Abstract
Background:
Does minor head impact without signs of structural brain damage cause short-term changes in vasogenic edema as measured by an increase apparent diffusion coefficient (ADC) using diffusion weighted imaging? If so, could the increase in vasogenic edema be treated with a vasopressin V1a receptor antagonist? We hypothesized that SRX251, a highly selective V1a antagonist, would reduce vasogenic edema in response to a single minor head impact.
Methods:
Lightly anesthetized male rats were subjected to a sham procedure or a single hit to the forehead using a closed skull, momentum exchange model. Animals recovered in five min and were injected with saline vehicle (n=8) or SRX251 (n=8) at 15 min post head impact and again 7–8 hrs later. At 2 hrs, 6 hrs, and 24 hrs post injury, rats were anesthetized and scanned for increases in ADC, a neurological measure of vasogenic edema. Sham rats (n=6) were exposed to anesthesia and scanned at all time points but were not hit or treated. Images were registered to and analyzed using a 3D MRI rat atlas providing site-specific data on 150 different brain areas. These brain areas were parsed into 11 major brain regions.
Results:
Untreated rats with brain injury showed a significant increase in global brain vasogenic edema as compared to sham and SRX251 treated rats. Edema peaked at 6 hrs in injured, untreated rats in three brain regions where changes in ADC were observed, but returned to sham levels by 24 hrs. There were regional variations in the time course of vasogenic edema and drug efficacy. Edema was significantly reduced in cerebellum and thalamus with SRX251 treatment while the basal ganglia did not show a response to treatment.
Conclusion:
A single minor impact to the forehead causes regional increases in vasogenic edema that peak at 6 hrs but return to baseline within a day in a subset of brain regions. Treatment with a selective V1a receptor antagonist can reduce much of the edema.
Keywords: traumatic brain injury, diffusion weighted imaging, apparent diffusion coefficient, magnetic resonance imaging, cerebellum
Background
Traumatic brain injury (TBI) affects more than 50 million people each year worldwide across all demographic and socioeconomic groups [39]. In the United States and Europe, TBI accounts for over 4 million emergency hospital visits annually [39, 40, 69]. Major traumatic brain injuries are a contributing factor to a third of injury-related deaths in the United States and is one of the leading causes of death and disability in persons under 35 and over 65 [69]. In the United States alone, the annual economic burden of TBI is conservatively estimated at $86 billion [39]. While these statistics point to the significance of TBI as a health issue, one form of brain injury, the effects of a single, minor impact that does not require hospitalization or necessitate immediate medical attention, are not well understood [10]. The health community lists guidelines for diagnosing these ”mild” head injuries that include self-reports of transient confusion, disorientation, impaired consciousness, or dysfunction in memory around the time of the injury and, importantly, no apparent structural damage as determined with imaging [1, 10, 65]. The self-reports invite a “bump on the head, ice pack” approach to the problem.
There are neurological complications following any closed head injury, including mild TBI [30, 70]. Edema is among the most significant complications because it is a critical component in the pathophysiology of brain injury and occurs in stages. Vasogenic edema is caused by disruption of the blood-brain barrier (BBB) and, in moderate and severe TBI, is followed by cytogenic edema from cellular swelling and lastly, by a combination of both [28]. The timing and severity of each is dependent upon the level of brain injury. The extent of edema occurring after TBI can be determined by measures of apparent diffusion coefficient (ADC) using diffusion weighted imaging (DWI) [70]. ADC is a quantitative measure of water mobility. Cellular swelling due to loss of homeostatic regulation of osmolarity across the plasma membrane results in lower ADC values. Vasogenic edema, characterized by higher ADC values, results from a change in permeability across the BBB, with an influx of water and solute into the interstitial space contributing to the extracellular volume. This increase in brain water contributes to parenchymal swelling and increase in intracranial pressure.
There are currently no approved pharmaceutical treatments that can prevent, minimize, or reverse brain edema following any level of brain damage [17, 23]. Several labs have studied the role of the neuropeptide arginine vasopressin (AVP) in intracranial water regulation [2, 15, 18, 32, 57]. AVP is a naturally occurring neuropeptide hormone involved in brain water permeability and vasoconstriction [33, 47]. The AVP V1a (V1a) receptor subtype is highly expressed in cortical and subcortical brain areas across all mammals [52, 77] and AVP signaling through this receptor is strongly tied to the pathology of cerebral edema in TBI. For instance, V1a receptor density increases following TBI and infusion of V1a antagonists block increased intracranial pressure after brain injury in animal models [6, 20, 43]. Knockout mice lacking V1a receptors show reduced edema, secondary injuries, and behavioral impairments in the days following brain damage [55]. We recently showed that systemic treatment with a highly selective V1a receptor antagonist for 5 days starting within 24 hrs of moderate/severe brain TBI significantly reduced edema and prevented cognitive deficits [46].
The present study was designed to address two questions: 1) Does minor head impact without signs of structural brain damage cause short-term changes in vasogenic edema as measured by an increase in ADC and, if so, 2) could these changes be mitigated by treatment with an AVP receptor V1a antagonist.
Methods
Animals
Male Sprague Dawley rats (n=22) weighing between 300 and 325 gm were obtained from Charles River Laboratories (Wilmington, Massachusetts, USA). Rats were housed in Optirat® GenII (Animal Care Systems, Inc., Centennial, CO) cages with Bed-o’Cobs® corn-cob bedding (Anderson lab bedding, Delphi, IN) mixed with Envirodri™ shredded paper (Shepards Specialty Papers, Richland, MI). The vivarium was maintained at an ambient temperature of (22–24°C), humidity (35–45%) on a 12:12 light:dark cycle (lights on at 07:00 a.m.). Rats were allowed access to food and fresh water ad libitum. Rats were imaged during the light phase of the circadian cycle. All rats were acquired and cared for in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals. All methods and procedures described below were pre-approved by the Northeastern University Institutional Animal Care and Use Committee and adhere to the ARRIVE guidelines for reporting in vivo experiments in animal research [31]
Treatment
Lightly isoflurane anesthetized rats were subjected to a sham procedure or a single mild hit to the forehead, midline and rostral to Bregma, using a closed skull, momentum exchange model [36]. Originally developed by Viano and colleagues to reliably inflict mild, medium, or severe rat brain injury [73], this same model was further refined by Mychasiuk et al., and used to test the behavioral effects of TBI controlling for the axis of injury, rotational force, and head acceleration in different directions [48]. All rats recovered from the sham or mild head impact within 5 min as demonstrated by righting and motor activity. There was no perioperative procedure for pain. Rats were injected with saline vehicle (n=8) or SRX251 (n=8) 15 min post head impact and again 7–8 hrs later. At 2 hrs, 6 hrs, and 24 hrs post head impact, rats were anesthetized and scanned for structural brain damage and increases in ADC, a neurological measure of vasogenic edema. Sham rats (n=6) were exposed to anesthesia and scanned at all time points but were not hit or treated. Scanning sessions lasted about 70 min, after which animals were returned to their respective home cage.
