Abstract
The intestinal epithelium represents the primary interface between the host, the gut microbiome and its external environment. Since the intestinal epithelium contributes to innate immunity as a first line of defense, understanding how the epithelium responds to microbial and host stimuli is an important consideration in promoting homeostasis. Human intestinal enteroids (HIEs) are primary epithelial cell cultures that can provide insights into the biology of the intestinal epithelium and innate immune responses. One potential limitation of using HIEs for innate immune studies is the relative lack of responsiveness to factors that stimulate epithelial cytokine production. We report technical refinements, including removal of extracellular antioxidants, to facilitate enhanced cytokine responses in HIEs. Using this new method, we demonstrate that HIEs have distinct cytokine profiles in response to pro-inflammatory stimuli derived from host and microbial sources. Overall, we found that host-derived cytokines TNF and IL-1α stimulated reactive oxygen species and a large repertoire of cytokines. In contrast, microbial stimuli LPS, LTA and flagellin stimulated a limited number of cytokines and histamine did not stimulate the release of any cytokines. Importantly HIE secreted cytokines were functionally active, as denoted by the ability of human blood-derived neutrophil to migrate towards HIE supernatant containing IL-8. These findings establish that the immune responsiveness of HIEs depends on medium composition and stimuli. By refining the experimental culture medium and creating an environment conducive to epithelial cytokine responses by human enteroids, HIEs can facilitate exploration of many experimental questions pertaining to the role of the intestinal epithelium in innate immunity.
Keywords: human intestinal enteroids (HIEs), cytokines, Toll-like receptors (TLR), histamine, tumor necrosis factor (TNF), flagellin
Introduction
The epithelium of the gastrointestinal tract represents a key interface between the luminal environment of the gut, which contains a diverse microbial community, and the host immune system. Epithelial cells are essential modulators of inflammation through sensing and responding to intestinal microbes and the luminal environment. In response to stimuli by luminal antigens, intestinal epithelial cells release cytokines, thereby regulating immune cell trafficking in the gut. Elucidating the molecular interactions between the gut microbiota, the intestinal epithelium and the immune system has become a topic of intense interest due to the growing relevance of these signaling cascades in both health and disease (Belkaid & Hand, 2014; Engevik & Versalovic, 2017; Cianci et al., 2018; Lazar et al., 2018).
Previous investigations of epithelial-immune system communication have relied on animal models or cancer-derived cell cultures to identify key molecular pathways. However, the development of human intestinal enteroids (HIEs) has significantly advanced our ability to examine cell-cell interactions in a non-transformed human model system. HIEs recapitulate intestinal structure and function, thereby circumventing key limitations of cancer-derived/immortalized cell lines or animal models. HIEs are derived from Lgr5+ stem cells in the intestinal crypts and harbor all the cell types normally found in the intestinal epithelium including enterocytes, stem cells, goblet cells, Paneth cells, tuft cells, and enteroendocrine cells (Sato et al., 2009). Moreover, HIEs maintain segment specificity and can be grown in 3D spheroids or as 2D monolayers, providing versatility for experimental manipulation (Zachos et al., 2016; Chen et al., 2017; Noben et al., 2017; Blutt et al., 2018b). Thus, HIEs provide a novel platform to recapitulate mature human intestinal epithelial function and to improve our understanding of the mechanisms that regulate intestinal homeostasis. Despite advances in HIE cultivation, challenges remain in using HIEs to study microbiota-epithelial or immune-epithelial communication; particularly the limited cytokine response to pro-inflammatory stimuli. Evaluating cytokine production by the intestinal epithelium has been a key method for studying the process of intestinal inflammation particularly in the understanding of disease processes such as inflammatory bowel disease (IBD). This lack of significant cytokine response to pro-inflammatory stimuli has impeded the study of intestinal epithelial inflammation.
Multiple studies have demonstrated that host cytokines and microbial stimuli elicit epithelial pro-inflammatory pathways (Gross et al., 1995; Kolios et al., 1996; Warhurst et al., 1998; Takeuchi et al., 2001; Hosoi et al., 2003; Rochon & Romling, 2006; Cheng et al., 2008; Angrisano et al., 2010; Sonnier et al., 2010). Using cancer-derived cell lines, these studies have shown that tumor necrosis factor (TNF), interlukin-1α (IL-1α), lipoteichoic acid (LTA), lipopolysaccharides (LPS), flagellin and histamine stimulate production of the neutrophil chemoattractant interleukin 8 (IL-8). However, recent studies using HIEs have not found similarly significant cytokine production in response to pro-inflammatory factors. For example, flagellin can mildly stimulate IL-8 and TNF in HIEs. Interestingly, no effects were observed with either toll like receptor 2 (TLR-2) or toll like receptor 4 (TLR-4) agonists (Samuel et al., 2017; Nishimura et al., 2019). In another study, Nishimura and colleagues reported that daily treatment with a combination of poly(I:C), flagellin, interluken-1 beta (IL-1β), and TNF for 12 weeks was required to stimulate significant IL-8 production in HIEs (Nishimura et al., 2019). These discrepancies in cytokine production in transitional cell culture models compared to HIEs may be explained by factors present in the HIE culture system that may suppress epithelial responses to pro-inflammatory stimuli.
Typical growth and maintenance HIE medium is complex and contains N-acetyl-cysteine, glutathione, B27, N2, and superoxide dismutase among other components; all of which suppress reactive oxygen species (Sato et al., 2009). Reactive oxygen species are short lived, highly electrophilic molecules that possess essential signaling roles (Jones et al., 2012). Certain receptors, including TLRs and cytokine receptors, physically interact with reactive oxygen species-generating NADPH oxidase/Dual oxidase (NOX/DUOX) enzymes (Grandvaux et al., 2007; Karrasch et al., 2007; Kim et al., 2007; Ogier-Denis et al., 2008; Sandoval et al., 2018). For example, TLR activation stimulates NOX, which generates reactive oxygen species (Grandvaux et al., 2007; Ogier-Denis et al., 2008; Yang et al., 2009). Reactive oxygen species then modifies redox-sensitive proteins by reversible H2O2-mediated oxidation of their active site cysteine (Jones et al., 2012). These oxidant-sensitive proteins include protein tyrosine phosphatases, such as lipid phosphatase (PTEN) and MAP kinase phosphatases (MAPK-P); which are important mediators of NFκB and inflammatory signaling (Kamata et al., 2005; Chiarugi & Buricchi, 2007; Ogier-Denis et al., 2008; Yang et al., 2009; Heneberg et al., 2010). In various cell types, host-derived stimuli such as TNF (O’Hara et al., 2009; Kim et al., 2010), IL-1α (Qin et al., 2011), LPS (DeForge et al., 1993; Sanlioglu et al., 2001), and microbial-stimuli such as LTA (Saetre et al., 2001) stimulate reactive oxygen species to promote pro-inflammatory cytokine release. As a result, we hypothesized that the antioxidants in HIE medium suppress secretion of select epithelial cytokines, and that modification of the experimental medium would facilitate investigation of epithelial cytokine production.
We have made technical refinements to remove the antioxidants from the HIE media to create experimental conditions that yield more responsive enteroid cultures capable of secreting multiple human cytokines. Using modified media, we demonstrate a robust cytokine response from HIE monolayers to pro-inflammatory stimuli. Herein, we characterize the response of HIEs derived from jejunum and colon to host derived stimuli (TNF, IL-1α), microbial stimuli (LPS, LTA, flagellin) and host/microbial stimuli (histamine). Our work indicates: (1) both host- and microbial-derived stimuli elicit cytokine production in HIEs; (2) HIEs secrete greater quantities of IL-8, Chemokine-X-C-Motif Ligand 1 (GRO), and monocyte chemoattractant protein 1 (MCP-1) in response to both host-derived and microbial derived products; (3) flagellin stimulates a greater variety of human cytokines when compared with other microbial-derived stimuli; (4) IL-8 secretion occurs in a similar pattern in jejunal and colonic HIEs, (5) host-derived and microbial-derived histamine had no effect on human cytokine production and (6) HIE secreted IL-8 is functionally active and capable of stimulating human neutrophil chemotaxis. This study demonstrates the enhanced responsiveness of HIE cultures to host and microbial signaling compounds and the potential of these cultures to more effectively mimic the human intestinal epithelium.
Methods
Ethical Approval
All HIE cultures were obtained through the Texas Medical Center Digestive Diseases Center (TMC DDC) Gastrointestinal Experimental Model Systems (GEMS) Core. All procedures used to generate HIE cultures were approved by the Baylor College of Medicine IRB committee and all cultures established from patient samples were de-identified. These experiments were conducted in accordance with the latest revision of the Declaration of Helsinki, except for registration in a database.
Human intestinal enteroid cultures
HIEs were generated previously from surgical specimens of adult jejunum obtained at Baylor College of Medicine through the Texas Medical Center Digestive Diseases Center (TMC DDC) Gastrointestinal Experimental Model Systems (GEMS) Core using complete medium with growth factors (CMGF+), as previously described (Sato et al., 2011; Saxena et al., 2016; Chang-Graham et al., 2019). We used jejunal HIEs from patients designated J2 and J3, and colonic HIE from patient designated C103, all adult females, obtained from the TMC DDC GEMS Core. CMGF+ consists of Advanced DMEM/F12 (Invitrogen), 100 U/mL penicillin-streptomycin (Invitrogen), 10 mM HEPES buffer (Invitrogen), and 1X GlutaMAX (Invitrogen) with 50% (v/v) Wnt3A-conditioned medium, 20% (v/v) R-spondin conditioned medium, 10% (v/v) Noggin-conditioned medium, 1X B-27 supplement (Invitrogen), 1X N-2 supplement (Invitrogen), 1 mM N-acetylcysteine (Sigma-Aldrich), 50 ng/mL mouse epidermal growth factor (EGF) (Invitrogen), 10 mM nicotinamide (Sigma-Aldrich, St. Louis, MO), 10 nM Leu-Gastrin I (Sigma-Aldrich), 500 nM A-83-01 (Tocris Bioscience), and 10 nM SB202190 (Sigma-Aldrich) (Table 1). Differentiation medium consisted of the same components as CMGF+ without Wnt3A conditioned medium, R-spondin conditioned medium, SB202190, and nicotinamide and only 5% (v/v) Noggin conditioned medium.
