Abstract
Objective
Non-alcoholic steatohepatitis (NASH) is the most common liver disease in the United States. Phase 3 clinical trials have stringent study criteria which may limit real-world generalizability. Thus, we studied whether a real-world, university-based cohort of patients could be eligible for a pivotal phase 3 NASH clinical trial.
Methods
We queried Yale New Haven Health System electronic medical records for patients with a diagnosis of NASH from 2013 to 2017. Of those who received liver biopsy, we extracted demographic, clinical, laboratory, and biopsy data. We compared patient characteristics to enrollment criteria of the Randomized Global Phase 3 Study to Evaluate the Impact on NASH with Fibrosis of Obeticholic Acid Treatment (REGENERATE).
Results
Of 14,403 patients with NASH, 478 (3.3%) completed liver biopsy, of whom 237 (49.6%) had histological confirmation by a gastrointestinal pathologist. Histologically-confirmed NASH patients were 51.1 ± 13.2 years old, 56.5% female, 69.6% White race, and 24.6% had cirrhosis. In this group, 68 (28.7%) patients met all inclusion criteria, 87 (36.7%) had no exclusions, and 34 (14.4%) met all enrollment criteria. Other than cirrhosis, the most common reasons for ineligibility were presence of medical comorbidity (n = 83) or laboratory abnormalities (n = 47). Multiple logistic regression did not reveal significant predictors of eligibility.
Conclusion
Within a university-based cohort of NASH patients, few met phase 3 clinical trial enrollment criteria, mostly due to low rates of liver biopsy. Of those with histologic confirmation, 14.4% met enrollment criteria. Validation of generalizability for safety and efficacy of NASH investigational agents in real-world populations is needed.
Keywords: Real-world, Generalizability, Clinical Trial, Non-alcoholic steatohepatitis, NASH
Introduction
Nonalcoholic steatohepatitis (NASH) prevalence is growing in tandem with the obesity epidemic, and has now become the most common chronic liver disease in the United States (U.S.) [1]. There have been recent breakthroughs in pharmacologic therapy to address NASH, with numerous clinical trials currently underway to evaluate the efficacy of novel investigational agents to improve steatohepatitis and reverse fibrosis. Phase 3 clinical trials are considered the standard to assess drug safety and efficacy, but some evidence suggests that the stringent inclusion and exclusion criteria of trials may lead to narrow enrollment of patients who are distinct from the intended treatment target population [2–4].
NASH clinical trials require histologically-established disease with liver biopsy, which is considered to be the necessary gold-standard for diagnosis of NASH [5,6]. However, there are limited data on the generalizability of these clinical trial results to the general population with NASH, who are mostly diagnosed by clinical and laboratory criteria without liver biopsy. Among those with biopsy-confirmed NASH, narrow eligibility criteria raise concern for the external validity of safety and efficacy findings to patients failing to meet these criteria or who do not receive liver biopsy. There are also limited current data to describe rates of NASH clinical trial screening, exclusion, and eligibility predictors. Thus, we aimed to characterize a university-based cohort of patients with a clinical and histological diagnosis of NASH and their eligibility for enrollment in a large, phase 3 registration trial.
Methods
Sample Data Source
We performed a cross-sectional study of patients receiving care in the Yale New Haven Health System (YNHHS), a major university health network of five hospitals and affiliated outpatient care sites in Connecticut, New York, and Rhode Island. Patient data were derived from query of the YNHHS Epic (Verona, WI, USA) electronic medical record to identify patients with diagnosis codes of NASH. All reported information was recorded in a de-identified manner. The Yale School of Medicine (New Haven, CT) Institutional Review Board deemed that this study, using secondary data from a de-identified database from chart review, met criteria for exemption.
