Summary
As a key precursor for nitrogenous compounds and fertilizer, ammonia affects our lives in numerous ways. Rapid and sensitive detection of ammonia is essential, both in environmental monitoring and in process control for industrial production. Here we report a novel and nonperturbative method that allows rapid detection of ammonia at low concentrations, based on the all-optical detection of surface-enhanced Raman signals. We show that this simple and affordable approach enables ammonia probing at selected regions of interest with high spatial resolution, making in situ and operando observations possible.
Subject Areas: Chemistry, Chemical Engineering, Analytical Chemistry, Electrochemistry
Graphical Abstract

Highlights
-
•
Novel method for detection of ammonia at concentrations below 1 ppm in just under 1 s
-
•
This approach allows local detection of ammonia amounts as low as 104–105 molecules
-
•
Method for sensitive direct monitoring of catalytic/electrocatalytic processes
-
•
The method allows following the dynamics of ammonia concentration change in real time
Chemistry; Chemical Engineering; Analytical Chemistry; Electrochemistry
Introduction
Ammonia is key to many chemical technologies that drive world economies (Haber, 1905; Bosch, 1908), being the major source for fertilizers for agriculture/food production (Ampuero and Bosset, 2003) and directly used in multitude of industrial processes (Chen et al., 2018). Ammonia analyses are critical for water quality control (Baird and Bridgewater, 1995), exhaust gas sensing (Docquier and Candel, 2002; Riegel et al., 2002), monitoring and process control in industries of new chemicals, plastics, and pharmaceutical. It is also critical in detection of combustibles (Kohl, 2001), detonation, and propulsion processes. Moreover, it is at heart of our quest to find alternative routes of nitrogen fixation mechanisms for large-scale ammonia generation that are more efficient, energy saving, and carbon dioxide footprint free (Andersen et al., 2019; Tang and Qiao, 2019; Qing et al., 2020; Cui et al., 2018). In all of these processes, rapid and sensitive detection of ammonia is critical, thus driving a need for reliable ammonia sensing technologies. Current detection concepts are based on various “wet chemistry” processes and devices—electrochemical sensor devices (Chang et al., 1993; Lundström et al., 1993; Timmer et al., 2005), metal oxides (Clifford and Tuma, 1982; Hübner and Drost, 1991; Imawan et al., 2000; Srivastava et al., 1994; Wang et al., 2000; Xu et al., 2000; Yamazoe, 1991; Zakrzewska, 2001), catalytic polymer (Cai et al., 2001; Chabukswar et al., 2001; Heiduschka et al., 1997; Kukla et al., 1996; Lähdesmäki et al., 1996, 2000; Nicolas-Debarnot and Poncin-Epaillard, 2003; Palmqvist et al., 1995), inkjet printing films (Lv et al., 2019), polymer hybrid framework (Ahmadi Tabr et al., 2019). Spectroscopic methods for ammonia detection include nuclear magnetic resonance (NMR) (Nielander et al., 2019; Andersen et al., 2019) and optical spectroscopy in almost every part of electro-magnetic spectrum, ranging from the UV (Mount et al., 2002) to the far IR branch (Giovannozzi et al., 2015; Max and Chapados, 2013; Patton and Crouch, 1977; Tzollas et al., 2010; Li and Keppler, 2014; Ujike and Tominaga, 2002), optoelectronic (Zilberman et al., 2014; Mahendran and Philip, 2013) and specially designed fiber sensors (Guo and Tao, 2007).
Among the full arsenal of ammonia detection methods (Timmer et al., 2005), spectroscopic approaches are of particular interest as they provide direct information on chemical content and unambiguously identify the presence of the target molecular compound even at low concentrations. Traditionally, NMR spectroscopy has been the method of choice for sensitive ammonia detection (Nielander et al., 2019; Andersen et al., 2019). NMR provides chemical selectivity and high sensitivity down to 3 μM of the target and is rightfully among the most trusted approaches. However, complex instrumentation and large required sampling volumes (>0.5 mL) constitute serious limitations. More importantly, sensitive to contamination, the NMR approach relies on using isotope labeling, which renders even routine experiments very expensive and limits applications mainly to fundamental research in laboratory environments.
