Abstract
Background and Aims:
Ukraine’s HIV epidemic remains concentrated in opioid dependent people who inject drugs (PWID) where opioid agonist therapies (OAT) like methadone (MMT) and buprenorphine (BMT) maintenance treatments are the most cost-effective HIV prevention strategy, but remain under-scaled. This study aimed to measure the association between dose and type of OAT prescribed and treatment retention.
Design:
Observational longitudinal cohort study.
Participants/Setting:
Patients (n=15,290) prescribed OAT throughout Ukraine from 2004 through 2016.
Measurements:
Data were analyzed using time-event strategies to estimate cumulative treatment retention, defined as time to OAT discontinuation. Cumulative retention proportions at 1, 12 and 36 months were assessed for outcomes. Cox-regression with log-rank likelihood assessed independent predictors of treatment discontinuation.
Findings:
The proportion prescribed high (MMT: >85mg; BMT: ≥16mg), medium (MMT: >40–85mg; BMT: >6–15mg), and low (MMT: ≤40mg; BMT: ≤6mg) dosages was 25%, 43% and 32%, respectively. Retention was significantly higher for BMT than MMT both at 12 (89% vs 75%) and 36 months (80% vs 56%). Though dosing levels for BMT did not influence retention, increasing dosages for MMT were significantly associated with higher retention rates at 1 (90%, 96%, 99%), 12 (59%, 78%, 91%) and 36 (34%, 59%, 79%) months, respectively. Independent predictors associated with 12-month OAT discontinuation were medium (adjusted hazards ratio: aHR=2.23; 95%CL=1.95–2.54) and low (aHR=4.96; 95%CL=4.37–5.63) OAT dosage relative to high dosage, male sex (aHR=1.27; 95%CL=1.14–1.41), MMT relative to BMT prescription (aHR=1.57; 95%CL=1.32–1.87), and receiving OAT in general (aHR=1.22; 95%CL=1.02–1.46) or tuberculosis (aHR=1.43; 95%CL=1.10–1.85) hospitals, relative to specialty addiction treatment and AIDS Center settings. Lower dosages contributed more to dropout especially at 1 month (aHR 3.12; 95%CL=2.21–4.41 and aHR 7.71; 95%CL=5.51–10.79 for medium and low dosages, respectively). Younger age was significantly associated with OAT discontinuation only at 36 months (aHR=1.08; 95%CI=1.02–1.15).
Conclusions:
Higher dosages of opioid agonist therapies, especially for methadone maintenance treatment patients, appear to be associated with higher levels of treatment retention in Ukraine.
Keywords: methadone, buprenorphine, dosing, treatment retention, treatment drop-out, HIV prevention, Ukraine, implementation science
Introduction
Eastern Europe and Central Asia (EECA) has the highest prevalence of opioid injection (1.6%) among the 17 million opioid users globally (1, 2). It is also the only region where HIV incidence and mortality are increasing due to low levels of HIV prevention and treatment (3). In the absence of effective treatment, opioid injection results in multiple adverse consequences, including transmission of blood-borne infections like HIV and viral hepatitis, overdose, increased crime, unemployment and lower quality of life (4). Ukraine’s HIV epidemic is volatile with the highest adult HIV prevalence (1.2%) in Europe (5), concentrated in people who inject drugs (PWID) (4, 6) with opioid use disorder (OUD), with evidence of a transitioning epidemic to the sexual partners of PWID (5). Maintenance on opioid agonist therapies (OAT) like methadone (MMT) and buprenorphine (BMT) are among the most effective and cost-effective HIV prevention strategies where the epidemic is concentrated in PWID, including in Ukraine (7). Systematic reviews and meta-analyses suggest OAT reduces primary HIV transmission by 54%(8) and secondary transmission by improving HIV treatment engagement along the HIV care continuum (9). Initiation on any or medications to treat opioid use disorder is associated with decreased mortality , yet longer retention on OAT is associated with increased HIV prevention benefits (10) and retention on OAT beyond 12 months is also associated with decreased mortality (11).
In 2016, OAT coverage was only 2.7% of the 346,000 PWID in Ukraine (>80% use opioids), far lower than the 40% coverage recommended by WHO to effectively reduce HIV transmission (7). New modeling from Ukraine suggests that scaling up OAT to 20% would markedly avert tens of thousands of new HIV infections and deaths over 10 years (10). Buprenorphine was first introduced in Ukraine in 2004 (12), and has been restricted to approximately 800 patients due to cost. In 2008, all new OAT slots were added using lower-cost MMT (13). New patients may receive BMT only when others leave treatment. Despite plans to scale-up OAT to 30,000 patients by 2015 (14), OAT scale-up stubbornly remained around 9,000 patients from 2010 to 2013 due to stringent governmental regulations, negative attitudes toward OAT by patients and providers (15–18), low political will and structural and policy factors like mandatory daily supervised dosing restricted to specialized settings (17, 18). Further, Russian annexation of Crimea and armed conflict between Ukraine and Russia in the Donbas region resulted in OAT discontinuation of over 800 patients in these regions in 2014, though some OAT patients were expatriated to receive OAT in nearby regions of Ukraine (19).
Scaling up OAT is a dynamic process that involves increasing treatment access by increasing OAT entry, maximizing retention, and thereby increasing capacity (i.e., the number of patients currently on treatment). Regarding retention, a 2008 assessment of the first 391 BMT and MMT patients in Ukraine found that 6-month retention was similar (~85%) for patients receiving either MMT or BMT (20). As capacity increased, however, an observational study of 13 large MMT sites showed lower 12-month retention (66%) through 2012, highest among those on moderate to high dosages, but was limited to only 2916 patients and did not allow assessment of other modifiable factors that could improve program delivery (21). Despite a number of individual and organizational factors that have been associated with treatment retention (or drop-out) in various settings, systematic reviews suggest that retention levels on OAT are higher for methadone than for buprenorphine (22) and the most potent factor influencing retention of patients on OAT is dosage levels (23–25).
We examined the entire national database of all patients ever to receive OAT in publicly-funded sites in Ukraine over a 13-year period and analyzed the relationship between dose and type of OAT prescribed on treatment retention. Results from this analysis are intended to guide OAT treatment programs in Ukraine and throughout EECA where policies for OAT are similar, to improve OAT scale-up to meet national targets and advance HIV prevention and treatment efforts.
Methods
Addiction treatment context
By December 31, 2016, all OAT for 9,214 patients in Ukraine was prescribed only by narcologists (addiction treatment specialists) at 174 licensed treatment centers, including at specialty addiction treatment clinics and at integrated sites at HIV and tuberculosis centers. Narcology, a discipline of addiction psychiatry in Ukraine and throughout the EECA region, is a vestige of the Soviet Union (26). International funders required that OAT be introduced in Ukraine to prevent HIV, not necessarily to treat opioid use disorder (OUD), but narcologists, the only ones who could prescribe OAT, were reluctant since they believed it not to be effective; nearby Russia bans all OAT and continues to exert influence on treatment in Ukraine. Consequently, negative attitudes toward OAT prevail among physicians, patients and policy makers (27, 28). Regulations regarding OAT are especially stringent, requiring a panel of three narcologists to confirm OUD and change OAT dosages, report patients to a national registry that markedly restricts their access to driving and certain employment opportunities (29) and OAT programs have limited hours of operation (18). Adjunctive counseling is provided at limited OAT centers by personnel who are not trained in evidence-based counseling. New legislation passed in November 2016 now allows OAT programs and pharmacies to dispense medications for up to 10 days if patients meet stringent take-home dosing criteria; in November 2019 take-criteria was relaxed so that patients must verify sobriety using monthly urine drug testing for 3 rather than 6 months. OAT can be prescribed by any physician who has completed supplemental training. Nearly all OAT is dosed using tablets. Patients who miss 10 consecutive days of OAT must be discontinued from treatment and may re-enroll if a slot becomes available.
