Abstract
All living beings have an optimal temperature for growth and survival. With the advancement of global warming, the search for understanding adaptive processes to climate changes has gained prominence. In this context, all living beings monitor the external temperature and develop adaptive responses to thermal variations. These responses ultimately change the functioning of the cell and affect the most diverse structures and processes. One of the first structures to detect thermal variations is the plasma membrane, whose constitution allows triggering of intracellular signals that assist in the response to temperature stress. Although studies on this topic have been conducted, the underlying mechanisms of recognizing thermal changes and modifying cellular functioning to adapt to this condition are not fully understood. Recently, many reports have indicated the participation of sphingolipids (SLs), major components of the plasma membrane, in the regulation of the thermal stress response. SLs can structurally reinforce the membrane or/and send signals intracellularly to control numerous cellular processes, such as apoptosis, cytoskeleton polarization, cell cycle arresting and fungal virulence. In this review, we discuss how SLs synthesis changes during both heat and cold stresses, focusing on fungi, plants, animals and human cells. The role of lysophospholipids is also discussed.
Keywords: sphingolipid, ceramide, heat shock, cold stress, heat shock protein, lysophospholipid, fungal virulence
1. Introduction
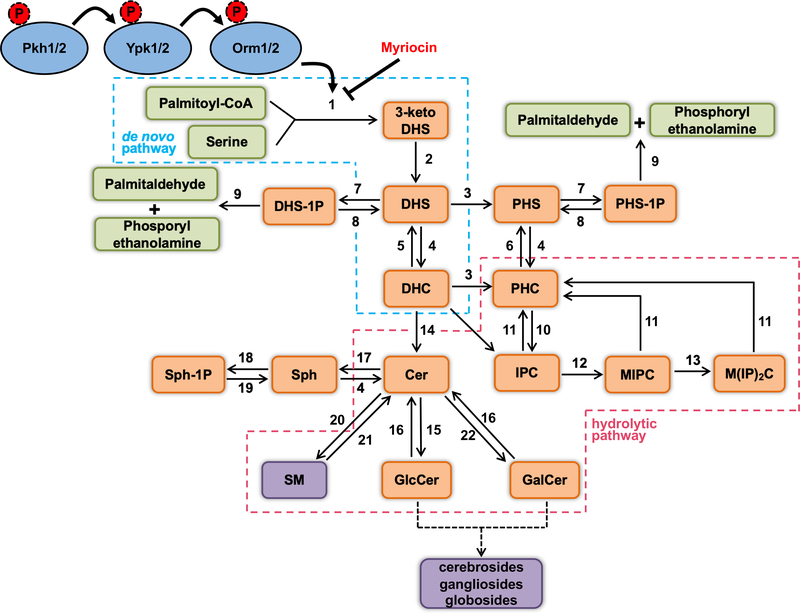
Biological membranes are indispensable for cell survival in view of the numerous structural and signaling functions they perform. Amongst the lipid molecules that make up the eukaryotic plasma membrane, the sphingolipids (SLs) are some of the most unique, functioning as both structural components and signaling molecules. In addition to being involved in processes such as stress response, cell cycle arrest, apoptosis, pathogenesis, cell growth and differentiation [1], SLs also associate with sterols in eukaryotic membranes to form the lipid rafts microdomains, which are fundamental for signal transduction and membrane trafficking [2]. Structurally, SLs are characterized by a long-chain sphingoid base linked to a fatty acid chain and to a polar head group. The de novo biosynthetic pathway of these bioactive compounds begins in the endoplasmic reticulum (ER) with the condensation of serine and palmitoyl-CoA by the activity of the serine palmitoyltransferase (SPT), and sequential reactions lead to the generation of dihydrosphingosine (DHS), dihydroceramide (DHC) and ceramide (Cer), as shown in Figure 1 [3]. Next, more complex and specific SLs are usually formed in the Golgi, such as glucosylceramide (GlcCer) in fungi, plants and mammals, phytoceramide (PHC) and glycosylinositol phosphoryl ceramide (GIPC) in fungi and plants, inositol phosphorylceramide (IPC), mannosylinositol phosphorylceramide (MIPC) and galactosylceramide (GalCer) in fungi, and sphingomyelin (SM), complex cerebrosides, gangliosides and globosides in mammalian cells [4, 5].
Figure 1.
Fungal sphingolipids biosynthetic pathway. The proteins kinases Pkh1/2, Ypk1/2 and Orm1/2 shown in blue circles are part of the regulatory module that controls the de novo sphingolipids synthesis, which is highlighted by a dashed blue line. The sphingolipids hydrolytic pathway is highlighted by a dashed red line. Sphingolipids species are shown in orange boxes. The purple boxes represent molecules found in animals. Enzymatic steps are numbered as follows: (1) Serine palmitoyltransferase (SPT) codified by the genes LCB1 and LCB2; (2) 3-ketosphinganine reductase codified by the TSC10 gene; (3) Dihydrosphingosine C4-hydroxylase codified by the SUR2 gene; (4) Ceramide synthase codified by the genes LAG1, LAC1 and LIP1; (5) Dihydro-ceramidase codified by the YDC1 gene; (6) Phyto-ceramidase codified by the YPC1 gene; (7) Sphingoid base kinases codified by the genes LCB4 and LCB5; (8) Sphingoid base phosphate phosphatases codified by the genes LCB3/YSR2 and YSR3; (9) Long-chain base phosphate lyase codified by the DPL1 gene; (10) IPC synthase codified by the AUR1/IPC1 gene; (11) Inositol phosphosphingolipid phospholipase codified by the ISC1 gene; (12) MIPC synthases codified by the genes SUR1, CSG2 and CSH1; (13) Inositolphosphotransferase codified by the IPT1 gene; (14) This step comprises the enzymes dihydroceramide C4 desaturase, ceramide C8 desaturase and sphingolipid C9 methyltransferase (Smt1); (15) Glucosilceramide synthase codified by the GCS1 gene; (16) Hexosyl ceramidase; (17) Ceramidase; (18) Sphingosine kinase; (19) Sphingosine-1P-phosphatase; (20) Sphingomyelin synthase; (21) Sphingomyelinase; (22) Galactosilceramide synthase. Abbreviations: 3-keto DHS: 3-keto dihydrosphingosine; DHS: dihydrosphingosine; PHS: phytosphingosine; DHC: dihydroceramide; PHC: phytoceramide; DHS-1P: dihydrosphingosine-1-phosphate; PHS-1P: phytosphingosine-1-phosphate; Cer: ceramide; GlcCer: glucosylceramide; GalCer: galacyosylceramide; IPC: inositol phosphorylceramide; MIPC: mannosylinositol phosphorylceramide; M(IP)2C: mannosyldiinositol phosphoryl ceramide; Sph: sphingosine; Sph-1P: sphingosine 1-phosphate; SM: sphingomyelin.
For cell survival, organisms must constantly monitor the environmental temperature, since the increase in temperature can lead to the unfolding and consequent nonspecific aggregation and malfunction of proteins, thus causing defects in the cytoskeleton, intracellular transport, translation process, plasma membrane morphology and cell cycle [6]. To cope with this condition and prevent these problems, most organisms exposed to increased temperatures activate an adaptive mechanism known as heat shock (HS) response, which is regulated by several crucial proteins, including the transcription factors Msn2/Msn4 and the HS transcription factors (HSFs) [7]. Upon HS, the HSFs change the gene expression profile by inducing the transcription of different genes, including those that encode the heat shock proteins (HSPs) [6]. These proteins are mostly molecular chaperones that aid the cell to survive at higher temperatures, helping to decrease the aggregation of denatured proteins and restoring their native conformations to ensure proteostasis. In addition, HSPs such as human Hsp70 and small HSPs from different organisms can be found associated with the plasma membrane and help to maintain the membrane stability under stress conditions [8, 9]. On the other hand, a number of cold stress response transcription factors, the so-called CBF-transcription factors, are critical for cold acclimation, especially in plants and fish, and also assist cell survival during temperature changes by activating the expression of cold-regulated (COR) genes [10]. Since the targets of all these proteins are broad, the adaptation to the environment temperature ends up impacting different cellular structures and functions.
Given the growing concerns about the imminent consequences of temperature changes arising from global warming in fields such as agriculture, medicine and conservation of endangered species susceptible to temperature variations (such as bats and frogs, which are at high risk of fungal colonization), understanding the impacts of temperature changes on cell physiology and survival is of great importance in dealing with these variations. Temperature shift has an outcome on the cellular macromolecules and the plasma membrane is perhaps the first cellular structure to detect temperature fluctuations, changing considerably its chemical composition, fluidity and other properties [11]. The phospholipids, for instance, ultimately dictate the fluid state of the biological membranes during temperature variations, being widely accepted that both the degree of saturation and the size of the phospholipid fatty acid chains increase when temperature raises [12–14]. In this scenario, Mga2 and Spt23 are transcription factors that might play a crucial role in the regulation of the physical state of the ER membranes via the Ole1 pathway, which is responsible for the synthesis of unsaturated fatty acids [15]. Through sensing mechanisms, Mga2 and Spt23 trigger changes in the synthesis pattern of membrane lipids according to the membrane fluidity, assisting cell survival in adverse conditions, including during temperature variations [15]. In addition, the temperature increase usually leads to the accumulation of sterylglucosides, ergosterol, specific classes of phospholipids and the second messenger lipid phosphatidylinositol-4,5-bisphosphate (PIP2) in different organisms [16–18]. Some species of bacteria can produce and accumulate more SLs at high temperatures and less at lower ones, which adjusts the fluidity and permeability of the plasma membrane [19, 20]. In fact, SLs have higher melting temperatures than phospholipids [21]. The levels of many classes of SLs also change during heat stress in different fungi and these molecules end up impacting several processes, such as the programmed cell death, the actin cytoskeletal polarization and the trehalose production [22–26]. Furthermore, many of these lipids also act as signaling molecules [e.g. long chain bases (LCBs) and Cer] in response to temperature stimulus, suggesting the existence of a lipid regulatory role in the temperature stress response [27]. Since several and specific implications of SLs in the eukaryotic HS response were recently raised, in this review we discuss the main changes and functions of this unique class of lipids during the thermal adaptation of fungi, plants and animal cells.
2. Sphingolipids in the fungal adaptation to temperature stress
2.1. The behavior of fungal sphingolipids in response to heat stress
One of the expected imminent consequences of global warming is the emergence of new diseases associated with the rise of thermotolerant species of pathogenic bacteria and fungi. An outstanding example of this current scenario is the extinction of numerous amphibian species as a result of infection with a chytrid fungus (reviewed in [28]). Phenotypic and biochemical studies of cells exposed to temperature stress can help to predict the impacts of these events. The yeast Saccharomyces cerevisiae has long served as a model for studying the eukaryotic HS response. The study of yeast thermotolerance is also of great economic importance due to its use in the fermentation process, in which the fungus must adapt to constant changes in temperature. Thanks to the yeast model, it is known that the most common cellular consequences in the face of a sudden increase in temperature (shifts from 24–30 °C to 37–50 °C) are the interruption of the cell cycle, the induction of the HSPs expression, the cell wall and plasma membrane restructuring and the metabolic reprogramming, which leads, for example, to the activation of proteolytic pathways and the accumulation of trehalose, a known thermoprotectant [29]. These changes occur in the early phase of HS response, in which yeasts acquire thermotolerance for approximately one hour; after this period, the cell resumes growth even at the increased temperature [22].
Regarding the metabolism of the yeast plasma membrane during the period of thermotolerance acquisition, the HS causes a fast and dramatic change in the SLs content, leading to the accumulation of many species of Cer, LCBs such as phytosphingosine (PHS) and DHS, and LCBs phosphates (LCB-Ps) such as phytosphingosine-1-phosphate (PHS-1P) and dihydrosphingosine-1-phosphate (DHS-1P) (Figure 1), specially through the transient activation of the de novo biosynthetic pathway (Figure 2A) [22, 25, 30]. This phenomenon suggests that SLs are necessary for the yeast HS response. In fact, mathematical models predicted that the first enzyme of the SLs pathway (SPT) is quickly and highly up-regulated by HS, and then falls again within a few minutes [31]. Since HS causes a reduction in the overall rate of protein synthesis and in theory leads to increased serine availability, this event could explain the increased SPT activity and, consequently, the increased SLs concentration during HS [32]. However, Cowart and colleagues showed that during HS, the serine used by the de novo pathway is actually from exogenous sources (uptook from the medium), contrary to the fatty acid component, which in turn is derived endogenously from the activity of fatty-acid synthases [33]. Curiously, while DHS and PHS levels are induced a few minutes after the initiation of HS (10 min), PHC levels increase later (after 20 min) and remain high for at least 60 min [25]. In addition, longer DHS and PHS molecules show a larger proportional increase in concentration than the shorter ones, in accordance with the adequacy of the plasma membrane fluidity [22, 25]. Interestingly, the concentration of Cer also begins to increase after 20 min of the initiation of HS, but keeps growing at least until 60 min after that, which points to an adaptation of the SLs biosynthesis towards the production of Cer in the long term heat stress, with the participation of other branches besides the de novo pathway.
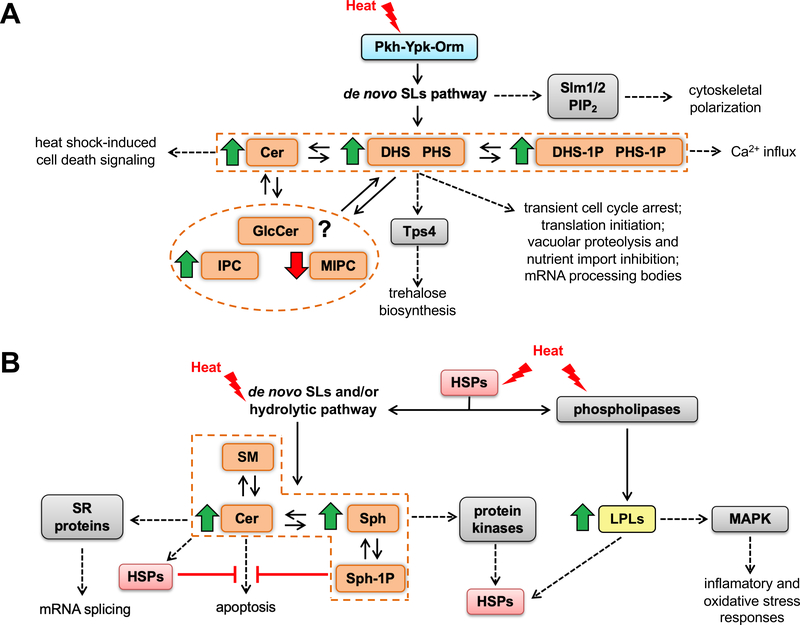
Figure 2.
