Abstract
Background:
This study evaluated the feasibility of a technology-enhanced group-based fitness intervention for adolescent and young adult (AYA) survivors of childhood cancer.
Procedure:
AYA survivors ages 13 to 25 were randomized to the intervention (8 in-person group sessions with mobile app and FitBit followed by 4 weeks of app and FitBit only) or waitlist control. Assessments were at 0, 2, 3, 6, and 9 months. Feasibility was evaluated by enrollment, retention, attendance, app engagement, and satisfaction. Secondary outcomes included physical activity, muscular strength/endurance, cardiorespiratory fitness, health-related quality of life, and fatigue.
Results:
A total of 354 survivors were mailed participation letters; 68 (19%) were screened, of which 56 were eligible and 49 enrolled (88% of those screened eligible, 14% of total potentially eligible). Forty-nine survivors (Mage = 18.5 years, 49% female) completed baseline assessments and were randomized (25 Intervention, 24 Waitlist). Thirty-seven (76%) completed the post-intervention assessment and 32 (65%) completed the final assessment. On average, participants attended 5.7 of 8 sessions (range 1-8). Overall intervention satisfaction was high (M = 4.3, SD = 0.58 on 1-5 scale). Satisfaction with the companion app was moderately high (M = 3.4, SD = 0.97). The intervention group demonstrated significantly greater improvement in lower body muscle strength compared to the waitlist post-intervention, and small but not statistically significant changes in other secondary measures.
Conclusions:
A group-based intervention with a mobile app and fitness tracker was acceptable but has limited reach due to geographical barriers and competing demands experienced by AYA survivors.
Keywords: adolescents and young adults, cancer survivors, physical activity, exercise, eHealth
Up to 62-95% of childhood cancer survivors develop a chronic health condition such as cardiovascular disease by age 45.1,2 Cardiovascular events are the leading cause of non-cancer related death among survivors of childhood cancer.3 Regular physical activity may mitigate the risk of some late effects.4 However, 42%−72% of childhood cancer survivors demonstrate insufficient rates of physical activity,5-9 with rates lower than the general population.10,11 Physical activity declines sharply in adolescence.12,13 Intervening to increase activity during this time may help adolescents and young adults (AYAs) change this trajectory and carry healthy lifestyle habits into adulthood.14,15
Increased exercise has been associated with a reduction in all-cause mortality among childhood cancer survivors16 and a dose-dependent reduction in the incidence of cardiovascular events.17 There is a growing body of evidence suggesting that participation in physical activity interventions can improve physical function (e.g., muscular strength, cardiorespiratory fitness) and psychosocial outcomes (e.g., health-related quality of life, fatigue).18,19 However, the literature as a whole is characterized by single-arm feasibility and pilot studies, with very few rigorously designed randomized trials.19,20
The majority of studies have been designed for child and adolescent pediatric survivors21-23 or young adults diagnosed as young adults.24-26 Few studies specifically target AYA survivors of pediatric cancers.27-32 Of those studies targeting AYA survivors of pediatric cancer, almost all found positive but not statistically significant improvements in physical activity and fitness outcomes, which may be attributed to small sample size. Of note, a group-based community intervention found significant improvements in physical activity and fitness outcomes post-intervention, but gains were not maintained at follow-up.30 Studies incorporating a FitBit or other electronic activity monitor found trackers to be acceptable to AYAs.27,28,31 Taken together, these studies suggest that an in-person supervised intervention may have good initial impact but could be enhanced by incorporating mobile interventions that are acceptable to AYA and more likely to be integrated into daily life. No studies to date have combined the in-person group-based approach enhanced with mobile technology to capitalize on the strengths of each approach.
To address this gap, we developed “FitSurvivor,” a group-based supervised fitness intervention with a companion mobile app. Guided by Social Cognitive Theory,33 the intervention consisted of eight weekly group-based sessions with four primary components: (1) exercise with a trainer, (2) goal setting and self-monitoring, (3) healthy lifestyle education, and (4) social support. The primary aim was to evaluate the feasibility and acceptability of FitSurvivor. The secondary aim was to examine effects on physical activity, muscular strength, cardiorespiratory fitness, fatigue, and health-related quality of life.
Methods
Study Design
This study evaluated the feasibility of FitSurvivor versus waitlist control, chosen to increase the acceptability of randomized assignment and to obtain intervention adherence and satisfaction data from all enrolled participants. The intervention was 12 weeks, including eight weeks of in-person group sessions followed by four weeks of mobile app and fitness tracker use alone. Baseline assessment occurred prior to randomization followed by assessments at 2 months (end of group sessions), 3 months (post-intervention), 6 months (short-term follow-up) and 9 months (end of intervention for waitlist and longer follow-up for intervention). This study was approved by the Institutional Review Board at both recruitment sites and performed in accordance with the ethical standards in the 1964 Declaration of Helsinki and its later amendments. The study was registered at ClinicalTrials.gov (NCT02688192).
Participants
AYA survivors were recruited from long-term follow-up clinics at two pediatric cancer centers in the northeastern US. Eligibility criteria were age 13 to 25 years old, diagnosed with any cancer prior to age 21, off treatment at least six months, physician approval, and self-reported physical activity levels below the CDC activity guidelines (i.e., for adolescents: <60 minutes of moderate-vigorous aerobic activity daily including three or more days of vigorous intensity activities, muscle-strengthening exercises, and bone strengthening exercises; for adults ≥ 18: <150 minutes of moderate-intensity aerobic activity or 75 minutes of vigorous-intensity aerobic activity and two or more days of muscle-strengthening exercises per week). Exclusion criteria were significant developmental delay, pregnancy, and non-English speaking.
FitSurvivor Intervention
FitSurvivor is a group-based supervised program enhanced with a custom-built mobile app and a commercially available activity tracker (FitBit Charge or Alta). FitSurvivor was developed grounded in social cognitive theory,33 with major components targeting self-efficacy, goal setting and self-monitoring, instruction in proper exercise form, increasing cancer-related and general health behavior knowledge, and social support. For example, the trainer taught participants how to set specific goals and reviewed progress weekly. The companion app prompted users to set goals during the first log-in. In training sessions, the trainer encouraged participants to work on the goals set. The FitBit provided self-monitoring of steps and active minutes, and participants could log workouts within the app. See Supporting Information Table S1 for more examples of how behavior change techniques were employed.
