Abstract
Objectives:
Torus Palatinus (TP) is a bony projection located on the oral surface of the hard palate. The trait is typically benign, has an unknown etiology, and varies widely in phenotypic expression. Prior studies suggest differences in TP prevalence by sex and population, but the reported rates vary, even within a single ancestral group. We assessed the prevalence of TP and its association with palatal shape in a large multi-ethnic cohort of normal individuals.
Methodology:
1102 adults were included (625 with European ancestry, 377 with West African anscestry, and 100 with East Asian ancestry). 3D digital dental casts were obtained and rated. TP frequencies were compared between sexes and/or ethnicities using Chi-squared tests. Dental cast models were then landmarked, and canonical variates analysis was performed to test for shape differences between those with and without TP.
Results:
Females had a significantly higher rate of TP than males across all three ancestral groups (p≤0.004). In males, no significant differences were found among ethnicities. Ancestral differences in TP frequency were driven by females, with East Asians having the highest rate (34.69%), followed by Europeans (24.88%) and West Africans (15.22%). Shape differences were found only in Asians and Africans, indicated a shorter and wider palate in presence of TP.
Conclusions:
Ethnic differences in TP frequency were present only in females. Further, females have considerably higher rates of TP than males in each population tested. Further studies of TP at earlier time-points and in connection to other aspects of craniofacial growth may shed light on these sex and ethnic differences.
Keywords: palatal tori, oral torus, oral bony exostosis, craniofacial biology
Introduction:
Torus Palatinus (TP) is a bony protuberance located at the midline of the oral surface of the hard palate (Figure 1) and can vary considerably in size and shape (Jeong, Kim, Jang, Kim, & Huh, 2019). TP is considered to be a slow growing benign lesion that can take decades to become noticeable (Hanafi & Alweis, 2019; Komori & Takato, 1998). Surgical removal is warranted if TP is interfering with the fitting of a dental prosthesis, or to be used as a bone graft donor site for alveolar bone reconstruction; otherwise complications arising from the presence of TP are rare (Hanafi & Alweis, 2019; Moraes Junior, Damante, & Araujo, 2010). Although poorly understood, both genetic and biomechanical factors may influence the presence of oral tori (Jainkittivong & Langlais, 2000; Jeong et al., 2019).
Figure 1.

Examples of torus palatinus on dental casts.
Population studies (see Table 1) report widely varying prevalence of TP with a range of 2–67% (Austin, Radford, & Banks, 1965; Chew & Tan, 1984; Eggen & Natvig, 1994; García-García, Martínez-González, Gómez-Font, Soto-Rivadeneira, & Oviedo-Roldán, 2010; Haugen, 1992; Keng & Ow, 1981; Seah, 1995; Vidic, 1966). Prior studies suggest differences in TP prevalence among sexes and populations, occurring most frequently in females (García-García et al., 2010; Jeong et al., 2019) and individuals with Asian and Inuit ancestry (García-García et al., 2010; Hanafi & Alweis, 2019). However, the reported rates of TP vary considerably, even within a single ethnic/racial group. Discrepancies in the method of TP assessment coupled with the fact that most prior studies include only a single ancestral group can complicate comparisons among populations. Our objectives in the current study include assessing the prevalence of TP in a multi-ethnic cohort of individuals from three ancestral groups: European, West African, and East Asian. We further investigate the relationship between the presence of TP and the palate shape using landmark-based morphometric approaches.
Table 1.