Table 1:
CMGF+ and CMGF− Media Components
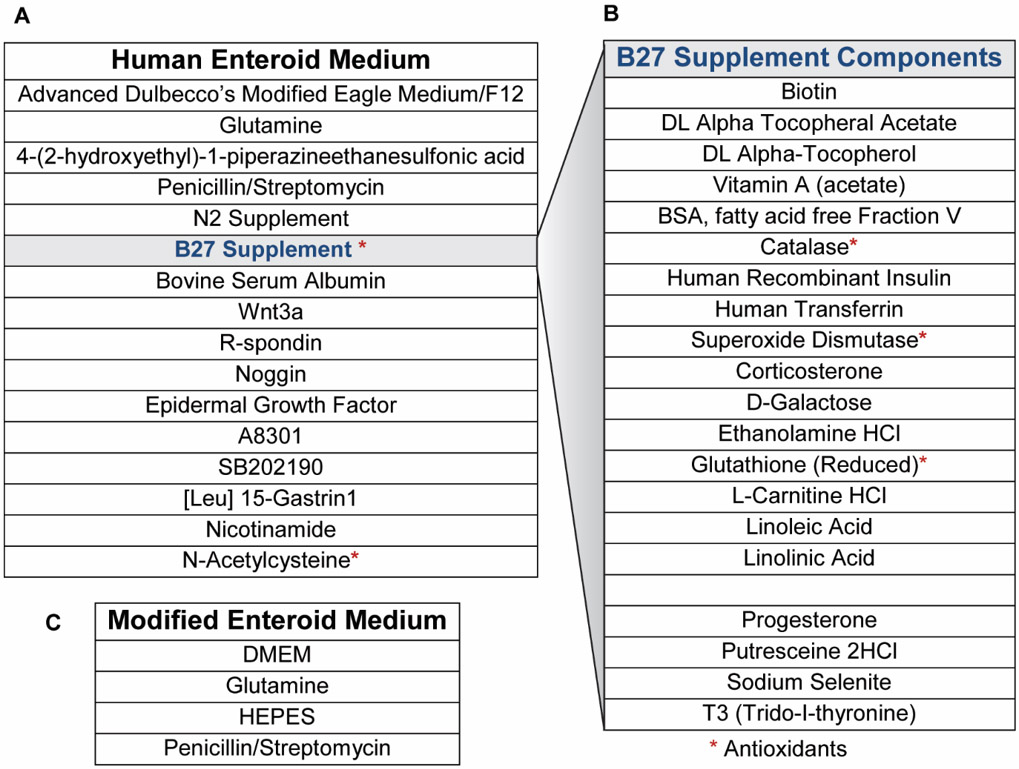 |
HIEs were propagated in phenol red-free, growth factor-reduced Matrigel (Corning), with passages every 7 to 10 days of growth. 2D HIEs were prepared from 3D cultures and seeded into flat 96 well plates that were pre-treated with collagen type IV diluted in H2O and incubated at 37°C with 5% CO2 as previously described (VanDussen et al., 2015; Ettayebi et al., 2016; Chang-Graham et al., 2019). Briefly, 3D HIEs were lifted from Matrigel and washed with an ice cold solution of 0.5 mM EDTA in 1X PBS and dissociated with 0.05% trypsin/0.5 mM EDTA for 4 min at 37°C. Trypsin was inactivated with CMGF− and 10% FBS and the cell solution was pipetted vigorously and filtered using a 40 μm nylon cell strainer (Falcon, Cat no. 352340) to dissociate into single cells. The cells were pelleted by centrifugation at 400xg for 5 min, resuspended in CMGF+ plus 10 μM Y-27632 Rock inhibitor, and added to the prepared wells. After 48 hours of monolayer development in CMGF+ supplemented with 10 μM Y-27632 Rock inhibitor, differentiation was induced by replacing the medium with differentiation media supplemented with 10 μM Y-27632 and incubated for 4-5 days. To create experimental conditions favorable for HIE response to pro-inflammatory stimuli, cultures were differentiated as described above, followed by incubation (3 hrs) in a CMGF− medium consisting of: Advanced Dulbecco’s modified Eagle medium (DMEM) (Invitrogen) supplemented with 100 U/mL penicillin-streptomycin (Invitrogen), 10 mM HEPES buffer (Invitrogen), and 1X GlutaMAX (Invitrogen). Prior to stimulation experiments, media was removed and replaced with simplified CMGF-media of Dulbecco’s modified Eagle medium (DMEM) (Invitrogen) supplemented with 100 U/mL penicillin-streptomycin (Invitrogen), 10 mM HEPES buffer (Invitrogen), and 1X GlutaMAX (Invitrogen). For stimulation experiments 1 μg/ml of lipopolysaccharide (LPS) (Santa Cruz Biotech, Cat SC-3535), lipoteichoic acid (LTA) (Sigma), flagellin (Sigma, Cat SRP8029), tumor necrosis factor (TNF) (Sigma T6674), recombinant IL-1α (PeproTech, Cat 200-01A), or histamine (Sigma, Cat H7250-5G) were added to the simplified media and incubated at 37°C with 5% CO2. Following a 16-hour incubation, the supernatants were collected for cytokine analysis.
Culture of Human Intestinal Enteroids for RNA Sequencing and Transcriptional Analysis
Human intestinal enteroids were cultured and processed according to previously published methods (Chang-Graham et al., 2019) to assess the transcriptional profile of HIEs. Briefly, monolayers of HIEs were prepared and differentiated as previously described in culture methods but seeded onto transwell (Corning) insert instead of a 96-well plate. The transwell insert was rinsed in ice cold Dulbecco’s PBS (lacking Ca2+ and Mg2+), and inserts were excised from the plate support and placed into 1 ml of TRizol reagent (Invitrogen) in a sterile microfuge tube. The tube was mixed thoroughly by vortex mixer for 30 seconds and 200 μL chloroform was added prior to another 30 seconds of vortex mixing. After a 5-minute incubation at room temperature, the phases were separated by centrifugation per manufacturer’s directions. The aqueous phase was moved to a new sterile microfuge tube and RNA was extracted following the standard protocol for the Qiagen RNeasy Isolation Kit. Agilent Bioanalyzer analysis was performed to validate the ribosomal RNA integrity (Agilent 2100 Bioanalyzer) from 6 biological replicates. Paired-end Illumina sequencing of mRNA enriched samples was conducted by Novogene (Sacramento, CA, USA) following the standard workflow. STAR software mapped reads using the human reference genome 19 to generate counts per gene and the number of transcripts per million reads.
Comparison of Jejunal HIE RNA-seq data with Human Protein Atlas Database
Publicly available RNA-seq data sets for human tissue and cell lines were downloaded from the Human Protein Atlas Database, section 4 “RNA gene data” (https://www.proteinatlas.org/about/download). To assess the enrichment of HIE transcripts in small intestinal genes, a cutoff of 1 count per million (CPM) was used and significance was determined using a hypergeometric test. For Gene Ontology analysis, a cutoff of 0.6 transcripts per million (TPM) was used and results from the human tissue set were filtered for genes expressed in the small intestine. Data analyses were performed in RStudio version 1.2.1335 and data is listed in Table 2.
Table 2:
GO Biological Process Grouping of Overlapping Genes
| GO Biological Process Grouping of Overlapping Genes n =9,359 | |||
|---|---|---|---|
| Category Name | Ascension Number | # Genes | % Total |
| Cellular Process | GO:0009987 | 2923 | 30.6 |
| Metabolic Process | GO:0008152 | 2389 | 25.0 |
| Biological Regulation | GO:0065007 | 1438 | 15.0 |
| Localization | GO:0051179 | 1101 | 11.5 |
| Multicellular Organismal Process | GO:0032501 | 410 | 4.3 |
| Response to Stimulus | GO:0050896 | 389 | 4.1 |
| Developmental Process | GO:0032502 | 178 | 1.9 |
| Cellular Component Organization | GO:0071840 | 152 | 1.6 |
| Immune System Process | GO:0002376 | 152 | 1.6 |
| Biological Adhesion | GO:0022610 | 122 | 1.3 |
| Reproduction | GO:0000003 | 72 | 0.8 |
| Cell Proliferation | GO:0008283 | 41 | 0.4 |
| Biological Phase | GO:0044848 | 8 | 0.1 |
| Signaling | GO:0023052 | 5 | 0.1 |
Human Cell Viability Assay
To assess the viability of HIE monolayers following stimulation, the cells were washed with 200 μl of pre-warmed sterile PBS (without Ca2+, Mg2+), prior to harvesting. To release the cells from the Matrigel coated surface, 5 mM EDTA, and 10 mM glucose was added to pre-warmed PBS (without Ca2+, Mg2+) and incubated for 10 minutes before removal by gentle pipetting. 10 μL of Trypan blue was mixed with 10 μL HIE cell suspension, and cell viability was quantified by Countess Automated Cell Counter (Invitrogen).
IL-8 ELISA
To determine if the HIE response to stimuli was different in the presence of CMGF+ versus simplified enteroid media prior to Magpix experiments, the concentration of IL-8 produced by HIEs was tested. A Quantitative DuoSet ELISA (R&D Systems) was used to determine IL-8 concentrations in HIE monolayer supernatants after incubation with stimuli in the presence of complete CMGF+ versus simplified CMGF-.
Magpix Human Cytokine Studies
Supernatants from HIE monolayers were collected after overnight (16 hr) incubation with treatment conditions. Cytokines were quantified using a MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel Assay (EMD Millipore cat. #HCYTOMAG) per standard manufacturer’s protocols. Cytokines included in the panel were IL-8, GRO, MCP-1, IL-1Rα, TNF, EGF, G-CSF, GM-CSF, IL-15, IL-1α, IL-1β, IL-7, IFN-α, INFγ, IP-10, MCP-3, MDC, and TGF-α. The assay was performed by the Functional Genomics and Microbiome Core of the TMC DDC. Raw data were obtained with Luminex xPONENT for MAGPIX, version 4.2 Build 1324 and analyzed with MILLIPLEX Analyst version 5.1.0.0 standard Build 10/27/2012.
HIE Reactive Oxygen Species Assay
Reactive oxygen species (ROS) were measured in HIEs pre-treated with the peroxynitrite indicator 20-70-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma-Aldrich, cat #D6883). Differentiated HIEs monolayers were washed 2x with PBS containing Mg2+ and Ca2+ and incubated with 10 μM DCFH-DA for 30 min at 37°C, 5% CO2. Non-stained HIEs monolayers served as a negative control. Following incubation, HIEs monolayers were washed twice and treated with stimuli (IL-1α, TNF, LPS, LTA, Flagellin, or histamine) diluted in CMGF− made with optically clear Fluorobrite DMEM (ThermoFisher, cat # A1896701). After 3 hrs of incubation, the fluorescence emission of DCFH-DA was measured using a Biotek microtitre plate reader at excitation/emission of 485 nm/535 nm. The background fluorescence of Fluorobrite DMEM and HIE monolayers incubated without the probe were measured to calculate the net fluorescence emitted. Experiments were conducted in triplicate, two independent times.
