Abstract
Objective:
People living with HIV (PLWH) have an increased risk of pulmonary vascular disease and pulmonary hypertension (PH). Endothelial cell dysfunction is thought to contribute, but human studies have been limited by the invasive nature of conventional measures of pulmonary artery endothelial function (PAEF). We report here a noninvasive MRI approach to measure nitric-oxide (NO) mediated PAEF by quantifying changes in PA area and blood flow during isometric handgrip exercise (IHE), an endothelial NO-dependent stressor. We used this to test the hypothesis that PLWH have impaired PAEF, even before development of PH.
Design:
Prospective cohort study.
Methods:
We enrolled 25 HIV+ viral-suppressed subjects on stable anti-retroviral therapy without known or suspected PH and 19 matched seronegative control subjects (HIV-). PA area and blood flow changes in response to IHE were measured with non-contrast MRI. Data previously collected during NO-synthase inhibition were analyzed to determine the role of NO in the PA response to IHE.
Results:
Seronegative subjects exhibited the anticipated PA vasodilatory response to IHE, but this was completely absent in HIV+ subjects who exhibited an impaired area change (−1.1±1.2% vs +7.7±2.2%, HIV+ vs HIV-, mean±SEM, respectively, P=0.002) and blood flow response (0.2±2.3% vs 13.5±4.8%, P=0.005). The PA vasodilatory effect of IHE in healthy subjects was fully blocked by NO-synthase, demonstrating this PA response is predominantly NO-mediated.
Conclusion:
Using noninvasive MRI methods to quantify PAEF, we observed significantly impaired PAEF in PLWH compared to matched HIV- controls. Noninvasive PAEF testing may be useful in evaluating early HIV-related pulmonary vascular disease.
Keywords: HIV, pulmonary hypertension, pulmonary vascular disease, endothelial function, MRI
INTRODUCTION
As therapies to treat human immunodeficiency virus (HIV) have improved over recent decades, people living with HIV (PLWH) are living longer and faced with chronic diseases, including cardiopulmonary disease and pulmonary hypertension (PH) [1, 2]. The prevalence of HIV-associated pulmonary hypertension (HIV-PH) is higher than in the general population, confers significant morbidity, and is an independent predictor of mortality [2–5]. Furthermore, survival rates are worse for those with HIV-PH compared to those with HIV without PH [2]. In addition to primary pulmonary arterial hypertension, PLWH more frequently develop secondary PH due to common comorbidities such as coronary atherosclerosis, heart failure, and chronic obstructive lung disease [6]. Although the mechanisms underlying PH are not well understood in PLWH, several factors have been implicated in the pathogenesis including viral proteins, coinfection with hepatitis C virus (HCV), inflammation, genetics, and social factors such as smoking and intravenous drug use [6]. What is needed is the ability to detect pulmonary artery (PA) injury at the earliest stages both as a tool to understand the natural history of the disease and to tease out the importance of factors contributing to injury as well as for early clinical detection when therapeutic options may have greater efficacy.
Endothelial dysfunction of the pulmonary vascular bed is an early, central underlying pathophysiologic mechanism in the development of PH which is characterized by diminished nitric oxide (NO) production and impaired relaxation of the PA to specific stressors [7, 8]. Moreover, studies in animal models of PH have shown that viral proteins can induce vascular oxidative stress and direct endothelial cell injury, which may progress to pulmonary vasculature remodeling and vasoconstriction [3, 9, 10]. Endothelial dysfunction in peripheral and coronary arteries of people with and without HIV is a marker of cardiovascular disease and is associated with adverse outcomes [11–13]. PA endothelial function (PAEF) has historically been assessed with invasive catheter-based measures of changes in PA cross-sectional area (CSA) and blood flow in response to an endothelial-dependent stressor [14, 15]. Previous studies showed that pulmonary vasoreactivity is impaired in patients with idiopathic PH [16], and predicts mortality in this population [14] [17, 18]. The invasive nature of prior techniques to quantify the PA vasodilatory and flow responses to endothelial-dependent stressors significantly limited the clinical utility of this technique, particularly in those requiring repeated studies and/or studies in clinically stable or healthy subjects. However, because of these invasive requirements, little is known about PAEF in PLWH. A noninvasive method to identify pulmonary endothelial dysfunction would represent a valuable tool to better understand the pathogenesis of pulmonary vascular disease and PH in HIV.
Previous work from our group utilizing the novel combination of 3T MRI methods with isometric handgrip exercise (IHE), a well-established endothelial-dependent stressor, demonstrated a noninvasive method of measuring coronary endothelial function with high reproducibility including in cohorts of PLWH [19, 20]. In the present study, we used MRI techniques to quantify PAEF by measuring stress-induced changes in both PA CSA and PA blood flow (PBF) [21]. We aimed to assess feasibility of this technique as well as to test the hypothesis that PLWH have impaired PAEF compared to age and sex matched participants without HIV. Furthermore, in order to determine whether the normal observed PA vasoreactive responses to IHE are mediated by NO and thus reflect PAEF, we studied the PA response to IHE before and during infusion of NG-monomethyl-l-arginine (l-NMMA), a NO-synthase inhibitor, in separate group of healthy volunteers.
