Abstract
Although romantic rejection and acceptance have a strong impact on mood in adults, their neural response patterns are relatively unexplored. The present study used functional magnetic resonance imaging (fMRI) to examine neural responses to romantic rejection and acceptance in 36 healthy men and women, ages 18–53 years. Activations during rejection showed extensive anatomical overlap with activations during acceptance in the ventrolateral prefrontal cortex (vlPFC) and anterior insula (AI). In an analysis of sex differences, men and women did not differ in behavioral responses, however men showed greater activation to romantic rejection and acceptance in the left vlPFC and AI compared to women. The vlPFC and AI may play a role in social cognition, tuned to detect the intentions and feelings of others whether they are positive or negative. In the context of romantic rejection and acceptance, this activation may signal the intent of others who are desired by the individual, leading to changes in mood, self-esteem, and social motivation.
Keywords: social rejection, social acceptance, romantic, depression, operculum, ventrolateral prefrontal cortex, anterior insula
Introduction
Forming strong, stable interpersonal relationships is a basic human need, critical for survival and emotional well being1. Social rejection – when one is not wanted or liked – is a potent threat to this need that can lead to sadness, anxiety, social withdrawal, impulsivity, and aggression2–10. Most neuroimaging studies of social rejection have examined peer rejection in adolescents and young adults11–13, given the heightened sensitivity to peer rejection in adolescence14. However, there is direct clinical relevance to examining romantic rejection in young to middle-aged adults. The median age of onset of mood disorders is 30 years15, and romantic rejection is one of the most stressful life events in adults, leading to distinct patterns of depressive symptoms (e.g., sadness, anhedonia, appetite loss, guilt), compared to chronic stress and failures, which are more strongly associated with other symptoms (fatigue, hypersomnia)16. In addition, a recent meta-analysis showed that the neural response to social exclusion differs in adolescents compared to young adults17. To address this gap in understanding, the present study examined neural responses to romantic feedback in healthy young to middle-aged adults.
A review of functional magnetic resonance imaging (fMRI) studies suggest that social rejection (both peer and romantic) activates the dorsal anterior cingulate cortex (dACC) and the anterior insula (AI)11. These areas, which serve multiple functions including adaptive task control18 and salience processing19,20. However, a meta-analysis also found that romantic rejection compared to peer rejection activated several additional areas outside of the dACC-AI network12. In addition, peer rejection studies have consistently found that opposite sex social feedback resulted in greater neural responses compared to same-sex feedback21–24, although the paradigms used in those studies were ostensibly within a non-romantic context. Thus, it is possible that romantic feedback, a clinically-relevant social stimulus in adults16, activates unique areas compared to peer feedback.
Sex differences in the neural response to social feedback (peer or romantic) are virtually unexplored and may inform how sex moderates the relationship between rejection and psychiatric disorders. In women, relationship problems and lower levels of social support have been shown to be more strongly predictive of depression than in men25,26. Two studies that examined behavioral sex differences using peer rejection23 or Cyberball, a virtual ball-tossing game in which the participant is socially excluded27, did not find differences between men and women. Thus, a primary aim of the present study was to examine sex differences in the behavioral and neural response to social feedback.
In summary, the present study examined the behavioral and neural responses to both romantic rejection and acceptance in healthy young to middle-aged men and women (ages 18–53), an understudied demographic in social feedback experiments, and examined sex differences in these responses. In addition to the dACC and AI, we also focused on the ventrolateral PFC (vlPFC), amygdala (AMY), and nucleus accumbens (NAcc). The vlPFC has been associated with the regulation of negative affect during rejection28–30, and the AMY and NAcc have been associated with both rejection21 and acceptance22. In light of a recent study in adolescents/young adults showing an anatomical overlap in the neural response to positive and negative social evaluation31, we performed a conjunction analysis for neural activation responding to both romantic rejection and acceptance.
Methods
Participants
Participants were 38 right-handed healthy adults (18 men, 20 women; ages 18–53 years; mean ± SD = 29.7 ± 10.6) recruited from the local community through advertisements. Men and women were similar with respect to age, racial/ethnic background, sexual orientation, and relationship status (Table 1). All participants were informed that they would be participating in an online dating scenario. Participants were screened for current or past psychiatric disorders in themselves or any first-degree relatives using the M.I.N.I. International Psychiatric Interview32. Other exclusion criteria included the use of psychotropic substances in the past four weeks, regular tobacco use, history of DSM-IV alcohol or drug dependence within the past five years, or drug or alcohol abuse in the past two years. Behavioral and neuroimaging data from the healthy women in the present study were also used to compare to data obtained from depressed women, who showed exaggerated responses to social feedback33. All protocols were approved by the University of Michigan Medical School Institutional Review Board, all methods were performed in accordance with the relevant guidelines and regulations, and written informed consent was obtained.
Table 1. Participant demographics.
Relationship status was not obtained from one male participant.
| Men (n = 18) | Women (n = 20) | |
|---|---|---|
| Age range | 18 – 53 | 18 – 53 |
| Age: mean years ± SD | 29.0 ± 10.4 | 30.3 ± 11.0 |
| Ethnicity: White, Black or African-American, Asian, Hispanic, Mixed | 14, 2, 1, 0, 1 | 14, 3, 2, 0, 1 |
| Sexual orientation: heterosexual, homosexual, bisexual | 15, 1, 2 | 19, 1, 0 |
| Relationship status: single, in a relationship, married | 12, 3, 2 | 12, 5, 3 |
Social Feedback Task during fMRI
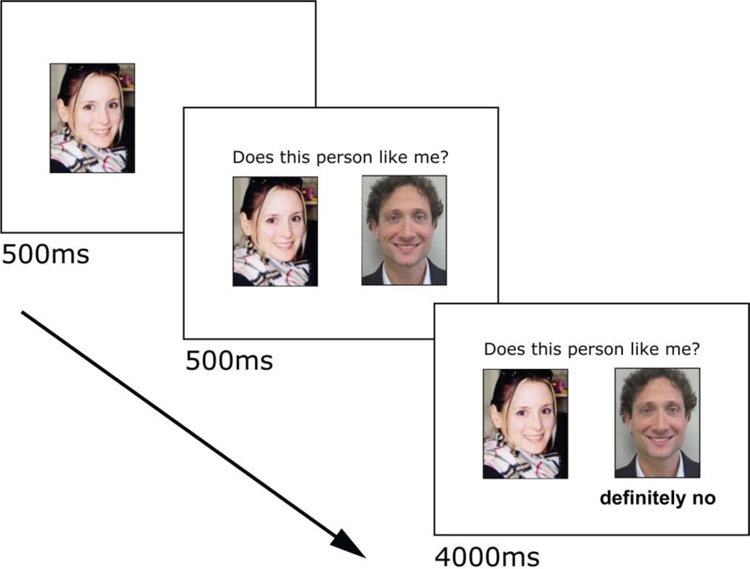
Several days prior to scanning, participants rated dating profiles from a set of over 400 profiles from the mass media compiled by the experimenters. Participants rated opposite or same sex profiles depending on their orientation. Two men reported to be bisexual and chose to rate women only. Participants rated profiles on how much they would like the potential partner and how much they thought that person would like them in return. To maximize saliency, only the highest rated profiles chosen by each participant was presented during the Social Feedback Task (SFT). As in our previous studies2,33–35, the SFT did not involve deception however participants were asked to immerse themselves in the experience and respond as if the feedback was real (see Supplemental Methods for additional detail). To increase engagement with the scenario, participants submitted photos of themselves and completed their own dating profile (age, height, major/occupation, interests, and positive qualities about themselves). During fMRI, the participant’s own photo was shown on the left, followed by the question “Does this person like me?” along with a picture from a highly-rated profile on the right, followed by feedback (Fig. 1).