Neuroimaging
Imaging sessions were conducted using a Bruker Biospec 7.0 T/20-cm USR horizontal magnet (Bruker, Billerica, MA, USA) and a 20-G/cm magnetic field gradient insert (ID=12 cm) capable of a 120-μs rise time. Radio frequency signals were sent and received with a quadrature volume coil built into the animal restrainer (Animal Imaging Research, Holden, Massachusetts). The design of the restraining system included a padded head support obviating the need for ear bars, helping to reduce discomfort while minimizing motion artifact. All rats were imaged under 1–2% isoflurane while keeping a respiratory rate of 40–50 breathes/min. At the beginning of each imaging session, a high-resolution anatomical data set was collected for assessment of structural damage using the RARE pulse sequence with following parameters, 35 slice of 0.7mm thickness; field of view [FOV] 3 cm; 256×256; repetition time [TR] 3900 msec; effective echo time [TE] 48 msec; NEX 3; 6 min 14 sec acquisition time.
Diffusion Weighted Imaging – Quantitative Anisotropy
DWI was acquired with a spin-echo echo-planar-imaging (EPI) pulse sequence having the following parameters: TR/TE = 500/20 msec, eight EPI segments, and 10 non-collinear gradient directions with a single b-value shell at 1000 s/mm 2 and one image with a B-value of 0 s/mm 2 (referred to as B 0) [8, 19, 36]. Geometrical parameters were: 48 coronal slices, each 0.313 mm thick (brain volume) and with in-plane resolution of 0.313×0.313 mm 2 (matrix size 96×96; FOV 30 mm3). The imaging protocol was repeated two times for signal averaging. Each DWI acquisition took 35 min and the entire MRI protocol including the anatomy lasted about 90 min. Image analysis included DWI analysis of the DWI-3D-EPI images to produce the maps of ADC. DWI analysis was completed with MATLAB and MedINRIA (1.9.0; http://www-sop.inria.fr/asclepios/software/MedINRIA/index.php) software. Because sporadic excessive breathing during DWI acquisition can lead to significant image motion artifacts that are apparent only in the slices sampled when motion occurred, each image (for each slice and each gradient direction) was screened prior to DWI analysis. If found, acquisition points with motion artifacts were eliminated from analyses.
For statistical comparisons among rats, each brain volume was registered to a 3D MRI Rat Brain Atlas (© 2012 Ekam Solutions LLC, Boston, MA) allowing voxel- and region-based statistics. All image transformations and statistical analyses were carried out using the in-house MIVA software (http://ccni.wpi.edu/). For each rat, the B 0 image was co-registered with the B 0 template (using a 6-parameter rigid-body transformation). The co-registration parameters were then applied on the DWI ADC maps. Normalization was performed on the maps because they provided the most detailed visualization of brain structures and allowed for more accurate normalization. The normalization parameters were then smoothed with a 0.3-mm Gaussian kernel. To ensure that ADC values were not affected significantly by the pre-processing steps, the ‘nearest neighbor’ option was used following registration and normalization. Statistical differences in measures of DWI between experimental groups for each of 11 brain regions were determined using two-way ANOVA followed by Bonferroni post hoc tests (alpha set at 5%).
The 3D MRI rat atlas has 173 segmented, annotated brain areas. For this study, 150 areas were chosen for analysis because they could be organized into well-defined neuroanatomical regions. Areas excluded were white matter tracts because they traverse several brain regions. Circumventricular organs, e.g. anterior and posterior pituitary, pineal gland, area postrema, median eminence and small adjacent areas like the arcuate and retrochiasmatic nuclei, were also excluded because of their larger, more fenestrated blood vessels. Also excluded were areas with no clear regional organization e.g., prerubral field. The remaining 150 brain areas were divided into 11 brain regions: cerebellum (20), cortex (19), thalamus (20), basal ganglia (10), hypothalamus (14), hippocampus (9), prefrontal cortex (9), olfactory bulb/cortex (8), amygdala (8), midbrain/pons (12), brainstem (21). The areas and their average ADC values for each experimental condition and for 2, 6 and 24 hrs, are provided in Supplementary Data Excel S1. The organization was based on conventional neuroanatomy and an effort to keep individual brain areas localized and contiguous within a region. The olfactory bulb/cortex is the exception as the piriform cortex extends some distant caudally along the ventral lateral cortex away from the bulbs.
Results
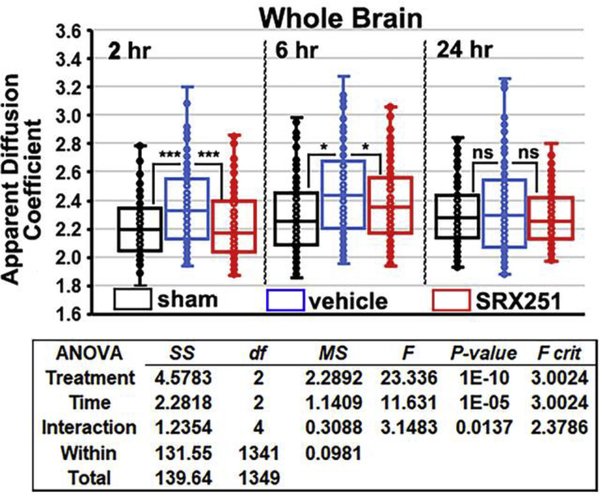
Shown in Fig 1 are box and whisker scatter plots for measures of ADC in the whole brain (150 areas) for each experimental condition over the three time points following brain injury. The horizontal line in each box denotes the median. The two-way ANOVA shows a significant main effect among treatments, time of measurement over the 24 hr period, and interaction between both. At 2 hrs post head injury, vehicle treated rats (blue box) showed a significant (p<0.001) increase over sham (black box) and SRX251 (red box). ADC values in injured vehicle-treated animals were still significantly greater than sham or SRX251 (p<0.05) at 6 hr. At the 24 hr time point, there were no significant differences among the groups in ADC values. There was no evidence of structural brain damage in the 16 rats exposed to a single mild head impact during the 24 hr study period Fig 2.
Figure 1. Edema in whole brain.