At present, the most popular and widely adopted optical methods are based on colorimetric approaches and their experimental derivatives (Searle, 1984). A popular solution employs the Berthelot reaction between ammonia, chlorine, and phenolic compounds, resulting in blue coloration of an indophenol dye that can be easily detected by conventional spectrometers (Patton and Crouch, 1977). This approach allows ammonia detection with an impressive sensitivity, down to several tens of ppb (parts per billion) (Bolleter et al., 1961; Patton and Crouch, 1977; Tzollas et al., 2010; Zhao et al., 2019). Although considered as the “gold standard,” the blue indophenol method requires time-consuming sample handling. An aliquot of >1 mL has to be collected from the chemical reactor and mixed with toxic, sample-altering reagents, followed by tens of minutes of aging prior to spectroscopic analysis.
In this context, the availability of a detection method for ammonia and its derivatives that is robust, affordable, and universally applicable would be highly desirable. We envision that simplicity, speed, sensitivity, affordability, and reliability are five crucial ingredients to advance ammonia sensing and making it more accessible for both laboratory and field use. Although it is not a simple task to compare radically different detection concepts in terms of a single set of consistent metrics, we would like to briefly touch on a few associated aspects. Existing spectroscopic methods do not allow tracking of small changes in the concentration of ammonia and intermediate species on-site, not to mention in situ or operando observation in situations where the reaction mechanisms are not known. Most spectroscopic techniques and sensor approaches are directly or indirectly vulnerable to external laboratory or field environment, jeopardizing reliability. Although NMR and colorimetric methods excel in detection limits for overall concentration, it is important to remember that both approaches rely on the need for relatively large sensing volumes. For instance, strong signals are observed for a 1 mL volume at <1 ppm (parts per million) concentrations, which contains 1015–1017 molecules of ammonia. For fundamental research, this means even greater number of molecules has to be produced at reactor heart to fulfill volume and sensitivity requirements for further analysis. This obstacle is elegantly overcome using surface-sensitive IR absorption spectroscopy, which is based on the principle of total attenuated reflection (ATR-FTIR) (Yao et al., 2018, 2020). This approach uniquely probes molecules produced right on the surface of electrode. It is a relatively fast and non-perturbative technique, requiring only tens of seconds for spectral acquisition. At the same time, the fixed experimental geometry, the necessity to isolate the IR light from the laboratory environment and the need for additional chemicals limit a broader implementation of the method.
In this work, we present a novel approach to ammonia detection, enabled through the use of surface-enhanced Raman scattering (SERS). This is a simple, fast, yet sensitive method that provides chemical selectivity to ammonia and does not rely on complex sensor design, specific beam path geometry, or chemical modifications of the solution. The method allows detection of sub-1 ppm ammonia concentrations in just under 1 s, which paves the way to ultrasensitive operando electrochemical experiments. Taking into account the short spatial reach of plasmonic enhancement, the observed signals correspond to as little as 104–105 molecules detected locally at the region of interest. SERS detection concept does not put any limitations on the identity or morphology of the electrode as long as it can be put close enough to SERS structure. The following can be easily engineered per specific experimental requirements to probe ammonia close to the electrode both on micro- and macro-scales—provide local reading of ammonia presence in closest proximity to the reaction site.
Results
Figure 1A shows a representative spontaneous Raman spectrum of a concentrated ammonia solution spanning the molecular fingerprint region and the stretching modes of N-H, O-H, and C-H-related complexes. To understand the signatures of this spectrum, we performed a normal mode frequency analysis for ammonia and its aqueous formations via density functional theory (DFT) calculations, visualized in Figure 1B. These formations are addressed thoroughly in previous works and are most widely accepted contributors to vibrational spectrum of ammonia aqueous solution (Buckley and Ryder, 2017; Gardiner et al., 1973; Simonelli and Shultz, 2001; Sosa et al., 1996; Ujike and Tominaga, 2002; Yeo and Ford, 1991; Aggarwal et al., 2016; Langseth, 1932; Li and Keppler, 2014; Mysen and Fogel, 2010)—ammonia (NH3), ammonium ion (NH4+), ammonia dimer (NH3-NH3), ammonia-water complex (NH3-H2O), and solvated ammonia complex (not shown in Figure 1).
Figure 1.
Raman Spectrum of Ammonia in Water and Calculated Spectral Positions Based on Various Stretching Modes
(A) Spontaneous Raman spectrum of bulk ammonia solution (0.3 wt%, 30,000 ppm). Exposure time 1 s. Column bars indicate positions of theoretically predicted bending, stretching, and rocking modes of ammonia-related formations.