Human Neutrophil Chemotaxis Assay
Human blood derived neutrophils were purchased from Astarte (cat# 1025-4387JY19). Neutrophils were washed in Hank’s Balanced Salt Solution (HBSS, Sigma cat # 55021C), centrifuged for 10 minutes at 200 x g and the neutrophil pellet was resuspended in HBSS containing 10 μM CFDA-SE (ThermoFisher, cat# C1157). Cells were incubated for 30 min at 37°C, 5% CO2 to fluorescently tag all living neutrophils. Following the incubation cells were washed three times in HBSS and resuspended in FluoroBrite DMEM. Neutrophils at a concentration of 1 x 105 cells were added to the top of a 96-well 3.0 μm pore size transwell chemotaxis chamber (Corning HTS Transwell Cat# 3385). On the bottom of the transwells, HIEs supernatant (Fluorobrite DMEM conditioned media after 16 hrs of incubation) was added. The chemotaxis plate was incubated for 3 hr at 37°C, 5% CO2 to allow neutrophils to translocate to the bottom wells containing HIE conditioned Fluorobrite media. As a control, Fluorobrite DMEM that had not been conditioned by HIEs was added to select wells. Neutrophil chemotaxis was examined by fluorescent plate reader at Excitation/Emission: 485 nm/535 nm and by microscopy.
Quantitative real time PCR (qRT-PCR)
HIE monolayers were collected in TRIZOL and RNA was extracted according to manufacturer details (ThermoFisher, cat# 15596026). RNA quality was examined by nano-drop (ThermoFisher) and 1 μg RNA was converted to cDNA using the SensiFAST cDNA synthesis kit (Bioline USA Inc.). Gene expression was examined by quantitative real-time PCR (qPCR) usingFastSYBR Green (Thermo-Fisher) and primers (Table 3) using a QuantStudio 3 qPCR machine (Applied Biosystems). Fold changes of relative mRNA levels were calculated using the ΔddCT method with the housekeeping gene 18S.
Table 3:
Quantitative Real Time PCR Primer Sequences.
| Primer | Forward | Reverse |
|---|---|---|
| 18S | GATATGCTCATGTGGTGTTG | AATCTTCTTCAGTCGCTCCA |
| Claudin-2 | ACACACAGCACAGGCATCAC | TCTCCAATCTCAAATTTCATGC |
| ZO-1 | TGCCATTACACGGTCCTCTG | GGTTCTGCCTCATCATTTCCTC |
| β -catenin | GCAGAGTGCTGAAGGTGCTA | TCTGTCAGGTGAAGTCCTAAAGC |
| Occludin | TTTGTGGGACAAGGAACACA | CAGGCGAAGTTAATGGAAG |
Statistical Analyses
Experimental results were tested for statistical significance using a One-way or Two-way ANOVA with Bonferroni’s Post hoc test using GraphPad Prism 8 (Prism Software San Diego, CA, USA). Statistical significance is denoted by *p<0.05, ** p<0.01, *** p<0.001, and **** p<0.0001. The data are presented as mean ± standard deviation unless otherwise noted. All authors had access to the study data, reviewed, and approved the final manuscript.
Results
Enteroids from Human Jejunum Recapitulate Transcriptional Profiles of the Human Small Intestine
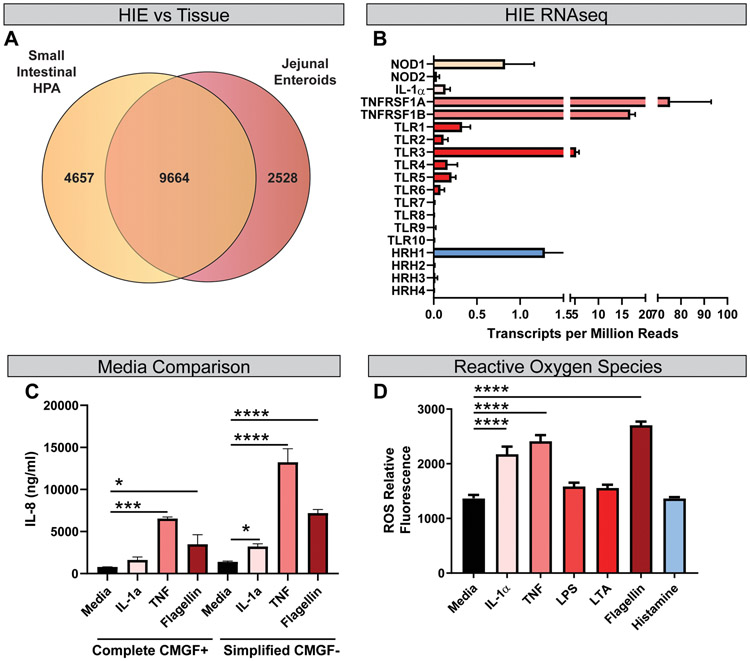
Characterization of epithelial responses to stimuli has been limited by the lack of human in vitro systems that faithfully recapitulate the complex nature of the native intestinal epithelium. Recently, multiple groups have demonstrated that human intestinal enteroids (HIEs) retain the tissue-specific transcriptional profiles, providing an improved model for examining microbe-host-immune crosstalk. To confirm that the cellular architecture necessary to sense and respond to pro-inflammatory signals were expressed in jejunal HIE monolayers, we compared the global transcriptional profiles of HIEs derived from the jejunum in control conditions (media alone) to the gene expression profiles of the small intestine in the publicly available database Human Protein Atlas. As expected, significant overlap was observed between the expression profiles of HIEs and that of the small intestine, with 9664 genes expressed in both datasets (P=6.7*10−97, hypergeometric test) (Fig. 1A). Further, overlapping genes were grouped into gene ontology classifications (Mi et al., 2019), and primary biological and molecular functions of epithelial cells were maintained between HIEs and small intestinal cells (Table 2). This analysis establishes that our jejunal enteroids accurately recapitulate human intestinal physiology. Importantly, further examination of the RNAseq data confirmed the presence of cell surface receptors known to play an important role in pro-inflammatory signaling (Fig. 1B), indicating the potential of HIEs to respond to external stimuli. We found that HIEs express microbial pattern recognition receptors such as TLR1-10 as well as microbial sensing nucleotide-binding oligomerization domain-containing proteins (NOD1 and NOD2). In addition, HIEs exhibited elevated expression of TNF superfamily members 1B and 1A and modest expression of cytokine receptors such as IL-1 receptors. Of the four known histamine receptors that may respond to host or microbe-generated histamine, only HRH1 was expressed in jejunal HIEs. These data suggest that HIEs have the capacity to respond to both host and microbial stimuli.
Figure 1. Human Jejunal Enteroids Recapitulate the Human Small Intestine and respond to physiologically revealing stimuli.
A. Transcripts from jejunal HIE monolayers are enriched in small intestinal genes in the Human Protein Atlas (HPA) (9664 genes shared, p= 6.7*10−97 by a hypergeometric test). B. Key epithelial receptors were significantly expressed in HIEs, enabling responses to extracellular stimuli (n =6 biological replicates). C. HIEs were seeded as 2D monolayers and differentiated for 4-5 days. Following differentiation, enteroids were treated with either complete CMGF+ or simplified CMGF− (lacking antioxidants with Advanced DMEM) for 3 hrs and stimulated with 1 μg/ml TNF, IL-1α, or flagellin for 16 hrs. Supernatants were examined by IL-8 ELISA (n=3-6; repeated 2 independent times). An interaction was observed between media and cytokine by Two-way ANOVA (P<0.001). D. Reactive oxygen species were measured in DCFH-DA treated HIEs after 3 hrs of stimulation in simplified CMGF− in response to stimuli (IL-1α, TNF, LPS, LTA, flagellin or histamine). One Way ANOVA, * p<0.05, **p< 0.01, ***p<0.001, ****p<0.0001. n= 3 replicates, repeated 2 independent times.
Simplified media yields more responsive HIE cultures when exposed to microbial and host stimuli
In order to create a more responsive HIE model system for cytokine analysis, HIEs were seeded as monolayers and differentiated for 4 −5 days as previously described (VanDussen et al., 2015; Ettayebi et al., 2016; Chang-Graham et al., 2019). Following differentiation, confluent monolayers were incubated with CMGF-, which lacks antioxidant compounds such as B-27 and N-acetylcysteine (see Table 1 for enteroid media recipes), for 3 hrs prior to stimulation. Because Advanced DMEM contains glutathione, we used a simplified CMGF− that contained DMEM rather than advanced DMEM for all stimulation assays. Monolayers were stimulated with 1 μg/ml of pro-inflammatory stimuli such as tumor necrosis factor (TNF) and interleukin (IL)-1α, or flagellin for 16 hrs in normal enteroid media (CMGF+) or our simplified CMGF (CMGF-, with regular DMEM). Following incubation, supernatants were collected, and an ELISA was performed to assess IL-8 cytokine concentrations. The simplified CMGF− medium resulted in HIEs that were more responsive to pro-inflammatory stimuli (Figure 1C). We found that the simplified CMGF− promoted increased IL-8 production for all stimuli (TNF, IL-1α, and flagellin) compared to antioxidant rich CMGF+ media. Analysis of the groups by 2-WAY ANOVA revealed effects of the culture medium on cytokine production by HIE cultures, suggesting that HIEs are modulated by the antioxidant composition of the medium.
Modified enteroid culture conditions enhance HIE responsiveness to host and microbe-derived stimuli
To characterize epithelial responses to physiologically relevant stimuli, we focused on HIE responses to host-derived cytokines such as IL-1α and TNF, as well as microbial-derived stimuli such as lipopolysaccharide (LPS), lipotechoic acid (LTA), and flagellin. For comparison, we also analyzed histamine, which can be generated by both mammalian cells and microbes (Rodwell, 1953; Pessione et al., 2005; Rossi et al., 2011; Thomas et al., 2012; Hemarajata et al., 2013; Krystel-Whittemore et al., 2015). In epithelial cells, reactive oxygen species (ROS) mediate inflammatory signals, including cytokine production (O’Hara et al., 2009; Rada et al., 2011; Choi et al., 2012; Chen et al., 2013; Ko et al., 2014). To assess ROS levels, HIE monolayers were incubated with the dye DCFH-DA and then treated with various stimuli in simplified CMGF− medium (Figure 1D). Host-derived cytokines IL-1α and TNF as well as microbial-derived flagellin elevated ROS levels in our DCFH-DA treated HIEs (Figure 1D). Interestingly, no significant changes in ROS were observed with microbial LPS and LTA or with host- and microbial-derived histamine. This data suggest that select stimuli activate ROS in HIEs.