The intervention was 12 weeks (eight weeks of in-person group-based exercise sessions followed by four weeks of using the companion app and FitBit alone). Each session was 90 minutes, with 60 minutes of trainer-led exercise and 30 minutes of personalized goal setting, feedback, and education. Education included strategies for behavior change (e.g., goal setting, overcoming barriers) and information about exercise and nutrition. Exercises were age-appropriate, and the trainers assisted participants in modifying intensity or form as needed based on participants’ ratings of perceived exertion or physical limitations due to cancer treatment (such as balance problems or limited use of a limb). Trainers taught participants to gradually increase resistance, reps, and minutes of moderate/vigorous exercise over time.
The FitSurvivor app (available for Apple and Android devices) included graphic feedback on activity from the FitBit tracker, goal setting, cancer-specific and general health information, full-, upper-, or lower-body strength workouts (including video and/or pictures), high intensity circuit workouts, achievements unlocked by meeting activity goals or completing workouts (e.g., “Strong Body” awarded for the first time meeting weekly strength goal), points for completing workouts, and a social feed to highlight participant successes (e.g., “[Nickname] completed Workout 1”). Participants were able to “like” or comment on others’ accomplishments but there was no private messaging. See Supporting Information Figure S1 for screenshots of the app. Participants could optionally join a private Facebook group to allow participants to message each other.
Procedures
Recruitment occurred in four waves between January 2016 and September 2017 at two sites. Potentially eligible patients were mailed recruitment letters/flyers. At Site 1, study staff made follow-up phone calls and/or approached potential participants during routine clinic visits. At Site 2, participants had to contact the study staff for information and screening. Interested participants were screened for eligibility via phone/in-person and scheduled for a consent/baseline visit. After obtaining informed consent/assent and parent permission for minors, participants completed the baseline assessment conducted by a qualified trainer in a gym setting. Participants completed anthropometric measures first followed by cardiorespiratory and muscular fitness tests and survey measures. Participants were asked to wear an ActiGraph wGT3X-BT accelerometer on their non-dominant wrist for 7 days following the baseline assessment. They were asked to wear 24-hours per day but to remove for water-related activities. For subsequent assessments, participants were mailed actigraphs and surveys prior to the visit.
Participants were randomized 1:1 to either the FitSurvivor intervention or waitlist control (WLC). The study biostatistician created the randomization scheme, stratifying by site and age (13-17 vs. 18-25), using a varying block size of 2-6 to ensure equal assignment between arms. Individual assignments were sealed in envelopes and opened sequentially according to baseline completion. Participants randomized to the intervention were invited to start the group sessions within a few weeks. All participants were asked to complete assessments at 2, 3, 6, and 9 months following randomization. Individuals randomized to the WLC received the intervention after the 6-month assessment. Participants were compensated with $20 gift cards for each assessment and given a FitBit free-of-charge. Participants who completed all study assessments were entered into a raffle for a $150 prize at the end of the study.
Measures
Feasibility and satisfaction.
Feasibility was evaluated by enrollment, retention, attendance at in-person sessions, engagement with the FitSurvivor companion app, engagement with the private Facebook group, and satisfaction with the intervention. Data regarding app usage was securely downloaded by study staff. Engagement with the app was measured by number of in-app exercises completed, points earned, achievements unlocked, optional posting of a “survivor story” (i.e., participants’ story/motivation for exercise), and likes/comments posted within the social feed. Engagement with the Facebook group was measured by joining the group, percentage of posts viewed, and number of likes/comments posted. In addition, participants completed a survey rating their satisfaction with various aspects of the intervention using a scale of 1 (not at all) to 5 (very much), and responded to open-ended questions about whether they would recommend the program to others, what they liked, what they disliked, and suggestions for improving the group sessions and the app.
Physical activity and sedentary behavior.
Objective physical activity data was collected in 1-second epochs using Actigraph wGT3X-BT initialized at 30 Hz. Self-reported minutes of moderate-vigorous activity (MVPA) in the past seven days was measured using the modified International Physical Activity Questionnaire – Short Form (IPAQ-SF34). Participants report the number of days of moderate and vigorous activity and number of minutes per day. Scoring yields a total weekly minutes of moderate-vigorous activity. Sedentary behavior was measured using two items from the PACE Adolescent Psychological and Sedentary Behavior Survey35 regarding the number of hours spent engaging in sedentary activity (e.g., watching TV) on weekdays and weekends. This measure yields a summary score ranging from 2 to 12, with higher scores indicating longer amounts of sedentary behavior.
Fitness.
All tests were performed by certified strength trainers. Lower and upper body muscular strength were measured via 10-repetition maximum (10-RM) tests following National Strength and Conditioning Association guidelines36 (leg press machine for lower body and barbell bench press for upper body). Following a warm-up, participants started with one set of 10 repetitions using a moderate load (~50% of estimated 10-RM based on rating of perceived exertion). Weight was progressively increased until a load allowing only 10 repetitions (not 11) in good form for both exercises. Participants generally completed two to four sets to determine the 10-RM, with about three minutes of rest between sets. Estimates of 1-RM leg and bench press were made using a linear prediction equation.37 Cardiorespiratory fitness was measured via submaximal treadmill testing, chosen over maximal testing because it was feasible at both sites, using a modified Bruce protocol.38 This standardized graded treadmill test adds two warm-up stages, with the first performed at a 1.7 mph and 0% grade and the second at 1.7 mph and 5% grade. Participants performed the graded exercise test until they reached 85% of their age-predicted maximum heart rate,39 which was measured using a chest-worn Polar heart rate monitor. Estimated VO2 max was calculated for each participant using the multi-stage model and American College of Sports Medicine metabolic equations.40 Heart rate was recorded from the treadmill interface for the first cohort of fifteen participants and a Polar heart rate watch for the remainder of the cohorts. Analyses were run with and without a covariate for device.
Anthropometrics.
Height, weight, and waist circumference were measured.