(B) Main ammonia-related vibrations presented in stretching modes spectral region.
Positions and relative intensities of the observed lines and their corresponding theoretical predictions are in excellent agreement. For more details on the DFT calculations and a table of the mode frequencies, see Methods and Supplementary Information. Although not very bright, several lines from monomer and dimer complexes are clearly visible in the fingerprint spectral region (Figure 1A, inset). Theory shows that, in contrast to the ammonia molecule, the ammonium ion should have the strongest presence in this frequency range through a 1,476 cm−1 contribution of an N-H deformation mode. Yet, the experimental absence of this line is confirmed through the evaluation of the NH3/NH4+ mole ratio by pH measurements (see Methods). It should be noted that even 100 ppb ammonia dissolved in neutral pH solution consists of 95% ammonia molecules compared with ammonium ions. This agrees well with spectroscopy data, where only a broad line comprising H-N-H bending of NH3, NH3-NH3, and NH3-H2O complexes is observed.
In contrast to the weak vibrational features in the fingerprint range, the stretching modes reveal clear signatures of ammonia's presence with intensities that are two orders of magnitude higher than fingerprint modes, in full agreement with DFT predictions. Although these ammonia-related peaks sit atop a strong background of water OH-stretching vibrations, we will show in this work that these signatures enable ammonia detection even at low concentration of the target. We expect the ammonia-related signals to remain clearly distinguishable even in other, more complex electrochemically relevant solutions. The CH-stretching mode of organic reagents (Figure S4) or different modes associated with dissolved salts are generally absent from the spectral region of interest.
The origin of the peaks in this spectral region and their positions has been thoroughly studied (Li and Keppler, 2014; Simonelli and Shultz, 2001; Srivastava et al., 1994; Ujike and Tominaga, 2002; Yeo and Ford, 1991) with the main peaks outlined in Table 1. From the perspective of relative intensities, the highest Raman signal corresponds to the N-H symmetric stretching mode that clearly stands out against the broad and complex O-H stretching band of water. According to DFT calculations, the asymmetric N-H stretching has 4 to 5 times smaller Raman intensity but strongly contributes to the broad profile of the overlapping water signals. Ujike et al. obtained the normal mode frequencies of the ammonia molecule, ammonia-ammonia dimer, and ammonia-water dimer by means of the GF matrix method (Ujike and Tominaga, 2002). In this method, the constants of the force matrix were determined from a direct fitting of the experimental data. In contrast, our approach utilizes force derivate matrices determined from first principles and as such relies on basic physical parameters of the compounds involved. For the ammonia monomer molecule, we obtained excellent agreement between this approach and previous works (Ujike and Tominaga, 2002; Zhao and Truhlar, 2008). However, for the complex dimer formations there are significant discrepancies. Although the lowest energies for ammonia and ammonia-water dimers have been determined at 3,217.4 and 3,044.1 cm−1, respectively (Ujike and Tominaga, 2002), first principles anharmonic frequency calculations for dimer modes of NH3-NH3 and NH3-H2O do not differ by more than 10 cm−1.
Table 1.
Main Ammonia-Related Vibrations Presented in Stretching Modes Spectral Region
| Formation | Spectral Position | Intensity | Assignment |
|---|---|---|---|
| NH3 | 3,409.0 cm−1 | 36.8 | NH asymmetric stretching |
| 3,309.8 cm−1 | 155.8 | NH symmetric stretching | |
| NH4+ | 3,380.0 cm−1 | 16.8 | NH asymmetric stretching |
| 3,276.8 cm−1 | 101.0 | NH symmetric stretching | |
| NH3-NH3 | 3,441.0 cm−1 | 76.2 | NH asymmetric stretching |
| 3,438.0 cm−1 | 7.8 | NH asymmetric stretching | |
| 3,402.3 cm−1 | 48.2 | NH asymmetric stretching | |
| 3,369.5 cm−1 | 15.6 | NH asymmetric stretching | |
| 3,315.4 cm−1 | 341.6 | NH symmetric stretching | |
| NH3-H2O | 3,545.6 cm−1 | 175.0 | OH stretching (H-bonded) |
| 3,410.9 cm−1 | 35.3 | NH asymmetric stretching | |
| 3,389.2 cm−1 | 33.0 | NH asymmetric stretching | |
| 3,305.0 cm−1 | 155.31 | NH symmetric stretching |
For full mode list, please, see Table S1.