Fig. 1. Social Feedback Task trial.
Each trial begins with the participant’s own picture presented for 500ms, the addition of the question “Does this person like me?” along with picture from a highly-rated profile (500ms), followed by the feedback (4000ms). A rejection trial is shown. Figure adapted from Yttredahl et al.33.
Participants viewed blocks of trials containing one of three types of feedback: rejection (Rej), acceptance (Acc), and neutral (Neu). Rej trials contained the feedback “very likely no” and “definitely no,” Acc trials contained the feedback “very likely yes” and “definitely yes,” and Neu trials contained the feedback “not completed” (participants were informed that this indicated that the other person had not yet completed profile ratings). Four trials (5s each) of the same type were presented in one block. Functional images were collected in 4 scan runs, each containing 6 pseudorandomized blocks (2 blocks each of Rej, Acc, and Neu). Cross-hair fixations (10–14s) were presented between blocks. The total duration of the task was 12 min, 48s, plus less than 30s shim time between runs. All stimuli were presented using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA).
fMRI Acquisition
Whole-brain scans were acquired on a 3.0 Tesla GE Signa 9.0 scanner (Milwaukee, WI, USA) with a standard frequency coil. Functional images (blood-oxygen-level dependent, BOLD signal) were obtained using a T2*-weighted pulse sequence (repetition time, TR, 2000ms; echo time, TE, 30 ms; flip angle, 90°; field of view, FoV, 20 × 20cm, 64 × 64 matrix; in-plane resolution, 3.12 × 3.12 mm; slice thickness, 4 mm) with single-shot combined spiral in/out sequence was used to reduce signal dropout in subcortical and around sinus regions36. A high-resolution T1-weighted pulse sequence provided anatomical localization (3D spoiled gradient recalled echo; TR, 12 ms; TE, 5 ms; TI, 500 ms; flip angle, 15°; FoV, 26 × 26 cm, 256 × 256 matrix; in-plane resolution, 1.02 × 1.02 mm; slice thickness, 1.2 mm). Participant motion was minimized with foam pads secured with a strap around the head. One male and one female participant were excluded from fMRI analyses due to excessive head movement (>3 mm translation or >3° rotation), leaving a total of 36 participants for fMRI data analysis.
fMRI Data Analysis
Functional images were preprocessed using FMRIB Software Library (FSL) and included slice-time correction, realignment, spatial smoothing (5 mm full-width at half maximum) using a Gaussian kernel, coregistration, and normalization to MNI standard space (Montreal Neurological Institute, Quebec, CA). First-level analyses were performed using the General Linear Model (Statistical Parametric Mapping v.8 (SPM8), Wellcome Institute of Cognitive Neurology, London, UK), and contrast t maps for each participant were created for the main contrasts of interest: Rej-Neu and Acc-Neu.
Group-level, voxel-wise one-sample two-tailed t-tests were conducted for each contrast in SPM8 to examine the effect of the task, controlling for sex and age. A two-way repeated measures ANOVA in SPM8 was used to examine the effect of sex between-subjects (men vs. women), contrasts within-subjects (Rej-Neu vs. Acc-Neu), and the interaction effect between them, controlling for age and responses to manipulation checks Q2 and Q3 (since sex differences were found – see Results/Behavior). In all tests, a whole-brain gray matter mask37 was first applied as an exclusion mask, followed by an a priori combined ROI mask that included the left and right dACC, AI, vlPFC, AMY, and NAcc, which were all anatomically defined using Harvard Brain Atlas38 probability masks, thresholded at 0.25 confidence, and binarized (Fig. 2). The anterior-posterior boundaries of the dACC was set at y = 0 to 36, and the posterior boundary of the AI at y = 8, based on a previous study that examined neural activation to social exclusion39. Within this mask, activation was considered significant at family-wise error within small volume (FWE-SVC)-corrected, P < 0.05 (two-tailed), followed by an additional minimum extent threshold of kE > 10 voxels.
Fig. 2. Anatomical regions of interest (ROIs).
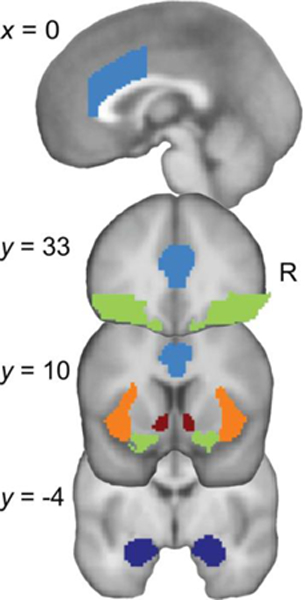
ROIs in the left and right dorsal anterior cingulate cortex (dACC, light blue), ventrolateral prefrontal cortex (vlPFC, green), anterior insula (AI, orange), nucleus accumbens (NAcc, red), and amygdala (AMY, dark blue), superimposed on T1-weighted template brain image (Montreal Neurological Institute). Top image shows a sagittal section, bottom 3 images show coronal sections. R, right
To determine voxels across the whole brain that were common to both rejection and acceptance, a logical “AND” conjunction analysis31,40–42 was performed. In SPM8, ImCalc was used to binarize clusters in each contrast (Rej-Neu, Acc-Neu) at a threshold of P < 0.001, uncorrected, kE > 10. Binarized images were used in the formula “i1 + 2(i2)”; the resulting image with a value of 3 indicated activated voxels that were common to both contrasts40,41. Brain regions were identified using the Anatomical Automatic Labeling software43, a human brain atlas44, and architectonic maps of the prefrontal cortex45. The center of mass coordinates of each conjunction was determined using MarsBar region-of-interest toolbox (v.0.38) for SPM8.