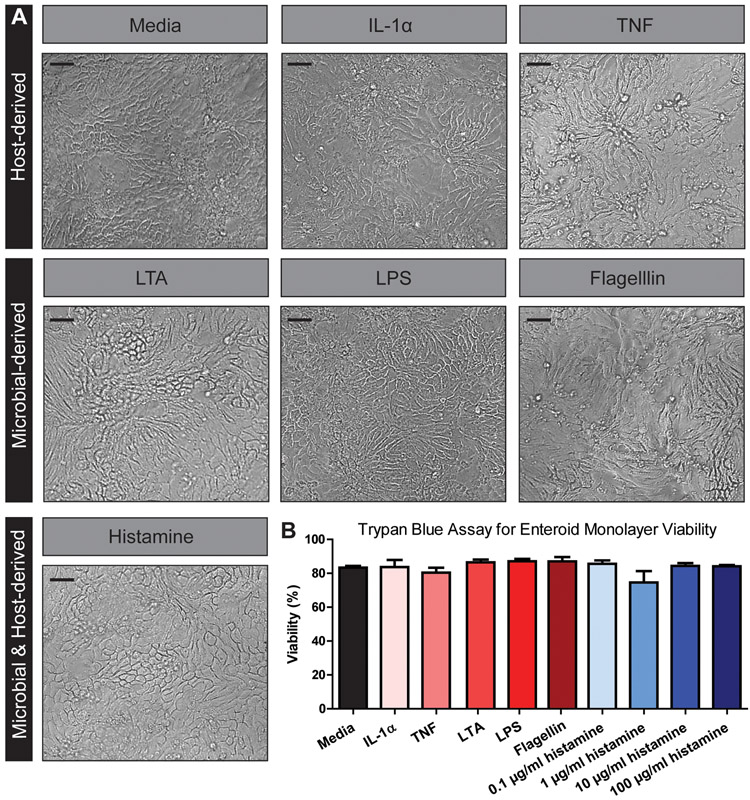
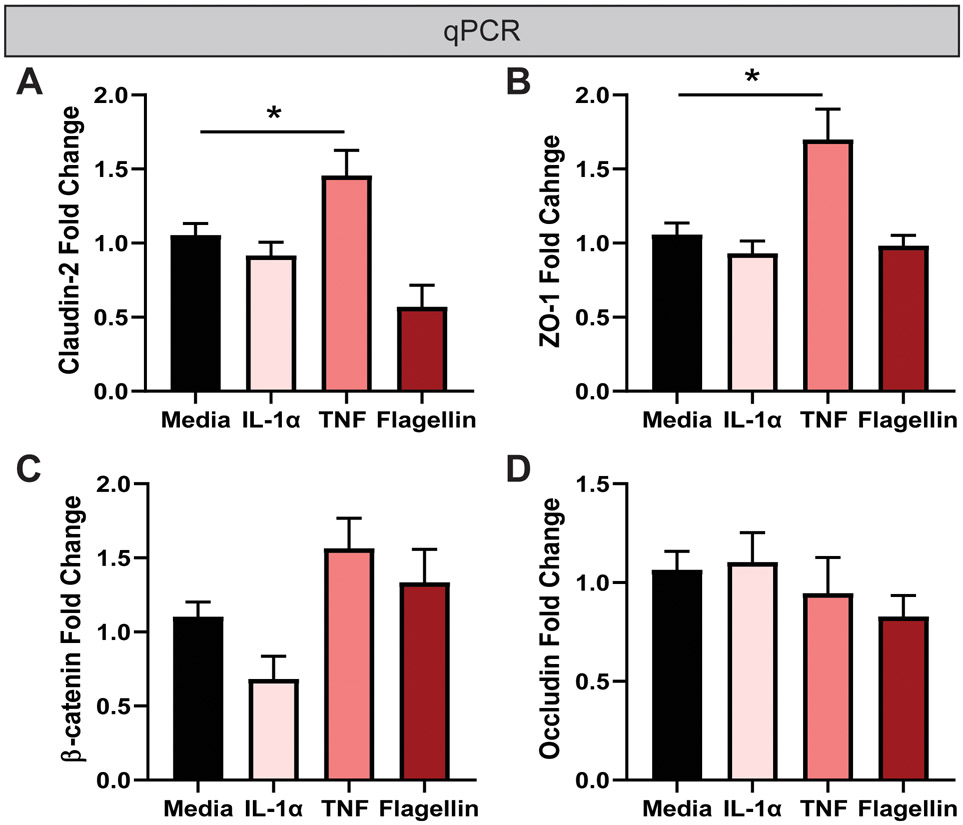
Pro-inflammatory stimuli can alter cell morphology in some cell types, such as fibroblasts, monocytes, and microglia (Zeng & Chen, 2010; Sheng et al., 2011; Ščigalková et al., 2019). Therefore, we first examined whether the cellular morphology of enteroid monolayers was affected by our selected treatments by light microscopy. No differences were visualized in the HIE architecture between control and treatment groups (Figure 2A). Importantly, the monolayer integrity remained intact in all conditions. Trypan blue staining confirmed that treatments (TNF, IL-1α, LPS, LPTA, flagellin, or histamine) did not alter cell viability (Figure 2B). Interestingly, mRNA levels of the tight junction proteins Claudin-2 and ZO-1 were increased in response to TNF by transcriptional analysis, even in the absence of morphological changes (Figure 3A, B). No changes in Claudin-2 or ZO-1 expression were found with IL-1α or flagellin, and no changes were observed in β-catenin or Occludin levels with any treatment (Figure 3C, D). These findings suggest that HIEs respond to TNF with tight junction modifications, but the overall integrity of the monolayer is maintained.
Figure 2. Host and microbial stimuli do not affect human jejunum enteroid viability.
A. Representative light microscopy images of human enteroid monolayers at 200x magnification, demonstrating no changes in cell morphology following stimulation. B. Trypan blue assay of enteroid monolayer viability following exposure to indicated stimuli below bar graph. There are no significant differences in enteroid viability after stimulation with different factors after performing one-way ANOVA testing for statistical differences. Scale bars = 100 μm. One Way ANOVA, No significance noted. n= 3 replicates, repeated 4 independent times.
Figure 3. TNF modulates tight junction gene expression by qPCR.
Real time quantitative PCR (qPCR) was used to examine the relative expression of junction markers A. Claudin-2, B. ZO-1, C. β-catenin, and D. Occludin. HIEs were incubated for 16hours in media, 1 μg/ml IL-1α, TNF and flagellin. The cells were then collected in TRIZOL and RNA isolated. qPCR was then formed to evaluate tight junctions. Data is presented as the ΔΔCT with the house-keeping gene 18S. n=3 wells/experiments, representative of 3 independent experiments. One-Way ANOVA. *p< 0.05.
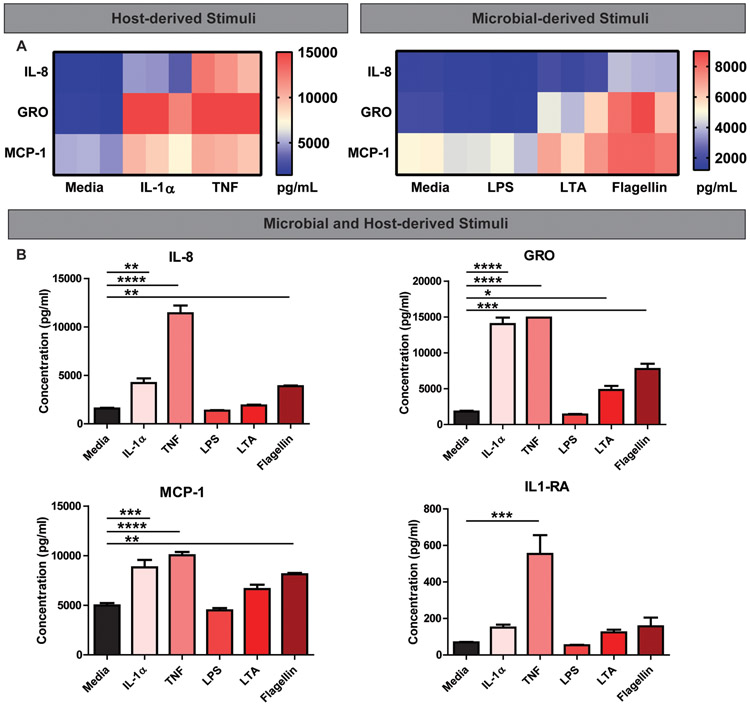
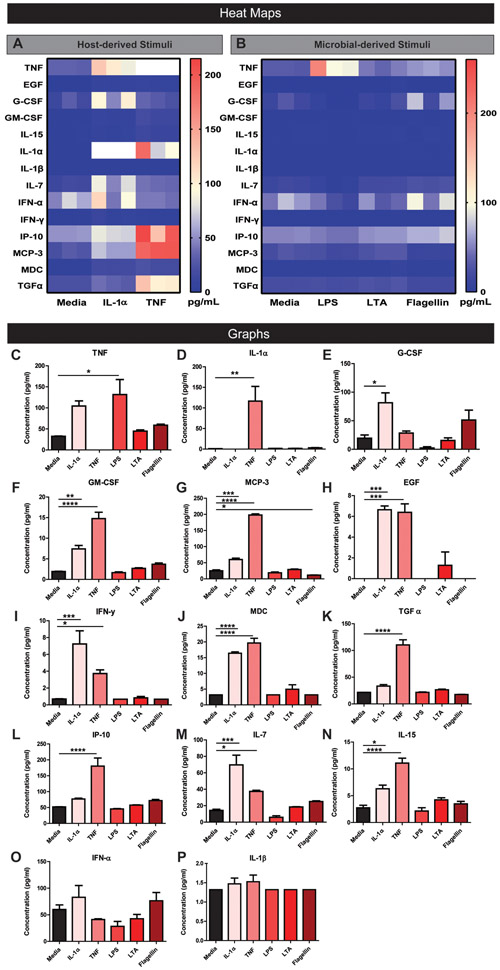
Next, we sought to characterize the capacity of stimulated HIEs to produce cytokines in simplified CMGF-. HIE monolayers were treated with indicated compounds, and secreted proteins in the supernatant were quantitated using a Magpix Luminex Cytokine panel. Analysis of cytokine levels revealed unique patterns of cytokine production in response to stimulation by host and microbial factors. Host-derived TNF stimulated the broadest array of epithelial cytokines produced by HIEs (Figure 4 and Figure 5). TNF-stimulated high levels of IL-8, growth-regulated oncogene (GRO, also known as chemokine (C-X-C motif) ligand 1), monocyte chemoattractant protein 1 (MCP-1; also known as CCL2), and IL-1Rα (Figure 4A-F). TNF also stimulated epidermal growth factor (EGF), Granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-15, IL-7, IL-1α, interferon gamma (IFN-γ), interferon gamma-induced protein 10 (IP-10, also known as C-X-C motif chemokine 10 (CXCL10)), monocyte-chemotactic protein 3 (MCP-3), macrophage-derived chemokine (MDC), and transforming growth factor alpha (TGF-α) (Figure 5A-P). Of the cytokines examined, TNF was the most potent inducer of IL-8, GRO, and MCP-1. Host-derived IL-1α also stimulated several cytokines including IL-8, GRO, MCP-1, EGF, G-CSF, GM-CSF, IL-15, IL-7, IFNγ, MCP-3, and MDC with the highest cytokine production being GRO, MCP-1, and IL-8 (Figure 4A, C-F, Figure 5A-P). Although IL-1α stimulated GRO to the same degree as TNF (Figure 4A &D), in general TNF was a more potent stimulator of cytokine secretion than IL-1α. This finding may reflect the cellular concentration of IL-1 receptors, which was far lower in relative abundance than TNF receptor gene expression (Figure 1B).
