Abstract
Background:
Skin is the organ most extensively exposed to light of a broad range of wavelengths. Several studies have reported that skin expresses photoreceptive molecules called opsins. However, the identity and functional role of opsins in the human skin remain elusive. We aim to summarize current scientific evidence on the types of opsins expressed in the skin and their biological functions.
Methods:
A primary literature search was conducted using PubMed to identify articles on dermal opsins found in nonhuman animals and humans.
Results:
Twenty-two articles, representing, however, a non-exhaustive selection of the scientific papers published in this specific field, met the inclusion criteria. In nonhuman animals, opsins and opsin-like structures have been detected in the skin of fruit fly, zebrafish, frog, octopus, sea urchin, hogfish, and mouse, and they mediate skin color change, light avoidance, shadow reflex, and circadian photoentrainment. In humans, opsins are present in various skin cell types, including keratinocytes, melanocytes, dermal fibroblasts, and hair follicle cells. They have been shown to mediate wound healing, melanogenesis, hair growth, and skin photoaging.
Conclusion:
Dermal opsins have been identified across many nonhuman animals and humans. Current evidence suggests that opsins have biological significance beyond light reception. In nonhuman animals, opsins are involved in behaviors that are critical for survival. In humans, opsins are involved in various functions of the skin although the underlying molecular mechanisms remain unclear. Future investigation on elucidating the mechanism of dermal opsins will be crucial to expand the therapeutic benefits of photobiomodulation for various skin disorders.
Keywords: dermal photoreceptor, extraocular photoreceptor, hair follicle, keratinocytes, melanocytes, opsin, photobiomodulation, skin
1 |. INTRODUCTION
The concept of dermal photoreception has started to gain wide-spread attention since the 1950s when Steven et al demonstrated that sea lamprey could respond to tail illumination even in the absence of the eyes.1 Shortly thereafter, more evidence from behavioral studies supported the presence of photoreceptive elements in the skin of animals by showing that localized illumination on the skin elicits locomotion (river lamprey, Lampetra fluviatilis, Chordata), skin color change (octopus, Octopus bimaculoides, Mollusca), withdrawal behavior (pond snail, Lymnaea stagnalis, Mollusca), light avoidance (fruit fly, Drosophila melanogaster, Arthropoda), and thermoregulatory behavior (lizard, Podarcis muralis, Chordata).2–9 Continuous investigations in dermal photoreception have identified mitochondrial cytochrome c oxidase (CCO), nitrosated proteins, flavoproteins generating reactive oxygen species (ROS), and light-activated calcium ion channels as endogenous photosensors.10 More recently, opsins, which are key phototransducing molecules found in the retina, have emerged as new photosensors in the skin as several lines of scientific evidence support their expression in both nonhuman animal and human skin.11,12
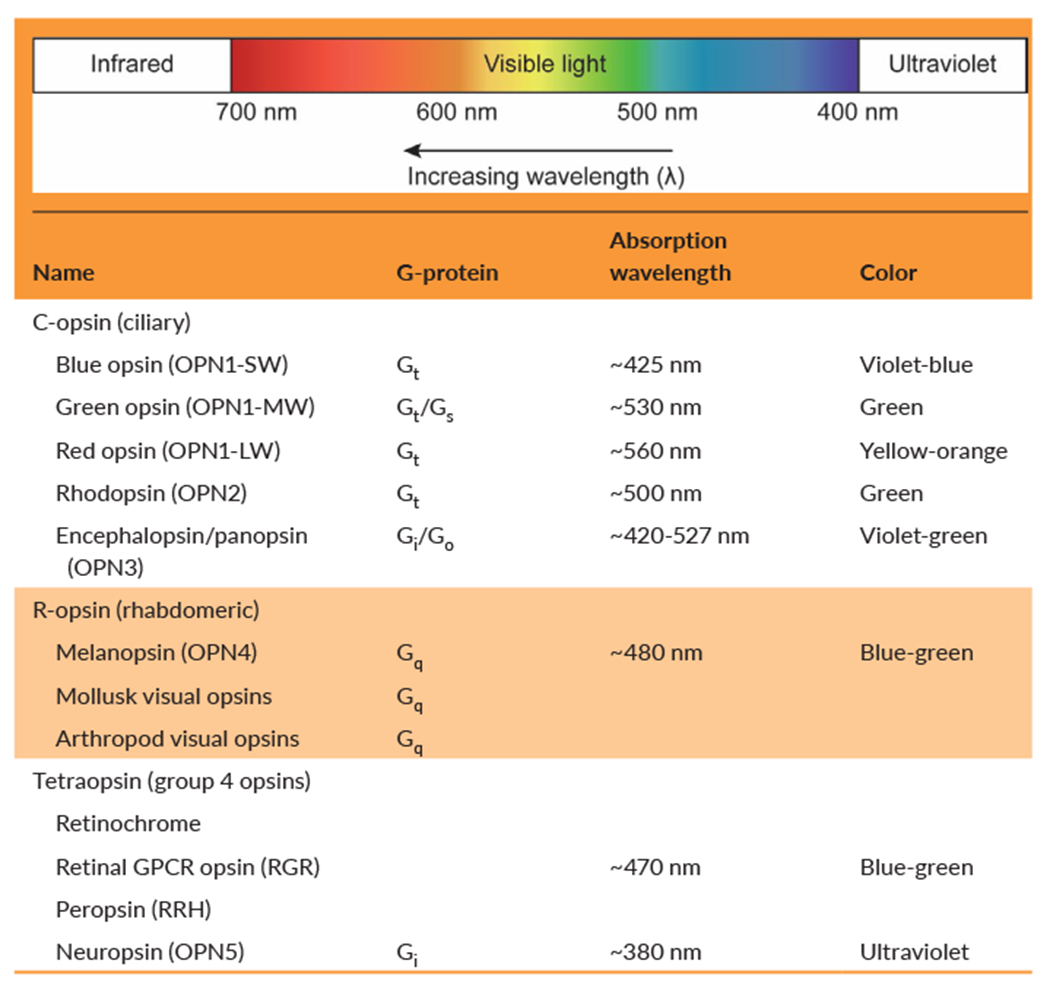
Opsins are a large group of light-sensitive G protein–coupled receptors (GPCRs) that use retina as a ligand and trigger signaling cascades upon distinct wavelength of light. Opsins, primarily found in light-detecting cells such as the retinal photoreceptors, are widely known for their key role in visual transduction.13–15 Opsins have evolved across animal phylogeny, and their diversity can be categorized into three large groups: ciliary opsins (c-opsins), rhabdomeric opsins (r-opsins), and tetraopsins (Table 1).16,17 The c-opsins, which are mostly present in vertebrates, are characterized by their expression in ciliary photoreceptor cells and cyclic nucleotide signaling cascade. On the other hand, r-opsins, which are expressed in invertebrates except melanopsins, are expressed in rhabdomeric photoreceptor cells and have phosphoinositol signaling cascade. Tetraopsins, also known as group 4 opsins, include retinal G protein–coupled receptor opsins (RGR), retinochrome, peropsin (RRH), neuropsin (OPN5), and Go-opsins. In contrast to c-opsins and r-opsins, many tetraopsins are relatively poorly characterized although more recent evidence suggests their functional role in photoisomerization of trans-form to cis-form of retinal.18–20
TABLE 1.
