Abstract
Thrombotic complications are frequent in COVID-19 and contribute significantly to mortality and morbidity. We review several mechanisms of hypercoagulability in sepsis that may be upregulated in COVID-19. These include immune-mediated thrombotic mechanisms, complement activation, macrophage activation syndrome, antiphospholipid antibody syndrome, hyperferritinemia, and renin-angiotensin system dysregulation. We highlight biomarkers within each pathway with potential prognostic value in COVID-19. Lastly, recent observational studies have evaluated a role for the expanded use of therapeutic anticoagulation in COVID-19. We review strengths and weaknesses of these studies, and we also discuss the hypothetical benefit and anticipated challenges of fibrinolytic therapy in COVID-19.
Keywords: COVID-19, thrombosis, hypercoagulability, cytokine storm, renin-angiotensin syndrome
Introduction
Coronavirus disease 2019 (COVID-19) frequently results in a hypercoagulable state that is strongly associated with mortality.1 Widespread micro- or macrovascular thrombosis could explain several disparate phenomena observed in COVID-19, and an exhaustive search for underlying mechanisms is now underway. Emerging reports have suggested a possible role for lupus anticoagulant as part of the antiphospholipid syndrome,2 which could plausibly link a highly pro-inflammatory state with thrombus formation. However, numerous pathogenic mechanisms exist that may be at play in COVID-19, and a great deal may be learned from current observations in COVID-19 and from related disease states such as acute respiratory distress syndrome or other viral infections. Recent reviews have highlighted important procoagulant mechanisms that may be upregulated in COVID-19.3,4 In this review, we aim to build on these concepts by 1) summarizing more recent evidence that micro- and macrovascular thrombosis is indeed prevalent in COVID-19, 2) reviewing data supporting and refuting the role of thrombosis as a contributor to COVID-19 multiorgan dysfunction, 3) summarizing pathogenic mechanisms of thrombosis seen in related disease states that may occur in COVID-19, 4) suggesting biomarker-based strategies that could identify elevated thrombotic risk and differentiate potentially heterogenous mechanisms of thrombosis in COVID-19, 5) highlighting observational data and upcoming randomized trials for the role of anticoagulation in COVID-19 that should inform best practice, and 6) discussing the rationale and anticipated challenges with fibrinolytic therapy in COVID-19.
Overview of COVID-19 Associated Hypercoagulopathy
COVID-19 induces a prothrombotic state, and a high frequency of reported major thrombotic events raises concern for unique prothrombotic pathophysiology.3 Micro- and macrovascular thromboembolic or in situ thrombotic complications have been observed in COVID-19 in the vasculature of the lungs, spleen, brain, gut, and periphery.5–8 There are reports of frequent thrombus formation in hemodialysis circuits, strokes as a presenting feature in young patients who were previously healthy, and arterial and venous thromboembolism formation in spite of prophylactic or fully therapeutic anticoagulation.8,9 Incident thrombotic events have been described in patients who were otherwise asymptomatic. Moreover, thromboses have been identified both in the acute setting and in the weeks following critical illness, suggesting that the pro-thrombotic state could last several weeks or even longer post-hospitalization. Similar thrombotic complications were observed in severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS).10,11
While many laboratory findings suggest overlap between inflammation and hypercoagulability, a principal unresolved question is whether the two are linked by causal mechanisms or simply indicative of severe illness activating parallel biologic pathways. If the former is true, anti-inflammatory interventions could plausibly mitigate thrombotic complications. If the latter is true, no such benefit is likely to be observed. Additionally, it is unknown whether conventional methods of anticoagulation are efficacious in COVID-19 or if COVID-19 coagulopathy bypasses mechanisms targeted by existing therapies.
Pulmonary Involvement
Pulmonary embolism (PE) and deep vein thrombosis are the most frequently noted thrombotic events in COVID-19, with initial reports noting an incidence of 20 to 30% in critically ill patients (Figure 1).12 In a Dutch cohort of 184 subjects with COVID-19 in the intensive care unit (ICU), the cumulative incidence of large-vessel thrombotic events was 49%, the majority of which were pulmonary emboli seen on computed tomography in segmental and subsegmental pulmonary arteries.8 This occurred in spite of universal thromboprophylaxis with Nadroparin at 2800 or 5700 IU once or twice daily, and the risk of all-cause death in that cohort was 5-fold higher among patients with a thrombotic event (HR 5.4; 95% CI 2.4 to 12). An Italian cohort of 388 patients observed a smaller but nevertheless sizable cumulative incidence of thromboembolic events at a rate of 21% (27.6% in the ICU, 6.6% on the general ward), half of which were diagnosed within 24 hours of hospital admission.13 A French cohort observed a similar cumulative incidence of thrombotic events and compared these to event rates from two different historical control populations: 1) ICU patients without COVID-19 admitted to the ICU in the Winter of 2019 and 2) patients with influenza admitted to the same ICU in 2019.14 Among COVID-19 ICU patients, 20.6% exhibited evidence of pulmonary embolism (diagnosed a median of 6 days from ICU admission), which was >2-fold higher compared to either historical control group. Therefore, it appears that COVID-19 may be uniquely prothrombotic compared to other severe viral respiratory pneumonias.
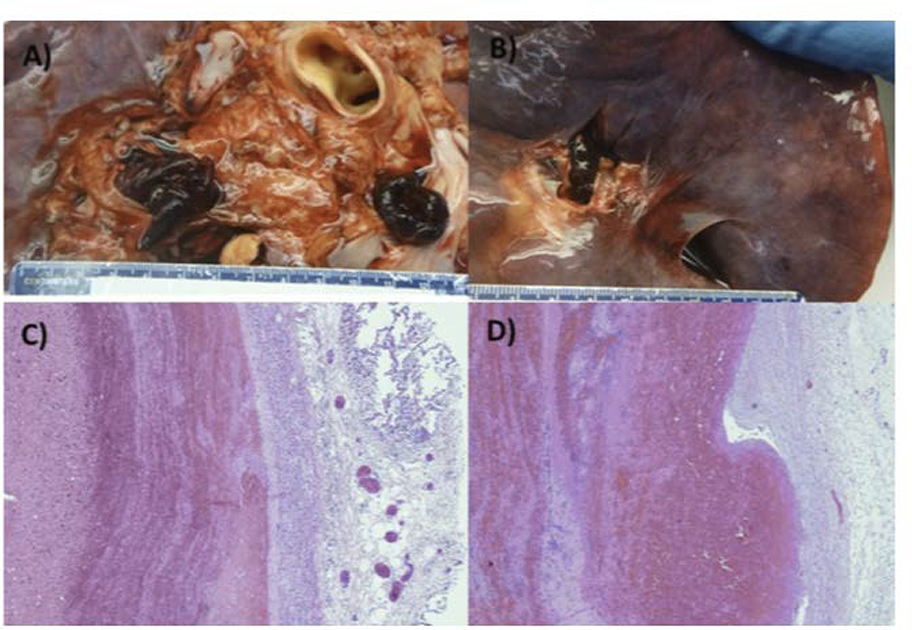
FIGURE 1:
Pulmonary emboli in the main pulmonary arteries of patients with COVID-19 (A and B). Characteristic “lines of Zahn” demonstrating alternating fibrin and erythrocyte deposition in pulmonary emboli from two patients (C and D). Reprinted from Grimes et al.99 with permission from the journal of Cardiovascular Pathology.
