Abstract
Background.
Excellent adherence to HIV antiretroviral therapy (ART) remains a cornerstone of HIV care. A three-item adherence self-report scale was recently developed and validated, but the scale has not been previously tested in a nationally representative sample.
Design.
We administered the adherence scale to participants in the CDC’s Medical Monitoring Project (MMP), which is a probability sample of U.S. adults with diagnosed HIV.
Methods.
We combined sociodemographic and clinical participant data from three consecutive cycles of the Medical Monitoring Project (6/2015–5/2018). We used medical record reviews to determine most recent viral load, and whether viral loads were suppressed at all measurement points in the past 12 months. We describe the relationship between adherence scale score and two measures of viral load suppression (most recent and sustained), and estimate linear regression models using sampling weights to determine independent predictors of ART adherence scores.
Results.
Of those using ART, the median adherence score was 93 (100=perfect adherence), and the standardized Cronbach’s alpha was 0.83. For both measures of viral load suppression, the relationship with the adherence score was generally linear; there was no “cutoff’ point indicating good vs. poor adherence. In the multivariable model, younger age, non-white race, poverty, homelessness, depression, binge-drinking, and both non-injection and injection drug use were independently associated with lower adherence.
Conclusions.
The adherence measure had good psychometric qualities and a linear relationship with viral load, supporting its use in both clinical care and research. Adherence interventions should focus on persons with the highest risk of poor adherence.
Keywords: Medication Adherence, Treatment Adherence and Compliance, Patient Compliance, HIV, Antiretroviral Therapy, Highly Active, Behavior Rating Scale
INTRODUCTION
The efficacy and tolerability of oral antiretroviral therapies (ART) has improved dramatically over the last 20 years [1, 2]. The recent approval of long acting injectable ART [3], and the possibility that depot delivery systems will be approved in the next few years offer hope that there will soon be an array of options available for those for whom daily oral therapy has proved too difficult. But oral ART will likely continue to be the mainstay of HIV treatment for most patients throughout the world for the foreseeable future, and with it the need to measure and monitor oral ART adherence. Adherence to ART can be measured many ways, including through pharmacy claims databases, self-report, electronic monitoring of pill container openings, pill counts, monitoring of drug levels in serum or hair, and a variety of other techniques [4, 5]. Clinicians and adherence researchers need to be flexible in how they measure adherence because the complexity, cost and intrusiveness of these methods differ dramatically. Recent guidelines have emphasized the importance of valid adherence measurement [6].
Wilson et al recently developed and validated a three-item adherence scale that can be used for any oral medication, including ART [7–9]. The scale has been translated into a number of different languages and has been successfully used in a number of health care settings. For example, in South Africa it was translated into Xhosa, and successfully used to examine adherence to ART in pregnant and post-partum women [10, 11]. In that context, scale scores were associated with viral load measures cross-sectionally, and predicted nonadherence using a longitudinal study design.
In this paper we report on the use of the three-item scale in a national probability sample of US adults with HIV who were part of the Centers for Disease Control and Prevention’s (CDC’s) Medical Monitoring Project (MMP) during 3 annual cycles spanning 6/2015–5/2018. We had three main study questions. First, what were the psychometric properties of the scale in a diverse, national sample of adults with HIV using ART? Second, what was the relationship between scale scores and patient viral load measurements? Third, how did adherence vary in different sociodemographic and clinical subsets of persons with HIV?
METHODS
Participants and data collection
MMP is a national surveillance system that collects interview and medical record data from a probability sample of US adults with diagnosed HIV [12]. MMP methods are described in full elsewhere [13], but MMP used a 2 stage sampling design. During the first stage, 23 jurisdictions were sampled from all U.S. states, the District of Columbia, and Puerto Rico. Second, simple random samples of persons with diagnosed HIV aged 18 years and older who were alive at the end of the year prior to the start of data collection were drawn for each participating state/territory from the National HIV Surveillance System (NHSS), a census of persons with diagnosed HIV in the United States. For this analysis, we combined data from 3 annual data collection cycles; data were collected via phone or face-to-face interviews and medical records were abstracted during June 2015 through May 2018. Response rates were 100% at the state/territory level and ranged from 40–46% at the person level across cycle years. Data were weighted based on known probabilities of selection and were adjusted for non-response. Then, data were post-stratified to known population totals from NHSS by sex, race/ethnicity, and age.
In accordance with the federal human subjects protection regulations [14] and guidelines for defining public health research [15], MMP has been determined to be a non-research, public health surveillance activity used for disease control program or policy purposes. Participating states or territories and facilities obtain local institutional review board approval to conduct MMP if required locally. Informed consent is obtained from all interviewed participants.
Sample
For this analysis, we included patients who were currently taking ART and who responded to all three adherence items. Of the full sample, 93.7% (11162/11914) responded to all three adherence items. Of these, 10.1% (1124/11162) responded to the Spanish language version of the survey.