To test for the degree to which activation during Rej was correlated with activation during Acc, mean signal intensities (raw values) were extracted from each ROI from each contrast (Rej-Neu, Acc-Neu) using MarsBar for SPM8. Pearson’s correlations were used with P threshold corrected for multiple ROIs (5 ROIs x 2 hemispheres = 10; Bonferroni-corrected P threshold = 0.005).
Behavior
In a separate room, participants were seated at a personal computer and performed an abbreviated version of the SFT. In this version, participants were reminded of the feedback they received during the scan in three blocks, each containing 18 trials of one feedback type. After each block, participants rated how “sad”, “rejected”, “happy”, and “accepted” they felt, their current level of self-esteem (state version of the Rosenberg Self-Esteem scale46), and their current desire for social interaction2. Responses were recorded using a 5-button response box, corresponding to 5-point Likert-type scales. As in our previous studies2,33–35, scores for “sad” were averaged with “rejected,” and scores for “happy” were averaged with “accepted.” The was done because ratings for “rejected” and “accepted” may be influenced by the objective feedback received (i.e., participants may report feeling “rejected” because they were ostensibly rejected). Therefore, the more affective items “sad” and “happy” were included in the overall score. Rej-Neu and Acc-Neu scores for individual items were significantly correlated (sadRej-Neu vs. rejectedRej-Neu: r36 = 0.63, P < 0.001; happyAcc-Neu vs. acceptedAcc-Neu: r36 = 0.38, P = 0.02) and not significantly different (P’s > 0.40), suggesting that “rejected” and “accepted” may be used interchangeably with “sad” and “happy.” A two-way repeated measures ANOVA was used to compare within-subjects behavior during Rej-Neu vs. Acc-Neu, between-subjects effect of sex, and interactions.
As a manipulation check, participants responded to three questions: Q1) “How much were you able to experience the profiles and feedback as if they were real?”; Q2) “How similar to a real-life situation was your emotional response to the positive feedback?”; and Q3) “How similar to a real-life situation was your emotional response to the negative feedback?”, rated on a 5-point Likert-type scale (1 = very slightly or not at all, 2 = a little, 3 = moderately, 4 = quite a bit, 5 = extremely). Two-way between-subjects ANOVAs tested if Sex x Relationship Status (i.e., single or in a relationship/married) had an effect on the responses to each of these questions.
Q2 and Q3 were explored for potential relationships with mean signal intensities in each ROI during Acc-Neu and Rej-Neu, respectively. To explore the possibility that higher Q2 may explain higher mean signal intensities during Acc-Neu compared to Rej-Neu, we examined if the differences in how real acceptance felt compared to rejection (Q2 – Q3) correlated with differences in mean signal intensities during acceptance compared to rejection [(Acc-Neu) – (Rej-Neu)] with Bonferroni-corrected P threshold for each ROI correlation = 0. 005.
Results
Neural response to romantic rejection and acceptance
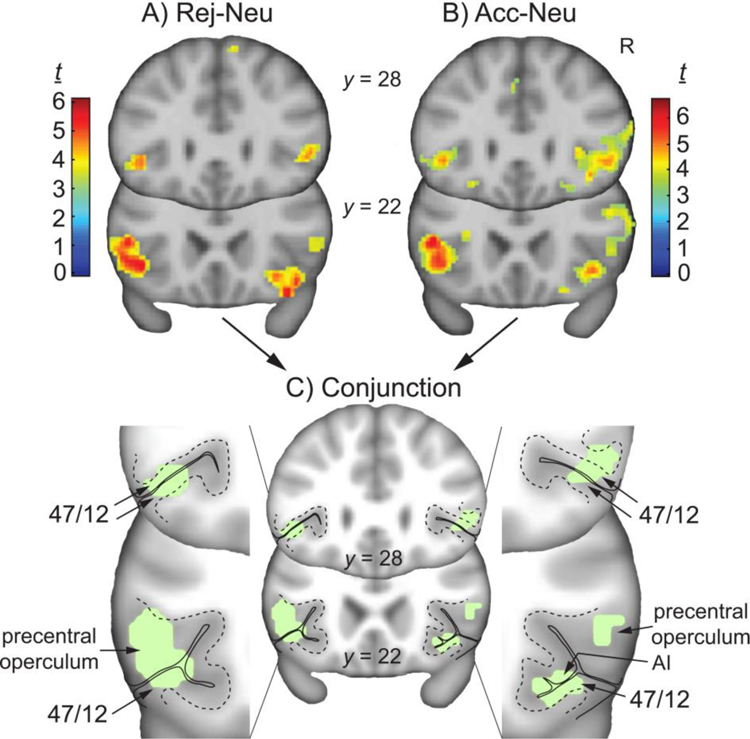
In the rejection minus neutral (Rej-Neu) contrast, family-wise error (FWE)-corrected voxel-wise analysis (using the combined ROI mask for small volume correction (SVC), P < 0.05, two-tailed, followed by an additional minimum extent threshold of kE > 10), significant activation was found within the left vlPFC specifically in the precentral part of the frontal operculu m45 (t = 5.46, PFWE-SVC = 0.017; kE = 143; peak activation [MNI x, y, z coordinates, mm]: −42, 22, −4), and within the right vlPFC specifically in area 47/12 as previously define d47, and in the right AI (t = 5.28, PFWE-SVC = 0.027; kE = 210; peak activation: 28, 18, −12) (Fig. 3A). In the Neu-Rej contrast, no significant clusters were found within the ROI mask at this threshold. For a list of additional clusters in the whole brain, and results from the Rej-Acc and Acc-Rej contrasts, see Supplemental Table S1 and Figs. S1 & S2. Analyses of relationship status is also reported in Supplemental Data, and data from ROI masks examined individually are also included in Supplemental Table S2.