While published autopsy studies have been relatively rare in the setting of the pandemic, small case series have noted frequent macrovascular and microvascular fibrinous thrombi in the lungs and occasionally within multiple other organs (Figure 2). In a German autopsy series of 12 consecutive COVID-19 deaths, 7 patients had venous thromboembolism and PE was considered the direct cause of death in 4.5 Swiss pathologists reviewing 21 autopsies of COVID-19 patients found microthrombi in alveolar capillaries in 5 out of 11 patients in whom fibrin immunohistochemistry was performed, potentially consistent with complement-mediated microvascular injury.15,16 Evidence of small pulmonary vein and capillary vasculitis was also seen in one patient, though systemic vasculitis was absent. A series of 21 autopsies from multiple sites in the United States (US) confirmed a similarly high frequency of pulmonary microthrombi, and major pulmonary thromboembolism was a common fatal complication.17 Disseminated pulmonary microthrombi have been suggested as a contributor to unique COVID-19 ARDS physiology that has relatively normal lung compliance, potentially requiring ventilation strategies different from those typically employed in ARDS due to other etiologies.18,19 However, the initial interest in novel ventilation strategies was misguided, as the pathologic correlate of ARDS—diffuse alveolar damage—has indeed been seen in most COVID-19 autopsies (Figure 2).17 Lastly, a final study compared lung autopsies from 7 patients who died from COVID-19 with 7 patients who died from ARDS from H1N1 influenza.20 In patients with COVID-19, alveolar capillary microthrombi were 9 times as prevalent as in patients with influenza (p<0.001), consistent with the increased incidence of thrombosis seen clinically in COVID-19 compared to other viral pneumonias. Severe endothelial injury and intracellular virus were also noted in patients with COVID-19 in areas associated with microthrombosis, suggesting that endothelial damage and inflammation may directly underlie thrombus formation.
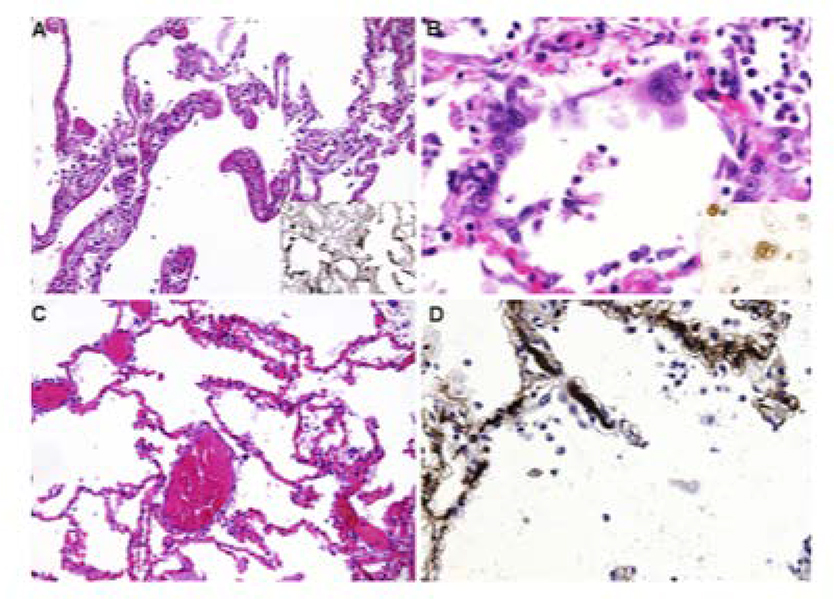
FIGURE 2:
Microscopic lung findings from patients with COVID-19. A) Diffuse alveolar damage, characteristic of acute respiratory distress syndrome, with insert showing immunohistochemistry (IHC) for fibrinogen; B) Syncitial cells, with insert showing IHC staining for thyroid transcription factor-1 that confirms pneumocyte II origin; C) Capillary congestion without diffuse alveolar damage; D) Microthrombi in alveolar capillaries, with IHC for fibrin. Reprinted from Menter et al.15 with permission from the journal of Histopathology.
It is highly likely that acute right ventricular dysfunction and cor pulmonale in COVID-19 stem, at least in part, from an abundance of central or segmental pulmonary emboli or a high burden of small vessel pulmonary microthrombi. These would be exacerbated by hypoxic vasoconstriction and increased intrathoracic pressure from mechanical ventilation, all of which would induce a sudden increase in right ventricular afterload leading to right ventricular-pulmonary artery uncoupling. Indeed, in a cohort of 120 patients with COVID-19, evidence of impaired right ventricular longitudinal strain and right ventricular dilatation was strongly associated with mortality,21 although these echocardiographic changes have not yet been directly linked to the presence of PE. Lastly, PE and right ventricular strain could also significantly contribute to troponin elevation, cardiogenic shock, and sudden death, which can develop suddenly even in patients with COVID-19 who were previously asymptomatic or otherwise recovering from respiratory failure.22,23
Extrapulmonary and Cardiac Involvement
Although thrombus has been identified most frequently in the lungs in patients with COVID-19, there is an increasing recognition of extrapulmonary thrombosis that may be a manifestation of de novo thrombus or exacerbation of previous atherosclerotic disease and endothelial dysfunction. Many patients with COVID-19 have presented with acute ischemic stroke or suffered a stroke while hospitalized, including young patients less than 50 years old with relatively few pre-existing risk factors.9 The incidence of stroke in ICU patients has been approximately 2.5% in several COVID-19 cohorts.8,13 Mesenteric ischemia, peripheral artery obstruction, and large vessel arteriosclerosis obliterans have also been reported,6,24–26 as has cerebral venous sinus thrombosis.27 Hemodialysis circuits have been observed to clot at very high rates (in one cohort, this occurred in 28 out of 29 patients receiving continuous renal replacement therapy, 96.6%).26 Lastly, phenomena such as acro-ischemia (i.e., “COVID Toe”) are concerning for digital microvascular thromboembolism,28,29 although it is possible that these represent microvascular inflammatory damage without microthrombosis per se.30
Some cardiac involvement in COVID-19 may be related to thrombotic complications. This includes myocardial infarction, in-stent thrombosis, and sudden left ventricular dysfunction, although the incidence of these are unclear and there are a number of non-thrombotic mechanisms that could also underlie cardiac pathophysiology. In a study of 416 patients hospitalized with COVID-19, the presence of cardiac injury detected by high sensitivity troponin I (TnI) was associated with a much higher risk of death (51.2% mortality with cardiac injury vs 4.5% without, p < 0.001).31 Moreover, the association with mortality was proportional to the magnitude of TnI elevation, and patients with cardiac injury also tended to have laboratory evidence of hypercoagulation (7.3% with cardiac injury vs 1.8% without, p = 0.02). One possibility is that there is a causal link between hypercoagulation, inflammation, and thrombus or plaque rupture, similar to that seen in influenza or perhaps related to novel mechanisms of hypercoagulation.32–34 However, it may also be the case that biomarkers of cardiac injury and hypercoagulation are simply confounded by the severity of illness and not directly linked. Several other pathogenic mechanisms for cardiac injury may also exist, including myocarditis, direct viral cardiomyocyte or endothelial damage, stress cardiomyopathy, type 2 myocardial infarction, or microvascular dysfunction, so the presentation of cardiac damage in COVID-19 is likely to be heterogeneous and potentially multifactorial.35,36 Moreover, there has been a paradoxical decrease in the volume of catheterization lab activation for acute coronary syndrome as patients delay care or systems navigate infectious control issues, so the actual impact of COVID-19 on myocardial infarction incidence and epicardial plaque rupture or thrombus has been challenging to assess.37