Psychosocial outcomes: health-related quality of life and fatigue.
Participants completed the PedsQL Generic Core,41,42 a 23-item multidimensional measure of physical, emotional, social, and cognitive functioning. Respondents indicate how much of a problem each item has been in the past week using a scale from 0 (never) to 4 (almost always). Scores are transformed on a 0-100 scale, with higher scores indicating better functioning. It yields two summary scores – Physical and Psychosocial. The PedsQL inventory is widely used and extensively validated.42-44 The PedsQL Multidimensional Fatigue Scale measures general, sleep/rest, and cognitive fatigue. Higher scores indicating better functioning.
Statistical Analyses
Feasibility was evaluated using descriptive statistics (percentages, means, frequencies) of enrollment rates, assessment completion rates, attendance at weekly sessions, engagement with the app, use of the Facebook groups, and satisfaction with the intervention. Responses to the open-ended satisfaction questions were thematically coded by two members of the research team, with any discrepancies resolved through discussion. We examined attendance at weekly sessions and engagement with the app separately by intervention group and the WLC after receiving the intervention; there were no significant differences between groups (data not shown) so pooled data on all participants who began the intervention is presented.
Data from Actigraph wGT3X-BT were re-integrated into 10-second epochs for scoring using the ActiLife software. Wear-time was determined using the wear sensor and the Choi algorithm.45,46 As has been recommended, valid days were defined as 10 or more hours of wear time and at least 4 valid days were required for use in analysis.47 Chandler cut points for MVPA were used.48 Daily minutes of MVPA were calculated by dividing the total number of moderate-to-vigorous activity minutes by the number of valid wear days.
While feasibility was the primary outcome, the a priori secondary outcomes were changes in fitness tests and self-reported outcomes at 3 months or immediately post-intervention. These were evaluated using group x time in a repeated measures analysis of variance with gender as a covariate, performed in SPSS 26.0, for participants with complete data at 3 months. Trainer, cohort, and device to read heart rate were initially included as covariates but did not substantially alter results, so were excluded. Missing data ranged from 25% to 37.5% across outcomes. In an exploratory analysis using baseline data carried forward for those who dropped out, we found the results were similar, but effect sizes were reduced (data not presented). Additional exploratory analyses evaluated whether there were any lasting short-term benefits by examining group x time interactions in repeated measures analyses of variance, covarying for gender, from baseline to 6 months (i.e., 3 months after the end of the intervention).
Results
Feasibility
Enrollment and retention.
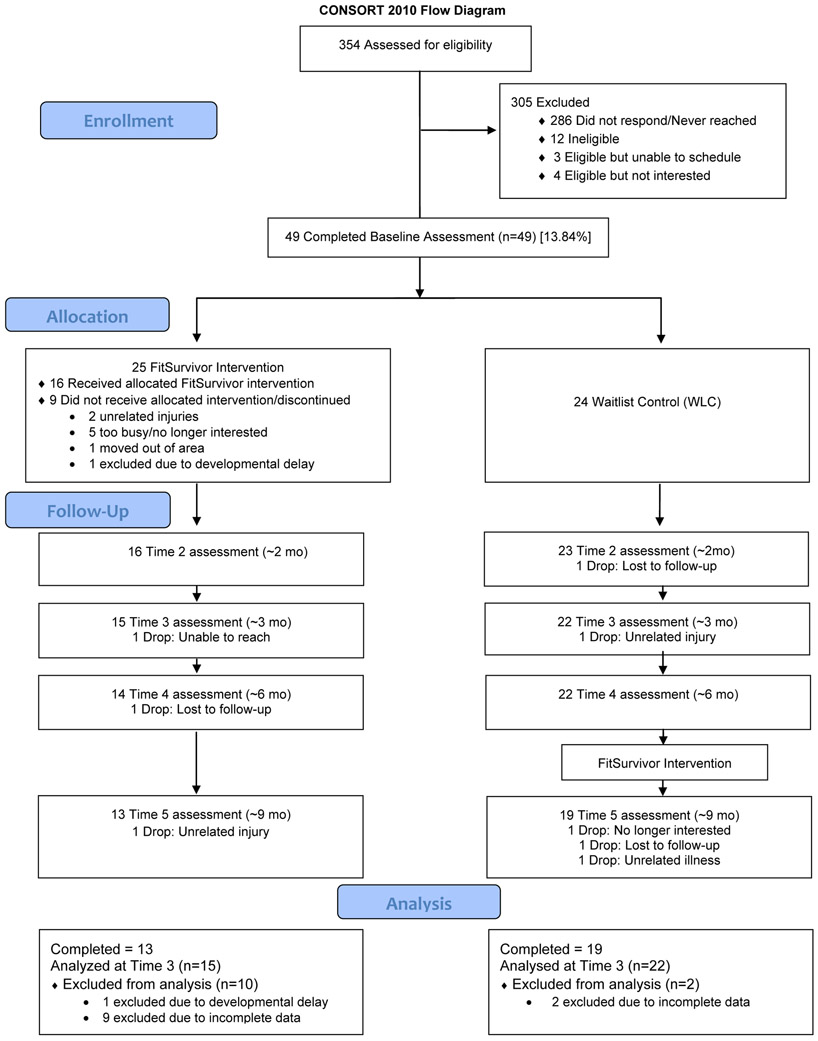
In total, 354 participants were mailed letters; 68 were contacted/screened, of which 56 were eligible and 49 enrolled (88% of those screened eligible, 14% of total potentially eligible pool). A total of 49 participants completed baseline assessments and were randomized to the intervention (n=25) or waitlist control (n=24). Of those assigned to the intervention, 16 (64%) completed the group sessions (see Figure 1 for CONSORT flow diagram). For the WLC, 19 (79%) completed the group sessions. For the intervention group, 16 (64%) completed assessments at 2 months, 15 (60%) completed assessments at 3 months, 14 (56%) completed assessments at 6 months, and 13 (52%) completed assessments at 9 months. For the WLC, 23 (96%) completed assessments at 2 months, 22 (92%) completed assessments at 3 months, 22 (92%) completed assessments at 6 months, and 19 (79%) completed assessments at 9 months. Overall, 32 of the 49 participants (65%) completed the study. Participants assigned to the intervention group were more likely to drop out by 3 months, χ2(1) = 5.78, p = .02, but this was not significant at 9 months, p = .12. Among participants assigned to the intervention group, there were no significant differences in baseline characteristics (i.e., age, sex, race, ethnicity, cancer diagnosis, time since treatment completion) between those who completed the intervention and those who did not (ps > .05). Participant characteristics are presented in Table 1. There were no serious adverse events and three related or possibly related non-serious adverse events during the study (i.e., minor wrist cut, muscle strain, dizziness during treadmill test).
