Abstract
Studies have demonstrated that diabetic (db/db) mice have increased susceptibility to myocardial ischemia–reperfusion (IR) injury and ventricular tachyarrhythmias (VA). We aimed to investigate the antiarrhythmic and molecular mechanisms of ranolazine in db/db mouse hearts with acute IR injury. Ranolazine was administered for 1 week before coronary artery ligation. Diabetic db/db and control db/+ mice were divided into ranolazine-given and -nongiven groups. IR model was created by 15-min left coronary artery ligation and 10-min reperfusion. In vivo electrophysiological studies showed that the severity of VA inducibility was higher in db/db mice than control (db/ +) mice. Ranolazine suppressed the VA inducibility and severity. Optical mapping studies in Langendorff-perfused hearts showed that ranolazine significantly shortened action potential duration, Cai transient duration, Cai decay time, ameliorated conduction inhomogeneity, and suppressed arrhythmogenic alternans induction. Western blotting studies showed that the expression of pThr17-phospholamban, calsequestrin 2 and voltage-gated sodium channel in the IR zone was significantly downregulated in db/db mice, which was ameliorated with ranolazine pretreatment and might play a role in the anti-arrhythmic actions of ranolazine in db/db mouse hearts with IR injury.
Subject terms: Experimental models of disease, Cardiovascular biology
Introduction
Studies have demonstrated that diabetic (db/db) mice have increased susceptibility to myocardial ischemia–reperfusion (IR) injury1,2, a longer duration of IR-induced ventricular tachycardia (VT) and more degeneration of VT into ventricular fibrillation (VF)3, and a greater mortality after IR compared with control (db/ +) mice4. However, the underlying electrophysiological and molecular mechanisms remain incompletely understood. Accumulation of intracellular Na+ occurs during IR5, and increased late sodium current (INa,L) has been linked to elevated intracellular Na+ during IR. Upon reperfusion of ischemic myocardium, the sudden availability of oxygen in the ischemic myocardium increases the formation of reactive oxygen species which are known to increase INa,L6, thereby worsening intracellular Na+ overload7. Subsequently, intracellular Ca2+ (Cai) overload occurs via reverse-mode Na+/Ca2+ exchanger (NCX), leading to cell damage, apoptosis, and lethal cardiac arrhythmias. In diabetic mice, it has been reported that phosphoinositide 3-kinase signaling is reduced, resulting in a higher INa,L in cardiomyocytes from db/db mice than in wild-type cardiomyocytes8. A higher intrinsic INa,L density would play a role in the increased susceptibility to IR arrhythmias in db/db mice. Ranolazine, a clinically used nonspecific blocker of INa,L9, was reported to reduce Ca2+ overload and oxidative stress, to improve mitochondrial integrity10, and to reduce ventricular tachyarrhythmia (VA) induced by IR injury11. In this study, we conducted simultaneous Cai and membrane voltage (Vm) optical mapping to investigate the arrhythmogenicity of db/db mice with acute IR injury and the antiarrhythmic mechanisms of ranolazine in these hearts. We also performed immunoblot studies to investigate the molecular remodeling in correlation with the electrophysiological remodeling by acute regional IR injury with or without ranolazine treatment.
Results
Ranolazine suppressed in vivo VA inducibility and severity in mouse hearts with acute regional IR injury
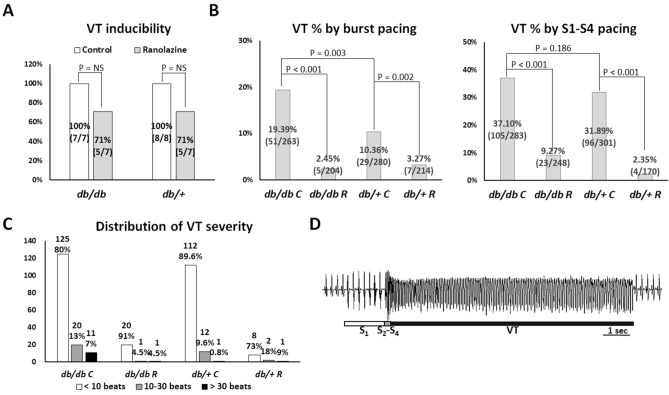
In the in vivo electrophysiological studies, we acquired data from 7, 7, 8, and 7 mice in the db/db C, db/db R, db/+ C, and db/+ R groups, respectively. The effective refractory period was significantly longer in the db/db C mice than in the db/db R, db/+ C, and db/+ R groups (74 ± 17 vs. 62 ± 18, 58 ± 18, and 60 ± 11 ms, respectively; P = 0.034). Figure 1 summarizes the result of VT inducibility and severity. VT was inducible in 7 of 7, 5 of 7, 8 of 8 and 5 of 7 mice in the db/db C, db/db R, db/+ C, and db/+ R groups, respectively (P = NS, Fig. 1A). But the percentage of VT-induced episodes by burst pacing protocol was higher in the db/db C group compared to the db/+ C group (P = 0.003). Pretreatment of ranolazine significantly reduced the percentage of VT episodes by both burst pacing and extrastimulus pacing protocols in both db/db and db/+ groups (Fig. 1B). The distribution of VT episodes shown in Fig. 1C suggests that db/db C mice were significantly more vulnerable to long VT (> 30 beats), which was 11 episodes induced in 5 of 7 db/db C mice (the longest 180 beats), 1 episode induced in 1 of 7 db/db R mice (the longest 50 beats), 1 episode induced in 1 of 8 db/+ C mice (the longest 72 beats), and 1 episode induced in 1 of 7 db/+ R mice (the longest 66 beats) hearts (P = 0.031). A representative example of pacing-induced long VT in a db/db C mouse heart is shown in Fig. 1D.
Figure 1.