Fig. 3. Activity during rejection and acceptance, and their conjunction.
Whole-brain contrast t maps for A) Rej-Neu, B) Acc-Neu), and C) conjunction of both contrasts (green), with magnified view of the vlPFC; R, right; AI, anterior insula; y coordinates in Montreal Neurological Institute stereotactic space. Contrast t maps displayed at uncorrected P < 0.001, kE > 10.
In the acceptance-neutral (Acc-Neu) contrast, within the combined ROI mask (FWE-corrected voxel-wise analysis, P < 0.05, two-tailed; followed by an additional minimum extent threshold of kE > 10), significant activation was found within the left vlPFC specifically in the precentral operculum (t = 5.53, PFWE-SVC = 0.014; kE = 184; peak activation: −42, 20, −4), and within the right vlPFC specifically in area 47/12, and in the right AI (t = 5.58, PFWE-SVC = 0.013; kE = 338; peak activation: 40, 24, −6) (Fig. 3B). For a list of these and additional clusters, see Supplemental Table S1 and Fig. S1. In the Neu-Acc contrast, no significant clusters were found within the ROI mask or whole brain. Analyses of relationship status is also reported in Supplemental Data, and data from ROI masks examined individually are included in Supplemental Table S2.
The conjunction analysis showed common areas of activation, defined as the overlap in voxels from independent significant contrasts (Rej-Neu and Acc-Neu)40–42. This analysis (as previously described31,40–42 is intended to identify this overlap, but does not imply similarities in the magnitude of effect. Conjunction was found in the left vlPFC (385 voxels; center of mass: −48, 20, 5), right vlPFC (92 voxels; center of mass: 49, 27, 4), and right vlPFC/AI (70 voxels; center of mass: 37, 24, −9) (Fig. 3C). More specifically, conjunction in the left vlPFC spanned the upper and lower bank of the lateral sulcus, in area 47/1247 (Fig. 3C, y = 28). More caudally, this cluster spread into the precentral operculum and the caudal part of area 47/12 (Fig. 3C, y = 22). Conjunction in the right rostral vlPFC was located in 47/12 (Fig. 3C, y = 28) and spread caudally into the precentral operculum (Fig. 3C, y = 22). The cluster in the right caudal vlPFC included area 47/12 and the AI (Fig. 3C, y = 28). Additional areas of conjunction outside the ROIs (i.e., in the whole brain without masking) are listed in Supplemental Table S3. Areas of non-overlapping clusters that were unique to Rej-Neu and Acc-Neu (whole brain without masking) are listed in Supplemental Table S4. In particular, clusters in the left and right caudate were found during Acc-Neu but not during Rej-Neu.
A two-way repeated measures ANOVA was used to test the effect of sex between-subjects (men vs. women), contrasts within-subjects (Rej-Neu vs. Acc-Neu), and the interaction effect between them, controlling for age and responses to manipulation checks Q2 and Q3 (since sex differences were found – see Results/Behavior). Same as above, a combined ROI mask was used for small volume correction (SVC), with P < 0.05, followed by an additional minimum extent threshold of kE > 10. This analysis revealed a single cluster for the main effect of sex (men > women) in the left vlPFC (area 47/12) that spread caudally into the AI (F = 22.83, PFWE-SVC = 0.032; kE = 18; peak activation: −32, 30, 0) (Fig. 4). No suprathreshold clusters were found for the main effect of contrast, or interactions.
Fig. 4. Sex differences.
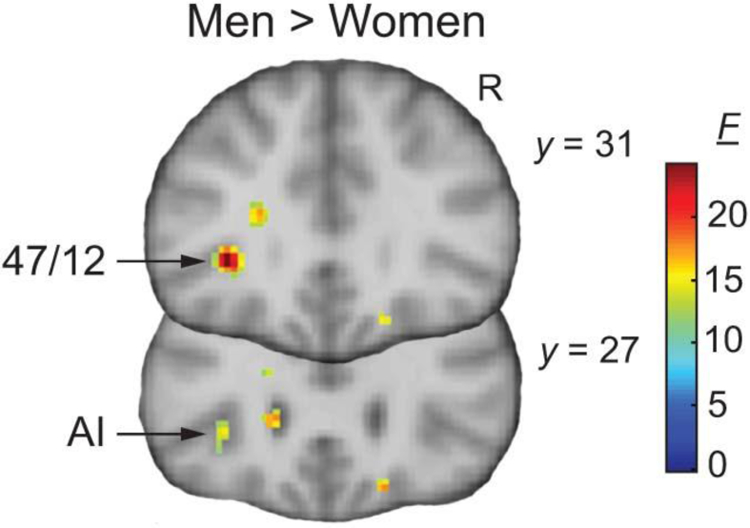
Whole-brain F maps for the main effect of sex revealed a significant cluster (men > women) in the vlPFC (area 47/12; y = 31), and AI (y = 27). R, right; y coordinates in Montreal Neurological Institute stereotactic space. F maps displayed at uncorrected P < 0.001, kE > 10.
Region-of-interest correlations
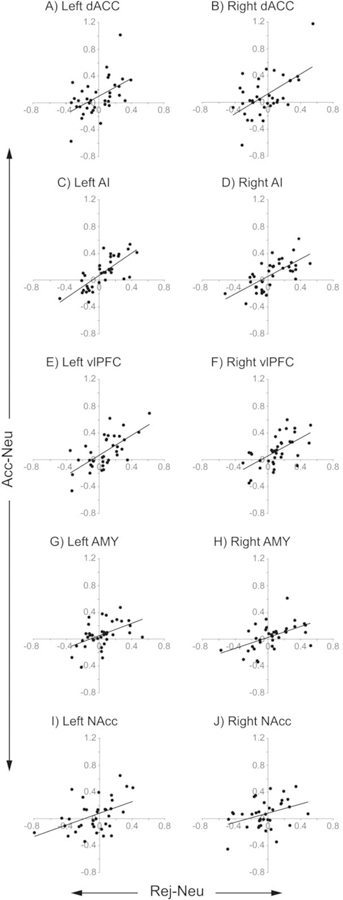
For all ROIs, activity in Rej-Neu was significantly correlated with activity in Acc-Neu, except in the NAcc (Bonferroni-corrected P threshold for each correlation = 0. 005) (Fig. 5). Activity was correlated in the dACC (left: r36 = 0.51, P = 0.001; right: r36 = 0.56, P = 0.0004), AI (left: r36 = 0.75, P = 1.2 × 10−7; right: r36 = 0.67, P = 8.8 × 10−6), vlPFC (left: r36 = 0.65, P = 1.5 × 10−5; right: r36 = 0.61, P = 0.0001), AMY (left: r36 = 0.47, P = 0.004; right: r36 = 0.51, P = 0.002), and NAcc (left: r36 = 0.44, P = 0.007; right: r36 = 0.37, P = 0.028). Outlier values from the same participant appeared in the Acc-Neu contrast in the left and right dACC (Figs. 5A, B). Spearman’s rank order correlation, which is less sensitive to strong outliers, confirmed a significant correlation in the left (ρ36 = 0.50, P = 0.002), but not the right dACC (ρ36 = 0.42, P = 0.01). After removing this data point, the Pearson correlation coefficient remained significant in the left dACC (r36 = 0.48, P = 0.003), but not in the right dACC (r36 = 0.42, P = 0.01).