The heat stress and the sphingolipids dynamics in fungi and animals. (A) General effects of heat stress in the sphingolipids biosynthesis and cellular consequences of these effects in fungi. Heat stress in fungi induces the de novo sphingolipid biosynthesis, mediated by the regulatory module Pkh-Ypk-Orm. As a result, the levels of most sphingolipid species increase, except for MIPC. The behavior of GlcCer is not clear. Intermediates of the biosynthesis pathway perform several signaling functions that impact on different processes, such as cell cycle arrest, proteolysis, accumulation of the trehalose and regulation of translation. More details in the text. (B) Consequences of heat stress for the sphingolipids synthesis and other processes in animal and human cells. Heat stress in animal and human cells induces the synthesis of Cer and Sph through the de novo biosynthesis pathway and / or the sphingomyelinase pathway, depending on the cell type. As a consequence, cellular processes such as apoptosis and dephosphorylation of proteins that participate in the regulation of mRNA splicing are activated. In parallel, the expression and activation of HSPs are induced by heat shock and these proteins connect with many points in the sphingolipids biosynthesis pathway. Additionally, heat shock favors the activity of phospholipases, increasing the levels of lysophospholipids, which also seem to play a role in the response to heat shock mediated by HSPs and MAPKs. More details in the text. SLs: sphingolipids; DHS: dihydrosphingosine; PHS: phytosphingosine; DHS-1P: dihydrosphingosine-1-phosphate; PHS-1P: phytosphingosine-1-phosphate; Cer: ceramide; GlcCer: glucosylceramide; IPC: inositolphosphorylceramide; MIPC: mannosylinositol phosphorylceramide; Sph: sphingosine; Sph-1P: sphingosine phosphate; HSPs: heat shock proteins; LPLs: lysophospholipids; SM: sphingomyelin.
Accordingly, other studies have suggested that some components of the SLs pathway are also synthetized through the activity of downstream branches of the pathway during the HS. Cowart and collaborators observed an increase in DHC and PHC synthesis in yeast 30 minutes after the start of the HS, and this increase in DHC content was mainly due to the activity of the inositol phosphosphingolipid phospholipase 1 (Isc1), which metabolizes IPC to DHC and PHC (enzyme #11 in Figure 1) [34]. However, at earlier time points and using the same genetic background, Montefusco and coworkers showed that DHC levels actually decrease after 15 minutes of heat stress and this decrease is not due to an inhibition of SPT [35]. In this report, the DHC decrease was likely due to the activation of a dihydroceramidase (Ydc1), which hydrolyzes DHC and releases free fatty acid and DHS (enzyme #5 in Figure 1) [35]. Interestingly, this phenomenon is not only present in S. cerevisiae but also conserved in other fungi such as in the filamentous mold Neurospora crassa, in which upon 15 minutes exposure to HS, PHC levels decrease [24]. However, the activity of Isc1 and other ceramidases has not been investigated in this system. Thus, while the short-term HS (up to 15 minutes) affects the breakdown of ceramides DHC and PHC, the long-term HS (after 30 minutes) affects the breakdown of IPC, which will ultimately lead to an increase of Cer levels. This response makes sense because most IPC are in fact made of long chain fatty acids, and the release of these fatty acids will help the adaptation of the plasma membrane during the HS response.
Mathematical modeling supports physical evidences that the majority of the SLs pathway enzymes are likely to be involved in the yeast well-coordinated adjustment of SLs metabolism under heat stress. Using a variety of pre-surveyed metabolic data, Chen and coworkers developed a mathematical modeling that predicted the behavior of yeast enzymes of the SLs biosynthetic pathway upon HS over time [36]. The authors proposed that the Cer synthases (enzyme #4 in Figure 1) are activated immediately following the initiation of heat stress and spike in a few minutes [36]. The model also suggests that upon heat stress, Cer and their intermediates can be formed from the de novo SLs biosynthesis pathway and from the hydrolysis of complex SLs [36]. Consistently, it was predicted that ceramidases (enzymes #5 and #6 in Figure 1) are similarly activated immediately upon HS, indicating that this kind of stress simultaneously triggers both the production and the utilization of SLs [36]. Interestingly, after reaching the spike, the activity of both group of enzymes (Cer synthases and ceramidases) varies depending on the lengths of the fatty acyl CoA chains used as substrates, as well as the type of product to be generated [Cer, DHC, PHC or sphingosine (Sph)] [36]. These results show that the SLs biosynthesis pathway during HS response is well-coordinated and undergoes a cooperative adaptation modulated by most enzymes in the pathway. However, the enzymatic regulatory mechanisms of the SLs pathway activity in this condition are currently unknown.
The levels of complex acidic SLs also change upon heat stress. The amount of IPC slightly increases in the initial moments of HS in yeast cells, while MIPC decreases (Figure 2A) [22]. In fact, IPC synthases (enzyme #10 in Figure 1) are activated by heat stress, but this activation dissipates within few minutes [36], therefore being a quick and transient response. This rapid and transient activation is in line with the results mentioned earlier showing that the Isc1 enzyme is activated in a later time point [36]. In summary, the first moments of HS are marked by the increase in Cer synthesis through the de novo pathway, but also by the increase in IPC levels through the activation of IPC synthases. But after approximately 15 minutes, when the IPC synthases are no longer activated, Isc1 starts to degrade IPC and keeps the Cer levels high. Together, these studies reinforce the coordinated adjustment of the SLs pathway with HS.
Other investigation in yeast showed that constant high temperature growth condition (37 °C) also favors the accumulation of Cer, IPC and MIPC and the lower production of mannosyldiinositol phosphorylceramides (M(IP)2C), which points to an adaptation of the biosynthetic pathway of complex SLs to the long-term exposure to high temperatures [16]. Similar results were also observed in Cryptococcus laurentii after growth at 28 °C followed by a continuous incubation at high temperature condition (40 °C) [37]. Besides that, in the same fungus and also in Schizosaccharomyces pombe and Wickerhamomyces anomalus, SLs with shorter fatty acid chains are more abundant after incubation at low temperature while the proportion of longer molecular species increases at higher temperatures, regardless of the type of polar head [37]. This correlation between the size of the fatty acid chain and the temperature is in agreement with the general notion that at high temperatures, longer lipid molecules are synthesized to adjust the fluidity of the plasma membrane [12], a function that relies on the participation of SLs. Considering that sterols have a greater affinity for SLs with long fatty acid chains and thus form a thicker, more compact and less permeable plasma membrane [38], changes in the overall size of lipid chains during HS suggest that the plasma membrane seeks a more stable conformation in adapting to heat.
Since S. cerevisiae cannot synthetize GlcCer, few investigations have scrutinized this group of SLs during HS. But other model yeasts, such as Pichia pastoris, Kluyveromyces lactis and S. pombe, do make GlcCer. Sakaki and coworkers showed that there is no significant change in the GlcCer levels in P. pastoris under HS [17]. Moreover, Tanji and colleagues reported that the levels of monoglycosylceramides (GlcCer and GalCer) in K. lactis increase with increasing growth temperature [39]. However, the behavior of GlcCer in other fungal species during HS awaits further clarification.
Genetic studies using yeast mutant strains have reinforced the conclusions that SLs are important for thermal adaptation. Firstly, mutants of S. cerevisiae lacking long chain base 1 (LCB1) gene, a subunit of the SPT enzyme, were hypersensitive to heat stress, and this phenotype was rescued by LCBs supplementation [22, 40, 41]. Recently, using an adaptive evolution approach, Randez-Gil and colleagues generated yeast strains resistant to the SPT inhibitor myriocin and found that, in addition to synthesizing more SLs, these strains exhibited an increased growth at higher temperature [42]. Furthermore, in S. pombe, the deletion of the LAC1 gene, which encodes a Cer synthase (enzyme #4 in Figure 1), increased the susceptibility to HS [43]. Cells with sensitivity to high temperature were also observed in S. pombe mutants for the CSS1 gene, a S. cerevisiae ISC1 homologue [44]. Moreover, mutants of the SLs pathway genes sdeA and gcsA, which act on the GlcCer synthesis, also displayed reduced growth at higher temperatures in the model organism Aspergillus nidulans [45]. Together, these studies show that the correct functioning of the SLs biosynthesis pathway is important and necessary for the fungal temperature adaptation.
Conversely, the deletion of the LCB3 gene that encodes a LCB-Ps phosphatase in yeast (converts DHS-1P and PHS-1P to DHS and PHS, respectively; enzyme #8 in Figure 1) conferred increased thermotolerance, while its overexpression diminished it [46], reinforcing the role of LCBs for HS adaptation. Since the deletion of this gene led to the accumulation of LCB-Ps (DHS-1P and PHS-1P) [46] and high levels of these molecules stimulated the Ca2+ influx [47], these results suggest that DHS-1P and PHS-1P also act as signals for resistance to heat stress, possibly through the calcineurin-signaling pathway. Accordingly, several studies have shown that both calcineurin and its activator calmodulin are necessary for Cryptococcus neoformans growth at the host higher temperature (37 °C), which is a prerequisite for fungal virulence [48–50]. Intracellular Ca2+ levels also control the activity of ceramidases in yeast [51]. These results then suggest a connection between temperature stress, SLs and the calcineurin-signaling pathway that can impact the virulence of pathogenic fungi, and future exploits of this connection may provide more information about the SLs signaling mechanisms.
In yeast, the de novo SLs biosynthesis is regulated by the protein kinases Ypk1 and Ypk2, two members of the AGC kinase subfamily [52]. Once activated by the upstream kinases Pkh1 and Pkh2, Ypk1 and Ypk2 phosphorylate the inhibitory regulators Orm1 and Orm2, which in turn activate SPT and the SLs biosynthesis [52]. In addition to be an essential regulatory part of the SLs biosynthetic pathway, the Pkh-Ypk-Orm module also responds to changes in temperature and performs a broad spectrum of functions during both heat and cold adaptation, and these responses partially explain the SLs content fluctuations under temperature stress. Sun and coworkers have found that Ypk1-mediated phosphorylation and activation of Orm2 is rapidly and transiently induced by HS [53]. As reported above, the content of the majority SLs species usually increases under HS, possibly through this Orm2 activation. However, a few minutes later, the accumulated SLs intermediates then trigger Orm2 dephosphorylation, which in turn down regulates SLs biosynthesis [53], suggesting that the dynamics of the de novo SLs biosynthesis is regulated by feedback during heat stress. This event may explain the transient activation of the SLs biosynthetic pathway enzymes discussed above. As a result of the Pkh-Ypk-Orm cascade activation and the associated heat stress-mediated increase in the SLs synthesis, enzymes involved in other cellular functions are activated. Some examples are the enzymes Slm1 and Slm2 that participate in the signaling pathway of the phospholipid PIP2, impacting the cell growth and actin cytoskeletal polarization (Figure 2A) [54]. In the pathogenic fungus Aspergillus fumigatus in turn, the expression of the Ypk1 homologue is increased by HS while the repression of this gene retarded the growth at higher temperatures [55], reiterating the possible function of this protein and the SLs biosynthesis pathway in the fungal adaptive response to thermal stress.
Yet, what are the physiological consequences of the HS-increased amount of SLs for fungal cells? Besides the participation in the adjustment of the permeability and fluidity of the plasma membrane, as well as the function in the actin cytoskeletal polarization mentioned above [23], heat induced-SLs have been implicated in mediating different responses, such as regulation of translation, cell cycle arrest and proteolysis (Figure 2A). Using a yeast strain with impaired SLs biosynthesis pathway, the LCB1–100 temperature-sensitive mutant, Meier and coworkers demonstrated that the Pkh1/2 kinases signaling cascade and the synthesis of LCBs are required for proper translation initiation during HS [32]. Besides that, during temperature stress, mRNA moves from active translation sites to cytoplasmic granules know as mRNA processing bodies (P-bodies), where they are enzymatically degraded [56]. Confocal microscopy analyses indicated that, in addition to assisting translation, the LCBs are required for the formation of P-bodies (Figure 2A) [56], thus revealing a novel SLs role in mediating mRNA degradation during HS.
Moreover, Chung and collaborators observed that the inactivation of SPT prevented ubiquitin-dependent proteolysis in yeast via the endocytosis vacuolar degradation during heat stress [57]. The addition of PHS not only restored proteolysis but also inhibited the import of nutrients, such as uracil, by inducing the degradation of the nutrient permeases Fur4 and Gap1 [58]. These results show that PHS are implicated in both vacuolar proteolysis and inhibition of growth and nutrient import in yeast cells. The same research group has found that the transient cell cycle arrest in the G0/G1 phase is another consequence of HS in yeast mediated by SLs. In this arrestment, budding and proliferation are decreased and cell’s energy is spent to adapt to the stress [59]. Through genetic and flow cytometry experiments, they found that the LCBs are the active species required for the cell cycle arrest during HS response, and the clearance of the LCBs through the activity of the LCBs kinases enables cell cycle recovery from the transient arrest [59]. In addition, Dickson and coworkers showed evidences that the accumulation of LCBs and Cer during heat stress also induces the transcription of the TPS2 gene, which encodes a subunit of trehalose synthase and increases trehalose production [25]. Since the promoter of this gene possess multiple copies of the stress response element (STRE), which is recognized by the transcription factors Msn2/4 during the HS response, this result supports the idea that SLs signals activate the STRE during heat adaptation [25]. Although the synthesis of trehalose is essential for high-temperature growth and virulence of several fungi [60, 61], this connection between SLs and trehalose synthesis during the HS has not been addressed in other species. Also, another study pointed out that Cer function as signal for HS-induced cell death in N. crassa [24], which coincides with the known lethal toxicity of high Cer concentrations. Together, these studies show that yeast SLs have the ability to function as signal messengers for stress response, cell cycle arrest and apoptosis during HS, as summarized in Figure 2A.
Regarding the possible role of SLs in the HS response mediated by the HSP molecular chaperones, although Friant and coworkers demonstrated that the heat-induction of the small Hsp26 is impaired in the LCB1 mutant [62], yeast mutants lacking SLs were able to induce wild-type levels of the major HSPs such as Hsp90 and Hsp70 under HS [25]. These findings suggest that SLs signaling may buffer differential recruitment of molecular chaperones during the HS response, favoring the expression of the less abundant HSPs, such as Hsp26 and the Hsp70 chaperonin Ssa4 [63], but not the major ones. Although the metabolism of SLs and the connections with HSPs have not been fully investigated in fungi, some additional information about this relationship have been described in mammals, which will be reported below.
Microarray analyzes showed additional details about the broad effects of the de novo SLs biosynthesis pathway in different yeast cellular processes after HS. Among the genes that require SLs biosynthesis through the enzymatic activity of the SPT enzyme during the HS response are those involved in the regulation of translation machinery and tRNA synthesis, amino acid metabolism, cell wall organization and biogenesis, transport and cycle control [63]. Besides, functions such as carbon source utilization and sexual reproduction are also regulated by the Isc1 enzyme during temperature stress [34]. These results reinforce the idea that SLs work as second messengers and play regulatory roles in the development of thermotolerance, mediating a diverse set of cellular responses.