Figure 1.
Consort flow diagram
Table 1.
Participant characteristics
| Intervention (n=25) | Waitlist (n=24) | |
|---|---|---|
| M (SD) or n (%) | M (SD) or n (%) | |
| Current Age (years) | 18.76 (3.9) | 18.25 (3.6) |
| Sex | ||
| Male | 10 (40.0%) | 15 (62.5%) |
| Female | 15 (60.0%) | 9 (37.5%) |
| Cancer Diagnosis | ||
| Blood Cancer | 19 (76.0%) | 21 (87.5%) |
| Brain Tumor | 3 (12.0%) | 0 (0.0%) |
| Solid Tumor | 3 (12.0%) | 3 (12.5%) |
| Age at diagnosis (years) range | 7.36 (5.5) [0-17] | 8.54 (6.1) [0-19] |
| Time since treatment completed (years) range | 9.46 (5.5) [1-22] | 7.63 (4.4) [1-16] |
| Treatment Received | ||
| Chemotherapy (yes) | 25 (100%) | 24 (100%) |
| Radiation | ||
| Yes | 7 (28.0%) | 7 (29.2%) |
| No | 18 (72.0%) | 17 (70.8%) |
| BMT/HSCT | ||
| Yes | 4 (16.0%) | 3 (12.5%) |
| No | 21 (84.0%) | 21 (87.5%) |
| Race/Ethnicity: | ||
| Non-Hispanic White | 13 (52.0%) | 14 (58.3%) |
| Non-Hispanic Black | 1 (4.0%) | 2 (8.3%) |
| Non-Hispanic Asian | 3 (12.0%) | 4 (16.7%) |
| More than 1 race | 3 (12.0%) | 1 (4.2%) |
| Other/unknown | 5 (20.0%) | 4 (16.7%) |
| Employment Status: | ||
| Student | 19 (76.0%) | 17 (70.8%) |
| Part-Time | 3 (12.0%) | 4 (16.7%) |
| Full-Time | 2 (8.0%) | 3 (12.5%) |
| Unemployed | 1 (4.0%) | 0 (0.0%) |
| Health Insurance: | ||
| None | 0 (0.0%) | 2 (8.3%) |
| Public | 7 (28.0%) | 7 (29.2%) |
| Private | 18 (72.0%) | 17 (70.8%) |
| Marital Status: | ||
| Single, never married | 24 (96.0%) | 25 (100.0%) |
| Married/Partnered | 1 (4.0%) | 0 (0.0%) |
Note. BMT = Bone Marrow Transplant; HSCT = Hematopoietic Stem Cell Transplant
Participant engagement and satisfaction.
On average, participants attended 5.7 out of eight group sessions (71%). There was wide variability in engagement with the companion app (Table 2). On average, participants completed 6.67 in-app workouts (SD = 10.77, range = 0 to 44). Participants earned an average of 169.5 points for completing workouts (SD = 290.1, range 0 to 1,195) and unlocked 8.71 achievements (SD = 4.5, range 0 to 20). Completion of in-app workouts appeared to decline over the course of the intervention, peaking at weeks 2 and 6. There was very little usage of the social features of the app where participants could “like” or comment on others’ activity (M=0.80 engagements per participant, SD = 1.63; range = 0 to 8). Only seven individuals (16%; 1 in cohort 1; 4 in cohort 2, and 2 in cohort 4) shared an optional “Survivor Story” about their cancer history and motivation for joining the intervention study. There were several technical problems where syncing between the FitBit app and FitSurvivor app was temporarily unavailable, typically lasting only a few hours but sometimes requiring technical support from study staff. Anecdotally, participants found this disruptive and frustrating.
Table 2.
Participant engagement with FitSurvivor group sessions and companion app
| Engagement | M (SD) | Range |
|---|---|---|
| # Group Sessions Completed | 5.67 (2.31) | 1-8 |
| # In-App Workouts | 6.50 (10.69) | 0-44 |
| Total In-App Points Earned | 165.48 (287.74) | 0-1,195 |
| # Achievements Unlocked | 8.50 (4.63) | 1-20 |
| # of Likes/Comments Posted | 0.62 (1.51) | 0-8 |
Note. Participants who dropped out prior to completing any sessions were excluded from these analyses (n = 42).
Overall, engagement with the optional Facebook private groups was low, with only 71% of participants joining the group. Given a low number of participants with Facebook accounts in the final waitlist group, we did not create a private Facebook group for that one group (n = 8). Participants who were younger were less likely to join due to not having a Facebook account (X2 = 5.99, p = .01). Of those who joined, participants viewed on average 50.9% of posts (SD = 38.5%, range 0-100%) and liked 1.1 posts (SD = 2.6). Group sizes were small (n = 5 to 9) and only two individuals posted comments in addition to moderators.
Participants reported greatest satisfaction with the trainer (M = 4.88, SD = 0.33), program overall (M = 4.27, SD = 0.57), types of exercises completed (M = 4.27, SD = 0.63), and length of group sessions (M = 4.21, SD = 0.65; see Table 3). Satisfaction with the FitSurvivor app was moderately high (M = 3.39, SD = 0.97), including the visual appeal of the app (M = 3.97, SD = 0.77), the workouts (M = 3.85, SD = 0.94), ease of use (M = 3.82, SD = 1.07), and the tips and information within the app (M = 3.79, SD = 0.77).
Table 3.