METHODS
Study Participants
Our study was approved by the Johns Hopkins institutional review board, and all participants provided written informed consent. There are two parts to this study: first, testing whether PLWH have impaired PA vasoreactivity with IHE as compared to matched controls and second, whether PA vasoreactivity to IHE exercise is predominantly NO-mediated. For the first part, HIV+ participants were prospectively recruited from outpatient clinics at the Johns Hopkins Hospital and were screened for contraindications to MRI. PLWH were on stable ART for at least 2 months with a CD4 count > 200 mm3. The majority had an undetectable viral load, and there was no self-reported recreational drug use for 2 months or more prior to enrollment. HIV seronegative subjects served as controls and were healthy adults prospectively recruited with no contraindications to MRI, no known history of cardiovascular disease or diabetes, and with at most one traditional cardiovascular risk factor. Control participants were intentionally matched to HIV patients based on age and sex. For the second part on whether the PA response to IHE is NO-mediated, we analyzed images previously acquired during NO-synthase inhibition.
MRI Protocol
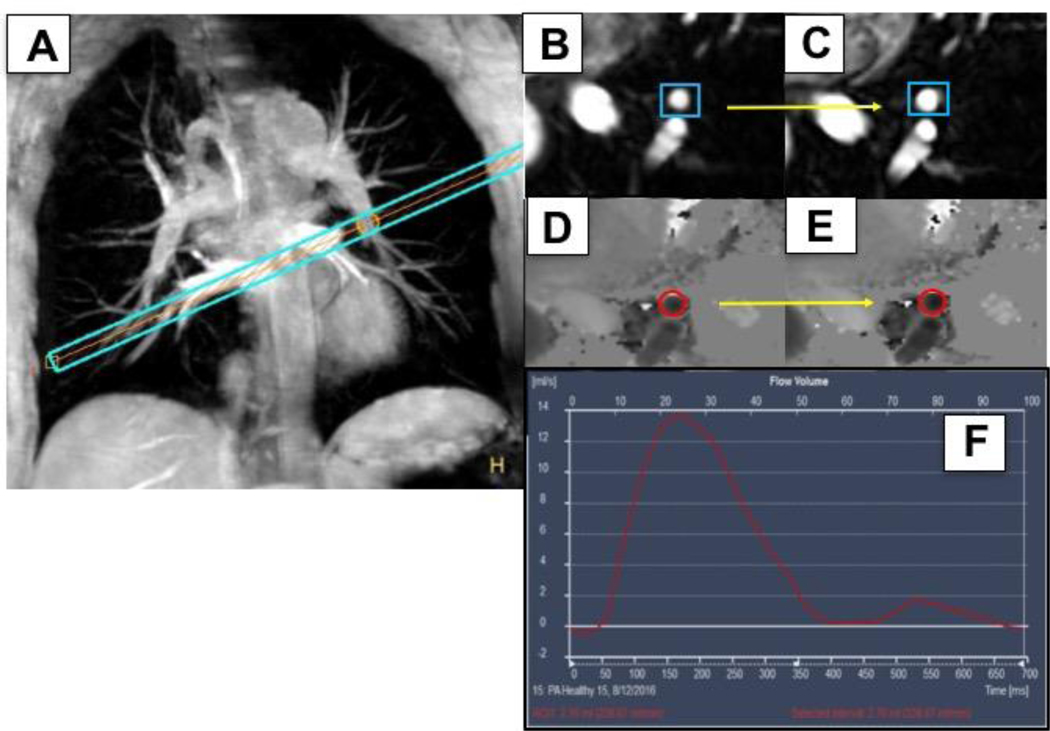
Each subject underwent non-contrast MRI using a commercial 3.0 Tesla (T) whole-body MR scanner (Achieva, Philips, Best, NL) with a 32-element cardiac coil for signal reception in a fasting state while in the prone position [21]. Based on an acquired field map, localized radiofrequency shimming was performed [22]. Baseline anatomical and velocity-encoded scout images were collected at rest and a high-resolution 3-dimensional (3D) volume using a segmented gradient echo sequence was used to localize PA segments during free breathing with real-time navigator gating and motion tracking as previously described [21]. Images were then acquired perpendicular to a linear segment of a branch PA best identified on scout images (Figure 1A).
Figure 1:
Example of a healthy adult coronal image of the pulmonary arterial tree. A slice perpendicular (A, blue bars) to the descending branch of the left pulmonary artery (PA) is shown with an arrow (yellow) pointing to the center of the imaging plane in a PA segment. (B) Corresponding PA cross sectional segment at rest (blue box) and (C) during isometric handgrip exercise (IHE), showing PA dilation (yellow arrow). (D) Phase contrast images of the same PA segment (red circle) at rest and during IHE (E). (F) Illustrates the corresponding blood flow profile of a PA segment illustrating blood flow integrated over time in the cardiac cycle (in msec, x-axis).