Notably, most environmental fungi are not pathogenic to animals and humans simply because they cannot grow and replicate at high temperatures (e.g. 37 °C and above). As these environmental fungi will adapt to the rise of environmental temperature (e.g. due to global warming), there also will be a potential increase in fungal virulence to animals and humans. It is anticipated that SLs will have a major role in the fungal temperature adaptation.
Lipidomic studies have already been conducted on some fungal species at different temperatures, but without addressing the SLs content. Moreover, HS also intensifies the SLs biosynthesis in the pathogen Aspergillus niger, but the dynamics of each specific SLs class is still unknown [26]. Despite the range of studies in yeast and a few other fungal species, the major consequences of HS for the SLs content and the role of these lipids during temperature stress were not addressed in most pathogenic and thermotolerant fungi of medical interest, such as Candida albicans, C. neoformans and A. fumigatus. Considering that SLs are implicated in the virulence of many of these fungi, the study of the relationship between thermotolerance and the SLs biosynthesis may bring new insights for the treatment of fungal diseases and the discovery of new promising therapeutic strategies.
2.2. The behavior of fungal sphingolipids in response to cold stress
Because most fungi are environmental microbes, they are also exposed to temperature stress caused by cold. While many species are adapted to live at lower temperatures and often under extreme cold conditions, others experience cycles of cold stress, such as the yeasts used in some wine, beer and bioethanol fermentation processes. However, studies focusing on cold stress and SLs homeostasis in fungi lag considerably compared to those focusing on HS response. In one of these few investigations, Puig-Castellvi and collaborators showed that, S. cerevisiae synthesizes less Cer at 15 °C than at the optimal growth temperature (30 °C) [64]. Moreover, the deletion of genes belonging to the SLs biosynthesis pathway, such as YSR3, CSG2, IPT1, SUR2, YDC1, LCB4 and LCB3 (Figure 1), decreased the yeast growth at 12 °C but not at 28 °C [65]. The overexpression of some of these genes, in turn, improved both growth and fermentation activity at lower temperatures, suggesting an important role of SLs also for cellular processes involved in fermentation activities during cold stress [66]. On the other hand, yeast strains resistant to the SPT inhibitor myriocin that synthesize more SLs showed lower growth rate at lower temperature [42], suggesting that the behavior and balance of SLs in the cold stress may be more complex.
Regarding the Pkh-Ypk-Orm regulatory cascade, it was recently found that the cold stress suppresses the expression of the protein Ypk1 and concomitantly induces the expression of Orm2 in yeast, suggesting that SLs biosynthesis is repressed under cold conditions [67]. In fact, the levels of DHS and PHS are decreased after cold stress, although their phosphate counterparts DHS-1P and PHS-1P are increased [67]. In addition, lack of the Orm1 homolog in A. fumigatus leads to hypersensitivity to low temperatures, reiterating the importance of SLs biosynthesis for cold adaptation [68]. Interestingly, García-Marqués and colleagues showed that the Sng1 transmembrane protein, which is associated with membrane fluidity and the cold tolerance phenotype, actively participates in a regulatory circuit that controls the activity of the Pkh-Ypk-Orm module handling SLs synthesis for cold adaptation [69]. Another protein that is likely to modulate the Pkh-Ypk-Orm signaling cascade in response to cold is the Pho85 cyclin dependent kinase, which among the numerous roles it plays, also regulates lipid metabolism [67]. These studies point to the existence of a regulatory cycle involving lipids and proteins: in the presence of cold stress, changes in the plasma membrane must be detected by regulatory proteins that signal to the Pkh-Ypk-Orm module in order to change the SLs synthesis, adjusting the plasma membrane status again and assisting adaptation to cold.
Furthermore, the cultivation of N. crassa at low temperature leads to the accumulation of glycosphingolipids, especially the monoglycosylceramides (GlcCer and GalCer) with shorter and saturated fatty acid chains, and these changes seem to compensate the reduced levels of phospholipids and to support the regulation of plasma membrane fluidity in this condition [70]. Besides that, both the fusaruside, an immunosuppressive fungal SLs with medical potentials for treating liver injury and colitis, and the Δ10(E)-sphingolipid desaturase that generates this molecule proved to be important for growth of the phytopathogen Fusarium graminearum at low temperatures [71]. These evidences indicate that perhaps fungi need to synthesize more complex SLs during the cold. However, more studies need to be carried out to confirm this hypothesis, especially by measuring the effects of cold stress on the SLs content of other fungal species.
Accordingly, although the lipidomic profile of psychrophilic yeasts Cryptococcus victoriae, Cystofilobasidium capitatum, Holtermaniella wattica, Mrakiella aquatica, Mrakiella cryoconiti, Rhodotorula lignophila, Kondoa malvinella and Trichosporon aggtelekiense have been obtained at a temperature range of 4–28 °C [72], the SLs levels have not been measured. This investigation would be interesting, since these oleaginous microorganisms have been explored as source of lipids for biodiesel production [72]. The filamentous and psychrophilic fungus Pseudogymnoascus destructans, responsible for the white-nose syndrome in bats in North America [73], may be also a potential model for the study of SLs during cold stress. Despite the scarcity of studies focusing on cold stress, it is notable that the biosynthesis of SLs in fungi is also modulated at low temperatures and may play a significant role in the adaption to this condition. Nevertheless, the exact behaviors of classes such as DHC, PHC, IPC and GlcCer, as well as the particular functions of these molecules during fungal adaptation to cold stress have yet to be unveiled.
3. Plant sphingolipids versus the environment temperature
The most famous plant model is the herbaceous Arabidopsis thaliana, whose optimal growth temperature of 22–23 °C allowed its dispersion in mild regions of Europe, Asia and North America [74]. However, like other organisms, there are plants capable of surviving extreme temperatures, with optimal growth at less than 10 °C or more than 30 °C (reviewed in [75]). In the context of contemporary climate change, any minimum change in global temperature can cause a decrease in plants germination, growth and reproduction rates, thus being a risk to crops productivity and even to the preservation of endangered species.
Plants SLs constitute four major classes: Cer, GlcCer, GIPC and free LCBs resulted from the de novo biosynthesis pathway. Besides their structural role, plants SLs also participate in essential signaling and regulatory mechanisms, such as programmed cell death, cell-to-cell interactions, Golgi and ER integrity and membrane stability, as well as cold response [76]. As discussed previously in this review, Orm proteins in yeasts have been shown to act as homeostatic negative regulators of SPT in response to intracellular SLs levels [52]. Chueasiri and collaborators have observed that suppression of ORM genes in rice results in pollen abnormalities and temperature sensitivity [77], which suggests the involvement of SLs in the thermal adaptation of plants, similarly to what was seen in fungi.
3.1. The plant heat stress response
Considering the general perspective of increasing the Earth temperature, all the environment changes in the patterns of rainfall, droughts and submergence stress negatively affect plant communities. To deal with heat stress, plants share with others eukaryotes a highly conserved arsenal of multiple pathways involved in the HS response that is triggered by different cellular sensors [78]. This response acts by reprogramming their transcriptome, proteome, metabolome and lipidome in order to allow plant survival and reproduction [78]. Without the correct HS response or under more severe thermal stress, plants can undergo growth inhibition, alteration of physiological processes such as respiration and photosynthesis, accumulation of reactive oxygen species (ROS) and consequently, oxidative damage [79]. Oxidative stress in turn results in greater lipid peroxidation. High temperatures alter the structure of the cell membrane, as well as the lipid and nucleic acid turnovers [79]. As previously reported in fungi, the membrane lipids saturation levels directly reflect the temperature changes. Not surprisingly, the heat stress tolerance in plants is linked to increased levels of saturated fatty acids [80].
Regarding the SLs contribution to HS in plants, few information is available. In A. thaliana, LCB-Ps are considered regulators of thermotolerance, since treatment with these molecules decreased apoptosis and increased cell survival after HS [81]. This fact is in agreement with the data from yeasts showing that the disruption of LCB-Ps phosphatase and consequent LCB-Ps accumulation confer heat resistance [82]. Alden and collaborators also suggested that plants Cer and LCB-Ps levels are determinant for cell viability [81], what is a well conserved dynamics in eukaryotic systems. However, the general effects of HS on specific cell levels of Cer, SPH, DHS, GlcCer and GIPC in plants await further verification.
3.2. The plant cold stress response
Despite the scarcity of studies during heat stress, the plant response to cold stress is better characterized. In agriculture, the process of adapting to low winter temperatures is called cold acclimation and has critical consequences to productivity. Physiological and morphological changes occur in plant cells depending on the regulation of the transcriptional response mediated by the CBF pathway to allow the survival of these organisms at low temperatures. The cold acclimation is marked by changes in the plasma membrane composition, whose adaptation is crucial not only for freezing tolerance acquisition but also for the accumulation of sugars to avoid the dehydration caused by the freezing process; the activation of antioxidant enzymes; and the adjustment of the photosynthetic machinery [83]. With respect to the latter, reduced membrane flexibility caused by low temperature exposure interferes in the photosynthesis process [84]. Although the photosynthesis light reactions are relatively stable, the activity of the dark reactions enzymes are reduced, which leads to photoinhibition of photosystems I and II and ROS production, culminating in lipid peroxidation and decreased membrane fluidity (reviewed in [85]).
Furthermore, in a comprehensive mode, plant membranes respond to cold stress by increasing lipid unsaturation, shortening chain lengths and modifying lipid composition, what in summary is very similar to fungi and mammals [86]. In a transcriptional analysis, Wang and coworkers observed that several genes involved in SLs biosynthesis are up-regulated in peach fruit during cold conditions, such as the homologs encoding the enzymes #1, #2, #4 and #15 in Figure 1 [87]. Interestingly, the authors believe these expression changes are due to the activity of transcription factors that are responsive to the ethylene production, which is a development and maturation hormone in plants [87]. In fact, the short three-day exposure of A. thaliana to the cold leads to an increase in the synthesis of total SLs, especially GIPC [88]. However, some reports have shown that long-term exposure to cold reduces the SLs content in different plant species. Tarazona and collaborators observed that, in general, ten days of cold acclimation lead to a reduction in the levels of the majority of the Cer, GlcCer and GIPC species in A. thaliana, especially those with saturated fatty acid chains [89]. Interestingly, some Cer species with long fatty acid chains (26C) are exceptions, since their contents significantly increase during cold stress [89]. Degenkolbe and colleagues also observed a decrease in GlcCer concentration in the same plant model after fourteen days of cold acclimation, but accompanied by an increase in Cer levels [90]. In wheat seedlings and oat and rye leaves, the GlcCer content also decreases after three or four weeks of cold acclimation [91, 92], and this change was more expressive in rye, which is a better-adapted plant to the winter cold [92], suggesting a strongly negative correlation between GlcCer levels and cold tolerance. The same results were obtained in potato species leaves after ten days of exposure to cold [93]. Studying different species of grapevines, Kawaguchi and coworkers found that the proportion of GlcCer with different sizes and hydroxylations also varies according to the ability to survive at lower temperatures [94]. Since lower levels of GlcCer prevent undesirable phase transitions in Arabidopsis plasma membrane under freezing temperatures [92], the abovementioned studies reinforce the role of SLs in remodeling the plasma membrane of plants during cold stress, similarly to other lipids molecules, possibly in order to assist the maintenance of photosynthetic capacity and to avoid dehydration, which contributes to increasing the cryostability.
Other examples of the SLs role in plants cold acclimation were also raised. Guillas and colleagues observed that short cold stress considerably reduces the content of LCBs in A. thaliana seedlings, and this phenotype was reversed when a non-symbiotic haemoglobin (AHb1) was overexpressed [95]. AHb1 is an element of nitric oxide (NO) turnover that converts NO in nitrate, being important for plant growth and protection against nitrosative stress [96]. Moreover, when A. thaliana subjected to chilling conditions is treated with NO, the formation of PHS-1P and Cer phosphate, which are rapidly and transiently accumulated upon cold exposure [97], is down-regulated [98]. These results indicate that the cold stress response of plants is possibly connected to both the nitrosative stress and the SLs content [95].
Besides, under low temperature treatment, Arabidopsis transiently synthetizes PHS-1P via the enhanced activity of LCBs kinase 2 (Lcbk2, homolog of enzyme #7 in Figure 1) [99]. Another kinase, Lcbk1, potentially acts in the plant freezing tolerance as well through regulation of ROS homeostasis [100]. As mentioned above, LCBs induce apoptosis during HS, and reports have shown that this signaling is dependent both on the concentration of cytosolic and nuclear Ca2+ in tobacco cells [101] and on the mitogen-activated protein kinase (MAPK) pathway involved in monitoring oxidative stress in A. thaliana [99]. Together, these studies indicate that plants control the synthesis of SLs to prevent cell death during cold stress, affecting not only the structure of the plasma membrane but also different signaling pathways.
The balance between the SLs saturation and unsaturation levels is also determinant for the response to environmental temperature variation, since Arabidopsis mutants lacking Δ8 LCBs unsaturations also presented a 50% decrease in GlcCer levels and were more sensitive to low temperature [102]. The increased need for the synthesis of unsaturated lipid chains is in line with the expected response to cold stress. Accordingly, the mRNA expression of the two desaturase enzymes responsible for these unsaturations is up-regulated by cold exposure, as well as Cer-modifying enzymes, LCBs hydroxylases and acyl lipid desaturases. [88]. These changes seem to be due to the cell death suppressor Bax inhibitor-1 (BI-1) [88], which once again connects the SLs metabolism with the cell death process triggered by temperature stress.
Interestingly, a glycosphingolipid (cerebroside C) isolated from the endophytic fungus Phyllosticta sp. was able to increase the chilling tolerance of wheat seedlings by causing growth induction, reduction of lipid peroxidation and other changes in lipid metabolism [103]. This study not only reinforced the regulatory role of SLs in cold adaptation but also showed that exogenous SLs originated from mutualistic organisms play roles in the cold acclimation of plants and may contribute to increase the productivity of the crops. Besides that, Takahashi and coworkers observed that the 2-hydroxy fatty acid composition of SLs species found in rye membrane microdomains also changes significantly during cold stress [104]. Accordingly, Minami and colleagues suggested that changes caused in SLs levels by cold acclimation result in a decrease of the lipid rafts in the plasma membrane, which affects the localization of membrane proteins involved in various processes, such as transport, cytoskeleton organization and endocytosis [105]. Therefore, lipid compositional changes seem to be important for maintaining basic cellular functions upon temperature variations.
Unfortunately, there is still lack of information about the importance of some complex SLs species, such as GIPC, for the membrane cold acclimation of plants. Also, no signaling role has been assigned to these and some others SLs molecules so far, which points to the need for further studies in the area. In addition, further studies of the specific differences in SLs content between plants adapted to lower temperatures and plants growing in warmer regions could highlight interesting adaptive patterns and help elucidate the gaps in the SLs participation in thermal adaptation.
