Abstract
Background.
Isolated limb infusion (ILI) is a minimally invasive procedure for delivering high-dose chemotherapy to extremities affected by locally advanced or in-transit melanoma. This study compared the outcomes of melanoma patients treated with ILI in the United States of America (USA) and Australia (AUS).
Methods.
Patients with locally recurrent in-transit melanoma treated with ILI at USA or AUS centers between 1992 and 2018 were identified. Demographic and clinicopathologic characteristics were collected. Primary outcomes of treatment response, in-field progression-free survival (IPFS), distant progression-free survival (DPFS), and overall survival (OS) were evaluated by the Kaplan-Meier method. Multivariable analysis evaluated whether availability of new systemic therapies affected outcomes.
Results.
More ILIs were performed in AUS (n = 411, 60 %) than in the USA (n = 276, 40 %). In AUS, more ILIs were performed for stage 3B disease than in the USA (62 % vs 46 %; p < 0.001). The reported complete response rates were similar (AUS 30 % vs USA 29 %). Among the stage 3B patients, AUS patients had better IPFS (p = 0.001), whereas DPFS and OS were similar between the two countries. Among the stage 3C patients, the USA patients had better OS (p < 0.001), whereas IPFS and DPFS were similar. Availability of new systemic therapies did not affect IPFS or DPFS in either country. However, the USA patients who received ILI after ipilimumab approval in 2011 had significantly improved OS (hazard ratio, 0.62; p = 0.013).
Conclusions.
AUS patients were treated at an earlier disease stage than the USA patients with better IPFS for stage 3B disease. The USA patients treated after the availability of new systemic therapies had a better OS.
Whereas early-stage melanoma remains effectively treated by surgical resection, patients with a more advanced stage of disease provide additional challenges for successful treatment. In-transit disease is defined as the presence of melanoma adjacent to the primary lesion or between the primary site and first-echelon regional lymph nodes. However, blockage of lymphatic channels by tumor may alter patterns of lymphatic flow so that in-transit disease also may appear distal to the primary lesion.1 Clinically, in-transit disease may cause pain, ulceration, bleeding, and restricted mobility, leading to a lower quality of life.2
Thompson et al.3 developed isolated limb infusion (ILI) in the 1990s as a minimally invasive approach to treat unresectable in-transit melanoma confined to an extremity, isolating the extremity through vascular occlusion with a tourniquet at the root of the limb. During ILI, percutaneously placed catheters are used to deliver high doses of chemotherapeutic agents (melphalan ± actinomycin-D). Due to its advantages of low procedural morbidity and negligible systemic drug toxicity, ILI may be used to treat elderly and frail patients who cannot tolerate other available therapies and can be repeated if only a partial response (PR) is seen or disease recurs.3–9
Previous studies have reported overall response rates (ORR = CR + PR) after ILI ranging from 50 % to 100 %, with complete response (CR) rates of 23 % to 44 %.1,4–6,10 In this international, multicenter study, direct comparison of patients treated in high-volume centers with long-term follow-up periods in Australia (AUS) and the United States of America (USA) was performed to elucidate differences in the use of ILI between the two countries. The study aimed to identify factors that achieve the best treatment response and long-term oncologic outcomes for in-transit melanoma patients treated with ILI.
MATERIAL AND METHODS
Patient Selection
Each of the AUS and USA centers obtained approval from their respective institutional review boards to identify patients who underwent ILI for the first time between 1992 and 2018. The centers provided a de-identified dataset in compliance with their institution’s regulatory requirements and data use agreements. Patients eligible for inclusion had recurrent stage 3B or 3C melanoma, in-transit lesions confined to a limb, and no evidence of distant metastatic disease as defined by the seventh edition of the American Joint Committee on Cancer (AJCC) staging manual.11
The clinicopathologic characteristics recorded were patient age, sex, stage of disease, involved extremity, and burden of disease (BOD). The BOD was reported according to the stratification described by Muilenburg et al.12 as follows: high BOD (≥10 lesions or any one lesion >2 cm) or low BOD (<10 lesions and no lesion >2 cm). A history of therapy given before the first ILI such as hyperthermic isolated limb perfusion, intralesional therapy, surgical resection, or systemic therapy were not reasons for exclusion from the study. Post-ILI therapies such as repeat ILI, surgical resection, and systemic therapy after disease progression were likewise not reasons for exclusion. Only the results and outcomes after the first ILI are presented in this report.
Description of Procedure
Dosing for ILI was determined preoperatively using limb volume measured by water displacement or sequential circumference measurements along the length of the limb.13 The doses of melphalan were 7.5 mg/L for lower extremities and 10 mg/L for upper extremities. The doses of actinomycin-D were 75 to 100 mcg/L.6 The maximum melphalan dose was 100 mg for lower and 50 mg for upper extremities. In most ILI performed within the USA, the dose of melphalan was corrected for the ideal body weight. This was not done at AUS centers. The ILI procedure was performed as previously described.6,14,15
After the procedure, the patients were monitored with daily physical examination and serum creatine phosphokinase (CPK) levels. Limb toxicity was assessed according to the Wieberdink grading scale.13 At the AUS centers, treatment response was measured using the World Health Organization (WHO) criteria, with CR defined as the disappearance of all measurable disease, and PR defined as a 50 % or greater decrease in total tumor size without the appearance of new lesions or progression of any known lesion, both determined by two postoperative observations 4 and 8 or more weeks apart.16 At the USA centers, treatment response was measured using the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) guidelines for cutaneous lesions, with CR defined as the disappearance of all lesions and PR defined as a 30 % or greater decrease in diameter of target lesions, recorded 3 months postoperatively.17
Influence of Systemic Therapies
The use of systemic chemotherapy, targeted therapy, or immunotherapy varied during the 26-year period of the study, and specific reporting of systemic therapy initiation for a majority of the patients was lacking. With this discrepancy, patients were instead stratified and evaluated according to the date of government approval of reimbursement for ipilimumab (Pharmaceutical Benefits Scheme [PBS], AUS 2013; Food and Drug Administration [FDA], USA, 2011). The dates of government approval were similar or subsequent to that for ipilimumab used in other systemic therapies, including targeted therapies (vemurafenib [PBS 2017, FDA 2011], vemurafenib/cobimetinib [PBS 2018, FDA 2015], dabrafenib [PBS 2013, FDA 2013], dabrafenib/trametinib [PBS 2016, FDA 2014], and encorafenib/binimetinib [PBS 2019, FDA 2018]), as well as for the checkpoint inhibitors pembrolizumab (PBS 2015, FDA 2014) and nivolumab (PBS 2016, FDA 2015).
