeutic biomarkers in cervical cancer. J Cell Physiol. 2019;234:17064–17099. doi: 10.1002/jcp.28457. [DOI] [PubMed] [Google Scholar]
- 27.Peta E., Sinigaglia A., Masi G., Di Camillo B., Grassi A., Trevisan M., Messa L., Loregian A., Manfrin E., Brunelli M. HPV16 E6 and E7 upregulate the histone lysine demethylase KDM2B through the c-MYC/miR-146a-5p axys. Oncogene. 2018;37:1654–1668. doi: 10.1038/s41388-017-0083-1. [DOI] [PubMed] [Google Scholar]
- 28.Feng D., Yan K., Zhou Y., Liang H., Liang J., Zhao W., Dong Z., Ling B. Piwil2 is reactivated by HPV oncoproteins and initiates cell reprogramming via epigenetic regulation during cervical cancer tumorigenesis. Oncotarget. 2016;7:64575–64588. doi: 10.18632/oncotarget.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P.M., Cheng Y.W., Wang Y.C., Wu T.C., Chen C.Y., Lee H. Up-regulation of FOXM1 by E6 oncoprotein through the MZF1/NKX2-1 axis is required for human papillomavirus-associated tumorigenesis. Neoplasia. 2014;16:961–971. doi: 10.1016/j.neo.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nahand J.S., Vandchali N.R., Darabi H., Doroudian M., Banafshe H.R., Moghoofei M., Babaei F., Salmaninejad A., Mirzaei H. Exosomal microRNAs: novel players in cervical cancer. Epigenomics. 2020;12:1651–1660. doi: 10.2217/epi-2020-0026. [DOI] [PubMed] [Google Scholar]
- 31.Shafabakhsh R., Reiter R.J., Mirzaei H., Teymoordash S.N., Asemi Z. Melatonin: a new inhibitor agent for cervical cancer treatment. J Cell Physiol. 2019;234:21670–21682. doi: 10.1002/jcp.28865. [DOI] [PubMed] [Google Scholar]
- 32.Wang L.H., Xu M., Fu L.Q., Chen X.Y., Yang F. The Antihelminthic Niclosamide Inhibits Cancer Stemness, Extracellular Matrix Remodeling, and Metastasis through Dysregulation of the Nuclear beta-catenin/c-Myc axis in OSCC. Sci Rep. 2018;8:12776. doi: 10.1038/s41598-018-30692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L., Su Y., Huang C., Yin Y., Zhu J., Knupp A., Chu A., Tang Y. FOXH1 Is Regulated by NANOG and LIN28 for Early-stage Reprogramming. Sci Rep. 2019;9:16443. doi: 10.1038/s41598-019-52861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muhammad N., Bhattacharya S., Steele R., Phillips N., Ray R.B. Involvement of c-Fos in the Promotion of Cancer Stem-like Cell Properties in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2017;23:3120–3128. doi: 10.1158/1078-0432.CCR-16-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsompana M., Gluck C., Sethi I., Joshi I., Bard J., Nowak N.J., Sinha S., Buck M.J. Reactivation of super-enhancers by KLF4 in human Head and Neck Squamous Cell Carcinoma. Oncogene. 2020;39:262–277. doi: 10.1038/s41388-019-0990-4. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero M., Sabate-Perez A., Francis V.A., Castrillon-Rodriguez I., Diaz-Ramos A., Sanchez-Feutrie M., Duran X., Palacin M., Moreno-Navarrete J.M., Gustafson B. TP53INP2 regulates adiposity by activating beta-catenin through autophagy-dependent sequestration of GSK3beta. Nat Cell Biol. 2018;20:443–454. doi: 10.1038/s41556-018-0072-9. [DOI] [PubMed] [Google Scholar]
- 38.Gao J.X. Cancer stem cells: the lessons from pre-cancerous stem cells. J Cell Mol Med. 2008;12:67–96. doi: 10.1111/j.1582-4934.2007.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wicha M.S., Liu S., Dontu G. Cancer stem cells: an old idea–a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895-1886. [DOI] [PubMed] [Google Scholar]
- 40.Donnenberg V.S., Donnenberg A.D. Stem cell state and the epithelial-to-mesenchymal transition: implications for cancer therapy. J Clin Pharmacol. 2015;55:603–619. doi: 10.1002/jcph.486. [DOI] [PubMed] [Google Scholar]