Case Definitions
Adults aged ≥18 years with a clinical diagnosis of NASH were identified with a composite definition based on the International Classification of Disease, 9th and 10th revision (ICD-9 & 10), from January 1st, 2013 to December 31st, 2017. Patients were included if they had a diagnosis code of K75.81 (“nonalcoholic steatohepatitis”) or K76.0 (“fatty [change of] liver, not elsewhere classified”). Among patients with a diagnosis code for NASH, those receiving a liver biopsy within YNHHS were identified, and the matching pathology reports produced by dedicated gastrointestinal pathologists were assessed for histological characteristics of NASH. Most samples were obtained by ultrasound-guided percutaneous liver biopsies, with some cases of transjugular and intraoperative laparoscopic liver biopsies. Histological diagnosis of NASH was defined by NASH Clinical Research Network (CRN) criteria as the presence of hepatic steatosis (≥5%), lobular inflammation, and hepatocyte ballooning degeneration, and NASH disease activity was quantified with the NAFLD Activity Score (NAS) [7–9]. Biopsy results describing “none”, “mild”, “moderate”, and “severe” were coded as 0, 1, 2, and 3 points for steatosis, lobular inflammation, and hepatocellular ballooning, respectively. For those who completed more than one biopsy during the study period, we evaluated histologic findings of the first biopsy for that patient. We then further analyzed those without biopsy-proven NASH to evaluate who underwent a subsequent percutaneous liver biopsy to determine whether the second sample confirmed NASH.
Patient Covariates and Eligibility Criteria
From patients with histologically-diagnosed NASH, we extracted demographic, clinical, medication, and laboratory data. We compared patient characteristics with the inclusion and exclusion criteria used for enrollment in the Randomized Global Phase 3 Study to Evaluate the Impact on NASH with Fibrosis of Obeticholic Acid Treatment (REGENERATE) study, a pivotal clinical trial evaluating a novel pharmacotherapy for patients with NASH (Clinical Trial Identifier NCT02548351, Supplemental Table 1) [10].
Statistical Analysis
Descriptive statistics were generated for individual and complete enrollment criteria in this patient cohort. We calculated cumulative proportions of inclusion and exclusion from clinical trial enrollment. We compared demographic characteristics between eligible and ineligible patients using independent sample t-tests, chi-square tests, or Fisher exact tests as appropriate. We also performed multiple logistic regression modeling to assess for predictors of eligibility based on cohort demographic characteristics. Statistical significance of all tests was defined as a two-tailed p<0.05. All analyses were performed with SAS 9.4 (Cary, NC, USA) and Microsoft Excel 2016 (Redmond, WA, USA). The institutional review board of Yale University School of Medicine reviewed and approved this study.
Results and Discussion
Sample Size and Characteristics
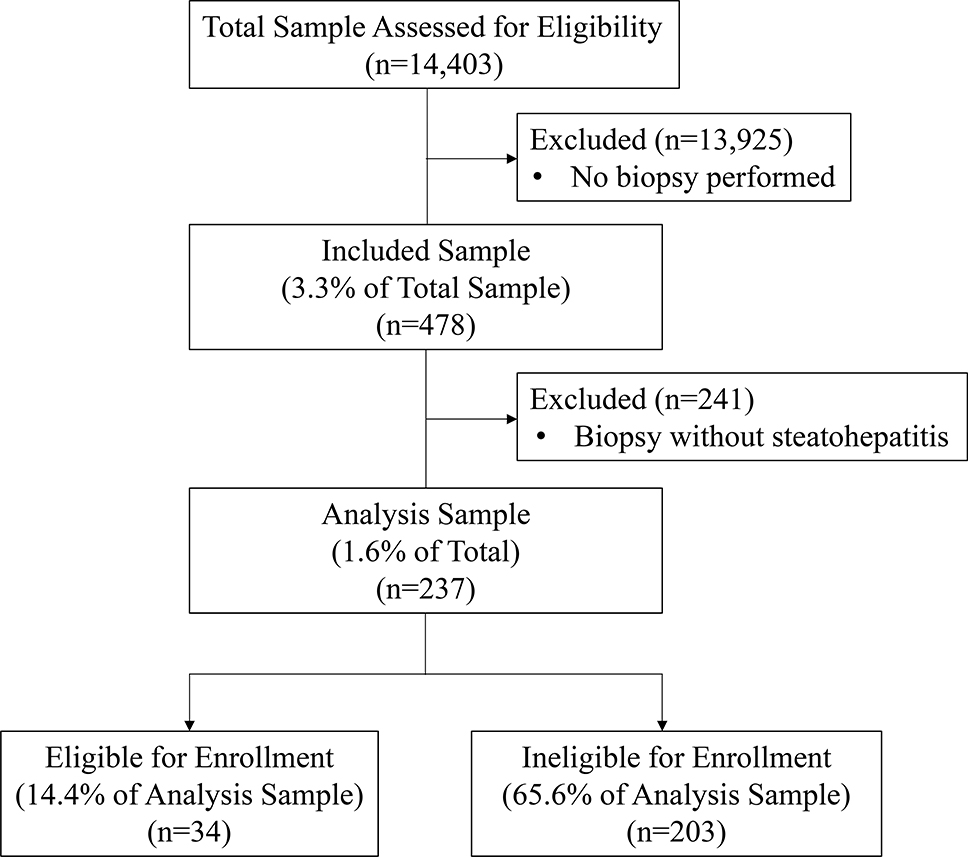