To obtain PAEF measurements, the imaging plane was localized perpendicular to a well-visualized, linear segment of the descending left and/or right PA without branches over a distance of approximately two centimeters. A high-resolution coronal 3D MRI angiogram and its transverse reformat were used to ensure that slice orientation was perpendicular to the PA (Figure 1B). For PA measurements, a Cartesian, retrospectively VCG-triggered, segmented gradient echo cine acquisition reconstructed to 40 cardiac phases was used. Following anatomical area images, PA blood flow images were acquired with gradient echo cine phase contrast velocity-encoded images that were reconstructed to 25 cardiac phases with velocity encoding set to 100 cm/s. The breath-hold duration was approximately 20–25 seconds for each anatomical and velocity-encoded sequence. Repeat imaging of the same anatomic location was then completed during 4–7 minutes of continuous IHE using an MRI-compatible dynamometer (Stoelting, Wood Dale, IL, USA) at 30% maximum grip strength as directed by a supervising research nurse. Heart rate and blood pressure were monitored throughout using the scanner’s vector electrocardiogram (VCG) and a MRI-compatible blood pressure cuff on the calf (Invivo, Precess, Orlando, FL, USA). The rate pressure product (RPP) was calculated from the product of systolic blood pressure x heart rate.
L-NMMA Study
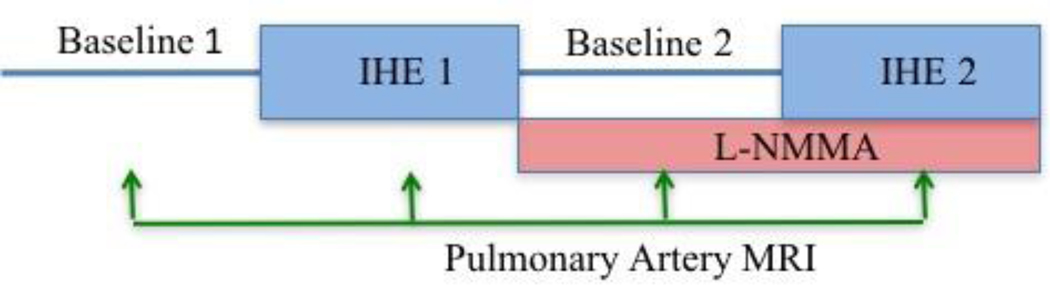
To determine the contribution of NO to the PA vasoreactive response, we analyzed previously acquired images collected before and during infusion of NG-monomethyl-L-arginine (l-NMMA, 0.3 mg·kg−1·min−1), a NO-synthase inhibitor, in healthy volunteers.[19] The prior study was performed to study NO-mediated coronary endothelial function detected by MRI during IHE but we re-analyzed the data for the responses of the pulmonary arteries. Each participant (n=7, adults without HIV) underwent a first IHE period during which intravenous saline (placebo) was infused. After post-exercise recovery, each subject then received an intravenous infusion of l-NMMA at a dose of 0.3 mg·kg−1·min−1, as previously described [19]. A new set of baseline images was obtained after 5 min of l-NMMA infusion. A second IHE period was then initiated while l-NMMA infusion continued, and imaging was repeated at the same location, with the average infusion lasting approximately 15–22 minutes. PA percent area change measures were obtained at each time point as shown in Figure 2.
Figure 2:
Protocol diagram illustrating MRI NG-monomethyl-l-arginine (l-NMMA) study of the pulmonary arteries in 7 healthy volunteers (mean age 42±3 years, 4 women). IHE=Isometric handgrip exercise.
Image Analysis
The images were analyzed at baseline and during IHE stress for PA CSA, in mm2 and PBF, in mL/min. The relative percent stress-induced change was calculated for each segment. The CSA was measured using semi-automated software (Cine Version 3.15.17, General Electric, Milwaukee, WI, USA), that utilized manual outline of the PA branch of interest in cross-section followed by automated adjustment of the vessel border according to full-width-half-maximum-algorithm. The CSA was taken during systole at peak vessel diameter and averaged over 3 image frames. For PBF measurement, a similar technique with automatic outline of each cross-sectional segment with manual correction was applied on phase contrast images, averaged throughout one cardiac cycle, and then integrated to obtain total flow in mL/min in the corresponding vessel using (QFLOW version 3.0, Medis, NL). Images with poor quality (due to artifact and/or motion) were excluded from the analysis (n=2, assessed by consensus of two blinded readers). In cases where greater than one PA were measured in the same subject, the percent change in CSA and/or PBF between baseline and IHE stress was averaged to perform per subject analysis. Intra- and interobserver measurements were performed by two independent readers blinded to participant group for PA CSA and PBF on a subset of 10 healthy subjects (20 segments total), and analysis was performed blinded in terms of state (rest vs. stress). Data analysis for the main PA study and l-NMMA study was performed by two independent investigators blinded to study group (placebo vs. l-NMMA) and stage of the protocol (rest vs. stress).