Reported prevalence of palatal tori among sexes in European, African, and Asian ancestral groups.a
| Study | Population | Ancestry | Prevalence in Males (%) | Prevalence in Females (%) | Total Prevalence in Population (%) |
|---|---|---|---|---|---|
| Haugen, 1992 | Norwegians (N = 5000) |
European | 6.7 | 11.2 | 9.2 |
| Austin et al., 1965 | Black Americans (USA) (N = 1509) |
African | 12.9 | 26.3 | 19.5 |
| Keng et al., 1981 | Chinese (N = 200) |
Asian | 41.6 | 33.8 | 36.5 |
| Woo, 1950 | Black Americans (USA) (N = 873) |
African | 36.0 | 40.6 | 37.0 |
| White Americans (USA) (N = 667) |
European | 42.4 | 47.2 | 45.0 | |
| Mongolians (N = 163) |
Asian | 44.0 | 50.0 | 47.0 | |
| Vidic, 1966 | Yugoslavians (N = 400) |
European | 42.0 | 57.5 | 45.5 |
| Chew et al., 1984 | Chinese (N = 200) |
Asian | 48.0 | 48.0 | 48.0 |
| Reichart et al., 1988 | German (N = 1317) |
European | 11.7 | 15.1 | 13.5 |
| Thai (N = 947) |
Asian | 15.8 | 28.5 | 23.1 | |
| Eggen et al., 1994 | Norwegian (N = 2010) |
European | 23.7 – 32.7 | 39.8 – 43.4 | 36.1 |
| Kerdpon et al., 1999 | Thai (N = 609) |
Asian | 48.1 | 67.6 | 61.7 |
| Sonnier et al., 1999 | Black Americans (USA) (N = 74) |
African | 4.7 | 22.6 | 12.2 |
| White Americans (USA) (N = 254) |
European | 18.5 | 26.7 | 22.8 | |
| Bruce et al., 2004 | Ghanaian (N = 926) |
African | 2.2 | 5.2 | 3.9 |
Adapted from Seah (Seah, 1995) and García-García et al. (García-García et al., 2010).
Methodology:
Study Population:
The study sample was comprised of 1102 individuals recruited as controls for a large international genetic study of orofacial clefting. All participants were at least 18 years of age (mean age = 36.6) and screened for a personal and family history of oral and craniofacial malformations and prior trauma or surgery involving the palatal region. Three ancestral groups were represented: 625 individuals of European ancestry recruited from the United States), 377 individuals of West African ancestry recruited from Nigeria), and 100 individuals of East Asian ancestry recruited from the Philippines and the United States). See Table 2 for sample demographics.
Table 2.
Sample size (N) and mean age of participants included in the current study.
| Ancestral Group | Sex | N | Mean Age |
|---|---|---|---|
| East Asian | Male | 51 | 24.1 |
| Female | 49 | 26.4 | |
| European | Male | 211 | 42.6 |
| Female | 414 | 42 | |
| West African | Male | 206 | 31.1 |
| Female | 171 | 29.2 |
Data acquisition, Phenotype Capture and Rating:
Individuals provided written consent prior to participation. The protocol was approved by Institutional Review Board (IRB) of the University of Pittsburgh. Demographic data was recorded as well as dental, medical and social history through in-person interviews. Maxillary impressions were taken by standard hydrocolloid impression materials, poured into plaster casts, and later digitized as 3D models by laser scanning (3Shape, Copenhagen, Denmark). The 3D digital casts were then visualized from multiple perspectives and evaluated for the presence or absence of TP by a single observer (AMES). The observer was blinded to both sex and ancestry.
Morphometric Data Collection:
Geometric morphometric analyses were performed in order to investigate the association between the presence of TP palate shape. Landmarking was performed directly on the 3D digital models using 3dMDVultus software (3dMD Inc., Atlanta, Georgia, USA). Five landmarks were placed at the tip of the incisive papilla (IP), the deepest points of the gingival crevice at the right and left canines (CR & CL) and the deepest points of the gingival crevice at the right and left first molars (6R & 6L) (Figure 2). For validation, a subset of 30 casts were landmarked twice, separated by at least 24 hours, yielding intraclass correlation coefficients for coordinates ranging from 0.983 to 0.998, indicating low error. Individuals with missing teeth were excluded from the morphometric analyses due to missing landmarks.
Figure 2.