Participant satisfaction with FitSurvivor intervention
| How satisfied were you with the… | Not at all % |
Slightly % |
Somewhat % |
Quite a bit % |
Very % |
M (SD) |
|---|---|---|---|---|---|---|
| Program overall | 0 | 0 | 6.1 | 60.6 | 33.3 | 4.27 (0.57) |
| Types of exercises completed | 0 | 0 | 9.1 | 54.5 | 63.6 | 4.27 (0.63) |
| Trainer | 0 | 0 | 0 | 12.1 | 87.9 | 4.88 (0.33) |
| Number of group sessions (8) | 0 | 0 | 12.1 | 54.5 | 33.3 | 4.21 (0.65) |
| FitSurvivor app overall | 3.0 | 9.1 | 48.5 | 24.2 | 15.2 | 3.39 (0.97) |
| Tips and info within app | 0 | 9.1 | 21.2 | 51.5 | 24.2 | 3.79 (0.96) |
| Workouts in the app | 3.0 | 3.0 | 24.2 | 45.5 | 24.2 | 3.85 (0.94) |
| Rate the… | Very hard/ unappealing |
Somewhat hard/ unappealing |
Neither | Somewhat easy/ appealing |
Very easy/ appealing |
M (SD) |
| Ease of using the app | 0 | 15.2 | 21.2 | 30.3 | 33.3 | 3.82 (1.07) |
| Visual appeal of the app | 0 | 3.0 | 21.2 | 51.5 | 24.2 | 3.97 (0.77) |
Note. Satisfaction questions were from participant surveys (n = 33).
When asked what they liked about the intervention, participants’ responses had four themes: encouragement and motivation, education, beneficial outcomes, and social enjoyment (see Table 4). Suggested improvements centered on refining technical aspects of the mobile app, adding functional features to the app, and adding more variety of training exercises as well as increasing the duration (more weeks) or dose (more frequent sessions) of the program.
Table 4.
Participant feedback about intervention benefits and suggestions for improvement.
| Positive Themes | Example quotes |
|---|---|
| Encouragement & motivation (n = 21) | “This program really gave me the push I needed to begin a more healthy lifestyle for me.” “I never felt this encouraged to work out. It’s very helpful and life changing.” “The sessions are more personable and gave more of a drive and motivation to push your limits.” “The app challenges you every day and kept me motivated (along w/the tips)” |
| Education (n = 18) | “Having [the trainer] directing workouts, because I don't really know how to exercise.” “Learning the correct way to do the exercise…” “I was able to check the app to make sure I was doing the workouts right.” “Lots of good educational information and recommendations.” |
| Beneficial Outcomes (n = 15) | “This program helped a lot.” “I felt better from exercising. I also lost a few pounds.” “It was very productive and I got a lot out of it, from like [the] physical aspect to the educational aspect as well.” “It helps you feel better about yourself.” |
| Social enjoyment (n = 7) | “You can meet people that have gone through the same thing as you.” “Exercising in a group was fun.” “I liked exercising in a group and learning new exercises.” “I like how people can share their stories.” |
| Problems/Suggested Improvements | Example quotes |
| App: Technical aspects (n = 20) | “Sometimes my workout data deleted before I completed the workout.” “It really didn't sync well for me.” “Make it work more efficiently…no crashing, load faster.” |
| App: Functional features (n = 11) | “Ability to set reminders. For example, setting yourself personal reminder to go workout.” “Please add Apple watch support.” “Adding a setting to plug music through the app would be useful.” “Add proposed meals to the app.” |
| Enhanced training (n = 7) | “More training on equipment to use alone after.” “Add small amounts of weight training.” “Having the sessions more often instead of once a week” “I would change the number of weeks. I would say 16-week program” |
Note. The n for each theme reflects the number of participants making a comment within that theme and participants could provide multiple comments that aligned with more than one them.
Secondary Outcomes
Physical activity and sedentary behavior.
Participants wore the Actigraph monitors for an average of 6.21 days (SD = 2.22) at baseline and 5.67 days (SD = 2.23) at 3 months, with 41 and 30 participants, respectively, providing at least 4 valid days for analysis. Although both groups self-reported increased weekly MVPA, Actigraph data indicated a slight decrease in MVPA for the intervention group and no change for the WLC post-intervention. There were no statistically significant group*time differences in physical activity or sedentary behavior post-intervention (Table 5) or at 6 months (Supporting Information Table S2).
Table 5.
Group means for physical activity, fitness, body composition, and psychosocial outcomes at baseline and post-intervention for participants with complete data.
| Interventiona | Waitlist Controlb | Group x Time p |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post- Intervention |
% change |
Baseline | Post- Intervention |
% change |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Lower Body 1-RM (kg) | 191.93 | 80.76 | 247.23 | 109.76 | 30.8% | 199.78 | 84.56 | 219.71 | 89.85 | 11.7% | .02 |
| Upper Body 1-RM (kg) | 35.78 | 22.76 | 40.50 | 24.26 | 23.5% | 45.51 | 25.83 | 48.48 | 25.40 | 10.9% | .34 |
| Estimated VO2 Max | 36.67 | 7.03 | 39.08 | 9.48 | 6.6% | 40.61 | 9.60 | 39.80 | 9.27 | 1.5% | .29 |
| BMI | 25.09 | 4.54 | 25.23 | 4.82 | 0.5% | 24.25 | 5.21 | 24.67 | 5.33 | 1.7% | .25 |
| Waist circumference (cm) | 81.17 | 11.48 | 82.16 | 8.65 | 1.9% | 80.71 | 13.13 | 81.90 | 12.84 | 1.9% | .95 |
| Daily MVPA – accelerometer (minutes) | 30.53 | 27.91 | 22.26 | 30.25 | -20.3% | 17.50 | 11.38 | 17.19 | 11.78 | 64.1% | .20 |
| Weekly MVPA – self-report (minutes) | 207.18 | 177.10 | 278.21 | 241.32 | 68.5% | 237.41 | 215.24 | 364.73 | 325.41 | 124.7% | .46 |
| Sedentary Behavior | 7.75 | 3.11 | 7.50 | 3.16 | 1.9% | 7.05 | 2.97 | 6.73 | 2.75 | 15.4% | .96 |
| HRQOL | |||||||||||
| Physical Summary | 72.10 | 21.10 | 74.11 | 20.74 | 14.9% | 79.75 | 8.32 | 81.68 | 9.56 | 2.9% | .98 |
| Psychosocial Summary | 72.64 | 12.28 | 70.00 | 16.17 | -2.3% | 77.92 | 12.14 | 75.92 | 11.98 | -1.7% | .85 |
| Fatigue | |||||||||||
| General | 60.12 | 25.72 | 65.18 | 23.60 | 37.9% | 71.02 | 12.76 | 71.40 | 13.44 | 2.0% | .39 |
| Sleep/Rest | 55.95 | 20.78 | 63.10 | 24.78 | 15.5% | 62.88 | 15.90 | 65.72 | 17.95 | 6.4% | .49 |
| Cognitive | 57.74 | 24.78 | 63.69 | 26.27 | 33.9% | 70.08 | 22.59 | 75.38 | 20.45 | 14.5% | .83 |
Note.