Study Population
A prospective, longitudinal national database (SyrEx) of all patients enrolled in OAT in Ukraine since 2004 (N=15,290), including receipt of a single dose, has been maintained. Each client is assigned a unique code without personal identifiers. SyrEx records individual- and program-level monitoring of OAT entry and retention dynamics (30). The following data are available for each client: age; sex; age at first injection; OAT initiation and discharge dates; type of OAT prescribed; OAT dosage; treatment location and facility type; and HIV test results (99% were tested); other psychiatric and substance use co-morbidity are not collected.
Data were censored through December 31, 2016 and data for patients from Crimea and Donbas conflict regions were excluded because patients discontinued treatment due to treatment facility closures (19). For the 2,748 (18%) participants with multiple (range: 2–22) treatment episodes, only one episode was selected at random and included in the analysis. Additionally, 1,114 (7%) OAT patients were excluded from the analysis due to death, leaving 14,176 in the analytic sample. Reason for death was not available.
Measures
The primary outcome was time to OAT treatment discontinuation (i.e., dropout), defined as missing 10 continuous days of OAT. The primary exposure was OAT dosage (current or at time of discharge) categorized based on levels from a recent Cochrane review, mostly from high-income settings (31), which suggested that higher dosages were associated with higher treatment retention. Dosing was categorized as high (MMT: >85mg; BMT: ≥16mg), medium (MMT: >40–85mg; BMT: >6–15mg), and low (MMT: ≤40mg; BMT: ≤6mg).
The remaining independent variables were analyzed as potential correlates of the time to OAT treatment discontinuation. Age and age of injection initiation, which were not normally distributed, were dichotomized by lowest quartile (≤33 vs. >33 years) and median (≤17 vs >17 years), respectively. OAT was categorized as receiving MMT or BMT, which can be administered at all facility types, including specialty addiction clinics, general hospitals, or integrated AIDS or TB centers. Finally, a variable denoting HIV status was categorized as HIV positive, HIV negative or unknown.
Statistical Analysis
Descriptive statistics were used to characterize OAT patients, stratified by OAT dosage level and type of OAT prescribed. Bivariate associations were tested using Chi-square for categorical variables and Wilcoxon-Mann-Whitney test for continuous variables, given their non-normal distribution. Kaplan-Meier survival curves were estimated for the 1-, 12- and 36-month follow-up periods, stratified by OAT type and dosage and compared using log-rank likelihood test.
We employed event-time approaches where for all patients, retention time started from the time of treatment initiation and ended at the time of discharge from OAT, or the last date while still on OAT at 1, 12 and 36 months; analyses at 24 months did not generally differ from findings at 12 and 36 months. Participants who were still on OAT at the end of the study period (December 31, 2016) but had insufficient time to assess the outcome at 1, 12 and 36 months, were censored but included in the analysis. We conducted univariate and multivariate Cox proportional regression analyses of factors associated with time-to-treatment discontinuation or “dropout” (32). Adjusted hazard ratios (aHR) and corresponding 95% CL were calculated. Backward elimination using Bonferroni correction was used to define the final multivariable model, which corresponded to the best goodness-of-fit. Variables were retained in the final model only if they were significantly (p<0.05) associated with the outcome in the adjusted model. The Cox model treats the instantaneous risk of treatment discontinuation for a given subject as the product of two terms: a time-dependent “baseline” hazard, which is assumed to be common for all subjects, and a function of the subject’s covariates. To address concerns about changes in temporal trends, year of OAT treatment initiation was controlled for in all analyses; not controlling for enrollment year did not influence the outcomes. The analyses were not pre-registered and considered exploratory.
Results
Tables 1 and 2 describe characteristics of OAT patients stratified by dosage levels and OAT type (MMT vs. BMT). Overall, patients were primarily male (82%), in their late 30s (median=37 years), and had injected drugs a median of 17 years. Most (91%) patients were prescribed MMT and the median dose and interquartile range (IQR) for MMT and BMT was 60mg (35mg–90mg) and 10mg (6mg–12mg), respectively. HIV prevalence was 40%. The proportion of patients prescribed high, medium and low OAT dosages was 25%, 43% and 32%, respectively, but the proportion differed for MMT and BMT patients. Over half (55%) received OAT at narcology clinics and 35% were treated at general hospitals. Bivariate analysis of characteristics significantly associated with receiving higher OAT dosages included: 1) longer duration of drug use; 2) being on MMT rather than on BMT; 3) positive HIV status; and 4) receiving OAT at AIDS center sites. Compared to patients on BMT, those on MMT were significantly more likely to be male (83% vs. 79%), with shorter drug use duration [17 vs 19 years], lower HIV prevalence (40% vs. 54%) and receiving OAT at narcology clinics (83% vs. 52%).
Table 1: Characteristics patients on opioid agonist treatments, stratified by dosage levels (N=14,176).
| Characteristic | Overall N=14,176 (%) |
OAT Dosage | P-value* | ||
|---|---|---|---|---|---|
| High N=3,531 (24.9%) |
Medium N=6,053 (42.7%) |
Low N=4,592 (32.4%) |
|||
| ≤33 years | 4256 (30.0) | 978 (23.0) | 1,889 (44.4) | 1,389 (32.6) | |
| Female | 2488 (17.6) | 580 (23.3) | 1,135 (45.6) | 773 (31.1) | |
| ≤17 years | 7,177 (50.6) | 1,611 (22.4) | 3,106 (43.3) | 2,460 (34.3) | |
| BMT | 1,331 (9.4) | 201 (15.1) | 794 (59.7) | 336 (25.2) | |
| Not known | 124 (0.9) | 23 (18.5) | 49 (39.5) | 52 (42.0) | |
| TB center | |||||
IQR: interquartile range; NA: not applicable; OAT: opioid agonist therapy; MMT: methadone maintenance therapy; BMT: buprenorphine; OAT dosage: high (MMT: >85mg; BMT: ≥16mg), medium (MMT: >40–85mg; BMT: >6–15mg), and low (MMT: ≤40mg; BMT: ≤6mg).
P-value for Chi-Square test.
Table 2: Characteristics of Patients Receiving Methadone and Buprenorphine Treatment in Ukraine (N=14,176).
| Characteristic | Type of Opioid Agonist Treatment | P-value* | |
|---|---|---|---|
| Methadone N=12,845 (91%) |
Buprenorphine N=1,331 (9%) |
||
| Median age, years (IQR) | 37 (33–43) | 38 (34–44) | <0.001 |
| Female | 2205 (88.6) | 283 (11.4) | |
| Median drug injection duration, years (IQR) | 17 (13–22) | 19 (15–23) | <0.001 |
| Median dose, mg (IQR) | 60 (35–90) | 10 (6–12) | NA |
| Unknown | 113 (91.1) | 11 (8.9) | |
| TB center | 425 (88.7) | 54 (11.3) | |
IQR: interquartile range; NA: not applicable; OAT: opioid agonist therapy.
P-value for Chi-Square test.
Figure 1 compares the retention levels for patients on MMT and BMT. Retention levels were significantly higher for BMT relative to MMT patients at 12 (89% vs 75%), 24 (84% vs 64%) and 36 months (80% vs 56%). Retention on MMT increased significantly for higher treatment dosages (Figure 2A), however, did not do so to the same extent for patients on BMT (Figure 2B). Table 3 presents results from the multivariate Cox regression models. Factors significantly and independently associated with lower 1-month, 12-month and 36-month retention (i.e., dropout) after controlling for year of enrollment include: 1) medium and low OAT dosage; 2) male sex; 3) being on MMT rather than BMT; and 4) receiving OAT at general hospitals or TB centers compared to treatment in specialty AIDS or narcology centers. Being HIV positive was not associated with higher retention. While age did not contribute to 1-month or 12-month OAT retention, younger age significantly reduced the likelihood of being retained in treatment at 24 (data not shown) and 36 months.