Fig 4. Representative Magpix analysis of HIE supernatants after stimulation by host and microbial factors showing robust cytokine production of IL-8, GRO, MCP-1 and IL-1RA.
Heat map representation of high cytokine concentrations in the supernatant after enteroids were stimulated with host cytokines TNF or IL-1α (1 μg/ml)(A) or microbial stimuli LPS, LTA, or flagellin (1 μg/ml)(B) in simplified CMGF− media for 16 hrs. Representative individual Magpix results comparing the amount of cytokine production between the stimulated group and simplified CMGF− media control group for C. IL-8, D. GRO, E. MCP-1, and F. IL-1RA. One Way ANOVA * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. n=3 wells/experiments, representative of 3 independent experiments.
Figure 5. Representative Magpix analysis of HIE supernatants after stimulation by host and microbial factors showing mild cytokine production.
Heat map representation of low cytokine concentrations in the supernatant after enteroids were stimulated with host cytokines TNF or IL-1α (1 μg/ml)(A) or microbial stimuli LPS, LTA, or flagellin (1 μg/ml)(B) in simplified CMGF− media for 16 hrs. Representative individual Magpix results comparing the amount of cytokine production between the stimulated group and simplified CMGF− media control group for C. TNF, D. IL-1α, E. G-CSF, F. GM-CSF, G. MCP-3, H. EGF, I. IFN-γ, J. MDC, K. TGF α, L. IP-10, M. IL-7, N. IL-15, O. IFN α, and P. IL-1β. One Way ANOVA * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. n=3 wells/experiments, representative of 3 independent experiments.
Of the microbial stimuli, flagellin stimulated the broadest repertoire of cytokines, including IL-8, GRO, and MCP-1 (Figure 4B-F, Figure 5B-P). LTA and LPS stimulate many cytokines in cancer-derived cell lines, including IL-8 (LTA stimulated GRO and MCP-1, and LPS only stimulated TNF (Figure 4B & D, Figure 5B & C). The data suggest that flagellin stimulation yields a broader spectrum of epithelial cytokine responses compared with other microbial components. In contrast to previous studies in cancer-derived cell lines (Schuerer-Maly et al., 1994; Sanlioglu et al., 2001; Angrisano et al., 2010; Li et al., 2014; Lin et al., 2015), HIEs did not release IL-1β or Interferon-alpha (IFNα) in response to any tested stimuli (Figure 5O,P).
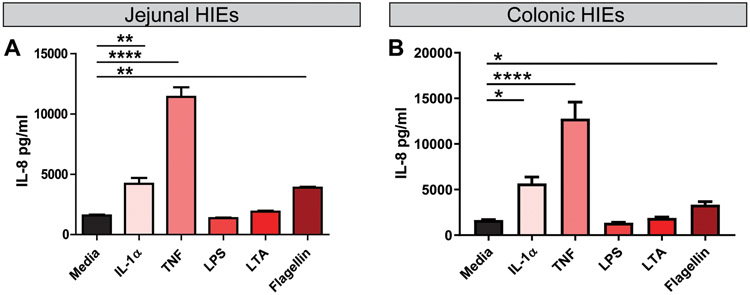
Since LPS has been shown to stimulate IL-8 production in colorectal cancer cell lines (Schuerer-Maly et al., 1994; Angrisano et al., 2010; Lin et al., 2015), we reasoned that the response of HIEs to LPS may be influenced by the intestinal segment from which the enteroid was generated. To address this question, we stimulated colonic HIE monolayers in the same conditions as the jejunal HIE experiments (Figure 6). Surprisingly, the colonic HIEs IL-8 secretion pattern was remarkably consistent with the observed pattern of IL-8 secretion in our jejunal HIEs (Figure 6A, B). While IL-1α, TNF, and flagellin stimulated IL-8, LPS and LTA had no effect on IL-8 (Figure 6B). These data suggest that non-cancer, non-transformed epithelium does not respond to LPS with IL-8 secretion.
Figure 6. Jejunal and colonic HIEs respond to IL-1α, TNF and flagellin with IL-8 production.
Differentiated HIE monolayers derived from A. jejunum or B. colon were treated with simplified CMGF− (lacking antioxidants) with 1 μg/ml TNF, IL-1α, or flagellin for 16 hrs. Supernatants were examined by IL-8 ELISA. One Way ANOVA * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. n=3-6
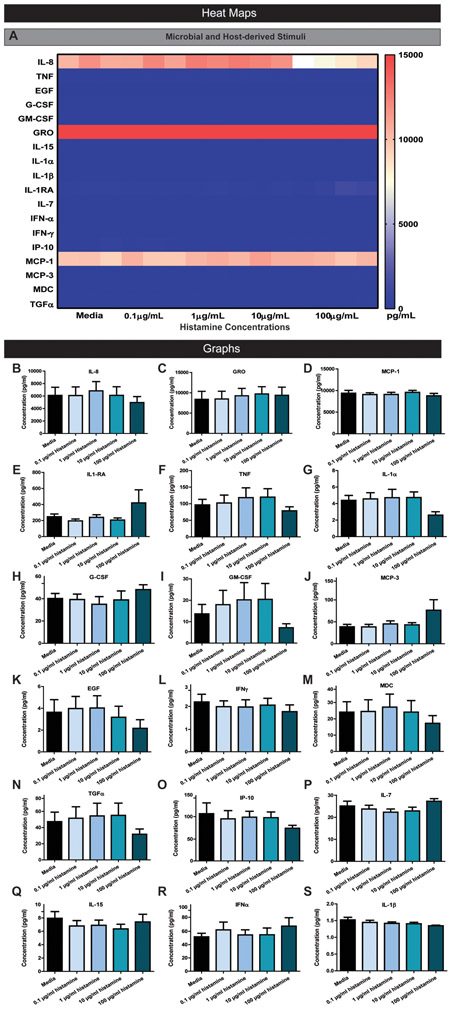
In addition to host- and microbial-derived stimuli, we also analyzed histamine, a microbial and host-produced biogenic amine which stimulates pro-inflammatory responses via H1R in the airway epithelium (Takizawa et al., 1995). In contrast to our selected host-derived and microbiota-derived stimuli, histamine had no effect on HIE cytokine production (Figure 7A-S). We tested various concentrations of histamine (0.1 μg/ml to 100 μg/ml) to confirm that histamine was unable to elicit a pro-inflammatory response. For all concentrations, we observed no statistically significant changes in cytokine production compared to simplified CMGF− media controls. This finding indicates that although the epithelium harbors histamine receptor 1 (H1R) which drives inflammation in airway epithelial cells, histamine does not influence epithelial cytokine production in the intestine.
Figure 7. Representative Magpix analysis of HIE supernatants after stimulation by various concentrations of histamine.
A. Heat map representation of cytokine concentrations in the supernatant after enteroids were stimulated with various concentrations of histamine in simplified CMGF− media for 16 hrs. Representative individual Magpix results comparing the amount of cytokine production between the stimulated group and simplified CMGF− media control group for B. IL-8, C. GRO, D. MCP-1, E. IL-1RA, F. TNF, G. IL-1α, H. G-CSF, I. GM-CSF, J. MCP-3, K. EGF, L. IFN-γ, M. MDC, N. TGF α, O. IP-10, P. IL-7, Q. IL-15, R. IFNα, and S. IL-1β. One Way ANOVA * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. n=3 wells/experiments, representative of 3 independent experiments.
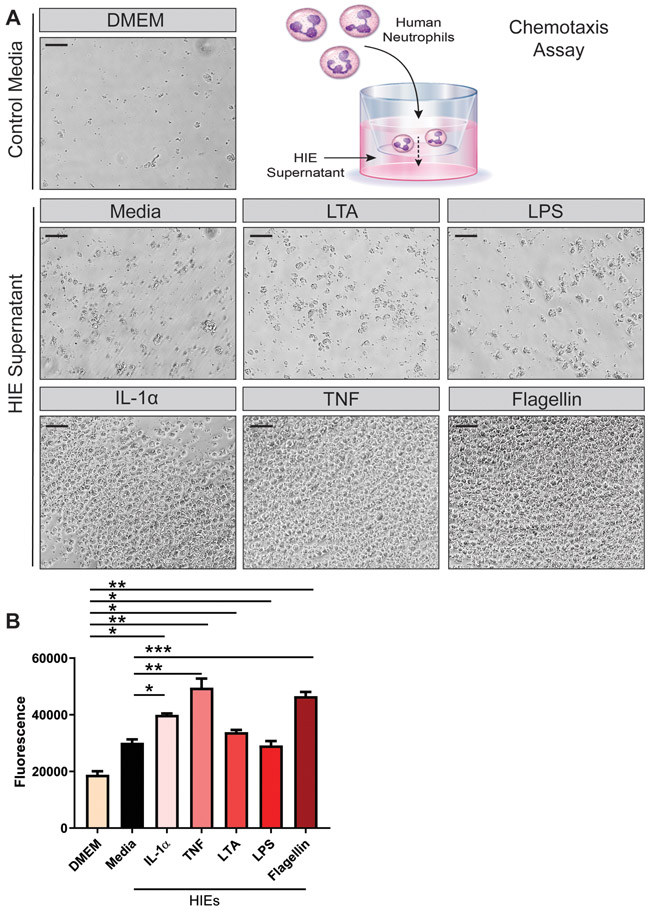
Finally, we sought to determine if HIE secreted cytokines were functionally active. It has been established that IL-8 both attracts and activates neutrophils (1993). Therefore, we examined chemotaxis of human neutrophils towards our HIE conditioned media. In this assay, human blood-derived neutrophils were fluorescently tagged with CFDA-SE and applied to the top wells of a 3 μm transwell chemotaxis plate. In the bottom wells, HIE conditioned medium was added. Following 3 hrs of incubation, the migration of neutrophils into the basal chamber was determined by light microscopy and neutrophil fluorescence. Compared to control DMEM medium, we found that neutrophils chemotaxed towards HIE conditioned media (Figure 8A,B). The highest chemotaxis was observed with IL-1α, TNF and flagellin, stimuli which also produced the highest IL-8 levels in our jejunal HIEs. Taken together, these findings indicate the IL-8 released by HIEs is functionally active and capable of chemoattracting immune cells.
Figure 8. Human Neutrophils Migrate toward HIE supernatant containing IL-8.
Human blood derived neutrophils were fluorescently tagged with CFDA-SE and added to the apical membrane of 96-well transwells with a 3 μm pore size. HIE conditioned media was added to the basal wells and chemotaxis was examined after 3 hrs incubation by A. light microscopy (scale bar =100 μm) and B. Fluorescent plate reader (Ex/Em: 485 nm/535 nm). One Way ANOVA * p<0.05, ** p<0.01, *** p<0.001. n=3-9, supernatant collected from 2 independent experiments.