n = 12-14
n = 20-22.
I = Intervention; WLC = Waitlist Control; SD = Standard Deviation; 1-RM = estimated 1-repetition maximum; VO2 Max = maximum consumption during incremental exercise (mL/kg/min); BMI = Body mass index; MVPA = Moderate/vigorous physical activity; HRQOL = Health-related quality of life. The p value is for the group x time interaction in repeated measures analysis of variance with gender as a covariate.
Fitness.
Repeated measures ANOVA demonstrated a significant group*time effect for lower body muscular strength, with the intervention group demonstrating a greater increase post-intervention (30.8% vs. 11.7% in WLC), F[1,32] = 6.15, p = .02, η2 = .16). This was not significant at 6 months. There were no statistically significant group*time differences in upper body strength or cardiorespiratory fitness post-intervention (Table 5) or at 6 months (Supporting Information Table S2).
Anthropometrics.
There were no statistically significant changes in BMI or waist circumference post-intervention (Table 5) or at 6 months (Supporting Information Table S2).
Psychosocial outcomes: HRQOL and fatigue.
Although the percent increase generally favored the Intervention group, there were no statistically significant group*time differences across psychosocial outcomes post-intervention (Table 5) or at 6 months (Supporting Information Table S2).
Discussion
The FitSurvivor intervention was designed to meet the unique needs of adolescent and young adult survivors of pediatric cancers by combining in-person group-based exercise sessions with a mobile application. Recruitment was challenging, primarily because we were only able to reach a small percentage of the potentially eligible pool (<20%). However, study acceptance amongst those who were eligible was high (88%). Retention was suboptimal, but consistent with other studies of adolescent and young adult survivors, where retention rates have ranged from 60% for a community-based intervention22 to 90% for a mobile intervention.27 A potential explanation is that interventions involving greater time and effort, like this intervention, result in lower retention due to competing time commitments. Satisfaction with the in-person training was very high, and attendance at group sessions averaged 71%. Satisfaction and ease of use of the app were rated moderately high, but engagement decreased over time for the majority of participants. This is similar to the general literature regarding mobile health applications, as many factors influence sustained engagement over time.49
Engagement with the social features of the app and the private Facebook group was poor, particularly among adolescent participants. Low utilization may have been due to a lack of interest in engaging with other participants outside of sessions, low perceived need to engage outside sessions given socialization during group sessions, or preference to engage with their existing social networks through other channels (e.g., Snap Chat, Instagram). Younger participants were less likely to have a Facebook account, suggesting that Facebook is not the optimal platform for engaging with adolescents. Engaging with other survivors is commonly endorsed by AYA survivors50 but often difficult to successfully create in intervention studies. Connecting survivors 1:1 instead of a general group, or using direct prompts to provide structure for responding, may facilitate engagement. More research is needed to identify the best ways to successfully foster peer interaction and support in mobile environments.
Given the goal was to determine feasibility, this study was not powered to detect small to medium effects in fitness, health-related quality of life, or fatigue outcomes, and we must interpret these results cautiously.51 However, there were some promising improvements in muscular strength that aligned with the intervention’s focus on strength training rather than aerobic training. To have a greater impact on cardiorespiratory fitness, future iterations should consider specific aerobic training and increasing the intensity or duration of the intervention. Future work could consider using maximal aerobic capacity testing to individually prescribe aerobic training.
Despite strengths of a randomized design, there were several weaknesses. The small sample size appropriate for feasibility testing limited power to detect differences in secondary outcomes, similar to the state of the field in this area.19 The study design did not allow examination of different components of the intervention (i.e., in-person groups vs. app/FitBit). Given that there is mixed literature on the impact of activity tracking devices like FitBit,27,52 future studies should consider including a device-only control group. The inclusion of a wide developmental range from 13 to 25 was a strength in representing the AYA group but made it difficult to foster group cohesion and present educational information in a way that engaged the full age range. Future work may consider different approaches for adolescents and young adults. Eligibility criteria required that participants did not meet the CDC guidelines for both aerobic and strength training, such that some participants may have met one guideline but not the other, which may have biased baseline fitness scores towards a slightly more active group but would not have differentially affected intervention or waitlist control groups. To reduce participant burden, we did not include a measure of self-efficacy; however, future work should explicitly evaluate self-efficacy as a potential mediator.
In sum, while the FitSurvivor intervention offered promise to those who were willing and able to attend in-person group sessions, such a program is likely to have limited reach due to geographical barriers and competing school, work, and social demands of AYA survivors.50 There are a number of potential ways to move forward. Given that the in-person group sessions showed promise, future studies might invite AYA survivors to join group sessions in community settings with a friend or family member to increase group size, recognizing a potential reduction in the focus on cancer survivorship specifically but harnessing natural social support networks. Alternatively, telehealth technology might be used to deliver group sessions to survivors at home, perhaps with family or other friends involved. Indeed, the healthcare system has rapidly expanded the use of telehealth in response to COVID-19,53 with seemingly high acceptance amongst patients. Although a stand-alone mobile app is more likely to have broader reach, there would be concerns about engagement and accountability over time. The use of factorial or adaptive designs could provide insight into which features are effective in producing behavior change.54 Given our participants’ high satisfaction with the trainer, another potential approach is individualized health coaching with a trainer or coach via an app or telehealth. Health coaching interventions have demonstrated positive effects among adults with chronic conditions.55 Future work should evaluate the efficacy of different approaches and characteristics of individuals most likely to benefit from one approach over another.