Figure 1:
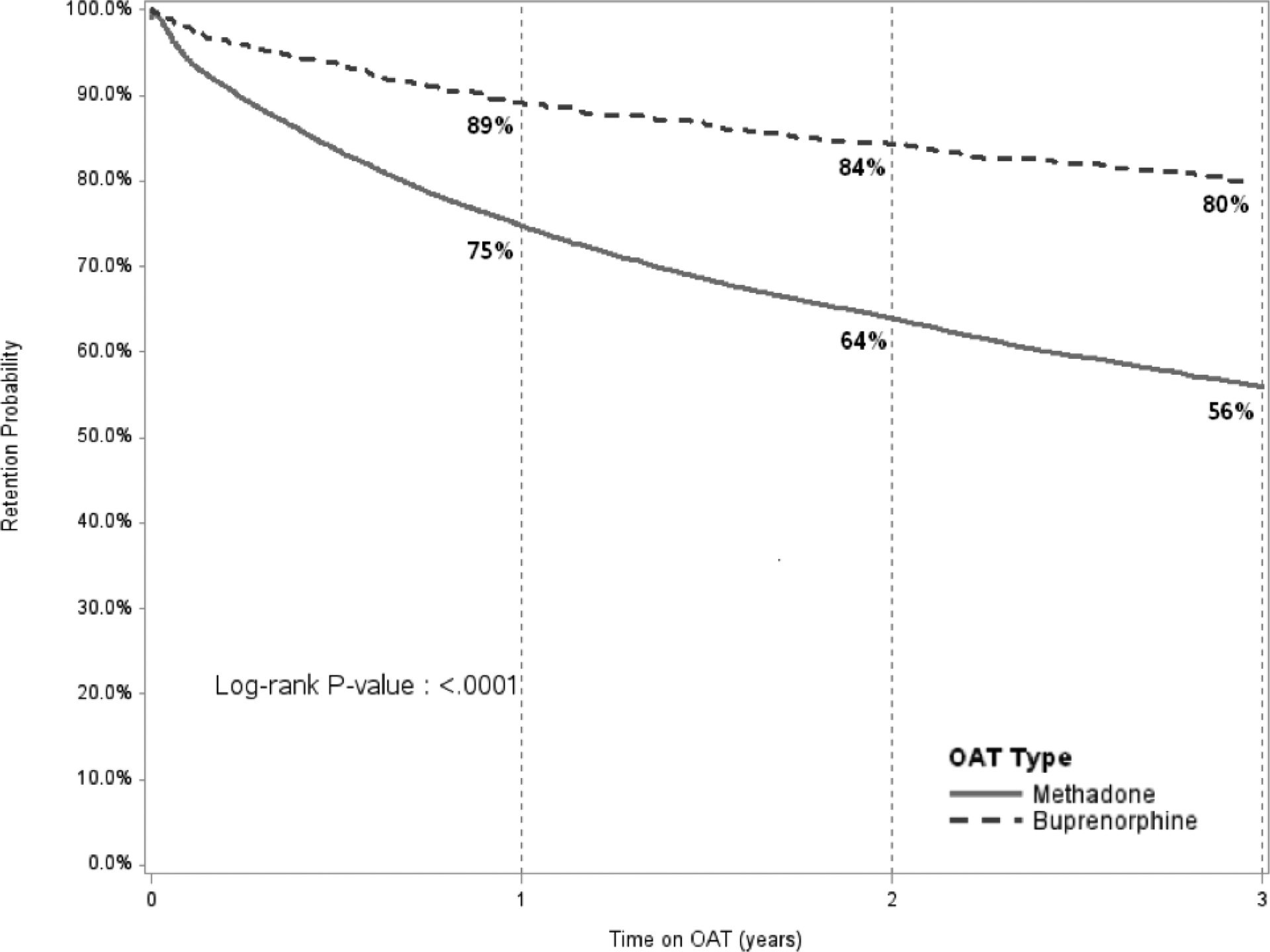
Comparison of Retention on Treatment for Methadone and Buprenorphine over 36 months
OAT: opioid agonist therapies
Figure 2:
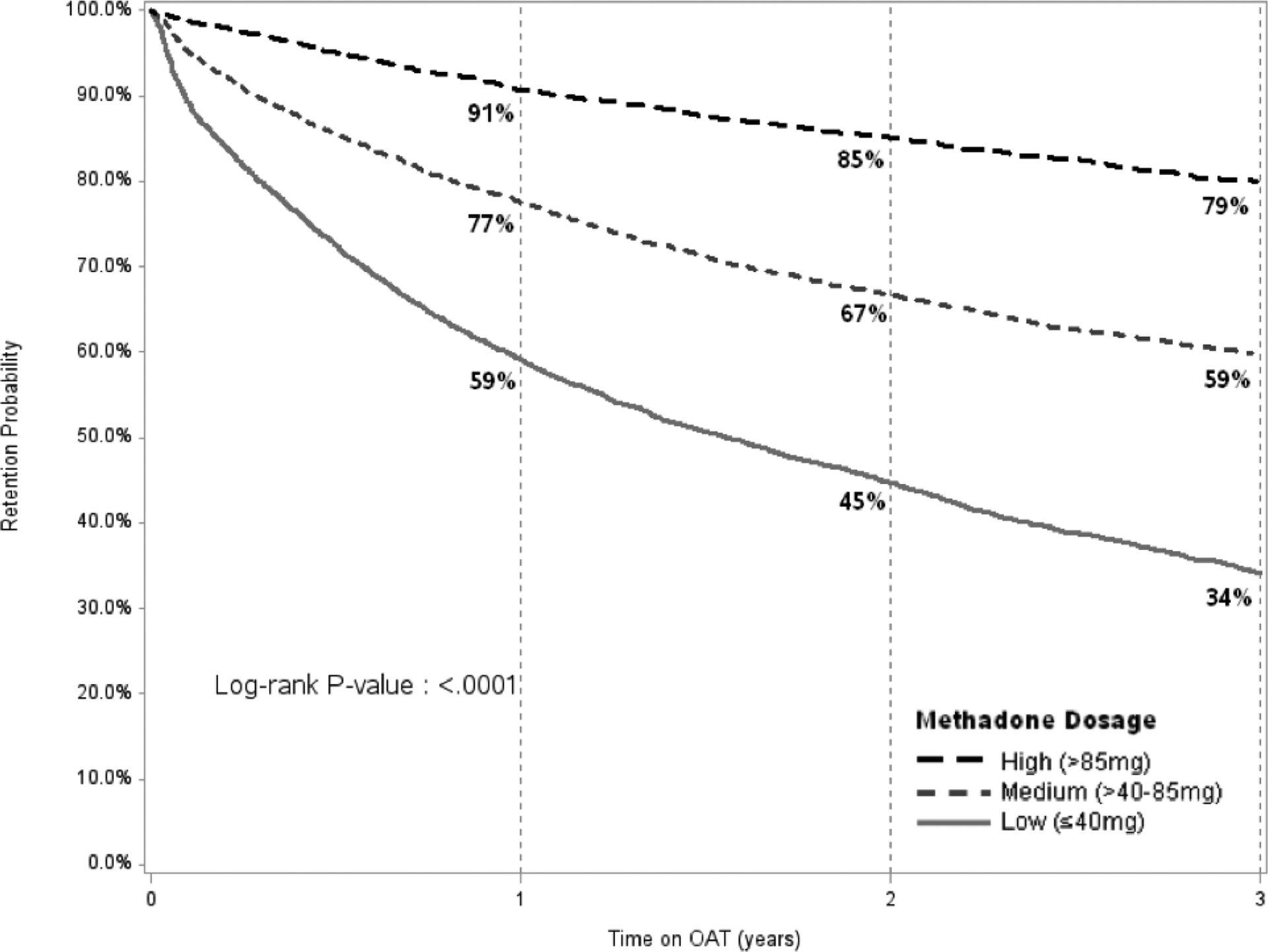
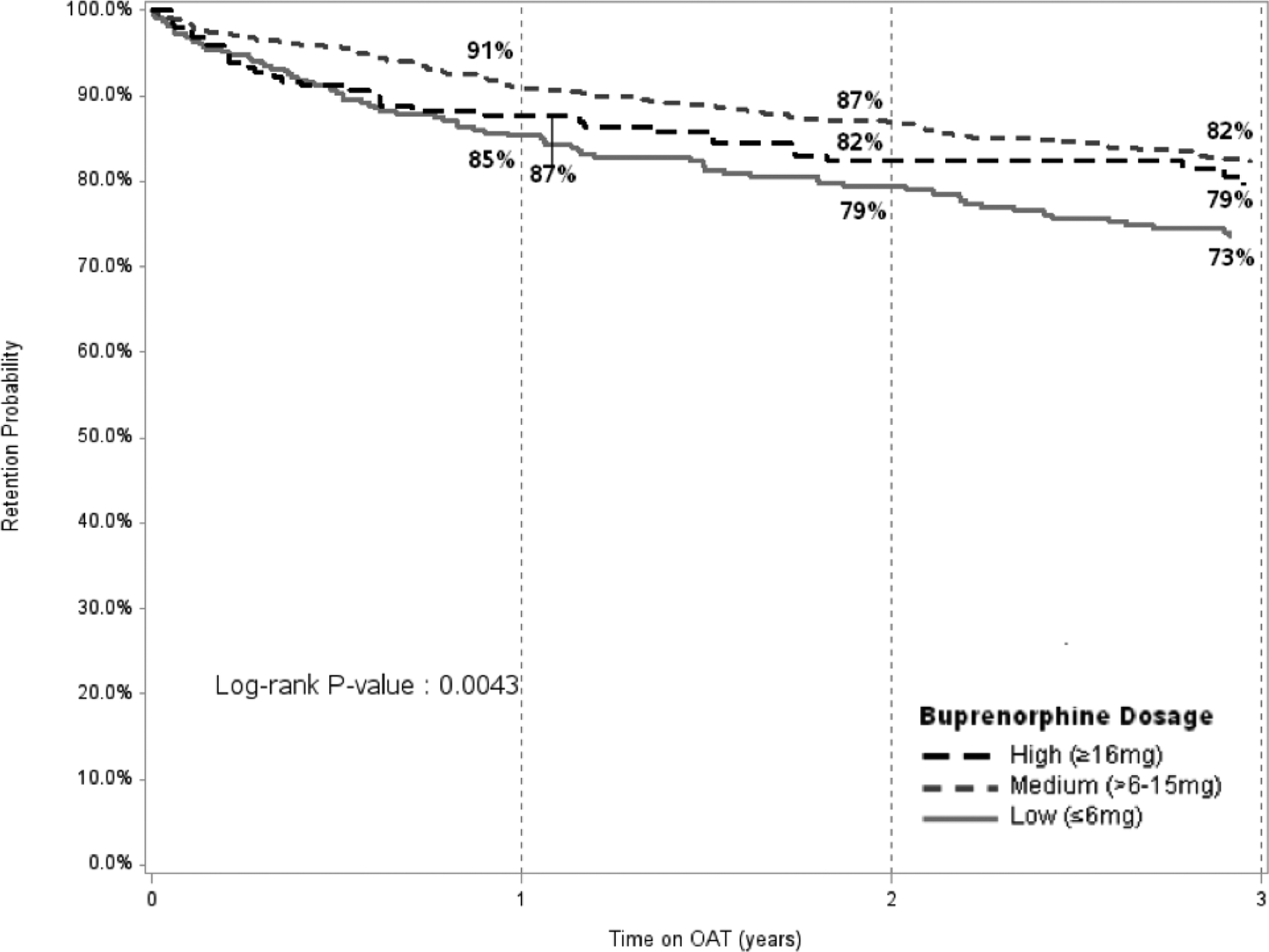
Retention on Methadone (Panel A) and Buprenorphine (Panel B) over 36 months, Stratified by Dosage Panel A (Methadone)
OAT: opioid agonist therapies
*** PLEASE PUT FIGURE A and B side by side in panels in FIGURE 2
Panel B (Buprenorphine)
OAT: opioid agonist therapies
Table 3. Cox regression model of factors associated with lower retention (dropout) on opioid agonist therapies, controlling for year of enrollment.
| 1-month | 12-month | 36-months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| aHR | 95% HR CL | p-value | aHR | 95% HR CL | p-value | aHR | 95% HR CL | p-value | |
| Low | 7.71 | 5.51–10.79 | <0.0001 | 4.96 | 4.37–5.63 | <0.001 | 4.80 | 4.36–5.28 | <0.001 |
| Male | 0.98 | 0.80–1.21 | 0.8688 | 1.27 | 1.14–1.41 | <0.001 | 1.32 | 1.21–1.44 | <0.001 |
| Methadone | 1.98 | 1.30–3.02 | 0.0015 | 1.57 | 1.32–1.87 | <0.001 | 1.77 | 1.55–2.03 | 0.001 |
| ≤33 years old | 0.77 | 0.65–0.92 | 0.1054 | 1.08 | 1.02–1.15 | 0.014 | |||
| TB center | 1.95 | 1.10–3.46 | 0.0224 | 1.43 | 1.10–1.85 | 0.007 | 1.44 | 1.15–1.80 | 0.002 |
I: aHR: adjusted Hazards Ratio; CL: Confidence Limits; OAT: opioid agonist therapy; MMT: methadone maintenance therapy; BMT: buprenorphine; OAT dosage: high (MMT: >85mg; BMT: ≥16mg), medium (MMT: >40–85mg; BMT: >6–15mg), and low (MMT: ≤40mg; BMT: ≤6mg).
Discussion
Aside from one systematic review that included 58 OAT programs and 27,047 patients in low/middle-income countries (LMIC)(33), findings here report the largest evaluation of OAT dosage on retention in any single LMIC. Findings from this study contribute new and important findings not found elsewhere, including longitudinal data over an extended time period (13 years), more robust patient-level data, inclusion of program-level variables, markedly higher levels of retention at 12 months reported in the systematic review and, importantly, an assessment of 36-month retention, which has previously not been assessed. Since being treated with any medication for opioid use disorder (methadone, buprenorphine or extended-release naltrexone) reduces mortality, and more so if retained on treatment beyond 12 months (11), it stands to reason that maintaining patients on higher OAT doses will result in reduced death through its retention benefit. Moreover, because OAT benefits other social, health and HIV prevention outcomes (10, 34), being retained on OAT is likely to extend these benefits, which was demonstrated in a recent mathematical modeling study for Ukraine (10). Unlike the systematic review of LMICs, this study found significantly higher retention levels in patients on BMT than on MMT.