By autopsy, microthrombi have been identified in several extrapulmonary organs, but at a much lower frequency than pulmonary involvement and less consistently across studies. In the kidney, glomerular capillary microthrombi were seen in 3 of the 21 patients from the Swiss series but in zero patients from the US or German series.5,15,17 In the spleen, two out of 10 patients in another early autopsy series identified small artery thromboses,7 but this finding has been inconsistent across studies. Indirectly, however, the spleen has shown consistent expansion of the red pulp, which could be an indicator of increased splenic vascular congestion. Prostate and testicular vein microthrombi have also been seen, though only rarely.5 Lastly, cardiac and liver microthrombi were reported in early unpublished autopsy findings, but the presence of microthrombi in these organs has not been confirmed in larger recent studies. On the other hand, individual cardiomyocyte damage has been identified, and the differential etiology for this includes endothelial or paravascular cell dysfunction versus direct viral cardiomyocyte damage.17,36 Varga and colleagues identified viral inclusion bodies within endothelial cells and sequestered mononuclear and polymorphonuclear cellular infiltration of the endothelium, along with evidence of endothelial apoptosis.38 This suggests the presence of endotheliitis, which could lead to microvascular dysfunction resulting in multiorgan dysfunction, including the heart, kidney, and liver. Anti-viral agents could directly mitigate viral inclusions in the endothelium, which may manifest as decreased thrombotic events in treatment arms of randomized trials for agents like remdesivir or lopinavir-ritonavir—although rates of thrombosis have not yet been reported from these trials.39 In the heart, the concern for microvascular dysfunction is corroborated by a relative paucity of evidence for myocarditis: although myocarditis has been observed by autopsy or endomyocardial biopsy, its incidence is much lower than suggested by elevated serum troponin levels.17,40
Mechanisms of Thrombosis in COVID-19
Disseminated Intravascular Coagulation
Disseminated intravascular coagulation (DIC) is common in critical illness. Typically, it represents activation of the tissue factor pathway of the coagulation cascade and deposition of platelet-fibrin thrombi in the microvasculature. This eventually leads to the consumption of platelets and procoagulant factors, resulting in an associated bleeding diathesis.41,42 Hypercoagulability in DIC can be exacerbated by features of critical illness itself, including hypoxia, dehydration, and relative immobility. In severe cases, DIC can lead to damage of the microvasculature and subsequent organ dysfunction. While DIC has no specific marker for its diagnosis, it is often characterized by the presence of markedly elevated fibrin degradation products, as is seen in COVID-19. On the other hand, DIC classically has an associated bleeding diathesis that follows from the secondary activation of fibrinolysis, a feature that is not common with other thrombotic microangiopathies (TMA) such as thrombotic thrombocytopenic purpura or catastrophic antiphospholipid antibody syndrome.43 The rate of life-threatening bleeding events in COVID-19 is not well established, but thus far it appears that major bleeding occurs much less frequently than thrombotic events. It could be that hemorrhagic events are less easily identified: for instance, filling defects on CT are more readily detected than alveolar hemorrhage, which requires bronchoscopic confirmation. Conversely, if bleeding truly occurs less frequently than thrombotic events, this suggests that COVID-19 coagulopathy may represent a type of TMA distinct from DIC (Figure 3).
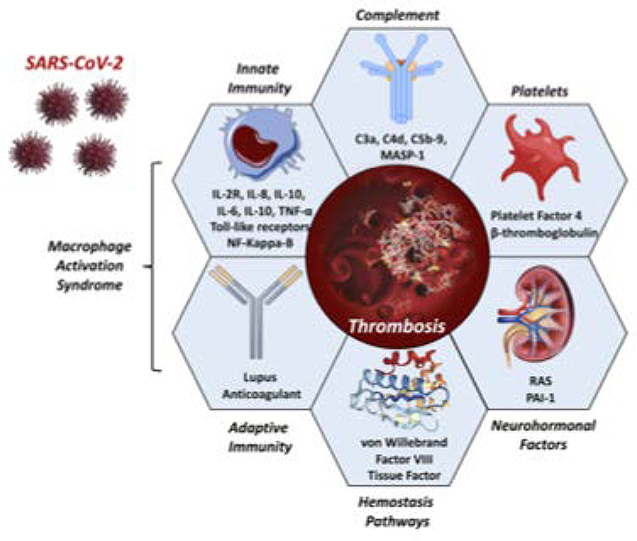
FIGURE 3: Components of COVID-19 Coagulopathy.
Several components of the innate immune system, platelet activation, coagulation system, complement system, renin-angiotensin system, and coagulation cascade have been observed in COVID-19 or SARS.
Acronyms: MASP-1, mannose binding lectin-associated serine protease; NF-Kappa-B, nuclear factor kappa B; PAI-1, plasminogen activator inhibitor 1; RAS, renin-angiotensin system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
COVID-19 biomarkers generally suggest idiosyncratic thrombotic pathophysiology that is distinct from DIC, whereas only a small number of laboratory findings in COVID-19 coagulopathy meet DIC criteria.44,45 Initial suspicion of DIC was based on marked elevations in D-dimer and fibrin degradation products (FDP). This can be seen in DIC, and in COVID-19 these biomarkers are strongly associated with morbidity and mortality.1 An elevated D-dimer at admission (≥ 1.0 mcg/mL) is associated with an increased mortality with a remarkably high odds ratio of 18.42 (95% CI 2.64–128.55), and D-dimer continues to rise throughout the course of hospitalization in non-surviving patients.46 This suggests an ongoing coagulopathic state that tracks with disease severity. On the other hand, elevations in the prothrombin time (PT) and activated partial thromboplastin time (aPTT) are only modest, and fibrinogen and factor VIII are increased,47 which is more typical of an acute phase response than DIC. Thrombocytopenia is another feature of COVID-19 that is linearly associated with the risk of death, but the degree of thrombocytopenia observed in late stages of COVID-19 is lower than that which is typically seen in DIC.48,49
Detailed hypercoagulability assays in COVID-19 indicate the existence of clot-predominant coagulopathy that peaks within one week of ICU admission without undergoing a transition to secondary hyperfibrinolysis. In one series, hypercoagulability measured by thromboelastometry was increased on ICU admission, continued to increase up to day 5, and then partially (but not completely) improved by day 10.50 Fibrinogen levels were highest upon admission to the ICU (895.7 mg/dL ± 110) and decreased thereafter to a nadir of 332.5 mg/dL ± 50 by day 10. These elevated fibrinogen levels may represent an acute phase response,51 or they may themselves have a more complex role in the hypercoagulable state seen in COVID-19. One major caveat of this study is that measurements on day 10 could only include surviving patients (n=33 of 40). If the 7 patients who died would otherwise have developed hyperfibrinolysis had they survived, this is unknown. Thus, one possibility is that a high mortality rate early in the natural history of COVID-19 precludes observation of the later fibrinolytic features of DIC. Conversely, COVID-19 coagulopathy may remain hypercoagulable, indicating the presence of a non-DIC TMA.
Several pathogenic mechanisms exist that may contribute to the hypercoagulability seen in COVID-19. We will review several possibilities, drawing insight from SARS and MERS, immunobiology, and activity of the renin-angiotensin system (Figure 3).
Cytokine Storm and Thrombosis
It is plausible that COVID-19 associated coagulopathy is a downstream consequence of the host inflammatory response to SARS-CoV-2 and innate immune activation (Central Figure). Activation of coagulation and subsequent fibrin deposition is presumably adaptive in the early phase of some other infections, but continued inflammation can quickly lead to a deleterious hyperinflammatory response mediated by cytokine storm and macrophage activation syndrome. Cytokine storm is an auto-amplifying syndrome of proinflammatory cytokine release that is a major contributor to ARDS and multiorgan dysfunction syndrome in several settings, including CAR T-cell therapy and Castleman disease.52–54 Macrophage activation syndrome is a related proinflammatory cascade that is associated with a high rate of thrombosis and death in sepsis.55,56 However, the exact progression from initial infection in COVID-19 to inflammatory response and hypercoagulable state is unknown.