- 41.Ostrin E.J., Little D.R., Gerner-Mauro K.N., Sumner E.A., Rios-Corzo R., Ambrosio E., Holt S.E., Forcioli-Conti N., Akiyama H., Hanash S.M. beta-Catenin maintains lung epithelial progenitors after lung specification. Development. 2018;145 doi: 10.1242/dev.160788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng Q., Li P., Che M., Liu J., Biswas S., Ma G., He L., Wei Z., Zhang Z., Yang Y. Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/beta-Catenin. Elife. 2019;8:e50208. doi: 10.7554/eLife.50208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu K., Li J., Li J., Sun J., Guo Y., Tian H., Li L., Zhang C., Shi M., Kong G. Ring1 promotes the transformation of hepatic progenitor cells into cancer stem cells through the Wnt/beta-catenin signaling pathway. J Cell Biochem. 2019;121:3941–3951. doi: 10.1002/jcb.29496. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen V.H.L., Hough R., Bernaudo S., Peng C. Wnt/beta-catenin signalling in ovarian cancer: insights into its hyperactivation and function in tumorigenesis. J Ovarian Res. 2019;12:122. doi: 10.1186/s13048-019-0596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antas P., Novellasdemunt L., Kucharska A., Massie I., Carvalho J., Oukrif D., Nye E., Novelli M., Li V.S.W. SH3BP4 regulates intestinal stem cells and tumorigenesis by modulating beta-catenin nuclear localization. Cell Rep. 2019;26:2266–2273.e2264. doi: 10.1016/j.celrep.2019.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan A.Q., Ahmed E.I., Elareer N.R., Junejo K., Steinhoff M., Uddin S. Role of miRNA-regulated cancer stem cells in the pathogenesis of human malignancies. Cells. 2019;8:840. doi: 10.3390/cells8080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmerli D., Cecconi V., Valenta T., Hausmann G., Cantu C., Restivo G., Hafner J., Basler K., van den Broek M. WNT ligands control initiation and progression of human papillomavirus-driven squamous cell carcinoma. Oncogene. 2018;37:3753–3762. doi: 10.1038/s41388-018-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran I., Thavathiru E., Ramalingam S., Natarajan G., Mills W.K., Benbrook D.M., Zuna R., Lightfoot S., Reis A., Anant S. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene. 2012;31:2725–2737. doi: 10.1038/onc.2011.455. [DOI] [PubMed] [Google Scholar]
- 49.Kahn M. Wnt signaling in stem cells and cancer stem cells: a tale of two coactivators. Prog Mol Biol Transl Sci. 2018;153:209–244. doi: 10.1016/bs.pmbts.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Lee J.H., Jung C., Javadian-Elyaderani P., Schweyer S., Schutte D., Shoukier M., Karimi-Busheri F., Weinfeld M., Rasouli-Nia A., Hengstler J.G. Pathways of proliferation and antiapoptosis driven in breast cancer stem cells by stem cell protein piwil2. Cancer Res. 2010;70:4569–4579. doi: 10.1158/0008-5472.CAN-09-2670. [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Shen R., Ye Y., Pu X.A., Liu X., Duan W., Wen J., Zimmerer J., Wang Y., Liu Y. Precancerous stem cells have the potential for both benign and malignant differentiation. PLoS ONE. 2007;2:e293. doi: 10.1371/journal.pone.0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taubert H., Wurl P., Greither T., Kappler M., Bache M., Bartel F., Kehlen A., Lautenschlager C., Harris L.C., Kaushal D. Stem cell-associated genes are extremely poor prognostic factors for soft-tissue sarcoma patients. Oncogene. 2007;26:7170–7174. doi: 10.1038/sj.onc.1210530. [DOI] [PubMed] [Google Scholar]
- 53.Li S., Lu X., He H., Cui R., Wang X., Wang X., Wu X. A novel culture system robustly maintained pluripotency of embryonic stem cells and accelerated somatic reprogramming by activating Wnt signaling. Am J Transl Res. 2017;9:4534–4544. [PMC free article] [PubMed] [Google Scholar]
- 54.Marson A., Foreman R., Chevalier B., Bilodeau S., Kahn M., Young R.A., Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lluis F., Pedone E., Pepe S., Cosma M.P. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell. 2008;3:493–507. doi: 10.1016/j.stem.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Ross J., Busch J., Mintz E., Ng D., Stanley A., Brafman D., Sutton V.R., Van den Veyver I., Willert K. A rare human syndrome provides genetic evidence that WNT signaling is required for reprogramming of fibroblasts to induced pluripotent stem cells. Cell Rep. 2014;9:1770–1780. doi: 10.1016/j.celrep.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P., Chang W.H., Fong B., Gao F., Liu C., Al Alam D., Bellusci S., Lu W. Regulation of induced pluripotent stem (iPS) cell induction by Wnt/beta-catenin signaling. J Biol Chem. 2014;289:9221–9232. doi: 10.1074/jbc.M113.542845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.