Laboratory Measurements
As part of routine clinical evaluation, the most recent CD4 count and HIV RNA were obtained from the electronic medical record (EMR, within one year of study), as were CD4 nadir, HCV testing, and lipid panels. When available, any prior clinically indicated echocardiographic data was obtained from the EMR to document right ventricular systolic pressure (RVSP) as a marker of pulmonary pressures.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism version 8.3.1 (GraphPad Software, San Diego, California, USA). The Shapiro-Wilk test was used to test the data for normality. Parametric Welch’s t-test was used to compare between-group differences in normally distributed data, and non-parametric testing (Wilcoxon rank sum or Mann-Whitney test) was used to make comparisons between skewed data, as appropriate, for differences in PA CSA and PBF in response to IHE. Linear regression analysis was performed to assess correlation between subject BMI and each PAEF parameter (percent change CSA and PBF from baseline to stress) as well as correlations in the PLWH group between ART duration, most recent CD4, CD4 nadir (if known), and each PAEF parameter. The Bland-Altman method was used to assess inter- and intraobserver agreement for PA CSA and PBF measurements, with p-values derived from Pitman’s test of differences. Intraclass correlation coefficients were also determined for inter and intraobserver results for PA CSA and PBF using a two-way mixed effects model where people effects are random and measure effects are fixed. Statistical significance was defined as a two-tailed p-value ≤ 0.05. Data are expressed as mean ± standard error of the mean (SEM).
RESULTS
Participant Characteristics
The characteristics of all subjects are reported in Table 1. There was no statistically significant difference between groups in terms of age or sex due to intentional matching. Conventional cardiovascular risk factors of type II diabetes mellitus, hypertension, hyperlipidemia, and tobacco smoking (combined current and former) tended to be higher in the PLWH group but did not meet statistical significance. Body mass index (BMI) was significantly higher in the PLWH group (p=0.004). Importantly, the PLWH participants were all on stable ART for >2 months prior to study enrollment with CD4 >200, and 92% with viral load <20 copies/mL (the remaining 2 subjects had detectable viral loads ≤60 copies/mL). All PLWH subjects were negative for HCV. Measurements of low-density lipoprotein (LDL) cholesterol within the preceding 12 months were clinically available for 23 of the PLWH subjects (mean LDL 102.8±6.4 mg/dL). Of those who had prior clinically indicated transthoracic echocardiograms (n=15, all from the PLWH group), the mean RVSP was within the normal range at 28.3±1.6 mmHg with only one subject with mildly elevated RVSP of 40 mmHg.
Table 1.
Baseline characteristics of subjects with HIV vs seronegative controls
| Characteristics | Controls (n=19) | PLWH (n=25) | P-value |
|---|---|---|---|
| Age (years), Mean ± SEM | 46.5 ± 2.9 | 49.6 ± 1.7 | 0.36 |
| Women, n (%) | 11 (58) | 16 (64) | 0.68 |
| BMI (kg/m2), Mean ± SEM | 25 ± 0.8 | 29 ± 1 | 0.004 |
| Diabetes, n (%) | 0 (0) | 2 (8) | 0.50 |
| HTN, n (%) | 4 (22) | 9 (36) | 0.49 |
| Smoker, n (%) | 3 (17) | 5 (20) | 0.72 |
| Statin use, n (%) | 2 (11) | 9 (36) | 0.053 |
| Years on ART, mean ± SEM | N/A | 12.6 ± 1.2 | - |
| Protease Inhibitor, n (%) | N/A | 5 (20) | - |
| NNRTI, n (%) | N/A | 4 (16) | - |
| NRTI, n (%) | N/A | 22 (88) | - |
| Integrase Inhibitor, n (%) | N/A | 21 (84) | - |
| CD4 count (mm3), mean ± SEM | N/A | 874 ± 72 | - |
| CD4 nadir (mm3), mean ± SEM | N/A | 288 ± 43 | - |
| Viral Load <20 copies/mL, n (%) | N/A | 23 (92) | - |
| Prior RVSP (mmHg), mean ± SEM | n = 15; 28.3±1.6 | - |
Abbreviations: HIV, human immunodeficiency virus; SE, standard error of the mean; BMI, body mass index; HTN, hypertension; ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; RVSP, right ventricular systolic pressure.
Hemodynamic Effect of IHE
There was no significant difference in baseline resting heart rate, systolic blood pressure, or RPP between the two groups (p=0.25, p=0.46, and p=0.22, respectively). There was a similar increase in RPP with IHE for both groups (15.4±3.7% for PLWH and 15.9±3.3% for controls, p=0.92).
Pulmonary Artery Vasoreactivity
Representative anatomical and velocity-encoded PA images are shown in Figure 1. Image quality was sufficient for analysis of PA CSA in all subjects (total of 30 PA segments from the control group and 31 PA segments from the PLWH group). The image quality for the PBF analysis was adequate for 29 PA segments in each group. Bland-Altman analyses for intraobserver and interobserver variability for PAEF measures are shown in the supplement (Supplemental Fig 1, showing Bland-Altman analyses).