Statistical Methods
Continuous variables are described as median and interquartile range (IQR), and categorical variables are described by frequency. The primary outcomes of treatment response, in-field progression-free survival (IPFS), distant progression-free survival (DPFS), and overall survival (OS) were evaluated by the Kaplan-Meier curve method. Time to in-field progression, distant progression, and death were calculated from the day of ILI. The logrank test was used to compare responders (patients with PR or CR) with non-responders (patients with stable disease [SD] or progressive disease [PD]) regarding distribution of time to event.
Multivariable Cox proportional hazards regression analysis with a backward variable elimination method was used for assessment of hazard ratios (HRs) to summarize the association of demographic and clinicopathologic characteristics with time-to-event outcome. The multivariable analysis also was used to evaluate whether availability of new systemic therapies in each country affected survival outcomes. Availability was defined as FDA approval in the USA and PBS reimbursement in AUS. A two-sided p value less than 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (copyright 2013, SAS Institute Inc., Cary, NC, USA).
RESULTS
Comparison of Patient and Tumor Characteristics
Of the 687 ILI procedures analyzed in this study, 411 (60 %) were performed in AUS centers and 276 (40 %) in USA centers. The majority of the patients were women (AUS: 59 % [n = 244], USA: 61 % [n = 168]), and most patients underwent ILI of the lower extremity (AUS: 92 % [n = 377], USA: 84 % [n = 233]). AUS patients were older (median age, 73 vs 68 years; p < 0.001), had lower median limb volume (6 vs 7.5 L; p < 0.001), and more often had stage 3B disease (62 % [n = 255] vs 46 % [n = 128]; p < 0.001). There was no significant difference in BOD between countries (p = 0.75; Table 1).
TABLE 1.
Comparison of patient and tumor characteristics
| Variable | AUS (n = 411) n (%) | USA (n = 276) n (%) | p Value | |
|---|---|---|---|---|
| Age (years) | Median (IQR) | 73 (59–76) | 68 (64–82) | <0.001 |
| Sex | Female | 244 (59) | 168 (61) | 0.694 |
| Male | 167 (41) | 108 (39) | ||
| Extremity | Lower | 377 (92) | 233 (84) | 0.003 |
| Upper | 34 (8) | 43 (16) | ||
| Stage | 3B | 255 (62) | 128 (46) | <0.001 |
| 3C | 156 (38) | 148 (54) | ||
| Burden of disease | High | 186 (45) | 127 (47) | 0.745 |
| Low | 225 (55) | 146 (53) | ||
| Breslow thickness of primary melanoma (mm) | n (with avaiable information) | 352 | 225 | 0.188 |
| Median (IQR) | 2.8 (2–4) | 2.5 (2–4) | ||
| Limb volume (L) | n (with avaiable information) | 411 | 235 | <0.001 |
| Median (IQR) | 6 (4.5–7.5) | 7.5 (5.6–9) |
Bold values indicate statistically significant
AUS Australia; USA United States of America; IQR interquartile range
Comparison in Procedure
The median melphalan dose used did not differ significantly between the two countries (AUS 44 mg vs USA 43 mg; p = 0.89), but AUS centers used actinomycin less frequently than the USA centers (73 % vs 97 %; p < 0.001). Drug circulation times varied among AUS centers, whereas all USA centers used a standardized time (21 min [IQR 15–30 min] vs 30 min; p < 0.001). AUS centers reported a significantly shorter tourniquet time, with a median ischemia time of 49 min compared with the USA centers (58 min) (p < 0.001). AUS centers also reported a significantly lower median pH at 30 min (7.13 vs 7.18; p < 0.001), while USA centers reported a significantly higher median limb temperature at 30 min (38.1 °C vs 38.9 °C; p < 0.001; Table 2).
TABLE 2.
Comparison of procedural technique
| Variable | AUS (n = 411) | USA (n = 276) | p Value | |
|---|---|---|---|---|
| Melphalan dose (mg) | n | 411 | 272 | 0.659 |
| Median (IQR) | 43.5 (32–50) | 42.8 (35–52) | ||
| Actinomycin given: n (%) | Yes | 302 (73) | 269 (97) | <0.001 |
| No | 109 (27) | 7 (3) | ||
| Actinomycin dose (ug) | n | 302 | 269 | <0.001 |
| Median (IQR) | 400 (300–500) | 570 (420–700) | ||
| Circulation time (min) | n | 411 | 276 | <0.001 |
| Median (IQR) | 21 (15–30) | 30 (30–30) | ||
| Ischemia time (min) | n | 410 | 269 | <0.001 |
| Median (IQR) | 49 (35–64) | 58 (50–71) | ||
| pH at 30 min | n | 184 | 261 | <0.001 |
| Median (IQR) | 7.12 (7.07–7.19) | 7.17 (7.13–7.23) | ||
| Limb temperature at 30 min | n | 364 | 266 | <0.001 |
| Median (IQR) | 38.1 (37.3–38.9) | 38.9 (38.2–39.7) |
Bold values indicate statistically significant
AUS Australia; USA United States of America; IQR interquartile range
Comparison of Toxicity and Clinical Outcomes
All patients experienced some degree of Wieberdink limb toxicity, without significant differences between the two countries (p = 0.14). The majority of patients in both countries experienced grade 2 limb toxicity (AUS 61 % [n = 250] vs USA 60 % [n = 161]). No patient experienced measurable systemic side effects or grade 5 limb toxicity necessitating amputation. The post-procedural serum CPK levels and trends were similar, with the median peak at 566 U/L in AUS centers and at 590 U/L in the USA centers (p = 0.75). The median time to peak CPK level occurred on postoperative day (POD) 5 for AUS patients and on POD 4 for the USA patients (Table 3).