Shown are scatter plots of average ADC values taken from whole brain (150 areas) for sham (black), vehicle (blue) and SRX251 (red) treatments 2, 6 and 24 hrs post head injury. The analysis from a two-way ANOVA is shown below. ns – not significant, *** p<0.001, * p<0.05
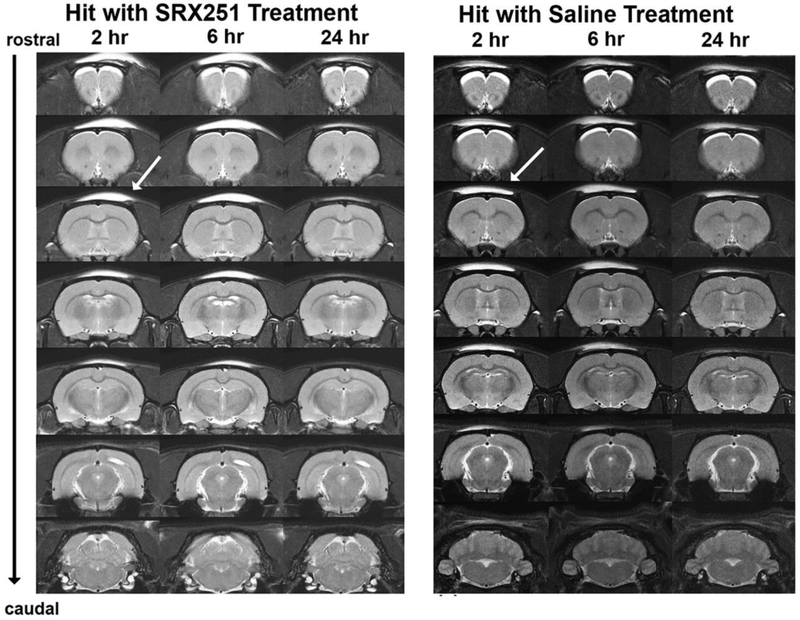
Figure 2. Mild head impact without evidence of brain damage.
Shown are serial coronal sections of T2-weighted anatomical images taken at 2, 6 and 24 hrs post mild head impact. The site of impact is shown by the arrow. The bright area above the skull is fluid from swelling under the skin. These images are from a single rat representative of each experimental treatment.
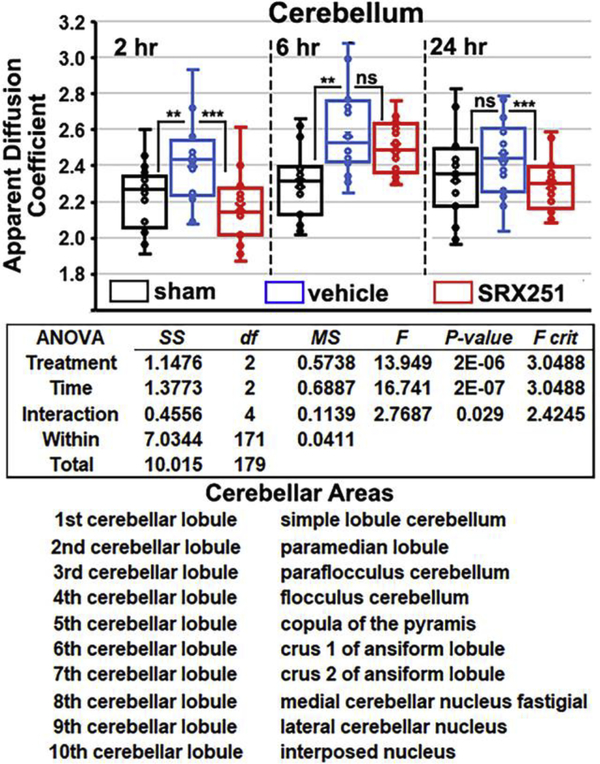
To determine which areas of the brain were most sensitive to this single mild head impact directed to the forebrain, ANOVA’s with post hoc analyses were run on the 11 brain regions. Only the cerebellum, thalamus, and basal ganglia showed a significant increase in ADC between experimental groups. Fig 3 shows scatter plots for ADC measures in the cerebellum. The two-way ANOVA results and list of twenty brain areas that comprise the cerebellum taken from the rat 3D MRI atlas are shown. At 2 and 6 hrs post impact, injured vehicle-treated rats present with significantly greater edema than sham (p<0.01), a difference that was no longer found at 24 hrs. At 6 hrs, ADC values peak in injured vehicle animals and significantly exceed the two hr levels (p< 0.02). SRX251 treatment significantly reduced edema at the 2 and 24 hr time points compared to vehicle (p< 0.001), but the effect was not significant at the 6 hr time point. There are no significant differences at any time point between SRX251 and sham rats.
Figure 3. Edema in cerebellum.
Shown are scatter plots of average ADC values taken from the cerebellum (20 areas shown below) for sham (black), vehicle (blue) and SRX251 (red) treatments 2, 6 and 24 hrs post head injury. ns – not significant, *** p<0.001, ** p<0.01
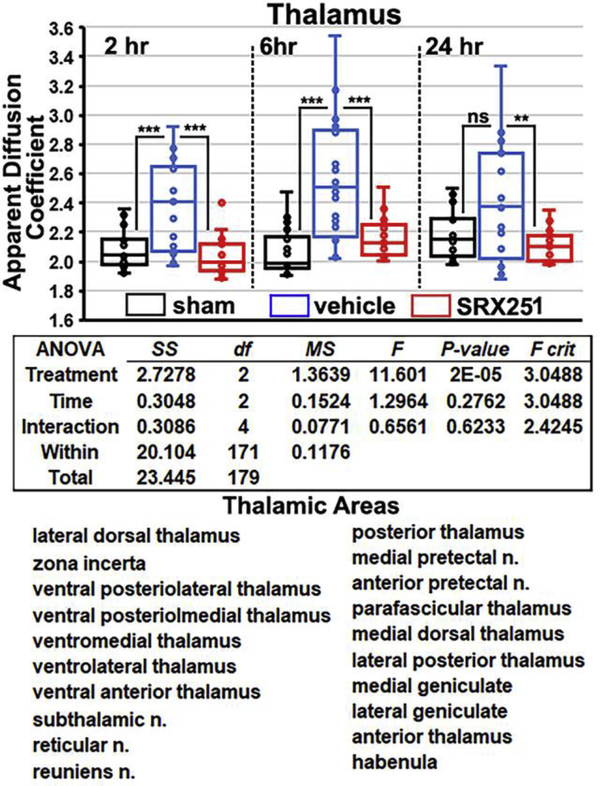
Fig 4 shows scatter plots for ADC measures in the thalamus, the two-way ANOVA table and a list of twenty brain areas that comprise the thalamus taken from the rat 3D MRI atlas. The profile of ADC values over the 24 hr period for each of the experimental conditions are similar to the cerebellum. At 2 and 6 hrs post impact, injured vehicle-treated rats showed significantly greater edema than sham (both p’s<0.001). Edema in the vehicle group peaked at 6 hrs and is significantly greater than both sham and SRX251 (p<0.001). The SRX251 treatment significantly reduced edema compared to vehicle at 2 and 6 hrs (p<0.001) and 24 h (p<0.01). There are no significant differences at any time point between SRX251 and sham rats.
Figure 4. Edema in thalamus.