Classification of selected opsins expressed in the skin
 |
Despite a crucial role in vision, a wide array of opsins has been identified in the skin of animals, including humans, suggesting their role as dermal photosensors. Studies in nonhuman animals suggest that dispersed photoreception through dermal opsins enables animals to quickly respond against potential dangers in a wild environment and increase their chance of survival.11,14 On the other hand, very little is known about the opsin expression profiles and physiological functions in human skin.
As photobiomodulation (PBM), a form of light therapy based on nonionizing forms of light sources, is becoming a promising therapeutic approach for the treatment of various dermatological conditions, such as psoriasis, atopic dermatitis, hair regrowth, wound healing, and tissue regeneration, it is particularly important to understand the types of opsins expressed in human skin and their downstream molecular mechanisms.10,21–24 Since each opsin has distinct absorption spectra and signaling transduction, the optimization of light therapy tailored to the features of each opsin will maximize the benefit that PBM can offer in the clinic.
In this review, we aim to examine the types of opsins expressed in human skin cell types and their elucidated role in skin physiology. To provide a background on how dermal opsin research has evolved over time, we will begin with a brief overview of the opsin expression in nonhuman animal skin.10,22,23,25–29
2 |. METHODS
We searched the PubMed database using the following key term combination: “(photoreceptors OR opsin OR opsin-like OR rhodopsin) AND (skin OR dermal OR melanocytes OR keratinocytes OR epidermis).” The search retrieved 484 studies published from August 1951 up to January 2020, and two independent reviewers screened all titles and abstracts in accordance with the Preferred Reporting System for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1). The following exclusion criteria were applied: (a) non–evidence-based studies including review articles, letters, and commentaries; (b) studies performed with non–human-derived immortal cell line; (c) studies written in languages other than English; (d) studies focusing on other primary photosensor molecules, such as cytochrome c oxidase (CCO), nitrosated proteins, flavoproteins, and calcium channels; and (e) studies about extraocular opsins that are not expressed in the skin.
FIGURE 1.
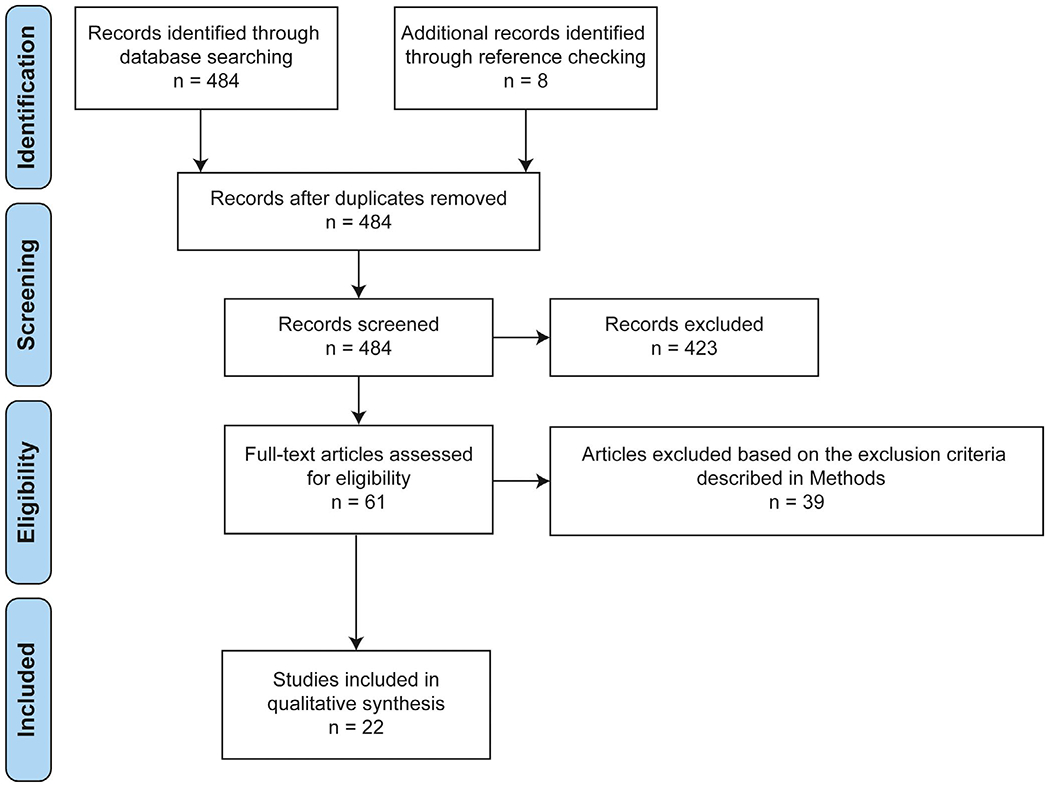
Literature search according to the PRISMA guideline [Colour figure can be viewed at wileyonlinelibrary.com]
On a further note, we acknowledge the potential bias in our literature search by using keywords, “skin” and specific skin cell types, that could result in the exclusion of literature focusing on opsins expressed in the integument of invertebrates.