Moreover, our theoretical study did not find any ammonia normal modes at the lower energy side from the main peak at 3,260 cm−1. The broad profile on which the main peak rests can be assigned to a distribution of additional contributions from solvated ammonia species, i.e., molecules that are engaged through multiple hydrogen bonds. At the high-energy side of the band, our predictions match previous estimates for ammonia-water complexes with non-bonded and bonded O-H stretches at 3,989 and 3,721 cm−1 (Yeo and Ford, 1991) (not shown in Figure 1B, see Table S1).
The strong peaks from the symmetric N-H stretch at ∼3,300 cm−1 provide an opportunity to employ these spectroscopic signatures for tracing ammonia molecules or related reaction precursors. However, owing to the presence of the substantial water background and the weak response in the fingerprint range, Raman spectroscopy has long been considered unsuitable for ammonia detection. Indeed, regular Raman scattering is blind to solutions with concentrations below 1,000 ppm (Figure S1). To heighten and discriminate molecular signatures from the strong aqueous signals we employed surface-enhanced Raman scattering (SERS), a well-known approach to detect analytes down to single molecule limit (Blackie et al., 2009; Yampolsky et al., 2014). The broadly accepted electromagnetic theory for enhancement relies on excitation of localized surface plasmons (LSP), which provide strongly localized fields that can enhance the optical response of molecules in their vicinity. Surprisingly, SERS has not been specifically utilized for ammonia detection, although some basic principles of the effect in relation to ammonia has been demonstrated in 1984 in work by Sanchez et al. (1984) Further studies on detection of ammonium nitride revealed sensitive detection limits in fingerprint branch (Farrell et al., 2014), even though all the observed and discussed lines are not related to NH3, but rather NO3-. Recently the possibility of ammonia detection with excitation on the slope of plasmonic resonance has been discussed (Nazemi et al., 2020; Nazemi, 2020). In this work, an elegant implementation of SERS detection for live reaction monitoring was discussed with acquisition time constants of ∼10 s. Although the authors focused mainly on the fingerprint region and sidebands from probable ammonia-water dimers, no stretching peak of the NH3 monomer has been observed in operando experiments.
Ammonia Detection with Commercial SERS Substrates
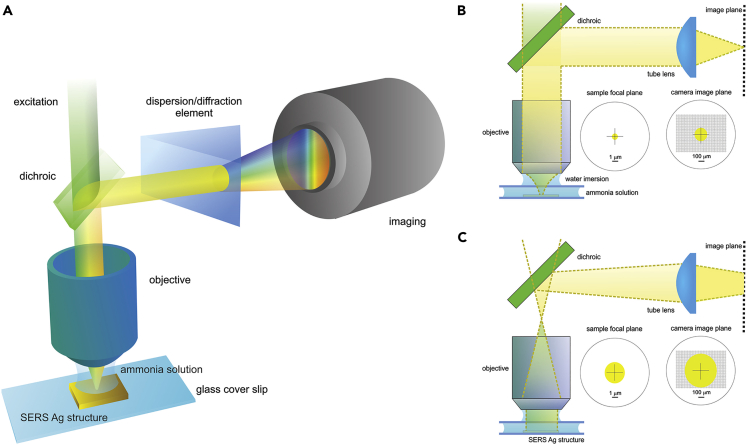
For our first initial demonstration of ultrasensitive ammonia detection via SERS we used a conventional confocal microscopy setup and commercially available SERS substrates (SERStrate, Silmeco, Denmark). The substrates consist of leaning silicon nanopillars covered with silver (Schmidt et al., 2012). The structure forms a rather dense and homogeneous distribution of metal covered clusters of 50–100 nm with corresponding plasmonic resonances that closely match the excitation wavelength of 532 nm. The aqueous ammonia solution was confined between the SERS substrate and the microscope slide, with the Raman signal collected in the epi-geometry by a water immersion objective and coupled into a CCD-based spectrometer (Figure 2A, see Methods for details). The setup was used in two regimes—confocal and wide-field approach (Figures 2B and 2C). The confocal collection geometry allows probing of fine local ammonia concentrations at the region of interest. For the given experimental conditions and the numerical aperture of the collection objective, this volume corresponds to only ∼1.2 μm3 or 1.2 fL of solution being probed. To enable excitation within a wider region of interest, the beam was focused at the back aperture of the excitation/collection objective and the imaging aperture/slit was effectively removed from the image plane. The latter allowed for an increased focal volume by almost an order of magnitude, as well as a more effective use of the detector area without significantly losing spectral resolution (4 cm−1). Hence, this wide-field imaging mode greatly improves detection sensitivity (Figure S2).