Variables
Adherence measurement
Adherence was measured using a 3-item adherence scale previously developed and validated by Wilson et al [7–9]. The scale was only administered to persons who reported currently taking ART. The first item asked, “In the past 30 days, on how many days did you miss at least one dose of any of your HIV medicines.” The response option was a number between 0 and 30. The second item asked, “In the past 30 days, how good a job did you do at taking your HIV medicines in the way you were supposed to?” Likert-type response options were very poor, poor, fair, good, very good, and excellent. The third item asked, “During the past 30 days, how often did you take your HIV medicines in the way you were supposed to?” Response options were never, rarely, sometimes, usually, almost always, and always.” Item responses for the three adherence items were linearly transformed to a 0–100 scale with zero being the worst adherence, and 100 the best. Summary scales were calculated as the mean of the three individual items (see Appendix 1 for details, http://links.lww.com/QAD/B844). Based on the distribution of the summary adherence score, the 0–100 adherence score was analyzed as a continuous variable.
HIV viral loads
Viral load measures were determined by medical record review, as described elsewhere [16]. We used two viral load measurements: whether the viral load was suppressed (documented as < 200 copies/mm3 or undetectable) at the most recent viral load test (“recent viral suppression”), and whether the viral load was suppressed at all tests over the past 12 months (“sustained viral suppression”). The mean (median) time difference between the time of the most recent viral load and the time of adherence assessment was 101 (79) days, and 22.5% (weighted, unweighted n=2261) were within 30 days of the self-report. There was no association (p=0.59) between recent viral load suppression and whether the viral load was done in 30 days prior to the self-report. The mean (median) number of viral load tests that were used to determine sustained viral suppression was 2.4 (1.8) with a range of 1–11 tests.
Covariates
Age was analyzed by decade (18–29, 30–39, 40–49, and ≥50 years). Current gender was classified as male, female, and transgender. Sexual orientation was self-reported, and classified as homosexual/gay, heterosexual/straight, bisexual, and other. Race/ethnicity was classified as White (non-Hispanic), Black (non-Hispanic), Hispanic or Latino, or other/multiracial. Health insurance status was classified as (1) any private insurance, (2) public insurance only, or (3) Ryan White HIV/AIDS Program (RWHAP) or uninsured. Those with RWHAP were grouped with uninsured individuals because the RWHAP provides some primary care services and covers HIV antiretroviral medications, but is not a substitute for health insurance. Household income was classified as above or below the federal poverty level. Poverty guidelines were determined using the U.S. Department of Health and Human Services poverty guidelines corresponding to the calendar year about which the combined household income was asked. Education was classified as less than high school, high school or equivalent, or more than high school. Housing status was a dichotomous variable assessing homelessness (i.e., living on the street, in a shelter, in a single room occupancy hotel, or in a car) at any time during the last 12 months. HIV transmission category was measured using information from the NHSS [17]. HIV transmission categories included men who have sex with men (MSM), injection drug use (IDU), both MSM and IDU, heterosexual contact, and other (including no indicated risk and no reported risk). Depression during the past two weeks was based on the patient health questionnaire depression scale (PHQ-8) [18]. Participants were classified as having “depression” if they had experienced 5 or more symptoms at least “more than half the days” in the preceding two weeks. Binge drinking in the 30 days before being interviewed was defined as having ≥5 alcoholic drinks for men and 4≥ alcoholic drinks for women in one sitting on at least one day. Drug use was classified as noninjection (yes/no) and injection (yes/no) drug use. The antiretroviral regimens used by these patients are reported elsewhere [19].
Analyses
Descriptive characteristics are presented both as raw numbers and weighted percentages, with the weighting done as described above. To illustrate the relationship between the 3-item scale and each of the two measures of viral suppression, we showed rates of viral suppression for each 5-point increment of the scale, and calculated the R2 for the relationship from a simple linear regression equation. To further demonstrate the value of the 3-item scale, we also used the R2 to compare the 3-item scale with three possible 2-item scales (items 1&2, 1&3, and 2&3).
For our multivariable model of adherence, we estimated a linear regression model with adherence as the dependent variable. Variables that were statistically significant (p-value for inclusion, <0.1) in bivariate tests were included in the model. Backward elimination was used to determine the final model, with all variables in the final model having a p-value of <0.05. All analyses were conducted using SAS (Version 9.4; SAS Institute Inc., NC, USA).
RESULTS
Characteristics of US adults with HIV who were taking ART
Characteristics of US adults with HIV who were taking ART are shown in Table 1. Approximately half were 50 years or older, 74.7% were male, 41.1% were Black, and 22.4% were Hispanic. A tenth were uninsured, 54.3% had public insurance, 43.4% had a household income below the Federal Poverty Level, 17.9% had less than a high school education, and 8.7% had been homeless at some time in the last year. Depression was reported by 21.6%, binge drinking by 15.6%, noninjection drug use by 29.8%, and injection drug use by 2.6%.
Table 1.