In vivo electrophysiological study. (A) Summary of in vivo ventricular tachycardia (VT) inducibility. (B) Percentage of VT episodes by pacing protocols. The number and percentage of pacing-induced VT were shown in the middle of each bar. Pretreatment of ranolazine significantly reduced the inducibility ratio of VT episodes by both burst pacing and extrastimulus pacing protocols in db/db and db/+ groups. (C) Distribution of the severity of VT, plotted as the number of beats of VT. The number and percentage of VT episodes were shown on the top of each bar. The db/db C group had higher percentage of long VT (> 30 beats) than other groups (P = 0.031). (D) A representative example of pseudo-electrocardiogram showing extrastimulus pacing-induced VT in a db/db C mouse heart.
The electrophysiologic mechanisms of ranolazine in suppressing VA inducibility
Ranolazine shortened and reduced dispersion of APD80 and CaiTD80
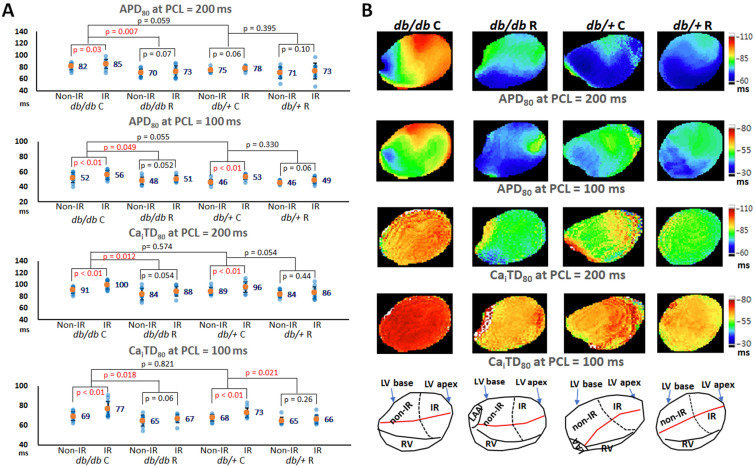
In the optical mapping studies, we acquired data from 11, 10, 11, and 10 mice in the db/db C, db/db R, db/+ C, and db/+ R groups, respectively. Figure 2A summarizes the results. The db/db C group tended to have a longer APD80 than the db/+ C group. APD80 in the db/db C group was significantly longer than that in the db/db R group, but there was no significant difference between the db/+ C and db/+ R groups. In addition, APD80 in the IR zone was significantly longer than that in the non-IR zone in the ranolazine non-given groups, but not in the ranolazine given groups (Table 1). The APD80 dispersion was significantly different among the four groups and the db/db C group had the largest APD80 dispersion: at PCL = 200 ms: 27 ± 8, 22 ± 7, 17 ± 5, 16 ± 3 ms in db/db C, db/+ C, db/db R, db/+ R groups, respectively (P = 0.002); at PCL = 100 ms: 17 ± 5, 17 ± 3, 13 ± 3, 13 ± 4 ms in db/db C, db/+ C, db/db R, db/+ R groups, respectively (P = 0.041). Similarly, CaiTD80 in the db/db C group was significantly longer than that in the db/db R group, and CaiTD80 in the IR zone was significantly longer than that in the non-IR zone in the ranolazine non-given groups, but not in the ranolazine given groups (Table 1). The difference of CaiTD80 dispersion was insignificant at PCL = 200 ms: 23 ± 7, 19 ± 7, 17 ± 8, and 16 ± 6 ms (P = 0.217); but was significant at PCL = 100 ms: 18 ± 7, 18 ± 7, 10 ± 5, and 11 ± 5 ms (P = 0.028) in the db/db C, db/+ C, db/db R, and db/+ R groups, respectively. These findings indicated that ranolazine shortened APD80 and CaiTD80, reduced the differences of APD80 and CaiTD80 between non-IR and IR zones, and attenuated the APD80 and CaiTD80 heterogeneity in both db/db and db/+ mouse hearts. Figure 2B shows representative examples of APD80 and CaiTD80 maps of the four groups. The db/db C mouse heart had the longest APD80 and CaiTD80, which were longer in the IR zone than in the non-IR zone.
Figure 2.
Effects of ranolazine therapy on APD80 and CaiTD80. (A) Scattered graphs of APD80 and CaiTD80 at pacing cycle length (PCL) of 200 ms and 100 ms. Orange dots and numbers indicate the mean values. Ranolazine significantly shortened APD80 and CaiTD80 in db/db mice. (B) Representative APD80 and CaiTD80 maps at pacing cycle length (PCL) = 200 and 100 ms.
Table 1.
Electrophysiological effects of ranolazine in isolated Langendorff-perfused mouse hearts after IR injury.
| APD80 (ms) | CaiTD80 (ms) | CV (cm/s) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 ms | 100 ms | 200 ms | 100 ms | 200 ms | 150 ms | 120 ms | 100 ms | 90 ms | 80 ms | 70 ms | 60 ms | |
| db/db C (N = 11) | ||||||||||||
| Non-IR | 82 ± 9* | 52 ± 7* | 91 ± 7* | 69 ± 5* | 78 ± 13* | 76 ± 13* | 74 ± 14* | 70 ± 13* | 64 ± 12* | 57 ± 13* | 51 ± 12* | 45 ± 12* |
| IR | 86 ± 9* | 56 ± 6* | 100 ± 9* | 77 ± 8* | 68 ± 18* | 61 ± 16* | 57 ± 15* | 54 ± 14* | 50 ± 12* | 47 ± 11* | 41 ± 10* | 37 ± 10* |
| db/db R (N = 10) | ||||||||||||
| Non-IR | 70 ± 7 | 48 ± 6 | 84 ± 11 | 65 ± 6 | 88 ± 5 | 84 ± 8 | 80 ± 9 | 75 ± 8 | 74 ± 11 | 72 ± 12 | 64 ± 12 | 62 ± 10 |
| IR | 73 ± 10 | 51 ± 6 | 89 ± 9 | 67 ± 5 | 82 ± 9 | 78 ± 8 | 76 ± 7 | 70 ± 6 | 67 ± 7 | 61 ± 6 | 58 ± 5 | 53 ± 6 |
| db/+ C (N = 11) | ||||||||||||
| Non-IR | 75 ± 4 | 46 ± 4* | 89 ± 7* | 68 ± 3* | 83 ± 15 | 78 ± 13* | 75 ± 10* | 70 ± 12* | 67 ± 13* | 60 ± 12* | 57 ± 13* | 52 ± 11* |
| IR | 78 ± 4 | 54 ± 4* | 96 ± 10* | 73 ± 6* | 69 ± 12 | 64 ± 13* | 59 ± 11* | 55 ± 9* | 51 ± 9* | 46 ± 8* | 43 ± 7* | 38 ± 7* |
| db/+ R (N = 10) | ||||||||||||
| Non-IR | 71 ± 10 | 46 ± 2 | 84 ± 6 | 65 ± 4 | 90 ± 25 | 89 ± 25 | 85 ± 27 | 83 ± 28 | 80 ± 25 | 75 ± 23 | 67 ± 19 | 61 ± 17 |
| IR | 73 ± 14 | 49 ± 5 | 86 ± 10 | 66 ± 5 | 86 ± 22 | 82 ± 21 | 80 ± 22 | 75 ± 24 | 72 ± 21 | 68 ± 22 | 64 ± 21 | 56 ± 16 |
Values are mean ± SD. APD80, action potential duration at 80% repolarization; CaiTD80, effective refractory period; CV, conduction velocity; IR, ischemia–reperfusion. * indicates P < 0.05 for non-IR vs. IR.