3 |. RESULTS
A total of 22 studies met our criteria and were included for review. Eleven studies provided the evidence of dermal photoreception and the opsin expression in nonhuman animal skin. Eleven studies describe the opsin expression and their potential function in human skin cell types including keratinocytes, melanocytes, hair follicle cells, and dermal fibroblasts. We organized our findings into two tables (Table 2 on nonhuman animals and Table 3 on humans).
TABLE 2.
List of studies providing evidence of opsin expression in nonhuman animal skin
| Year | Author | Species | Photosensitive protein | Detection method | Potential role | Major finding |
|---|---|---|---|---|---|---|
| 1998 | Provencio et al | Frog (Xenopus laevis) | OPN4 | In situ hybridization, Western blot | Mediates melanosome migration for color change | OPN4 is detected in dermal melanophores |
| 2005 | Isoldi et al | Frog (Xenopus laevis) | OPN4 | RT-qPCR | Mediates melanosome migration for color change | Light activates melanopsin phosphoinositide cascade, resulting in melanosome granule dispersion within melanophores |
| 2010 | Xiang et al | Fruit fly (Drosophila melanogaster) | Gr28b (rhodopsin-like protein) | Electrophysiology, light-activated signaling assay (GCaMP3 imaging) | Mediates light-avoidance behavior | Ablation of class IV dendritic arborization neurons decreases light-avoidance behavior |
| 2010 | Mäthger et al | Cuttlefish (Sepia officinalis) | r-opsin | RT-PCR | Mediates distributed light sensing | r-opsin mRNA is found in the skin |
| 2013 | Ullrich-Lüter et al | Sea urchin (Strongylocentrotus purpuratus) | c-opsin | Immunohistology, in situ hybridization | Mediates shadow reflex | c-opsin mRNA and protein are found in the epidermal cells |
| 2015 | Ramirez et al | Octopus (Octopus bimaculoides) | r-opsin | Immunofluorescence, gene expression analysis | Mediates the skin color change | Isolated skin tissue shows color alteration upon light illumination |
| 2015 | Kingston et al | Octopus, cuttlefish, longfin squid (Doryteuthis pealeii) | r-opsin, RGR | Immunofluorescence, Western blot, RT-qPCR | Mediates skin color change | Phototransduction components of the retina are found in the skin |
| 2015 | Davies et al | Zebrafish (Danio rerio) | OPN5 | RT-PCR | Not shown | OPN5 is detected in the skin |
| 2018 | Schweikert et al | Hogfish (Lachnolaimus maximus) | SWS1 | De novo transcriptome | Mediates color change | Skin expresses distinct phototransduction signaling cascade from that found in the retina |
| 2019 | Delroisse et al | Velvet belly lanternshark (Etmopterus spinax) | OPN3 | De novo transcriptome | Mediates color change | Opsins are differentially expressed in the retina and skin |
| 2019 | Buhr et al | Mouse (mus musculus) | OPN5 | Immunofluorescence, RT-PCR | Synchronizes the circadian rhythm | OPN5 regulates the amplitude of clock gene expression |
Abbreviations: Gqα, Gq α-subunit; Gr28b, gustatory G protein–coupled receptor 28b; OPN3, encephalopsin; OPN4, melanopsin; OPN5, neuropsinRGR, retinochrome; r-opsin, rhabdomeric opsin; RT-PCR, reverse transcriptase PCR; RT-qPCR, reverse transcription-quantitative PCR; SWS1, short-wavelength sensitive opsin.
TABLE 3.
List of studies providing evidence of opsin expression in human skin
| Year | Author | Cell type | Photosensitive protein | Detection method | Potential role | Major finding |
|---|---|---|---|---|---|---|
| 2009 | Tsutsumi et al | Keratinocytes | OPN2, OPN1-LW, OPN1-MW | Immunofluorescence, RT-qPCR | Not shown | First study to identify the expression of rhodopsin-like and opsin-like genes in human keratinocytes |
| 2011 | Wicks et al | Melanocytes | OPN2 | Western blot, immunofluorescence, RT-qPCR | Mediates melanin production | UV radiation induces calcium influx and melanin synthesis in melanocytes; OPN2 knockdown abrogates the effect |
| 2013 | Kim et al | Keratinocytes | OPN2 | Western blot, immunofluorescence | Regulates cell differentiation | Violet light (410 nm) increases the expression of OPN2 mRNA, while decreasing the expression levels of keratinocyte differentiation markers |
| 2015 | Haltaufderhyde et al | Keratinocytes, melanocytes | OPN1-SW, OPN2, OPN3, OPN5 | RT-qPCR, Western blot | Not shown | Various opsin molecules are expressed in epidermal keratinocytes and melanocytes |
| 2017 | Toh et al | Keratinocytes | RRH | Immunofluorescence, RT-qPCR, Western blot | Mediates light-induced phototransduction | Irradiation with 380 nm light elicits intracellular calcium influx in cell culture; RRH knockdown downregulates the genes involved in phototransduction |
| 2017 | Buscone et al | Hair follicle stem cells | OPN2 and OPN3 | Immunofluorescence, RT-qPCR | Mediates hair growth regulation | Blue light (453 nm) activation of hair follicles prolongs anagen hair growth phase. OPN3 knockdown abrogates the effect |
| 2018 | Regazzetti et al | Melanocytes | OPN3 | RT-qPCR | Induces melanin production | Violet light (415 nm) irradiation induces calcium signaling and upregulation of melanogenesis-associated proteins. OPN3 knockdown abrogates the effect |
| 2018 | Pellicena et al | Keratinocytes, dermal fibroblasts | OPN3 | Immunocytochemistry, RT-qPCR | Mediates cutaneous wound healing | Blue light (447 nm) induces early differentiation in keratinocytes culture and activation of OPN3 in keratinocytes OPN3 mRNA increased in irradiated keratinocyte culture in vitro migration assay |
| 2019 | Ozdeslik et al | Melanocytes | OPN3 | RT-qPCR, Western blot | Negatively modulates melanin production | OPN3 inhibits MC1R-mediated cAMP response that leads to melanin production. This regulation is not mediated by calcium-dependent pathway |
| 2019 | Lan et al | Dermal fibroblasts | OPN1–5; focus on OPN3 | RT-qPCR, Western blot | Mediates UVA-induced MMP production and skin photoaging | UVA induces OPN3 and phototransduction as well as an increase in the level of MMP |
| 2020 | Wang et al | Melanocytes | OPN3 | RT-qPCR, Western blot, immunofluorescence | Regulates the survival of melanocytes | Knockdown of OPN3 triggers the apoptosis through a calcium-dependent G protein-coupled signaling and mitochondrial pathway |
Abbreviations: Gqα, Gq α-subunit; MC1R, melanocortin 1 receptor; MMP, matrix metalloprotease; OPN1-LW, long-wavelength sensitive cone opsin; OPN1-MW, medium-wavelength sensitive cone opsin; OPN1-SW, short-wavelength sensitive cone opsin; OPN2, rhodopsin; OPN3, encephalopsin; RGR, retinochrome; RRH, peropsin; RT-qPCR, reverse transcription-quantitative PCR; UVA, ultraviolet A.