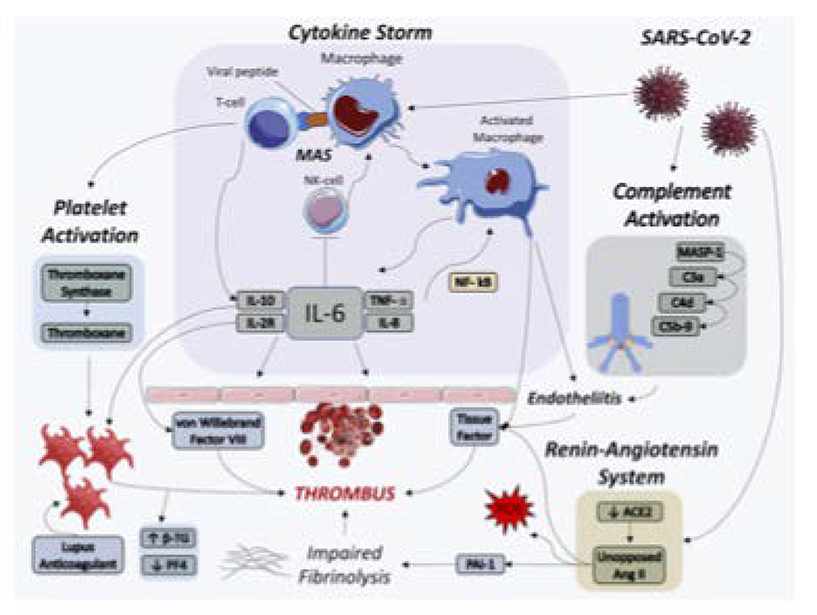
CENTRAL FIGURE: Thrombotic Pathways Implicated in COVID-19.
SARS-CoV-2 infection may lead to thrombus formation via cytokine storm, macrophage activation syndrome, complement activation, platelet activation, and renin-angiotensin system dysregulation. Several of these pathways are inter-related and auto-amplifying.
Acronyms: ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II, β-TG, beta thromboglobulin; IL, interleukin; MAS, macrophage activation syndrome; MASP-1, mannose binding lectin-associated serine protease; NK-cell, natural killer cell; NF-κB, nuclear factor kappa B; PAI-1, plasminogen activator inhibitor 1; PF4, platelet factor 4; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF-α, tumor necrosis factor alpha
Several nonspecific inflammatory biomarkers are greatly increased in hospitalized COVID-19 patients, including C-reactive protein, erythrocyte sedimentation rate, and ferritin, as are several procoagulant factors such as von Willebrand factor and Factor VIII (Table 1).47,57 In addition, numerous proinflammatory cytokines are increased including tumor necrosis factor alpha (TNF-α) and interleukin (IL)-2R, IL-6, IL-8, and IL-10.58,59 TNF-α and IL-6, in particular, are elevated to a degree not typically seen in bacterial sepsis or influenza.60 Many of these cytokines demonstrate a prothrombotic effect in other settings, alone or in combination, though a causal link in COVID-19 has not yet been established. Nevertheless, an association between elevated IL-6 and increased fibrinogen has been observed at the time of ICU admission.61 If IL-6 is mechanistically linked to a hypercoagulable state, tocilizumab and sarilumab, specific IL-6 antagonists, may reduce thrombotic risk. This should be explored in ongoing trials of these agents. Lastly, COVID-19 patients with prolonged aPTT were found to have lupus anticoagulant present at a high rate (91% of tested patients) compared to a historical control of patients with elevated aPTT without COVID-19 (26%).2 The clinical significance of lupus anticoagulant in COVID-19 is unclear, but this raises the possibility of the antiphospholipid antibody syndrome as a potential contributor to thrombogenesis in some patients.
Table 1:
Biomarkers of Inflammation, Coagulopathy, and Renin-Angiotensin System Activity of Interest in COVID-19
| Inflammation | Coagulopathy | Renin Angiotensin System Activity |
|---|---|---|
| IL-2R | Prothrombin time | Angiotensin II |
| IL-6 | Activated partial-thromboplastin time | Angiotensin converting enzyme 2 |
| IL-8 | D-dimer | |
| IL-10 | Plasminogen activator inhibitor 1 | |
| TNF-α | Platelet count | |
| Ferritin | Fibrinogen (↑ or ↓)* | |
| Transforming growth factor-β | Fibrin degradation products | |
| Erythrocyte Sedimentation Rate | von Willebrand factor | |
| C-reactive Protein | Factor VIII |
Biomarkers expected to be increased in severe COVID-19 except where otherwise noted.
Causal mechanisms for immune-mediated thrombosis could potentially be inferred from other severe infections. In SARS-CoV human in vitro models, infected mononuclear cells expressed a high level of procoagulant genes including fibrinogen, SERPINS, Tissue Factor, and factors II and X.11,62 These factors would induce hypercoagulability. The cells also expressed genes for Toll-like receptor 9 and thromboxane synthase, which promote platelet activation and aggregation, endothelial dysfunction, and vasoconstriction. This could tie into evidence of endothelial damage that was observed in the aforementioned COVID-19 autopsy series, which could underlie aspects of multiorgan dysfunction. Other mechanisms of platelet activation were independently associated with worse prognosis in a proteomic analysis of SARS-CoV, including decreased serum platelet factor 4 and increased beta-thromboglobulin (Central Figure).63 It is possible that overlapping prothrombotic mechanisms exist in COVID-19, mediated by procoagulant factors and platelet dysfunction.
At the same time, viral infections and sepsis in general have several mechanisms by which innate immune system activity triggers coagulation, including activation of tissue factor, complement system C3a and C5a, and von Willebrand factor.64–66 In particular, viruses can trigger the extrinsic coagulation pathway mediated by Tissue factor and factor VIIa. Normally, tissue factor/VIIa complexes form at the site of endothelial damage.67 However, monocytes and macrophages can be induced to express tissue factor in the setting of viral infection, largely via activity of TNF-α and nuclear factor kappa B.64 Thus, increased TNF-α levels measured in COVID-19 could implicate tissue-factor dependent thromboses, although this is relatively non-specific—TNF-α is a pleiotropic proinflammatory cytokine with multiple potentially prothrombotic downstream effects. Moreover, it is notable that TNF-α blockade has been attempted as a therapy in sepsis on numerous occasions without ever establishing a mortality benefit in any single trial, although a modest but significant mortality benefit across studies has been observed by meta-analysis.68
Complement Activation
Complement cascade activation may also recruit and activates leukocytes, leading to greatly amplified local release of the pro-inflammatory cytokines IL-1, IL-6, IL-8, and interferon-γ and subsequent microvascular damage. Complement activation is robust in sepsis, and inhibition of the complement system can ameliorate coagulopathy and endothelial dysfunction in animal models of sepsis.65 In lung tissue from patients with severe COVID-19 pneumonia and skin biopsies from COVID-19 patients with purpuric rash, there was evidence of catastrophic microvascular injury accompanied by terminal components of complement activation: C5b-9, C4d, and mannose binding lectin-associated serine protease (Central Figure).16 Anti-compliment C5 therapy with eculizumab has been considered for COVID-19. In a single-arm, open label study, four patients with COVID-19 given eculizumab experienced decreased C-reactive protein and all patients successfully recovered from COVID-19.69,70 Further randomized study of this therapy is warranted to compare this effect to placebo.