A total of 14,403 patients with NASH were identified with diagnosis codes, of whom 478 patients completed 540 liver biopsies. Of those who completing a liver biopsy, 237 (49.6%) were confirmed to have histologic NASH (Figure 1) based on the presence of all three histological components of NASH. This group had median age 51.0 ± 13.1 years, and comprised 56.4% female, 75.3% white race, and 22.0% Hispanic ethnicity (Table 1). No significant statistical differences were observed between eligible and non-eligible groups. There were 48 patients who underwent a second liver biopsy whose initial biopsy did not confirm NASH. Of these, 8 (16.6%) were subsequently confirmed to have NASH.
Figure 1.
Flow Diagram of Patients for Inclusion in REGENERATE Clinical Trial
Table 1.
Demographic Characteristics of Patients with Biopsy-Proven Nonalcoholic Steatohepatitis, by Enrollment Eligibility Status
| Characteristic | Total (n = 237) | Eligible (n = 34) | Non-Eligible (n = 203) | p-value* |
|---|---|---|---|---|
| Age, mean ± SD | 51.0 ± 13.1 | 51.4 ± 11.6 | 51.0 ± 13.4 | 0.87 |
| Gender, n (%) Female Male |
134 (56.5) 103 (43.5) |
23 (67.7) 11 (32.4) |
111 (54.7) 92 (45.3) |
0.16 |
| Race, n (%) White Black Asian/PI Other |
165 (75.3) 12 (5.5) 10 (4.6) 32 (14.6) |
55 (64.7) 1 (2.9) 2 (5.9) 9 (26.5) |
143 (77.3) 11 (6.0) 8 (4.3) 23 (14.2) |
0.16 |
| Ethnicity, n (%) Hispanic Non-Hispanic |
53 (22.4) 182 (76.8) |
9 (26.5) 25 (73.5) |
44 (21.7) 157 (77.3) |
0.71 |
| Body Mass Index, mean ± SD | 32.6 ± 7.8 | 30.9 ± 5.7 | 32.9 ± 8.1 | 0.09 |
p-value for t-test for continuous variables, chi-square or Fisher’s exact test for categorical variables
Inclusion and Exclusion Criteria
Of the study inclusion criteria, histologic findings on liver biopsy was the most common cause of patient ineligibility (Table 2). Of those who completed a liver biopsy for histologic confirmation of their NASH clinical diagnosis, 40.1% did not have a NAS score ≥ 4. Additionally, 41.8% of patients did not meet hepatic fibrosis criteria (which requires stage 2 or 3 fibrosis or stage 1 with associated comorbidity). Overall, 169 (71.3%) patients were ineligible for clinical trial enrollment based on not meeting all inclusion criteria, independent of the presence of exclusion criteria.
Table 2.
Inclusion Criteria Applied to Patient Sample, for REGENRATE Clinical Trial
| Inclusion Criteria | Inclusion met | Inclusion not met |
|---|---|---|
| Biopsy with all 3 features and NAS Score ≥ 4 | 142 (59.9%) | 95 (40.1%) |
| Stage 2 or 3 fibrosis, or 1 with obesity, DM2, or ALT >1.5x ULN | 138 (58.2%) | 99 (41.8%) |
| Not on, or on stable dose TZD or vitamin E for 6 mo | 234 (98.7%) | 3 (1.3%) |
| Stable body weight (<10% variation for 3 mo) | 224 (94.5%) | 13 (5.5%) |
| Met all inclusion criteria | 68 (28.7%) | 169 (71.3%) |
Acronyms: NAS, nonalcoholic fatty liver disease activity score
In assessment of study exclusion criteria (Table 3), 150 (63.3%) patients met at least one exclusion criteria, resulting in trial ineligibility. The most common reasons for exclusion included the presence of other chronic liver diseases (n=59), histologic cirrhosis (n=58), and serum platelet count <100,000 (n=54). Overall, 87 patients (36.7%) were deemed eligible for enrollment by meeting no exclusion criteria.