3.1 |. The expression and function of dermal opsins in nonhuman animals
The earliest evidence of opsin in the skin is the discovery of melanopsin (OPN4) in dermal melanophores in frogs (Xenopus laevis, Chordata).18,30 In 1998, Provencio et al identified OPN4 in the frog dermal melanophores by in situ hybridization and Western blot analysis. Their goal was to identify the light-detecting molecule that is responsible for melanosome migration, which enables frogs to change their skin colors to maintain body temperature or avoid the predators.30 Despite a discovery of OPN4 in melanophores, whether the OPN4 is the target light-sensing receptor-mediating melanosome migration remained uncertain until 2005. In 2005, Isoldi et al showed that molecular components of OPN4 signaling pathway also exist in the cultured Xenopus dermal melanophores and that light increases the intracellular level of inositol trisphosphate and activity of phospholipase C in dermal melanophores during the melanosome migration, suggesting that OPN4 is the key sensor mediating melanosome dispersion in Xenopus.31
In 2010, Xiang et al identified rhodopsin-like protein called gustatory G protein–coupled receptor 28b (Gr28b) in class IV dendritic neurons on the body surface of fruit fly larvae (Drosophila melanogaster, Arthropoda). Although Gr28b is not exactly an opsin, it has a rhodopsin-like structure and likewise converts the light into electrical signals transmitting to the brain.9 The group found that Gr28b enables Drosophila larvae to sense light over their entire bodies and move away from the light, which is critical for the survival of Drosophila larvae to minimize predation risk since they spend most of the time feeding by digging their heads into food.
In 2010, Mäthger et al detected the mRNA of r-opsin, also known as rhabdomeric opsin responsible for vision in mollusks, in the skin of cuttlefish (Sepia officinalis, Mollusca), suggesting its possible role in dermal photoreception and skin color change mediated by dermal chromatophores.32 In 2015, other groups also detected the expression of r-opsin protein in the skin of several cephalopod species, including cuttlefish, longfin squid (Doryteuthis pealeii, Mollusca), and octopus (Octopus bimaculoides, Mollusca).3,33 Additionally, Kingston et al identified retinochrome, a type of tetraopsin, in the skin of these species. In cephalopod eyes, the retinochrome is known to isomerize all-trans-retinal to 11-cis-retinal upon illumination and contributes to the formation of visual pigments by counterbalancing isomerization of r-opsin.34,35 The role of retinochrome in the skin is not clear, but several lines of evidence reported that retinochrome might be involved in light-activated chromatophore expansion (LACE), which causes skin color change.33
In 2013, Ullrich-Lüter et al reported that purple sea urchin (Strongylocentrotus purpuratus, Echinodermata) expresses c-opsin in the epidermal cells of the body wall.36 The role of c-opsins in the sea urchin remained ambiguous, but authors hypothesized that they might be involved in the so-called shadow reflex, a rapid spine movement in response to overhead shadow. Another body of studies has provided compelling evidence that dermal opsins expressed in zebrafish (Danio rerio) and mice (Mus musculus) contribute to the synchronization of the circadian rhythm.37–39 It has been previously suggested that neuropsin (OPN5) appeared to be expressed in the hypothalamus of birds and contribute to seasonal reproduction.40–43 However, Buhr et al demonstrated that OPN5 is also expressed in mice melanocytes and synchronize the circadian clock of the skin to the light and dark cycle.38 Interestingly, the photic circadian entrainment by OPN5 in the skin was independent of the retina and suprachiasmatic nuclei (SCN).38
More recently, next-generation sequencing has emerged as a useful tool for detecting novel phototransduction genes, allowing the comparison of the differential expression of opsins in the retina and skin, and helps determine whether they share the same evolutionary history. Dissecting the evolutionary history of skin opsins can provide insights into their physiological significance in the skin. The transcriptomic analysis on the skin and retina of hogfish (Lachnolaimus maximus, Chordata) showed that the skin and retina have distinct phototransduction signaling cascade.44 In contrast to two visual opsin genes and cGMP-dependent phototransduction component expression in the retina, only a single short-wavelength opsin (SWS1) and cAMP-dependent phototransduction components were expressed in the skin. The transcriptome analysis on the skin and eye of velvet belly lanternshark (Etmopterus spinax, Chordata), likewise, showed distinct expression pattern in the eye and skin.45 In the eye, rhodopsin (OPN2) and peropsin (RRH) were enriched, whereas in the skin, encephalopsin (OPN3) was most abundant. The transcriptomic data suggest that the molecular mechanism of opsins in the skin may be distinct from that of opsins found in the eye although further investigations need to be conducted.
Here, we showed several evidence of dermal opsins found in nonhuman animals to understand their biological significance. Current findings from nonhuman animal studies support that dermal opsins mediate dispersed photoreception across the body surface and allow the animals to instantly respond to changes in irradiation of local surrounding.6,11,46 In animals that face a constant threat of unpredictable environment and predators, dermal photoreception allows them to move away from potential danger, conceal themselves by camouflage or shadow reflex, and control circadian rhythms. However, it is still unclear to what extent dermal opsins mediate such behaviors, and would require further studies to answer this question.