Fig. 5. Correlated activity in anatomical regions of interest (ROIs).
In each of 10 ROIs, extracted mean signal intensities from Rej-Neu contrasts (x axis) were correlated with extracted mean signal intensities from Acc-Neu contrasts (y axis). All correlations were significant (except for the left and right NAcc) at Bonferroni-adjusted P threshold = 0.005.
Behavior
After fMRI scanning, participants were seated at a personal computer in a separate room and performed an abbreviated version of the fMRI task (SFT) (see Methods). The purpose here was to measure subjective responses to the SFT in a separate behavior-only testing session. Performing subjective ratings of emotionally salient stimuli in the scanner has been shown to attenuate activity in areas such as the AI and AMY48. Our primary interest was in the neural response social feedback, not the explicit evaluation of internal mood states. Responses to 5-point Likert-type scales were recorded using a 5-button response box. A two-way repeated measures ANOVA was used to compare within-subjects behavior during Rej-Neu vs. Acc-Neu, between-subjects effect of sex, and interactions. Results showed significant within-subject effects of Condition (Rej-Neu vs. Acc-Neu) for “sad and rejected” (F1,36 = 10.98, P = 0.002, Rej-Neu > Acc-Neu), “happy and accepted” (F1,36 = 9.87, P = 0.003, Acc-Neu > Rej-Neu), and Desire for Social Interaction (F1,36 = 5.14, P = 0.03, Acc-Neu > Rej-Neu). There was no significant main effect of Sex for any behaviors, and no significant interactions.
For all subjects, mean scores ± SD for manipulation checks were Q1 (“How much were you able to experience the profiles and feedback as if they were real?”): 3.34 ± 1.07; Q2 (How similar to a real-life situation was your emotional response to the positive feedback?”): 3.74 ± 1.03; Q3 (How similar to a real-life situation was your emotional response to the negative feedback?”): 3.03 ± 1.13. These scores were further explored as a function of Sex or Relationship Status. For Q1 and Q3, there were no significant main effects of Sex or Relationship Status, and no significant interactions. For Q2, there was a significant main effect of Sex (F1,33 = 4.71, P = 0.04, men > women), but no significant main effect of Relationship Status, and no significant interactions. Q2 did not correlate with Acc-Neu mean signal intensities extracted from any ROIs in men (P’s > 0.41).
Q2 was significantly higher than Q3 (t37 = 5.05, P < 0.001). Q2 correlated with the left NAcc during Acc-Neu (r36 = 0.35, P = 0.04), and Q3 correlated with the left NAcc during Rej-Neu (r36 = 0.40, P = 0.02), although not significant with Bonferroni-corrected P threshold = 0.005. No significant correlations in any ROIs were found (P’s > 0.31) between the differences in how real acceptance felt compared to rejection (Q2 – Q3) and differences in mean signal intensities during acceptance compared to rejection [(Acc-Neu) – (Rej-Neu)].
Discussion
Romantic rejection and acceptance are powerful cues affecting emotional well-being and, from an evolutionary perspective, the chance for reproductive success. We found extensive overlap between neural activation during romantic rejection (Rej-Neu) and acceptance (Acc-Neu) in healthy adult men and women, primarily in the vlPFC and AI, areas that were previously shown to be involved in social rejection/exclusion. Novel findings in the present study include 1) the significant activation and extensive overlap in the left/right precentral operculum and the right AI in response to rejection and acceptance 2) the high degree of correlated activity between rejection and acceptance, and 3) significantly greater activation in the left vlPFC and AI in men compared to women during social feedback.
There are several functional implications for the extensive overlap between Rej-Neu and Acc-Neu in the vlPFC (which includes the precentral operculum and area 47/12), and in the AI. The vlPFC is involved in emotion regulation49,50 and has been suggested to reduce social distress during social exclusion29,51, however it also appears to be activated when regulating both pleasant and unpleasant emotions52. The precentral operculum, on the other hand, plays a role in making inferences about other people’s intentions and goals53,54 and may have been activated as a participant’s response to perceiving others’ intentions of rejection and acceptance in the present study. In line with this idea, the precentral operculum and the adjacent AI together have been shown to be involved in emotional awareness, empathy, and understanding other people’s feeling states and intentions55–58. Another possibility is that overlap in the right AI may result from general activation during awareness of emotionally salient information59.
In summary, the vlPFC (including precentral operculum and area 47/12), and AI may be involved in evaluating others’ intentions – whether positive or negative – in order to inform how one should behave in that situation. Consistent with this interpretation, a recent study showed that AI activation was not significantly different between rejection vs. acceptance, and that the AI responded more strongly when the feedback was directed towards the participant compared to when the feedback is directed towards others60. This suggests involvement of the AI in processing both positive and negative salient information, which may include social feedback directed at the self in social situations.
Activations in the vlPFC and AI were also highly correlated during Rej-Neu vs. Acc-Neu (Fig. 5C,E), providing further support for their similar functions. Our previous PET study showed that endogenous opioid release in the AI was also highly correlated during rejection and acceptance (left AI, r = 0.79; right AI, r = 0.62). Indeed, common neural pathways are activated during both pain and pleasure, involving endogenous opioid and dopamine neurotransmitter systems61, however, the functional relationship between blood-oxygen-level dependent (BOLD) signal and these neurotransmitter systems are not known. In the present study, significantly correlated activations were also found in the dACC and AMY, but not in the NAcc (after strict Bonferroni correction).