Collectively, this work is among the first to demonstrate that modification of the enteroid media by removal of antioxidants, may generate HIEs that are more responsive to pro-inflammatory stimuli. Our results indicate that HIEs may be informative for dissecting the responsiveness of the intestinal epithelium and its role in intestinal pathophysiology.
Discussion
Our work demonstrates that modification of the HIE culture medium creates a permissive environment for the human small intestinal epithelium to respond to host and microbial stimuli. We reasoned that antioxidant compounds in HIE media (e.g., N-acetylcysteine, glutathione, superoxide dismutase, catalase) likely dampen the production of cytokines that require reactive oxygen species (ROS) for activation. ROS are small, highly reactive, unstable radicals (Aviello & Knaus, 2017). Low short-term concentrations of ROS are vital for regulating intracellular signaling pathways, such as cytokine production. Epithelial ROS is activated by both microbial stimuli (Jones et al., 2012; Matziouridou et al., 2018) and pro-inflammatory cytokines (Babbar & Casero, 2006; Kim et al., 2008; Kim et al., 2010; Lee et al., 2011). In the intestine, ROS have been shown to activate the PKD-IKK-NF-κB signaling pathway (Storz et al., 2004; Shin et al., 2017) and induces the production of pro-inflammatory cytokines such as IL-8 and TNF (Babbar & Casero, 2006; Mittal et al., 2014; Shin et al., 2017). Consistent with these findings, we demonstrated that removal of antioxidants from HIE media enhances the activation of HIEs by microbial and host-derived stimuli.
This study is the first to systematically characterize HIE cytokine production in response to relevant microbial and inflammatory stimuli. Our work indicates that HIEs are highly sensitive to host-derived TNF, and to a slightly lesser degree, IL-1α. Consistent with previous studies, we detected pro-inflammatory cytokines in response to flagellin, a TLR-5 agonist that has previously been used in HIE studies (Samuel et al., 2017; Nishimura et al., 2019). However, our simplified CMGF− medium increases the HIE cytokine production. We show a relatively limited response of HIEs to LPS and LTA, indicating that these compounds may not be dominant factors in the interactions of the human jejunal epithelium with the small intestinal microbiome. These findings indicate that HIEs are capable of robust cytokine production in response to inflammatory stimuli, an effect which may have been masked in previous studies due to culture media components. Using a simplified culture media, HIEs are a biologically relevant system for examining microbial- and immune-mediated epithelial responses.
Classically cytokine production, particularly IL-8, has been used to characterize inflammatory responses in the intestinal epithelium. IL-8 is a chemokine produced by intestinal epithelial cells that promotes neutrophil chemotaxis to areas of mucosal inflammation. Excessive recruitment and accumulation of neutrophils to the site of inflammation in IBD is associated with mucosal injury. IL-8 is present in inflamed mucosa and in the sera of patients with Crohn’s Disease (Jones et al., 1993; Mazzucchelli et al., 1994; Grimm et al., 1996; Ina et al., 1997; Uguccioni et al., 1999; Subramanian et al., 2008). IL-8 and neutrophil accumulation also correlate with disease severity (Fournier & Parkos, 2012; Wera et al., 2016). Our work showed that human TNF and IL-1α significantly induced IL-8 secretion by human jejunal enteroid cultures. We also demonstrated that microbial flagellin (which activates TLR-5), but not LPS or LTA (which bind TLR-4 and TLR-2 respectively), stimulated IL-8 production. Contrary to what has been reported for HT29, Caco2 and T84 cell lines (Schuerer-Maly et al., 1994; Sanlioglu et al., 2001; Angrisano et al., 2010; Li et al., 2014; Lin et al., 2015), stimulation with LPS or LTA did not result in IL-8 secretion by either jejunal or colonic HIEs. This finding highlights a key difference between HIEs and cancer-derived colonic epithelial cell lines. Additionally, the concentrations used in this study (1 μg/ml) are far above the concentrations required to produce IL-8 from cancer-derived cell lines (10 ng/ml). We also tested 10 ng/ml and 100 ng/ml of LPS and LTA in HIE cultures and did not detect IL-8 secretion (data not shown). Although IL-8 production was not stimulated by LTA or LPS, we did observe that LPS can stimulate TNF secretion, a key cytokine known to be upregulated in IBD. We speculate that such differences may be attributable to the intrinsic differences between cancer-derived tissue and non-cancerous, non-transduced human epithelial cells.
We also found that HIEs produce elevated quantities of GRO and MCP-1 compared to other commonly examined cytokines. Similar to IL-8, growth-regulated oncogene-alpha (GRO or CXCL1) recruits and activates neutrophils in response to tissue injury or microbial infection (Rudack et al., 2003; Zaja-Milatovic & Richmond, 2008). GRO signals primarily through G protein-coupled receptor CXCR2 (Ahuja & Murphy, 1996). In contrast, monocyte chemoattractant protein-1 (MCP-1 or CCL2) regulates migration and infiltration of monocytes and macrophages via activation of the CCR2 receptor (Deshmane et al., 2009). Migration of monocytes and conversion of monocytes to macrophages from the bloodstream into the intestinal mucosa is required for routine surveillance and inflammation in mucosal immunity. Previous work has shown that GRO-alpha and MCP-1 are expressed in epithelial cells and are induced during inflammation and wound healing (Zaja-Milatovic (Standiford et al., 1991; Zaja-Milatovic & Richmond, 2008). Our work confirms these studies as we show that GRO and MCP-1 are highly expressed in HIEs in response to TNF and IL-1α. Our work also indicates that human neutrophils migrate towards HIE conditioned medium containing IL-8. As a result, we speculate that expression of these cytokines by the epithelium is likely integral to immune-epithelial crosstalk.
TNF- and TNFR-mediated signaling pathways are involved in a multitude of cell events, including cell survival, proliferation, morphogenetic changes, wound repair and cell death (Aggarwal et al., 2012; Ruder et al., 2019). Studies suggest that TNF exerts both beneficial as well as harmful functions in the gut depending on the inflammatory context (Kojouharoff et al., 1997; Neurath et al., 1997; Kontoyiannis et al., 1999; Naito et al., 2003; Arrieta et al., 2009; Noti et al., 2010). Marchiando et al. demonstrated that TNF promoted intestinal epithelial cell shedding, but the epithelial integrity was maintained by the redistribution of tight junction proteins ZO-1 and Occludin (Marchiando et al., 2011). Although we did not observe disruption of HIE monolayers following 16 hr exposure to TNF, we did find increased levels of ZO-1 and Claudin-2, which is consistent with previous work in cell lines(Prasad et al., 2005; Mankertz et al., 2009). We speculate the HIE may maintain their monolayer integrity through tight junction redistribution and upregulation of key proteins, which is the subject of future research. Further, we note that no changes in TLR or cytokine receptor gene expression in response to any of our stimuli were observed (data not shown). It is possible that these receptors could have been endocytosed from the cell surface, rendering HIEs non-responsive to further stimulation. Although these studies are outside the scope of the current work, we think HIEs provide a novel method for addressing these questions and others in the future.
In addition to both host- and microbe-derived compounds, we included a co-metabolite produced by both host and microbes, notably histamine. Histamine is a biogenic amine that is generated via decarboxylation of dietary L-histidine. Certain gut microbes use histamine production to harness the proton gradient and generate energy and/or increase the cytoplasmic pH inside microbes, boosting acid resistance (Rodwell, 1953; Molenaar et al., 1993; Pessione et al., 2005; Rossi et al., 2011; Tabanelli et al., 2012; Thomas et al., 2012; Hemarajata et al., 2013). In the host, histamine is used by immune cells, particularly mast cells, as a mechanism of eliciting allergic immune responses. Histamine is known to exert pro-inflammatory and anti-inflammatory effects. Activation of histamine receptor type 1 (H1R) or 3 (H3R) has classically been associated with pro-inflammatory effects. In contrast, activation of histamine receptor type 2 (H2R) or 4 (H4R) is associated with anti-inflammatory responses. Our RNA-seq data indicates that the HIEs only express H1R in the human jejunal epithelium. Although this receptor is traditionally viewed as pro-inflammatory, we found that exogenous histamine had no detectable effect on any cytokine quantities. These data indicate that while histamine may play important roles in immune and other cell types, histamine does not appear to directly modulate production of epithelial cytokines by the human small intestinal epithelium.
HIEs offer many advantages for studying the interactions between the gut microbiota and the host. HIEs are human derived, can be grown as monolayers which allows easy access to the apical membrane, harbor TLRs and NOD proteins, and contain multiple cell types of the native intestine. Additionally, it is possible to cultivate patient stool microbes and pair these microbes with matching HIEs: potentially enabling the study of patient-specific microbe interactions (Blutt et al., 2018a). However, mechanistic investigations of host-microbe interactions are limited in HIEs due to challenges of oxygen supply and luminal flow. A large hurdle in in studying microbe-host interactions is that the majority of gut microbiota are obligate anaerobes, while the HIEs are oxygen dependent. Generating physiologically-relevant oxygen gradients across the epithelium has posed a noteworthy design challenge for HIE studies (Zeitouni et al., 2016). Additionally, luminal contents are in a continual state of movement as they progress down the intestinal tract and this is movement not reflected in the static HIE monolayer model. Some groups have begun to develop “gut-on-a-chip” technology for HIEs (Bein et al., 2018; Jalili-Firoozinezhad et al., 2019; Sontheimer-Phelps et al., 2019), which combines flow and compartment specific oxygenation. In addition, it is possible to add immune cells to culture system, allowing for epithelial-immune interactions (Kim et al., 2016). In the future, we believe these “gut-on-a-chip” devices will be instrumental in dissecting the cross-talk between the gut microbiome, epithelium and immune cells.
Conclusions
Human intestinal enteroids provide a unique model system for studies of human intestinal health and disease. The utility of human intestinal enteroids has been hindered by the relative lack of cellular responsiveness and cytokine production when challenged by different stimuli. Using modified media deficient in antioxidants, this study is among the first to demonstrate that HIEs can be highly responsive to host and microbial signals. We show that antioxidants in culture medium suppressed the innate immune responses of HIEs. This refinement opens new possibilities for applications of HIEs to studies of intestinal inflammation and microbial- host interactions. This new system represents a valuable tool for investigating therapeutics in human IBD and dissecting intestinal pathophysiology.
Supplementary Material
Key Points:
Enteroids are a physiologically relevant model to examine the human intestine and its functions.
Previously, the measurable cytokine response of human intestinal enteroids has been limited following exposure to host or microbial pro-inflammatory stimuli.
Modifications to enteroid culture conditions facilitated robust human cytokine responses to pro-inflammatory stimuli.
This new human enteroid culture methodology refines the ability to study microbiome:human intestinal epithelium interactions in the laboratory.