TABLE 3.
Comparison of perioperative and clinical outcomes
| Variable | AUS (n = 411) n (%) | USA (n = 276) n (%) | p Value | |
|---|---|---|---|---|
| Wieberdink Toxicity Scale Peak Score | n | 411 | 268 | 0.132 |
| 1 (no visible effect) | 1 | 44 (11) | 32 (12) | |
| 2 (slight erythema and/or edema) | 2 | 250 (61) | 161 (60) | |
| 3 (considerable erythema and/or edemawith blistering) | 3 | 106 (26) | 59 (22) | |
| 4 (epidermolysis and/or obvious damage to deep tissues with a threatened or actual compartment syndrome) | 4 | 11 (2) | 16 (6) | |
| 5 (severe tissue damage necessitating amputation) | 5 | 0 | 0 | |
| Hospital stay (days) | n | 398 | 272 | <0.001 |
| Median (IQR) | 7 (6–9) | 6 (5–8) | ||
| CPK peak (U/L) | n | 335 | 276 | 0.513 |
| Median (IQR) | 566 (148–1976) | 590 (171–1785) | ||
| Treatment response | CR | 123 (30) | 76 (29) | <0.001 |
| PR | 177 (43) | 65 (24) | ||
| SD | 66 (16) | 34 (13) | ||
| PD | 45 (11) | 91 (34) | ||
| Overall response rate | Respondera | 300 (73) | 141 (53) | <0.001 |
| Nonresponderb | 111 (27) | 125 (47) | ||
| ILI performed before or after ipilimumab approval | After | 23 (6) | 124 (45) | <0.001 |
| USA 2011 | Before | 388 (94) | 152 (55) | |
| AUS 2013 | ||||
| Stage 3B (median; 95 % CI) | In-field PFS (months) | 20.7 (13–30) | 8.2 (6–10) | 0.001 |
| Distant PFS (months) | 65 (41–166) | 69 (29-NE) | 0.55 | |
| Overall survival (months) | 45 (38–56) | 60 (41–151) | 0.07 | |
| Stage 3C (median; 95 % CI) | In-field PFS (months) | 8 (6–11) | 6 (4–8) | 0.88 |
| Distant PFS (months) | 17 (11–20) | 14 (8–22) | 0.99 | |
| OS (months) | 21 (15–26) | 38 (27–63) | <0.0001 |
Bold values indicate statistically significant
AUS Australia; USA United States of America; IQR interquartile range; CPK creatine phosphokinase; CR complete response; PR partial response; SD stable disease; PD protressive disease; ILI isolated limb infusion; PFS progression-free survival; CI confidence interval; NE not estimable; OS overall survival
Patients who demonstrated complete or partial response to treatment
Patients who exhibited SD or PD in response to treatment
The reported CR rates were similar between the two countries, observed to be 30 % (n = 123) in AUS patients and 29 % (n = 76) in USA patients. The ORR was better among AUS patients (73 % vs 53 %; p < 0.001), as was the disease control rate (DCR = CR+PR+SD, 89 % vs 66 %; p < 0.001). The study findings showed SD in 16 % (n = 66) of AUS patients and 13 % (n = 34) of the USA patients, with PD occurring in 11 % (n = 45) of AUS patients and 34 % (n = 91) of the USA patients.