Figure 2.
Concept of Experimental Approach
(A–C) (A) Epi detection of Raman signal, (B) confocal detection for high spatial resolution, (C) wide-field detection approach for large volume probing.
A typical SERS spectrum of an aqueous ammonia solution is shown in Figure 3, acquired using confocal geometry and at only 200 ppm concentration (Figure 2B). Strong ammonia SERS signals clearly overcome the water background compared with the spontaneous Raman signal of the bulk solution. The bright line around ∼3,260 cm−1 indicates high sensitivity to the symmetric N-H stretching mode with an improvement of the detection limit by a 1000-fold relative to regular Raman spectroscopy. The spectral shift (∼50 cm−1) and line broadening are anticipated owing to the molecule-surface interaction, with the main line of the NH3 monomer linewidth changed from 14 (for spontaneous Raman) to 30 cm−1 (for SERS). Overall, the spectral shape corroborates theoretical predictions and can be closely reproduced with a simple Gaussian broadening of the NH3 monomer and NH3-H2O dimer modes (see Table S2). Note the rather strong presence of additional spectral structures around 2,930 cm−1 that were not visible in spontaneous Raman measurements of bulk solution (Figure S2A) but are found at certain locations on the substrate through SERS (Figure S3). Further experiments confirmed that these signals do not scale similarly with ammonia concentration and N-H-related bands. These lines were also occasionally observed in SERS of pure Millipore water. Additional experiments and simple line shape analysis suggest that these signals can be assigned to the strong C-H stretching modes of occasional organic contamination on the plasmonic structure (Figure S4). These characteristic modes in the C-H stretching spectral range are well known and can be confidently used for identifying organic species in bio-related environments (Faiman and Larsson, 1976; Yu et al., 2013).
Figure 3.
Calculated Modes for Ammonia-Related Structure Overlaid with SERS Spectrum of 200 ppm Ammonia Solution
Red curve is visualization through using Gaussian line shapes at predicted theoretical positions for NH3 and NH3-H2O only. Exposure time 0.3 s.
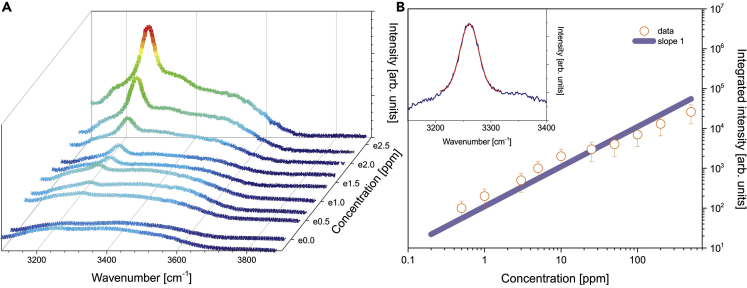
The SERS signal dependence on the ammonia concentration gives rather important insights into the actual chemical content of the solution. As observed in Figure 4A, for higher-concentration solutions the ammonia-related bands dominate the bulk water background with broad and rich spectral features in the stretching mode region. With a decrease of concentration, the spectrum evolves into a steady plateau—the broad O-H spectrum of bulk water molecules. A detailed analysis of the integrated intensity for the bright spectral feature around 3,260 cm−1 reveals clear linear behavior as a function of ammonia concentration (Figure 4B). The following means that signals in this range are dependent on complexes that contain a single NH3 molecule, i.e., the NH3 monomer and the NH3-H2O dimer, as any signal response from NH3-NH3 dimers is anticipated to be quadratic. We stress that all observations are fully reversible when concentration levels are changed on the same SERS substrate, as shown in Figure S5. This points to the absence of a strong chemical interaction with the metal surface and thus allows reversible use of the sensor over a large dynamic range of the ammonia concentration.
Figure 4.
Concentration Dependence Studies
(A) Spectral evolution as a function of ammonia concentration.