Receiving and being stabilized on OAT is crucial for both primary and secondary HIV prevention. Noteworthy is that Ukraine’s HIV epidemic is concentrated in PWID with OUD, and like other countries in the EECA region, suboptimal HIV prevention and treatment coverage in PWID remains concerning (5, 35, 36). The EECA region, whose legacy of addiction treatment evolves from the Narcology system developed by the Soviet Union, remains the only region globally where HIV incidence and mortality are increasing (5). Ukraine is emblematic of most of these countries for its suboptimal prevention and treatment response to an HIV epidemic concentrated in PWID. OAT is completely banned in Russia and Turkmenistan and is available mostly as pilot projects elsewhere in EECA (2, 34, 36, 37). Mathematical modeling for Ukraine suggests that the combination of OAT and ART scale-up reduces HIV transmission the most, but in poorly-resourced settings, OAT scale-up is the most cost-effective intervention (7), making OAT scale-up and retention a crucial factor in addressing the HIV epidemic in this region (10). A recent modeling study from Ukraine suggested that scaling up OAT to 20% coverage could prevent 10,864 new HIV infections and 17,863 deaths over a 10-year horizon (10).
Several important findings emerge that have major implications for scaling up OAT in Ukraine, and potentially throughout EECA. First, consistent with international data (31) but not from LMICs (33), higher OAT doses, especially when methadone dosages exceed 85mg per day, resulted in significantly higher treatment retention at 1, 12 and 36 months. Second, lower OAT dosages played a more pronounced role in dropout at 1 month relative to 12 and 36 months, suggesting the need to safely help patients obtain adequate but higher dosages early. Third, several reviews suggest that OAT with either MMT or BMT is superior to supervised withdrawal (detox) or counseling strategies, finding no differences in retention between MMT or BMT (2, 22, 38, 39). OAT dosing levels, however, were not addressed in these reviews and in one, only opioid dependent persons taking prescription pain medications were included. Finally, a 2014 Cochrane Review suggests that compared to supervised opioid withdrawal alone, both BMT and MMT are more effective at low, medium, and high doses in reducing illicit opioid use, but that treatment retention is increased only at the medium and high OAT dose levels, especially for OAT patients prescribed the highest OAT dosage range (40).
Findings from this real-world observational study differ from these reviews in several important ways. First, the sample size from the real-world retention data in Ukraine is more than 15-fold higher than that of studies that focus on highly selected patients in prospective controlled trials. Second, retention was significantly higher in BMT than in MMT patients in Ukraine, which suggests that the local context where patient selection may be involved could play an important role in retention. One potential explanation for this difference is that PWID in Ukraine substantially prefer BMT over MMT (16, 17), making BMT a more valued commodity as a limited resource. Because buprenorphine is often prescribed at pharmacies and taken unsupervised elsewhere, Ukraine’s requirement of supervised daily dosing for both medications may influence retention outcomes between the medications. Third, while retention rates increased with increasing doses in MMT patients, such a trend did not hold for BMT patients, again suggesting a contextual difference in treatment. Finally, most OAT retention studies, especially in prospective trials, do not report retention beyond 12 months. Here, we provide retention findings over a 36-month period for methadone (56%) and buprenorphine (80%), but with marked differences based on dosages prescribed for individuals receiving methadone.
While systematic reviews and meta-analyses support higher OAT dosages based on higher retention levels, many clinicians and experts recommend that OAT dosages be individualized and flexible because each patient presents a unique clinical challenge as no single dose is best for all patients. Dosage induction and subsequent dosage adjustments have usually been needed in clinical practice (41) and individualized flexible dosing is supported by a systematic review (42).
Flexible dosing algorithms to individualize therapy often include dosage increases based on urine toxicology, craving and withdrawal symptoms (43, 44). Dosing adequacy has emerged as an individualized strategy to promote retention (45) for patients with OUD. Dosing adequacy is an independent contributor to optimal treatment outcomes for OUD and balances the amount needed to alleviate withdrawal symptoms, eliminate ongoing illicit opioid use, and markedly reduce craving while avoiding signs of opioid excess. While absolute dose and dosing adequacy constructs should be differentiated from each other (46), dosing adequacy can be guided using objective and validated instruments (47, 48). One longitudinal study found that absolute dose, when supplemented with dosing adequacy measures, not only increased methadone dosages, but also improved addiction severity indicators (49). Such strategies are patient-centered and can optimize treatment outcomes, like retention, by minimizing or removing the often-paternalistic management strategies by physicians caring for patients with OUD and improve patient satisfaction by guiding treatment decisions using validated measures and involving patients in their own treatment.
Dosing in any context can be capricious and influenced by patient (e.g., keeping dose low to allow for euphoria if still injecting) and physicians (e.g., concerns that there is a maximum dose needed). One alternative to align patient and physician decision-making is using shared decision-making (SDM) aids where patient preferences are linked to true evidence (e.g., higher retention leads to better outcomes). SDM aids are evidence-based strategies that balance patient autonomy with physician judgment and information sharing (50–52), and remove the influence of cultural practice. The retention findings generated by these nation-wide data offer a unique opportunity to shift the informed decision-making process between the provider and prospective patients in Ukraine. A simple tool to guide OAT preference and dosing combined with outlining the specific benefits of retention could be developed from these local data; such a pragmatic and Ukraine-specific tool could guide clinical induction processes to optimize higher doses early, while maintaining safety standards, in the induction process. This shared decision-making aid could guide the discussion regarding treatment expectations and include optimal and adequate dosing, and further address anticipated duration of OAT maintenance. Such strategies would not only support OAT scale-up and HIV prevention efforts, which have been thwarted by personal, clinical setting and policy factors in Ukraine (18), but also provide clear guidance at multiple levels to curtail the HIV epidemic.
Of interest, and contrary to the international experience, OAT retention in Ukraine is higher for patients on buprenorphine rather than on methadone. Though this finding cannot be fully explained, potential explanations exist. First, Ukraine’s initial experience with OAT was with BMT in 2004 (12), with public perceptions that it was safer. Second, OAT was not introduced as addiction treatment – instead it was introduced for HIV prevention, but its delivery required that it be prescribed by Narcologists who believed it to be ineffective for treating opioid use disorder. Third, “ideal” patients, those deemed likely to succeed with treatment, were initially selected to ensure OAT sustainability, evidenced by extraordinarily high retention in the period soon after BMT and MMT introduction (20). Fourth, methadone was perceived by patients as an inferior treatment because it was cheaper and accounted for most OAT slots starting in 2008 (53). Fifth, both MMT and BMT were supervised daily over this observation period, which is unlike the international experience where the safer buprenorphine profile typically allows its dispensation by prescription. While year of OAT enrollment was controlled for in the analysis, enrollment in BMT early in the OAT treatment era only partially explains higher retention relative to MMT. BMT patients, however, had significantly higher retention even for controlling for enrollment year, but retention did decrease over time for both BMT and MMT. Sixth, higher retention in BMT patients might also be explained by patient preferences, which has recently been documented in PWID in Ukraine (17). This cannot, however, fully explain the retention differences because surveys of PWID elsewhere show that patients often prefer buprenorphine over methadone (54, 55). Last, though not recorded in our database, BMT patients may have received ‘intermittent’ dosing based on its longer pharmacological half-life, allowing increased flexibility (e.g., for work, weekends) for BMT patients that promoted retention. While we cannot fully disentangle the reasons for these findings, future studies should include patient-centered strategies. Such strategies should incorporate patient preferences in the treatment decision process so that patients may truly weigh various treatment attributes (e.g., cost, degree of supervision, pharmacological properties, retention on treatment), which have all become options with the new legislation. Informed or shared decision-making aids provide a structured and consistent strategy to guide this process to better engage the patient in treatment and recognize that controversies in OAT exist at both the patient and provider level, and promote a guided deliberation process where uncertainty in treatment outcomes exist (52).