Descriptive characteristics of persons with diagnosed HIV who are taking antiretroviral therapy—United States, 2015–2017. (N=11914)
| Characteristics | na | Col % (95% CI)b |
|---|---|---|
| Total | 11914 | |
| Age (years) | ||
| 18–29 | 989 | 9.0 (8.2–9.8) |
| 30–39 | 1947 | 16.6 (15.7–17.4) |
| 40–49 | 2894 | 24.8 (23.8–25.8) |
| >=50 | 6084 | 49.7 (48.2–51.1) |
| Current gender | ||
| Male | 8681 | 74.7 (73.2–76.3) |
| Female | 3049 | 23.8 (22.2–25.3) |
| Transgender | 169 | 1.5 (1.2–1.8) |
| Sexual orientation (selfreported) | ||
| Homosexual/gay | 4974 | 41.9 (39.7–44.2) |
| Heterosexual/straight | 5594 | 46.5 (44.2–48.8) |
| Bisexual | 987 | 8.4 (7.7–9.0) |
| Others | 359 | 3.2 (2.7–3.6) |
| Race and ethnicity | ||
| White (non-Hispanic) | 3537 | 29.6 (26.1–33.1) |
| Black (non-Hispanic) | 4983 | 41.1 (35.6–46.5) |
| Hispanic or Latino | 2594 | 22.4 (18.0–26.8) |
| Other/Multiracialc | 800 | 6.9 (6.0–7.9) |
| Healthcare insurance | ||
| No (including Ryan White only) | 1025 | 10.2 (8.4–12.0) |
| Yes | 10795 | 89.8 (88.0–91.6) |
| Healthcare coverage type | ||
| Any Private Insurance | 4113 | 35.4 (33.6–37.2) |
| Public Insurance Only | 6623 | 54.3 (52.1–56.5) |
| Ryan White Coverage Only/Uninsured | 1025 | 10.3 (8.5–12.1) |
| Poverty level | ||
| Above poverty level | 6198 | 56.6 (54.1–59.2) |
| At or below poverty level | 4902 | 43.4 (40.8–45.9) |
| Education | ||
| <High school | 2136 | 17.9 (16.6–19.1) |
| High school diploma or equivalent | 3061 | 25.7 (24.6–26.9) |
| >High school | 6664 | 56.4 (54.6–58.2) |
| Homeless at any time (past 12 months) | ||
| No | 10811 | 91.3 (90.6–92.0) |
| Yes | 1061 | 8.7 (8.0–9.4) |
| Depression | ||
| No depression | 9297 | 78.4 (77.2–79.6) |
| Any depression | 2459 | 21.6 (20.4–22.8) |
| HIV Transmission Category | ||
| MSM | 5966 | 50.9 (48.8–53.0) |
| IDU | 1260 | 9.5 (8.6–10.5) |
| MSM-IDU | 735 | 5.8 (5.1–6.5) |
| Heterosexual contact | 2654 | 21.0 (19.3–22.7) |
| Other (including NIR/NRR) | 1283 | 12.7 (10.8–14.7) |
| Cigarette smoking | ||
| No | 7800 | 65.1 (63.8–66.4) |
| Yes | 4021 | 34.9 (33.6–36.2) |
| Binge drinking (during past 30 days) | ||
| No | 9924 | 84.4 (83.3–85.5) |
| Yes | 1839 | 15.6 (14.5–16.7) |
| Noninjection drug use | ||
| No | 8230 | 70.2 (68.8–71.6) |
| Yes | 3569 | 29.8 (28.4–31.2) |
| Injection drug use | ||
| No | 11475 | 97.4 (96.8–97.9) |
| Yes | 340 | 2.6 (2.1–3.2) |
| Surveillance year | ||
| 2015 | 3654 | 32.8 (30.3–35.3) |
| 2016 | 4038 | 33.2 (30.9–35.6) |
| 2017 | 4222 | 34.0 (31.3–36.6) |
HIV, human immunodeficiency virus; CI, confidence interval; ART, antiretroviral therapy
Numbers are unweighted
Percentages and corresponding CIs are weighted percentages
Includes American Indian/Alaska Native, Asian, Native Hawaiian/Other Pacific Islander, or multiple races
Distribution and psychometric characteristics of the three-item adherence scale
Descriptive characteristics of the three items and the scale are shown in Table 2. The median of the final scale was 93, and the mean was 90.2 (sd 14.4). The 25th and 75th percentiles were 84 and 100. A histogram of the scores for the three-item scale is shown in the Appendix 2, http://links.lww.com/QAD/B845. Forty-four percent were at the ceiling (score of 100), 49.8% had adherence of 95 or higher, 66.3% had adherence of 90 or higher, and 75.9% had adherence of 85 or higher. The correlations between items 1 and 2, and 1 and 3 were both 0.60, and the correlation between items 2 and 3 was 0.69. The raw Cronbach’s alpha was 0.81, and the standardized alpha was 0.83. The weighted percent scoring at the ceiling (100) was 43.6% using all 3 items. For each two-item combination, it was 44.9% for items 1 and 2, 53.7% for items 1 and 3, 50.1 for items 2 and 3. For items 1, 2, and 3 individually, the percents were 59.6, 59.5, and 53.5, respectively.
Table 2.