(B) Integrated intensity of 3,260 cm-1 spectral line. Blue straight line represents linear function with slope 1. Inset: Gaussian fit of ammonia-related line for 200 ppm solution. Exposure time 1 s. Integrated signal intensity is determined with under 10% error for the smallest concentrations and the signal fluctuations are considered negligible compared with spatial reproducibility within the same substrate, that varies on average by the factor of 2 (error over 10 experiments for each given concentration).
Figures 4 and 5 illustrate the detection sensitivity of the approach. We note that all spectra have been acquired without any signal processing, modulation or electronic filtering. Acquisition times did not exceed 2 s to reveal the true and unperturbed potential of the method.
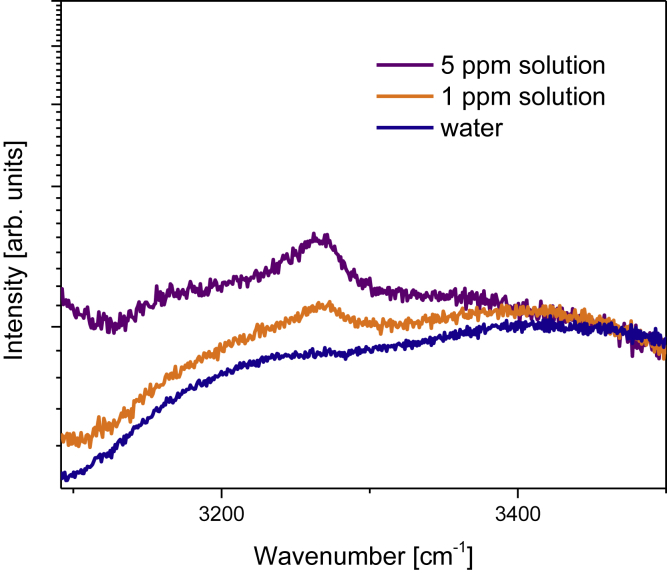
Figure 5.
Fast Detection of Low Amount of Ammonia Using Commercial Ag-Coated Si Nanopillars Substrate
Exposure time 1 s.
For the given plasmonic structure, collection efficiencies, and experimental geometries, 1-ppm concentrations of ammonia can be clearly observed for measurement times of 1 s or less. For longer acquisition times of 1–2 s the detection limit of few hundreds of ppb is easily attainable (Figure S6).
In this work, we report the method's sensitivity in terms of molecular concentration of ammonia in bulk solution. At the same time, owing to specifics of SERS-based method, i.e., sensitivity to local molecular presence, it is helpful to express sensitivity in terms of an alternative metric, namely, the estimated number of molecules probed. This metric relies on the assumption that (1) the plasmonic enhancement is short ranged (Bouhelier and Novotny, 2007) and (2) ammonia signals from bulk solutions are negligible at these concentrations (Figure S1). For the confocal geometry experiments with a 1.2-fL probe volume, a 1-ppm concentration corresponds to only 104–105 molecules—the amount necessary for spectroscopic observation (Table S3). The local sensitivity limit, and thus the molecular detection limit, is already significantly smaller compared with competing methods; these numbers are still conservative estimates. The estimation relies on the total amount of molecules in the confocal volume with Rayleigh range of about ∼1.5 μm. In reality, only those molecules that are in near-field proximity (<1 nm) to the metal structure are contributing to the signal. Thus, the actual number of sampled molecules may still be a few orders of magnitude smaller. In the wide-field illumination approach, the surface area efficiently increases just under ten times, thus probing a greater number of molecules contained within the detection area.