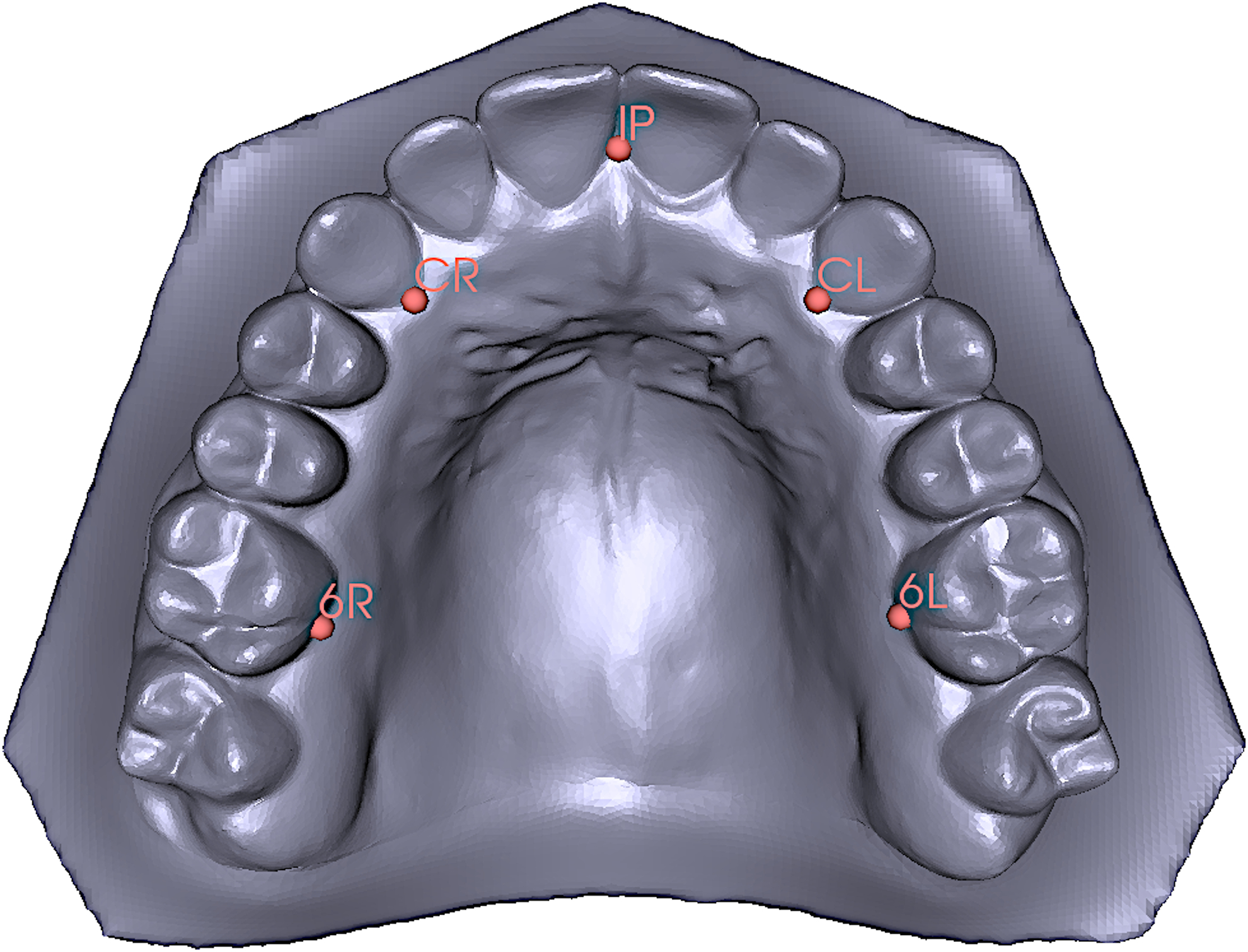
Landmarks used in the morphometric analysis; Incisive Papilla (IP), Right Canine (CR), Left Canine (CL), Right First Molar (6R) and Left First Molar (6L).
Statistical Analysis & Visualization:
Statistical analysis was performed by conducting chi-squared tests of independence to compare frequencies of TP among groups using R programming software (Vienna, Austria). For the geometric morphometric analyses, canonical variates analysis (CVA) was used to compare palate shape between individuals with TP and to those without TP. This was done separately for each of the three ancestral groups. P-values were determined based on permutation testing of Procrustes and Mahalanobis distances. Morphometric tests and subsequent visualizations were generated with MorphoJ, R packages geomorph and Morpho, and 3D Slicer (Adams, Collyer, & JKaliontzopoulou, 2020; Fedorov et al., 2012; Klingenberg, 2011; Schlager, 2017). The nominal threshold for statistical significance was set at p ≤ 0.05. The Bonferroni adjusted threshold for study-wide significance was set at p ≤ 0.005.