4. Sphingolipids in the animal adaptation to temperature stress
With the evolution, animal cells developed new processes and acquired new functions and specializations. Not surprisingly, some specific lipid molecules are found in these higher animals, such as SM, complex cerebrosides, gangliosides and globosides (Figure 1). However, the global environment temperature also impacts the SLs dynamic of animals, with implications for their lifestyle. In this context, invertebrate animals are no exception. For instance, coral reefs, which are formed by cnidarians and algae living under symbiotic association, are very important for the O2 production, but are highly susceptible to increased temperature due to global warming. Detournay and coworkers found that exposure to elevated temperatures in combination with the exogenous addition of Sph increased the activity of caspases in sea anemones and the phenomenon of coral bleaching, which is a consequence of cell death [106]. In contrast, the exogenous addition of sphingosine 1-phosphate (Sph-1P) reduced these deleterious effects [106]. The same research group also found that the long-term exposure to higher temperatures caused an increase in intracellular Sph levels [107], suggesting that the balance of these SLs participates in the response to temperature stress and ensures the survival of these animals. In addition, in cell lines of the bug Spodoptera frugiperda, HS causes a three-fold increase in SM levels [108], but the consequences of this change are unknown.
SLs also play important roles in vertebrates during temperature stress. Fishes are examples of poikilothermic animals that constantly undergo thermal acclimatization, which is intrinsically related to the lipid plasma membrane composition. Studying the brain of the fish Carassius carassius, Kakela and collaborators found that, although there are no significant differences in the composition of SM and GalCer at temperatures of 4 °C, 16 °C and 30 °C, both cold and heat cause variations in the levels of some species of Cer [109]. Accordingly, HS increases Cer levels and reduces SM levels in zebrafish embryonic cells by activating the enzyme neutral sphingomyelinase 1, and these changes can trigger a proapoptotic signaling [110]. In another study, Kappel and coworkers observed that fishes synthesize more alkaline brain gangliosides and less polar brain gangliosides than other animals, and these differences are more evident in fishes living in colder places [111]. The same research group also reported that gangliosides affect the activity of membrane channels according to the environment temperature [112]. Considering that gangliosides modulate neuronal calcium homeostasis, these results suggest that these molecules probably assist the neuronal membrane functioning under temperature fluctuations, especially with regard to Ca2+-mediated synaptic transmission [113]. The constitution of lipid-rafts from the rainbow trout (Oncorhynchus mykiss) hepatocytes also undergo major changes with increasing or decreasing temperature, but, although the SM content does not vary, the effects on other SLs have not been investigated [114]. On the other hand, the erythrocyte ghost membranes of temperate-water fishes are richer in SM in comparison to those from Antarctic fishes [115], which suggests an adaptation of SM synthesis to the environmental temperature in order to assist the fluidity of the plasma membrane.
In the modern farming industry, animal breeding and reproductive biotechnology play a fundamental role in raising animals for human consumption of meat and animal products, what makes temperature control a prerequisite in this field. For bovine reproduction, for example, temperature control is important to guarantee the highest fertilization efficiency and, consequently, higher profit and lower losses. It is known that HS in bovine oocytes leads to Cer accumulation via activation of the sphingomyelinase pathway, which forms Cer through the hydrolysis of SM [116]. Similar to zebrafish, Cer then acts as a second messenger of apoptosis, affecting the oocyte development and consequently animal reproduction [116]. In contrast, the addition of Sph-1P in the oocyte maturation medium in order to modulate the Cer/Sph-1P balance was able to block the deleterious effects caused by HS [117]. In fact, Sph-1P is associated with proliferation and cell survival in mammals [117]. Since Sph-1P is formed by the phosphorylation of Sph, which, in turn, originates from the hydrolysis of Cer, these studies have shown that the SLs balance is essential for cell thermal adaptation during animal reproduction, and the modulation of the SLs biosynthetic pathway may be preponderant for the success of the animal breeding business.
As mentioned above, Cer can function as a second messenger for apoptosis and cell differentiation in animals in general. Interestingly, although HS leads to the accumulation of Cer in mouse fibroblast cells [118], and this event can trigger an apoptosis signaling, the cellular death of mouse embryo fibroblasts as a result of Cer treatment was less evident when these cells were previously exposed to pre-conditioning HS [119]. In addition, Chang and collaborators showed that both the exogenous addition of Cer and the increased endogenous levels of Cer by inhibiting the glucosylceramide synthase (enzyme #15 in Figure 1) were able to induce the synthesis of a small HSP [118], suggesting a possible participation of SLs in mediating and controlling the HS response throughout HSPs expression, something that seems to be conserved from fungi [62]. Accordingly, Sph-1P was able to induce both Hsp27 gene and protein expression in mouse osteoblasts, and this effect has been shown to be mediated via p38 MAPK [120]. Sphingosylphosphorylcholine, a bioactive SLs in blood plasma that mediates cellular signaling (such as MAPK cascades and Ca2+ signaling), proliferation and death, was also able to activate the small Hsp27 in rat cerebral arteries [121]. Furthermore, Megidish and colleagues showed there are indirect interactions between HSPs and SLs as well [122]. They demonstrated that a specific group of protein kinases (Sph-dependent kinases) were activated by Sph, thus inducing the phosphorylation and activation of a range of other proteins in mouse fibroblasts, including the Hsp84 and Hsp86 chaperones [122]. Interestingly, the activation of these protein kinases was not triggered by any other tested lipid [123].
In fact, physic connections between SLs molecules and HSPs were also found. Hsp90, Hsp70, Hsp60 and Hsp40, for instance, could be found associated with cholesterol and SLs that make up lipid rafts of different animals and cell types, and these interactions are likely to be important for signal transduction, neuronal synaptic transmission and other processes [9, 124]. Interestingly, lipid rafts of human macrophages were also able to recognize and bind to exogenous Hsp70, which stimulated phagocytosis and triggered the immune response [125]. Noteworthy, Hsp70 has often been found associated with SLs metabolism. Harada and colleagues demonstrated in vitro that a mouse Hsp70 formed complexes with gangliosides [126]. Such complexes seem to be specific for acidic SLs and not for neutral SLs [126], and may indicate that Hsp70 chaperone assists the correct folding of enzymes involved in lipid metabolism. Besides, Niimura and collaborators reported that long-term HS in canine kidney cells also increases the Cer, GlcCer, GalCer and lactosylceramide content but reduces ganglioside levels [127]. Working with monkey and canine kidney cells, they also observed that transfection of a plasmid expressing the Hsp70 protein increased glycosphingolipid synthesis [127]. Accordingly, Zhu and coworkers found that Hsp70 is able to regulate the activation of acid sphingomyelinase in monkey brain tissue, thus contributing to neuronal survival by inhibiting lysosomal destabilization [128]. A similar result has been reported in human bone osteosarcoma cells [129], showing that Hsp70 promotes cell survival by modulating SM levels and inhibiting lysosomal membrane permeabilization in tumor cells. This could be also a promising approach for the treatment of patients with the genetic disorder called Niemann-Pick disease, in which the acid sphingomyelinase activity is reduced [130]. Considering that HS induces the synthesis of HSPs, these results suggest that Hsp70 regulates the synthesis of glycosphingolipids during HS, but the mechanism of this regulation still needs to be investigated. In agreement, Hsp90 inhibition by treatment with geldanamycin and analogs reduced GlcCer levels in rat pheochomocytoma cells, but the metabolic importance of this regulation awaits further elucidation as well [131]. Such findings corroborate the notion that SLs are implicated in the response to temperature stress in mammals (Figure 2B), and this is an open field of investigation across all life kingdoms.
The metabolism of SLs in mammals also changes during cold stress. The study of the mammal adaptation to cold usually aims to unveil the thermogenic capacity associated with the hibernation process, which can also be affected by global warming. In a study with C57BL6/J mice, Xu and colleagues observed that inguinal white adipose tissue accumulates Cer, Sph and SM after three days of exposure to cold [132]. In fact, qPCR analysis revealed that cold induces mRNA expression of the SLs biosynthesis cascade enzymes (such as the homologs of enzymes #1 and #4 in Figure 1) and suppressed the expression of the SLs-breakdown-related genes (such as the homologs of enzymes #5 and #6 in Figure 1) [132], suggesting that these SLs species could be involved in cold adaption. Since cold also leads to the accumulation of free fatty acids in adipose tissue and these can activate Cer synthesis, the authors concluded that increased SLs levels during cold stress might be a secondary result of increased availability of free fatty acids [132]. In contrast, three days of cold exposure did not change the total levels of Cer and SM in brown adipose tissue from mice [133], suggesting that the SLs metabolism may be different according to the type of tissue and organ, in this case linked to energy production. Moreover, Bouma and colleagues have demonstrated that hamsters in hibernation have a lower concentration of Sph-1P in blood plasma, and this change controls the lymphocyte dynamics during hypothermic condition [134]. They observed that the drop in Sph-1P levels is detected by the receptor of this molecule in the plasma membrane, which induces the storage of lymphocytes in secondary lymphoid organs, reducing immune function as a way to avoid energy expenditure [134]. This result shows that SLs such as Sph-1P can regulate important physiological functions in response to temperature variation.
Unlike yeast, changes in the levels of LCBs were not observed during HS in mammals, suggesting that although Cer is involved in mediating the response to temperature stress, LCBs are apparently not as important for this process as it is in fungi. This has also been seen in human cells, as reported below.
4.1. Sphingolipids and temperature stress in human cells
When it comes to human beings, the study of lipid molecules is often the interest of research on diseases, such as cancer for example, aiming to understand the molecular basis of such problems. Interestingly, hyperthermia is one of the existing cancer therapy procedures used to kill tumor cells [135]. In this scenario, some findings regarding the relationship between SLs metabolism and temperature stress have already been observed. As reported in other organisms, the HS also promotes the accumulation of Cer in human cells, as observed for the HL-60 and Molt-4 human leukemia cell lines subjected to HS from 37 °C to 40–44 °C [135, 136]. A decrease in SM levels accompanied by an increase in sphingomyelinase activity was noticed in HL-60 cells after HS, suggesting that the increase in Cer content derives from the SM hydrolysis [135]. However, the study of HS in Molt-4 cells using the pulse labeling technique and the SPT-inhibitor myriocin concluded that increased levels of Cer in these cells derive from the activation of de novo biosynthetic pathway, similarly to the yeast model [136]. Besides, HS also increased the incorporation of palmitate into Cer, SM, cerebrosides and gangliosides, suggesting that the temperature stress also induces the flux throughout the de novo pathway [136].
Regardless the source, the accumulation of Cer during and after heat stress brings consequences for human cells (Figure 2B). One example is that the dephosphorylation of proteins that regulate mRNA splicing, the so called SR proteins, is dependent on the Cer accumulated in the HS, suggesting the participation of SLs in the regulation of RNA editing [136]. As proposed in other organisms, Cer also signal for programmed cell death, inducing the expression and activity of proteins of the apoptotic signaling cascade, such as caspases [135]. Interestingly, the treatment of lymphoma-derived Jurkat cells with D609, a SM/Cer pathway inhibitor, prevented the apoptosis induced by HS, reaffirming the importance of Cer in activating the signaling cascade for cell death [137]. Another molecule known to cause apoptosis is the Sph analog fingolimod, a drug derived from myriocin and used to treat multiple sclerosis (reviewed in [138]). Interestingly, the process of cell death of human neutrophils triggered by this molecule is characterized by the cellular externalization of the small chaperone Hsp27 [139], which connects once again the SLs metabolism to the HS response.
In fact, the exposure of HSPs such as Hsp70, Hsp60 and Hsp27 on the cell surface is a characteristic of tumor cells, especially the highly aggressive ones [8, 140, 141]. Gehrmann and coworkers observed that around 20% of the total Hsp70 content of carcinoma cells is present on the cell surface, associated with cholesterol rich microdomains through anchoring mediated by the SL globotriaoslyceramide [142]. The superficial exposure of Hsp70 in tumor cells has immunological relevance, since this characteristic makes such cells more easily recognized and susceptible to natural killer (NK) cells, stimulating tumor cell death [142, 143]. An ER homolog of Hsp90 (Grp96) also induces the immune response against tumor cells when associated with the plasma membrane [141]. In both cases, HSPs exposure works as a mechanism for elimination of damage cells. However, concomitantly, it is believed that membrane-bound Hsp90 could increase the plasma membrane integrity and protect the cancer cells from different stresses [141]. This is because both in tumor or normal cells, the membrane-associated HSPs can alter the fluidity and permeability of the membrane lipid phase, which maintains cell membrane stability and functioning under stress conditions such as HS, providing thermotolerance [9, 144]. Another role of these membrane-associated HSPs is to avoid lysosomal destabilization, as discussed in the previous section, especially for Hsp70 [8]. On the other hand, the expression of HSPs is also partially controlled by the physical state of membrane microdomains through the HS response activation mediated by the small GTPase Rac1, suggesting that the HSPs expression pattern may be regulated by membrane sensors [8, 144]. These findings show that lipids are important dictators of chaperone expression, and the modulation of membrane composition/structure, and consequently HSPs expression, might be useful for the treatment of cancer, diabetes and other diseases [144].
Besides that, the apoptosis of T-cell hybridoma induced by increased levels of Cer was prevented by the constitutive expression of cytosolic Hsp70 [145], which suppressed the activation of the cell death signaling cascade, enhancing cell survival [146]. Similarly, Hsp90 inhibition induced apoptosis of hepatic stellate cells via sphingomyelinase-dependent signaling [147]. These results suggest the existence of a protective cellular function of HSPs linked to the metabolism of SLs. Moreover, Hsp60 is also able to regulate sphingomyelinase activity in HEK293 cells, similarly to the Hsp70 chaperone in monkeys. In this case, however, Hsp60 negatively regulates the enzyme, since the use of Hsp60 siRNA increased the sphingomyelinase enzymatic activity and consequently increased Cer levels [148].
Altogether, the abovementioned examples suggest that cell survival is guaranteed in part due to an intricate cooperative association of molecular chaperones and the SLs biosynthesis pathway (Figure 2B). Additional consequences of increased levels of Cer in human cells due to HS have yet to be investigated, as well as the effect of cold stress on these molecules.
5. Temperature impacts on other lipid molecules
Lysophospholipids (LPLs) are bioactive molecules usually produced by phospholipases’ activity on SLs or glycerophospholipids [149, 150]. In general, LPLs are simple lipids characterized by a single carbon chain and a polar head group. Depending on their base backbone, LPLs can be classified in two subcategories: lysosphingolipids (sphingoid base backbone) or lysoglycerophospholipids (glycerol backbone). Lysophosphatidic acid (LPA), lysophosphatidylcholine (LPC), lysophosphatidylinositol (LPI) and lysophosphatidylethanolamine (LPE) are the most common lysoglycerophospholipids. Amongst the LPLs derived from the sphingoid backbone, the abovementioned Sph-1P stands out, but other species still lack deeper characterization such as sphingosylphosphorylcholine (SPC) [150–152]. LPA and Sph-1P are the best characterized LPLs so far [153, 154], and it is known that they act in different cellular processes, such as transmembrane signals through G-protein-coupled receptors (GPCRs), regulation of cell survival, differentiation, cytokine secretion, proliferation, cytoskeletal architecture, cell–cell contact and adhesion, Ca2+ homeostasis and Ca2+-dependent functions [155–157]. The broad distribution of these bioactive molecules points to their relevance in many physiological processes in animals, such as lymphocyte trafficking, vascularization and functioning of the nervous system [158–161].