Table 3.
Exclusion Criteria Applied to Patient Sample, for REGENERATE Clinical Trial
| Exclusion Criteria | Excluded from Study | Not Excluded |
|---|---|---|
| Significant alcohol use | 25 (10.6%) | 212 (89.5%) |
| Ileal resection of bariatric surgery in antecedent 5 years | 1 (0.4%) | 236 (99.6%) |
| HbA1c > 9.5% within 60 days | 10 (4.2%) | 227 (95.8%) |
| Other chronic liver disease | 59 (24.9%) | 178 (75.1%) |
| Histological cirrhosis | 58 (24.6%) | 179 (75.4%) |
| MELD score > 12 | 9 (3.8%) | 228 (96.2%) |
| Known or suspected hepatocellular carcinoma | 10 (4.2%) | 227 (95.8%) |
| Total bilirubin >1.5 | 21 (8.9%) | 216 (91.1%) |
| Conjugated bilirubin ≥ 1.5 ULN | 21 (8.9%) | 216 (91.1%) |
| AST or ALT ≥ 10x ULN | 5 (2.1%) | 232 (97.9%) |
| Creatinine ≥ 1.5 | 7 (3.0%) | 230 (97.0%) |
| Creatine phosphokinase >5x ULN | 0 (0.0%) | 237 (100.0%) |
| Platelet count < 100,000 | 54 (22.8%) | 183 (77.2%) |
| LDL ≥ 190 on statin or PCSK9 inhibitor | 1 (0.4%) | 236 (99.6%) |
| History of biliary diversion | 0 (0.0%) | 237 (100.0%) |
| HIV Infection | 1 (0.4%) | 236 (99.6%) |
| Significant atherosclerotic cardiovascular disease in past year | 4 (1.7%) | 233 (98.3%) |
| Known substance abuse in past 1 year | 6 (2.5%) | 231 (97.5%) |
| Pregnancy | 0 (0.0%) | 237 (100.0%) |
| Receipt of any investigational product not for DM2, weight loss, or NASH in past 6 months | 2 (0.8%) | 235 (99.2%) |
| Previous exposure to OCA | 0 (0.0%) | 237 (100.0%) |
| Acute cholecystitis or acute biliary obstruction | 0 (0.0%) | 237 (100%) |
| BMI >45 with comorbid high BP, HLD, or DM2 | 7 (3.0%) | 230 (97.1%) |
| Meeting any exclusion criteria | 150 (63.3%) | 87 (36.7%) |
Cause of Clinical Trial Ineligibility
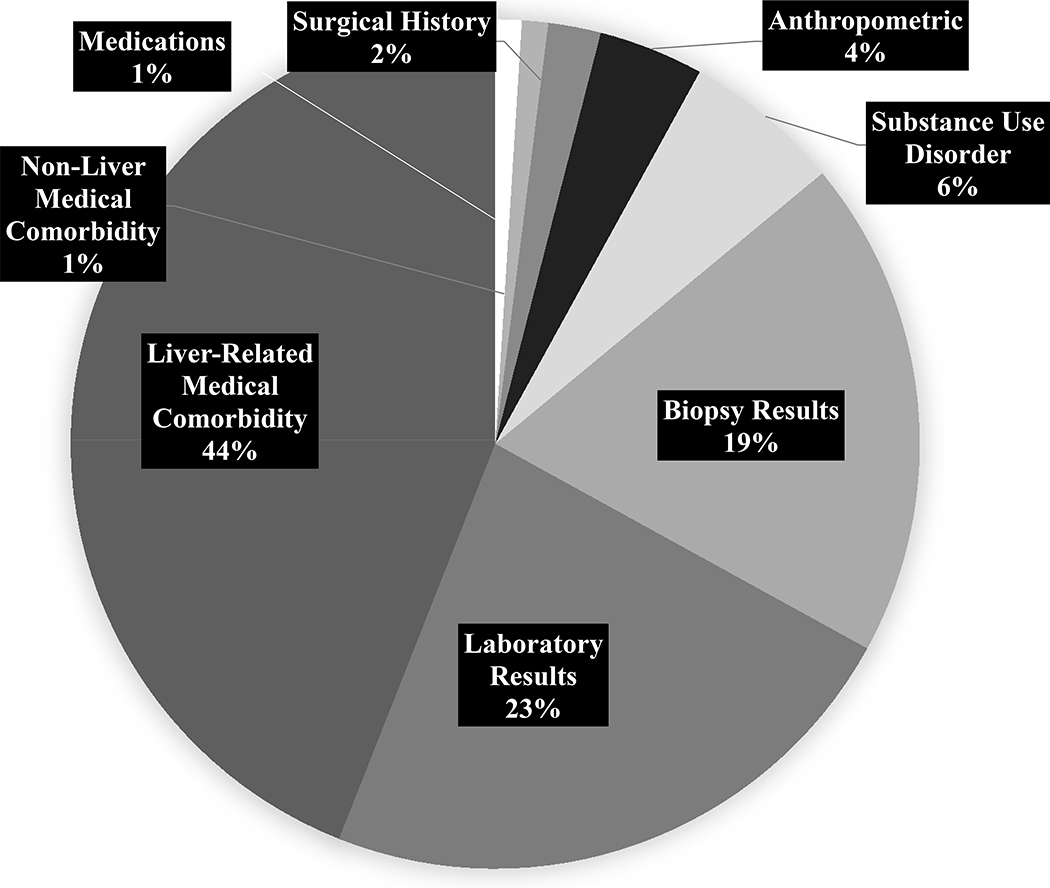