Ranolazine fastened Cai decay
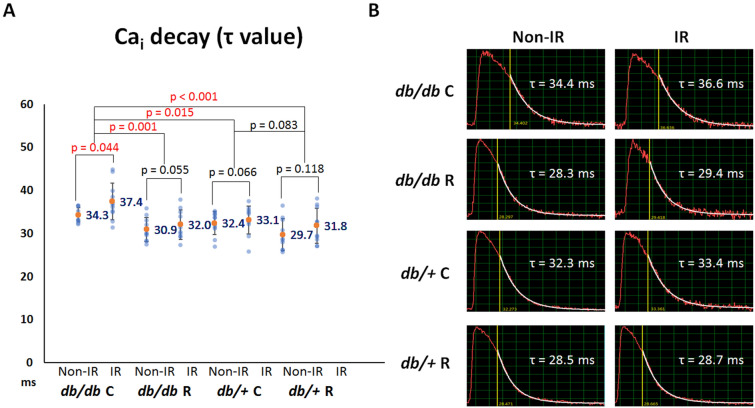
Dysregulation of Cai homeostasis plays a role in the development of IR-induced VA. Figure 3A summarizes the results of Cai decay tau value among the four groups. Cai decay time was the longest in the db/db C group among the four groups (P = 0.013). The post hoc analysis shows that ranolazine shortened the tau value significantly in db/db mouse hearts (from 35.8 ± 3.8 ms to 31.5 ± 3.4 ms; P = 0.001) but insignificant in db/+ mouse hearts (from 32.8 ± 3.5 ms to 30.8 ± 3.9 ms; P = 0.083). Furthermore, Cai decay time was longer in the IR zone than in the non-IR zone. Ranolazine ameliorated the differences in the tau values between the non-IR and IR zones in db/db mice. As shown in Fig. 3A, the P values were increased from 0.044 to 0.055 (db/db C vs. db/db R). But the differences in the tau values between the non-IR and IR zones were insignificant in the db/+ C and db/+ R groups (P = 0.066 and 0.118, respectively). A representative example of Cai decay at the non-IR and IR zones in the four groups is shown in Fig. 3B.
Figure 3.
Effects of ranolazine therapy on intracellular Ca2+ (Cai) decay. (A) Scattered graphs of Cai decay tau values among the four groups and between the ischemia–reperfusion (IR) and non-IR zones. Orange dots and numbers indicate the mean values. Ranolazine therapy shortened the tau value in db/db mice and ameliorated the differences of tau values between the non-IR and IR zones in db/db mice. (B) Representative examples of Cai decay in the non-IR and IR zones among the four groups.
Ranolazine ameliorated conduction inhomogeneity
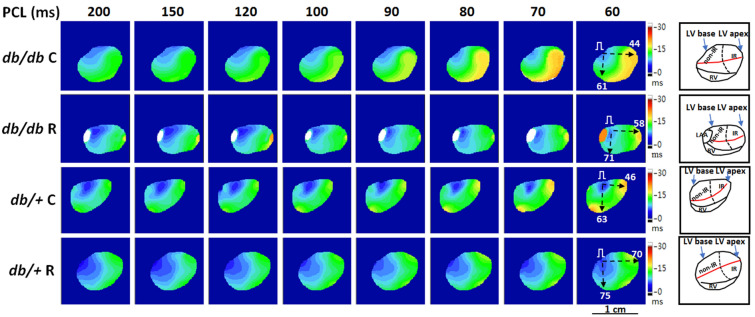
Table 1 summarizes the effects of 1-week ranolazine pretreatment on CV in mouse hearts with acute regional IR injury. The difference between CVIR and CVnon-IR was significant in the db/db C and db/+ C groups, but was insignificant in the db/db R and db/+ R groups, suggesting that ranolazine ameliorated CVIR slowing to ameliorate conduction inhomogeneity. Figure 4 shows an example of isochrone maps in the four groups. At PCL = 60 ms, the CVIR was slower in the db/db C (44 cm/s) and db/+ C (46 cm/s) mice than in the db/db R (58 cm/s) and db/+ R (70 cm/s) mice; and the difference between CVIR and CVnon-IR was also greater in the db/db C (17 cm/s) and db/+ C (17 cm/s) mice than in the db/db R (13 cm/s) and db/+ R (5 cm/s) mice.
Figure 4.
Representative examples of isochrone maps at a pacing cycle length (PCL) from 200 to 60 ms in four groups. Black dashed arrows indicate the directions of CVnon-IR (conduction velocity (CV) along the atrioventricular ring) and CVIR (CV pointing to the apical ischemia–reperfusion zone) measurements; numbers in right subpanels are CV (cm/s). Right subpanels show the anatomical structure and orientation of optical maps. Ranolazine therapy ameliorated CVIR slowing to ameliorate conduction inhomogeneity (see Table 1). Red line, left coronary artery; dashed line, margin of IR zone; LAA, left atrial appendage; LV, left ventricle; RV, right ventricle.