In the manipulation checks, participants reported that acceptance felt more real compared to rejection, raising the possibility that NAcc activation during Acc-Neu may reflect greater salience regardless of valence. However, in testing this possibility, we found that differences in how real the acceptance felt compared to rejection (manipulation check Q2 scores – Q3 scores) were not significantly correlated with differences in activity during acceptance or rejection [(Acc-Neu) – (Rej-Neu)], in the NAcc or any other ROI. In addition, activity in the left NAcc in Acc-Neu and Rej-Neu contrasts were correlated with how real the acceptance felt (manipulation check Q2: r36 = 0.35, P = 0.04) and how real the rejection felt (manipulation check Q3: r36 = 0.40, P = 0.02) to the participant (although not significant after strict Bonferroni correction). Taken together, data from the manipulation checks suggest that salience (i.e., how real the feedback felt) did not explain greater activity in the NAcc during acceptance compared to rejection, but salience was associated with the magnitude of activity within each type of feedback in the left NAcc. To further specify the role of the NAcc in processing salience and valence during social feedback, future fMRI studies may examine NAcc functional connectivity to unique networks during each condition. Neurotransmitter systems in the NAcc may also be explored for their role in valence and salience of social feedback including oxytocin and serotonin, which play a role in social reward62, or endogenous opioids in the NAcc, which play a role in both social reward/acceptance34,63 and rejection2.
The conjunction that was found in the right superior frontal gyrus and the left middle and superior temporal gyrus (Supplemental Table S3) should also be highlighted. In a previous study13, these two areas (identified as the rostromedial prefrontal cortex, and posterior superior temporal sulcus, respectively) were activated when participants were unexpectedly liked or disliked after a “speed-dating” paradigm, suggesting involvement of these areas in expectancy violation. However, in the present study, only the first of four trials in each block of the SFT were unpredictable, since each of the 3 subsequent trials in that block contained the same type of feedback. Thus, an alternative explanation is that activation of these two areas in the present study may instead be associated with “mentalizing” the intentions of others, since both of these areas are part of a network shown to be involved in encoding and updating beliefs about the intentions and feelings of others13,64, and the rostromedial prefrontal cortex has also been shown to be involved in relating one’s own self-image with the mental state of others65. Of clinical relevance, patients with borderline personality disorder showed stronger engagement of dACC and mPFC (compared to healthy controls) to all conditions (inclusion, exclusion, control condition) of a social exclusion paradigm, suggesting that patients “hypermentalize”66 during social interactions regardless of inclusion or exclusion.
In an analysis of sex differences, we found greater activation in the left vlPFC (area 47/12) and AI in men compared to women during social feedback (combined effect of Rej-Neu and Acc-Neu). Consistent with this, a previous study found that men and women recruit a different set of brain regions in response to emotion-evoking images, with men exhibiting higher activity within the insula and cingulate cortex67. Significant sex differences in the subjective responses to the SFT were not found, however in the manipulation check, men more than women reported that acceptance felt similar to a real-life situation. This difference was not related to differences in neural activity, since there was no Sex x Condition interaction, nor a correlation in Q2 scores vs. any ROIs during Acc-Neu in men (P’s > 0.41). Although sex differences in brain activation were not accompanied by differences in behavior, other behavioral measures may have revealed potential sex differences in behavior. For example, men have been shown to be more risk-seeking than women following physical stress68, and the vlPFC and AI appears to be involved in this process69. Thus, identifying these regions in the present study may form the basis for future investigations to include measurements of risk-taking behavior to test if the left vlPFC and AI in men mediates the relationship between emotionally salient social cues and risk-taking behavior. This has implications for why, for example, men more often than women take externalizing paths (impulsivity, risk-taking) towards risky behaviors such as heavy alcohol use70,71.
The present findings are consistent with overlapping activations found during Rej-Neu and Acc-Neu in two recent studies from different labs, using different social feedback paradigms albeit in younger age groups than in the present study. Importantly, similar to the present study, these studies examined Rej and Acc relative to a Neu condition, allowing for the analysis of overlapping and unique activations. In the first study72, young healthy adults (ages 18–27 years) were informed that peers didn’t like (Rej), liked (Acc), or didn’t know what to think (Neu) of their personal profile. Rej-Neu and Acc-Neu contrasts showed overlapping activation in the bilateral vlPFC/AI, and mPFC, similar to the overlapping activation in the present study (Fig. 3C), although overlapping mPFC activity in the present study was found more anteriorly (BA 9/10, Supplemental Table S3). That study72, also found increased activity in the striatum that was unique to Acc-Neu, consistent with our finding that a cluster of activation unique to Acc-Neu was found in the striatum (i.e., bilateral caudate, Supplemental Table S4). In the second study31, young healthy adults (ages 17–20 years) received Rej, Acc, and Neu feedback from a group of judges about a pre-recorded video of themselves. Both Rej-Neu and Acc-Neu contrasts showed overlapping activations in the dACC and AI, and Acc-Neu feedback specifically activated the ventral striatum and ventral mPFC31. In summary, these prior studies31,72 along with the present study – three independent labs using three different social feedback tasks – converge to demonstrate that the vlPFC and AI respond to both rejection and acceptance, with the striatum showing more activation during acceptance. As described above, novel findings in the present study include the extensive overlap specifically in the left precentral operculum, the high degree of correlated activity between Rej-Neu and Acc-Neu, and sex differences in the vlPFC (area 47/12) and AI response to social feedback. Interestingly, no study (including the present study) found greater activation during rejection compared to acceptance.