3.2 |. The expression and function of dermal opsins in humans
Humans live in a vastly different environment in comparison with the aforementioned nonhuman animal species. For instance, humans do not need or have the ability to camouflage for survival as frogs and octopuses do. Nevertheless, a wide array of opsins is expressed throughout different skin cell types including keratinocytes, melanocytes, dermal fibroblasts, and hair follicle cells (Figure 2).24,47 It is still unknown whether these opsins have biological functions beyond light reception in human skin cells or they are vestigial evolutionary remnants from mechanisms developed in prehistoric times. To interrogate the potential function of these opsins expressed in the human skin, it is important to first understand the physiological role of each skin cell type. Human skin consists of three basic layers–the epidermis (outermost), the dermis (middle), and the hypodermis (innermost).48 The epidermis is comprised mainly of keratinocytes and melanocytes. Keratinocytes synthesize keratin, acting as the first line of innate immune defense against infection and external factors. Melanocytes produce a pigment called melanin, which protects the skin from UV rays.49 The dermis contains dermal fibroblasts, which generate connective tissue allowing the skin to recover from injury, and hair follicle cells, which regulate hair growth.49 The hypodermis is mostly made of fat and connective tissue that attaches the dermis to the body and regulates body temperature.49 Here, we compile studies reporting the identity and the elucidated role of opsins in each cell type.
FIGURE 2.
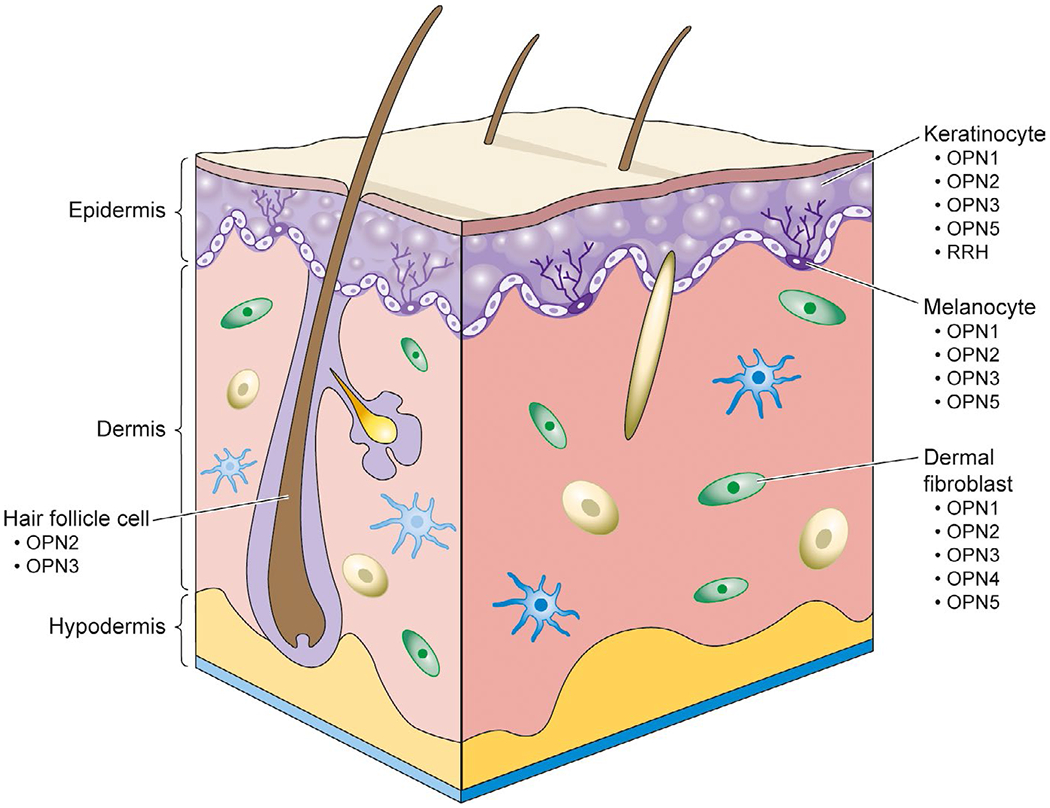
The expression of opsins in human skin cell types [Colour figure can be viewed at wileyonlinelibrary.com]
3.2.1 |. Keratinocytes
In 2009, Tsutsumi et al first identified the expression of visual opsins, cone opsin (OPN1) and rhodopsin (OPN2), in both human facial skin tissue and cultured normal human epidermal keratinocytes (NHEKs) by RT-PCR and immunohistochemistry.50 In 2013, Kim et al confirmed the expression of OPN2 in cultured human epidermal keratinocytes and suggested that OPN2 could be involved in the regulation of keratinocyte differentiation by showing that violet light (410 nm) irradiation decreases keratinocyte differentiation markers with a corresponding increase in OPN2 expression.51 However, the difference in the absorption spectrum of OPN2 (λmax, 480-530 nm) raises a question whether the effect is mediated through OPN2. In 2015, Haltaufderhyde et al identified two other opsin types, encephalopsin (OPN3) and neuropsin (OPN5), from neonatal foreskin-derived epidermal keratinocytes in addition to OPN1 and OPN2.47 In 2017, Toh et al identified peropsin (RRH), a type of tetraopsin which had previously been known to be exclusively expressed in the retinal pigmented epithelial (RPE) cells of the eye, in both human skin tissue and cultured NHEKs.52 They reported that irradiation of cultured keratinocytes with violet light (380 nm) elicits the highest amplitude of Ca2+ transients only in the presence of the all-trans-retinal ligand, suggesting that RRH may contribute to the phototransduction of violet light in keratinocytes. The spectral sensitivity of RRH is unknown, but neuropsin and RGR, two other phylogenetically related tetraopsins, have maximal absorption spectra at 380 and 470 nm, respectively.19,53 However, the pathophysiological relevance of this RRH in human skin warrants further investigation.