Supplementary Material
Supporting Information Figure S1 Screen shots of the FitSurvivor app showing the menu, dashboard, and a workout
ACKNOWLEDGMENTS
The authors would like to acknowledge Susan Stephens, MSW, Dawn Carey, RN, Raymond Calicchio, CPT, and Timothy Marshall, PhD for their work in facilitating the study.
FUNDING INFORMATION
This work was supported in part by the National Cancer Institute of the National Institutes of Health under award number K07CA174728 to the first author. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AYA
Adolescent and young adult
- WLC
Waitlist control
- MVPA
Moderate-vigorous physical activity
Footnotes
Conflicts of Interest Statement: The authors declare that they have no conflict of interest.
Availability of Data: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. Jama. 2013;309(22):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oeffinger K, Mertens A, Sklar C, et al. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 3.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ: British Medical Journal. 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones LW, Liu Q, Armstrong GT, et al. Exercise and Risk of Major Cardiovascular Events in Adult Survivors of Childhood Hodgkin Lymphoma: A Report From the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2014;32:3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: A comparison of long-term outcomes, health care utilization, and health-related behaviors from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2005;23(27):6499–6507. [DOI] [PubMed] [Google Scholar]
- 6.Devine KA, Mertens AC, Whitton JA, et al. Factors associated with physical activity among adolescent and young adult survivors of early childhood cancer: A report from the childhood cancer survivor study (CCSS). Psycho-Oncology. 2018;27(2):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness KK, Leisenring WM, Huang SJ, et al. Predictors of Inactive Lifestyle Among Adult Survivors of Childhood Cancer A Report From the Childhood Cancer Survivor Study. Cancer-Am Cancer Soc. 2009;115(9):1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson CL, Stratton K, Leisenring WL, et al. Decline in Physical Activity Level in the Childhood Cancer Survivor Study Cohort. Cancer Epidem Biomar. 2014;23:1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berdan CA, Tangney CC, Scala C, Stolley M. Childhood cancer survivors and adherence to the American Cancer Society Guidelines on Nutrition and Physical Activity. J Cancer Surviv. 2014;8(4):671–679. [DOI] [PubMed] [Google Scholar]
- 10.Stolley MR, Restrepo J, Sharp LK. Diet and Physical Activity in Childhood Cancer Survivors: A Review of the Literature. Ann Behav Med. 2010;39(3):232–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang FF, Kelly MJ, Saltzman E, Must A, Roberts SB, Parsons SK. Obesity in Pediatric ALL Survivors: A Meta-Analysis. Pediatrics. 2014;133(3):E704–E715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Medicine & Science in Sports & Exercise. 2000;32(5):963–975. [DOI] [PubMed] [Google Scholar]
- 13.Dumith SC, Gigante DP, Domingues MR, Kohl HW. Physical activity change during adolescence: a systematic review and a pooled analysis. International Journal of Epidemiology. 2011;40(3):685–698. [DOI] [PubMed] [Google Scholar]
- 14.Hallal PC, Victora CG, Azevedo MR, Wells JCK. Adolescent physical activity and health - A systematic review. Sports Med. 2006;36(12):1019–1030. [DOI] [PubMed] [Google Scholar]
- 15.Juan AFS, Wolin K, Lucia A. Physical Activity and Pediatric Cancer Survivorship. Recent Results Canc. 2011;186:319–347. [DOI] [PubMed] [Google Scholar]
- 16.Scott J, Li N, Liu Q, Yasui Y, Leisenring W, Nathan PC. Association of exercise with late mortality in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor study. Journal of Clinical Oncology. 2018;36(15):10512. [Google Scholar]
- 17.Jones LW, Liu Q, Armstrong GT, et al. Exercise and Risk of Major Cardiovascular Events in Adult Survivors of Childhood Hodgkin Lymphoma: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2014;32(32):3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizrahi D, Wakefield CE, Fardell JE, et al. Distance-delivered physical activity interventions for childhood cancer survivors: A systematic review and meta-analysis. Critical reviews in oncology/hematology. 2017;118:27–41. [DOI] [PubMed] [Google Scholar]
- 19.Braam KI, van der Torre P, Takken T, et al. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database of Systematic Reviews. 2016(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.San Juan AF, Wolin K, Lucía A. Physical activity and pediatric cancer survivorship. Recent Results Cancer Res. Physical Activity and Cancer. 2011:319–347. [DOI] [PubMed] [Google Scholar]
- 21.Huang JS, Dillon L, Terrones L, et al. Fit4Life: a weight loss intervention for children who have survived childhood leukemia. Pediatr Blood Cancer. 2014;61(5):894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilliam MB, Ross K, Futch L, et al. A pilot study evaluation of a web-based token economy to increase adherence with a community-based exercise intervention in child and adolescent cancer survivors. Rehabil Oncol. 2011;29(2):16–22. [Google Scholar]
- 23.Takken T, van der Torre P, Zwerink M, et al. Development, feasibility and efficacy of a community based exercise training program in pediatric cancer survivors. Psycho Oncol. 2009;18(4):440–448. [DOI] [PubMed] [Google Scholar]
- 24.Valle CG, Tate DF, Mayer DK, Allicock M, Cai J. A randomized trial of a Facebook-based physical activity intervention for young adult cancer survivors. J Cancer Surviv. 2013;7(3):355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabin C, Dunsiger S, Ness KK, Marcus BH. Internet-based physical activity intervention targeting young adult cancer survivors. J Adolesc Young Adult Oncol. 2011;1(4):188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabin C, Pinto B, Fava J. Randomized trial of a physical activity and meditation intervention for young adult cancer survivors. J Adolesc Young Adult Oncol. 2016;5(1):41–47. [DOI] [PubMed] [Google Scholar]