The most common reasons for ineligibility for trial enrollment included liver-related medical comorbidity (44.0%), laboratory abnormalities (23.0%), and histologic findings (19.0%) (Figure 2). Most of this patient sample met some component of the clinical trial exclusion criteria (63.3%). The more common exclusion criteria that applied to our NASH-biopsied patient cohort were evidence of chronic liver disease (24.9%), histological cirrhosis (24.6%), and significant alcohol use (10.6%) (Table 3). Figure 2 summarizes classification of the 511 instances of ineligibility observed in this patient sample.
Figure 2.
Classification of Reasons for Ineligibility for REGENERATE Clinical Trial (n = 511 Instances of Ineligibility)
Enrollment Eligibility and Predictors
Accounting for both inclusion and exclusion criteria, 34 patients (14.4%) of 237 met all enrollment criteria for the REGENERATE trial and thus could enroll. Univariate and multiple logistic regression models revealed no statistically significant demographic predictors of eligibility.
Patients Receiving Second Liver Biopsy
There were 48 patients with first liver biopsy not showing steatohepatitis who received a second ultrasound-guided percutaneous biopsy. Assessment of second biopsy revealed histological NASH in 8 (16.7%) patients. However, these patients also had alternative acute or chronic liver disease (hepatocellular carcinoma, status-post liver transplant for Budd-Chiari, hemochromatosis, concern for drug-related toxic injury) which would have excluded them from eligibility.
Discussion
Within a real-world, academic medical center-based cohort of 14,403 patients with a clinical diagnosis of nonalcoholic steatohepatitis (NASH), we found that a very small fraction completed a liver biopsy, of whom only half were histologically-confirmed to have NASH. Among those with histological confirmation, we found a low trial eligibility rate with only 34 patients (14.4%) meeting all study criteria for enrollment in REGENERATE, a pivotal phase 3 NASH drug trial. These findings reveal several important observations in the research of NASH therapeutics which query whether current clinical trial criteria would be generalizable to their intended target population.