Shown are scatter plots of average ADC values taken from thalamus (20 areas shown below) for sham (black), vehicle (blue) and SRX251 (red) treatments 2, 6 and 24 hrs post head injury. ns – not significant, *** p<0.001, ** p<0.01
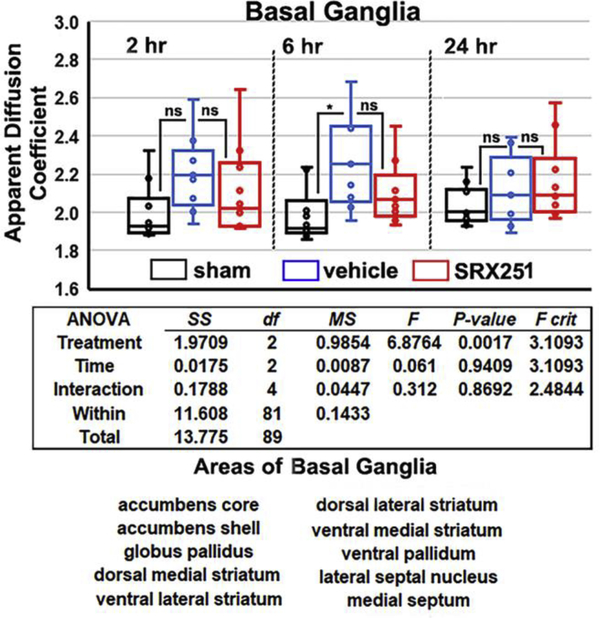
Fig 5 shows scatter plots for ADC measures in the basal ganglia, the two-way ANOVA table, and a list of ten brain areas that comprise the basal ganglia taken from the rat 3D MRI atlas. The change in ADC measures at 2 hr is not significantly different among conditions. By 6 hrs, injured vehicle-treated rats show significantly greater edema than sham but not SRX251. At 24 hrs, ADC values show no significant differences between conditions. At no time point is SRX251 significantly different from sham.
Figure 5. Edema in the basal ganglia.
Shown are scatter plots of average ADC values taken from the basal ganglia (10 areas shown below) for sham (black), vehicle (blue) and SRX251 (red) treatments 2, 6 and 24 hrs post head injury. ns – not significant, * p<0.05
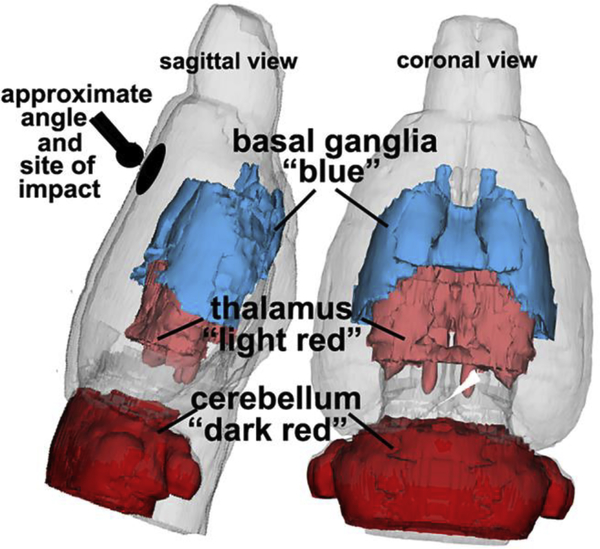
The 3D reconstruction shown in Fig 6 summarizes the brain regions with significant increases in edema over the 24 hr period following impact to the forebrain (location depicted in black). The thalamus (light red) and cerebellum (dark red) were responsive to SRX251 treated and we found significantly reduced edema in these areas. While the basal ganglia only showed an increase in edema at 6 hrs, as noted above, a decrease in ADC was present in response to SRX2521 but did not differ significantly when compared to vehicle (area in blue).
Figure 6. 3D reconstruction of affected brain areas.
Shown is a color-coded reconstruction of brain areas showing significant increases in edema following a single mild hit to the forehead (black ball and circle)
Discussion
This was an initial study examining the effects of a single, minor head impact on the development of early edema in a closed head, momentum exchange model of brain injury and the ability of a V1a receptor antagonist to reduce or block observed changes. The ADC from diffusion weighted imaging was used as a proxy for vasogenic edema. In this model of mild head impact, edema was significantly increased in whole brain and a subset of the 11 regions that were tested, including basal ganglia, cerebellum, and thalamus, without evidence of structural damage. The observed edema was transient because at 24 hours post-injury, vehicle-treated rats with mild head impact did not differ from sham controls in ADC. V1a antagonist treatment significantly reduced edema compared to vehicle treatment in whole brain, thalamus, and cerebellum.
Edema is a critical component in the pathophysiology of TBI and can occur within the first few hrs post-injury [3, 68, 71]. The type of edema occurring after TBI can be determined using DWI with ADC, a quantitative measure of water mobility [3, 25, 26, 56, 61]. Vasogenic edema, characterized by higher ADC values, results from a change in permeability across the BBB with an influx of water and solute into the interstitial space contributing to the extracellular volume. This increase in brain water contributes to parenchymal swelling and an increase in intracranial pressure. In the present study, only higher ADC values were found in the affected regions, indicating that a single, mild head impact can induce vasogenic edema.
An important question is why the cerebellum and thalamus, regions caudal to the site of impact on the forehead, but not the prefrontal cortex immediately beneath the site of impact, show the greatest increases in ADC? The transmission of mechanical force down white matter tracts and along brain areas abutting the ventricular system may offer an explanation [21]. The cerebellum has been recognized as being particularly vulnerable to mTBI [44, 49, 54, 72] and neuroradiological evidence of cerebellar dysfunction has been advanced as a diagnostic biomarker of TBI [72]. Because this is the only study, we know of that used a closed-head, mild injury protocol, we cannot readily compare our findings to the other rodent models with structural brain damage.
The increase in ADC, which reflects vasogenic edema, suggests a change in the permeability of the BBB with a single mild head impact. Using extravasation of blood biomarkers, e.g. Evan blue, plasma immunoglobulins, horse radish peroxidase, and albumin, there is an increase in BBB permeability within 1–4 hr hrs after brain damage in rodent models of TBI [4, 22, 24, 67]. Similar results have been reported in rats using dynamic contrast enhanced (DCE) MRI to measure BBB permeability [38, 78]. In humans, DCE MRI shows an increase in BBB permeability around damage tissue within 24 hrs of injury that persists for days [29]. However, all the work cited above follows moderate TBI with brain damage. Athletes playing American football and thought to have mild, sub-concussive head injuries show evidence of increased BBB permeability with DCE MRI [74]. Using DCE-MRI, O’Keeffe and colleagues reported BBB disruption in response to repetitive mild concussions in rugby players after a season and mixed martial arts fighters within days after a competitive fight [51]. There were no significant differences in self-reported concussions between these players and control subjects (track and field athletes). Players with multiple sub concussive collisions in American football show elevated blood levels of astrocytic protein S100B thought to be due to BBB disruption [42]. Interestingly, professional boxers show an increase in ADC values in large white matter tracts and a general increase in brain diffusivity thought to be the cumulative effect of multiple non-severe head impacts [11, 79, 80]. To our knowledge, these are the first data reporting evidence of global and region-specific increases in brain edema to a single, mild head impact in the absence of structural brain damage.