Ranolazine suppressed induction of spatially concordant and discordant alternans
Alternans represents a phenomenon of electrophysiological instability which may lead to conduction block, re-entry, and tachyarrhythmias. Although spatially concordant alternans (SCA) could be provoked in all hearts in the four groups, the longest PCL required to provoke SCA was significantly shorter in the ranolazine groups (107 ± 15, 86 ± 5 ms in the db/db C, db/db R groups, respectively; 102 ± 15, 79 ± 17 ms in the db/+ C, db/+ R groups, respectively; P < 0.001). Similarly, spatially discordant alternans (SDA) could be provoked in all hearts, and the longest PCL required to provoke SDA was significantly shorter in the ranolazine groups (90 ± 12, 69 ± 11 ms in the db/db C, db/db R groups, respectively; 80 ± 14, 67 ± 9 ms in the db/+ C, db/+ R groups, respectively; P < 0.001). A representative example was shown in Supplementary Fig. S1.
Ranolazine suppressed VA inducibility
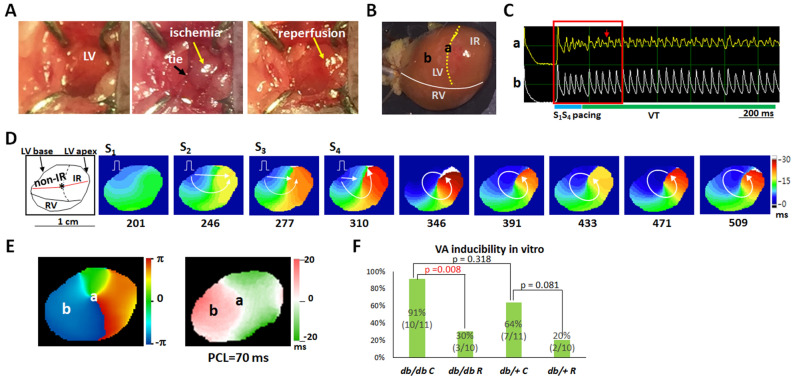
The db/db C group had the highest VA inducibility among the four groups: VA was induced in 10 of 11 (91%, db/db C), 3 of 10 (30%, db/db R), 7 of 11 (64%, db/+ C) and 2 of 10 (20%, db/+ R) hearts (P = 0.004). The VA inducibility was significantly different between the db/db C and db/db R groups (P = 0.008) but insignificantly different between the db/+ C and db/+ R groups (P = 0.081). Figure 5 illustrates VT induction in a db/db C mouse heart. Figure 5A,B show images of IR creation and the mapping field, respectively. Figure 5C shows the Vm recordings at sites “a” (rotor anchoring site on a nodal line, Fig. 5E) and “b” (left ventricular base) during VT induction. Extrastimulus pacing led to dispersion of refractoriness and unidirectional conduction block (frame 310; Fig. 5D), and reentrant wavefronts were initiated after pacing (frames 346–509). During the initiation of VT, the core of reentrant wavefronts anchored at site “a,” where fragmented Vm transient is shown (Fig. 5C). Post hoc analysis revealed that ranolazine effectively suppressed the VA inducibility in both db/db and db/+ mouse hearts with acute regional IR injury (Fig. 5F).
Figure 5.
Mechanisms of ventricular tachycardia (VT) induction in a db/db C mouse heart with ischemia–reperfusion (IR) injury. (A) IR creation. Ischemia zone distal to the tie is shown in gray, and recovered after removal of the ligature in red. (B) Mapping field. Yellow dotted line indicates the margin of the IR zone. LV, left ventricle; RV, right ventricle. (C) Membrane voltage (Vm) traces showing the initiation of VT by S1-S4 pacing. Red arrow indicates fragmented Vm transient during rotor anchoring at site “a”. (D) Isochrone maps corresponding to the period marked by a red square in C. The number below each frame is the time (ms) with the onset of data acquisition as time zero. White arrows indicate the directions of wavefront propagation. Left subpanel shows the anatomical structure of the mapping filed. Red line, left coronary artery; dashed line, margin of IR zone. (E) Phase singularity (left) and Vm alternans (right) maps. A phase singularity (site “a”) was formed on a nodal line during VT. (F) Summary of the VA inducibility result.
Alteration of protein expression after acute regional IR injury
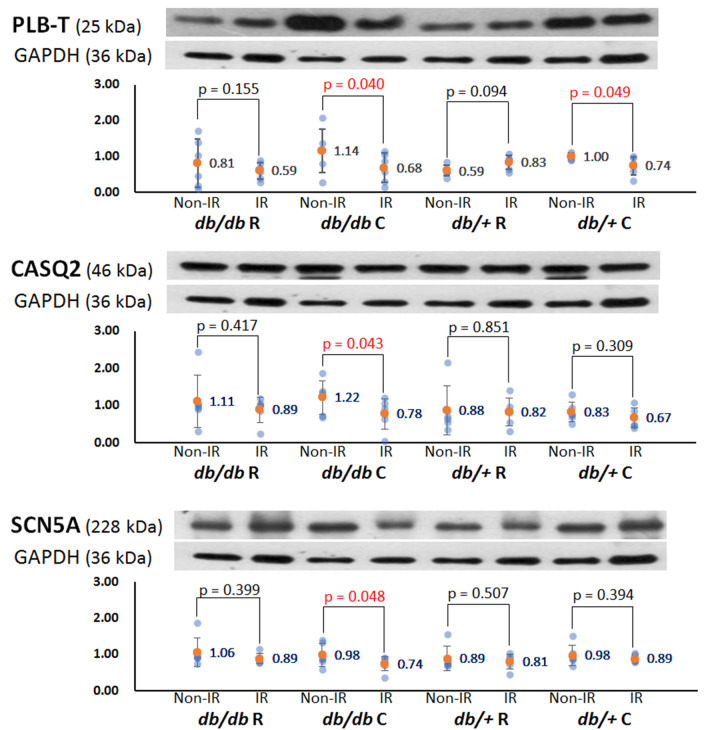
To elucidate the roles of Ca2+-handling proteins, Na+ channel, and Cx43 in the antiarrhythmic mechanisms of ranolazine, we measured and compared the levels of the associated proteins between the IR and non-IR zones. The results are presented in Fig. 6 and supplementary Fig. S2, and all analyzed proteins with fuller-length blots are shown in Supplementary Fig. S3. In db/db C hearts, the expression levels of pThr17-phospholamban, calsequestrin 2, and voltage-gated sodium channel (SCN5A) in the IR zone were significantly lower than those in the non-IR zone. Ranolazine pretreatment attenuated the downregulation of these proteins in the IR zone by acute IR injury. In db/+ C hearts, the expression level of pThr17-phospholamban was significantly lower than that in the non-IR zone, which was attenuated by ranolazine.