Patients who responded to ILI (n = 300) at AUS centers demonstrated an improved median IPFS, with a near doubling of time to in-field progression (25 vs 13 months; p = 0.05) and a similar median DPFS (58 vs 46 months; p = 0.39), yet a significantly shorter median OS (42 vs 79 months; p < 0.001) than those who responded to ILI at the USA centers (n = 141). Non-responders treated at AUS centers (n = 111) had a similar median IPFS (3 vs 3 months; p = 0.48), a similar median DPFS (14 vs 10 months; p = 0.74), and a significantly shorter median OS (16 vs 33 months; p < 0.001) than the non-responders at the USA centers (n = 125) (Fig. 1).
FIG. 1.
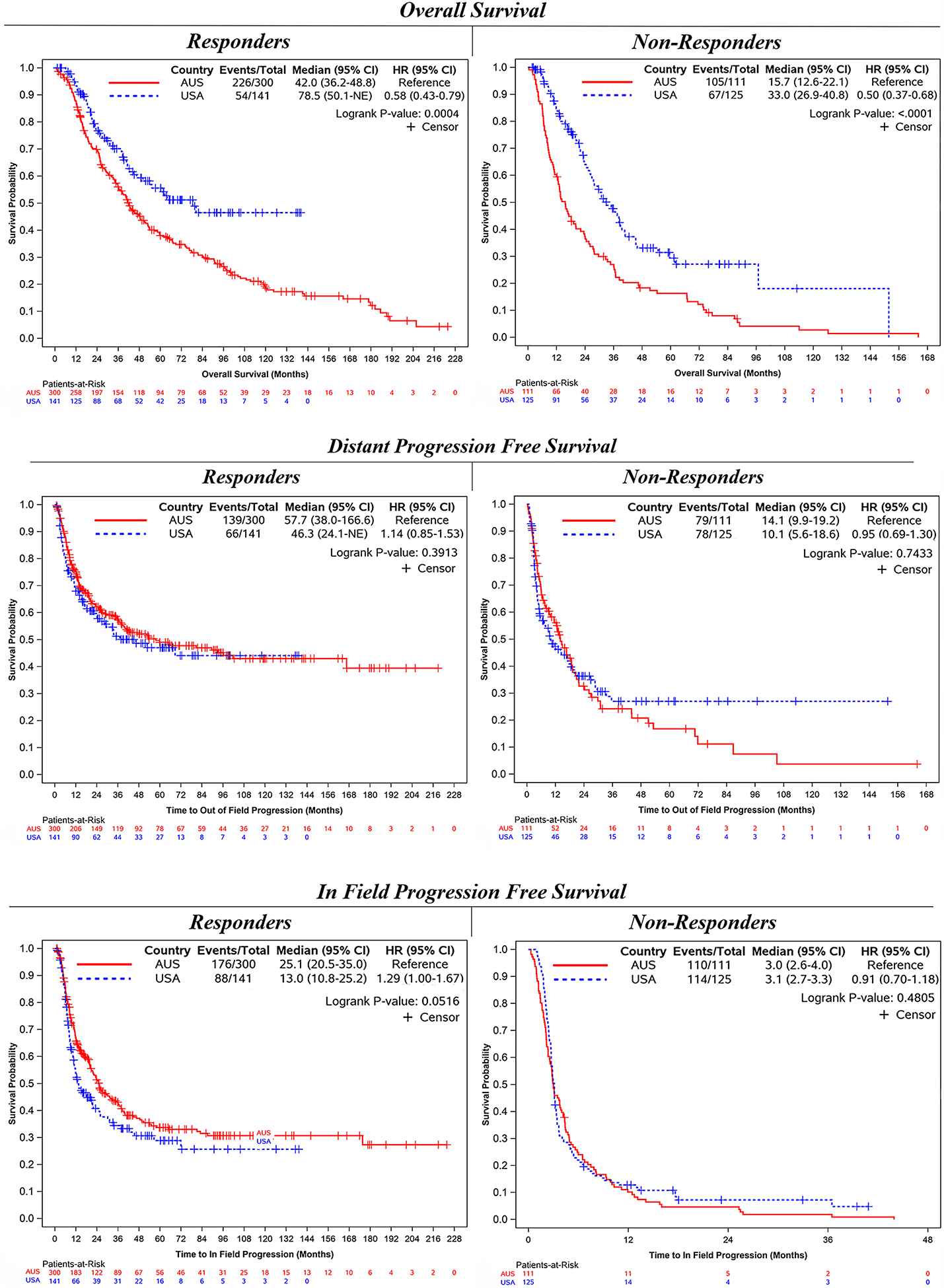
Outcomes of responders versus non-responders, stratified by country
The stage 3B patients treated at AUS centers (n = 255) had a significantly longer median IPFS (21 vs 8 months; p < 0.001), but a similar median DPFS (65 vs 69 months; p = 0.55) and median OS (45 vs 60 months; p = 0.07) compared with those who had stage 3B disease treated at the USA centers (n = 128). The stage 3C patients treated at AUS centers (n = 156) had a similar median IPFS (8 vs 6 months; p = 0.88) and DPFS (17 vs 14 months; p = 0.99), but a significantly shorter median OS (21 vs 38 months; p < 0.001) compared with those who had stage 3C disease treated at the USA centers (n = 148; Fig. 2).
FIG. 2.
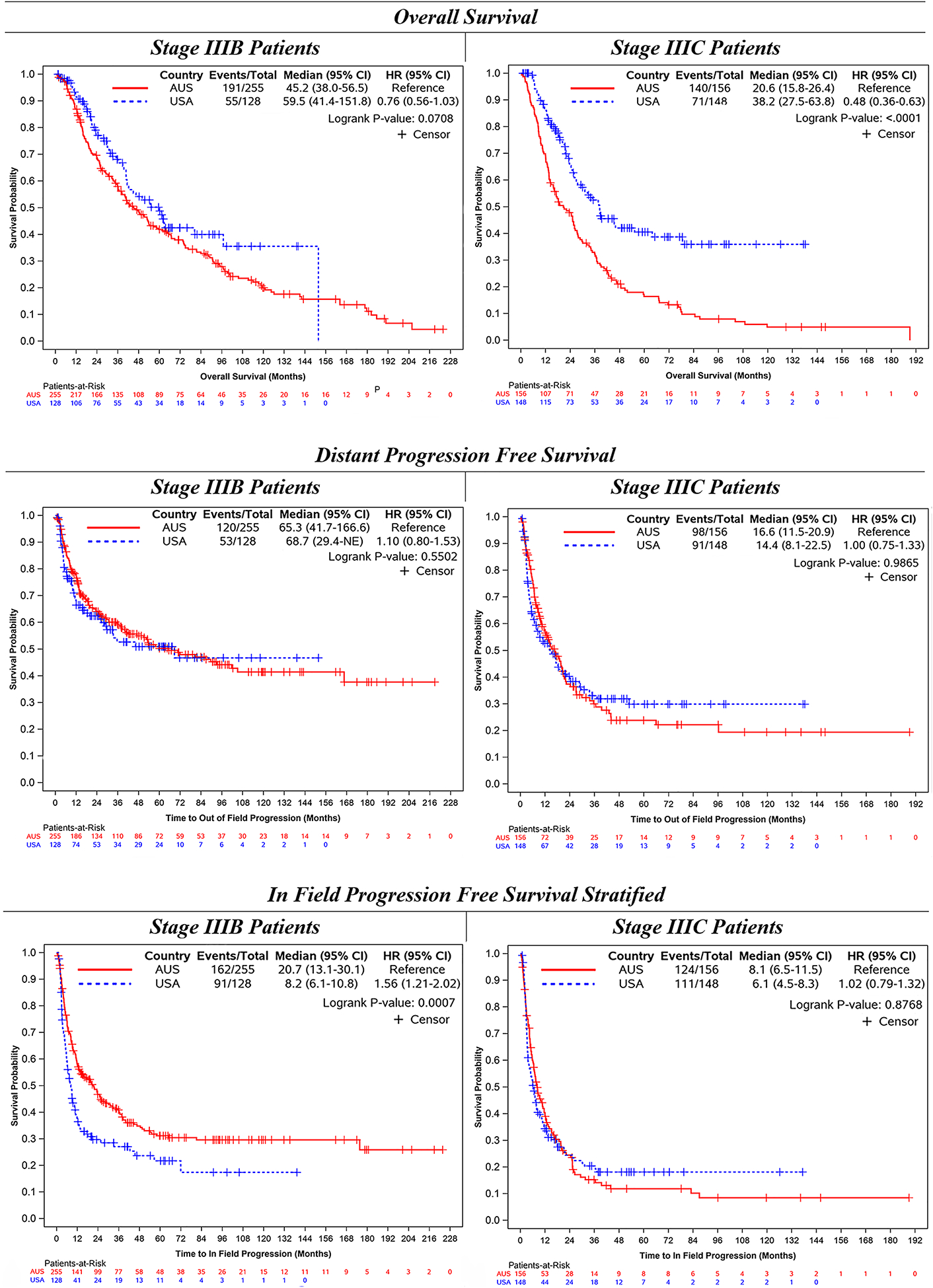
Outcomes of stage IIIB versus stage IIIC patients, stratified by country
Comparison by Availability of New Systemic Therapies
The majority of ILIs were performed in AUS before (n = 388) versus after (n = 23) availability of ipilimumab. For the patients treated at AUS centers, median IPFS (10 vs 12 months; p = 0.23), median DPFS (30 vs 35 months; p = 0.95), and median OS (27 vs 34 months; p = 0.99) were similar between the two countries. A comparison of the patients treated before (n = 152) and after (n = 124) availability of ipilimumab at the USA centers showed no significant difference in median IPFS (8 vs 6 months; p = 0.16) or median DPFS (15 vs 34 months; p = 0.13), but those treated with ILI after availability of ipilimumab demonstrated a significantly longer median OS (40 vs NE months [46.5 – NE months]; p = 0.01; Fig. 3).