Ammonia Detection on Ag Ink
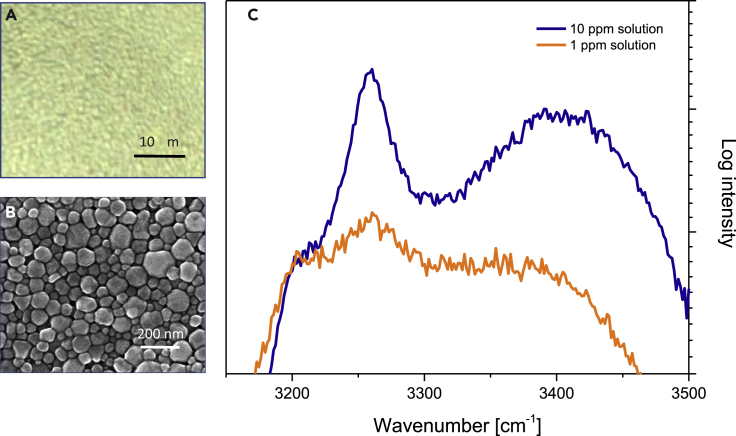
We have performed further experiments based on a similar detection concept, but that employs less sophisticated plasmonic structures to explore more affordable sensor alternatives. For this purpose, we used a commercially available Ag ink (Sigma Aldrich), based on metal nanoparticles. This material is widely used for printable conductors, antibacterial material filters, thin film electronics, etc. This particular ink is based on silver particles (d90 = 115 nm, d50 = 70 nm) with plasmonic resonances that match our previous experiments. When drop casted and dried on mica glass slide, the colloidal suspension forms a homogeneous mirror-like film. Optical microscopy and SEM images of the film are shown in Figures 6A and 6B, respectively. Despite its simplicity, the ink-based SERS substrate permits clear observations of signals with a sub-1 ppm detection limit for ammonia (Figure 6C). Although the enhancement, spatial distribution, and reproducibility are on par with commercial SERS substrates, the approach can be improved by adopting better controlled and cleaner printing procedures.
Figure 6.
Fast Detection of Low Amount of Ammonia Using Drop Casted Ag Ink on a Glass Slide
(A–C) (A) Optical image of dried Ag ink, (B) SEM image of dried Ag ink, (C) SERS signal on custom made SERS substrate. Exposure time 1 s.
Discussion
The SERS method discussed in this work offers a new approach for detecting ammonia and related complexes with a chemical sensitivity that is competitive with that of other spectroscopic techniques, while at the same time being straightforward and affordable in its implementation.
For the low limit of detection, the method is on par with other spectroscopic techniques that allow detection of concentration under 1 ppm. Note that the physical principle of plasmonic enhancement does not put any fundamental limitations on the phase of the sample, allowing trace detection in both liquid and gaseous phases. Using basic assumptions for the spatial extent of the local fields and the size of the illumination spot, 1 ppm overall solution concentration corresponds to 104–105 molecules in probed volume. However, such sensitivity does not come at the expense of long acquisition time. The acquisition speed easily allows live observations—sub-second detection of only 1 ppm concentration or a few thousands of molecules locally at the region of interest makes in situ monitoring of chemical reaction possible.
As an optical-based approach, it allows distant, nonperturbative (non-consuming) probing, thus offering clean and reliable experiments. Moreover, molecules do not appear to strongly bind to the surface, making observations reversible as the molecular content is changed.
The practical implementation of the concept introduced in this work can be significantly improved, in particular in terms of speed and sensitivity. The SERS signal enhancement factors rely on the interplay between spectral position of the plasmonic resonance, excitation source wavelength, and the intrinsic spectral response of the molecule. Thus, fine-tuning of the metal particle size and the structure arrangement may result in a significant improvement of detection limits. Although commercially available SERS substrates are excellent initial choice, they require complex production and are currently too expensive for up-scaling. We demonstrate that simple deposition of colloidal silver ink on the substrate of choice already gives comparable results. Hence, the method does not require special facilities and procedures to produce affordable SERS sensors.
Lastly, the simplicity of the method is of key significance to broad implementation. With flexible beam positioning, measurements can be performed at any region of interest within a given environment or chemical cell. Through point scanning or a wide-field imaging mode, the method allows sampling of the spatial distribution of ammonia content. This flexibility has the potential to provide important insights in underlying reactions mechanisms, which is especially relevant for solid-liquid interfaces. In addition, in our experiments with confocal signal collection we have used no more than 1.5 mW CW laser light, light doses that are easily attained with compact and inexpensive laser diode modules. Furthermore, the method can be adapted for use with lower numerical aperture lenses, whereby the lower collection efficiency is offset by a larger illumination area. By probing a greater number of molecules in larger spot sizes, long focusing approach may prove to be advantageous for sensing needs that do not require spatial confinement and resolution.
As underlined in this work, the Raman spectrum in the N-H stretching region is rich in NH3-related signals. Detection of this broadband spectral region, as opposed to the narrow NH3 monomer line alone, will result in drastic improvements of detection limits. The large Stokes shift of this spectral window makes it possible to use simple colored glass Schott filters, rather than expensive sharp-edge Raman dichroic mirrors. Finally, conventional single-pixel Si detectors can be used. Although cheap and affordable, such detectors can match standard charged-coupled devices, when using photo-multipliers and avalanche mode detectors can even boost the sensitivity. All these potential simplifications will allow the design of a simple and robust device to be used for field research or in-line production.