Descriptive characteristics of items and three-item summary scale.
| Percentile | |||||
|---|---|---|---|---|---|
| Variable | Mean (sd) | 25th | 50th (median) | 75th | Range |
| Item 1 | 95.3 (116) | 93 | 100 | 100 | 1 to 100 |
| Item 2 | 84.3 (21.1) | 80 | 100 | 100 | 1 to 100 |
| Item 3 | 90.8 (17.0) | 80 | 100 | 100 | 1 to 100 |
| Scale | 90.2 (14.4) | 84 | 93 | 100 | 1 to 100 |
Items:
In the past 30 days, on how many days did you miss at least one dose of any of your HIV medicines (response option was a number between 0 and 30)
In the past 30 days, how good a job did you do at taking your HIV medicines in the way you were supposed to? (response options were very poor, poor, fair, good, very good, and excellent)
During the past 30 days, how often did you take your HIV medicines in the way you were supposed to? (response options were never, rarely, sometimes, usually, almost always, and always)
Rates of viral load suppression
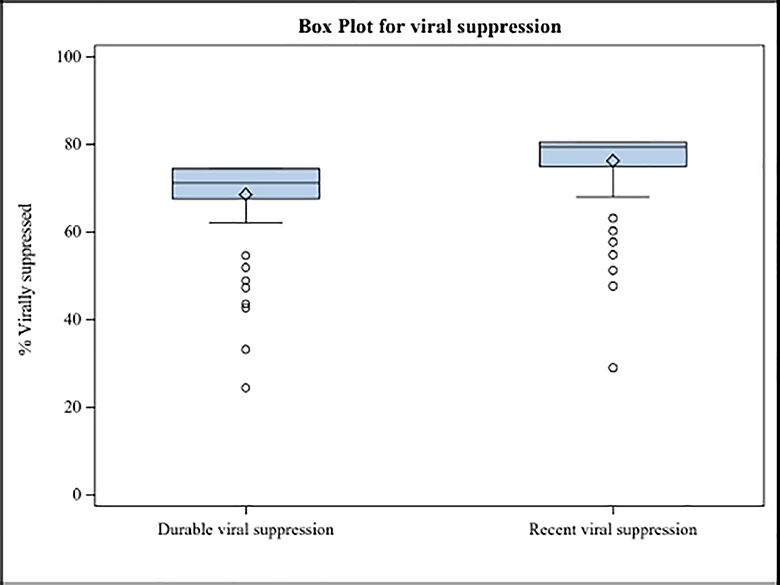
The distributions of rates of viral load suppression - both most recent and sustained – are shown in Figure 1. The mean (median) rate of suppression for the last viral load was 76.2% (78.7%) (25th and 75th percentile, 73.9% and 79.8%, results not presented in Figure 1). The mean (median) rate of sustained viral load suppression was 68.5% (71.1%) (25th and 75th percentile, 63.2% and 72.8%).
Figure 1.
Distribution of viral load suppression, for most recent viral load and sustained viral load suppression (last 12 months). This plot uses values from weighted sample.
Bivariate relationship between viral load measures and adherence
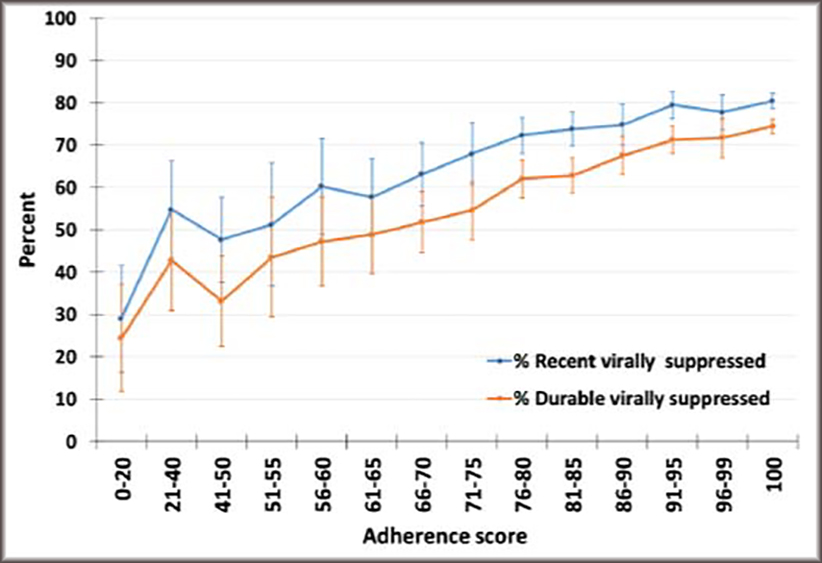
Figure 2 shows a plot of the adherence score by recent and sustained viral suppression, which indicates that the relationship between the score and both outcomes is generally linear. The Pearson correlation coefficients for the relationship between the adherence score and most recent and sustained viral suppression were 0.81 and 0.88, respectively. The R2s for the 3-item scale and recent and sustained viral suppression were 0.874 and 0.945, respectively. The R2s for recent viral suppression and 2-item scales composed of items 1&2, 1&3, and 2&3 were all lower, at 0.853, 0.732, and 0.735, respectively; for sustained viral load suppression the R2s were also all lower, at 0.929, 0.745, and 0.828, respectively.
Figure 2. Relationship between viral load suppression and adherence score among persons with diagnosed HIV who are taking antiretroviral therapy—United States, 2015–2017.
Notes: Estimates are weighted; recent viral suppression defined as most recent HIV viral load documented undetectable or <200 copies/mL; sustained viral suppression defined as all viral load measurements in the past 12 months documented undetectable or <200 copies/mL; adherence score based on 3-item scale with 0 indicating the worst adherence and 100 indicating the best adherence.