Several limitations should be noted. Unlike most social feedback tasks, our task did not use deception to inform participants that they were receiving feedback from “real” people; instead, participants were asked to imagine that they were real. It is not known if deception would have produced greater emotional responses, however we have demonstrated here and in previous studies2,33–35 that our task without deception produced significant changes in emotional states following rejection or acceptance (see Supplemental Methods for additional detail). It is also not known whether our task resulted in more cognitive reappraisal (i.e., participants may have been reminding themselves that the feedback was not real), although participants under deception may also use reappraisal or other cognitive strategies unless explicitly instructed not to do so. Nevertheless, we found patterns of neural activation that were similar to those in studies using deception31,72. On the other hand, a task that does not use deception may be valuable for studying social feedback in vulnerable populations (e.g., suicidal ideation, borderline personality disorder) in which deception is unfeasible or unethical. A second limitation is that behavioral responses to the task were measured after the scanning session. Thus, it was not possible to associate real-time subjective experiences to neural activity. In addition, the salience of the task may have decreased after the scan session. Nevertheless, we found significant effects of the task on several behavioral measures after the scan session. A third limitation is that each participant’s task used different photos of potential partners and different self-photos, which may have introduced noise. Photos of potential partners were different because they were self-selected by each participant for increased saliency of the feedback, and self-photos were presented in every trial to increase engagement of the online dating scenario (the same self-photo was used in every trial including the neutral condition, which was subtracted from rejection and acceptance conditions). Lastly, the present study did not uniquely identify brain areas activated during social feedback vs. other social stimuli (e.g., facial expressions) or non-social stimuli. Future studies will need to determine differences between these types of stimuli, and control for valence and/or salience. As an example, one study in adolescents controlled for the valence and salience of social feedback and found that the dACC and AI were involved only when feedback was directed towards the participant, but not when the feedback was directed towards others60. These types of fine-grained social feedback paradigms may be used to better understand psychiatric disorders such as borderline personality disorder, which is characterized by extreme sensitivity to social feedback and a heightened sense of self-awareness73.
In conclusion, the present study found a common neural response to romantic rejection and acceptance in adults in the vlPFC and AI. The results contribute to the social feedback literature by showing that romantic rejection and acceptance activated a common neural network (including the vlPFC, AI, RMPFC, and pSTS) that may not be specific to negative or positive emotional responses, but rather serve a social cognitive function to evaluate others’ intentions, or to detect socially salient information. Men appeared to respond more strongly to romantic feedback at the neural level compared to women, however it will be important to examine sex-specific emotion regulation strategies. Romantic rejection is one of the most salient life events in adults, and future studies may examine how this pattern of activation found in healthy adults is different in psychiatric disorders, perhaps becoming more strongly activated during rejection compared to acceptance in depressione.g.,33, anxiety, or borderline personality disorder, all of which exhibit high sensitivity to rejection74. Novel treatments may involve neuromodulation of the right vlPFC, which has been shown to reduce the negative impact of social rejection29,30, potentially bringing responses to rejection and acceptance back into balance in these disorders.
Supplementary Material
Acknowledgement.
We are deeply thankful to Ben Sanford for participant recruitment, MRI scanning, and assistance with data processing.
This research was supported by the National Institute of Mental Health under Grant K01 MH085035 (DTH) and R01 MH102264 (DTH).
Footnotes
Data Availability. The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Disclosure of interest. The authors report no conflict of interest.
References
- 1.Baumeister RF & Leary MR The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull 117, 497–529 (1995). [PubMed] [Google Scholar]
- 2.Hsu DT et al. Response of the μ-opioid system to social rejection and acceptance. Mol. Psychiatry 18, 1211–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu DT et al. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol. Psychiatry 20, 193–200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platt B, Cohen Kadosh K & Lau JYF The role of peer rejection in adolescent depression. Depress. Anxiety 30, 809–821 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Slavich GM, O’Donovan A, Epel ES & Kemeny ME Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci. Biobehav. Rev 35, 39–45 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spielberg JM et al. Anticipation of peer evaluation in anxious adolescents: divergence in neural activation and maturation. Soc. Cogn. Affect. Neurosci 10, 1084–1091 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmer-Gembeck MJ & Nesdale D Anxious and angry rejection sensitivity, social withdrawal, and retribution in high and low ambiguous situations. J. Pers 81, 29–38 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Baumeister RF, DeWall CN, Ciarocco NJ & Twenge JM Social exclusion impairs self-regulation. J. Pers. Soc. Psychol 88, 589–604 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Leary MR, Twenge JM & Quinlivan E Interpersonal rejection as a determinant of anger and aggression. Personal. Soc. Psychol. Rev. Off. J. Soc. Personal. Soc. Psychol. Inc 10, 111–132 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Romero-Canyas R, Downey G, Berenson K, Ayduk O & Kang NJ Rejection sensitivity and the rejection-hostility link in romantic relationships. J. Pers 78, 119–148 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Eisenberger NI The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci 13, 421–434 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Cacioppo S et al. A quantitative meta-analysis of functional imaging studies of social rejection. Sci. Rep 3, 2027 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper JC, Dunne S, Furey T & O’Doherty JP The role of the posterior temporal and medial prefrontal cortices in mediating learning from romantic interest and rejection. Cereb. Cortex N. Y. N 1991 24, 2502–2511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somerville LH The Teenage Brain: Sensitivity to Social Evaluation. Curr. Dir. Psychol. Sci 22, 121–127 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler RC et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Matthew Keller, Ph. D., Michael Neale, Ph. D. & Kenneth Kendler, M. D.. Association of different adverse life events with distinct patterns of depressive symptoms. Am. J. Psychiatry 164, 1521–1529 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Vijayakumar N, Cheng TW & Pfeifer JH Neural correlates of social exclusion across ages: A coordinate-based meta-analysis of functional MRI studies. NeuroImage 153, 359–368 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dosenbach NUF et al. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A 104, 11073–11078 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uddin LQ Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci 16, 55–61 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Iannetti GD, Salomons TV, Moayedi M, Mouraux A & Davis KD Beyond metaphor: contrasting mechanisms of social and physical pain. Trends Cogn. Sci 17, 371–378 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Silk JS et al. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc. Cogn. Affect. Neurosci (2014) 10.1093/scan/nst175. [DOI] [PMC free article] [PubMed]
- 22.Davey CG, Allen NB, Harrison BJ, Dwyer DB & Yücel M Being liked activates primary reward and midline self-related brain regions. Hum. Brain Mapp 31, 660–668 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somerville LH, Kelley WM & Heatherton TF Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cereb. Cortex N. Y. N 1991 20, 3005–3013 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolling DZ, Pelphrey KA & Vander Wyk BC Differential brain responses to social exclusion by one’s own versus opposite-gender peers. Soc. Neurosci 7, 331–346 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendler KS & Gardner CO Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am. J. Psychiatry 171, 426–435 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalgard OS et al. Negative life events, social support and gender difference in depression. Soc. Psychiatry Psychiatr. Epidemiol 41, 444–451 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM & Irwin MR An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. NeuroImage 47, 881–890 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenberger NI, Lieberman MD & Williams KD Does rejection hurt? An fMRI study of social exclusion. Science 302, 290–292 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Riva P, Lauro LJR, DeWall CN & Bushman BJ Buffer the Pain Away Stimulating the Right Ventrolateral Prefrontal Cortex Reduces Pain Following Social Exclusion. Psychol. Sci 23, 1473–1475 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Riva P, Romero Lauro LJ, Vergallito A, DeWall CN & Bushman BJ Electrified emotions: Modulatory effects of transcranial direct stimulation on negative emotional reactions to social exclusion. Soc. Neurosci 10, 46–54 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Dalgleish T et al. Social pain and social gain in the adolescent brain: A common neural circuitry underlying both positive and negative social evaluation. Sci. Rep 7, 42010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheehan DV et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–33;quiz 34–57 (1998). [PubMed] [Google Scholar]
- 33.Yttredahl AA et al. Abnormal emotional and neural responses to romantic rejection and acceptance in depressed women. J. Affect. Disord 234, 231–238 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu DT et al. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol. Psychiatry 20, 193–200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sankar A et al. Dissociable Neural Responses to Monetary and Social Gain and Loss in Women With Major Depressive Disorder. Front. Behav. Neurosci 13, 149 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glover GH & Law CS Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn. Reson. Med 46, 515–522 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Wager T help:core:brain_masks [Cognitive and Affective Neuroscience Lab - Tor D. Wager, Ph.D.] https://canlabweb.colorado.edu/wiki/doku.php/help/core/brain_masks (2018).