Acknowledgments
Funding:
This study was supported by the NIH T32 grant 5T32DK007664-28 awarded to WR, AG, and KAE. Trainee support was provided by NIH grant F30DK112563 (ACG), BCM Medical Scientist Training Program (ACG), NIH grant F32AI136404 (HAD), NLM Training Program in Biomedical Informatics and Data Science T15LM007093 (SCD), NIH P30-DK-56338 (MAE). Experiments were also made possible through the NIH U01CA170930 grant (JV), the NLM Training Program in Biomedical Informatics and Data Science T15LM007093 (SCD), and the Digestive Diseases Center which is funded by NIH/NIDDK P30 DK56338-06A2.
Abbreviations:
- HIE
Human Intestinal Enteroids
- TNF
Tumor necrosis factor
- IL
interleukin
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- MCP
monocyte chemoattractant protein
- GRO
(Chemokine-X-C-Motif Ligand 1)
- EGF
(Epidermal Growth Factor)
- G-CSF
(Granulocyte-Colony Stimulating Factor)
- GM-CSF
(Granulocyte-macrophage colony-stimulating factor)
- IFNγ
(Interferon Gamma)
- IP-10
(Chemokine-X-C-Motif Ligand 10)
- MDC
(C-C Motif Chemokine Ligand 22)
- TGFα
(Transforming Growth Factor Alpha)
- 2D
two-dimensional
- 3D
three-dimensional
- CMGF+
complete media with growth factors
Footnotes
Competing Interests:
JV and RAB receive unrestricted research support from BioGaia AB, a Swedish probiotics company. JV serves on the scientific advisory board of Seed, a USA- based probiotics/prebiotics company. JV also serves on the scientific advisory board of Biomica, an Israeli informatics enterprise and on the scientific advisory board of Plexus Worldwide, a USA-based nutrition company. R.A. Britton serves on the scientific advisory board of Tenza, is a co-founder of Mikrovia, and consults for Takeda and Probiotech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability:
All data supporting the results presented in the manuscript is included in the manuscript figures as n were ≤30. The data that support the findings of this study are available on request from the corresponding author.
References
- (1993). Cytokine-induced pathology. Part B: inflammatory cytokines, receptors and disease. Workshop. Basel, Switzerland, August 1991. Int Rev Exp Pathol 34 Pt B, 1–216. [PubMed] [Google Scholar]
- Aggarwal BB, Gupta SC & Kim JH. (2012). Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 119, 651–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja SK & Murphy PM. (1996). The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem 271, 20545–20550. [DOI] [PubMed] [Google Scholar]
- Angrisano T, Pero R, Peluso S, Keller S, Sacchetti S, Bruni CB, Chiariotti L & Lembo F. (2010). LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol 10, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta MC, Madsen K, Doyle J & Meddings J. (2009). Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 58, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviello G & Knaus UG. (2017). ROS in gastrointestinal inflammation: Rescue Or Sabotage? Br J Pharmacol 174, 1704–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbar N & Casero RA Jr. (2006). Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res 66, 11125–11130. [DOI] [PubMed] [Google Scholar]
- Bein A, Shin W, Jalili-Firoozinezhad S, Park MH, Sontheimer-Phelps A, Tovaglieri A, Chalkiadaki A, Kim HJ & Ingber DE. (2018). Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell Mol Gastroenterol Hepatol 5, 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y & Hand TW. (2014). Role of the microbiota in immunity and inflammation. Cell 157, 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutt SE, Crawford SE, Ramani S, Zou WY & Estes MK. (2018a). Engineered Human Gastrointestinal Cultures to Study the Microbiome and Infectious Diseases. Cell Mol Gastroenterol Hepatol 5, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutt SE, Crawford SE, Ramani S, Zou WY & Estes MK. (2018b). Engineered Human Gastrointestinal Cultures to Study the Microbiome and Infectious Diseases. Cellular and Molecular Gastroenterology and Hepatology 5, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Graham AL, Danhof HA, Engevik MA, Tomaro-Duchesneau C, Karandikar UC, Estes MK, Versalovic J, Britton RA & Hyser JM. (2019). Human Intestinal Enteroids With Inducible Neurogenin-3 Expression as a Novel Model of Gut Hormone Secretion. Cell Mol Gastroenterol Hepatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li M, Li B, Wang W, Lin A & Sheng M. (2013). Effect of reactive oxygen species generation in rabbit corneal epithelial cells on inflammatory and apoptotic signaling pathways in the presence of high osmotic pressure. PLoS One 8, e72900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhou W, Roh T, Estes MK & Kaplan DL. (2017). In vitro enteroid-derived three-dimensional tissue model of human small intestinal epithelium with innate immune responses. PloS one 12, e0187880–e0187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Shivshankar P, Zhong Y, Chen D, Li Z & Zhong G. (2008). Intracellular interleukin-1alpha mediates interleukin-8 production induced by Chlamydia trachomatis infection via a mechanism independent of type I interleukin-1 receptor. Infect Immun 76, 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi P & Buricchi F. (2007). Protein tyrosine phosphorylation and reversible oxidation: two cross-talking posttranslation modifications. Antioxid Redox Signal 9, 1–24. [DOI] [PubMed] [Google Scholar]
- Choi SY, Lim JW, Shimizu T, Kuwano K, Kim JM & Kim H. (2012). Reactive oxygen species mediate Jak2/Stat3 activation and IL-8 expression in pulmonary epithelial cells stimulated with lipid-associated membrane proteins from Mycoplasma pneumoniae. Inflamm Res 61, 493–501. [DOI] [PubMed] [Google Scholar]
- Cianci R, Pagliari D, Piccirillo CA, Fritz JH & Gambassi G. (2018). The Microbiota and Immune System Crosstalk in Health and Disease. Mediators Inflamm 2018, 2912539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA & Remick DG. (1993). Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem 268, 25568–25576. [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S & Sawaya BE. (2009). Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik MA & Versalovic J. (2017). Biochemical Features of Beneficial Microbes: Foundations for Therapeutic Microbiology. Microbiol Spectr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng XL, Qu L, Kou B, Opekun AR, Burrin D, Graham DY, Ramani S, Atmar RL & Estes MK. (2016). Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier BM & Parkos CA. (2012). The role of neutrophils during intestinal inflammation. Mucosal Immunol 5, 354–366. [DOI] [PubMed] [Google Scholar]
- Grandvaux N, Soucy-Faulkner A & Fink K. (2007). Innate host defense: Nox and Duox on phox's tail. Biochimie 89, 1113–1122. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Elsbury SK, Pavli P & Doe WF. (1996). Interleukin 8: cells of origin in inflammatory bowel disease. Gut 38, 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross V, Andus T, Daig R, Aschenbrenner E, Scholmerich J & Falk W. (1995). Regulation of interleukin-8 production in a human colon epithelial cell line (HT-29). Gastroenterology 108, 653–661. [DOI] [PubMed] [Google Scholar]
- Hemarajata P, Gao C, Pflughoeft KJ, Thomas CM, Saulnier DM, Spinler JK & Versalovic J. (2013). Lactobacillus reuteri-specific immunoregulatory gene rsiR modulates histamine production and immunomodulation by Lactobacillus reuteri. J Bacteriol 195, 5567–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneberg P, Draberova L, Bambouskova M, Pompach P & Draber P. (2010). Down-regulation of protein-tyrosine phosphatases activates an immune receptor in the absence of its translocation into lipid rafts. J Biol Chem 285, 12787–12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T, Hirose R, Saegusa S, Ametani A, Kiuchi K & Kaminogawa S. (2003). Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto). Int J Food Microbiol 82, 255–264. [DOI] [PubMed] [Google Scholar]
- Ina K, Kusugami K, Yamaguchi T, Imada A, Hosokawa T, Ohsuga M, Shinoda M, Ando T, Ito K & Yokoyama Y. (1997). Mucosal interleukin-8 is involved in neutrophil migration and binding to extracellular matrix in inflammatory bowel disease. Am J Gastroenterol 92, 1342–1346. [PubMed] [Google Scholar]
- Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, Camacho DM, Fadel CW, Bein A, Swenor B, Nestor B, Cronce MJ, Tovaglieri A, Levy O, Gregory KE, Breault DT, Cabral JMS, Kasper DL, Novak R & Ingber DE. (2019). A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng 3, 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Mercante JW & Neish AS. (2012). Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications. Curr Med Chem 19, 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SC, Evans SW, Lobo AJ, Ceska M, Axon AT & Whicher JT. (1993). Serum interleukin-8 in inflammatory bowel disease. J Gastroenterol Hepatol 8, 508–512. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H & Karin M. (2005). Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120, 649–661. [DOI] [PubMed] [Google Scholar]
- Karrasch T, Kim JS, Muhlbauer M, Magness ST & Jobin C. (2007). Gnotobiotic IL-10−/−;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol 178, 6522–6532. [DOI] [PubMed] [Google Scholar]
- Kim H, Hwang JS, Woo CH, Kim EY, Kim TH, Cho KJ, Kim JH, Seo JM & Lee SS. (2008). TNF-alpha-induced up-regulation of intercellular adhesion molecule-1 is regulated by a Rac-ROS-dependent cascade in human airway epithelial cells. Exp Mol Med 40, 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Li H, Collins JJ & Ingber DE. (2016). Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A 113, E7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee SB, Park JK & Yoo YD. (2010). TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L). Cell Death Differ 17, 1420–1434. [DOI] [PubMed] [Google Scholar]
- Kim YS, Morgan MJ, Choksi S & Liu ZG. (2007). TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell 26, 675–687. [DOI] [PubMed] [Google Scholar]
- Ko JW, Lim SY, Chung KC, Lim JW & Kim H. (2014). Reactive oxygen species mediate IL-8 expression in Down syndrome candidate region-1-overexpressed cells. Int J Biochem Cell Biol 55, 164–170. [DOI] [PubMed] [Google Scholar]
- Kojouharoff G, Hans W, Obermeier F, Mannel DN, Andus T, Scholmerich J, Gross V & Falk W. (1997). Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol 107, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolios G, Robertson DA, Jordan NJ, Minty A, Caput D, Ferrara P & Westwick J. (1996). Interleukin-8 production by the human colon epithelial cell line HT-29: modulation by interleukin-13. Br J Pharmacol 119, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F & Kollias G. (1999). Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398. [DOI] [PubMed] [Google Scholar]
- Krystel-Whittemore M, Dileepan KN & Wood JG. (2015). Mast Cell: A Multi-Functional Master Cell. Front Immunol 6, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L & Chifiriuc MC. (2018). Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front Immunol 9, 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EN, Choi YW, Kim HK, Park JK, Kim HJ, Kim MJ, Lee HW, Kim KH, Bae SS, Kim BS & Yoon S. (2011). Chloroform extract of aged black garlic attenuates TNF-alpha-induced ROS generation, VCAM-1 expression, NF-kappaB activation and adhesiveness for monocytes in human umbilical vein endothelial cells. Phytother Res 25, 92–100. [DOI] [PubMed] [Google Scholar]