Figure 6.
Representative examples of the Western blots result of pThr17-phospholamban (PLB-T), calsequestrin 2 (CASQ2) and voltage-gated sodium channel (SCN5A). Scattered graphs represent densitometric values normalized to the corresponding GAPDH. Orange dots and numbers indicated the mean values.
Ranolazine effects on INa,L in cardiomyocytes from db/db and db/+ mice with acute regional IR injury
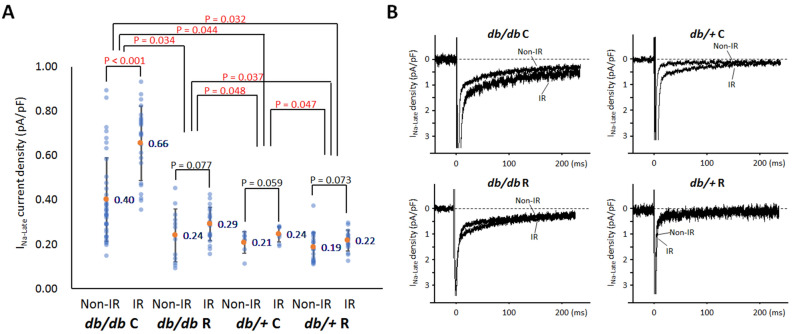
The db/db cardiomyocytes expressed a greater INa,L density (0.420 ± 0.214 pA/pF, n = 93) than the db/+ cells (0.209 ± 0.056 pA/pF, n = 51, P < 0.001). As shown in Fig. 7A, ranolazine therapy significantly decreased the density of INa,L in both db/db mice (0.497 ± 0.219 pA/pF, n = 61 in db/db C vs. 0.272 ± 0.096 pA/pF, n = 32 in db/db R, P = 0.034) and db/+ mice (0.229 ± 0.044 pA/pF, n = 15 in db/+ C vs. 0.201 ± 0.059 pA/pF, n = 36 in db/+ R, P = 0.047), but the density of INa,L in db/db R group was still higher than those in db/+ C (P = 0.048) and db/+ R (P = 0.037) groups. There was significant difference of INa,L density between IR and non-IR cardiomyocytes in the db/db C group (0.655 ± 0.168 pA/pF, n = 23 vs 0.402 ± 0.189 pA/pF, n = 38, P < 0.001), but not in the db/db R group (0.291 ± 0.076 pA/pF, n = 20 vs 0.240 ± 0.120 pA/pF, n = 12, P = 0.077). Representative INa,L recordings were shown in Fig. 7B.
Figure 7.
Whole-cell late Na+ currents (INa,L) recording. (A) Scattered graphs of mean INa,L. The graph shows the comparisons of the current density among four groups and between the ischemia–reperfusion (IR) and non-IR zones. Orange dots and numbers indicated the mean values. Student’s t-test for comparisons between the IR and non-IR zones and one-way repeated measures analysis of variance with post hoc least significant difference analysis for comparisons among the four groups. The db/db cardiomyocytes expressed a greater INa,L density than the db/+ cells. Ranolazine therapy significantly decreased the density of INa,L in both db/db mice and db/+ mice. (B) Representative INa,L traces of the cardiomyocytes among the four groups.
Discussion
Previously Ogawa et al. showed that acute ranolazine perfusion facilitated the termination of ischemic VT/VF by the suppression of INa,L-dependent focal arrhythmogenic activity in isolated rabbit hearts12. Dhalla et al. reported that ranolazine markedly reduced IR-induced VAs possibly via its INa,L inhibitor property to reduce afterdepolarizations11. In this study our optical mapping results showed that 1-week ranolazine pretreatment significantly reduced VA inducibility via amelioration of IR injury-induced conduction inhomogeneity and impaired Cai decay, reduction of APD80 and CaiTD80 prolongation and dispersion, and suppression of arrhythmogenic alternans induction. The in vivo electrophysiological studies show that the db/db C group was more vulnerable to long VT compared with the other three groups, suggesting that ranolazine pretreatment is effective in protecting db/db mice from IR-induced life-threatening VA. Western blotting showed that protein expression of pThr17-phospholamban, calsequestrin 2, and SCN5A was significantly decreased in the IR zone in diabetic mouse hearts, and ranolazine ameliorated the downregulation of these proteins. These molecular mechanisms may play a role in the antiarrhythmic actions of ranolazine in diabetic mouse hearts with regional IR injury. The whole-cell patch clamp study further confirmed that db/db cardiomyocytes expressed a greater INa,L density, which was significantly higher in the IR zone than the non-IR zone. Ranolazine significantly decreased the density of INa,L and reduced the difference of INa,L density between the IR and non-IR zones in db/db mouse hearts.
Ranolazine administration improves Cai dynamics in the IR zone
During IR injury, Cai overload can result from the impaired ability of sarcoendoplasmic reticular Ca2+-ATPase (SERCA2a) to sequester cytosolic Ca2+ in stunned myocardium13, and from the enhanced INa,L to increase Na+ influx, and via reverse-mode NCX, to increase Cai9. INa,L enhancement may also result in calcium/calmodulin protein kinase II (CaMKII) activation, which may induce proarrhythmic sarcoplasmic reticulum (SR) Ca2+ leak14. It has been reported that mitochondrial Ca2+ uptake, binding of the L-type Ca2+ channel to the sarcolemma, and Ca2+ intake by SR are decreased in diabetic hearts15. In conjunction with an intrinsic higher INa,L, cellular dysregulation of Ca2+ homeostasis would be more pronounced in post-IR myocardial dysfunction and arrhythmogenicity in db/db mouse hearts.