Though OAT may be prescribed in a number of clinical settings, findings here suggest that retention is significantly higher at 12 and 36 months for patients treated in either narcology or AIDS specialty treatment settings and lower in general hospital or TB clinics. Lower retention in TB clinics, also observed for 1-month retention, may be due to a number of factors like drug interactions with rifampicin that can precipitate opioid withdrawal symptoms (56), or patients successfully completing TB treatment and either not interested in transitioning to alternative care sites (53). Lower retention in general hospital clinics might be related to the generally negative feelings toward drug users and the perception that PWID should be treated outside mainstream medicine and in specialty addiction treatment settings (57). Recent pilot data from Ukraine suggest that 6-month retention outcomes of MMT prescribed in primary care clinics was similar to those prescribed in addiction specialty clinics; findings support that clinicians in primary care clinics reduced their likelihood to discriminate against PWID and patients felt less stigma over time (57). The 2016 legislation now allows for such strategies, which provides more treatment options for patients while expanding OAT cost-effectively (58). While the SyrEx database could differentiate the site of the OAT delivery, it could not differentiate which of these were truly integrated care settings, which in Ukraine, have been associated with better HIV and addiction treatment outcomes (59). Importantly, being treated in AIDS Centers, but not having HIV itself, was independently associated with higher retention. Because HIV patients treated at AIDS Centers were more likely to be prescribed ART (60), OAT dosages were likely increased to overcome known pharmacokinetic drug interactions between ART (mostly efavirenz) and OAT (56) through better communication onsite between OAT and ART prescriber (61).
Concerning in Ukraine, however, is that only 25% of OAT patients were dosed at the level associated with highest retention. Strategies that focus on achieving OAT dosing adequacy, especially at higher dosages early in the treatment initiation process, is supported by markedly different retention outcomes even within the first month of treatment. Escalating dosages quickly and early in the treatment process may be one strategy to improve retention, yet the long half-life of methadone requires careful monitoring for opioid excess and polysubstance use initially to guide treatment retention improvements and OAT scale-up in Ukraine. More rapid dose escalation when prescribing buprenorphine is less concerning due to its partial agonist/antagonist properties.
Though the findings here are important for HIV prevention and treatment, they are not without limitations. First, temporal trends may influence the outcomes with OAT programs initially selecting more suitable patients than those enrolled later. Though there was some evidence that earlier enrolled patients had better outcomes, year of enrollment was controlled in all analyses to reduce this bias. Second, there was a lack of granularity of the data, both at the individual but also in the clinic setting that does not allow us to fully disentangle some unmeasured factors that may influence treatment retention. Moving forward, the new 2016 legislation allows for more expanded treatment options, including the ability to pay out of pocket and to choose their preference for BMT or MMT, to receive treatment in primary care settings and to receive OAT for up to 10 days if they maintain sobriety or six months.
Conclusions
Data from Ukraine confirm that increased OAT dosages of OAT are associated with higher levels of retention throughout 36 months. Given OAT’s role in primary and secondary HIV prevention and treatment in the only region where HIV incidence and mortality are increasing, it stands that strategies that promote higher OAT dosages to foster treatment retention, including during the first month of treatment, would greatly contribute to curbing the HIV epidemic and make progress toward meeting the UNAIDS 95–95-95 treatment strategy.
Figure 3.
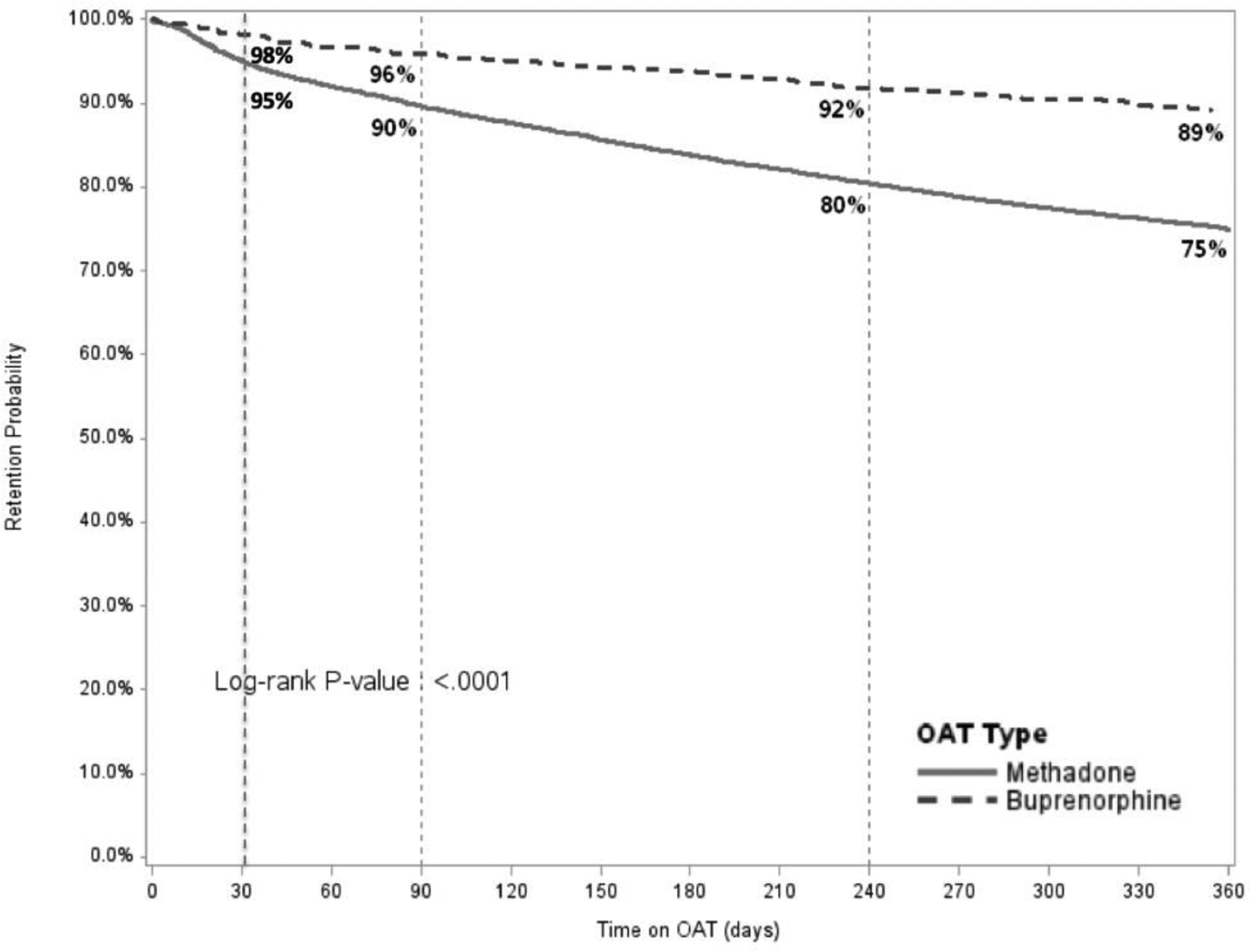
Comparison of Retention on Treatment for Methadone and Buprenorphine over 12 months
OAT: opioid agonist therapies
Figure 4.