Macrophage Activation Syndrome and Hyperferritinemia
Macrophage activation syndrome (MAS) may be contributing to aspects of the cytokine storm and hypercoagulable state seen in COVID-19. MAS is thought to occur when activated antigen presenting cells cannot be lysed by CD8 T cells or natural killer (NK) cells.56 Following an initial inflammatory trigger, elevated IL-6 has been shown to decrease NK cell cytolytic function (Central Figure). As a consequence, there is prolonged interaction between innate and adaptive immune cells that further promotes cytokine storm, hemophagocytosis, and multi-organ dysfunction. Two biomarkers in COVID-19 suggest the potential presence of MAS. The first, as previously mentioned, is elevated IL-6 that is seen in COVID-19 at levels higher than are typical in other viral pneumonias. Second, ferritin elevation is a hallmark sign of MAS, and sustained fever and liver dysfunction are frequently seen in MAS though these are nonspecific. In a combined analysis of 653 COVID-19 patients across two studies, patients with severe vs non-severe COVID-19 had higher serum ferritin by 408 ng/mL (95% CI 311 to 505), and non-surviving versus surviving patients had a ferritin level that was 760 ng/mL higher (95% CI 561 to 959).59 Thus, key MAS biomarkers have been identified in severe COVID-19, potentially implicating MAS in a pro-inflammatory, prothrombotic, hyperferritinemic syndrome.71
Renin Angiotensin System Overactivation
In comparison to other bacterial and viral pneumonias, inflammation and hypercoagulability in COVID-19 may be uniquely related to its interaction with the renin-angiotensin system (RAS). Infection with SARS-CoV-2 is triggered when the virus binds to angiotensin-converting enzyme 2 (ACE2),72 similar to what was seen with SARS-CoV.73 ACE2 is a membrane bound protein found in many areas of the body including the lungs, small intestine, heart, brain, adipose tissue, and endothelium.74 Its distribution is particularly high in the lungs, heart, arteries, and veins.75 In the RAS, angiotensinogen is converted to angiotensin (Ang) I by renin, Ang I is converted to Ang II by angiotensin-converting enzyme (ACE), and Ang II promotes pleiotropic vasoconstrictive, proinflammatory, and prothrombotic downstream effects via the Angiotensin II receptor type I (AT1R) and Angiotensin II receptor type IV (AT4R).76–78 ACE2 opposes the activity of the RAS via two mechanisms. First, ACE2 leads to degradation of Ang I and Ang II, depleting the substrate available for activation of AT1R via the classical RAS cascade. Secondly, Ang II is directly degraded into Ang(1–7), which is a vasoactive peptide that has vasodilatory and anti-inflammatory effects via the Mas receptor.
SARS-CoV-2 exploits ACE2 for cellular entry after using the serine protease TMPRSS2 to prime the viral spike protein.79 Through this process, it is possible that the pulmonary expression of membrane-bound ACE2 is downregulated. Virus-mediated downregulation of ACE2 would then shift the normal balance towards proinflammatory and prothrombotic effects mediated by Ang II and AT1R (Central Figure). This would potentially be reflected by an increased local or circulating ratio of Ang II to Ang(1–7) or absolute level of Ang II. Indeed, increased plasma Ang II was observed by Liu and colleagues in a sample of COVID-19 patients compared to healthy controls, although the sample size of this study was only 12 patients and the range of Ang II was wide.80
Ang II has several proinflammatory and prothrombotic effects that could be amplified in COVID-19. Accelerated microvascular thrombosis has been demonstrated in mouse models infused with Ang II.81 Ang II plays a role in vascular and endothelial cell dysfunction, hypertrophy, and oxidative stress,82–84 all of which may be prothrombotic. AT1R activation by Ang II enhances platelet activation and impairs fibrinolysis, resulting in hypercoagulability.82,85 In several models, Ang II has also been shown to increase expression of tissue factor, which would activate the extrinsic coagulation pathway, in addition to plasminogen-activator inhibitor 1 (PAI-1), which is the principal endogenous inhibitor of tissue plasminogen activator and urokinase (Central Figure).86 Increased tissue factor and PAI-1 downstream of Ang II could both lead to a prothrombotic and hypofibrinolytic state. In ARDS, PAI-1 levels are elevated, but the pathophysiologic implication of this has not been firmly established.87 Some experimental evidence indicates that tissue factor and PAI-1 are neither necessary nor sufficient for accelerated microvascular thrombosis from chronic hypertension,81 but their role in the acute setting of COVID-19 downstream of Ang II activation is unknown.
Attenuated fibrinolysis has been observed in a high proportion of patients admitted to the ICU with COVID-19, and this is strongly correlated with thrombotic events.88 PAI-1, as the principal inhibitor of plasminogen activation, could lead to such a state of impaired fibrinolysis if overactivated in COVID-19. PAI-1 has primarily been implicated in chronic thrombus deposition or atherosclerosis, as seen in mendelian-randomization studies confirming a causal relationship between PAI-1 and coronary artery disease. However, in models of other viral infections such as influenza and cytomegalovirus, increased IL-6 is acutely associated with increased PAI-1,89 and PAI-1 increases coagulation and decreases fibrinolysis in mouse models of influenza.90 In mouse models of SARS-CoV, PAI-1 expression led to increased pulmonary fibrosis mediated by transforming growth factor-β.91 Moreover, PAI-1 is expressed by multiple cell types, including adipose tissue,92 which offers a possible connection between obesity and death in COVID-19—particularly in younger patients without any other preexisting comorbidities. However, the presence of elevated fibrin degradation products in COVID-19 could potentially contradict a potential role of PAI-1, which would be expected to induce a state of hypofibrinolysis with low levels of fibrin degradation. On the other hand, this same feature in later stages of DIC could also explain the absence of a shift to secondary fibrinolysis, perhaps explaining the lack of reported bleeding complications typical of DIC.
Therapeutic Anticoagulation in COVID-19
At present, compelling evidence for an expanded role of therapeutic anticoagulation in COVID-19 does not exist, although the high burden of thrombotic events and relationship of thrombotic events to COVID-19 mortality raise the urgency of this question and the need for randomized trial data (Table 2). Equipoise regarding universal therapeutic anticoagulation currently exists, and caution is warranted with this strategy as it would invariably lead to increased bleeding events that must be weighed against uncertain benefit. Current guidelines support venous thromboembolism prophylaxis for all admitted patients absent specific contraindications for bleeding risk. Likewise, all patients with direct evidence of venous or arterial thrombus, atrial fibrillation, mechanical cardiac valves, or need for secondary venous thromboembolism prophylaxis should be initiated on or maintained on therapeutic anticoagulation unless specific contraindications arise. The mode of therapeutic anticoagulation is worth considering at the time of hospital admission, including concerns for the burden of therapeutic monitoring and the potential role for therapies with a shorter half-life or reversal potential in case bleeding events arise.
Table 2:
Existing Anticoagulation Clinical Trials in Adults
| Clinical Trial Number | Country of Sponsor | Target Sample | Biomarker Inclusion Criteria | Experimental Arm | Control Arm | Primary Outcome |
|---|---|---|---|---|---|---|
| NCT04362085 | Canada | 462 | D-dimer ≥2 × ULN | Full dose LMWH or UFH | Prophylactic Dose Anticoagulation | Composite of ICU admission, vent, all-cause mortality |
| NCT04359277 | United States | 1000 | D-dimer > 2 & < 10 μg/mL | Full dose LMWH or UFH | Prophylactic Dose Anticoagulation | All-cause mortality up to 1 year, Incidence of major thrombotic events |
| NCT04377997 | United States | 300 | D-dimer > 1.5 μg/mL | Full dose LMWH or UFH | Prophylactic Dose Anticoagulation | Composite of all-cause mortality, cardiac arrest, and major thrombotic events |
| NCT04367831 | United States | 100 | - | Intermediate dose LMWH or UFH | Prophylactic Dose Anticoagulation | Alive and discharged from ICU without major thrombotic event |
| NCT04345848 | Geneva | 200 | D-dimer > 1 μg/mL | Full dose LMWH or UFH | Prophylactic Dose Anticoagulation | Composite of major thrombotic events, DIC, and all-cause mortality |
| NCT04344756 | France | 808 | - | Full Dose LMWH or UFH | Prophylactic Dose Anticoagulation | Ventilation-free survival |
| NCT04394377 | Brazil | 600 | D-dimer > 3 × ULN | Full dose LMWH or rivaroxiban 20mg daily | Prophylactic Dose Anticoagulation | Hierarchical composite endpoint of mortality, days alive, days in the hospital, and days on oxygen |
| NCT04360824 | United States | 170 | Modified ISTH Overt DIC score ≥ 3 | Intermediate dose LMWH | Prophylactic Dose Anticoagulation | All-cause mortality |
| NCT04372589 | Canada | 3000 | - | Full dose LMWH or UFH | Prophylactic Dose Anticoagulation | Intubation and mortality |
Two observational studies have retrospectively assessed the mortality rate of patients hospitalized for COVID-19 who received therapeutic anticoagulation versus those who did not. Both reported a significantly lower mortality rate in the group receiving therapeutic anticoagulation, but these findings may be impacted by bias and confounding given their observational nature. In a study by Tang and colleagues, 449 adult patients hospitalized for COVID-19 longer than 7 days were evaluated, of whom 99 received heparin (unfractionated or low molecular weight) for greater than 7 days.93 No association with mortality was seen in the overall cohort in patients, although a significant mortality difference was measured in a post-hoc subgroup analysis of patients with evidence of sepsis-induced coagulopathy. More recently, Paranjpe and colleagues evaluated 2,773 patients hospitalized with COVID-19, among whom 28% received therapeutic anticoagulation at some point during admission.94 In a subset of anticoagulated patients who required mechanical ventilation, in-hospital mortality was 29.1% compared to mortality of 62.7% of ventilated patients who never received therapeutic anticoagulation. After multivariable adjustment controlling for important baseline differences, a significant mortality difference was still observed (hazard ratio 0.86 per day, 95% CI 0.82 to 0.89).