The biology of AVP contributes to the edema associated with TBI. How this neuropeptide promotes edema remains unclear, but the evidence of its involvement is substantial. Following a moderate impact to the brain, the tissue surrounding the damage parenchyma increases the expression of V1a receptors on neurons, astrocytes and endothelium of microvessels, an effect that occurs within hrs and persists for days [53, 62]. AVP appears to have a role in post-traumatic neuroinflammation as the proinflammatory chemokines in endothelial cells and astrocytes of damaged tissue are reduced in AVP-deficient Brattleboro rats as compared to wild-type controls [63, 64]. With brain damage, the water content from vasogenic edema goes up dramatically in the first 5 to 24 hrs [43, 68, 71]. The increase in brain edema can be reduced by the continuous intravenous infusion of SR49059, a V1a receptor antagonist, immediately after brain injury [20, 43, 68]. The intracerebroventricular injection of V1a receptor antagonists e.g., SR-49059, V1880 [35] or the peptidic deamino-Pen(1), O-Me-Tyr(2), Arg(8)]-vasopressin [34, 71] are also effective but only when given within the first few hrs of injury [35]. Moderate brain injury is associated with elevated levels of the water channel AQP4, GFAP, and V1a receptor, and disruption in sodium/potassium balance, all of which can be corrected by continuous exposure to SR49059 [20, 43, 68]. The edema in the these moderate models of TBI is reduced in V1a null mice [55]. Szczygielski and coworkers used DWI to measure changes in brain edema 24 hrs post head trauma in a closed-head mouse model of moderate TBI[60]. Impact to one side of the cortex increased AQP4 levels in remote brain areas rather than the trauma epicenter itself. Interestingly, treating the core site of brain injury with focal hypothermia reduced the remote expression of AQP4 levels. In a recent study, we reported that AVN576, an orally active V1a receptor antagonist, given ip 24 hrs post injury for 5 days, could effectively reduce edema following TBI with brain damage [46].
Limitations and Data Interpretation
This was an initial study with a limited number of animals to explore the possibility that we could measures significant changes in ADC following a single mild head impact. As such there were several limitations: 1) ADC is a proxy for vasogenic edema and while there was no evidence of structural brain damage, post mortem histology looking at site-specific or regional signs of inflammation or alterations in BBB permeability would have helped to interpret the findings [59]. 2) The sensitivity of DWI was such that it did not allow us to examine 150 individual brain areas; instead, we were only able to observe change in ADC values in the whole brain and in three brain regions, cerebellum, thalamus, and basal ganglia. 3) Would there have been sex differences if we ran female rats? We are aware of the many studies reporting sex differences in response to TBI, e.g. [5, 7, 12–14, 58, 75]. Recent reviews of the literature, report there are male/female differences in morbidity and mortality following TBI [9, 45]. However, the data collected over decades of preclinical and clinical research are inconsistent, certainly as it relates to the role of estrogen and progesterone [76]. These initial studies were not designed to address the issues surrounding hormones and head injury. 4) 5) Animals were exposed to light isoflurane anesthesia four times over a 24 hr period. This necessary condition over this abbreviated time span precluded taking any meaningful measures of motor or cognitive behavior.
These data reinforce concerns expressed by others about the long-term effect of mild brain injury [51]. For example, mild brain injury begins with an impact that by itself may be recorded but not pursued as needing medical attention. Yet here we show there are global changes in vasogenic edema presumably caused by disruption in the BBB. Newsome et al. [50] reported modest neurocognitive abnormalities and increased functional connectivity in adolescent athletes with sports related concussions as compared to age matched orthopedically injured controls. Despite these signs of altered cortical function, post-concussive symptoms were judged to be resolved and athletes could return to competition. Indeed, more adolescent players are suffering from undetected neurological problems than previously thought [66]. A single season of high school American football produces DWI changes in diffusivity in the absence of clinical concussion [16]. Multiple mild head injuries are a risk factor for cognitive, and emotion impairment and neurodegenerative disorders in athletes [41].
Conclusion
We were interested in two primary questions 1) could we detect global changes in brain edema using ADC, and 2) could these changes be mitigated by drug treatment with a vasopressin 1a antagonist? This study differs from previous animal TBI studies as it relies on imaging, without animal behavior, to evaluate the consequences of a mild head impact. The Glasgow Coma Scale of 13–15 defines a brain injury as “mild” based on measures of motor behavior, verbal responses and eye opening, with loss of consciousness and short hospitalization [27]. With CT and MRI, physical damage to the brain can be confirmed, adjusting the classification to “mild with complication” [37]. Organized sports in adolescence added another dimension to the characterization of “mild.” The Centers for Disease Control and Prevention, World Health Organization and the Veteran’s Administration have included self-reports of transient confusion, disorientation, impaired consciousness or dysfunction in memory around the time of the injury - importantly there should be no structural damage as determined with imaging [1, 10, 65]. This study in rats used a single head impact that caused no structural damage to the brain but clearly showed global and regional increases in vasogenic edema that appear within 2 hrs, increase at 6 hrs, but return to baseline within a day. Systemic treatment with a selective V1a receptor antagonist that can cross the BBB reduces much of the vasogenic edema. These findings highlight the potential risk to a single mild head impact and the role AVP in acute vasogenic edema.
Supplementary Material
Highlights.
A single, mild head impact causes a rise in vasogenic edema 2–6 hrs post impact.
Areas most affected, like the cerebellum, are remote from the site of impact.
The edema peaks within 6 hrs of head impact but resolves within 24 hrs.
A vasopressin V1a receptor antagonist blocks much of the early vasogenic edema.
Acknowledgments
Funding
Supported in part by NINDS SBIR award 1R43NS110343 to M.J.B, (C.F. and N.G.S., co-Investigators) and Azevan Pharmaceuticals, Inc.
List of Abbreviations
- EPI
echo planar imaging
- MRI
magnetic resonance imaging
- AVP
arginine vasopressin
- V1a
vasopressin V1a receptor
- FOV
field of view
- RARE
rapid acquisition relaxation enhanced
- BBB
blood-brain barrier
- ADC
apparent diffusion coefficient
- DWI
diffusion weighted imaging
- TBI
traumatic brain injury
- DCE
dynamic contrast enhanced
- TR
repetition time
- TE
echo time
- NEX
number of excitations
Footnotes
Competing interests. C.F.F has a financial interest in Animal Imaging Research, the company that makes the RF electronics and holders for animal imaging. C.F.F, S.L., MJB, and N.G.S. hold equity in Azevan Pharmaceuticals, Inc. M.J.B. and N.G.S receive compensation from Azevan.