Results:
TP was found to be significantly more frequent in females than males, both overall and within each ancestral group. Comparing across ancestral groups, TP was present at about twice the rate (20% vs 10.1%) in individuals of East Asian ancestry compared with individuals of West African ancestry (p = 0.0008) (Table 3). When males and females were treated separately, the ancestral differences were observed to be limited to females (p = 0.005), while males showed a 6% rate in all three ancestral groups. (Table 4).
Table 3.
Combined Prevalences of Palatal Tori.
| By Sex (Ancestries Combined)a | By Ancestry (Sexes Combined)b | ||||
|---|---|---|---|---|---|
| Male (n = 468) |
Female (n = 634) |
East Asian (n = 100) |
European (n = 625) |
West African (n = 377) |
|
| Tori Present | 5.98% | 23.34% | 20% | 18.56% | 10.08% |
| Tori Absent | 94.02% | 76.66% | 80% | 81.44% | 89.92% |
X2 = 57.56, p = 3.28×10−14*
X2 = 14.185, p = 0.0008*
Table 4.
Palatal tori prevalence by sex and ancestry.
| East Asian | European | West African | X2 | p-value | |
|---|---|---|---|---|---|
| Male | 5.88% | 6.16% | 5.82% | 0.021936 | 0.99 |
| Female | 34.69% | 24.88% | 15.20% | 10.467 | 0.005* |
| X2 | 11.227 | 31.171 | 8.064 | ||
| p-value | 0.0008* | 2.36×10−08* | 0.004* |
Because there were some differences in mean age among groups, we tested the relationship between age and the presence of TP. The point-biserial correlation between TP and age was 0.02 (95% CI = −0.05, 0.09) for the entire sample, −0.02 (95% CI = −0.14, 0.09) among males, and 0.009 (95% CI = −0.09, 0.11) among females, indicating that age was not a major factor driving our results.
Because females showed higher TP prevalence than males and the observed ancestral differences were limited to females, we focused our morphometric analyses on females (n = 136 with TP vs. n = 470 without TP). Significant shape differences were noted in both the East Asian and West African samples, but not in individuals of European ancestry (Table 5). In both East Asians and West Africans, females with TP showed shorter and wider palates (Figure 3). Adjusting for age in our morphometric analysis did not impact these results.
Table 5.
Results of canonical variates analysis for testing palate shape differences between females with and without palatal tori.a
| Procrustes distance (p-value) | Mahalanobis distance (p-value) | |
|---|---|---|
| East Asian (n = 49) | 0.0503 (0.0006)* | 1.2344 (0.0011)* |
| European (n = 388) | 0.0056 (0.5779) | 0.2108 (0.5412) |
| West African (n = 169) | 0.0253 (0.0064)b | 0.7953 (0.0083)b |
p-values based on permutation testing (10000 resamples)
p-value achieving nominal significance level (p < 0.05)
Figure 3.

Canonical variate effects of the presence of TP on palatal morphology. Warp scale factor = 4.
Green = East Asian females with TP. Red = West African females with TP. Grey = Demographically matched female controls without TP.
Discussion:
The reported prevalence of TP varies greatly, even within a single ancestral group. There is wide agreement that TP is more common in females and, generally, the reported prevalence seems to be highest among Asians and lowest among Africans, with Europeans falling somewhere in the middle. This general ancestral gradient has been shown in other multi-ethnic studies (Table 1). Our findings largely support these prior claims; however, in our study, the ancestral differences were driven entirely by females, which has not been previously reported. In a prior multi-ethnic study, Woo showed roughly similar ancestral prevalence patterns in males and females (Woo, 1950). In another study, Reichart et al. reported that Thai men and women both had a higher TP prevalence than Germans, but they did find the ancestral difference was greater for women (Reichart, Neuhaus, & Sookasem, 1988). Our finding, therefore, awaits independent replication.