These analyses have key limitations that weaken the strength of the reported results. In the study by Tang and colleagues, heparin exposure was defined as administration of a minimum of 7 days of heparin. Thus, patients who received heparin had to survive for a minimum of 7 days, whereas this restriction did not apply equally to the non-heparin group. This introduces an immortal time bias that could explain all or some of the apparent survival benefit. Moreover, the assignment of heparin was non-random, allowing for significant between-group confounding, yet no adjustment for baseline differences occurred. Paranjpe and colleagues had a more robust analysis that controlled for baseline differences, although residual confounding by indication for anticoagulation could remain. However, the results of the study were still subject to significant immortal time bias. Specifically, all patients who received anticoagulation, by definition, had to survive long enough to receive this therapy, introducing immortal time bias as mentioned above. This type of bias can lead to potentially erroneous results or results that exaggerate the apparent protective effect from anticoagulation therapy. The impact of this bias can be large, so these results should be considered hypothesis-generating rather than conclusive.
Several randomized trials are currently enrolling patients in order to clarify the role of therapeutic anticoagulation in COVID-19 (Table 2). Within these and future trials, an important strategy would be a pre-specified analysis of therapeutic anticoagulation within a substratum of patients with biomarker-based evidence of hypercoagulability or excess inflammation. D-dimer cutoffs have been applied in the inclusion criteria for most of these studies, but several other biomarkers may help select patients at particularly high risk of thrombotic events. The biomarkers listed in Table 1 are noted to be deranged in severe COVID-19 and mechanistically related to hypercoagulability. We would hypothesize that the patients with alterations in one or several of these biomarkers would be at the greatest risk for venous or arterial thrombotic events, which may lead to a favorable risk/benefit balance with therapeutic anticoagulation.
Fibrinolytics in COVID-19
There is some interest in the use of fibrinolytic therapy as a potential treatment for systemic thrombotic events in COVID-19 as well as severe ARDS. Historically, porcine models of ARDS treated with systemic urokinase or tissue plasminogen activator demonstrated improved respiratory mechanics, oxygen exchange, and survival.95 The mechanism may be related to the lysis of fibrin-rich hyaline membranes that form in the lungs in ARDS, which have been similarly observed in COVID-19. To this end, tissue plasminogen activator was used off-label in three patients with COVID-19 and ARDS requiring invasive-mechanical ventilation, given as a 25mg bolus over 2 hours followed by an additional 25mg infusion over the following 22 hours.96 In each patient, some initial improvement in oxygenation was observed, but this was transient in 2 of the 3 patients. Thus, it is not clear if there was any meaningful benefit overall, though no robust conclusions can be drawn from this small non-randomized series.
Fibrinolytic therapy in severe COVID-19 may confer a significant risk of major hemorrhagic events, including intracranial hemorrhage and diffuse alveolar hemorrhage. In general, the risk of major bleeding with systemic fibrinolytic therapy is consistently 1–3% across a wide range of indications, including ST elevation myocardial infarction, acute ischemic stroke, and severe PE.97,98 The risk of major bleeding in COVID-19 may be further exacerbated by underlying coagulopathy, and the potential for hemorrhagic conversion of unrecognized subacute stroke should be considered. Given the high risk of bleeding events with fibrinolytic therapy, its universal application in severe COVID-19 is not presently justified, even with biomarkers suggesting a hypercoagulable state. However, select use of fibrinolytics in carefully selected patients in a research setting may be warranted.
Conclusion
Thrombotic complications are frequent in COVID-19 and significantly contribute to mortality and morbidity. PE have been commonly observed in several autopsy series, and the occurrence of acute PE may explain some cases of sudden right ventricular dysfunction, cardiogenic shock, and sudden death in addition to troponin elevation. Whether long term complications such as pulmonary hypertension may complicate post-COVID recovery remains to be seen. Several thrombogenic mechanisms are potentially implicated in COVID-19 thrombosis, including thrombosis triggered by cytokine storm, antiphospholipid antibody syndrome, macrophage activation syndrome, the complement cascade, and RAS dysregulation.
At present, it is not clear which set of mechanisms are predominant in COVID-19, although cytokine storm and IL-6 in particular are particularly elevated in COVID-19 compared to other septic etiologies, and these are mechanistically upstream of multiple thrombogenic pathways. The incidence of thrombotic events in trials of anti-inflammatory and antiviral therapies will be of particular important from a therapeutic and mechanistic standpoint, and several novel therapies such as eculizumab, tocilizumab, and sarilumab may directly target upstream mediators of COVID-19 hypercoagulability. In the meantime, longitudinal studies are needed to clarify the role of these mechanisms and their suitability as therapeutic targets. An expanded role for therapeutic anticoagulation has also been considered based on recent observational studies, but the interpretation of these data is impeded by immortal time bias and confounding. Randomized trials of therapeutic anticoagulation are urgently needed to determine optimal antithrombotic regimens in COVID-19.
Acknowledgments
Funding: This work was supported by the National Institute of Health [T32-AI007433 to AMM] and National Heart Lung and Blood Institute at the National Institute of Health [T32-HL007891 to TCH; K23-HL133843 to JBC; R01-HL 121510-01A1, R61-HL-146390, R01-AG058969, 1R01-HL104106, P01-HL094307, R03-HL146874-01, and R56-HL136730 to JAC].
Disclosures: JAC has received honoraria from Sanifit, Microsoft, Fukuda-Denshi, Bristol Myers Squibb, OPKO Healthcare, Ironwood Pharmaceuticals, Pfizer, Akros Pharma, Merck, Edwards Lifesciences, Bayer and JNJ and research grants from Microsoft, Fukuda-Denshi and Bristol Myers Squibb. JG is on the advisory boards of Inari Medical and Astra Zeneca and has received grants to the University of Pennsylvania from St. Jude Medical and Recor Medical. All other authors have no disclosures.