Recent studies have suggested a role for LPLs during thermotolerance in several organisms in addition to those previously mentioned for Sph-1P. Both the HS and the constant high temperature growth condition lead to an increase in the synthesis of LPE and a decrease in the levels of phosphatidylethanolamine in the pathogenic and psychrotrophic bacteria Yersinia pseudotuberculosis [162]. HS also increases the LPE content in Escherichia coli [163]. In addition, Kern and colleagues showed in vitro that different E. coli LPLs can assist the refolding and solubilization of chemically unfolded proteins [163]. Furthermore, the same authors observed that LPLs possess chaperone-like properties, since LPE, LPC, LPI and LPA prevented the aggregation of the protein citrate synthase at 42 °C [163]. These results hint that in addition to participating in the remodeling of the bacteria plasma membrane, LPLs help to recover the structure of proteins during HS.
Vesicles of S. cerevisiae exporting cargo molecules from ER to Golgi are also rich in LPLs [164]. The protein Sec12 is an important regulator of the formation of these vesicles [164]. Interestingly, the overexpression of a phospholipase B (Plb3), which increases the cellular levels of LPLs, rescued the temperature-sensitive SEC12 mutant phenotype, restoring growth and protein transport at non-permissive temperatures [164]. This finding suggests that LPLs are preponderant for the yeast vesicular transport, especially at higher temperatures.
Moreover, heat stress is known to induce phospholipase activity and to increase intracellular levels of phosphatidic acid in tobacco BY-2 cells, A. thaliana and rice seedlings [165]. In another perspective, the membrane lipids also work as second messengers for heat stress-induced signaling [166]. Mishkind and coworkers showed that after a HS (changing from 20–25 °C to 35 °C) of 20 minutes, the phosphatidylinositol phosphate kinase (PIPK) and the phospholipase D (PLD) are activated, which leads to the accumulation of PIP2 and phosphatidic acid [165]. The accumulation of PIP2 then culminate in the inositol-1,4,5-trisphosphate (IP3) formation, which controls the release of intracellular Ca2+ and activates transcription of HS genes [167]. This reaction is another example of how plants lipids help to promote the response to temperature stress. Accordingly, the knockout of the phospholipase C NPC1 in A. thaliana increased the sensitivity to HS, reaffirming the involvement of these enzymes in the response to heat [168]. In fact, increased activity of several phospholipases under heat stress has also been reported in fungi, invertebrates, mammals and human cells [165, 169–172]. The phospholipase A2 (PLA2) is responsible for increasing the levels of LPI and LPC in HeLa cells in the first minutes of HS, by mechanisms other than through Ca2+ mobilization [173]. Accordingly, in the algae Chlamydomonas reinhardtii, HS repressed the gene expression of LPLs acyltransferases, which metabolize LPLs into phospholipids [174]. In contrast, long-term heat stress caused a decrease in the concentration of LPC in the milk of cows, which may affect its nutritional value [175]. Together, these results suggest that LPLs participate in the homeostatic response to HS.
LPLs receptors also play an important role on MAPK signaling pathways during heat stress. Studying mouse myoblast cells C2C12, Chei and coworkers reported that LPA is recognized by LPA-receptors present in the cell membrane, which activates GPCRs [176]. This activation induces the extracellular signal-regulated kinase (ERK) activity, which starts the inflammatory response, increasing the secretion of cytokines and the expression of glutathione reductase and catalase for the oxidative stress response [176]. This specific example shows how lipid molecules connect the detection of thermal stress with signal-mediated cellular responses in order to maintain cellular homeostasis.
Based on the thermosensitive properties of these lipids, several recent researches have been dedicated to the construction of temperature sensitive liposomes for the delivery of different types of molecules, such as drugs and therapeutic agents, especially for cancer therapy [177–180]. These thermosensitive liposomes are bilayers of LPLs and other lipids organized in the form of vesicles containing encapsulated drugs of interest that can be released in solid tumors when the compromised area is exposed to heating [181]. This disturbance alters the melting phase transition of the liposome lipid bilayer, increasing its permeability and facilitating the leakage of the internal content [182]. This is therefore another example of how the knowledge of the behavior of lipids during thermal variations can be useful in the discovery of new and promising strategies for the medicine field.
In addition, different studies have shown that LPLs metabolism often links to the expression and activity of the Hsp70 chaperone. For instance, Blondeau and colleagues showed that LPI treatment induces Hsp70 expression in the brain of rats, assisting the process of acquiring tolerance and protection against cerebral ischemia [183]. Besides that, Hsp70 from MLE12 murine lung epithelial cells also physically interacts with the LPA receptor 1, a protein that regulates cell proliferation [184]. Moreover, human Hsp70 was able to activate the phospholipase A2 (PLA2) in vitro, which again connects LPLs metabolism with the HS response [185].
LPLs synthesis also seems to be triggered by cold stress. Psychrophilic yeasts such as Cryptococcus vishniacii and Dioszegia cryoxerica naturally synthesize more LPE and LPC than the mesophile S. cerevisiae, but the potential modulatory roles that these molecules may play still wait investigation [186]. Similar to HS, cold stress causes the accumulation of phosphatidic acid and increases the activity of phospholipases in plants, in addition to increase the phospholipid proportion, especially of the LPLs species, and these changes also interfere in the activity of the plasma membrane Ca2+ channels [187, 188]. In agreement, a mutation in the gene encoding LPA acyltransferase 1 (LPAT1) suppressed the sensitivity of A. thaliana fatty acid biosynthesis mutants to low temperature [189]. Considering that LPAT1 impairment favors the accumulation of LPLs, this result suggests that LPLs cope with cold tolerance. Interestingly, Andersson and coworkers observed that LPLs positively modulate the activity of a coldsensitive ion channel of Chinese hamster ovary cells, indicating that these molecules may have signaling roles as cold sensors in mammalian cells [190].
6. Concluding remarks
In this review, we gathered evidences that the temperature stress causes a significant change in the SLs content of different organisms and cellular types. The levels of Cer and its derivatives are induced by heat stress in fungi, plants, animals and human cells. In yeast, these molecules are formed firstly through the de novo pathway and later from the hydrolysis of complex SLs, in a constant and coordinated enzymatic balance. However, IPC and MIPC are also increased when yeasts grow continuously at a high temperature. During cold stress, in turn, much less is known, but the few investigations suggest a decrease in the levels of the simplest SLs and an increase in the synthesis of the more complex ones. When it comes to plants, the LCB-Ps are likely to be the most important for the response to both heat and cold stresses, while the concentration of the other SLs seems to decrease, especially during long cold acclimation. In animals in general, the levels of Cer, SM and Sph-1P seem to be constantly balanced to deal with HS, while the complex SLs (gangliosides, for example) are determinant for cold adaptation.
The studies have also shown that SLs then act as temperature stress sensors, linking an essential metabolic process to a range of different cellular functions necessary for the temperature stress response. Among these functions, the process of programmed cell death signaled mainly by the levels of Cer species upon HS seems to be the most conserved from fungi to humans. In addition, fungi SLs are also involved in processes such as cell cycle arrest, translation initiation, trehalose biosynthesis, proteolysis and calcium signaling during the HS response. Beyond that, temperature stress is generally associated with oxidative stress, and a clear example of this relationship is that plants SLs have been shown to be important in regulating the response to cold stress in partnership with NO metabolism. In animals, in turn, several researches show that HSPs activity is more related to SLs than previously thought, including in humans. LPLs also respond to temperature stress and assist the adaptive response signaling.
With the advancement of molecular biology, bioinformatics and biochemical techniques, new discoveries will help to elucidate the gaps between the SLs pathway and the cellular processes it influences during temperature stress. As a whole, further studies about the relationship between lipid molecules and the adaptation to environmental temperature will be fundamental in the health and economics fields, where they could aid the treatment of diseases and the improvement of production processes, respectively. In addition, in view of the climate changes arising from global warming, these investigations also acquire importance for the adoption of strategies to protect endangered species, as well as for the control of the emergence and dispersion of pathogenic microorganisms.
Acknowledgements
We thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for the Research Internship Abroad (BEPE 2018/22755-7) fellowship awarded to JHTMF.
Funding
This work was supported by NIH grants AI136934, AI116420, and AI125770, and by the Merit Review Grant I01BX002924 from the Veterans Affairs Program to MDP. Maurizio Del Poeta is a Burroughs Welcome Investigator in Infectious Diseases. This study was also supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2017/19694-3 and FAPESP 2016/07870-9) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 462383/2014-8) to IM.
Abbreviations:
- Cer
ceramides
- COR
cold-regulated
- DHC
dihydroceramide
- DHS
dihydrosphingosine
- DHS-1P
dihydrosphingosine-1-phosphate
- ER
endoplasmic reticulum
- GalCer
galactosylceramide
- GIPC
glycosylinositol phosphoryl ceramide
- GlcCer
glucosylceramide
- GPCRs
G-protein-coupled receptors
- HS
heat shock
- HSFs
heat shock transcription factors
- HSPs
heat shock proteins
- IPC
inositol phosphorylceramide
- LCBs
long chain bases
- LCB-Ps
long chain base phosphates
- LPLs
lysophospholipids
- LPA
lysophosphatidic acid
- LPC
lysophosphatidylcholine
- LPE
lysophosphatidylethanolamine
- LPI
lysophosphatidylinositol
- MAPK
mitogen-activated protein kinase
- MIPC
mannosylinositol phosphorylceramide
- M(IP)2C
mannosyldiinositol phosphoryl ceramide
- NO
nitric oxide
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PHC
phytoceramide
- PHS
phytosphingosine
- PHS-1P
phytosphingosine-1-phosphate
- ROS
reactive oxygen species
- SLs
sphingolipids
- SM
sphingomyelin
- SPC
sphingosylphosphorylcholine
- Sph
sphingosine
- Sph-1P
sphingosine 1-phosphate
- SPT
serine palmitoyltransferase
- STRE
stress response element
Footnotes
Conflict of interest
Dr. Maurizio Del Poeta, M.D. is a Co-Founder and Chief Scientific Officer (CSO) of MicroRid Technologies Inc.