In 2018, Pellicena et al confirmed the expression of OPN1-SW, OPN3, and OPN5 in cultured epidermal keratinocytes of human facial and abdominal skin by immunofluorescence analysis and also showed that irradiation with blue light (447 nm) accelerates wound closure in the regenerating epithelial tongue of an ex vivo human skin wound-healing model with a corresponding increase in OPN3 expression.54 To further explore how OPN3 mediates wound healing, they evaluated the migration, proliferation, and differentiation, which are vital for wound repair, in cultured keratinocytes following blue light irradiation. They found that low levels of blue light did not affect migration or proliferation, but stimulated differentiation of keratinocytes, which was abrogated by OPN3 knockdown. Overall, the current findings on keratinocytes suggest that OPN3 is involved in cell differentiation regulation and restoration of the barrier function.54
3.2.2 |. Melanocytes
In 2011, Wicks et al first identified the expression of OPN2 in cultured human epidermal melanocytes (HEMs) and reported that UV irradiation induces calcium influx and melanin production in HEMs.55 The same group later proposed that melanin synthesis occurs via calcium-mediated Gαq/11 pathway, the same mechanism of visual phototransduction in the retina.56 In 2015, Haltaufderhyde et al detected three other opsins expressed in cultured normal human melanocytes (NHMs), OPN1-SW, OPN3, and OPN5, although OPN2 and OPN3 were expressed most abundantly.47 In 2018, Regazzetti et al proposed that OPN3 might be a key receptor responsible for visible light-induced hyperpigmentation by demonstrating that violet light (415 nm) irradiation on cultured NHMs activates calcium-dependent microphthalmia-associated transcription factor (MITF) pathway and upregulates melanogenesis-associated enzymes, whereas the violet light-induced effect was abrogated by silencing OPN3.57 In 2019, Ozdeslik et al also suggested that OPN3 might play a key role in the regulation of melanogenesis, but proposed a different mechanism of action.58 Ozdeslik et al found that OPN3 acts as a negative modulator of melanin production via coupling to Gαi pathway, which inhibits melanocortin 1 receptor (MC1R)-mediated cAMP response leading to melanin production.
In 2020, Wang et al reported that OPN3 is a key receptor responsible for survival of human epidermal melanocytes.59 They observed that downregulation of OPN3 markedly reduces the intracellular calcium level and triggers the conventional apoptosis pathway via mitochondria.
Taken all together, the existing evidence supports the expression of several opsin types in human melanocytes with OPN3 being most abundantly expressed. The mechanism of action of OPN3 in melanogenesis is debated and will require further investigations.
3.2.3 |. Hair follicle cells
The presence of opsins in the hair follicle stem cells has recently gained attention as many reports have shown that PBM has positive effects on hair growth.23 In 2017, Buscone et al detected OPN2 and OPN3 in anagen hair follicles and demonstrated that blue light (453 nm), which corresponds to the absorption spectra of OPN3, prolongs anagen hair growth phase. In contrast, red light did not affect hair growth and silencing OPN3 abrogated stimulatory effects of blue light.60 Unfortunately, there is scarce evidence on the functional role of opsin in hair follicle cells. Elucidating the molecular target and mechanism will open new door for utilization of light therapy in alopecia patients.
3.2.4 |. Dermal fibroblasts
In 2018, Pellicena et al reported for the first time that cultured dermal fibroblasts from human facial and abdominal skin tissue express OPN1-SW, OPN2, and OPN3.54 Although the function of these opsins in dermal fibroblasts was not elucidated in their study, they inferred from the previous finding showing the anti-proliferative effect of blue light (450-490 nm) on fibroblasts in vitro that opsins might be involved in the regulation of cell proliferation.61–63 The modulation of dermal fibroblasts by blue light has the potential for the treatment of hypertrophic scarring, such as keloids and other fibrotic skin diseases, and the role of opsins in fibroblasts is worthy of further investigations.64
In 2019, Lan et al provided the first evidence that OPN3 is the key sensor responsible for upregulating matrix metalloproteases (MMPs) in dermal fibroblasts upon ultraviolet A (UVA) exposure and contributes to the skin photoaging. It has been well known that chronic exposure to UVA radiation induces an increase in MMPs, which leads to the degradation of fibrous connective tissue and skin photoaging. The group detected all five opsins, OPN1-OPN5, in normal human dermal fibroblasts (NHDFs) by qPCR and Western blot analysis, but UVA exposure particularly increased the expression level of OPN3 and triggered the phototransduction and the expression of MMPs.
4 |. DISCUSSION
Many studies have supported the existence of skin photosensors across different species with the evidence of behavioral, electrophysiological, and genetic studies. At the beginning, most studies supported the existence of dermal photosensitivity through animal behavior and electrophysiology analysis. Advances in molecular biology techniques eventually led to a discovery of opsins as potential photosensors in the skin and inspired great scientific interest concerning the role of opsins in the skin. A number of studies demonstrated that opsins modulate various physiological processes of the skin, including wound healing, melanogenesis, photoaging, and hair growth.54,58
Despite compelling evidence, further investigations are needed to validate the current findings in the literature. Many studies have been performed on cultured skin cells, which might not display the exact morphological and physiological properties of native tissue in vivo. Cultured cells may alter the native expression profiles and lose their specific phenotypes after multiple passages. Such changes can potentially provide misleading information on the gene expression in native tissue.
The research in dermal opsins has great potentials for advancing the clinical applications of PBM. There has been a growing interest in the application of light therapy to clinical cases owing to the advantages of a cost-effective and noninvasive approach. In order to maximize the benefit of light therapy, it is critical to understand the underlying mechanisms of opsins in modulating physiological processes in the skin and define light parameters that elicit such responses. Several studies have already demonstrated the great potentials of PBM for treating dermatological conditions by targeting these opsins.21 Recent studies, which demonstrated the stimulatory effect on hair follicle stem cells and melanocytes with specific wavelengths of light irradiation, suggest that PBM could be a new promising treatment for hair loss and skin pigmentation disorders in near future.58,60
To enhance understanding of the mechanistic role of opsins in the skin, future investigations using Cre-based knockout mice will be a valuable tool to confirm the therapeutic efficacy and safety of PBM. Moreover, optimization of light exposure time, wavelength, intensity, and treatment interval will accelerate the clinical application of PBM. Although unlocking the therapeutic value of PBM still requires a deeper understanding of biochemical processes, the current literature strongly supports the notion that PMB is a promising therapeutic modality in clinical dermatology.
Acknowledgments
Funding information
This study was partially supported by the NIH grants F30EY029136, T32EY024236, and T32GM007250 to SS.