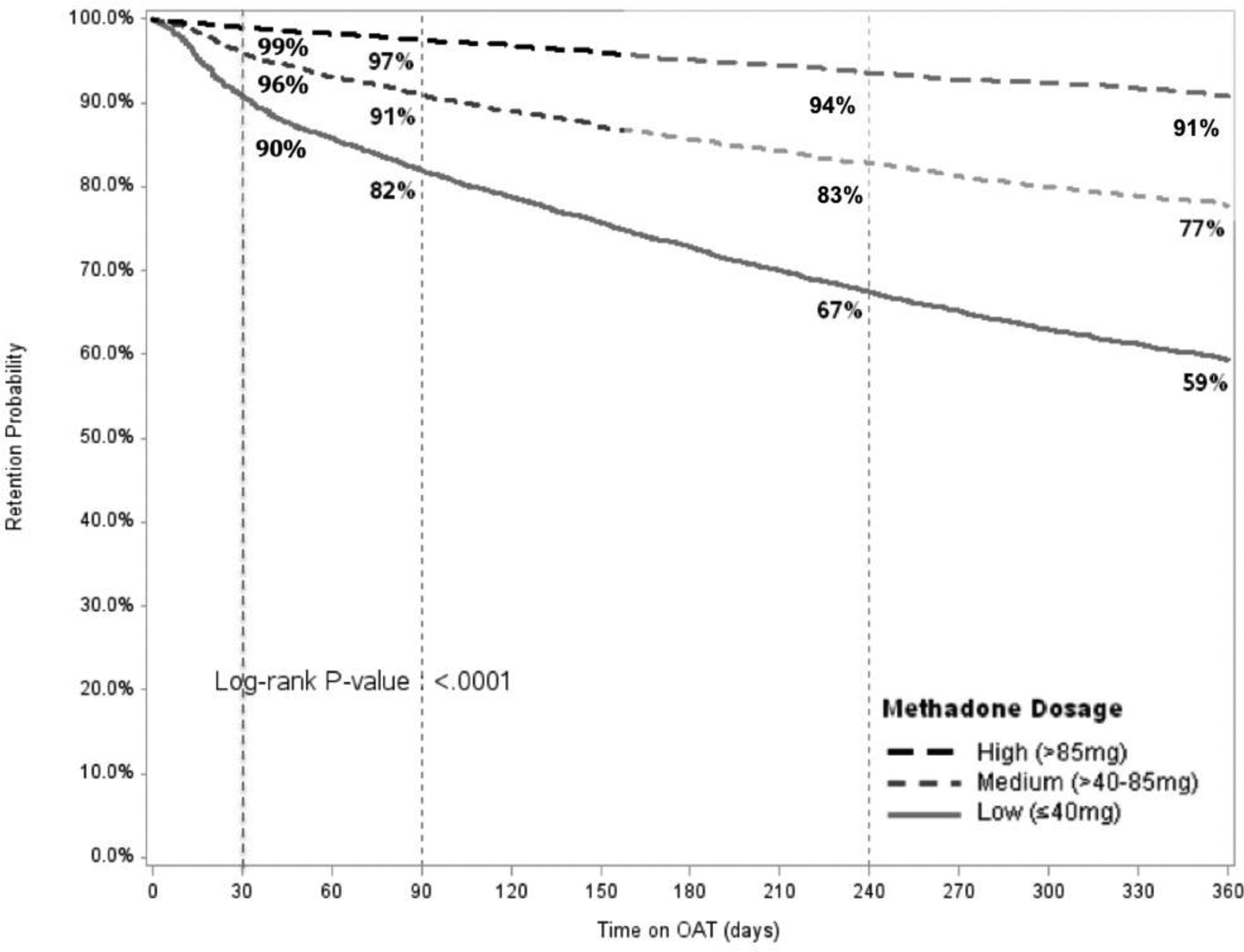
Retention on Methadone over 12 months, Stratified by Dosage
OAT: opioid agonist therapies
Acknowledgements
The authors would like to acknowledge funding from the National Institutes on Drug Abuse for research (R01 DA033679 and R01 DA029910 for FLA) and career development (K24 DA017072 for FLA and K01 DA 047194 for JR). The Global Health Equity Scholars Program funded by the Fogarty International Center and the National Institute of Allergy and Infectious Diseases (Research Training Grant R25 TW009338), and the New York State International Training and Research Program through an in-country training grant funded by the Fogarty International Center (D43TW000233) that provided support for some coauthors. Additional support was provided by the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFFATM) and the President’s Emergency Plan for AIDS Relief (PEPFAR).
Conflicts of Interest and Source of Funding:
No conflicts of interest were declared. The authors would like to acknowledge the National Institute on Drug Abuse for funding for research (R01 DA029910, R01 DA043125 and R01 DA033679) and career development (K24 DA017072), the Global Health Equity Scholars Program funded by the Fogarty International Center and the National Institute of Allergy and Infectious Diseases (Research Training Grant R25 TW009338), and the New York State International Training and Research Program through an in-country training grant funded by the Fogarty International Center (D43TW000233).
References
- 1.United Nations Office on Drugs and Crime (UNODC) World Drug Report, 2016. Vienna: 2017. p. Accessed on March 27, 2017 at: https://www.unodc.org/doc/wdr6/WORLD_DRUG_REPORT_6_web.pdf. [Google Scholar]
- 2.Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS). The Gap Report. Geneva, Switzerland: 2014. p. Accessed on 24 August 2014 at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication//unaids_gap_report_en.pdf. [Google Scholar]
- 4.United Nations Office on Drugs and Crime (UNODC). Word Drug Report 2016. Vienna, Austria: 2016. p. Accessed on 27 June 2016 at: http://www.unodc.org/doc/wdr/WORLD_DRUG_REPORT__web.pdf. [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS Update 2016. Geneva, Switzerland: 2016. p. Accessed on May 28, 2016 at: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-_en.pdf. [Google Scholar]
- 6.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–45. [DOI] [PubMed] [Google Scholar]
- 7.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med. 2011;8(3):e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low AJ, Mburu G, Welton NJ, May MT, Davies CF, French C, et al. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016;63(8):1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan J, Altice FL, Madden LM, Zelenev A. Effect of expanding opioid agonist therapies on the HIV epidemic and mortality in Ukraine: a modelling study. Lancet HIV. 2020;7(2):e121–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Bao YP, Wang RJ, Su MF, Liu MX, Li JQ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2018. [DOI] [PubMed] [Google Scholar]
- 12.Bruce RD, Dvoryak S, Sylla L, Altice FL. HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine--programme description and policy implications. Int J Drug Policy. 2007;18(4):326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaub M, Subata E, Chtenguelov V, Weiler G, Uchtenhagen A. Feasibility of buprenorphine maintenance therapy programs in the Ukraine: first promising treatment outcomes. Eur Addict Res. 2009;15(3):157–62. [DOI] [PubMed] [Google Scholar]
- 14.VRU. National Programme on HIV/AIDS for 2014–2018. Verkhovna Rada of Ukraine; 2014.