References
- [1].Bartke N, Hannun YA, Bioactive sphingolipids: metabolism and function, J Lipid Res 50 Suppl (2009) S91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Simons K, Sampaio JL, Membrane organization and lipid rafts, Cold Spring Harb Perspect Biol 3(10) (2011) a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fernandes CM, Goldman GH, Del Poeta M, Biological Roles Played by Sphingolipids in Dimorphic and Filamentous Fungi, mBio 9(3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gault CR, Obeid LM, Hannun YA, An overview of sphingolipid metabolism: from synthesis to breakdown, Adv Exp Med Biol 688 (2010) 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zauner S, Ternes P, Warnecke D, Biosynthesis of sphingolipids in plants (and some of their functions), Adv Exp Med Biol 688 (2010) 249–63. [DOI] [PubMed] [Google Scholar]
- [6].Richter K, Haslbeck M, Buchner J, The heat shock response: life on the verge of death, Mol Cell 40(2) (2010) 253–66. [DOI] [PubMed] [Google Scholar]
- [7].Gomez-Pastor R, Burchfiel ET, Thiele DJ, Regulation of heat shock transcription factors and their roles in physiology and disease, Nat Rev Mol Cell Biol 19(1) (2018) 4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Balogi Z, Multhoff G, Jensen TK, Lloyd-Evans E, Yamashima T, Jaattela M, Harwood JL, Vigh L, Hsp70 interactions with membrane lipids regulate cellular functions in health and disease, Prog Lipid Res 74 (2019) 18–30. [DOI] [PubMed] [Google Scholar]
- [9].Toth ME, Vigh L, Santha M, Alcohol stress, membranes, and chaperones, Cell Stress Chaperones 19(3) (2014) 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shi Y, Ding Y, Yang S, Molecular Regulation of CBF Signaling in Cold Acclimation, Trends Plant Sci 23(7) (2018) 623–637. [DOI] [PubMed] [Google Scholar]
- [11].Ernst R, Ballweg S, Levental I, Cellular mechanisms of physicochemical membrane homeostasis, Curr Opin Cell Biol 53 (2018) 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Okuyama H, Saito M, Joshi VC, Gunsberg S, Wakil SJ, Regulation by temperature of the chain length of fatty acids in yeast, J Biol Chem 254(24) (1979) 12281–4. [PubMed] [Google Scholar]
- [13].Leach MD, Cowen LE, Membrane fluidity and temperature sensing are coupled via circuitry comprised of Ole1, Rsp5, and Hsf1 in Candida albicans, Eukaryot Cell 13(8) (2014) 1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shapiro RS, Cowen LE, Thermal Control of Microbial Development and Virulence: Molecular Mechanisms of Microbial Temperature Sensing, 3(5) (2012) e00238–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ballweg S, Ernst R, Control of membrane fluidity: the OLE pathway in focus, Biol Chem 398(2) (2017) 215–228. [DOI] [PubMed] [Google Scholar]
- [16].Klose C, Surma MA, Gerl MJ, Meyenhofer F, Shevchenko A, Simons K, Flexibility of a eukaryotic lipidome--insights from yeast lipidomics, PLoS One 7(4) (2012) e35063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sakaki T, Zahringer U, Warnecke DC, Fahl A, Knogge W, Heinz E, Sterol glycosides and cerebrosides accumulate in Pichia pastoris, Rhynchosporium secalis and other fungi under normal conditions or under heat shock and ethanol stress, Yeast 18(8) (2001) 679–95. [DOI] [PubMed] [Google Scholar]
- [18].Desrivieres S, Cooke FT, Parker PJ, Hall MN, MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae, J Biol Chem 273(25) (1998) 15787–93. [DOI] [PubMed] [Google Scholar]
- [19].Ogawa S, Tachimoto H, Kaga T, Elevation of ceramide in Acetobacter malorum S24 by low pH stress and high temperature stress, J Biosci Bioeng 109(1) (2010) 32–6. [DOI] [PubMed] [Google Scholar]
- [20].Sollich M, Yoshinaga MY, Hausler S, Price RE, Hinrichs KU, Buhring SI, Heat Stress Dictates Microbial Lipid Composition along a Thermal Gradient in Marine Sediments, Front Microbiol 8 (2017) 1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rietveld A, Neutz S, Simons K, Eaton S, Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains, J Biol Chem 274(17) (1999) 12049–54. [DOI] [PubMed] [Google Scholar]
- [22].Jenkins GM, Richards A, Wahl T, Mao C, Obeid L, Hannun Y, Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae, J Biol Chem 272(51) (1997) 32566–72. [DOI] [PubMed] [Google Scholar]
- [23].Friant S, Zanolari B, Riezman H, Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis, EMBO J 19(12) (2000) 2834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Plesofsky NS, Levery SB, Castle SA, Brambl R, Stress-induced cell death is mediated by ceramide synthesis in Neurospora crassa, Eukaryot Cell 7(12) (2008) 2147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dickson RC, Nagiec EE, Skrzypek M, Tillman P, Wells GB, Lester RL, Sphingolipids are potential heat stress signals in Saccharomyces, J Biol Chem 272(48) (1997) 30196–200. [DOI] [PubMed] [Google Scholar]
- [26].Tereshina VM, Memorskaia AS, Kotlova ER, [Lipid metabolism in Aspergillus niger under conditions of heat shock], Mikrobiologiia 82(5) (2013) 528–33. [PubMed] [Google Scholar]
- [27].Dickson RC, Sumanasekera C, Lester RL, Functions and metabolism of sphingolipids in Saccharomyces cerevisiae, Prog Lipid Res 45(6) (2006) 447–65. [DOI] [PubMed] [Google Scholar]
- [28].Casadevall A, Don’t Forget the Fungi When Considering Global Catastrophic Biorisks, Health Secur 15(4) (2017) 341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Verghese J, Abrams J, Wang Y, Morano KA, Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system, Microbiol Mol Biol Rev 76(2) (2012) 115–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wells GB, Dickson RC, Lester RL, Heat-induced elevation of ceramide in Saccharomyces cerevisiae via de novo synthesis, J Biol Chem 273(13) (1998) 7235–43. [DOI] [PubMed] [Google Scholar]
- [31].Chen PW, Fonseca LL, Hannun YA, Voit EO, Coordination of rapid sphingolipid responses to heat stress in yeast, PLoS Comput Biol 9(5) (2013) e1003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meier KD, Deloche O, Kajiwara K, Funato K, Riezman H, Sphingoid base is required for translation initiation during heat stress in Saccharomyces cerevisiae, Mol Biol Cell 17(3) (2006) 1164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cowart LA, Hannun YA, Selective substrate supply in the regulation of yeast de novo sphingolipid synthesis, J Biol Chem 282(16) (2007) 12330–40. [DOI] [PubMed] [Google Scholar]
- [34].Cowart LA, Okamoto Y, Lu X, Hannun YA, Distinct roles for de novo versus hydrolytic pathways of sphingolipid biosynthesis in Saccharomyces cerevisiae, Biochem J 393(Pt 3) (2006) 733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Montefusco DJ, Chen L, Matmati N, Lu S, Newcomb B, Cooper GF, Hannun YA, Lu X, Distinct signaling roles of ceramide species in yeast revealed through systematic perturbation and systems biology analyses, Sci Signal 6(299) (2013) rs14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen PW, Fonseca LL, Hannun YA, Voit EO, Dynamics of the Heat Stress Response of Ceramides with Different Fatty-Acyl Chain Lengths in Baker’s Yeast, PLoS Comput Biol 11(8) (2015) e1004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rezanka T, Kolouchova I, Gharwalova L, Dolezalova J, Nedbalova L, Sigler K, Sphingolipidomics of Thermotolerant Yeasts, Lipids 53(6) (2018) 627–639. [DOI] [PubMed] [Google Scholar]
- [38].Lester RL, Withers BR, Schultz MA, Dickson RC, Iron, glucose and intrinsic factors alter sphingolipid composition as yeast cells enter stationary phase, Biochim Biophys Acta 1831(4) (2013) 726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tanji M, Kinoshita M, Yada H, Yamane M, Kakuta Y, Motoshima H, Oda Y, Ohnishi M, Effects of Growth Temperature on Cerebroside Content and Chemical Composition in Kluyveromyces lactis, Journal of Oleo Science 53(3) (2004) 127–33. [Google Scholar]
- [40].Patton JL, Srinivasan B, Dickson RC, Lester RL, Phenotypes of sphingolipid-dependent strains of Saccharomyces cerevisiae, Journal of bacteriology 174(22) (1992) 7180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zanolari B, Friant S, Funato K, Sutterlin C, Stevenson BJ, Riezman H, Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae, EMBO J 19(12) (2000) 2824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Randez-Gil F, Prieto JA, Rodriguez-Puchades A, Casas J, Sentandreu V, Estruch F, Myriocin-induced adaptive laboratory evolution of an industrial strain of Saccharomyces cerevisiae reveals its potential to remodel lipid composition and heat tolerance, Microb Biotechnol (2020) 1066–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dawson K, Toone WM, Jones N, Wilkinson CR, Loss of regulators of vacuolar ATPase function and ceramide synthesis results in multidrug sensitivity in Schizosaccharomyces pombe, Eukaryot Cell 7(6) (2008) 926–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Feoktistova A, Magnelli P, Abeijon C, Perez P, Lester RL, Dickson RC, Gould KL, Coordination between fission yeast glucan formation and growth requires a sphingolipase activity, Genetics 158(4) (2001) 1397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fernandes CM, de Castro PA, Singh A, Fonseca FL, Pereira MD, Vila TV, Atella GC, Rozental S, Savoldi M, Del Poeta M, Goldman GH, Kurtenbach E, Functional characterization of the Aspergillus nidulans glucosylceramide pathway reveals that LCB Delta8-desaturation and C9-methylation are relevant to filamentous growth, lipid raft localization and Psd1 defensin activity, Molecular microbiology 102(3) (2016) 488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mao C, Saba JD, Obeid LM, The dihydrosphingosine-1-phosphate phosphatases of Saccharomyces cerevisiae are important regulators of cell proliferation and heat stress responses, Biochem J 342 Pt 3 (1999) 667–75. [PMC free article] [PubMed] [Google Scholar]
- [47].Birchwood CJ, Saba JD, Dickson RC, Cunningham KW, Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation, J Biol Chem 276(15) (2001) 11712–8. [DOI] [PubMed] [Google Scholar]
- [48].Fox DS, Cruz MC, Sia RA, Ke H, Cox GM, Cardenas ME, Heitman J, Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans, Mol Microbiol 39(4) (2001) 835–49. [DOI] [PubMed] [Google Scholar]
- [49].Kraus PR, Nichols CB, Heitman J, Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth, Eukaryot Cell 4(6) (2005) 1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J, Calcineurin is required for virulence of Cryptococcus neoformans, EMBO J 16(10) (1997) 2576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mao C, Obeid LM, Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate, Biochim Biophys Acta 1781(9) (2008) 424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Roelants FM, Breslow DK, Muir A, Weissman JS, Thorner J, Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae, Proc Natl Acad Sci U S A 108(48) (2011) 19222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sun Y, Miao Y, Yamane Y, Zhang C, Shokat KM, Takematsu H, Kozutsumi Y, Drubin DG, Orm protein phosphoregulation mediates transient sphingolipid biosynthesis response to heat stress via the Pkh-Ypk and Cdc55-PP2A pathways, Mol Biol Cell 23(12) (2012) 2388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Daquinag A, Fadri M, Jung SY, Qin J, Kunz J, The yeast PH domain proteins Slm1 and Slm2 are targets of sphingolipid signaling during the response to heat stress, Mol Cell Biol 27(2) (2007) 633–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fabri J, Godoy NL, Rocha MC, Munshi M, Cocio TA, von Zeska Kress MR, Fill TP, da Cunha AF, Del Poeta M, Malavazi I, The AGC Kinase YpkA Regulates Sphingolipids Biosynthesis and Physically Interacts With SakA MAP Kinase in Aspergillus fumigatus, Front Microbiol 9 (2018) 3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cowart LA, Gandy JL, Tholanikunnel B, Hannun YA, Sphingolipids mediate formation of mRNA processing bodies during the heat-stress response of Saccharomyces cerevisiae, Biochem J 431(1) (2010) 31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chung N, Jenkins G, Hannun YA, Heitman J, Obeid LM, Sphingolipids signal heat stress-induced ubiquitin-dependent proteolysis, J Biol Chem 275(23) (2000) 17229–32. [DOI] [PubMed] [Google Scholar]
- [58].Chung N, Mao C, Heitman J, Hannun YA, Obeid LM, Phytosphingosine as a specific inhibitor of growth and nutrient import in Saccharomyces cerevisiae, J Biol Chem 276(38) (2001) 35614–21. [DOI] [PubMed] [Google Scholar]
- [59].Jenkins GM, Hannun YA, Role for de novo sphingoid base biosynthesis in the heat-induced transient cell cycle arrest of Saccharomyces cerevisiae, J Biol Chem 276(11) (2001) 8574–81. [DOI] [PubMed] [Google Scholar]
- [60].Petzold EW, Himmelreich U, Mylonakis E, Rude T, Toffaletti D, Cox GM, Miller JL, Perfect JR, Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans, Infect Immun 74(10) (2006) 5877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Puttikamonkul S, Willger SD, Grahl N, Perfect JR, Movahed N, Bothner B, Park S, Paderu P, Perlin DS, Cramer RA Jr., Trehalose 6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pathogen Aspergillus fumigatus, Mol Microbiol 77(4) (2010) 891–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Friant S, Meier KD, Riezman H, Increased ubiquitin-dependent degradation can replace the essential requirement for heat shock protein induction, EMBO J 22(15) (2003) 3783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cowart LA, Okamoto Y, Pinto FR, Gandy JL, Almeida JS, Hannun YA, Roles for sphingolipid biosynthesis in mediation of specific programs of the heat stress response determined through gene expression profiling, J Biol Chem 278(32) (2003) 30328–38. [DOI] [PubMed] [Google Scholar]
- [64].Puig-Castellvi F, Bedia C, Alfonso I, Pina B, Tauler R, Deciphering the Underlying Metabolomic and Lipidomic Patterns Linked to Thermal Acclimation in Saccharomyces cerevisiae, J Proteome Res 17(6) (2018) 2034–2044. [DOI] [PubMed] [Google Scholar]
- [65].Lopez-Malo M, Chiva R, Rozes N, Guillamon JM, Phenotypic analysis of mutant and overexpressing strains of lipid metabolism genes in Saccharomyces cerevisiae: implication in growth at low temperatures, Int J Food Microbiol 162(1) (2013) 26–36. [DOI] [PubMed] [Google Scholar]
- [66].Lopez-Malo M, Garcia-Rios E, Chiva R, Guillamon JM, Functional analysis of lipid metabolism genes in wine yeasts during alcoholic fermentation at low temperature, Microb Cell 1(11) (2014) 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Prieto JA, Estruch F, Corcoles-Saez I, Del Poeta M, Rieger R, Stenzel I, Randez-Gil F, Pho85 and PI(4,5)P2 regulate different lipid metabolic pathways in response to cold, Biochim Biophys Acta Mol Cell Biol Lipids 1865(2) (2020) 158557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhai P, Song J, Gao L, Lu L, A sphingolipid synthesis-related protein OrmA in Aspergillus fumigatus is responsible for azole susceptibility and virulence, Cell Microbiol 21(12) (2019) e13092. [DOI] [PubMed] [Google Scholar]