Our findings suggest that need for liver biopsy and multiple criteria for trial enrollment may result in limitations to generalizability for those trials. Co-morbid disease contributed to exclusion of a large proportion of candidates: severe obesity, diabetes, alcohol or other substance use. Nonalcoholic fatty liver disease is considered the hepatic manifestation of the metabolic syndrome, and so metabolic comorbidities would be expected in real-world populations with NASH and may exclude otherwise-treatable NASH patients [11]. Additionally, we found a low rate of minority patients, including Black and Hispanic patients, despite the New Haven population being considered the most representative American city in the United States [12]. Finally, we found that over 40% did not meet strict histologic inclusion criteria even with biopsy, thus suggesting further potential attrition even after screening and liver biopsy in patient who were likely to have NASH but did not capture all histological findings to make a formal diagnosis. This additionally does not account for patients who subsequently would not desire to enroll in a clinical trial, which may occur even if a given patient otherwise meets trial eligibility criteria.
Reliance on liver biopsy to define NASH may result in a biased sample due to limitations of liver biopsy as a gold-standard test for NASH. Liver biopsy, although considered the gold standard test, may suffer from sampling variability, thus biasing enrollment efforts and reducing eligibility rates for those with histological NASH [13]. Although fibrosis staging can be accurately performed with liver biopsy, sampling variability may result in failing to make a formal histological diagnosis of NASH with the presence of all three hallmark findings (steatosis, lobular inflammation, ballooning degeneration) and NAS score >4, needed for trial eligibility.
Confounding by indication for liver biopsy may also result in limited generalizability of eligibility trial patients to the general NASH population. From a clinical standpoint, for most patients suspected of having NASH, a clinical diagnosis is made in the presence of metabolic risk factors after excluding alternative causes of chronic liver disease such as alcoholic steatohepatis, viral hepatitis, and other autoimmune or metabolic diseases. Indication for liver biopsy may greatly vary depending on the provider, the hospital practice, and the regional practices. In our hospital system, liver biopsy is generally reserved for patients in the setting of diagnostic uncertainty or for definitive fibrosis staging. Current guidance from the American Association for the Study of Liver Diseases recommend liver biopsy for those “in whom competing etiologies for hepatic steatosis and the presence and/or severity of coexisting chronic liver diseases cannot be excluded without liver biopsy” [5]. Multinational data is necessary to assess current phase 3 clinical trial criteria including liver biopsy protocols and histological disease confirmation, as population differences cannot be drawn from a single-center study.
Our sample of patients with biopsy-proven disease likely differs from those not receiving liver biopsy, as often there is diagnostic uncertainty resulting in liver biopsy for these patients. Minimum size of liver biopsy acceptable for study eligibility may also be limiting, as biopsies smaller than 25mm may underdiagnose NASH inflammation and fibrosis stage [13]. This is evidenced by nearly one-quarter of our sample excluded due to identification of an alternative liver disease diagnosis, either with co-morbid NASH or as an alternative identified on liver biopsy. The low rate of eligible NASH patients in our study may reflect the true population of individuals who require targeted drug therapy, as compared to the greater population of NAFL patients who would benefit most through weight loss efforts. More than ever, the underlying issue is how to identify true NASH in the real world, and then subsequently design criteria for phase 3 trials based upon those identified characteristics.
It is clearly important that inferences made from clinical trials results be generalizable to the underlying population from which the sample was drawn. Our study suggests that while reliance on a patient sample with histologically-diagnosed NASH may be effective for establishing causality between treatment and clinical outcomes, this method may ultimately lead to decreased effectiveness in the larger population due to poor generalizability of the sample under study to the intended greater population seeking treatment.
Narrow trial enrollment criteria may also have ramifications beyond population generalizability. Patient demographic disparities have been reported in the setting of cancer clinical trials, which may lead to populations having reduced access to therapeutics [14]. In our study, patients were racially diverse in what has been considered a representative American city [12]. However, the prevalence of NASH varies on a global scale due to genetic traits, race, gender, and age differences result in distinct disease risk, which may not be reflected in enrollment of patients solely based on liver biopsy [15–18]. Our study observed that the demographic characteristics between patients were found to be similar between the eligible and non-eligible groups. This may suggest that in these demographic characteristics did not differ in frequency, though this study is likely underpowered to detect small differences.