DECLARATIONS
Consent for publication
Yes
Ethics approval and consent to participate
Not applicable
Availability of data and material
All data can be accessed through a link to Mandeley. DOI to follow.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1].Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention, Centers for Disease Control and Prevention, Atlanta, GA, in, 2018. [Google Scholar]
- [2].Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A, An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain, Proc Natl Acad Sci U S A, 100 (2003) 2106–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barzo P, Marmarou A, Fatouros P, Hayasaki K, Corwin F, Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging, Journal of neurosurgery, 87 (1997) 900–907. [DOI] [PubMed] [Google Scholar]
- [4].Baskaya MK, Rao AM, Dogan A, Donaldson D, Dempsey RJ, The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats, Neurosci Lett, 226 (1997) 33–36. [DOI] [PubMed] [Google Scholar]
- [5].Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP, Sex differences in outcome after mild traumatic brain injury, Journal of neurotrauma, 27 (2010) 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bemana I, Nagao S, Treatment of brain edema with a nonpeptide arginine vasopressin V1 receptor antagonist OPC-21268 in rats, Neurosurgery, 44 (1999) 148–154; discussion 154–145. [DOI] [PubMed] [Google Scholar]
- [7].Broshek DK, Kaushik T, Freeman JR, Erlanger D, Webbe F, Barth JT, Sex differences in outcome following sports-related concussion, Journal of neurosurgery, 102 (2005) 856–863. [DOI] [PubMed] [Google Scholar]
- [8].Cai X, Qiao J, Knox T, Iriah S, Kulkarni P, Madularu D, Morrison T, Waszczak B, Hartner JC, Ferris CF, In search of early neuroradiological biomarkers for Parkinson’s Disease: Alterations in resting state functional connectivity and gray matter microarchitecture in PINK1 −/− rats, Brain Res, 1706 (2019) 58–67. [DOI] [PubMed] [Google Scholar]
- [9].Caplan HW, Cox CS, Bedi SS, Do microglia play a role in sex differences in TBI?, Journal of neuroscience research, 95 (2017) 509–517. [DOI] [PubMed] [Google Scholar]
- [10].Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, Kraus J, Coronado VG, W.H.O.C.C.T.F.o.M.T.B. Injury, Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury, Journal of rehabilitation medicine : official journal of the UEMS European Board of Physical and Rehabilitation Medicine, (2004) 28–60. [DOI] [PubMed] [Google Scholar]
- [11].Chappell MH, Ulug AM, Zhang L, Heitger MH, Jordan BD, Zimmerman RD, Watts R, Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study, Journal of magnetic resonance imaging : JMRI, 24 (2006) 537–542. [DOI] [PubMed] [Google Scholar]
- [12].Colantonio A, Harris JE, Ratcliff G, Chase S, Ellis K, Gender differences in self reported long term outcomes following moderate to severe traumatic brain injury, BMC Neurol, 10 (2010) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colvin AC, Mullen J, Lovell MR, West RV, Collins MW, Groh M, The role of concussion history and gender in recovery from soccer-related concussion, The American journal of sports medicine, 37 (2009) 1699–1704. [DOI] [PubMed] [Google Scholar]
- [14].Covassin T, Schatz P, Swanik CB, Sex differences in neuropsychological function and post-concussion symptoms of concussed collegiate athletes, Neurosurgery, 61 (2007) 345–350; discussion 350–341. [DOI] [PubMed] [Google Scholar]
- [15].Cui T, Zhu G, Ulinastatin attenuates brain edema after traumatic brain injury in rats, Cell Biochem Biophys, 71 (2015) 595–600. [DOI] [PubMed] [Google Scholar]
- [16].Davenport EM, Whitlow CT, Urban JE, Espeland MA, Jung Y, Rosenbaum DA, Gioia GA, Powers AK, Stitzel JD, Maldjian JA, Abnormal white matter integrity related to head impact exposure in a season of high school varsity football, Journal of neurotrauma, 31 (2014) 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Diaz-Arrastia R, Kochanek PM, Bergold P, Kenney K, Marx CE, Grimes CJ, Loh LT, Adam LT, Oskvig D, Curley KC, Salzer W, Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup, Journal of neurotrauma, 31 (2014) 135–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Donkin JJ, Vink R, Mechanisms of cerebral edema in traumatic brain injury: therapeutic developments, Curr Opin Neurol, 23 (2010) 293–299. [DOI] [PubMed] [Google Scholar]
- [19].Ferris CF, Nodine S, Pottala T, Cai X, Knox TM, Fofana FH, Kim S, Kulkarni P, Crystal JD, Hohmann AG, Alterations in brain neurocircuitry following treatment with the chemotherapeutic agent paclitaxel in rats, Neurobiol Pain, 6 (2019) 100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Filippidis AS, Liang X, Wang W, Parveen S, Baumgarten CM, Marmarou CR, Real-time monitoring of changes in brain extracellular sodium and potassium concentrations and intracranial pressure after selective vasopressin-1a receptor inhibition following focal traumatic brain injury in rats, Journal of neurotrauma, 31 (2014) 1258–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Finan JD, Biomechanical simulation of traumatic brain injury in the rat, Clin Biomech (Bristol, Avon), 64 (2019) 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fukuda K, Tanno H, Okimura Y, Nakamura M, Yamaura A, The blood-brain barrier disruption to circulating proteins in the early period after fluid percussion brain injury in rats, Journal of neurotrauma, 12 (1995) 315–324. [DOI] [PubMed] [Google Scholar]
- [23].Gultekin R, Huang S, Clavisi O, Pattuwage L, Konig TC, Gruen R, Pharmacological interventions in traumatic brain injury: Can we rely on systematic reviews for evidence?, Injury, 47 (2016) 516–524. [DOI] [PubMed] [Google Scholar]
- [24].Habgood MD, Bye N, Dziegielewska KM, Ek CJ, Lane MA, Potter A, Morganti-Kossmann C, Saunders NR, Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice, Eur J Neurosci, 25 (2007) 231–238. [DOI] [PubMed] [Google Scholar]
- [25].Hudak AM, Peng L, Marquez C de la Plata, Thottakara J, Moore C, Harper C, McColl R, Babcock E, Diaz-Arrastia R, Cytotoxic and vasogenic cerebral oedema in traumatic brain injury: assessment with FLAIR and DWI imaging, Brain injury : [BI], 28 (2014) 1602–1609. [DOI] [PubMed] [Google Scholar]
- [26].Ito J, Marmarou A, Barzo P, Fatouros P, Corwin F, Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury, Journal of neurosurgery, 84 (1996) 97–103. [DOI] [PubMed] [Google Scholar]
- [27].Jennett B, Teasdale G, Aspects of coma after severe head injury, Lancet, 1 (1977) 878–881. [DOI] [PubMed] [Google Scholar]
- [28].Jha RM, Kochanek PM, Simard JM, Pathophysiology and treatment of cerebral edema in traumatic brain injury, Neuropharmacology, 145 (2019) 230–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jungner M, Siemund R, Venturoli D, Reinstrup P, S.C. W, Bentzer P, Blood-brain barrier permeability following traumatic brain injury, Minerva Anestesiol, 82 (2016) 525–533. [PubMed] [Google Scholar]