- [69].Garcia-Marques S, Randez-Gil F, Dupont S, Garre E, Prieto JA, Sng1 associates with Nce102 to regulate the yeast Pkh-Ypk signalling module in response to sphingolipid status, Biochim Biophys Acta 1863(6 Pt A) (2016) 1319–33. [DOI] [PubMed] [Google Scholar]
- [70].Aaronson LR, Martin CE, Temperature-induced modifications of glycosphingolipids in plasma membranes of Neurospora crassa, Biochim Biophys Acta 735(2) (1983) 252–8. [DOI] [PubMed] [Google Scholar]
- [71].Tian Y, Zhao GY, Fang W, Xu Q, Tan RX, Delta10(E)-Sphingolipid Desaturase Involved in Fusaruside Mycosynthesis and Stress Adaptation in Fusarium graminearum, Sci Rep 5 (2015) 10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rezanka T, Kolouchova I, Sigler K, Lipidomic analysis of psychrophilic yeasts cultivated at different temperatures, Biochim Biophys Acta 1861(11) (2016) 1634–1642. [DOI] [PubMed] [Google Scholar]
- [73].Verant ML, Bohuski EA, Richgels KLD, Olival KJ, Epstein JH, Blehert DS, Determinants of Pseudogymnoascus destructans within bat hibernacula: implications for surveillance and management of white-nose syndrome, J Appl Ecol 55 (2018) 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kipp E, Heat stress effects on growth and development in three ecotypes of varying latitude of Arabidopsis, Appl. Ecol. Env. Res. 6 (2008) 1–14. [Google Scholar]
- [75].Hatfield JL, Prueger JH, Temperature extremes: effect on plant growth and development, Environmental Science 10 (2015) 4–10. [Google Scholar]
- [76].Ali U, Li H, Wang X, Guo L, Emerging Roles of Sphingolipid Signaling in Plant Response to Biotic and Abiotic Stresses, Mol Plant 11(11) (2018) 1328–1343. [DOI] [PubMed] [Google Scholar]
- [77].Chueasiri C, Chunthong K, Pitnjam K, Chakhonkaen S, Sangarwut N, Sangsawang K, Suksangpanomrung M, Michaelson LV, Napier JA, Muangprom A, Rice ORMDL controls sphingolipid homeostasis affecting fertility resulting from abnormal pollen development, PLoS One 9(9) (2014) e106386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mittler R, Finka A, Goloubinoff P, How do plants feel the heat?, Trends Biochem Sci 37(3) (2012) 118–25. [DOI] [PubMed] [Google Scholar]
- [79].Hossain MA, Li ZG, Hoque TS, Burritt DJ, Fujita M, Munne-Bosch S, Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: key regulators and possible mechanisms, Protoplasma 255(1) (2018) 399–412. [DOI] [PubMed] [Google Scholar]
- [80].Grover A, Mittal D, Negi M, Lavania D, Generating high temperature tolerant transgenic plants: Achievements and challenges, Plant Sci 205–206 (2013) 38–47. [DOI] [PubMed] [Google Scholar]
- [81].Alden KP, Dhondt-Cordelier S, McDonald KL, Reape TJ, Ng CK, McCabe PF, Leaver CJ, Sphingolipid long chain base phosphates can regulate apoptotic-like programmed cell death in plants, Biochem Biophys Res Commun 410(3) (2011) 574–80. [DOI] [PubMed] [Google Scholar]
- [82].Mandala SM, Thornton R, Tu Z, Kurtz MB, Nickels J, Broach J, Menzeleev R, Spiegel S, Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response, Proc Natl Acad Sci U S A 95(1) (1998) 150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Barrero-Gil J, Salinas J, Gene Regulatory Networks Mediating Cold Acclimation: The CBF Pathway, Adv Exp Med Biol 1081 (2018) 3–22. [DOI] [PubMed] [Google Scholar]
- [84].Hall TD, Chastain DR, Horn PJ, Chapman KD, Choinski JS Jr., Changes during leaf expansion of PhiPSII temperature optima in Gossypium hirsutum are associated with the degree of fatty acid lipid saturation, J Plant Physiol 171(6) (2014) 411–20. [DOI] [PubMed] [Google Scholar]
- [85].Awasthi R, Bhandari K, Nayyar H, Temperature stress and redox homeostasis in agricultural crops, Front Environ Sci 3(11) (2015) 11. [Google Scholar]
- [86].Alonso A, Queiroz CS, Magalhaes AC, Chilling stress leads to increased cell membrane rigidity in roots of coffee (Coffea arabica L.) seedlings, Biochim Biophys Acta 1323(1) (1997) 75–84. [DOI] [PubMed] [Google Scholar]
- [87].Wang K, Yin XR, Zhang B, Grierson D, Xu CJ, Chen KS, Transcriptomic and metabolic analyses provide new insights into chilling injury in peach fruit, Plant Cell Environ 40(8) (2017) 1531–1551. [DOI] [PubMed] [Google Scholar]
- [88].Nagano M, Ishikawa T, Ogawa Y, Iwabuchi M, Nakasone A, Shimamoto K, Uchimiya H, Kawai-Yamada M, Arabidopsis Bax inhibitor-1 promotes sphingolipid synthesis during cold stress by interacting with ceramide-modifying enzymes, Planta 240(1) (2014) 77–89. [DOI] [PubMed] [Google Scholar]
- [89].Tarazona P, Feussner K, Feussner I, An enhanced plant lipidomics method based on multiplexed liquid chromatography-mass spectrometry reveals additional insights into cold- and drought-induced membrane remodeling, Plant J 84(3) (2015) 621–33. [DOI] [PubMed] [Google Scholar]
- [90].Degenkolbe T, Giavalisco P, Zuther E, Seiwert B, Hincha DK, Willmitzer L, Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana, Plant J 72(6) (2012) 972–82. [DOI] [PubMed] [Google Scholar]
- [91].Bohn M, Luthje S, Sperling P, Heinz E, Dorffling K, Plasma membrane lipid alterations induced by cold acclimation and abscisic acid treatment of winter wheat seedlings differing in frost resistance, J Plant Physiol 164(2) (2007) 146–56. [DOI] [PubMed] [Google Scholar]
- [92].Uemura M, Steponkus PL, A Contrast of the Plasma Membrane Lipid Composition of Oat and Rye Leaves in Relation to Freezing Tolerance, Plant Physiol 104(2) (1994) 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Palta JP, Whitaker BD, Weiss LS, Plasma Membrane Lipids Associated with Genetic Variability in Freezing Tolerance and Cold Acclimation of Solanum Species, Plant Physiol 103(3) (1993) 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kawaguchi M, Imai H, Naoe M, Yasui Y, Ohnishi M, Cerebrosides in grapevine leaves: distinct composition of sphingoid bases among the grapevine species having different tolerances to freezing temperature, Biosci Biotechnol Biochem 64(6) (2000) 1271–3. [DOI] [PubMed] [Google Scholar]
- [95].Guillas I, Guellim A, Reze N, Baudouin E, Long chain base changes triggered by a short exposure of Arabidopsis to low temperature are altered by AHb1 non-symbiotic haemoglobin overexpression, Plant Physiol Biochem 63 (2013) 191–5. [DOI] [PubMed] [Google Scholar]
- [96].Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M, Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity, Plant Cell 16(10) (2004) 2785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Dutilleul C, Chavarria H, Reze N, Sotta B, Baudouin E, Guillas I, Evidence for ACD5 ceramide kinase activity involvement in Arabidopsis response to cold stress, Plant Cell Environ 38(12) (2015) 2688–97. [DOI] [PubMed] [Google Scholar]
- [98].Cantrel C, Vazquez T, Puyaubert J, Reze N, Lesch M, Kaiser WM, Dutilleul C, Guillas I, Zachowski A, Baudouin E, Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana, New Phytol 189(2) (2011) 415–27. [DOI] [PubMed] [Google Scholar]
- [99].Dutilleul C, Benhassaine-Kesri G, Demandre C, Reze N, Launay A, Pelletier S, Renou JP, Zachowski A, Baudouin E, Guillas I, Phytosphingosine-phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling, New Phytol 194(1) (2012) 181–91. [DOI] [PubMed] [Google Scholar]
- [100].Huang X, Zhang Y, Zhang X, Shi Y, Long-chain base kinase1 affects freezing tolerance in Arabidopsis thaliana, Plant Sci 259 (2017) 94–103. [DOI] [PubMed] [Google Scholar]
- [101].Lachaud C, Da Silva D, Cotelle V, Thuleau P, Xiong TC, Jauneau A, Briere C, Graziana A, Bellec Y, Faure JD, Ranjeva R, Mazars C, Nuclear calcium controls the apoptotic-like cell death induced by d-erythro-sphinganine in tobacco cells, Cell Calcium 47(1) (2010) 92–100. [DOI] [PubMed] [Google Scholar]
- [102].Chen M, Markham JE, Cahoon EB, Sphingolipid Delta8 unsaturation is important for glucosylceramide biosynthesis and low-temperature performance in Arabidopsis, Plant J 69(5) (2012) 769–81. [DOI] [PubMed] [Google Scholar]
- [103].Li HX, Xiao Y, Cao LL, Yan X, Li C, Shi HY, Wang JW, Ye YH, Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots, PLoS One 8(9) (2013) e73380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Takahashi D, Imai H, Kawamura Y, Uemura M, Lipid profiles of detergent resistant fractions of the plasma membrane in oat and rye in association with cold acclimation and freezing tolerance, Cryobiology 72(2) (2016) 123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Minami A, Fujiwara M, Furuto A, Fukao Y, Yamashita T, Kamo M, Kawamura Y, Uemura M, Alterations in detergent-resistant plasma membrane microdomains in Arabidopsis thaliana during cold acclimation, Plant Cell Physiol 50(2) (2009) 341–59. [DOI] [PubMed] [Google Scholar]
- [106].Detournay O, Weis VM, Role of the sphingosine rheostat in the regulation of cnidarian-dinoflagellate symbioses, Biol Bull 221(3) (2011) 261–9. [DOI] [PubMed] [Google Scholar]
- [107].Kitchen SA, Weis VM, The sphingosine rheostat is involved in the cnidarian heat stress response but not necessarily in bleaching, J Exp Biol 220(Pt 9) (2017) 1709–1720. [DOI] [PubMed] [Google Scholar]
- [108].Gerbal M, Fournier P, Barry P, Mariller M, Odier F, Devauchelle G, Duonor-Cerutti M, Adaptation of an insect cell line of Spodoptera frugiperda to grow at 37 degrees C: characterization of an endodiploid clone, In Vitro Cell Dev Biol Anim 36(2) (2000) 117–24. [DOI] [PubMed] [Google Scholar]
- [109].Kakela R, Mattila M, Hermansson M, Haimi P, Uphoff A, Paajanen V, Somerharju P, Vornanen M, Seasonal acclimatization of brain lipidome in a eurythermal fish (Carassius carassius) is mainly determined by temperature, Am J Physiol Regul Integr Comp Physiol 294(5) (2008) R1716–28. [DOI] [PubMed] [Google Scholar]
- [110].Yabu T, Imamura S, Yamashita M, Okazaki T, Identification of Mg2+ -dependent neutral sphingomyelinase 1 as a mediator of heat stress-induced ceramide generation and apoptosis, J Biol Chem 283(44) (2008) 29971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kappel T, Hilbig R, Rahmann H, Variability in brain ganglioside content and composition of endothermic mammals, heterothermic hibernators and ectothermic fishes, Neurochem Int 22(6) (1993) 555–66. [DOI] [PubMed] [Google Scholar]
- [112].Kappel T, Anken RH, Hanke W, Rahmann H, Gangliosides affect membrane-channel activities dependent on ambient temperature, Cell Mol Neurobiol 20(5) (2000) 579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rahmann H, Jonas U, Kappel T, Hilderbrandt H, Differential involvement of gangliosides versus phospholipids in the process of temperature adaptation in vertebrates. A comparative phenomenological and physicochemical study, Ann N Y Acad Sci 845 (1998) 72–91. [DOI] [PubMed] [Google Scholar]
- [114].Zehmer JK, Hazel JR, Thermally induced changes in lipid composition of raft and non-raft regions of hepatocyte plasma membranes of rainbow trout, J Exp Biol 208(Pt 22) (2005) 4283–90. [DOI] [PubMed] [Google Scholar]
- [115].Palmerini CA, Mazzoni M, Giovinazzo G, Arienti G, Blood lipids in Antarctic and in temperate-water fish species, J Membr Biol 230(3) (2009) 125–31. [DOI] [PubMed] [Google Scholar]
- [116].Kalo D, Roth Z, Involvement of the sphingolipid ceramide in heat-shock-induced apoptosis of bovine oocytes, Reprod Fertil Dev 23(7) (2011) 876–88. [DOI] [PubMed] [Google Scholar]
- [117].Roth Z, Hansen PJ, Sphingosine 1-phosphate protects bovine oocytes from heat shock during maturation, Biol Reprod 71(6) (2004) 2072–8. [DOI] [PubMed] [Google Scholar]
- [118].Chang Y, Abe A, Shayman JA, Ceramide formation during heat shock: a potential mediator of alpha B-crystallin transcription, Proc Natl Acad Sci U S A 92(26) (1995) 12275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Buzzard KA, Giaccia AJ, Killender M, Anderson RL, Heat shock protein 72 modulates pathways of stress-induced apoptosis, J Biol Chem 273(27) (1998) 17147–53. [DOI] [PubMed] [Google Scholar]
- [120].Kozawa O, Niwa M, Matsuno H, Tokuda H, Miwa M, Ito H, Kato K, Uematsu T, Sphingosine 1-phosphate induces heat shock protein 27 via p38 mitogen-activated protein kinase activation in osteoblasts, J Bone Miner Res 14(10) (1999) 1761–7. [DOI] [PubMed] [Google Scholar]
- [121].Mathieson FA, Nixon GF, Sphingolipids differentially regulate mitogen-activated protein kinases and intracellular Ca2+ in vascular smooth muscle: effects on CREB activation, Br J Pharmacol 147(4) (2006) 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Megidish T, Takio K, Titani K, Iwabuchi K, Hamaguchi A, Igarashi Y, Hakomori S, Endogenous substrates of sphingosine-dependent kinases (SDKs) are chaperone proteins: heat shock proteins, glucose-regulated proteins, protein disulfide isomerase, and calreticulin, Biochemistry 38(11) (1999) 3369–78. [DOI] [PubMed] [Google Scholar]
- [123].Megidish T, White T, Takio K, Titani K, Igarashi Y, Hakomori S, The signal modulator protein 14–3-3 is a target of sphingosine- or N,N-dimethylsphingosine-dependent kinase in 3T3(A31) cells, Biochem Biophys Res Commun 216(3) (1995) 739–47. [DOI] [PubMed] [Google Scholar]
- [124].Chen S, Bawa D, Besshoh S, Gurd JW, Brown IR, Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain, J Neurosci Res 81(4) (2005) 522–9. [DOI] [PubMed] [Google Scholar]
- [125].Wang R, Kovalchin JT, Muhlenkamp P, Chandawarkar RY, Exogenous heat shock protein 70 binds macrophage lipid raft microdomain and stimulates phagocytosis, processing, and MHC-II presentation of antigens, Blood 107(4) (2006) 1636–42. [DOI] [PubMed] [Google Scholar]
- [126].Harada Y, Sato C, Kitajima K, Complex formation of 70-kDa heat shock protein with acidic glycolipids and phospholipids, Biochem Biophys Res Commun 353(3) (2007) 655–60. [DOI] [PubMed] [Google Scholar]
- [127].Niimura Y, Moue T, Takahashi N, Nagai K, Modification of sphingoglycolipids and sulfolipids in kidney cell lines under heat stress: activation of monohexosylceramide synthesis as a ceramide scavenger, Glycobiology 20(6) (2010) 710–7. [DOI] [PubMed] [Google Scholar]
- [128].Zhu H, Yoshimoto T, Yamashima T, Heat shock protein 70.1 (Hsp70.1) affects neuronal cell fate by regulating lysosomal acid sphingomyelinase, J Biol Chem 289(40) (2014) 27432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kirkegaard T, Roth AG, Petersen NH, Mahalka AK, Olsen OD, Moilanen I, Zylicz A, Knudsen J, Sandhoff K, Arenz C, Kinnunen PK, Nylandsted J, Jaattela M, Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology, Nature 463(7280) (2010) 549–53. [DOI] [PubMed] [Google Scholar]
- [130].Petersen NH, Kirkegaard T, Olsen OD, Jaattela M, Connecting Hsp70, sphingolipid metabolism and lysosomal stability, Cell Cycle 9(12) (2010) 2305–9. [DOI] [PubMed] [Google Scholar]