- Li W, Yang S, Kim SO, Reid G, Challis JR & Bocking AD. (2014). Lipopolysaccharide-Induced Profiles of Cytokine, Chemokine, and Growth Factors Produced by Human Decidual Cells Are Altered by Lactobacillus rhamnosus GR-1 Supernatant. Reprod Sci 21, 939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TY, Fan CW, Maa MC & Leu TH. (2015). Lipopolysaccharide-promoted proliferation of Caco-2 cells is mediated by c-Src induction and ERK activation. Biomedicine (Taipei) 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankertz J, Amasheh M, Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M & Schulzke JD. (2009). TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res 336, 67–77. [DOI] [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR & Watson AJ. (2011). The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 140, 1208–1218 e1201-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matziouridou C, Rocha SDC, Haabeth OA, Rudi K, Carlsen H & Kielland A. (2018). iNOS- and NOX1-dependent ROS production maintains bacterial homeostasis in the ileum of mice. Mucosal Immunol 11, 774–784. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue JA & Mueller C. (1994). Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol 144, 997–1007. [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Ebert D, Huang X & Thomas PD. (2019). PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 47, D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M, Siddiqui MR, Tran K, Reddy SP & Malik AB. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20, 1126–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar D, Bosscher JS, ten Brink B, Driessen AJ & Konings WN. (1993). Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J Bacteriol 175, 2864–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Takagi T, Handa O, Ishikawa T, Nakagawa S, Yamaguchi T, Yoshida N, Minami M, Kita M, Imanishi J & Yoshikawa T. (2003). Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol 18, 560–569. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Buschenfelde KH, Strober W & Kollias G. (1997). Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol 27, 1743–1750. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Shirasaki T, Tsuchiya K, Miyake Y, Watanabe Y, Hibiya S, Watanabe S, Nakamura T & Watanabe M. (2019). Establishment of a system to evaluate the therapeutic effect and the dynamics of an investigational drug on ulcerative colitis using human colonic organoids. J Gastroenterol. [DOI] [PubMed] [Google Scholar]
- Noben M, Vanhove W, Arnauts K, Santo Ramalho A, Van Assche G, Vermeire S, Verfaillie C & Ferrante M. (2017). Human intestinal epithelium in a dish: Current models for research into gastrointestinal pathophysiology. United European Gastroenterology Journal 5, 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noti M, Corazza N, Mueller C, Berger B & Brunner T. (2010). TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. J Exp Med 207, 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara AM, Bhattacharyya A, Bai J, Mifflin RC, Ernst PB, Mitra S & Crowe SE. (2009). Tumor necrosis factor (TNF)-alpha-induced IL-8 expression in gastric epithelial cells: role of reactive oxygen species and AP endonuclease-1/redox factor (Ref)-1. Cytokine 46, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier-Denis E, Mkaddem SB & Vandewalle A. (2008). NOX enzymes and Toll-like receptor signaling. Semin Immunopathol 30, 291–300. [DOI] [PubMed] [Google Scholar]
- Pessione E, Mazzoli R, Giuffrida MG, Lamberti C, Garcia-Moruno E, Barello C, Conti A & Giunta C. (2005). A proteomic approach to studying biogenic amine producing lactic acid bacteria. Proteomics 5, 687–698. [DOI] [PubMed] [Google Scholar]
- Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT & Collins JE. (2005). Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85, 1139–1162. [DOI] [PubMed] [Google Scholar]
- Qin Y, Ekmekcioglu S, Liu P, Duncan LM, Lizee G, Poindexter N & Grimm EA. (2011). Constitutive aberrant endogenous interleukin-1 facilitates inflammation and growth in human melanoma. Mol Cancer Res 9, 1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B, Gardina P, Myers TG & Leto TL. (2011). Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol 4, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon M & Romling U. (2006). Flagellin in combination with curli fimbriae elicits an immune response in the gastrointestinal epithelial cell line HT-29. Microbes Infect 8, 2027–2033. [DOI] [PubMed] [Google Scholar]
- Rodwell AW. (1953). The histidine decarboxylase of a species of Lactobacillus; apparent dispensability of pyridoxal phosphate as coenzyme. J Gen Microbiol 8, 233–237. [DOI] [PubMed] [Google Scholar]
- Rossi F, Gardini F, Rizzotti L, La Gioia F, Tabanelli G & Torriani S. (2011). Quantitative analysis of histidine decarboxylase gene (hdcA) transcription and histamine production by Streptococcus thermophilus PRI60 under conditions relevant to cheese making. Appl Environ Microbiol 77, 2817–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudack C, Maune S, Eble J & Schroeder JM. (2003). The primary role in biologic activity of the neutrophil chemokines IL-8 and GRO-alpha in cultured nasal epithelial cells. J Interferon Cytokine Res 23, 113–123. [DOI] [PubMed] [Google Scholar]
- Ruder B, Atreya R & Becker C. (2019). Tumour Necrosis Factor Alpha in Intestinal Homeostasis and Gut Related Diseases. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetre T, Kahler H, Foster SJ & Lyberg T. (2001). Aminoethyl-isothiourea inhibits leukocyte production of reactive oxygen species and proinflammatory cytokines induced by streptococcal cell wall components in human whole blood. Shock 15, 455–460. [DOI] [PubMed] [Google Scholar]
- Samuel G, Gadeock S, Schultz M & Butt G. (2017). The Differential Response of Human Epithelial Derived Colonic Organoids to TLR Agonists. The FASEB Journal Vol. 31, 1049.1046. [Google Scholar]
- Sandoval R, Lazcano P, Ferrari F, Pinto-Pardo N, Gonzalez-Billault C & Utreras E. (2018). TNF-alpha Increases Production of Reactive Oxygen Species through Cdk5 Activation in Nociceptive Neurons. Front Physiol 9, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB Jr., Ritchie TC, Hunninghake GW, Zandi E & Engelhardt JF. (2001). Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J Biol Chem 276, 30188–30198. [DOI] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD & Clevers H. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ & Clevers H. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Saxena K, Blutt SE, Ettayebi K, Zeng XL, Broughman JR, Crawford SE, Karandikar UC, Sastri NP, Conner ME, Opekun AR, Graham DY, Qureshi W, Sherman V, Foulke-Abel J, In J, Kovbasnjuk O, Zachos NC, Donowitz M & Estes MK. (2016). Human Intestinal Enteroids: a New Model To Study Human Rotavirus Infection, Host Restriction, and Pathophysiology. J Virol 90, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerer-Maly CC, Eckmann L, Kagnoff MF, Falco MT & Maly FE. (1994). Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology 81, 85–91. [PMC free article] [PubMed] [Google Scholar]
- Ščigalková I, Bystroňová J, Pravda M, Velebný V, Riabov V, Klüter H, Kzhyshkowsk J & Vrana NE. (2019). The effect of healing phenotype-inducing cytokine formulations within soft hydrogels on encapsulated monocytes and incoming immune cells. Royal Society of Chemistry Advances 9, 21396–21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W, Zong Y, Mohammad A, Ajit D, Cui J, Han D, Hamilton JL, Simonyi A, Sun AY, Gu Z, Hong JS, Weisman GA & Sun GY. (2011). Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA(2)-IIA expression in astrocytes and microglia. J Neuroinflammation 8, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HS, Satsu H, Bae MJ, Totsuka M & Shimizu M. (2017). Catechol Groups Enable Reactive Oxygen Species Scavenging-Mediated Suppression of PKD-NFkappaB-IL-8 Signaling Pathway by Chlorogenic and Caffeic Acids in Human Intestinal Cells. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnier DI, Bailey SR, Schuster RM, Lentsch AB & Pritts TA. (2010). TNF-alpha induces vectorial secretion of IL-8 in Caco-2 cells. J Gastrointest Surg 14, 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer-Phelps A, Chou DB, Tovaglieri A, Ferrante TC, Duckworth T, Fadel C, Frismantas V, Sutherland AD, Jalili-Firoozinezhad S, Kasendra M, Stas E, Weaver JC, Richmond CA, Levy O, Prantil-Baun R, Breault DT & Ingber DE. (2019). Human Colon-on-a-Chip Enables Continuous In Vitro Analysis of Colon Mucus Layer Accumulation and Physiology. Cell Mol Gastroenterol Hepatol 9, 507–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford TJ, Kunkel SL, Phan SH, Rollins BJ & Strieter RM. (1991). Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem 266, 9912–9918. [PubMed] [Google Scholar]
- Storz P, Doppler H & Toker A. (2004). Activation loop phosphorylation controls protein kinase D-dependent activation of nuclear factor kappaB. Mol Pharmacol 66, 870–879. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Rhodes JM, Hart CA, Tam B, Roberts CL, Smith SL, Corkill JE, Winstanley C, Virji M & Campbell BJ. (2008). Characterization of epithelial IL-8 response to inflammatory bowel disease mucosal E. coli and its inhibition by mesalamine. Inflamm Bowel Dis 14, 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabanelli G, Torriani S, Rossi F, Rizzotti L & Gardini F. (2012). Effect of chemico-physical parameters on the histidine decarboxylase (HdcA) enzymatic activity in Streptococcus thermophilus PRI60. J Food Sci 77, M231–237. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Kishioka C, Ishinaga H, Sakakura Y & Majima Y. (2001). Histamine alters gene expression in cultured human nasal epithelial cells. J Allergy Clin Immunol 107, 310–314. [DOI] [PubMed] [Google Scholar]
- Takizawa H, Ohtoshi T, Kikutani T, Okazaki H, Akiyama N, Sato M, Shoji S & Ito K. (1995). Histamine activates bronchial epithelial cells to release inflammatory cytokines in vitro. Int Arch Allergy Immunol 108, 260–267. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M & Versalovic J. (2012). Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 7, e31951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M & Baggiolini M. (1999). Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol 155, 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA & Stappenbeck TS. (2015). Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64, 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst AC, Hopkins SJ & Warhurst G. (1998). Interferon gamma induces differential upregulation of alpha and beta chemokine secretion in colonic epithelial cell lines. Gut 42, 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wera O, Lancellotti P & Oury C. (2016). The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J Clin Med 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, Bae YS & Jo EK. (2009). NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol 182, 3696–3705. [DOI] [PubMed] [Google Scholar]
- Zachos NC, Kovbasnjuk O, Foulke-Abel J, In J, Blutt SE, de Jonge HR, Estes MK & Donowitz M. (2016). Human Enteroids/Colonoids and Intestinal Organoids Functionally Recapitulate Normal Intestinal Physiology and Pathophysiology. J Biol Chem 291, 3759–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S & Richmond A. (2008). CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol 23, 1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitouni NE, Chotikatum S, von Kockritz-Blickwede M & Naim HY. (2016). The impact of hypoxia on intestinal epithelial cell functions: consequences for invasion by bacterial pathogens. Mol Cell Pediatr 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q & Chen W. (2010). The functional behavior of a macrophage/fibroblast co-culture model derived from normal and diabetic mice with a marine gelatin-oxidized alginate hydrogel. Biomaterials 31, 5772–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.