FIG. 3.
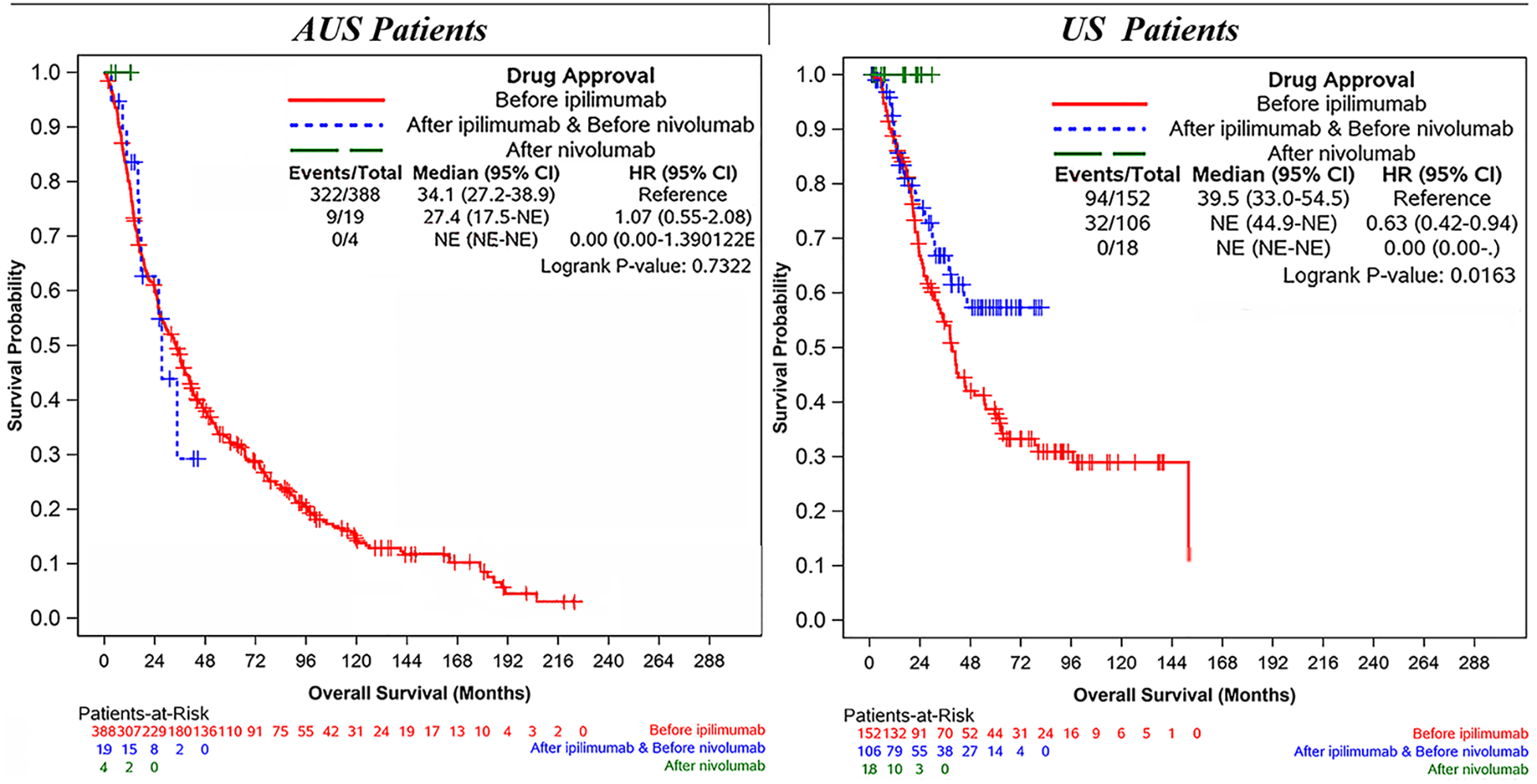
Overall survial stratified by immunotherapy availability
Systemic therapy was administered before ILI in 1 % (5/ 411) of the patients treated at AUS centers and included chemotherapy (n = 2), pembrolizumab (n = 2), and pembrolizumab/ipilimumab (n = 1). Unfortunately, pre-ILI systemic therapy was poorly documented therefore these numbers may not reflect the reality. At USA centers, 20 % (54/276) of the patients received systemic therapy before ILI, which consisted of interferon (n = 36), chemotherapy (n = 10), temozolomide (n = 6), interleukin (IL)-2 (n = 5), pembrolizumab (n = 4), and ipilimumab (n = 4), with some patients receiving multiple sequential agents (n = 11).