- 27.Mendoza JA, Baker KS, Moreno MA, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: a pilot study. Pediatr Blood Cancer. 2017;64(12):e26660. [DOI] [PubMed] [Google Scholar]
- 28.Howell CR, Krull KR, Partin RE, et al. Randomized web-based physical activity intervention in adolescent survivors of childhood cancer. Pediatr Blood Cancer. 2018;65(8):e27216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Järvelä LS, Kemppainen J, Niinikoski H, et al. Effects of a home-based exercise program on metabolic risk factors and fitness in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59(1):155–160. [DOI] [PubMed] [Google Scholar]
- 30.Keats MR, Culos-Reed SN. A community-based physical activity program for adolescents with cancer (project trek): Program feasibility and preliminary findings. J of Pediatr Hematol/Oncol. 2008;30(4):272–280. [DOI] [PubMed] [Google Scholar]
- 31.Le A, Mitchell H-R, Zheng DJ, et al. A home-based physical activity intervention using activity trackers in survivors of childhood cancer: A pilot study. Pediatr Blood Cancer. 2017;64(2):387–394. [DOI] [PubMed] [Google Scholar]
- 32.Berg CJ, Stratton E, Giblin J, Esiashvili N, Mertens A. Pilot results of an online intervention targeting health promoting behaviors among young adult cancer survivors. 2014;23(10):1196–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandura A. Self-efficacy: The exercise of control. USA: Freemann and Company; 1997. [Google Scholar]
- 34.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med & Sci in Sports & Exercise.35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 35.Norman GJ, Vaughn AA, Roesch SC, Sallis JF, Calfas KJ, Patrick K. Development of decisional balance and self-efficacy measures for adolescent sedentary behaviors. 2004;19(5):561–575. [Google Scholar]
- 36.Baechle TR, Earle RW. Essentials of strength training and conditioning. Human kinetics; 2008. [Google Scholar]
- 37.Lander J. Maximum based on reps. NSCA journal. 1985;6(6):60–61. [Google Scholar]
- 38.Lerman J, Bruce RA, Sivarajan E, Pettet GE, Trimble S. Low-level dynamic exercises for earlier cardiac rehabilitation: aerobic and hemodynamic responses. Archives of physical medicine and rehabilitation. 1976;57(8):355–360. [PubMed] [Google Scholar]
- 39.Gellish RL, Goslin BR, Olson RE, McDONALD A, Russi GD, Moudgil VK. Longitudinal modeling of the relationship between age and maximal heart rate. Medicine & Science in Sports & Exercise. 2007;39(5):822–829. [DOI] [PubMed] [Google Scholar]
- 40.Heyward VH. Advanced fitness assessment and exercise prescription. 6th ed. Champagne, IL: Human Kinetics; 2010. [Google Scholar]
- 41.Varni JW, Limbers CA. The PedsQL™ 4.0 generic core scales young adult version: feasibility, reliability and validity in a university student population. Journal of health psychology. 2009;14(4):611–622. [DOI] [PubMed] [Google Scholar]
- 42.Varni JW, Seid M, Kurtin PS. PedsQL (TM) 4.0: Reliability and validity of the pediatric quality of life Inventory (TM) Version 4.0 generic core scales in healthy and patient populations. Medical care. 2001;39(8):800–812. [DOI] [PubMed] [Google Scholar]
- 43.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL™ in pediatric cancer. Cancer. 2002;94(7):2090–2106. [DOI] [PubMed] [Google Scholar]
- 44.Wakefield CE, Patterson P, McDonald FE, Wilson HL, Davis E, Sansom-Daly UM. Assessment of psychosocial outcomes in adolescents and young adults with cancer: a systematic review of available assessments. Clin Oncol Adol Young Adult. 2013;3:13–27. [Google Scholar]
- 45.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of Accelerometer Wear and Nonwear Time Classification Algorithm. Medicine & Science in Sports & Exercise. 2011;43(2):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi L, Ward SC, Schnelle JF, Buchowski MS. Assessment of Wear/Nonwear Time Classification Algorithms for Triaxial Accelerometer. Medicine & Science in Sports & Exercise. 2012;44(10):2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tudor-Locke C, Camhi S, Troiano R. A Catalog of Rules, Variables, and Definitions Applied to Accelerometer Data in the National Health and Nutrition Examination Survey, 2003–2006. 2012;9:110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandler JL, Brazendale K, Beets MW, Mealing BA. Classification of physical activity intensities using a wrist-worn accelerometer in 8–12-year-old children. Pediatric Obesity. 2016;11(2):120–127. [DOI] [PubMed] [Google Scholar]
- 49.Yardley L, Spring BJ, Riper H, et al. Understanding and Promoting Effective Engagement With Digital Behavior Change Interventions. Am J of Prev Med. 2016;51(5):833–842. [DOI] [PubMed] [Google Scholar]
- 50.Rabin C, Simpson N, Morrow K, Pinto B. Intervention format and delivery preferences among young adult cancer survivors. Int J of behav med. 2013;20(2):304–310. [DOI] [PubMed] [Google Scholar]
- 51.Abbott JH. The Distinction Between Randomized Clinical Trials (RCTs) and Preliminary Feasibility and Pilot Studies: What They Are and Are Not. Journal of Orthopaedic & Sports Physical Therapy. 2014;44(8):555–558. [DOI] [PubMed] [Google Scholar]
- 52.Ridgers ND, McNarry MA, Mackintosh KA. Feasibility and Effectiveness of Using Wearable Activity Trackers in Youth: A Systematic Review. JMIR Mhealth Uhealth. 2016;4(4):e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wosik J, Fudim M, Cameron B, Gellad ZF, Cho A, Phinney D, Curtis S, Roman M, Poon EG, Ferranti J, Katz JN, Tcheng J. Telehealth transformation: COVID-19 and the rise of virtual care, J Am Med Info Assoc. 2020; ocaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michie S, Yardley L, West R, et al. Developing and Evaluating Digital Interventions to Promote Behavior Change in Health and Health Care: Recommendations Resulting From an International Workshop. JMIR. 2017;19:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kivelä K, Elo S, Kyngäs H, Kääriäinen M. The effects of health coaching on adult patients with chronic diseases: A systematic review. Patient Ed Counseling. 2014;97(2):147–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1 Screen shots of the FitSurvivor app showing the menu, dashboard, and a workout