The prevalence of NAFLD and NASH are expected to increase in the coming years, given the worsening obesity pandemic [18]. As more patients exhibiting typical presentations of metabolic syndrome receive clinical diagnosis for NASH, the growing population will become less represented by clinical studies using histological confirmation of disease. Overall, researchers may benefit from improved accrual rates with less stringent enrollment criterial. Though the FDA recommends liver biopsy confirmation within NASH drug development trials due to concerns for reliable NASH diagnosis and staging, identification and validation of biomarkers can potentially accelerate drug development efforts [19]. Given that any academic institution would be concurrently enrolling in multiple clinical trials, having a sufficient pool of patients from which to draw would likely improve study enrollment rates for NASH patients.
There are several arguments that can be made for inclusion of patients for NASH studies without requirement for histological diagnosis, including better generalizability, improved patient access to research studies, anticipated availability of non-invasive tests to assess for both disease and treatment response, as well as associated risks and sampling variability of liver biopsy. By recruiting patients with clinical diagnosis of NASH, the study sample will more likely represent the desired underlying population. Although current phase 3 studies can help to establish treatment efficacy, removing histological requirement may allow researchers the ability to determine effectiveness in a real-world population of patients receiving NASH diagnosis as would be obtained in a community setting. As efficacy and effectiveness should be viewed as a continuum rather than a dichotomy, enrollment of non-biopsy NASH patients would also shift clinical trial design towards effectiveness assessment [20].
We recommend the inclusion of non-invasive testing for fibrosis in combination with clinical data to better capture the intended target population. We anticipate that with increasing availability and high performance of non-invasive tests for fibrosis including composite indices (NAFLD fibrosis score, FIB-4 index, ELF panel) in combination with imaging modalities (transient elastography and magnetic resonance elastography), liver biopsy will be supplanted for most patients in clinical practice and in future clinical trials [21,22]. This will be especially the case for patients for whom testing can help to identify NASH even without abnormalities on standard liver enzyme testing [23].
This study has some notable limitations. All patients were drawn at a single center at a large academic institution, which may not represent the characteristics of patients in the community or at other academic centers. However, patients being enrolled in clinical trials are likely to derive from academic centers that have inherently similar characteristics to conduct such studies. We assessed enrollment capability in one clinical trial, though a given institution may concurrently be enrolling for multiple studies, potentially increasing patient accrual rates. Additionally, as patients were initially identified through diagnosis codes, and given that there is no established validated diagnosis code composite definition for NASH, some unidentified patients with NASH were likely not included in our study. More patients could potentially be eligible for clinical trial enrollment if a liver biopsy was obtained, but as a result they do not have histologic criteria to be included in this analysis. Furthermore, only a small number of subjects were eligible for study and thus included in multivariable modeling, resulting in limited statistical power. Restriction to adult patients results in inability to make inferences regarding the pediatric population. Underestimation of eligibility rates may occur if those patients who become eligible upon screening have agreed but not yet received liver biopsy, or for whom non-invasive fibrosis markers for stage 2 fibrosis were not performed. Conversely, overestimation may occur with additional exclusion criteria being met during screening such as unwillingness to undergo screening or liver biopsy, or having an anticipated pregnancy precluding birth control use.
However, this study is strengthened by inclusion of a well-characterized cohort of patients with clinically-diagnosed NASH from a major university health network, and by use of real-world, individual-level data. We confirmed NASH diagnosis using histopathologic assessment by gastrointestinal pathologists, thus minimizing the risk for misclassification. The patient sample was unselected, without restriction to those receiving care from gastroenterology or hepatology specialists. Our approach of a population-based patient identification method likely mirrors a similar process by which patients would be identified for potential NASH clinical trial enrollment. The academic medical setting from which the sample was drawn also likely represents the clinical context from which most NASH trial patients are drawn.
Further assessment using electronic medical record methods to facilitate identification of patients with clinically-diagnosed NASH and validation studies are needed. This is particularly important in the context of the need for non-invasive indicators (laboratory tests, composite and predictive scores, and non-invasive imaging) of both NASH diagnosis and as therapeutic endpoints, in lieu of liver biopsy. An additional strategy to exclude patients with alternative chronic liver disease would be incorporating assessment of serological tests of chronic liver disease, as diagnosis of NASH includes exclusion of alternative liver disease. Future efforts may allow application of population-based methods for identifying patients with disease through use of clinical information systems like those developed for other chronic disease such as hypertension, diabetes, and dyslipidemia [24].