- [30].Katz DI, Cohen SI, Alexander MP, Mild traumatic brain injury, Handbook of clinical neurology edited by Vinken PJ and Bruyn GW, 127 (2015) 131–156. [DOI] [PubMed] [Google Scholar]
- [31].Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research, PLoS biology, 8 (2010) e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kleindienst A, Dunbar JG, Glisson R, Marmarou A, The role of vasopressin V1A receptors in cytotoxic brain edema formation following brain injury, Acta neurochirurgica, 155 (2013) 151–164. [DOI] [PubMed] [Google Scholar]
- [33].Kozniewska E, Romaniuk K, Vasopressin in vascular regulation and water homeostasis in the brain, Journal of physiology and pharmacology : an official journal of the Polish Physiological Society, 59 Suppl 8 (2008) 109–116. [PubMed] [Google Scholar]
- [34].Krieg SM, Sonanini S, Plesnila N, Trabold R, Effect of small molecule vasopressin V1a and V2 receptor antagonists on brain edema formation and secondary brain damage following traumatic brain injury in mice, Journal of neurotrauma, 32 (2015) 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Krieg SM, Trabold R, Plesnila N, Time-Dependent Effects of Arginine-Vasopressin V1 Receptor Inhibition on Secondary Brain Damage after Traumatic Brain Injury, Journal of neurotrauma, 34 (2017) 1329–1336. [DOI] [PubMed] [Google Scholar]
- [36].Kulkarni P, Morrison TR, Cai X, Iriah S, Simon N, Sabrick J, Neuroth L, Ferris CF, Neuroradiological Changes Following Single or Repetitive Mild TBI, Frontiers in systems neuroscience, 13 (2019) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Levin HS, Williams DH, Eisenberg HM, High WM Jr., Guinto FC Jr., Serial MRI and neurobehavioural findings after mild to moderate closed head injury, Journal of neurology, neurosurgery, and psychiatry, 55 (1992) 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li W, Watts L, Long J, Zhou W, Shen Q, Jiang Z, Li Y, Duong TQ, Spatiotemporal changes in blood-brain barrier permeability, cerebral blood flow, T2 and diffusion following mild traumatic brain injury, Brain Res, 1646 (2016) 53–61. [DOI] [PubMed] [Google Scholar]
- [39].Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Buki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, van Essen TA, Feigin VL, Gao G, Giacino J, Gonzalez-Lara LE, Gruen RL, Gupta D, Hartings JA, Hill S, Jiang JY, Ketharanathan N, Kompanje EJO, Lanyon L, Laureys S, Lecky F, Levin H, Lingsma HF, Maegele M, Majdan M, Manley G, Marsteller J, Mascia L, McFadyen C, Mondello S, Newcombe V, Palotie A, Parizel PM, Peul W, Piercy J, Polinder S, Puybasset L, Rasmussen TE, Rossaint R, Smielewski P, Soderberg J, Stanworth SJ, Stein MB, von Steinbuchel N, Stewart W, Steyerberg EW, Stocchetti N, Synnot A, Te Ao B, Tenovuo O, Theadom A, Tibboel D, Videtta W, Wang KKW, Williams WH, Wilson L, Yaffe K, In TP, Investigators, Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research, Lancet neurology, 16 (2017) 987–1048. [DOI] [PubMed] [Google Scholar]
- [40].Majdan M, Plancikova D, Maas A, Polinder S, Feigin V, Theadom A, Rusnak M, Brazinova A, Haagsma J, Years of life lost due to traumatic brain injury in Europe: A cross-sectional analysis of 16 countries, PLoS medicine, 14 (2017) e1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Manley G, Gardner AJ, Schneider KJ, Guskiewicz KM, Bailes J, Cantu RC, Castellani RJ, Turner M, Jordan BD, Randolph C, Dvorak J, Hayden KA, Tator CH, McCrory P, Iverson GL, A systematic review of potential long-term effects of sport-related concussion, Br J Sports Med, 51 (2017) 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J, Zhu T, Blackman E, Stewart D, Ellis J, Butler R, Janigro D, Consequences of repeated blood-brain barrier disruption in football players, PLoS One, 8 (2013) e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Marmarou CR, Liang X, Abidi NH, Parveen S, Taya K, Henderson SC, Young HF, Filippidis AS, Baumgarten CM, Selective vasopressin-1a receptor antagonist prevents brain edema, reduces astrocytic cell swelling and GFAP, V1aR and AQP4 expression after focal traumatic brain injury, Brain Res, 1581 (2014) 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Meabon JS, Huber BR, Cross DJ, Richards TL, Minoshima S, Pagulayan KF, Li G, Meeker KD, Kraemer BC, Petrie EC, Raskind MA, Peskind ER, Cook DG, Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction, Science translational medicine, 8 (2016) 321ra326. [DOI] [PubMed] [Google Scholar]
- [45].Mollayeva T, Mollayeva S, Colantonio A, Traumatic brain injury: sex, gender and intersecting vulnerabilities, Nature reviews. Neurology, 14 (2018) 711–722. [DOI] [PubMed] [Google Scholar]
- [46].Morrison TR, Kulkarni P, Cai X, Iriah S, Aggarwal D, Lu SF, Simon NG, Madularu D, Ferris CF, Treating head injury using a novel vasopressin 1a receptor antagonist, Neurosci Lett, 714 (2020) 134565. [DOI] [PubMed] [Google Scholar]
- [47].Morrison TR, Melloni RH Jr., The role of serotonin, vasopressin, and serotonin/vasopressin interactions in aggressive behavior, Current topics in behavioral neurosciences, 17 (2014) 189–228. [DOI] [PubMed] [Google Scholar]
- [48].Mychasiuk R, Hehar H, Candy S, Ma I, Esser MJ, The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes, Journal of neuroscience methods, 257 (2016) 168–178. [DOI] [PubMed] [Google Scholar]
- [49].Nathan DE, Oakes TR, Yeh PH, French LM, Harper JF, Liu W, Wolfowitz RD, Wang BQ, Graner JL, Riedy G, Exploring variations in functional connectivity of the resting state default mode network in mild traumatic brain injury, Brain connectivity, 5 (2015) 102–114. [DOI] [PubMed] [Google Scholar]
- [50].Newsome MR, Li X, Lin X, Wilde EA, Ott S, Biekman B, Hunter JV, Dash PK, Taylor BA, Levin HS, Functional Connectivity Is Altered in Concussed Adolescent Athletes Despite Medical Clearance to Return to Play: A Preliminary Report, Front Neurol, 7 (2016) 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].O’Keeffe E, Kelly E, Liu Y, Giordano C, Wallace E, Hynes M, Tiernan S, Meagher A, Greene C, Hughes S, Burke T, Kealy J, Doyle N, Hay A, Farrell M, Grant GA, Friedman A, Veksler R, Molloy MG, Meaney JF, Pender N, Camarillo D, Doherty CP, Campbell M, Dynamic Blood-Brain Barrier Regulation in Mild Traumatic Brain Injury, Journal of neurotrauma, 37 (2020) 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ostrowski NL, Lolait SJ, Young WS 3rd, Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature, Endocrinology, 135 (1994) 1511–1528. [DOI] [PubMed] [Google Scholar]
- [53].Pascale CL, Szmydynger-Chodobska J, Sarri JE, Chodobski A, Traumatic brain injury results in a concomitant increase in neocortical expression of vasopressin and its V1a receptor, Journal of physiology and pharmacology : an official journal of the Polish Physiological Society, 57 Suppl 11 (2006) 161–167. [PubMed] [Google Scholar]