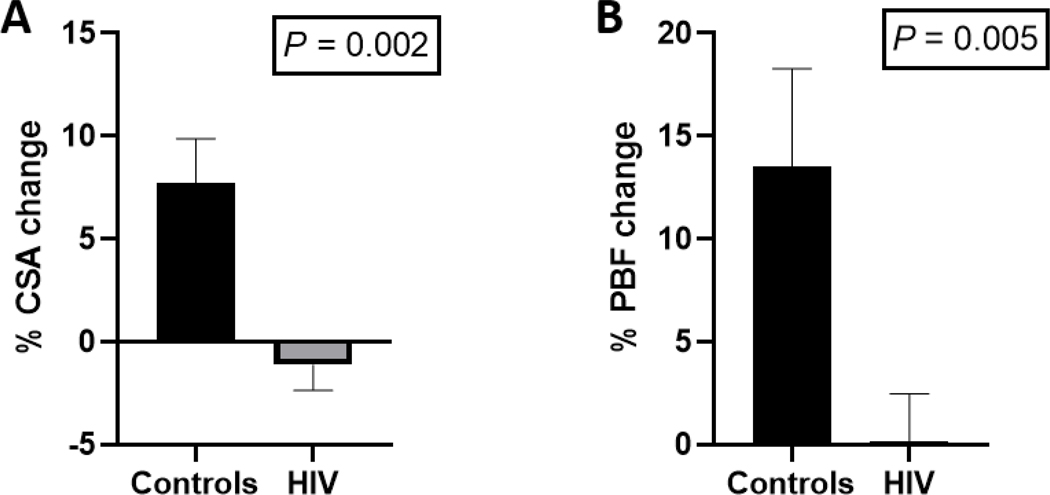
Baseline PA CSA and PBF in controls showed no significant difference from PLWH subjects (PA CSA 35.6±2.4 mm2 vs 30.3±2.8 mm2, p=0.07; PBF 322±47 mL/min vs 236±28 mL/min, p=0.27). In healthy, seronegative controls, the PA responded to IHE with vasodilation and an increase in blood flow. However, those normal responses were significantly attenuated in PLWH: PA CSA (7.7±2.2% vs −1.1±1.2%, p=0.002) and PBF (13.5±4.8% vs 0.2±2.3%, p=0.005, Figure 3).
Figure 3:
Percent change in pulmonary artery (PA) cross-sectional area (CSA, A) with isometric handgrip exercise (IHE), and percent change in PA blood flow (PBF, B) with IHE is demonstrated in 25 subjects with HIV (black bars) and 19 controls (gray bars).
Linear regression analysis revealed no significant correlation between BMI in all participants and each PAEF parameter (p=0.19 for CSA, p=0.17 for PBF). A separate analysis of PAEF in a subset of subjects matched for BMI (PLWH [n=23 for CSA, n=25 for PBF] vs. controls [n=14 for CSA, n=13 for PBF]) revealed a consistently significant difference in PA CSA and PBF change with IHE between groups (7.6±2.3% vs −1.1±1.2%, p=0.004 and 12.2±5.5% vs 0.2±2.3%, p=0.02, respectively). Linear regression was performed in the PLWH group to evaluate the association between CD4 count (n=21) and PAEF and revealed a positive association between CD4 and IHE-induced PBF change (r2=0.32, p=0.0096), however this was no longer significant after removing an outlier (r2=0.007, p=0.73). Similarly, there was no significant relationship between CD4 count and CSA change (r2=0.11, p=0.14, Figure 4). Additional linear regression analysis on ART duration, nadir CD4, and LDL cholesterol versus each PAEF parameter in the PLWH cohort revealed no significant correlations.