Blockade of INa,L may reverse the impaired Ca2+ cycling in conditions of increased INa,L. It has been shown that enhancement of INa,L increases the vulnerability to Cai alternans during rapid pacing16. Fukaya et al. reported that ranolazine reduces diastolic Cai and mitigates cardiac alternans, representing a mechanism underlying the antiarrhythmic benefit of INa,L blockade in heart failure17. Consistently, our data showed that ranolazine suppressed the induction of SCA and SDA in mouse hearts with IR injury.
In addition to INa,L blockade to ameliorate Cai overload, the presented data reveal some possible molecular mechanisms underlying the antiarrhythmic effects of ranolazine in the IR zone of diabetic hearts. Phospholamban is a key phosphorylation-dependent modulator of SERCA2a activity, and phospholamban dephosphorylation has been reported to account for myocardial stunning18. Our data show that ranolazine attenuated the downregulation of pThr17-phospholamban in the IR zone, which played a role in accelerating Cai decay and shortening CaiTD80. The Cai alternans suppression and CaiTD80 shortening were reported to reduce the susceptibility to subsequent refibrillation in a long-standing VF rabbit model19. Additionally, calsequestrin 2 is the main Ca2+-binding protein of the SR, serving as an important regulator of Ca2+ to protect the heart against premature Ca2+ release and triggered arrhythmias20. Downregulation of calsequestrin 2 increases SR Ca2+ leak and arrhythmia susceptibility under stress21. Our data show decreased expression of calsequestrin 2 in the IR zone of diabetic hearts, which may partly account for the increased VA inducibility in the db/db C group. Parikh et al. reported that ranolazine stabilizes cardiac ryanodine receptors to inhibit Cai oscillations and early afterdepolarizations22. The amelioration of calsequestrin 2 downregulation in the IR zone by ranolazine may contribute to the reduced VA inducibility in ranolazine-pretreated db/db mice.
It was reported that cardiac IR injury is accompanied by a marked reduction in SR Ca2+-pump ATPase, Ca2+-uptake and Ca2+-release activities, and the mRNA levels for SR Ca2+-handling proteins such as SERCA2a, ryanodine receptor, calsequestrin and phospholamban were decreased in the ischemia-reperfused heart as compared with the non-ischemic control23. Our data also shows that protein expression of pThr17-phospholamban, calsequestrin 2, and SCN5A was significantly decreased in the IR zone in diabetic mouse hearts, and ranolazine ameliorated the downregulation of these proteins. We do not know the exact mechanisms. Upon reperfusion of ischemic myocardium, the sudden availability of oxygen in the ischemic myocardium increases the formation of reactive oxygen species and intracellular Ca2+ overload, which cause cell damage and apoptosis. Because ranolazine was reported to reduce oxidative stress and Ca2+ overload, and to improve mitochondrial integrity during IR10, these actions may underlie the mechanism of ameliorating IR injury, including the downregulation of pThr17-phospholamban, calsequestrin 2, and SCN5A by ranolazine pretreatment.
Ranolazine administration ameliorates conduction inhomogeneity in regional IR injury
Studies have shown reduced cardiac conduction reserve in diabetic animal models24–26. Therefore, propagation of activity through the myocardium in diabetic hearts is more sensitive to conditions influencing cellular excitability or intercellular electrical coupling. For example, more pronounced activation of Ca2+-independent phospholipase A2 in response to acute ischemia was reported to contribute to arrhythmogenic conduction slowing in the diabetic rat heart27. In the regional IR model, the elevated Cai in the IR myocardium may prolong refractoriness by stimulating NCX current and thereby prolong APD28, which interferes with wavefront propagation. The effect of ranolazine on APD depends on the relative contributions of INa,L and rapidly activating delayed rectifier potassium current to repolarization9. Ranolazine abbreviates APD and thereby refractoriness in conditions when INa,L is enhanced. Our data show that ranolazine shortened APD80, especially in the IR zone, which may conjoin with the attenuated downregulation of SCN5A in the IR zone to improve CVIR in db/db mouse hearts. In addition, ranolazine, by shifting myocardial utilization of fatty acid to glucose during reperfusion, reduces deleterious lipid metabolites29. These lipid metabolites have been shown to cause uncoupling of gap junctions30. It is possible that ranolazine improves CVIR via its beneficial effects on myocardial metabolism.
Methods
This study protocol was approved by the Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital (approval no. 2015092401) and conformed to the current NIH guidelines for the care and use of laboratory animals. The C57BL/KsJ strain was obtained from Jackson Laboratories (Bar Harbor, ME, USA) and grew in Taiwan. The mice were divided into four groups: diabetic mice not given ranolazine (db/db C, n = 22, 12 female, age 23.7 ± 3.6 weeks, body weight 55.0 ± 7.8 g), diabetic mice given ranolazine (db/db R, n = 21, 11 female, age 23.7 ± 5.5 weeks, body weight 59.6 ± 12.0 g), control mice not given ranolazine (db/+ C, n = 23, 11 female, age 23.2 ± 3.5 weeks, body weight 30.1 ± 3.9 g), and control mice given ranolazine (db/+ R, n = 21, 10 female, age 24.7 ± 2.9 weeks, body weight 31.9 ± 4.2 g). Ranolazine (R6152; Sigma-Aldrich, Munich, Germany) was administered orally at 305 mg/kg/d (dose comparable with that used clinically in humans of 750 mg twice daily)31 for 7 days.
In-vivo IR model creation and electrophysiological studies
Mice were anesthetized with intra-peritoneal injection of xylazine (10 mg/kg) and zoletil (25 mg/kg). After mice appeared fully unconscious, endotracheal intubation was performed for gas general anesthesia with isoflurane (1%). Regional myocardial ischemia was induced by left coronary artery ligation at the midway between the atrio-ventricular junction and the apex. Ischemia was confirmed by the appearance of hypokinesis and pallor distal to the occlusion. After 15 min of ischemia, the ligature was removed, and reperfusion was visually confirmed (Fig. 5A).