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Steven DM. Some properties of the photoreceptors of the brook lamprey. J Exp Biol. 1950;27(3-4):350–364. [DOI] [PubMed] [Google Scholar]
- 2.Ullen F, Orlovsky GN, Deliagina TG, Grillner S. Role of dermal photoreceptors and lateral eyes in initiation and orientation of locomotion in lamprey. Behav Brain Res. 1993;54(1):107–110. [DOI] [PubMed] [Google Scholar]
- 3.Ramirez MD, Oakley TH. Eye-independent, light-activated chromatophore expansion (LACE) and expression of phototransduction genes in the skin of Octopus bimaculoides. J Exp Biol. 2015;218(Pt 10):1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chono K, Fujito Y, Ito E. Non-ocular dermal photoreception in the pond snail Lymnaea stagnalis. Brain Res. 2002;951(1):107–112. [DOI] [PubMed] [Google Scholar]
- 5.Sunada H, Lukowiak K, Sakakibara M. Repetitive noxious stimulus altered the shadow-induced withdrawal behavior in Lymnaea. Acta Biol Hung. 2012;63(Suppl 2):179–189. [DOI] [PubMed] [Google Scholar]
- 6.Sunada H, Sakaguchi T, Horikoshi T, Lukowiak K, Sakakibara M. The shadow-induced withdrawal response, dermal photoreceptors, and their input to the higher-order interneuron RPeDll in the pond snail Lymnaea stagnalis. J Exp Biol. 2010;213(Pt 20):3409–3415. [DOI] [PubMed] [Google Scholar]
- 7.Tosini G, Avery RA. Dermal photoreceptors regulate basking behaveior in the lizard Podarcis muralis. Physiol Behav. 1996;59(1):195–198. [DOI] [PubMed] [Google Scholar]
- 8.Pankey S, Sunada H, Horikoshi T, Sakakibara M. Cyclic nucleotide-gated channels are involved in phototransduction of dermal photoreceptors in Lymnaea stagnalis. J Comp Physiol [B], 2010;180(8):1205–1211. [DOI] [PubMed] [Google Scholar]
- 9.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468(7326):921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrage H, Heiskanen V, Palin WM, et al. Under the spotlight: mechanisms of photobiomodulation concentrating on blue and green light. Photochem Photobiol Sci. 2019;18(8):1877–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley JL, Davies WIL. The biological mechanisms and behavioral functions of opsin-based light detection by the skin. Front Ecol Evol. 2016;4:106. [Google Scholar]
- 12.Ramirez MD, Speiser Dl, Pankey MS, Oakley TH. Understanding the dermal light sense in the context of integrative photoreceptor cell biology. Vis Neurosci. 2011;28(4):265–279. [DOI] [PubMed] [Google Scholar]
- 13.Terakita A The opsins. Genome Biol. 2005;6(3):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shichida Y, Matsuyama T. Evolution of opsins and phototransduction. Philos Trans R Soc Lond B Biol Sci. 2009;364(1531):2881–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palczewski K, Kumasaka T, Hori T, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science (New York, NY). 2000;289(5480):739–745. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez MD, Pairett AN, Pankey MS, et al. The last common ancestor of most bilaterian animals possessed at least nine opsins. Genome Biol Evol. 2016;8(12):3640–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter ML, Blasic JR, Bok MJ, et al. Shedding new light on opsin evolution. Proc Royal Soc B: Biol Sci. 2012;279(1726):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung NY, Montell C. Unconventional roles of opsins. Annu Rev Cell Dev Biol. 2017;33(l):241–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Choi EH, Tworak A, et al. Photic generation of 11-cis-retinal in bovine retinal pigment epithelium. J Biol Chem. 2019;294(50):19137–19154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morshedian A, Kaylor JJ, Ng SY, et al. Light-driven regeneration of cone visual pigments through a mechanism involving RGR opsin in muller glial cells. Neuron. 2019;102(6):1172–U83.e1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aragona SE, Grassi FR, Nardi G, et al. Photobiomodulation with polarized light in the treatment of cutaneous and mucosal ulcerative lesions. J Biol Regul Homeost Agents. 2017;31(2 Suppl. 2):213–218. [PubMed] [Google Scholar]
- 22.Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mignon C, Botchkareva NV, Uzunbajakava NE, Tobin DJ. Photobiomodulation devices for hair regrowth and wound healing: a therapy full of promise but a literature full of confusion. Exp Dermatol. 2016;25(10):745–749. [DOI] [PubMed] [Google Scholar]
- 24.Narla S, Kohli I, Hamzavi IH, Lim HW. Visible light in photodermatology. Photochem Photobiol Sci. 2020;19(1):99–104. [DOI] [PubMed] [Google Scholar]
- 25.Lim HW, Silpa-archa N, Amadi U, Menter A, Van Voorhees AS, Lebwohl M. Phototherapy in dermatology: A call for action. J Am Acad Dermatol. 2015;72(6):1078–1080. [DOI] [PubMed] [Google Scholar]
- 26.Pfaff S, Liebmann J, Born M, Merk HF, von Felbert V. Prospective randomized long-term study on the efficacy and safety of UV-free blue light for treating mild psoriasis vulgaris. Dermatology. 2015;231(l):24–34. [DOI] [PubMed] [Google Scholar]
- 27.Stern RS. Psoralen and ultraviolet a light therapy for psoriasis. N Engl J Med. 2007;357(7):682–690. [DOI] [PubMed] [Google Scholar]
- 28.Garza ZCF, Born M, Hilbers PAJ, van Riel NAW, Liebmann J. Visible blue light therapy: molecular mechanisms and therapeutic opportunities. Curr Med Chem. 2018;25(40):5564–5577. [DOI] [PubMed] [Google Scholar]
- 29.Anders JJ, Lanzafame RJ, Arany PR. Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg. 2015;33(4):183–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95(l):340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isoldi MC, Rollag MD, Castrucci AM, Provencio I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Natl Acad Sci USA. 2005;102(4):1217–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathger LM, Roberts SB, Hanlon RT. Evidence for distributed light sensing in the skin of cuttlefish, Sepia officinalis. Biol Let. 2010;6(5):600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kingston ACN, Kuzirian AM, Hanlon RT, Cronin TW. Visual phototransduction components in cephalopod chromatophores suggest dermal photoreception. J Exp Biol. 2015;218(Pt 10):1596–1602. [DOI] [PubMed] [Google Scholar]