We investigated the relationship between the presence of TP and palate shape, choosing to focus on females. The presence of TP was associated with a wider and shorter palate in both individuals of East Asian and West African ancestry, but not in Europeans. It is unclear why this pattern was present in only two of our three populations. To our knowledge, this is the first study to examine the relationship between TP and quantitative palate shape. Several prior studies have connected oral tori to different factors including functional responses due to well-developed masticatory muscles, superficial injuries or in individuals with abraded teeth suggestive of parafunctional habits and temporomandibular disorders (Al-Bayaty, Murti, Matthews, & Gupta, 2001; Bruce, Ndanu, & Addo, 2004; García-García et al., 2010; Jainkittivong & Langlais, 2000; Kerdpon & Sirirungrojying, 1999; Morrison & Tamimi, 2013; Sirirungrojying & Kerdpon, 1999; Sonnier, Horning, & Cohen, 1999). While this link to occlusal stresses has been significantly associated with the presence and size of mandibular tori, a similar connection has not been made with TP (García-García et al., 2010; Jeong et al., 2019; Seah, 1995). The association of TP with a specific pattern of palate shape still requires an explanation.
Despite how common the trait is, the etiology of TP is poorly understood. Genetic factors have been widely theorized to play a role (Antoniades, Belazi, & Papanayiotou, 1998; Castro Reino, Perez Galera, Perez Cosio Martin, & Urbon Caballero, 1990; Eggen, Natvig, & Gåsemyr, 1994; García-García et al., 2010). Gorsky et al. reported an increased rate of familial segregation with evidence of vertical transmission, suggesting an autosomal dominance pattern (Gorsky, Bukai, & Shohat, 1998). While an autosomal dominant inheritance pattern has not been consistently shown, it is believed that there may be a dominant type linked to the X-chromosome, given the high preponderance towards females (Castro Reino et al., 1990; García-García et al., 2010). In addition, multiple studies reported a strong link between TP and other conditions involving bony dysostoses of a genetic origin such as familial osteosclerosis and hyperostosis where there is increased bone mass (Auškalnis et al., 2015; Whyte et al., 2019). Interestingly, those studies reported that the condition was consistently being passed down along with the TP trait across generations from mother to daughter. Unfortunately, large-scale population-based genetic studies of TP are currently lacking.
TP size has been correlated with bone density in postmenopausal women (Belsky, Hamer, Hubert, Insogna, & Johns, 2003), suggesting that advanced age could be an important factor in TP assessment. However, we found no relationship between TP status and age in our sample. Further, our assessment was limited to presence or absence of the trait, not specific features such as size or prominence which may be more sensitive to factors such as age-related changes in bone-density. Advanced morphometric approaches that would further characterize TP size and its association with other phenotypic traits and demographic features may shed more light on these factors.
There are several important limitations of this study. First, we do not have any functional or biomechanical data available to correlate with the presence of TP. Second, our landmark-based approach was limited to hand full of points placed around the dental arch, which limited our ability to model some aspects of shape. Adding additional points or landmark-free approaches could provide additional information in future analyses. Third, our sample sizes varied greatly by population, and the number of eligible males with TP was particularly low in our East Asian and West African groups, which could have impacted the power of some of our statistical shape analyses. Nevertheless, the present study represents one of the largest studies conducted to date on TP.
Conclusions:
Individuals of East Asian ancestry showed the highest prevalence of TP, while individuals of West African ancestry displayed the lowest prevalence. Those ancestral differences in TP frequency were present only in females. Further, females have considerably higher rates of TP than males in each population tested. In addition, morphological findings were found in an ethnicity-specific manner in females; the presence of TP was associated with shorter sagittal and wider transverse dimensions of the dental arch. These shape differences were present in East Asians and West Africans, but not in Europeans. Further studies on TP biology may shed light on these sex and ethnic differences.