- 15.Bojko MJ, Madden L, Farnum S, Mazhnaya A, Fomenko T, Marcus R, et al. Using Nominal Group Technique to Assess Barriers to Scale Up of Opioid Agonist Therapy (OAT) in Ukraine: The Providers’ Perspective International J Drug Policy. 2017: in press. [DOI] [PubMed] [Google Scholar]
- 16.Makarenko I, Mazhnaya A, Marcus R, Bojko MJ, Madden L, Filippovich S, et al. Willingness to pay for opioid agonist treatment among opioid dependent people who inject drugs in Ukraine. Int J Drug Policy. 2017;45:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makarenko I, Mazhnaya A, Polonsky M, Marcus R, Bojko MJ, Filippovych S, et al. Determinants of willingness to enroll in opioid agonist treatment among opioid dependent people who inject drugs in Ukraine. Drug Alcohol Depend. 2016;165:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bojko MJ, Mazhnaya A, Marcus R, Makarenko I, Islam Z, Filippovych S, et al. The Future of Opioid Agonist Therapies in Ukraine: A Qualitative Assessment of Multilevel Barriers and Ways Forward to Promote Retention in Treatment. J Subst Abuse Treat. 2016;66:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippovych S Impact of armed conflicts and warfare on opioid substitution treatment in Ukraine: responding to emergency needs. Int J Drug Policy. 2015;26(1):3–5. [DOI] [PubMed] [Google Scholar]
- 20.Lawrinson P, Ali R, Buavirat A, Altice FL, Chiamwongpaet S, Dvoryak S, et al. Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103(9):1484–92. [DOI] [PubMed] [Google Scholar]
- 21.Dumchev K, Dvoriak S, Vitek C, Altice FL. Retention in Medication-Assisted Treatment Programs in Ukraine - Identifying Factors Contributing to a Continuing HIV Epidemic International J Drug Policy. 2017: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis. 2016;35(1):22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao YP, Liu ZM, Epstein DH, Du C, Shi J, Lu L. A meta-analysis of retention in methadone maintenance by dose and dosing strategy. Am J Drug Alcohol Abuse. 2009;35(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph H, Stancliff S, Langrod J. Methadone maintenance treatment (MMT): a review of historical and clinical issues. Mt Sinai J Med. 2000;67(5–6):347–64. [PubMed] [Google Scholar]
- 25.Farre M, Mas A, Torrens M, Moreno V, Cami J. Retention rate and illicit opioid use during methadone maintenance interventions: a meta-analysis. Drug Alcohol Depend. 2002;65(3):283–90. [DOI] [PubMed] [Google Scholar]
- 26.Latypov AB. The Soviet doctor and the treatment of drug addiction: “A difficult and most ungracious task”. Harm Reduct J. 2011;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polonsky M, Rozanova J, Azbel L, Bachireddy C, Izenberg J, Kiriazova T, et al. Attitudes Toward Addiction, Methadone Treatment, and Recovery Among HIV-Infected Ukrainian Prisoners Who Inject Drugs: Incarceration Effects and Exploration of Mediators. AIDS Behav. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polonsky M, Azbel L, Wickersham JA, Taxman FS, Grishaev E, Dvoryak S, et al. Challenges to implementing opioid substitution therapy in Ukrainian prisons: Personnel attitudes toward addiction, treatment, and people with HIV/AIDS. Drug Alcohol Depend. 2015;148:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bojko MJ, Dvoriak S, Altice FL. At the crossroads: HIV prevention and treatment for people who inject drugs in Ukraine. Addiction. 2013;108(10):1697–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ICF International AIDS Alliance in Ukraine. Automated records management system in harm reduction programs: SyrEx2 Kyiv, Ukraine: 2015. [Available from: http://www.aidsalliance.org.ua/cgi-bin/index.cgi?url=/en/library/syrex/index.htm. [Google Scholar]
- 31.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society Series B (Methodological) 1972(34 (2)):187–220. [Google Scholar]
- 33.Feelemyer J, Des Jarlais D, Arasteh K, Abdul-Quader AS, Hagan H. Retention of participants in medication-assisted programs in low- and middle-income countries: an international systematic review. Addiction. 2014;109(1):20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altice FL, Azbel L, Stone J, Brooks-Pollock E, Smyrnov P, Dvoriak S, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016;388(10050):1228–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joint United Nations Programme on HIV/AIDS (UNAIDS). Prevention Gap Report. Geneva, Switzerland: 2016. p. Accessed on July 14, 2016 at: http://www.unaids.org/sites/default/files/media_asset/-prevention-gap-report_en.pdf. [Google Scholar]
- 36.Malyuta R, Krausz RM. No excuses left to delay opioid agonist treatment roll-out. Lancet HIV. 2019. [DOI] [PubMed] [Google Scholar]
- 37.LaMonaca K, Dumchev K, Dvoriak S, Azbel L, Morozova O, Altice FL. HIV, Drug Injection, and Harm Reduction Trends in Eastern Europe and Central Asia: Implications for International and Domestic Policy. Curr Psychiatry Rep. 2019;21(7):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst Rev. 2016(5):CD011117. [DOI] [PubMed] [Google Scholar]
- 39.Bell J, Strang J. Medication Treatment of Opioid Use Disorder. Biol Psychiatry. 2020;87(1):82–8. [DOI] [PubMed] [Google Scholar]
- 40.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2(2):CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trafton JA, Minkel J, Humphreys K. Determining effective methadone doses for individual opioid-dependent patients. PLoS Med. 2006;3(3):e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. [DOI] [PubMed] [Google Scholar]
- 43.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343(18):1290–7. [DOI] [PubMed] [Google Scholar]
- 44.Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: a randomized trial. Jama. 1999;281(11):1000–5. [DOI] [PubMed] [Google Scholar]
- 45.Reimer J, Boniakowski E, Bachner C, Weber B, Tietje W, Verthein U, et al. When higher doses in opioid replacement treatment are still inadequate - association to multidimensional illness severity: a cohort study. Subst Abuse Treat Prev Policy. 2014;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trujols J, Sinol N, de los Cobos JP. Methadone maintenance treatment: the need to distinguish between holding dose, dose adequacy, satisfaction with methadone as a medication, and satisfaction with treatment. J Clin Psychopharmacol. 2010;30(1):95–6; author reply 6. [DOI] [PubMed] [Google Scholar]
- 47.Gardini A, Poehlke T, Reimer J, Walcher S, Weber B. Cultural and Linguistic Validation of the questionnaire ODAS (EADO) used to determine the adequacy of the daily dose of methadone as part of the maintenance programme fo rhte treatment of opioid dependence [German]. Suchttherapie. 2010;11:138–45. [Google Scholar]
- 48.González-Saiz F, Lozano Rojas O, Ballesta Gómez R, Bilbao Acedos I, Galiana Martínez J, García Collantes MA. Evidence of reliability and validity of the Opiate Dosage Adequacy Scale (ODAS) in a sample of methadone maintenance patients. Heroin Addict Relat Clin Probl. 2008;10(1):25–38. [Google Scholar]
- 49.Walcher S, Koc J, Reichel V, Schlote F, Verthein U, Reimer J. The opiate dosage adequacy scale for identification of the right methadone dose--a prospective cohort study. BMC Pharmacol Toxicol. 2016;17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elwyn G, Fisher E. Higher integrity health care: evidence-based shared decision making. Circ Cardiovasc Qual Outcomes. 2014;7(6):975–80. [DOI] [PubMed] [Google Scholar]
- 52.Elwyn G, Frosch DL, Kobrin S. Implementing shared decision-making: consider all the consequences. Implement Science. 2016;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumchev K, Dvoryak S, Chernova O, Morozova O, Altice FL. Retention in medication-assisted treatment programs in Ukraine-Identifying factors contributing to a continuing HIV epidemic. Int J Drug Policy. 2017;48:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherjee TI, Wickersham JA, Desai MM, Pillai V, Kamarulzaman A, Altice FL. Factors associated with interest in receiving prison-based methadone maintenance therapy in Malaysia. Drug Alcohol Depend. 2016;164:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gryczynski J, Mitchell SG, Jaffe JH, Kelly SM, Myers CP, O’Grady KE, et al. Retention in methadone and buprenorphine treatment among African Americans. J Subst Abuse Treat. 2013;45(3):287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morozova O, Dvoriak S, Pykalo I, Altice FL. Primary healthcare-based integrated care with opioid agonist treatment: First experience from Ukraine. Drug Alcohol Depend. 2017;173:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morozova O, Crawford FW, Cohen T, Paltiel AD, Altice FL. Cost-effectiveness of expanding the capacity of opioid agonist treatment in Ukraine: dynamic modeling analysis. Addiction. 2020;115(3):437–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 2014;134:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazhnaya A, Marcus R, Bojko MJ, Zelenev A, Makarenko I, Pykalo I, et al. Opioid Agonist Treatment and Improved Outcomes at Each Stage of the HIV Treatment Cascade in People Who Inject Drugs in Ukraine. J Acquir Immune Defic Syndr. 2018;79(3):288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 2007;18(4):306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