Acronyms:
- ACE
angiotensin converting enzyme
- Ang
Angiotensin
- aPTT
activated partial-thromboplastin time
- AT1R
angiotensin II receptor type I
- AT4R
angiotensin II receptor type IV
- ARDS
acute respiratory distress syndrome
- COVID-19
coronavirus disease 2019
- DIC
disseminated intravascular coagulation
- FDP
fibrin degradation products
- ICU
intensive care unit
- IL
interleukin
- MAS
macrophage activation syndrome
- MERS
Middle East respiratory syndrome (MERS)
- PAI-1
plasminogen activator inhibitor 1
- PT
prothrombin time
- SARS
severe acute respiratory syndrome
- SARS-CoV
severe acute respiratory syndrome coronavirus
- SIC
sepsis-induced coagulopathy
- TMA
thrombotic microangiopathy
- TNF-α
tumor necrosis factor alpha
- TnI
troponin I
References
- 1.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, MacDonald V, Green L, Sivapalaratnam S, Pasi KJ, MacCallum P. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. N Engl J Med 2020:NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 2020;7:e438–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, Heer G de, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, Weerth A de, Paschen H-R, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vulliamy P, Jacob S, Davenport RA. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. Br J Haematol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Chang XN, Pan HX, Su H, Huang B, Yang M, Luo DJ, Weng MX, Ma L, Nie X. Pathological changes of the spleen in ten patients with new coronavirus infection by minimally invasive autopsies. Chinese J Pathol 2020;49:E014. [DOI] [PubMed] [Google Scholar]
- 8.Klok FA, Kruip MJHA, Meer NJM Van Der, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, Paassen J Van, Stals MAM, Huisman M V, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 2020:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, Leacy RA De, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med 2020:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong RSM, Wu A, To KF, Lee N, Lam CWK, Wong CK, Chan PKS, Ng MHL, Yu LM, Hui DS, Tam JS, Cheng G, Sung JJY. Haematological manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. Br Med J 2003;326:1358–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 2020;127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middeldorp S, Coppens M, Haaps TF van, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, Es N van. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020:jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Alexia B, Sandri MT, Barco S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poissy J, Goutay J, Caplan; Morgan, Parmentier E, Duburcq; Thibault, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S. Pulmonary Embolism in COVID-19 Patients: Awareness of an Increased Prevalence. Circulation 2020;epub ahead. [DOI] [PubMed] [Google Scholar]
- 15.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020:his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buja LM, Wolf D, Zhao B, Akkanti B, Do MM, Lelenwa L, Do NR, Ottaviani G, Tarek M, Trujillo DO, Aisenberg GM, Madjid M, Kar B, Buja M. Emerging Spectrum of Cardiopulmonary Pathology of the Coronavirus Disease 2019 (COVID-19): Report of Three Autopsies from Houston, Texas and Review of Autopsy Findings from other United States Cities. Cardiovasc Pathol 2020;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciceri F, Beretta L, Scandroglio AM, Colombo S, Landoni G, Ruggeri A, Peccatori J, D’Angelo A, Cobelli F De, Rovere-Querini P, Tresoldi M, Dagna L, Zangrillo A. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Coppola S, Cressoni M, Busana M, Chiumello D. Covid-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020:NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Li H, Zhu S, Xie Y, Wang B, He L, Zhang D, Zhang Y, Yuan H, Wu C, Sun W, Zhang Y, Li M, Cui L, Cai Y, Wang J, Yang Y, Lv Q, Zhang L, Xie M. Prognostic Value of Right Ventricular Longitudinal Strain in Patients with COVID-19. JACC Cardiovasc Imaging 2020:3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polat V, Bostancı Gİ. Sudden death due to acute pulmonary embolism in a young woman with COVID-19. J Thromb Thrombolysis 2020:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou B, She J, Wang Y, Ma X. Venous thrombosis and arteriosclerosis obliterans of lower extremities in a very severe patient with 2019 novel coronavirus disease: a case report. J Thromb Thrombolysis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin DO, Jensen A, Khan M, Chin J, Chin K, Parnell R, Awwad C, Patel D. Arterial thromboembolic complications in COVID-19 in low risk patients despite prophylaxis. Br J Haematol 2020:bjh.16792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes P-M, Meziani F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020:epub online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral Venous Sinus Thrombosis as a Presentation of COVID-19. Eur J Case Reports Intern Med 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Cao W, Xiao M, Li YJ, Yang Y, Zhao J, Zhou X, Jiang W, Zhao YQ, Zhang SY, Li TS. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue Za Zhi 2020;41:E006. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, Moreno-Arrones OM, Saceda-Corralo D, Arana-Raja A, Ortega-Quijano D. Characterization of acute acro-ischemic lesions in non-hospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cordoro KM, Reynolds SD, Wattier R, McCalmont TH. Clustered Cases of Acral Perniosis: Clinical Features, Histopathology and Relationship to COVID-19. Pediatr Dermatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020:Advance Online Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, Katz K, Ko DT, McGeer AJ, McNally D, Richardson DC, Rosella LC, Simor A, Smieja M, Zahariadis G, Gubbay JB. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018;378:345–353. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura M, Hiraoka K, Ohkawa S ichiro, Ueda K, Matsuda T, Murakami M. A Clinicopathological Study on Cardiac Lesions in 64 Cases of Disseminated Intravascular Coagulation. Jpn Heart J 1977;18:57–69. [DOI] [PubMed] [Google Scholar]
- 34.Dominguez-Erquicia P, Dobarro D, Raposeiras-Roubín S, Bastos-Fernandez G, Iñiguez-Romo A. Multivessel coronary thrombosis in a patient with COVID-19 pneumonia. Eur Heart J 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in covid-19. Heart 2020:heartjnl-2020–317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, Huang PP, Henry TD. Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States during COVID-19 Pandemic. J Am Coll Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 2020;382:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhainaut J-F, Shorr AF, Macias WL, Kollef MJ, Levi M, Reinhart K, Nelson DR. Dynamic evolution of coagulopathy in the first day of severe sepsis: Relationship with mortality and organ failure*. Crit Care Med 2005;33:341–348. [DOI] [PubMed] [Google Scholar]
- 42.Müller MC, Meijers JCM, Vroom MB, Juffermans NP. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: A systematic review. Crit Care 2014;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada H Disseminated intravascular coagulation. Clin Chim Acta 2004;344:13–21. [DOI] [PubMed] [Google Scholar]
- 44.Iba T, Levy JH, Raj A, Warkentin TE. Advance in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. J Clin Med 2019;8:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cipolloni L, Sessa F, Bertozzi G, Baldari B, Cantatore S, Testi R, D’Errico S, Mizio G Di, Asmundo A, Castorina S, Salerno M, Pomara C. Preliminary Post-Mortem COVID-19 Evidence of Endothelial Injury and Factor VIII Hyperexpression. Diagnostics (Basel, Switzerland) 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lippi G, Plebani M, Michael Henry B. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thiery-Antier N, Binquet C, Vinault S, Meziani F, Boisramé-Helms J, Quenot JP. Is Thrombocytopenia an Early Prognostic Marker in Septic Shock? Crit Care Med 2016;44:764–772. [DOI] [PubMed] [Google Scholar]
- 50.Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fish RJ, Neerman-Arbez M. Fibrinogen gene regulation. Thromb Haemost 2012;108:419–426. [DOI] [PubMed] [Google Scholar]
- 52.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 2017;39:517–528. [DOI] [PubMed] [Google Scholar]