Figure 4:
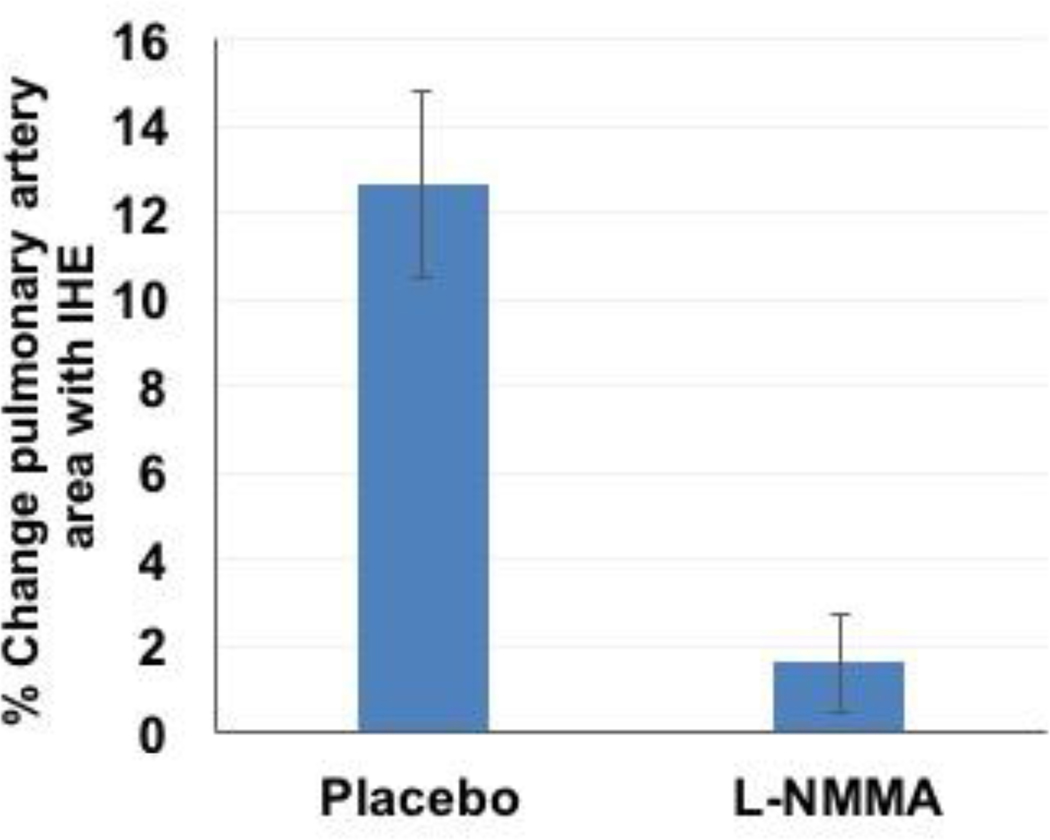
Intravenous infusion of L-NMMA blocks IHE-induced pulmonary artery (PA) vasodilation in 7 healthy volunteers measured by MRI. Summary changes during isometric handgrip exercise (% CSA increase from baseline values) for placebo (saline infusion) and during L-NMMA infusion showing that L-NMMA significantly blunts the IHE-induced increase in PA area in response to IHE (p=0.001 response during placebo vs l-NMMA).
Pulmonary Artery Area Changes with l-NMMA
For the PA analysis of previously acquired l-NMMA images in seven healthy adults (age 42±3 years, 4 women), there was significant PA dilation in response to the first IHE, consistent with the observations above (Figure 4). There was no difference in resting CSA of the PA before the first IHE episode (during placebo infusion) and before the second IHE episode (during l-NMMA infusion, p=0.4). However, in contrast to the vasodilatory PA response to IHE during placebo, there was no significant increase in PA area when IHE was repeated during l-NMMA infusion (Figure 4, p=0.3 vs. baseline). When comparing the IHE response between placebo and l-NMMA conditions, the normal PA area increase with IHE (as % baseline) was completely blocked by l-NMMA infusion (12.4±5.8% vs. 1.6±3%, placebo vs. l-NMMA; p=0.001, Figure 4).
DISCUSSION
In this study, we demonstrate that noninvasive, non-contrast 3T MRI measures of pulmonary artery cross-sectional area and blood flow are feasible with high temporal and spatial resolution as well as reproducible with good intraobserver and interobserver agreement for vasoreactivity. Moreover, the normal response to IHE of PA vasodilatation and increased flow are primarily NO-dependent and that normal response is nearly completely lost in otherwise healthy, virally-suppressed PLWH. This new ability to noninvasively characterize NO-dependent PAEF provides an opportunity to expand our understanding of the dynamic pathophysiologic factors contributing to pulmonary endothelial dysfunction in PLWH who are at risk for PH and, in the clinical setting, to potentially assess the response to interventions with noninvasive serial studies.
Pulmonary endothelial dysfunction is a proposed common mechanism underlying the development of PH due to attenuated NO release and the resulting impaired relaxation of the pulmonary arteries [9, 10]. Studies have directly demonstrated reduced endothelial NO production and decreased responsiveness of pulmonary vascular smooth muscle to vasodilators in animal models of severe PH [9, 23]. One study showed that intrapulmonary NO levels were significantly lower in patients with PH (due to pulmonary arterial hypertension) compared to healthy control subjects, and NO reaction product levels were inversely correlated with PA pressures [24]. Moreover, one of the mainstay treatment categories for PH targets the NO pathway to soluble guanylate cyclase. Taken together with several biochemical studies investigating the expression of factors and co-factors for NO in patients with PH, it has been concluded that impaired synthesis and/or activation of NO may contribute to the development and progression of PH.[25] Thus, our findings of impaired NO-mediated vasodilation are particularly compelling in a patient population known to be at increased risk of developing PH.
This MRI method of vasomotor testing has previously shown that PLWH have profound abnormalities in coronary endothelial function despite being on typical ART regimens with low viral loads and adequate CD4 counts, which is consistent with the findings in PAEF in low risk PLWH in this study [26]. Importantly, prior studies have shown that the reported prevalence of HIV-PH did not change significantly after the introduction of ART in PLWH [6, 27, 28]. This is supported by our observation that there was no significant relationship between either CD4 count or ART duration and PAEF.