Bivariate and multivariable correlates of adherence
Bivariate and multivariable relationships are shown in Table 3. Comparisons of mean adherence scores indicated that all examined covariates had a significant association with adherence except for type of healthcare coverage. In the final model, factors associated with nonadherence included younger age (age 18–29 had 3.29 points lower adherence compared with ≥50 years, p<0.0001), non-white race/ethnicity (Blacks and Hispanics had 3.9 and 2.7 points lower adherence than Whites, both p<0.0001), poverty (poverty associated with 0.99 points lower adherence, p=0.0034), homelessness (4.12 points lower, p<0.0001), depression (3.65 points lower, p<0.0001), binge drinking (2.7 points lower, p<0.0001), and injection (3.69 points lower, p<0.0001) and noninjection drug use (5.93 points lower, p<0.0001).
Table 3.
Bivariate and multivariable linear regression models predicting adherence score among person with diagnosed HIV who are taking antiretroviral therapy—United States, 2015–2017.
| Adherence score | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| Characteristics | Least Squares mean | Difference in Least Squares mean | pvalue | Difference in Least Squares mean | pvalue |
| Total | |||||
| Age (years) | • | ||||
| 18–29 | 86.13 | −5.57 | <.0001 | −3.29 | <.0001 |
| 30–39 | 87.93 | −3.77 | <.0001 | −2.30 | <.0001 |
| 40–49 | 90.42 | −1.28 | 0.0006 | −0.56 | 0.1631 |
| >=50 | 91.70 | Ref. | . | Ref. | |
| Current gender | • | ||||
| Male | 90.63 | Ref. | . | .... | |
| Female | 89.44 | −1.19 | 0.0092 | .... | |
| Transgender | 89.92 | −0.71 | 0.6034 | .... | |
| Sexual orientation (selfreported) | • | ||||
| Homosexual/gay | 90.83 | Ref. | . | .... | |
| Heterosexual/straight | 90.07 | −0.76 | 0.0273 | .... | |
| Bisexual | 90.16 | −0.67 | 0.2637 | .... | |
| Other | 87.63 | −3.20 | 0.0047 | .... | |
| Race and ethnicity | • | ||||
| White (non-Hispanic) | 92.81 | Ref. | . | Ref. | |
| Black (non-Hispanic) | 88.72 | −4.09 | <.0001 | −3.90 | <.0001 |
| Hispanic or Latino | 89.94 | −2.87 | <.0001 | −2.70 | <.0001 |
| Other/Multiracial | 90.01 | −2.80 | 0.0001 | −1.62 | 0.0093 |
| Healthcare coverage(past 12 months) | . | ||||
| No | 89.77 | −0.62 | 0.407 | .... | |
| Yes | 90.39 | Ref. | . | .... | |
| Healthcare coverage type (past 12 months) | • | ||||
| Any Private Insurance | 91.85 | Ref. | . | .... | |
| Public Insurance Only | 89.42 | −2.43 | <.0001 | .... | |
| Ryan White HIV/AIDS Program Coverage Only/Uninsured | 89.77 | −2.08 | 0.0081 | .... | |
| Household poverty level (past 12 months) | . | ||||
| Above poverty level | 91.36 | Ref. | . | Ref. | |
| At or below poverty level | 89.04 | −2.32 | <.0001 | −0.99 | 0.0034 |
| Education | . | ||||
| <High school | 89.33 | −1.55 | 0.0032 | .... | |
| High school diploma or equivalent | 89.8 | −1.08 | 0.0053 | .... | |
| >High school | 90.88 | Ref. | . | .... | |
| Homeless at any time (past 12 months) | . | ||||
| No | 90.86 | Ref. | . | Ref. | |
| Yes | 84.08 | −6.78 | <.0001 | −4.12 | <.0001 |
| Depression (past 2 weeks) | . | ||||
| No depression | 91.34 | Ref. | . | Ref. | |
| Any depression | 86.70 | −4.64 | <.0001 | −3.65 | <.0001 |
| HIV transmission category | • | ||||
| MSM | 90.80 | Ref. | . | .... | |
| IDU | 89.37 | −1.43 | 0.0134 | .... | |
| MSM-IDU | 88.46 | −2.34 | 0.0011 | .... | |
| Heterosexual contact | 90.12 | −0.68 | 0.0744 | .... | |
| Other (including NIR/NRR) | 90.37 | −0.43 | 0.5602 | .... | |
| Cigarette smoking | • | ||||
| No | 91.48 | Ref. | . | .... | |
| Yes | 88.11 | −3.37 | <.0001 | .... | |
| Binge drinking (during past 30 days) | • | ||||
| No | 90.99 | Ref. | . | Ref. | |
| Yes | 87.07 | −3.93 | <.0001 | −2.72 | <.0001 |
| Noninjection drug use | • | ||||
| No | 91.76 | Ref. | . | Ref. | |
| Yes | 86.85 | −4.91 | <.0001 | −3.69 | <.0001 |
| Injection drug use | • | ||||
| No | 90.55 | Ref. | . | Ref. | |
| Yes | 81.93 | −8.62 | <.0001 | −5.93 | <.0001 |
| Surveillance year | • | ||||
| 2015 | 90.06 | Ref. | . | .... | |
| 2016 | 90.11 | −0.71 | 0.0482 | .... | |
| 2017 | 90.83 | Ref. | . | .... | |
Notes: All estimates are weighted; all variables assessed via self-report.