- 53.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, Across Speciality Collaboration H. COVID-19: consider cytokine storm syndromes and immunosuppression. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.England JT, Abdulla A, Biggs CM, Lee AYY, Hay KA, Hoiland RL, Wellington CL, Sekhon M, Jamal S, Shojania K, Chen LYC. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev 2020:100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karakike E, Giamarellos-Bourboulis EJ. Macrophage activation-like syndrome: A distinct entity leading to early death in sepsis. Front Immunol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front Immunol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis. J Thromb Haemost 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henry BM, Oliveira MHS De, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chem Lab Med 2020. [DOI] [PubMed] [Google Scholar]
- 60.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ranucci M, Ballotta A, Dedda U Di, Bayshnikova E, Dei Poli M, Resta M, Falco M, Albano G, Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020:jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng LFP, Hibberd ML, Ooi EE, Tang KF, Neo SY, Tan J, Krishna Murthy KR, Vega VB, Chia JM, Liu ET, Ren EC. A human in vitro model system for investigating genome-wide host responses to SARS coronavirus infection. BMC Infect Dis 2004;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poon TCW, Pang RTK, Chan KCA, Lee NLS, Chiu RWK, Tong YK, Chim SSC, Ngai SM, Sung JJY, Lo YMD. Proteomic analysis reveals platelet factor 4 and beta-thromboglobulin as prognostic markers in severe acute respiratory syndrome. Electrophoresis 2012;33:1894–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Subramaniam S, Scharrer I. Procoagulant activity during viral infections. Front Biosci - Landmark 2018;23:1060–1081. [DOI] [PubMed] [Google Scholar]
- 65.Lupu F, Keshari RS, Lambris JD, Mark Coggeshall K. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res 2014;133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gragnano F, Sperlongano S, Golia E, Natale F, Bianchi R, Crisci M, Fimiani F, Pariggiano I, Diana V, Carbone A, Cesaro A, Concilio C, Limongelli G, Russo M, Calabrò P. The Role of von Willebrand Factor in Vascular Inflammation: From Pathogenesis to Targeted Therapy. Mediat Inflamm 2017;Epub:5620314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Gorp ECM, Suharti C, ten Cate H, Dolmans WMV, van der Meer JWM, ten Cate JW, Brandjes DPM. Review: Infectious Diseases and Coagulation Disorders. J Infect Dis 1999;180:176–186. [DOI] [PubMed] [Google Scholar]
- 68.Qiu P, Cui X, Sun J, Welsh J, Natanson C, Eichacker PQ. Antitumor necrosis factor therapy is associated with improved survival in clinical sepsis trials: A meta-analysis. Crit Care Med 2013;41:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diurno F, Numis FG, Porta G, Cirillo F, Maddaluno S, Ragozzino A, Negri P De, Gennaro C Di, Pagano A, Allegorico E, Bressy L, Bosso G, Ferrara A, Serra C, Montisci A, D’Amico M, Schiano Lo Morello S, Costanzo G Di, Tucci AG, Marchetti P, Vincenzo U Di, Sorrentino I, Casciotta A, Fusco M, Buonerba C, Berretta M, Ceccarelli M, Nunnari G, Diessa Y, Cicala S, Facchini G. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci 2020;24:4040–4047. [DOI] [PubMed] [Google Scholar]
- 70.Campbell CM, Kahwash R. Will Complement Inhibition be the New Target in Treating COVID-19 Related Systemic Thrombosis? Circulation 2020;epub. [DOI] [PubMed] [Google Scholar]
- 71.Colafrancesco S, Alessandri C, Conti F, Priori R. COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev 2020:102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020;181:281–292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: A functional receptor for SARS coronavirus. Cell Mol Life Sci 2004;61:2738–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, Goor H van. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Senchenkova EY, Russell J, Esmon CT, Granger DN. Roles of Coagulation and Fibrinolysis in Angiotensin II-Enhanced Microvascular Thrombosis. Microcirculation 2014;21:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res 2016;118:1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Senchenkova EY, Russell J, Almeida-Paula LD, Harding JW, Granger DN. Angiotensin II-mediated microvascular thrombosis. Hypertension 2010;56:1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nadar S, Lip G. The Prothrombotic State in Hypertension and the Effects of Antihypertensive Treatment. Curr Pharm Des 2005;9:1715–1732. [DOI] [PubMed] [Google Scholar]
- 82.Brown N, Vaughan D. Prothrombotic effects of angiotensin. Adv Intern Med 2000;45:419–429. [PubMed] [Google Scholar]
- 83.Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlöf B, Deanfield J, Diez J, Drexler H, Ferrari R, Gilst W Van, Hansson L, Hornig B, Husain A, Johnston C, Lazar H, Lonn E, Lüscher T, Mancini J, Mimran A, Pepine C, Rabelink T, Remme W, Ruilope L, Ruzicka M, Schunkert H, Swedberg K, Unger T, Vaughan D, Weber M. Pathophysiologic and therapeutic importance of tissue ACE: a consensus report. Cardiovasc drugs Ther 2002;16:149–60. [DOI] [PubMed] [Google Scholar]
- 84.Vaughan DE, Lazos SA, Tong K. Angiotensin II Regulates the Expression of Plasminogen Activator Inhibitor-1 in Cultured Endothelial Cells: A Potential Link between the Renin-Angiotensin System and Thrombosis. J Clin Invest 1995;95:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gromotowicz-Poplawska A, Stankiewicz A, Kramkowski K, Gradzka A, Wojewodzka-Zelezniakowicz M, Dzieciol J, Szemraj J, Chabielska E. The acute prothrombotic effect of aldosterone in rats is partially mediated via angiotensin II receptor type 1. Thromb Res 2016;138:114–120. [DOI] [PubMed] [Google Scholar]
- 86.Sun T, Ghosh AK, Eren M, Miyata T, Vaughan DE. PAI-1 contributes to homocysteine-induced cellular senescence. Cell Signal 2019;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bos LD, Schouten LR, Vught LA van, Wiewel MA, Ong DSY, Cremer O, Artigas A, Martin-Loeches I, Hoogendijk AJ, Poll T van der, Horn J, Juffermans N, Calfee CS, Schultz MJ. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 2017;72:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer M V., Urban S, Nydam TL, Moore PK, McIntyre RC. Fibrinolysis Shutdown Correlates to Thromboembolic Events in Severe COVID-19 Infection. J Am Coll Surg 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bouwman JJM, Diepersloot RJA, Visseren FLJ. Intracellular infections enhance interleukin-6 and plasminogen activator inhibitor 1 production by cocultivated human adipocytes and THP-1 monocytes. Clin Vaccine Immunol 2009;16:1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keller TT, Sluijs KF Van Der, Kruif MD De, Gerdes VEA, Meijers JCM, Florquin S, Poll T Van Der, Gorp ECM Van, Brandjes DPM, Büller HR, Levi M. Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ Res 2006;99:1261–1269. [DOI] [PubMed] [Google Scholar]
- 91.Zhao X, Nicholls JM, Chen YG. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-β signaling. J Biol Chem 2008;283:3272–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lundgren CH, Brown SL, Nordt TK, Sobel BE, Fujii S. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation 1996;93:106–10. [DOI] [PubMed] [Google Scholar]
- 93.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paranjpe I, Fuster V, Lala A, Russak A, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN. Association of Treatment Dose Anticoagulation with In-Hospital Survival Among Hospitalized Patients with COVID-19. J Am Coll Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardaway RM, Williams CH, Marvasti M, Farias M, Tseng A, Pinon I, Yanez D, Martinez M, Navar J. Prevention of adult respiratory distress syndrome with plasminogen activator in pigs. Crit Care Med 1990;18:1413–1418. [DOI] [PubMed] [Google Scholar]
- 96.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Moore HB, Barrett CD. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J Thromb Haemost 2020:jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brass LM, Lichtman JH, Wang Y, Gurwitz JH, Radford MJ, Krumholz HM. Intracranial hemorrhage associated with thrombolytic therapy for elderly patients with acute myocardial infarction: Results from the cooperative cardiovascular project. Stroke 2000;31:1802–1811. [DOI] [PubMed] [Google Scholar]
- 98.Chatterjee S, Weinberg I, Yeh RW, Chakraborty A, Sardar P, Weinberg MD, Kabrhel C, Barnes GD, Mukherjee D, Kumbhani D, Bashir R, Vaidya A, Smith A, Fuchs B, Groeneveld P, Giri J. Risk factors for intracranial haemorrhage in patients with pulmonary embolism treated with thrombolytic therapy development of the PE-CH score. Thromb Haemost 2017;117:246–251. [DOI] [PubMed] [Google Scholar]
- 99.Grimes Z, Bryce C, Sordillo EM, Gordon RE, Reidy J, Mondolfi AEP, Fowkes M. Fatal Pulmonary Thromboembolism in SARS-CoV-2-Infection. Cardiovasc Pathol 2020;48. [DOI] [PMC free article] [PubMed] [Google Scholar]