Systemic therapy was administered after ILI in 9 % (35/ 411) of the patients at AUS centers and included pembrolizumab (n = 18), ipilimumab (n = 8), chemotherapy (n = 7), ipilimumab/nivolumab (n = 3), and BRAF-targeted therapies (n = 3). However, because no adjuvant or recurrence treatment data could be reported for 333 patients at the AUS centers, these numbers may not reflect the reality.
At USA centers, 39 % (08/276) of patients received systemic therapy after ILI, which included ipilimumab (n = 43), pembrolizumab (n = 18), chemotherapy (n = 17), BRAF-targeted therapies (n = 16), IL-2 (n = 12), temozolomide (n = 11), nivolumab (n = 9), interferon (n = 4), ipilimumab/pembrolizumab (n = 3), and ipilimumab/nivolumab (n = 1).
Notably, an additional difference was found in use of the intralesional agent talimogene laherparepvec (T-VEC). Although this therapy was not administered to any AUS patients, it was administered to one patient before ILI and to 5 % (13/276) of patients after ILI in the USA. Intralesional therapy (GM-CSF, BCG, PV-10, Rose Bengal, T-VEC) was administered to 9.5 % (39/411) of AUS patients and 2.5 % (7/276) of USA patients before ILI, while 3.2 % (n = 13) of AUS patients and 8.7 % (n = 24) of USA patients received some type of intralesional therapy (GM-CSF, PV-10, Rose Bengal, T-VEC) after ILI.
Multivariable Analysis
In the multivariable Cox regression analysis, no factors that provided a long-term oncologic outcome benefit were identified regarding differences in procedure between the two countries. The patients treated in USA centers had a similar IPFS (HR, 1.08; p = 0.70) and DPFS (HR 1.00, p=0.99) but a significantly longer OS (HR, 0.69; p = 0.003) compared with the patients treated in AUS centers. In both countries, patients with stage 3C disease were at a higher risk for a significantly shorter IPFS (HR, 1.45; p < 0.001), DPFS (HR, 1.97; p < 0.001) and OS (HR, 1.52; p < 0.001) than the patients with stage 3B disease. Also in both countries, a significantly longer OS was associated with lower BOD (HR, 0.69; p < 0.001) and treatment after ipilimumab became available (HR,0.62; p = 0.013; Table 4).
TABLE 4.
Multivariable cox proportional hazard model
| Covariate | Level | HR (range) | HR p Value |
|---|---|---|---|
| Time to in-field progression | |||
| Country | USA | 1.08 (0.74–1.57) | 0.704 |
| AUS | Reference | — | |
| Stage | 3C | 1.45 (1.18–1.78) | <0.001 |
| 3B | Reference | — | |
| Other nonsignificant variables included: burden of disease, upper or lower extremity, actinomycin use, actinomycin dose, circulation time, ischemia time, ipilimumab availability | |||
| Time to distant field progression | |||
| Country | USA | 1.00 (0.64–1.57) | 0.992 |
| AUS | Reference | — | |
| Stage | 3C | 1.97 (1.55–2.50) | <0.001 |
| 3B | Reference | — | |
| Other nonsignificant variables included: burden of disease, upper or lower extremity, actinomycin use, actinomycin dose, circulation time, ipilimumab availability | |||
| Overall survival | |||
| Country | USA | 0.69 (0.54–0.88) | 0.003 |
| AUS | Reference | — | |
| Drug approval | After ipilimumab | 0.62 (0.43–0.91) | 0.013 |
| Prior to ipilimumab | Reference | — | |
| Stage | 3C | 1.51 (1.23–1.89) | <0.001 |
| 3B | Reference | — | |
| Burden of disease | Low | 0.69 (0.55–0.85) | <0.001 |
| High | Reference | — | |
| Other nonsignificant variables included: upper or lower extremity, actinomycin use, actinomycin dose, circulation time, ischemia time | |||
Bold values indicate statistically significant
HR hazard ratio; USA United States of America; AUS Australia
DISCUSSION
This study compared 687 patients from Australia (n = 411) and the United States (n = 276) who underwent first-time ILI for the treatment of stage 3B and 3C melanoma. These data have previously been analyzed together in a single cohort, with a significantly longer OS reported for patients who achieve a CR after undergoing ILI for in-transit melanoma confined to a limb.18 The current study sought to characterize similarities and differences in the use of ILI between the two countries. In both countries, ILI was an effective treatment with generally low toxicity. The ORR differed significantly between the two countries, yet the CR rate was similar, consistent with previous studies.4,6,7 Understanding the factors that may have contributed to a higher PR rate for the AUS patients garnered key interest because the patients in both countries who responded to ILI had a significantly longer median IPFS, DPFS, and OS than those who did not respond.
Using two distinct systems to evaluate response could explain the difference in PR rates. The AUS patients were evaluated earlier, approximately 4 and 8 weeks postoperatively according to the WHO guidelines, compared with later evaluation 3 months postoperatively using the RECIST criteria in the USA. This difference in practice might have led to an observer bias where patients were evaluated before disease progression (4 or 8 weeks) or after an early response that then failed by 3 months.
Response rates also could be attributed to differences in melphalan dose and limb volume. Although AUS patients had lower limb volumes, the dose of melphalan administered was similar between the two countries. This was attributed to routine correction of the melphalan dose for ideal body weight in the USA centers, an uncommon practice in AUS centers.6 Although some studies have associated dosing modification with similar response rates and lower rates of toxicity,19,20 others have failed to reproduce this result.21 In a large, multi-institutional study of ILI comparing melphalan dosing, patients had a significantly better PR rate with an uncorrected melphalan dose and lower limb volume, albeit with higher toxicity and no additional improvement in CR.6
Additionally, the response rates may have been due to differences in circulation and ischemia times. The better PR observed with shorter circulation and ischemia times in AUS centers is inconsistent with prior studies reporting a better PR rate with longer duration of circulation and ischemia.5,22 Evidence from one in vitro study may account for this, having demonstrated an elimination half-life of 15 to 25 min for melphalan and a plateau of melphalan uptake by melanoma cells after approximately 10 min.23 In the current study, despite variation in melphalan dose, limb volume, circulation, and ischemia times, the findings showed no significant differences in Wieberdink limb toxicity, median CPK levels or CR rates between the two countries.