DISCUSSION
There are three main findings from this analysis. First, the three-item self-report adherence scale in this national probability sample of persons with HIV showed good internal consistency reliability, with a standardized Cronbach’s alpha of 0.83. Second, the adherence scale showed a strong relationship to both most recent and sustained viral load suppression (Pearson correlation coefficients of 0.81 and 0.88), and the relationship appears to be linear, which does not support the establishment of any cut point between acceptable and poor adherence. Third, in multivariable models, younger age, non-white race/ethnicity, poverty, homelessness, depression, binge drinking, and both non-injection and injection drug use were independently associated with non-adherence.
In a web-based pilot test, the Cronbach’s alpha of the three-item scale was 0.89 [9]. In the sample used for the original validity testing of the scale, which was taken from a single HIV care site, the Cronbach’s alpha was 0.84. In this diverse, nationally representative sample the Cronbach’s alpha was 0.81. Cronbach’s alphas ≥ 0.8 are generally considered “good” [20]. The distributional characteristics of a scale depend on the true, underlying adherence of the population sampled. In this sample, 44% reported perfect adherence, the median was 93, and the 25th percentile was 84. These findings suggest that adherence to ART in the US is far from optimal. However, the true test of how much adherence is “enough” depends on the relationship of the measure in question to relevant clinical, or in this case virological, outcomes.
There is debate about whether it is appropriate to dichotomize measures of adherence for purposes of analysis [21–24]. Ultimately, this question is empirical and can only be resolved through a careful examination of the relationship between the adherence measure in question and the clinical outcome that is the goal of therapy, implying that there is no “one size fits all” answer, because there are many different measurement approaches, and many different ways to assess clinical goals. Viral load is a powerful and widely agreed upon measure of ART treatment effect that is universally used to guide therapeutic decision making (e.g., whether to continue or change ART regimens) because of its strong association with morbidity, mortality, and transmissibility [2, 25]. Our data show that there is a linear relationship between the adherence scale and both suppression at the most recent viral load measurement and sustained viral suppression. This relationship is strong evidence that—using this adherence measurement approach—there is no cut point that identifies acceptable vs. unacceptable ART adherence.
While for some this result may be counterintuitive, there are a variety of reasons to believe that the idea of an adherence cut off is empirically unlikely. First, there is tremendous person-to-person variation in drug metabolism, which is further complicated by the fact that many PLWH have comorbid conditions that are often being treated by other medications that have pharmacokinetic interactions with ART [26]. Second, many PLWH have long and complex treatment histories, and often have some level of viral resistance [27]. Finally, it has long been recognized that even excellent adherence does not produce 100% rates of viral suppression; and, conversely, even poor adherence does not produce 100% rates of non-suppressed viral loads (e.g, Paterson et al. [28] Figure 1).
For both clinicians and researchers measuring adherence using this 3-item scale, the message is that better adherence will produce a higher probability of viral load suppression. Or conversely, less than 100% adherence increases the risk of viral load non-suppression compared with persons with 100% adherence. Our data show that even in the groups that report perfect (score of 100) adherence, rates of viral suppression were between 74% (most recent VL, data not shown) and 80% (consistently suppressed). There are several reasons why those with adherence scores of 100 might not be fully suppressed. First, the adherence score may overestimate actual adherence (socially desirable response bias) [29]. Second, the adherence score only examines the last 30 days, and adherence prior to that may differ. Third, because viral loads were determined by medical record review, the time gap between the date of the interview and the date of the most recent viral load test varied from person to person, with an average of 101 days. Fourth, as noted above, some patients may have viral resistance, preventing viral suppression even in the presence of excellent adherence.
Regardless of why there might be discordance between adherence and viral load measures, in this national probability sample of treated persons, rates of viral load suppression were suboptimal. There is evidence that modern ART regimens are more forgiving to nonadherence that prior regimens [30], but challenges clearly remain. Continued efforts to improve ART adherence, which is consistently identified as a barrier to better virological outcomes [31], are indicated.
Our multivariable model suggests directions that these efforts should take. Age and race/ethnicity are not “modifiable” risk factors for poor adherence, but the effects seen for both in our models are highly statistically significant. Studies have consistently shown that younger and non-White patients have worse adherence [32–34], and deserve special attention from care teams. It is likely that age and race in our model are surrogates or markers for more specific and potentially modifiable health behaviors (e.g., concerns about medication side effects) [35]. Clinicians and care teams should be alert to the existence of such factors. In a similar vein, poverty and homelessness are very difficult to intervene on, and are often identified as risk factors for nonadherence [36]. Programs such as the Ryan White HIV/AIDS Program that address critical social determinants of nonadherence are invaluable complements to high quality medical care. It has long been understood that depression, and other mental and behavioral health problems, are risk factors for nonadherence [37], and more aggressive efforts to identify and treat these medical problems in PLWH are needed. Finally, substance use is strongly associated with non-adherence in most studies [38, 39], as we found in this analysis, and the diagnosis and treatment of substance use problems remains a critical element of high-quality HIV care.
There are several study limitations. First, the three-item adherence scale could not be validated in this study by any other direct measure of adherence, such as electronic drug monitoring. Second, adherence self-reports and viral loads could not be assessed contemporaneously, which necessarily introduces some error into measures of their relationship. Third, these findings may not be generalizable to persons with HIV in other countries. Strengths of this study include the fact that it is a true probability sample of persons with diagnosed HIV in the United States, which includes samples from 2015, 2016, and 2017.