Acknowledgements:
This study has been possible through funding support of NIH/NIDCR grants: R01-DE016148 & R00-DE022378. The authors declare no conflicts of interests.
References
- Adams D, Collyer M, & JKaliontzopoulou A (2020). Geomorph: Software for geometric morphometric analyses. R package version 3.2.1. [Google Scholar]
- Al-Bayaty HF, Murti PR, Matthews R, & Gupta PC (2001). An epidemiological study of tori among 667 dental outpatients in Trinidad & Tobago, West Indies. Int Dent J, 51(4), 300–304. doi: 10.1002/j.1875-595x.2001.tb00842.x [DOI] [PubMed] [Google Scholar]
- Antoniades DZ, Belazi M, & Papanayiotou P (1998). Concurrence of torus palatinus with palatal and buccal exostoses: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 85(5), 552–557. doi: 10.1016/s1079-2104(98)90290-6 [DOI] [PubMed] [Google Scholar]
- Auškalnis A, Bernhardt O, Putnienė E, Šidlauskas A, Andriuškevičiūtė I, & Basevičienė N (2015). Oral bony outgrowths: prevalence and genetic factor influence. Study of twins. Medicina (Kaunas), 51(4), 228–232. doi: 10.1016/j.medici.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Austin JE, Radford GH, & Banks SO (1965). Palatal And Mandibular Tori In The Negro. N Y State Dent J, 31, 187–191. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14280608 [PubMed] [Google Scholar]
- Belsky JL, Hamer JS, Hubert JE, Insogna K, & Johns W (2003). Torus palatinus: a new anatomical correlation with bone density in postmenopausal women. J Clin Endocrinol Metab, 88(5), 2081–2086. doi: 10.1210/jc.2002-021726 [DOI] [PubMed] [Google Scholar]
- Bruce I, Ndanu TA, & Addo ME (2004). Epidemiological aspects of oral tori in a Ghanaian community. Int Dent J, 54(2), 78–82. doi: 10.1111/j.1875-595x.2004.tb00259.x [DOI] [PubMed] [Google Scholar]
- Castro Reino O, Perez Galera J, Perez Cosio Martin J, & Urbon Caballero J (1990). [Surgery of palatal and mandibular torus]. Rev Actual Odontoestomatol Esp, 50(394), 47–50, 53–46. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2206647 [PubMed] [Google Scholar]
- Chew CL, & Tan PH (1984). Torus palatinus. A clinical study. Aust Dent J, 29(4), 245–248. doi: 10.1111/j.1834-7819.1984.tb06066.x [DOI] [PubMed] [Google Scholar]
- Eggen S, & Natvig B (1994). Concurrence of torus mandibularis and torus palatinus. Scand J Dent Res, 102(1), 60–63. doi: 10.1111/j.1600-0722.1994.tb01154.x [DOI] [PubMed] [Google Scholar]
- Eggen S, Natvig B, & Gåsemyr J (1994). Variation in torus palatinus prevalence in Norway. Scand J Dent Res, 102(1), 54–59. doi: 10.1111/j.1600-0722.1994.tb01153.x [DOI] [PubMed] [Google Scholar]
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, … Kikinis R (2012). 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging, 30(9), 1323–1341. doi: 10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-García AS, Martínez-González JM, Gómez-Font R, Soto-Rivadeneira A, & Oviedo-Roldán L (2010). Current status of the torus palatinus and torus mandibularis. Med Oral Patol Oral Cir Bucal, 15(2), e353–360. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19767716 [PubMed] [Google Scholar]
- Gorsky M, Bukai A, & Shohat M (1998). Genetic influence on the prevalence of torus palatinus. Am J Med Genet, 75(2), 138–140. doi: [DOI] [PubMed] [Google Scholar]
- Hanafi A, & Alweis R (2019). Images in medicine: torus palatinus. J Community Hosp Intern Med Perspect, 9(4), 367–368. doi: 10.1080/20009666.2019.1643219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen LK (1992). Palatine and mandibular tori. A morphologic study in the current Norwegian population. Acta Odontol Scand, 50(2), 65–77. doi: 10.3109/00016359209012748 [DOI] [PubMed] [Google Scholar]