The prevalence of PH in PLWH is higher than in the general population [2, 4, 5, 28], and the development of PH is considered to be an independent predictor of mortality [2, 3]. The degree of PA endothelial dysfunction observed in PLWH may explain in part why this population is at greater risk for PH. Prior studies have suggested that early diagnosis and treatment of PH may lead to improved long-term outcomes, therefore the earlier detection of PA endothelial dysfunction that contributes to PH is important [29–31]. Thus, our noninvasive method of assessing PAEF may provide additional information for evaluating PLWH who are at higher risk for developing PH. Importantly, as the MRI-IHE technique is safe and reproducible, this method may be used to follow patient response to therapy over time and therefore warrants further study.
Limitations
The main limitation of the study is the relatively modest sample size. However, the sample size was adequate to test our hypotheses, and importantly, we detected a significant difference in PAEF between PLWH and the control group with 44 participants total. It is difficult to determine from this cross-sectional study whether the mechanistic abnormalities in PAEF in the PLWH are due to the HIV infection itself, ART or another confounding factor. However, the groups were well matched in terms of age, sex and CV comorbidities such as hypertension and diabetes. Although the BMI was significantly higher in the PLWH group, regression analysis showed no significant correlation between BMI and PAEF suggesting that the abnormal endothelial response in the PLWH group cannot be explained by the higher BMI alone. This study was not conducted with patients who have known HIV-PH, which could provide further insight into the pathophysiology underlying the disease. Further studies are needed with such cohorts. Another limitation of the study is that the MRI measurements of PAEF were not compared with invasive methods of PA vasomotor testing, as this was not clinically indicated in relatively healthy subjects. However, given our goal of measuring CSA and blood flow, MRI has been previously validated for these measures (with the gold standard of invasive angiography) in other vascular beds [32, 33].
Conclusions
In summary, the present study demonstrates that 1) high-resolution MRI combined with IHE, provides an innovative reproducible noninvasive approach to the assessment of NO-mediated endothelial-dependent vasoreactivity of the pulmonary vascular bed and that 2) PLWH who have no known pulmonary vascular disease have significantly impaired PAEF compared to matched controls without HIV. Importantly, we demonstrated that the degree of PA endothelial dysfunction was not related to duration of ART and present despite well-controlled HIV infection. Our findings suggest that this noninvasive approach to measure PAEF may complement the risk assessment, be used to test new therapies for pulmonary vascular health, and to follow progression for PLWH who are at risk for pulmonary hypertension.
Supplementary Material
Acknowledgements:
Author contributions: R.G.W. and A.G.H. conceived the study and obtained regulatory approval. A.G.H., M.S., and G.B. collected data. A.G.H., E.G., and M.M. analyzed data. E.G. and M.M. both drafted the first manuscript in close collaboration with R.G.W. and A.G.H. All of the authors reviewed the manuscript.
Funding:
This work was supported by the Ruth L. Kirschstein Institutional National Research Service Award; T32HL007227-Pathophysiology of Myocardial Disease. This work was supported by the National Institute of Health (1R01HL147660, HL007227, HL125059).
Footnotes
Conflicts of interest
There are no conflicts of interest.
List of Supplemental Digital Content
Supplemental Digital Content 1.doc
References:
- 1.Staitieh B, Guidot DM. Noninfectious pulmonary complications of human immunodeficiency virus infection. Am J Med Sci 2014,348:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henriques-Forsythe M, Annangi S, Farber HW. Prevalence and hospital discharge status of human immunodeficiency virus-associated pulmonary arterial hypertension in the United States. Pulm Circ 2015,5:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correale M, Palmiotti GA, Lo Storto MM, Montrone D, Foschino Barbaro MP, Di Biase M, et al. HIV-associated pulmonary arterial hypertension: from bedside to the future. Eur J Clin Invest 2015,45:515–528. [DOI] [PubMed] [Google Scholar]
- 4.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest 1991,100:1268–1271. [DOI] [PubMed] [Google Scholar]
- 5.Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest 2011,139:128–137. [DOI] [PubMed] [Google Scholar]
- 6.Basyal B, Jarrett H, Barnett CF. Pulmonary Hypertension in HIV. Can J Cardiol 2019,35:288–298. [DOI] [PubMed] [Google Scholar]
- 7.Dinh-Xuan AT, Higenbottam TW, Clelland CA, Pepke-Zaba J, Cremona G, Butt AY, et al. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl J Med 1991,324:1539–1547. [DOI] [PubMed] [Google Scholar]
- 8.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004,109:159–165. [DOI] [PubMed] [Google Scholar]