In conclusion, this study has both methodologic and clinical implications. These data support the assertion that adherence to ART in persons who are actively using ART (i.e., persisting with therapy) can be validly measured using the three-item adherence scale developed by Wilson and colleagues [7–11, 40–43]. In addition, the relationship between implementation and viral load suppression is linear, which implies that there is no empirically justifiable cutpoint between “enough” and “not enough” adherence as measured by the three-item scale. Despite more potent regimens with improved side effect profiles, adherence to ART is still a problem in the United States. Only 66.3% of patients had adherence of 90% or higher, and the median rate of suppression for the most recent viral load was 78.7% and the median rate of sustained suppression was 71.1%. A critical step in the federal strategy to end the HIV epidemic is rapid and effective treatment with ART such that viral suppression is achieved [44]. Clearly, additional progress needs to be made for this to happen. Adherence interventions should focus on persons with the greatest need, which in our analysis included younger age, non-white race, poverty, homelessness, depression, binge drinking, and both non-injection and injection drug use. The three-item scale used in this analysis is a simple tool that can be used in clinical and research settings to easily and accurately evaluate patient adherence to ART.
Supplementary Material
Acknowledgements:
We thank MMP participants, project area staff, and Provider and Community Advisory Board members. We also acknowledge the contributions of the Clinical Outcomes Team and Behavioral and Clinical Surveillance Branch at CDC.
Sources of Funding: Funding for the Medical Monitoring Project is provided by the Centers for Disease Control and Prevention. Dr. Wilson is partially supported by the Providence/Boston Center for AIDS Research (P30AI042853) and by Institutional Development Award Number U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds Advance Clinical and Translational Research (Advance-CTR) from the Rhode Island IDeA-CTR award (U54GM115677).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Trickey A, May MT, Vehreschild J-J, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. The Lancet HIV 2017; 4(8):e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services; . https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0. 2019. Accessed: December 26, 2019. [Google Scholar]
- 3.Gulick RM, Flexner C. Long-acting HIV drugs for treatment and prevention. Annual review of medicine 2019; 70:137–150. [DOI] [PubMed] [Google Scholar]
- 4.Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring medication adherence in research. AIDS and Behavior 2013; 17(1):284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann A, Aslani P, Ahmed R, Celio J, Gauchet A, Bedouch P, et al. Assessing medication adherence: options to consider. International journal of clinical pharmacy 2014; 36(1):55–69. [DOI] [PubMed] [Google Scholar]
- 6.De Geest S, Zullig LL, Dunbar-Jacob J, Helmy R, Hughes DA, Wilson IB, et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med 2018; 169(1):30–35. doi: 10.7326/M7318-0543. Epub 2018 Jun 7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson IB, Lee Y, Michaud J, Fowler FJ, Rogers WH. Validation of a new three-item self-report measure for medication adherence. AIDS and Behavior 2016; 20(11):2700–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler FJ Jr, Lloyd SJ, Cosenza CA, Wilson IB. Coding cognitive interviews: an approach to enhancing the value of cognitive testing for survey question evaluation. Field Methods 2016; 28(1):3–20. [Google Scholar]
- 9.Wilson IB, Fowler FJ, Cosenza CA, Michaud J, Bentkover J, Rana A, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS and Behavior 2014; 18(12):2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips TK, Wilson IB, Brittain K, Zerbe A, Mellins CA, Remien RH, et al. Decreases in self-reported ART adherence predict HIV viremia among pregnant and postpartum South African women. JAIDS Journal of Acquired Immune Deficiency Syndromes 2019; 80(3):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips T, Brittain K, Mellins CA, Zerbe A, Remien RH, Abrams EJ, et al. A self-reported adherence measure to screen for elevated HIV viral load in pregnant and postpartum women on antiretroviral therapy. AIDS and Behavior 2017; 21(2):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beer L, Johnson CH, Fagan JL, Frazier EL, Nyaku M, Craw JA, et al. A national behavioral and clinical surveillance system of adults with diagnosed HIV (the medical monitoring project): protocol for an annual cross-sectional interview and medical record abstraction survey. JMIR research protocols 2019; 8(11):e15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention, Medical Monitoring Project (MMP). https://www.cdc.gov/hiv/statistics/systems/mmp/. Accessed: January 13.
- 14.Office for Human Research Protections. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html. Accessed: April 18, 2020.
- 15.DISTINGUISHING PUBLIC HEALTH RESEARCH AND PUBLIC HEALTH NONRESEARCH. https://www.cdc.gov/od/science/integrity/docs/cdc-policy-distinguishing-public-health-research-nonresearch.pdf. Accessed: April 18, 2020.
- 16.Centers for Disease Control and Prevention, Behavioral and Clinical Characteristics of Persons with Diagnosed HIV Infection—Medical Monitoring Project, United States, 2015 Cycle (June 2015-May 2016). https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-20.pdf 2019. Accessed: April 9, 2020.
- 17.Centers for Disease Control and Prevention, Terms, Definitions, and Calculations Used in CDC HIV Surveillance Publications. https://www.cdc.gov/hiv/pdf/statistics/systems/nhbs/cdc-hiv-terms-surveillance-publications-2014.pdf 2016. Accessed: April 17, 2020.