- Jainkittivong A, & Langlais RP (2000). Buccal and palatal exostoses: prevalence and concurrence with tori. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 90(1), 48–53. doi: 10.1067/moe.2000.105905 [DOI] [PubMed] [Google Scholar]
- Jeong CW, Kim KH, Jang HW, Kim HS, & Huh JK (2019). The relationship between oral tori and bite force. Cranio, 37(4), 246–253. doi: 10.1080/08869634.2017.1418617 [DOI] [PubMed] [Google Scholar]
- Keng SB, & Ow R (1981). A clinical study of the oral status of edentulous patients in the local Chinese population. Singapore Dent J, 6(2), 71–77. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6953598 [PubMed] [Google Scholar]
- Kerdpon D, & Sirirungrojying S (1999). A clinical study of oral tori in southern Thailand: prevalence and the relation to parafunctional activity. Eur J Oral Sci, 107(1), 9–13. doi: 10.1046/j.0909-8836.1999.eos107103.x [DOI] [PubMed] [Google Scholar]
- Klingenberg CP (2011). MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour, 11(2), 353–357. doi: 10.1111/j.1755-0998.2010.02924.x [DOI] [PubMed] [Google Scholar]
- Komori T, & Takato T (1998). Time-related changes in a case of torus palatinus. J Oral Maxillofac Surg, 56(4), 492–494. doi: 10.1016/s0278-2391(98)90720-0 [DOI] [PubMed] [Google Scholar]
- Moraes Junior EF, Damante CA, & Araujo SR (2010). Torus palatinus: a graft option for alveolar ridge reconstruction. Int J Periodontics Restorative Dent, 30(3), 283–289. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20386785 [PubMed] [Google Scholar]
- Morrison MD, & Tamimi F (2013). Oral tori are associated with local mechanical and systemic factors: a case-control study. J Oral Maxillofac Surg, 71(1), 14–22. doi: 10.1016/j.joms.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Reichart PA, Neuhaus F, & Sookasem M (1988). Prevalence of torus palatinus and torus mandibularis in Germans and Thai. Community Dent Oral Epidemiol, 16(1), 61–64. doi: 10.1111/j.1600-0528.1988.tb00557.x [DOI] [PubMed] [Google Scholar]
- Schlager S (2017). Morpho and Rvcg – Shape Analysis in R In Zheng G, Li S, & Szekely G (Eds.), Statistical Shape and Deformation Analysis (pp. 217–256): Academic Press. [Google Scholar]
- Seah YH (1995). Torus palatinus and torus mandibularis: a review of the literature. Aust Dent J, 40(5), 318–321. doi: 10.1111/j.1834-7819.1995.tb04820.x [DOI] [PubMed] [Google Scholar]
- Sirirungrojying S, & Kerdpon D (1999). Relationship between oral tori and temporomandibular disorders. Int Dent J, 49(2), 101–104. doi: 10.1111/j.1875-595x.1999.tb00516.x [DOI] [PubMed] [Google Scholar]
- Sonnier KE, Horning GM, & Cohen ME (1999). Palatal tubercles, palatal tori, and mandibular tori: prevalence and anatomical features in a U.S. population. J Periodontol, 70(3), 329–336. doi: 10.1902/jop.1999.70.3.329 [DOI] [PubMed] [Google Scholar]
- Vidic B (1966). Incidence of torus palatinus in Yugoslav skulls. J Dent Res, 45(5), 1511–1515. doi: 10.1177/00220345660450054101 [DOI] [PubMed] [Google Scholar]
- Whyte MP, McAlister WH, Zhang F, Bijanki VN, Nenninger A, Gottesman GS, … Mumm S (2019). New explanation for autosomal dominant high bone mass: Mutation of low-density lipoprotein receptor-related protein 6. Bone, 127, 228–243. doi: 10.1016/j.bone.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Woo JK (1950). Torus palatinus. Am J Phys Anthropol, 8(1), 81–111. doi: 10.1002/ajpa.1330080114 [DOI] [PubMed] [Google Scholar]