- 9.Christou H, Hudalla H, Michael Z, Filatava EJ, Li J, Zhu M, et al. Impaired Pulmonary Arterial Vasoconstriction and Nitric Oxide-Mediated Relaxation Underlie Severe Pulmonary Hypertension in the Sugen-Hypoxia Rat Model. J Pharmacol Exp Ther 2018,364:258–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mermis J, Gu H, Xue B, Li F, Tawfik O, Buch S, et al. Hypoxia-inducible factor-1 alpha/platelet derived growth factor axis in HIV-associated pulmonary vascular remodeling. Respir Res 2011,12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mezoh G, Crowther NJ. Deciphering Endothelial Dysfunction in the HIV-Infected Population. Adv Exp Med Biol 2019,1134:193–215. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation 2002,105:546–549. [DOI] [PubMed] [Google Scholar]
- 13.Anand AR, Rachel G, Parthasarathy D. HIV Proteins and Endothelial Dysfunction: Implications in Cardiovascular Disease. Front Cardiovasc Med 2018,5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apitz C, Zimmermann R, Kreuder J, Jux C, Latus H, Pons-Kuhnemann J, et al. Assessment of pulmonary endothelial function during invasive testing in children and adolescents with idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 2012,60:157–164. [DOI] [PubMed] [Google Scholar]
- 15.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function: from research into clinical practice. Circulation 2012,126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latus H, Werz A, Kock I, Rupp S, Kerst G, Kreuder J, et al. Systemic arterial endothelial function in children and young adults with idiopathic pulmonary arterial hypertension: is there a relation to pulmonary endothelium-dependent relaxation? Pediatr Cardiol 2014,35:844–850. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra R, Hess D, Lewis GD, Bloch KD, Waxman AB, Semigran MJ. Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension. Pulm Circ 2011,1:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu QX, Yang YH, Geng J, Zhai ZG, Gong JN, Li JF, et al. Clinical Study of Acute Vasoreactivity Testing in Patients with Chronic Thromboembolic Pulmonary Hypertension. Chin Med J (Engl) 2017,130:382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hays AG, Iantorno M, Soleimanifard S, Steinberg A, Schar M, Gerstenblith G, et al. Coronary vasomotor responses to isometric handgrip exercise are primarily mediated by nitric oxide: a noninvasive MRI test of coronary endothelial function. Am J Physiol Heart Circ Physiol 2015,308:H1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol 2010,56:1657–1665. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee M, Schär M, Bonanno G, Gerstenblith G, Weiss RG, Hays AG. Noninvasive Assessment of Pulmonary Artery Vasoreactivity at Rest in Healthy Subjects Using 3T MRI. SCMR Abstract 2017. [Google Scholar]
- 22.Schär M, Ding H, Herzka DA. Improvement in B1+ Homogeneity and Average Flip Angle Using Dual-Source Parallel RF Excitation for Cardiac MRI in Swine Hearts. PLoS One 2015,10:e0139859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mam V, Tanbe AF, Vitali SH, Arons E, Christou HA, Khalil RA. Impaired vasoconstriction and nitric oxide-mediated relaxation in pulmonary arteries of hypoxia- and monocrotaline-induced pulmonary hypertensive rats. J Pharmacol Exp Ther 2010,332:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, et al. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 1998,158:917–923. [DOI] [PubMed] [Google Scholar]
- 25.Klinger JR, Kadowitz PJ. The Nitric Oxide Pathway in Pulmonary Vascular Disease. Am J Cardiol 2017,120:S71–S79. [DOI] [PubMed] [Google Scholar]
- 26.Iantorno M, Schär M, Soleimanifard S, Brown TT, Moore R, Barditch-Crovo P, et al. Coronary artery endothelial dysfunction is present in HIV-positive individuals without significant coronary artery disease. AIDS 2017,31:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sitbon O. HIV-related pulmonary arterial hypertension: clinical presentation and management. AIDS 2008,22 Suppl 3:S55–62. [DOI] [PubMed] [Google Scholar]
- 28.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 2008,177:108–113. [DOI] [PubMed] [Google Scholar]
- 29.Humbert M, Sitbon O, Yaici A, Montani D, O’Callaghan DS, Jais X, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010,36:549–555. [DOI] [PubMed] [Google Scholar]
- 30.Humbert M, Gerry Coghlan J, Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev 2012,21:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, et al. Therapy for Pulmonary Arterial Hypertension in Adults: Update of the CHEST Guideline and Expert Panel Report. Chest 2019,155:565–586. [DOI] [PubMed] [Google Scholar]
- 32.Hundley WG, Hillis LD, Hamilton CA, Applegate RJ, Herrington DM, Clarke GD, et al. Assessment of coronary arterial restenosis with phase-contrast magnetic resonance imaging measurements of coronary flow reserve. Circulation 2000,101:2375–2381. [DOI] [PubMed] [Google Scholar]
- 33.Kelle S, Hays AG, Hirsch GA, Gerstenblith G, Miller JM, Steinberg AM, et al. Coronary artery distensibility assessed by 3.0 Tesla coronary magnetic resonance imaging in subjects with and without coronary artery disease. Am J Cardiol 2011,108:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.