- 34.Hara T, Hara R. Rhodopsin and retinochrome in the squid retina. Nature. 1967;214(5088):573–575. [DOI] [PubMed] [Google Scholar]
- 35.Hara T, Hara R. Regeneration of squid retinochrome. Nature. 1968;219(5153):450–454. [DOI] [PubMed] [Google Scholar]
- 36.Ullrich-Lüter EM, D’Aniello S, Arnone Ml. C-opsin expressing photoreceptors in echinoderms. Integr Comp Biol. 2013;53(l):27–38. [DOI] [PubMed] [Google Scholar]
- 37.Wl Davies, Tamai TK, Zheng L, et al. An extended family of novel vertebrate photopigments is widely expressed and displays a diversity of function. Genome Res. 2015;25(11):1666–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buhr ED, Vemaraju S, Diaz N, Lang RA, Van Gelder RN. Neuropsin (OPN5) mediates local light-dependent induction of circadian clock genes and circadian photoentrainment in exposed murine skin. Curr Biol. 2019;29(20):3478–3487.e3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kojima D, Mori S, Torii M, Wada A, Morishita R, Fukada Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS One. 2011;6(10):e26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakane Y, Ikegami K, Ono H, et al. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci USA. 2010;107(34):15264–15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohuchi H, Yamashita T, Tomonari S, et al. A non-mammalian type opsin 5 functions dually in the photoreceptive and non-photoreceptive organs of birds. PLoS One. 2012;7(2):e31534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson TJ, Ball GF. Disruption of neuropsin mRNA expression via RNA interference facilitates the photoinduced increase in thyrotropin-stimulating subunit beta in birds. Eur J Neuorsci. 2012;36(6):2859–2865. [DOI] [PubMed] [Google Scholar]
- 43.Nakane Y, Shimmura T, Abe H, Yoshimura T. Intrinsic photosensitiveity of a deep brain photoreceptor. Curr Biol. 2014;24(13):R596–597. [DOI] [PubMed] [Google Scholar]
- 44.Schweikert LE, Fitak RR, Johnsen S. De novo transcriptomics reveal distinct phototransduction signaling components in the retina and skin of a color-changing vertebrate, the hogfish (Lachnolaimus maximus). J Comp Physiol A. 2018;204(5):475–485. [DOI] [PubMed] [Google Scholar]
- 45.Delroisse J, Duchatelet L, Flammang P, Mallefet J. De novo transcriptome analyses provide insights into opsin-based photoreception in the lanternshark Etmopterus spinax. PLoS One. 2019;13(12):e0209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takigami S, Sunada H, Horikoshi T, Sakakibara M. Morphological and physiological characteristics of dermal photoreceptors in Lymnaea stagnalis. Biophysics (Nagoyashi, Japan). 2014;10:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol. 2015;91(1):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prost-Squarcioni C Histology of skin and hair follicle. Med Sci. 2006;22(2):131–137. [DOI] [PubMed] [Google Scholar]
- 49.Sharma S, Yousef H. Anatomy, Skin (Integument), Epidermis. 2017. [PubMed] [Google Scholar]
- 50.Tsutsumi M, Ikeyama K, Denda S, et al. Expressions of rod and cone photoreceptor-like proteins in human epidermis. Exp Dermatol. 2009;18(6):567–570. [DOI] [PubMed] [Google Scholar]
- 51.Yousef H, Alhajj M, Sharma S. Skin (Integument), Epidermis. In: StatPearls. 2020. Treasure Island (FL): StatPearls Publishing; https://pubmed.ncbi.nlm.nih.gov/29262154/ [PubMed] [Google Scholar]
- 52.Toh PP, Bigliardi-Qi M, Yap AM, Sriram G, Stelmashenko O, Bigliardi P. Expression of peropsin in human skin is related to phototransduction of violet light in keratinocytes. Exp Dermatol. 2016;25(12):1002–1005. [DOI] [PubMed] [Google Scholar]
- 53.Kumbalasiri T, Provencio I. Melanopsin and other novel mammalian opsins. Exp Eye Res. 2005;81(4):368–375. [DOI] [PubMed] [Google Scholar]
- 54.Castellano-Pellicena I, Uzunbajakava NE, Mignon C, Raafs B, Botchkarev VA, Thornton MJ. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg Med. 2018;51(4):370–382. [DOI] [PubMed] [Google Scholar]
- 55.Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol. 2011;21(22):1906–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellono NW, Najera JA, Oancea E. UV light activates a Gαq/11-coupled phototransduction pathway in human melanocytes. J Gen Physiol. 2014;143(2):203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Regazzetti C, Sormani L, Debayle D, et al. Melanocytes sense blue light and regulate pigmentation through opsin-3. J Invest Dermatol. 2018;138(1):171–178. [DOI] [PubMed] [Google Scholar]
- 58.Ozdeslik RN, Olinski LE, Trieu MM, Oprian DD, Oancea E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc Natl Acad Sci. 2019; 116(23): 11508–11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Lan Y, Lu H. Opsin3 downregulation induces apoptosis of human epidermal melanocytes via mitochondrial pathway. Photochem Photobiol. 2020;96(l):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buscone S, Mardaryev AN, Raafs B, et al. A new path in defining light parameters for hair growth: Discovery and modulation of photoreceptors in human hair follicle. Lasers Surg Med. 2017;49(7): 705–718. [DOI] [PubMed] [Google Scholar]
- 61.Opländer C, Hidding S, Werners FB, Born M, Pallua N, Suschek CV. Effects of blue light irradiation on human dermal fibroblasts. J Photochem Photobiol, B. 2011;103(2):118–125. [DOI] [PubMed] [Google Scholar]
- 62.Taflinski L, Demir E, Kauczok J, et al. Blue light inhibits transforming growth factor-β1-induced myofibroblast differentiation of human dermal fibroblasts. Exp Dermatol. 2014;23(4):240–246. [DOI] [PubMed] [Google Scholar]
- 63.Masson-Meyers DS, Bumah VV, Enwemeka CS. Blue light does not impair wound healing in vitro. J Photochem Photobiol, B. 2016;160:53–60. [DOI] [PubMed] [Google Scholar]
- 64.Mamalis A, Garcha M, Jagdeo J. Light emitting diode-generated blue light modulates fibrosis characteristics: fibroblast proliferation, migration speed, and reactive oxygen species generation. Lasers Surg Med. 2015;47(2):210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]