- 18.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of affective disorders 2009; 114(1–3):163–173. [DOI] [PubMed] [Google Scholar]
- 19.Vu QM, Shouse RL, Brady K, Brooks JT, Weiser J. Changes in HIV antiretroviral prescribing practices in the United States . International journal of STD & AIDS 2020; 31(1):22–29. [DOI] [PubMed] [Google Scholar]
- 20.Nunnally JC, Bernstein IH. Psychometric Theory. New York: McGraw-Hill, Inc.; 1994. [Google Scholar]
- 21.Baumgartner PC, Haynes RB, Hersberger KE, Arnet I. A Systematic Review of Medication Adherence Thresholds Dependent of Clinical Outcomes. Frontiers in Pharmacology 2018; 9(1290). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnier M Is There a Threshold for Medication Adherence? Lessons Learnt From Electronic Monitoring of Drug Adherence. Frontiers in Pharmacology 2019; 9(1540). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allemann SS, Dediu D, Dima AL. Beyond adherence thresholds: A simulation study of the optimal classification of longitudinal adherence trajectories from medication refill histories. Frontiers in pharmacology 2019; 10:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haynes RB. Strategies to Improve Compliance with Referrals, Appointments, and Prescribed Medical Regimens In: Compliance in Health Care. Haynes RB, Taylor DW, Sackett DL (editors). Baltimore: The Johns Hopkins University Press; 1979. pp. 121–143. [Google Scholar]
- 25.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. New England Journal of Medicine 2016; 375(9):830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong Y, Haque S, Chowdhury P, Cory TJ, Kodidela S, Yallapu MM, et al. Pharmacokinetics and pharmacodynamics of cytochrome P450 inhibitors for HIV treatment. Expert Opinion on Drug Metabolism & Toxicology 2019; 15(5):417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCluskey SM, Siedner MJ, Marconi VC. Management of Virologic Failure and HIV Drug Resistance. Infectious Disease Clinics 2019; 33(3):707–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. AnnInternMed 2000; 133(1):21–30. [DOI] [PubMed] [Google Scholar]
- 29.Steenkamp J-BE, De Jong MG, Baumgartner H. Socially desirable response tendencies in survey research. Journal of Marketing Research 2010; 47(2):199–214. [Google Scholar]
- 30.Byrd KK, Hou JG, Hazen R, Kirkham H, Suzuki S, Clay PG, et al. Antiretroviral Adherence Level Necessary for HIV Viral Suppression Using Real-World Data. JAIDS Journal of Acquired Immune Deficiency Syndromes 2019; 82(3):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viswanathan S, Justice AC, Alexander GC, Brown TT, Gandhi NR, McNicholl IR, et al. Adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). Journal of acquired immune deficiency syndromes (1999) 2015; 69(4):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nance RM, Delaney JC, Simoni JM, Wilson IB, Mayer KH, Whitney BM, et al. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015: a cohort study. Annals of internal medicine 2018; 169(6):376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn B, Shireman TI, Lee Y, Galârraga O, Wilson IB. Trends in medication adherence in HIV patients in the US, 2001 to 2012: an observational cohort study. Journal of the International AIDS Society 2019; 22(8):e25382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youn B, Shireman TI, Lee Y, Galârraga O, Rana AI, Justice AC, et al. Ten-year trends in antiretroviral therapy persistence among US Medicaid beneficiaries. AIDS (London, England) 2017; 31(12):1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manly JJ. Deconstructing race and ethnicity: implications for measurement of health outcomes. Medical care 2006:S10–S16. [DOI] [PubMed] [Google Scholar]
- 36.Burch LS, Smith CJ, Phillips AN, Johnson MA, Lampe FC. Socioeconomic status and response to antiretroviral therapy in high-income countries: a literature review. Aids 2016; 30(8):1147–1162. [DOI] [PubMed] [Google Scholar]
- 37.Bing EG, Burnam A, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry 2001; 58(8):721–728. [DOI] [PubMed] [Google Scholar]
- 38.Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The impact of alcohol use and related disorders on the HIV continuum of care: a systematic review. Curr HIV/AIDSRep 2015; 12(4):421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Socias ME, Milloy M. Substance use and adherence to antiretroviral therapy: what is known and what is unknown. Current infectious disease reports 2018; 20(9):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrestha R, Altice FL, Karki P, Copenhaver MM. Integrated bio-behavioral approach to improve adherence to pre-exposure prophylaxis and reduce HIV risk in people who use drugs: a pilot feasibility study. AIDS and Behavior 2018; 22(8):2640–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook R, Zhou Z, Kelso-Chichetto N, Janelle J, Morano J, Somboonwit C, et al. Alcohol consumption patterns and HIV viral suppression among persons receiving HIV care in Florida: an observational study. Addiction science & clinical practice 2017; 12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Downing MJ Jr, Millar BM, Hirshfield S. Changes in Sleep Quality and Associated Health Outcomes among Gay and Bisexual Men Living with HIV. Behavioral sleep medicine 2019:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunne EM, Cook RL, Ennis N. Non-planning Impulsivity But Not Behavioral Impulsivity is Associated with HIV Medication Non-adherence. AIDS and Behavior 2019; 23(5):1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.What is ‘Ending the HIV Epidemic: A Plan for America’? https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview 2020. Accessed: 02/27/2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.