In vivo electrophysiological study was performed after reperfusion for 10 min24. We first measured effective refractory period by giving a premature stimulus after 8 beats of S1S1 pacing at a pacing cycle length (PCL) of 200 ms. Extrastimulus pacing (S1-S4) and burst pacing (PCL = 50 ms, 2 s) were used to test VT (≥ three consecutive premature ventricular beats) inducibility to all mice. The severity of inducible VT was classified as < 10 beats, between 10 to 30 beats, and > 30 beats32.
Western blotting
Cardiac tissues were sampled from the non-IR and IR zones of the left ventricle at the end of in vivo electrophysiological studies for protein quantification as previously described (n = 6 per group)33. See the online supplement for detailed descriptions.
Langendorff heart preparation and optical mapping studies
Details of the experimental procedure for dual optical mapping of Vm and Cai transients have been described previously34. Briefly, the hearts were excised after reperfusion for 10 min and then subjected to Langendorff-perfusion with Rhod-2AM (Cai indicator), RH237 (Vm indicator) and 15 μM blebbistatin (Tocris Bioscience, MN, USA). Epifluorescence was acquired simultaneously using two high-speed cameras (MiCAM Ultima; BrainVision, Tokyo, Japan) at 1 ms/frame. Action potential duration APD80 (APD at 80% repolarization) and Cai alternans were induced and conduction velocity (CV) were studied by a dynamic pacing protocol. VA inducibility was defined as the ability to provoke VT/VF with the dynamic pacing protocol and/or programmed extra stimuli (up to S4).
Cardiomyocyte isolation and whole-cell patch clamp
Cardiomyocytes from the non-IR and IR zones of the left ventricle were isolated using a modified enzymatic digestion protocol (n = 4 per group)33. Whole-cell mode of the patch-clamp technique was used to measure INa as described previously35. See the online supplement for detailed descriptions.
Data analysis
APD80 and Cai transient duration at 80% decay (CaiTD80) were measured at two PCLs of 200 and 100 ms24. The differences between the longest and shortest APD80 and CaiTD80 were used to represent APD80 and CaiTD80 dispersion. To estimate CV, we measured the distance and conduction time between the earliest activation point and two epicardial points: one was from the pacing site to the left ventricular apex (CVIR), and the other was along an axis parallel to the atrioventricular ring (CVnon-IR)36.
Statistics
Continuous variables are expressed as mean ± standard deviation and categorical variables are represented by numbers and percentages. One-way analysis of variance (ANOVA) with post hoc least significant difference analysis was performed to calculate statistical significance of differences in continuous variables among four groups. Student’s t-test was performed to compare continuous variables between the non-IR and IR zones. Categorical variables were tested using Fisher’s exact test. Differences were considered significant at P < 0.05.
Ethics approval
The present study was approved by Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital (Reference number: 2015092401).
Supplementary information
Acknowledgements
This study was supported by a Grant from Chang Gung Medical Research Program, Taiwan, CMRPG3F035 to C.C. Chou. The present study was approved by Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital (Reference Number: 2015092401).
Author contributions
C.-C.C. performed optical mapping experiments, interpreted mapping data and was a major contributor in writing the manuscript. H.-L.L. assisted in animal model creation and helped analyzed mapping data. G.-J.C. helped performing patch-clamp experiments. H.-T.W., T.-H.Y., and H.-T.L. were engaged in Western blot experiments. M.-S.W. and Y.C. supervised Western blot experiments and interpreted data. P.-C.C. helped performing all parts of experiments and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-77014-0.
References
- 1.Jones SP, Girod WG, Granger DN, Palazzo AJ, Lefer DJ. Reperfusion injury is not affected by blockade of P-selectin in the diabetic mouse heart. Am. J. Physiol. Heart Circul. Physiol. 1999;277:H763–H769. doi: 10.1152/ajpheart.1999.277.2.H763. [DOI] [PubMed] [Google Scholar]
- 2.Lefer DJ, et al. HMG-CoA reductase inhibition protects the diabetic myocardium from ischemia-reperfusion injury. FASEB J. 2001;15:1454–1456. doi: 10.1096/fj.00-0819fje. [DOI] [PubMed] [Google Scholar]
- 3.Anzawa R, et al. Intracellular sodium increase and susceptibility to ischaemia in hearts from type 2 diabetic db/db mice. Diabetologia. 2006;49:598–606. doi: 10.1007/s00125-005-0091-5. [DOI] [PubMed] [Google Scholar]
- 4.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H146–153. doi: 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- 5.Tani, M. & Neely, J. R. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+–Na+ and Na+–Ca2+ exchange. Circul. Res.65, 1045–1056 (1989). [DOI] [PubMed]
- 6.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J. Physiol. 1997;500:631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma, J. H., Luo, A. T. & Zhang, P. H. Effect of hydrogen peroxide on persistent sodium current in guinea pig ventricular myocytes. Acta Pharmacol. Sin.26, 828–834 (2005). [DOI] [PubMed]
- 8.Lu Z, et al. Increased persistent sodium current due to decreased PI3K signaling contributes to QT prolongation in the diabetic heart. Diabetes. 2013;62:4257–4265. doi: 10.2337/db13-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antzelevitch C, Burashnikov A, Sicouri S, Belardinelli L. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8:1281–1290. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldakkak M, Camara AK, Heisner JS, Yang M, Stowe DF. Ranolazine reduces Ca2+ overload and oxidative stress and improves mitochondrial integrity to protect against ischemia reperfusion injury in isolated hearts. Pharmacol. Res. 2011;64:381–392. doi: 10.1016/j.phrs.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhalla AK, et al. Ranolazine, an antianginal agent, markedly reduces ventricular arrhythmias induced by ischemia and ischemia-reperfusion. Am. J. Physiol. Heart Circul. Physiol. 2009;297:H1923–H1929. doi: 10.1152/ajpheart.00173.2009. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa, T. et al. Ranolazine facilitates termination of ventricular tachyarrhythmia associated with acute myocardial ischemia through suppression of late INa-mediated focal activity. Circul. J., CJ-17–0128 (2017). [DOI] [PubMed]