- [54].Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D, Hart K, Yu CE, Raskind MA, Cook DG, Minoshima S, Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms, NeuroImage, 54 Suppl 1 (2011) S76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rauen K, Trabold R, Brem C, Terpolilli NA, Plesnila N, Arginine vasopressin V1a receptor-deficient mice have reduced brain edema and secondary brain damage following traumatic brain injury, Journal of neurotrauma, 30 (2013) 1442–1448. [DOI] [PubMed] [Google Scholar]
- [56].Ren H, Lu H, Dynamic features of brain edema in rat models of traumatic brain injury, Neuroreport, 30 (2019) 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rossi-Mossuti F, Fisch U, Schoettker P, Gugliotta M, Morard M, Schucht P, Schatlo B, Levivier M, Walder B, Fandino J, Surgical Treatment of Severe Traumatic Brain Injury in Switzerland: Results from a Multicenter Study, Journal of neurological surgery. Part A, Central European neurosurgery, 77 (2016) 36–45. [DOI] [PubMed] [Google Scholar]
- [58].Sandel NK, Schatz P, Goldberg KB, Lazar M, Sex-Based Differences in Cognitive Deficits and Symptom Reporting Among Acutely Concussed Adolescent Lacrosse and Soccer Players, The American journal of sports medicine, 45 (2017) 937–944. [DOI] [PubMed] [Google Scholar]
- [59].Shultz SR, Sun M, Wright DK, Brady RD, Liu S, Beynon S, Schmidt SF, Kaye AH, Hamilton JA, O’Brien TJ, Grills BL, McDonald SJ, Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 35 (2015) 1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Szczygielski J, Glameanu C, Muller A, Klotz M, Sippl C, Hubertus V, Schafer KH, Mautes AE, Schwerdtfeger K, Oertel J, Changes in Posttraumatic Brain Edema in Craniectomy-Selective Brain Hypothermia Model Are Associated With Modulation of Aquaporin-4 Level, Front Neurol, 9 (2018) 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Szczygielski J, Hubertus V, Kruchten E, Muller A, Albrecht LF, Mautes AE, Schwerdtfeger K, Oertel J, Brain Edema Formation and Functional Outcome After Surgical Decompression in Murine Closed Head Injury Are Modulated by Acetazolamide Administration, Front Neurol, 10 (2019) 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Szmydynger-Chodobska J, Chung I, Kozniewska E, Tran B, Harrington FJ, Duncan JA, Chodobski A, Increased expression of vasopressin v1a receptors after traumatic brain injury, Journal of neurotrauma, 21 (2004) 1090–1102. [DOI] [PubMed] [Google Scholar]
- [63].Szmydynger-Chodobska J, Fox LM, Lynch KM, Zink BJ, Chodobski A, Vasopressin amplifies the production of proinflammatory mediators in traumatic brain injury, Journal of neurotrauma, 27 (2010) 1449–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Szmydynger-Chodobska J, Gandy JR, Varone A, Shan R, Chodobski A, Synergistic interactions between cytokines and AVP at the blood-CSF barrier result in increased chemokine production and augmented influx of leukocytes after brain injury, PLoS One, 8 (2013) e79328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].T.B.I.W.G., Management of concussion/m, VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury, J. Rehabil. Res. Dev, 46 (2009) 1–68. [PubMed] [Google Scholar]
- [66].Talavage TM, Nauman EA, Breedlove EL, Yoruk U, Dye AE, Morigaki KE, Feuer H, Leverenz LJ, Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion, Journal of neurotrauma, 31 (2014) 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tanno H, Nockels RP, Pitts LH, Noble LJ, Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1: Distribution and time course of protein extravasation, Journal of neurotrauma, 9 (1992) 21–32. [DOI] [PubMed] [Google Scholar]
- [68].Taya K, Gulsen S, Okuno K, Prieto R, Marmarou CR, Marmarou A, Modulation of AQP4 expression by the selective V1a receptor antagonist, SR49059, decreases trauma-induced brain edema, Acta neurochirurgica. Supplement, 102 (2008) 425–429. [DOI] [PubMed] [Google Scholar]
- [69].Taylor CA, Bell JM, Breiding MJ, Xu L, Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013, MMWR Surveill Summ, 66 (2017) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Toth A, Magnetic Resonance Imaging Application in the Area of Mild and Acute Traumatic Brain Injury: Implications for Diagnostic Markers?, in: Kobeissy FH (Ed.) Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects, Boca Raton (FL), 2015. [PubMed] [Google Scholar]
- [71].Trabold R, Krieg S, Scholler K, Plesnila N, Role of vasopressin V(1a) and V2 receptors for the development of secondary brain damage after traumatic brain injury in mice, Journal of neurotrauma, 25 (2008) 1459–1465. [DOI] [PubMed] [Google Scholar]
- [72].Vergara VM, Mayer AR, Damaraju E, Kiehl KA, Calhoun V, Detection of Mild Traumatic Brain Injury by Machine Learning Classification Using Resting State Functional Network Connectivity and Fractional Anisotropy, Journal of neurotrauma, 34 (2017) 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Viano DC, Hamberger A, Bolouri H, Saljo A, Concussion in professional football: animal model of brain injury--part 15, Neurosurgery, 64 (2009) 1162–1173; discussion 1173. [DOI] [PubMed] [Google Scholar]
- [74].Weissberg I, Veksler R, Kamintsky L, Saar-Ashkenazy R, Milikovsky DZ, Shelef I, Friedman A, Imaging blood-brain barrier dysfunction in football players, JAMA neurology, 71 (2014) 1453–1455. [DOI] [PubMed] [Google Scholar]
- [75].Wright DK, O’Brien TJ, Shultz SR, Mychasiuk R, Sex matters: repetitive mild traumatic brain injury in adolescent rats, Ann Clin Transl Neurol, 4 (2017) 640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wright DW, Yeatts SD, Silbergleit R, Progesterone in traumatic brain injury, The New England journal of medicine, 372 (2015) 1766–1767. [DOI] [PubMed] [Google Scholar]
- [77].Young LJ, Toloczko D, Insel TR, Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain, J Neuroendocrinol, 11 (1999) 291–297. [DOI] [PubMed] [Google Scholar]
- [78].Yu M, Wang M, Yang D, Wei X, Li W, Dynamics of blood brain barrier permeability and tissue microstructure following controlled cortical impact injury in rat: A dynamic contrast-enhanced magnetic resonance imaging and diffusion kurtosis imaging study, Magn Reson Imaging, 62 (2019) 1–9. [DOI] [PubMed] [Google Scholar]
- [79].Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM, Diffusion anisotropy changes in the brains of professional boxers, AJNR. American journal of neuroradiology, 27 (2006) 2000–2004. [PMC free article] [PubMed] [Google Scholar]
- [80].Zhang L, Ravdin LD, Relkin N, Zimmerman RD, Jordan B, Lathan WE, Ulug AM, Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury?, AJNR. American journal of neuroradiology, 24 (2003) 52–57. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.