- 38.The Whole Brain Atlas http://www.med.harvard.edu/aanlib/ (2017).
- 39.Way BM, Taylor SE & Eisenberger NI Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc. Natl. Acad. Sci. U. S. A 106, 15079–15084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramaniam K et al. Neural signal during immediate reward anticipation in schizophrenia: Relationship to real-world motivation and function. NeuroImage Clin 9, 153–163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramaniam K et al. Neural Mechanisms of Positive Mood Induced Modulation of Reality Monitoring. Front. Hum. Neurosci 10, 581 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichols T, Brett M, Andersson J, Wager T & Poline J-B Valid conjunction inference with the minimum statistic. NeuroImage 25, 653–660 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Tzourio-Mazoyer N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Mai JK, Paxinos G & Assheuer JK Atlas of the Human Brain, Second Edition. (Academic Press, 2004). [Google Scholar]
- 45.Öngür D, Ferry AT & Price JL Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol 460, 425–449 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg M Society and the adolescent self-image (Princeton University Press, 1965). [Google Scholar]
- 47.Petrides M & Pandya DN Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci 16, 291–310 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Taylor SF, Phan KL, Decker LR & Liberzon I Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage 18, 650–659 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Etkin A, Büchel C & Gross JJ The neural bases of emotion regulation. Nat. Rev. Neurosci 16, 693–700 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Morawetz C, Bode S, Derntl B & Heekeren HR The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci. Biobehav. Rev 72, 111–128 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Eisenberger NI & Lieberman MD Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn. Sci 8, 294–300 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Seo D et al. Neural correlates of preparatory and regulatory control over positive and negative emotion. Soc. Cogn. Affect. Neurosci 9, 494–504 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ & Haxby JV Two takes on the social brain: a comparison of theory of mind tasks. J. Cogn. Neurosci 19, 1803–1814 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Iacoboni M et al. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol 3, e79 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craig ADB How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci 10, 59–70 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Critchley HD, Wiens S, Rotshtein P, Öhman A & Dolan RJ Neural systems supporting interoceptive awareness. Nat. Neurosci 7, 189–195 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Lamm C & Singer T The role of anterior insular cortex in social emotions. Brain Struct. Funct 214, 579–591 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Liljeholm M, Dunne S & O’Doherty JP Anterior insula activity reflects the effects of intentionality on the anticipation of aversive stimulation. J. Neurosci. Off. J. Soc. Neurosci 34, 11339–11348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Critchley HD, Mathias CJ & Dolan RJ Neuroanatomical basis for first- and second-order representations of bodily states. Nat. Neurosci 4, 207–212 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Perini I et al. The salience of self, not social pain, is encoded by dorsal anterior cingulate and insula. Sci. Rep 8, 6165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leknes S & Tracey I A common neurobiology for pain and pleasure. Nat. Rev. Neurosci 9, 314–320 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Dölen G, Darvishzadeh A, Huang KW & Malenka RC Social reward requires coordinated activity of accumbens oxytocin and 5HT. Nature 501, 179–184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trezza V, Damsteegt R, Achterberg EJM & Vanderschuren LJMJ Nucleus accumbens μ-opioid receptors mediate social reward. J. Neurosci 31, 6362–6370 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frith CD The social brain? Philos. Trans. R. Soc. Lond. B. Biol. Sci 362, 671–678 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitchell JP, Macrae CN & Banaji MR Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50, 655–663 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Domsalla M et al. Cerebral processing of social rejection in patients with borderline personality disorder. Soc. Cogn. Affect. Neurosci 9, 1789–1797 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Filkowski MM, Olsen RM, Duda B, Wanger TJ & Sabatinelli D Sex differences in emotional perception: Meta analysis of divergent activation. NeuroImage 147, 925–933 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Lighthall NR, Mather M & Gorlick MA Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PloS One 4, e6002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lighthall NR et al. Gender differences in reward-related decision processing under stress. Soc. Cogn. Affect. Neurosci 7, 476–484 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Becker JB, McClellan ML & Reed BG Sex differences, gender and addiction. J. Neurosci. Res 95, 136–147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaplin TM, Hong K, Bergquist K & Sinha R Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol. Clin. Exp. Res 32, 1242–1250 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Achterberg M, van Duijvenvoorde ACK, Bakermans-Kranenburg MJ & Crone EA Control your anger! The neural basis of aggression regulation in response to negative social feedback. Soc. Cogn. Affect. Neurosci 11, 712–720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winter D, Koplin K & Lis S Can’t stand the look in the mirror? Self-awareness avoidance in borderline personality disorder. Borderline Personal. Disord. Emot. Dysregulation 2, 13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.American Psychiatric Association, American Psychiatric Association & DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. (American Psychiatric Association, 2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.