- [131].Toyomura K, Saito T, Emori S, Matsumoto I, Kato E, Kaneko M, Okuma Y, Nakamura H, Murayama T, Effects of Hsp90 inhibitors, geldanamycin and its analog, on ceramide metabolism and cytotoxicity in PC12 cells, J Toxicol Sci 37(5) (2012) 1049–57. [DOI] [PubMed] [Google Scholar]
- [132].Xu Z, You W, Zhou Y, Chen W, Wang Y, Shan T, Cold-induced lipid dynamics and transcriptional programs in white adipose tissue, BMC Biol 17(1) (2019) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Marcher AB, Loft A, Nielsen R, Vihervaara T, Madsen JG, Sysi-Aho M, Ekroos K, Mandrup S, RNA-Seq and Mass-Spectrometry-Based Lipidomics Reveal Extensive Changes of Glycerolipid Pathways in Brown Adipose Tissue in Response to Cold, Cell Rep 13(9) (2015) 2000–13. [DOI] [PubMed] [Google Scholar]
- [134].Bouma HR, Kroese FG, Kok JW, Talaei F, Boerema AS, Herwig A, Draghiciu O, van Buiten A, Epema AH, van Dam A, Strijkstra AM, Henning RH, Low body temperature governs the decline of circulating lymphocytes during hibernation through sphingosine-1-phosphate, Proc Natl Acad Sci U S A 108(5) (2011) 2052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kondo T, Matsuda T, Kitano T, Takahashi A, Tashima M, Ishikura H, Umehara H, Domae N, Uchiyama T, Okazaki T, Role of c-jun expression increased by heat shock- and ceramide-activated caspase-3 in HL-60 cell apoptosis. Possible involvement of ceramide in heat shock-induced apoptosis, J Biol Chem 275(11) (2000) 7668–76. [DOI] [PubMed] [Google Scholar]
- [136].Jenkins GM, Cowart LA, Signorelli P, Pettus BJ, Chalfant CE, Hannun YA, Acute activation of de novo sphingolipid biosynthesis upon heat shock causes an accumulation of ceramide and subsequent dephosphorylation of SR proteins, J Biol Chem 277(45) (2002) 42572–8. [DOI] [PubMed] [Google Scholar]
- [137].Moulin M, Carpentier S, Levade T, Arrigo AP, Potential roles of membrane fluidity and ceramide in hyperthermia and alcohol stimulation of TRAIL apoptosis, Apoptosis 12(9) (2007) 1703–20. [DOI] [PubMed] [Google Scholar]
- [138].Aktas O, Kury P, Kieseier B, Hartung HP, Fingolimod is a potential novel therapy for multiple sclerosis, Nat Rev Neurol 6(7) (2010) 373–82. [DOI] [PubMed] [Google Scholar]
- [139].Skrzeczynska-Moncznik J, Bzowska M, Nogiec A, Sroka A, Zarebski M, Vallieres L, Guzik K, Rapid externalization of 27-kDa heat shock protein (HSP27) and atypical cell death in neutrophils treated with the sphingolipid analog drug FTY720, J Leukoc Biol 98(4) (2015) 591–9. [DOI] [PubMed] [Google Scholar]
- [140].Kleinjung T, Arndt O, Feldmann HJ, Bockmuhl U, Gehrmann M, Zilch T, Pfister K, Schonberger J, Marienhagen J, Eilles C, Rossbacher L, Multhoff G, Heat shock protein 70 (Hsp70) membrane expression on head-and-neck cancer biopsy-a target for natural killer (NK) cells, Int J Radiat Oncol Biol Phys 57(3) (2003) 820–6. [DOI] [PubMed] [Google Scholar]
- [141].Shevtsov M, Balogi Z, Khachatryan W, Gao H, Vigh L, Multhoff G, Membrane-Associated Heat Shock Proteins in Oncology: From Basic Research to New Theranostic Targets, Cells 9(5) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Gehrmann M, Liebisch G, Schmitz G, Anderson R, Steinem C, De Maio A, Pockley G, Multhoff G, Tumor-specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3, PLoS One 3(4) (2008) e1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G, The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells, J Immunol 172(2) (2004) 972–80. [DOI] [PubMed] [Google Scholar]
- [144].Escriba PV, Busquets X, Inokuchi J, Balogh G, Torok Z, Horvath I, Harwood JL, Vigh L, Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment, Prog Lipid Res 59 (2015) 38–53. [DOI] [PubMed] [Google Scholar]
- [145].Ahn JH, Ko YG, Park WY, Kang YS, Chung HY, Seo JS, Suppression of ceramide-mediated apoptosis by HSP70, Mol Cells 9(2) (1999) 200–6. [PubMed] [Google Scholar]
- [146].Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B, Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis, Mol Cell Biol 17(9) (1997) 5317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Myung SJ, Yoon JH, Kim BH, Lee JH, Jung EU, Lee HS, Heat shock protein 90 inhibitor induces apoptosis and attenuates activation of hepatic stellate cells, J Pharmacol Exp Ther 330(1) (2009) 276–82. [DOI] [PubMed] [Google Scholar]
- [148].Ahn KH, Kim SK, Choi JM, Jung SY, Won JH, Back MJ, Fu Z, Jang JM, Ha HC, Kim DK, Identification of Heat Shock Protein 60 as a Regulator of Neutral Sphingomyelinase 2 and Its Role in Dopamine Uptake, PLoS One 8(6) (2013) e67216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Torkhovskaya TI, Ipatova OM, Zakharova TS, Kochetova MM, Khalilov EM, Lysophospholipid receptors in cell signaling, Biochemistry (Mosc) 72(2) (2007) 125–31. [DOI] [PubMed] [Google Scholar]
- [150].D’Arrigo P, Servi S, Synthesis of lysophospholipids, Molecules 15(3) (2010) 1354–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Kabarowski JH, Zhu K, Le LQ, Witte ON, Xu Y, Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A, Science 293(5530) (2001) 702–5. [DOI] [PubMed] [Google Scholar]
- [152].Ge D, Yue HW, Liu HH, Zhao J, Emerging roles of sphingosylphosphorylcholine in modulating cardiovascular functions and diseases, Acta Pharmacol Sin 39(12) (2018) 1830–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kihara Y, Mizuno H, Chun J, Lysophospholipid receptors in drug discovery, Exp Cell Res 333(2) (2015) 171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Suckau O, Gross I, Schrotter S, Yang F, Luo J, Wree A, Chun J, Baska D, Baumgart J, Kano K, Aoki J, Brauer AU, LPA1, LPA2, LPA4, and LPA6 receptor expression during mouse brain development, Dev Dyn 248(5) (2019) 375–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Anliker B, Chun J, Lysophospholipid G protein-coupled receptors, J Biol Chem 279(20) (2004) 20555–8. [DOI] [PubMed] [Google Scholar]
- [156].Binder BY, Williams PA, Silva EA, Leach JK, Lysophosphatidic Acid and Sphingosine-1-Phosphate: A Concise Review of Biological Function and Applications for Tissue Engineering, Tissue Eng Part B Rev 21(6) (2015) 531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Tigyi G, Parrill AL, Molecular mechanisms of lysophosphatidic acid action, Prog Lipid Res 42(6) (2003) 498–526. [DOI] [PubMed] [Google Scholar]
- [158].Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, Uemura A, Kiyonari H, Abe T, Fukamizu A, Hirashima M, Sawa H, Aoki J, Ishii M, Mochizuki N, The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice, J Clin Invest 122(4) (2012) 1416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Poitevin S, Cussac D, Leroyer AS, Albinet V, Sarlon-Bartoli G, Guillet B, Hubert L, Andrieu-Abadie N, Couderc B, Parini A, Dignat-George F, Sabatier F, Sphingosine kinase 1 expressed by endothelial colony-forming cells has a critical role in their revascularization activity, Cardiovasc Res 103(1) (2014) 121–30. [DOI] [PubMed] [Google Scholar]
- [160].O’Sullivan S, Dev KK, Sphingosine-1-phosphate receptor therapies: Advances in clinical trials for CNS-related diseases, Neuropharmacology 113(Pt B) (2017) 597–607. [DOI] [PubMed] [Google Scholar]
- [161].Zhao Y, Hasse S, Zhao C, Bourgoin SG, Targeting the autotaxin - Lysophosphatidic acid receptor axis in cardiovascular diseases, Biochem Pharmacol 164 (2019) 74–81. [DOI] [PubMed] [Google Scholar]
- [162].Davydova L, Bakholdina S, Barkina M, Velansky P, Bogdanov M, Sanina N, Effects of elevated growth temperature and heat shock on the lipid composition of the inner and outer membranes of Yersinia pseudotuberculosis, Biochimie 123 (2016) 103–9. [DOI] [PubMed] [Google Scholar]
- [163].Kern R, Joseleau-Petit D, Chattopadhyay MK, Richarme G, Chaperone-like properties of lysophospholipids, Biochem Biophys Res Commun 289(5) (2001) 1268–74. [DOI] [PubMed] [Google Scholar]
- [164].Melero A, Chiaruttini N, Karashima T, Riezman I, Funato K, Barlowe C, Riezman H, Roux A, Lysophospholipids Facilitate COPII Vesicle Formation, Curr Biol 28(12) (2018) 1950–1958 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Mishkind M, Vermeer JE, Darwish E, Munnik T, Heat stress activates phospholipase D and triggers PIP accumulation at the plasma membrane and nucleus, Plant J 60(1) (2009) 10–21. [DOI] [PubMed] [Google Scholar]
- [166].Torok Z, Crul T, Maresca B, Schutz GJ, Viana F, Dindia L, Piotto S, Brameshuber M, Balogh G, Peter M, Porta A, Trapani A, Gombos I, Glatz A, Gungor B, Peksel B, Vigh L Jr., Csoboz B, Horvath I, Vijayan MM, Hooper PL, Harwood JL, Vigh L, Plasma membranes as heat stress sensors: from lipid-controlled molecular switches to therapeutic applications, Biochim Biophys Acta 1838(6) (2014) 1594–618. [DOI] [PubMed] [Google Scholar]
- [167].Liu HT, Gao F, Cui SJ, Han JL, Sun DY, Zhou RG, Primary evidence for involvement of IP3 in heat-shock signal transduction in Arabidopsis, Cell Res 16(4) (2006) 394–400. [DOI] [PubMed] [Google Scholar]
- [168].Krckova Z, Brouzdova J, Danek M, Kocourkova D, Rainteau D, Ruelland E, Valentova O, Pejchar P, Martinec J, Arabidopsis non-specific phospholipase C1: characterization and its involvement in response to heat stress, Front Plant Sci 6 (2015) 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Calderwood SK, Stevenson MA, Inducers of the heat shock response stimulate phospholipase C and phospholipase A2 activity in mammalian cells, J Cell Physiol 155(2) (1993) 248–56. [DOI] [PubMed] [Google Scholar]
- [170].Koelmel JP, Jones CM, Ulmer CZ, Garrett TJ, Yost RA, Schock TB, Bowden JA, Examining heat treatment for stabilization of the lipidome, Bioanalysis 10(5) (2018) 291–305. [DOI] [PubMed] [Google Scholar]
- [171].Liu YN, Lu XX, Chen D, Lu YP, Ren A, Shi L, Zhu J, Jiang AL, Yu HS, Zhao MW, Phospholipase D and phosphatidic acid mediate heat stress induced secondary metabolism in Ganoderma lucidum, Environ Microbiol 19(11) (2017) 4657–4669. [DOI] [PubMed] [Google Scholar]
- [172].Calderwood SK, Stevenson MA, Price BD, Activation of phospholipase C by heat shock requires GTP analogs and is resistant to pertussis toxin, J Cell Physiol 156(1) (1993) 153–9. [DOI] [PubMed] [Google Scholar]
- [173].Calderwood SK, Bornstein B, Farnum EK, Stevenson MA, Heat shock stimulates the release of arachidonic acid and the synthesis of prostaglandins and leukotriene B4 in mammalian cells, J Cell Physiol 141(2) (1989) 325–33. [DOI] [PubMed] [Google Scholar]
- [174].Legeret B, Schulz-Raffelt M, Nguyen HM, Auroy P, Beisson F, Peltier G, Blanc G, Li-Beisson Y, Lipidomic and transcriptomic analyses of Chlamydomonas reinhardtii under heat stress unveil a direct route for the conversion of membrane lipids into storage lipids, Plant Cell Environ 39(4) (2016) 834–47. [DOI] [PubMed] [Google Scholar]
- [175].Liu Z, Ezernieks V, Wang J, Arachchillage NW, Garner JB, Wales WJ, Cocks BG, Rochfort S, Heat Stress in Dairy Cattle Alters Lipid Composition of Milk, Sci Rep 7(1) (2017) 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Chei S, Song JH, Oh HJ, Lee K, Jin H, Choi SH, Nah SY, Lee BY, Gintonin-Enriched Fraction Suppresses Heat Stress-Induced Inflammation Through LPA Receptor, Molecules 25(5) (2020) 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Al-Ahmady Z, Lozano N, Mei KC, Al-Jamal WT, Kostarelos K, Engineering thermosensitive liposome-nanoparticle hybrids loaded with doxorubicin for heat-triggered drug release, Int J Pharm 514(1) (2016) 133–141. [DOI] [PubMed] [Google Scholar]
- [178].O’Neill HS, Herron CC, Hastings CL, Deckers R, Lopez Noriega A, Kelly HM, Hennink WE, McDonnell CO, O’Brien FJ, Ruiz-Hernandez E, Duffy GP, A stimuli responsive liposome loaded hydrogel provides flexible on-demand release of therapeutic agents, Acta Biomater 48 (2017) 110–119. [DOI] [PubMed] [Google Scholar]
- [179].Wang C, Wang X, Zhong T, Zhao Y, Zhang WQ, Ren W, Huang D, Zhang S, Guo Y, Yao X, Tang YQ, Zhang X, Zhang Q, The antitumor activity of tumor-homing peptide-modified thermosensitive liposomes containing doxorubicin on MCF-7/ADR: in vitro and in vivo, Int J Nanomedicine 10 (2015) 2229–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Woo J, Chiu GN, Karlsson G, Wasan E, Ickenstein L, Edwards K, Bally MB, Use of a passive equilibration methodology to encapsulate cisplatin into preformed thermosensitive liposomes, Int J Pharm 349(1–2) (2008) 38–46. [DOI] [PubMed] [Google Scholar]
- [181].de Matos MBC, Beztsinna N, Heyder C, Fens M, Mastrobattista E, Schiffelers RM, Leneweit G, Kok RJ, Thermosensitive liposomes for triggered release of cytotoxic proteins, Eur J Pharm Biopharm 132 (2018) 211–221. [DOI] [PubMed] [Google Scholar]
- [182].Zhang X, Luckham PF, Hughes AD, Thom S, Xu XY, Towards an understanding of the release behavior of temperature-sensitive liposomes: a possible explanation of the “pseudoequilibrium” release behavior at the phase transition temperature, J Liposome Res 23(3) (2013) 167–73. [DOI] [PubMed] [Google Scholar]
- [183].Blondeau N, Lauritzen I, Widmann C, Lazdunski M, Heurteaux C, A potent protective role of lysophospholipids against global cerebral ischemia and glutamate excitotoxicity in neuronal cultures, J Cereb Blood Flow Metab 22(7) (2002) 821–34. [DOI] [PubMed] [Google Scholar]
- [184].Zhao J, Wei J, Bowser RK, Dong S, Xiao S, Zhao Y, Molecular regulation of lysophosphatidic acid receptor 1 trafficking to the cell surface, Cell Signal 26(11) (2014) 2406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Mahalka AK, Code C, Jahromi BR, Kirkegaard T, Jaattela M, Kinnunen PK, Activation of phospholipase A2 by Hsp70 in vitro, Biochim Biophys Acta 1808(10) (2011) 2569–72. [DOI] [PubMed] [Google Scholar]
- [186].Dalluge JJ, Connell LB, On the potential of mass spectrometry-based metabolite profiling approaches to the study of biochemical adaptation in psychrophilic yeast, Extremophiles 17(6) (2013) 953–61. [DOI] [PubMed] [Google Scholar]
- [187].Vaultier MN, Cantrel C, Vergnolle C, Justin AM, Demandre C, Benhassaine-Kesri G, Cicek D, Zachowski A, Ruelland E, Desaturase mutants reveal that membrane rigidification acts as a cold perception mechanism upstream of the diacylglycerol kinase pathway in Arabidopsis cells, FEBS Lett 580(17) (2006) 4218–23. [DOI] [PubMed] [Google Scholar]
- [188].Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X, Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis, J Biol Chem 277(35) (2002) 31994–2002. [DOI] [PubMed] [Google Scholar]
- [189].Kim HU, Vijayan P, Carlsson AS, Barkan L, Browse J, A mutation in the LPAT1 gene suppresses the sensitivity of fab1 plants to low temperature, Plant Physiol 153(3) (2010) 1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Andersson DA, Nash M, Bevan S, Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids, J Neurosci 27(12) (2007) 3347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]