The median IPFS was consistently shorter than the median DPFS in both countries and both staging groups, indicating a trend of locoregional progression and recurrence. Progression of tumors in this fashion may indicate a disease biology amenable to repeat ILI for patients who previously demonstrated CR or PR, as prior studies have shown similar toxicity and response for repeat ILI compared with first-time ILI.8,24 BOD and limb volume were significantly lower for the AUS patients, therefore a better IPFS, DPFS, and OS than USA patients would be expected, as demonstrated by previous studies that lower BOD and lower limb volume are predictors of better ORR.5,6 Additionally, the AUS centers treated significantly more stage 3B patients than the USA centers. Nevertheless, the AUS patients had a worse median OS.
One explanation for the difference in median OS may be that AUS patients were significantly older than the USA patients. Another may be that systemic therapies for treatment of unresectable, metastatic melanoma have expanded during the last 10 years. Immunotherapy and targeted therapy have provided patients with improved response rates and longer OS.25–30
The study analysis stratified patients based on the availability of effective systemic therapies to further characterize long-term oncologic outcomes after ILI. Although ipilimumab was selected, other immunotherapy and targeted therapy agents were similar in chronologic comparison. Patients in the USA centers treated with ILI after the availability of these systemic therapies demonstrated a longer median OS than the patients who underwent ILI before their availability. This difference was not observed in patients treated at the AUS centers, possibly accounted for by not enough patients treated after availability of the new systemic therapies to adequately show a significant result. This trend toward preferential use of systemic therapies in the treatment of in-transit disease has also occurred in the United States.
Current National Comprehensive Cancer Network guidelines have detailed this shift in treatment utilization, stating that institutions have increasingly considered the use of the new systemic therapies as first-line treatment for in-transit disease, although no guideline recommendation for either ILI or systemic therapy as first-line treatment has been reported and no direct comparison between the therapies has been performed.31 Moreover, the phase 3 trials which led to the approval of ipilimumab,32 nivolumab,33 and pembrolizumab34 had marginal representation of patients with stage 3 unresectable in-transit melanoma.
Treatment with ILI has been established for more than two decades as a method for managing in-transit disease, with an ORR reaching 75 % in low BOD and 50 % in high BOD as well as minimal associated adverse events and no treatment-related deaths. Findings have proven ILI to be well tolerated by octo- and nonagenarians,35 who otherwise could not tolerate the adverse effects of systemic therapy. Because no large scale studies have investigated the efficacy of systemic therapies for in-transit melanoma, the abandoning of ILI (rather abrupt and consistent over time in AUS centers) seems inappropriate and may be explained only by a recall bias for recent therapy innovation present in both community and academic centers.
The ability of ILI to achieve a durable CR in 30 % of patients alongside the long-term remission oberved with systemic therapy offers a difficult clinical decision for optimal disease management. Therefore, each patient would benefit from multidisciplinary discussion to determine their best method of treatment. If eligible for ILI, patients should be given the opportunity for disease control using a single well-tolerated procedure, with the understanding that if progression occurs at the 3-month postoperative evaluation, new systemic treatment options are readily available. The use of ILI in concert with systemic immunotherapy is a reasonable next step in evaluation of treatment for in-transit melanoma, of special interest as descriptions of melphalan have shown its synergism with systemic immunotherapy and do not appear to preclude the effectiveness of adjuvant immunotherapy.36–42 The finalized results from two trials currently exploring ILI with ipilimumab in the neoadjuvant (NCT02115243) and adjuvant settings (NCT01323517) are eagerly anticipated.
This study included a selected group of stage 3B and 3C patients treated at high-volume tertiary referral centers to reduce selection bias. However, the retrospective nature of the study could not be avoided. The study also could not account for other limitations, including a lack of reporting on BRAF status and treatments in the adjuvant or recurrent setting that 333 patients may or may not have received during the course of their disease. These other therapies could have had an impact on clinical outcomes, and use of the ipilimumab approval date as a surrogate for drug availability limits the conclusions that could be made regarding OS.
Additionally, differences between the WHO and RECIST criteria for assessing outcomes may have introduced variability between centers. Despite this discrepancy in reporting of PR, the study did show that response to treatment is independently associated with improved survival in both countries.
CONCLUSIONS
This study is the first to directly compare ILI use between AUS and the USA. AUS patients were older, had lower limb volumes, underwent ILI at an earlier stage of disease, and had a better IPFS. The USA patients had longer procedural ischemia times and a longer median OS. Overall, the best long-term oncologic outcomes were associated with lower BOD, earlier stage of disease, and ILI administered after the availability of new systemic immunotherapies. Despite minor differences in procedural technique, the use of ILI in both countries was similarly well tolerated and offered potential for control of in-transit melanoma confined to an extremity. Yet, the use of ILI has markedly shifted in favor of new systemic therapies for an undefined reason. Future studies should focus on the role of ILI in conjunction with systemic therapies and further defining the response rates and adverse effects of each treatment option currently available.
ACKNOWLEDGMENTS
The study was supported by the Biostatistics and Bioinformatics Shared Resources at Moffitt Cancer Center, Tampa, FL (P30-CA076292).
Footnotes
DISCLOSURE Jonathan S. Zager was issued USA patent no. 10,583,246 for high-flow isolated limb infusion and has an additional high-flow ILI patent pending. John F. Thompson has received honoraria for advisory board participation from Merck Sharpe Dohme Australia and Bristol Myers Squibb Australia. He also has received honoraria and travel expenses from GSK and Provectus Inc. Consultant Regeneron 2019. The remaining authors have no conflicts of interest.