Conclusion
Within a major university-based cohort of 14,403 patients with a diagnosis of NASH, only a minority completed a liver biopsy for histologic confirmation and met eligibility criteria for enrollment in a phase 3 NASH trial. Studies on precise non-invasive diagnostic markers of NASH are needed to better inform eligibility criteria for investigational drug trials. As the benefits and risks of NASH therapy may vary in the one in four patients with alternative liver disease identified in this study, further investigations into the effects of treatment in a non-biopsied cohort of patients with potential NASH may be warranted prior to broad usage.
Supplementary Material
Supplemental Table 1. Inclusion and Exclusion Criteria of REGENERATE Study of Patients with Nonalcoholic Steatohepatitis
Acknowledgments
Source of Funding:
YI received support from NIH-NIDDKD student research grant (T35DK104689), the William U. Gardner Memorial Student Research Fellowship, and the Yale School of Medicine Medical Student Research Fellowship. AD is currently receiving NIH grant support (T32 DK007017-41) and support from the Yale Liver Center-Clinical Translational Core Award (P30KD034989). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest:
JKL reports research contracts to Yale University from Allergan, Conatus, Genfit, Gilead, and Intercept, and consulting honoraria from Gilead. For the remaining authors none were declared.
References
- 1.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377(21):2063–2072. [DOI] [PubMed] [Google Scholar]
- 2.Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. Journal of clinical oncology. 2001;19(6):1728–1733. [DOI] [PubMed] [Google Scholar]
- 3.Yamanouchi M, Skupien J, Niewczas MA, et al. Improved clinical trial enrollment criterion to identify patients with diabetes at risk of end-stage renal disease. Kidney Int. 2017;92(1):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kragholm K, Goldstein SA, Yang Q, et al. Trends in enrollment, clinical characteristics, treatment, and outcomes according to age in non-ST-segment-elevation acute coronary syndromes clinical trials. Circulation. 2016;133(16):1560–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 6.Patel YA, Imperial JC, Muir AJ, et al. Baseline Parameters in Clinical Trials for Nonalcoholic Steatohepatitis: Recommendations From the Liver Forum. Gastroenterology. 2017;153(3):621–625. e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. [DOI] [PubMed] [Google Scholar]
- 8.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. [DOI] [PubMed] [Google Scholar]
- 9.Romero-Gómez M, Zelberg-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846. [DOI] [PubMed] [Google Scholar]
- 10.Ratziu V, Sanyal AJ, MacConell L, et al. Regenerate: A Phase 3, Double-Blind, Randomized, Placebo-Controlled Multicenter Study of Obeticholic Acid Therapy for Nonalcoholic Steatohepatitis. Journal of Hepatology. 2016;64(2):S294–S295. [Google Scholar]
- 11.Yki-Jarvinen H Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014. November;2(11):901–10. [DOI] [PubMed] [Google Scholar]
- 12.Kolko J ‘Normal America’ Is Not a Small Town of White People. ABC News FiveThirtyEight; https://fivethirtyeight.com/features/normal-america-is-not-a-small-town-of-white-people/ Accessed November 28, 2019. [Google Scholar]
- 13.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–1906. [DOI] [PubMed] [Google Scholar]
- 14.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–397 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clinical Trials. 2015;12(3):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature genetics. 2008;40(12):1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider AL, Lazo M, Selvin E, Clark JM. Racial differences in nonalcoholic fatty liver disease in the US population. Obesity. 2014;22(1):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Center for Drug Evaluation and Research. Noncirrhotic Nonalcoholic Steatohepatitis with Liver Fibrosis: developing Drugs for Treatment. Dec 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/noncirrhotic-nonalcoholic-steatohepatitis-liver-fibrosis-developing-drugs-treatment Accessed November 28, 2019.
- 20.Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clinical and translational gastroenterology. 2014;5(1):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152(3):598–607. e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedossa P Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60(2):565–75. [DOI] [PubMed] [Google Scholar]
- 23.Portillo-Sanchez P, et al. High prevalence of nonalcoholic fatty liver disease in patients with Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J Clin Endocrinol Metab. 2015; 100(6):22231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. Jama. 2002;288(14):1775–1779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Inclusion and Exclusion Criteria of REGENERATE Study of Patients with Nonalcoholic Steatohepatitis