- 13.Limbruno U, et al. Sarcoplasmic reticulum function in the “stunned” myocardium. J. Mol. Cell. Cardiol. 1989;21:1063–1072. doi: 10.1016/0022-2828(89)90804-3. [DOI] [PubMed] [Google Scholar]
- 14.Sag CM, et al. Enhanced late INa induces proarrhythmogenic SR Ca leak in a CaMKII-dependent manner. J. Mol. Cell. Cardiol. 2014;76:94–105. doi: 10.1016/j.yjmcc.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Pereira L, et al. Calcium signaling in diabetic cardiomyocytes. Cell Calcium. 2014;56:372–380. doi: 10.1016/j.ceca.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Wasserstrom JA, et al. Ranolazine antagonizes the effects of increased late sodium current on intracellular calcium cycling in rat isolated intact heart. J. Pharmacol. Exp. Ther. 2009;331:382–391. doi: 10.1124/jpet.109.156471. [DOI] [PubMed] [Google Scholar]
- 17.Fukaya H, et al. Arrhythmogenic cardiac alternans in heart failure is suppressed by late sodium current blockade by ranolazine. Heart rhythm. 2019;16:281–289. doi: 10.1016/j.hrthm.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Kim S-J, et al. A novel mechanism for myocardial stunning involving impaired Ca2+ handling. Circ. Res. 2001;89:831–837. doi: 10.1161/hh2101.098547. [DOI] [PubMed] [Google Scholar]
- 19.Azam, M. A. et al. Effects of late sodium current blockade on ventricular refibrillation in a rabbit model. Circul. Arrhythmia Electrophysiol.10, e004331 (2017). [DOI] [PubMed]
- 20.Novák P, Soukup T. Calsequestrin distribution, structure and function, its role in normal and pathological situations and the effect of thyroid hormones. Physiol. Res. 2011;60:439. doi: 10.33549/physiolres.931989. [DOI] [PubMed] [Google Scholar]
- 21.Chopra N, et al. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ. Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- 22.Parikh A, et al. Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm. 2012;9:953–960. doi: 10.1016/j.hrthm.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeo S, et al. Role of cardiac renin-angiotensin system in sarcoplasmic reticulum function and gene expression in the ischemic-reperfused heart. Mol. Cell. Biochem. 2000;212:227–235. doi: 10.1023/A:1007174803993. [DOI] [PubMed] [Google Scholar]
- 24.Chou CC, et al. Roles of impaired intracellular calcium cycling in arrhythmogenicity of diabetic mouse model. Pacing Clin. Electrophysiol. 2017;40:1087–1095. doi: 10.1111/pace.13166. [DOI] [PubMed] [Google Scholar]
- 25.Nygren A, et al. Propagation of the cardiac impulse in the diabetic rat heart: reduced conduction reserve. J. Physiol. 2007;580:543–560. doi: 10.1113/jphysiol.2006.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen KB, et al. Myocardial impulse propagation is impaired in right ventricular tissue of Zucker diabetic fatty (ZDF) rats. Cardiovasc. Diabetol. 2013;12:19. doi: 10.1186/1475-2840-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahnema P, Shimoni Y, Nygren A. Reduced conduction reserve in the diabetic rat heart: role of iPLA2 activation in the response to ischemia. Am. J. Physiol. Heart Circul. Physiol. 2010;300:H326–H334. doi: 10.1152/ajpheart.00743.2010. [DOI] [PubMed] [Google Scholar]
- 28.Shiferaw Y, Watanabe M, Garfinkel A, Weiss J, Karma A. Model of intracellular calcium cycling in ventricular myocytes. Biophys. J . 2003;85:3666–3686. doi: 10.1016/S0006-3495(03)74784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996;93:135–142. doi: 10.1161/01.CIR.93.1.135. [DOI] [PubMed] [Google Scholar]
- 30.Arnsdorf MF, Sawicki GJ. The effects of lysophosphatidylcholine, a toxic metabolite of ischemia, on the components of cardiac excitability in sheep Purkinje fibers. Circ. Res. 1981;49:16–30. doi: 10.1161/01.RES.49.1.16. [DOI] [PubMed] [Google Scholar]
- 31.Tocchetti CG, et al. Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. Eur. J. Heart Fail. 2014;16:358–366. doi: 10.1002/ejhf.50. [DOI] [PubMed] [Google Scholar]
- 32.Rajab M, et al. Increased inducibility of ventricular tachycardia and decreased heart rate variability in a mouse model for type 1 diabetes: effect of pravastatin. Am. J. Physiol. Heart Circul. Physiol. 2013;305:H1807–H1816. doi: 10.1152/ajpheart.00979.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang PC, et al. Inhomogeneous down-regulation of INa underlies piceatannol pro-arrhythmic mechanism in regional ischemia-reperfusion. Pacing Clin. Electrophysiol. 2018;41:1116–1122. doi: 10.1111/pace.13424. [DOI] [PubMed] [Google Scholar]
- 34.Chou CC, Chang PC, Wei YC, Lee KY. Optical mapping approaches on muscleblind-like compound knockout mice for understanding mechanistic insights into ventricular arrhythmias in myotonic dystrophy. J. Am. Heart Assoc. 2017;6:e005191. doi: 10.1161/JAHA.116.005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang G-J, Chang C-J, Chen W-J, Yeh Y-H, Lee H-Y. Electrophysiological and mechanical effects of caffeic acid phenethyl ester, a novel cardioprotective agent with antiarrhythmic activity, in guinea-pig heart. Eur. J. Pharmacol. 2013;702:194–207. doi: 10.1016/j.ejphar.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Chou C-C, et al. Piceatannol facilitates conduction block and ventricular fibrillation induction in ischemia-reperfused rabbit hearts with pacing-induced heart failure. Int. J. Cardiol. 2014;171:250–258. doi: 10.1016/j.ijcard.2013.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.