REFERENCES
- 1.Greene FL CC, Fritz AG, Shah JP, Winchester DP (eds). AJCC Cancer staging Atlas: melanoma of the skin. vol 6 Springer, New York: 2006. [Google Scholar]
- 2.Sanki A, Kroon HM, Kam PC, Thompson JF. Isolated limb perfusion and isolated limb infusion for malignant lesions of the extremities. Curr Prob Surg. 2011;48:371–430. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14:238–47. [DOI] [PubMed] [Google Scholar]
- 4.O’Donoghue C, Perez MC, Mullinax JE, et al. Isolated limb infusion: a single-center experience with over 200 infusions. Ann Surg Oncol. 2017;24:3842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma: a 14-year experience. Ann Surg Oncol. 2008;15:3003–13. [DOI] [PubMed] [Google Scholar]
- 6.Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg. 2009;208:706–15. (discussion 715–707) [DOI] [PubMed] [Google Scholar]
- 7.Kroon HM, Coventry BJ, Giles MH, et al. Australian multicenter study of isolated limb infusion for melanoma. Ann Surg Oncol. 2016;23:1096–103. [DOI] [PubMed] [Google Scholar]
- 8.Chai CY, Deneve JL, Beasley GM, et al. A multi-institutional experience of repeat regional chemotherapy for recurrent melanoma of extremities. Ann Surg Oncol. 2012;19:1637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teras J, Kroon HM, Miura JT, et al. International multi-center experience of isolated limb infusion for in-transit melanoma metastases in octogenarian and nonagenarian patients. Ann Surg Oncol. 2020; 27:1420–9 [DOI] [PubMed] [Google Scholar]
- 10.Mian R, Henderson MA, Speakman D, Finkelde D, Ainslie J, McKenzie A. Isolated limb infusion for melanoma: a simple alternative to isolated limb perfusion. Can J Surg. 2001;44:189–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muilenburg DJ, Beasley GM, Thompson ZJ, Lee JH, Tyler DS, Zager JS. Burden of disease predicts response to isolated limb infusion with melphalan and actinomycin D in melanoma. Ann Surg Oncol. 2015;22:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–10. [DOI] [PubMed] [Google Scholar]
- 14.Kroon HM, Huismans A, Waugh RC, Kam PC, Thompson JF. Isolated limb infusion: technical aspects. J Surg Oncol. 2014;109:352–6. [DOI] [PubMed] [Google Scholar]
- 15.Carr MJ, Sun J, Zager JS. Isolated limb infusion: institutional protocol and implementation. J Surg Oncol. 2020; 122: 99–105 [DOI] [PubMed] [Google Scholar]
- 16.Hunter RD. World Health Organization (WHO) Handbook for Reporting Results of Cancer Treatment. vol 48 WHO, Geneva, Switzerland, 1979. [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 18.Miura JT, Kroon HM, Beasley GM, et al. Long-term oncologic outcomes after isolated limb infusion for locoregionally metastatic melanoma: an international multicenter analysis. Ann Surg Oncol. 2019;26: 2486–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–205. [DOI] [PubMed] [Google Scholar]
- 20.McMahon N, Cheng TY, Beasley GM, et al. Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann Surg Oncol. 2009;16:953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huismans AM, Kroon HM, Haydu LE, Kam PCA, Thompson JF. Is melphalan dose adjustment according to ideal body weight useful in isolated limb infusion for melanoma? Ann Surg Oncol. 2012;19:3050–6. [DOI] [PubMed] [Google Scholar]
- 22.Lindner P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–36. [DOI] [PubMed] [Google Scholar]
- 23.Parsons PG, Carter FB, Morrison L, Regius Mary S. Mechanism of melphalan resistance developed in vitro in human melanoma cells. Cancer Res. 1981;41:1525–34. [PubMed] [Google Scholar]
- 24.Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer. 2009;115:1932–40. [DOI] [PubMed] [Google Scholar]
- 25.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. [DOI] [PubMed] [Google Scholar]
- 27.Kroon HM, Huismans AM, Kam PC, Thompson JF. Isolated limb infusion with melphalan and actinomycin D for melanoma: a systematic review. J Surg Oncol. 2014;109:348–51. [DOI] [PubMed] [Google Scholar]
- 28.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–8. [DOI] [PubMed] [Google Scholar]
- 29.Perez MC, Miura JT, Naqvi SMH, et al. Talimogene laherparepvec (TVEC) for the treatment of advanced melanoma: a single-institution experience. Ann Surg Oncol. 2018;25:3960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long GV, Dummer R, Ribas A, et al. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(15 Suppl):9568. [Google Scholar]
- 31.Coit DG, Thompson JA, Albertini MR, et al. Cutaneous melanoma, version 2. 2019. NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:367–402. [DOI] [PubMed] [Google Scholar]
- 32.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 34.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- 35.Teras J, Kroon HM, Miura JT, et al. International multicenter experience of isolated limb infusion for in-transit melanoma metastases in octogenarian and nonagenarian patients. Ann Surg Oncol. 2020;27:1420–9. [DOI] [PubMed] [Google Scholar]
- 36.Lesterhuis WJ, Salmons J, Nowak AK, et al. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PloS One. 2013;8:e61895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gyorki DE, Yuan J, Mu Z, et al. Immunological insights from patients undergoing surgery on ipilimumab for metastatic melanoma. Ann Surg Oncol. 2013;20:3106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shetty G, Beasley GM, Sparks S, et al. Plasma cytokine analysis in patients with advanced extremity melanoma undergoing isolated limb infusion. Ann Surg Oncol. 2013;20:1128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang BS, Beasley GM, Speicher PJ, et al. Immunotherapy following regional chemotherapy treatment of advanced extremity melanoma. Ann Surg Oncol. 2014;21:2525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol. 2012;22:319–26. [DOI] [PubMed] [Google Scholar]
- 41.Gajewski TF, Woo SR, Zha Y, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268–76. [DOI] [PubMed] [Google Scholar]
- 42.McCarter MD, Baumgartner J, Escobar GA, et al. Immunosuppressive dendritic and regulatory T cells are upregulated in melanoma patients. Ann Surg Oncol. 2007;14:2854–60. [DOI] [PubMed] [Google Scholar]