Ammonia detection method through SERS converges simplicity and affordability with speed and sensitivity. It has potential to be greatly popularized and help to overcome several roadblocks that have been slowing fundamental and applied chemical research in these areas for many decades.
Limitations of the Study
Thin Ag film is expected to easily oxidize. Although no oxidation has been observed within the time frame of the experiments (several hours) for ink-based substrates, strong coloration, hence oxidation, of film becomes visible if left for several days in open laboratory environment. The following put the restrictions on substrate handling if considered for long-term use.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dmitry Fishman (dmitryf@uci.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability Statements
No code has been used to analyze or process the data. Original data are available from the corresponding author on request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported in part by a subcontract from DOE-EERE Advanced Manufacturing Office award to Sandia National Laboratories (AOP 34920). D.A.F. would like to thank Prof. Vartkess A. Apkarian for inspiring discussions. D.A.F. acknowledges NSF grant CHE-0960179. I.M. wishes to thank the UNM Center for Advanced Research Computing for computational resources. Authors are thankful to Dr. Andrea Perego, National Fuel Cell Research Center (NFCRC), University of California Irvine for the SEM images obtained at Irvine Materials Research Institute (IMRI).
Author Contributions
D.A.F. and P.A. conceived the idea and supervised the study. Y.L. prepared ammonia solutions. Y.L., T.A., and Y.C. directed general logistics of chemical experiments. I.M. performed DFT calculations. D.A.F. designed experimental concepts, conducted optical experiments, analyzed the data, and wrote initial manuscript. All authors took part in thorough discussions of data and contributed to its understanding. All authors contributed to manuscript editing.
Declaration of Interests
All authors declare no competing interests.
Published: November 20, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101757.
Supplemental Information
∗OH that is not H-bonded
References
- Aggarwal R.L., Farrar L.W., Cecca S.D., Jeys T.H. Raman spectra and cross sections of ammonia, chlorine, hydrogen sulfide, phosgene, and sulfur dioxide toxic gases in the fingerprint region 400-1400 cm−1. AIP Adv. 2016;6:025310. [Google Scholar]
- Ahmadi Tabr F., Salehiravesh F., Adelnia H., Gavgani J.N., Mahyari M. High sensitivity ammonia detection using metal nanoparticles decorated on graphene macroporous frameworks/polyaniline hybrid. Talanta. 2019;197:457–464. doi: 10.1016/j.talanta.2019.01.060. [DOI] [PubMed] [Google Scholar]
- Ampuero S., Bosset J.O. The electronic nose applied to dairy products: a review. Sensor. Actuat. B Chem. 2003;94:1–12. [Google Scholar]
- Andersen S.Z., Čolić V., Yang S., Schwalbe J.A., Nielander A.C., McEnaney J.M., Enemark-Rasmussen K., Baker J.G., Singh A.R., Rohr B.A. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature. 2019;570:504–508. doi: 10.1038/s41586-019-1260-x. [DOI] [PubMed] [Google Scholar]
- Baird R., Bridgewater L. American Public Health Association; 1995. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Blackie E.J., Le Ru E.C., Etchegoin P.G. Single-molecule surface-enhanced Raman spectroscopy of nonresonant molecules. J. Am. Chem. Soc. 2009;131:14466–14472. doi: 10.1021/ja905319w. [DOI] [PubMed] [Google Scholar]
- Bolleter W.T., Bushman C.J., Tidwell P.W. Spectrophotometric determination of ammonia as indophenol. Anal. Chem. 1961;33:592–594. [Google Scholar]
- Bosch C. U.S. Patent No. 990; 1908. Process of Producing Ammonia. [Google Scholar]
- Bouhelier A., Novotny L. Springer; 2007. Near-field Optical Excitation and Detection of Surface Plasmons', Surface Plasmon Nanophotonics; pp. 139–153. [Google Scholar]
- Buckley K., Ryder A.G. Applications of Raman spectroscopy in biopharmaceutical manufacturing: a short review. Appl. Spectrosc. 2017;71:1085–1116. doi: 10.1177/0003702817703270. [DOI] [PubMed] [Google Scholar]
- Cai Q.Y., Jain M.K., Grimes C.A. A wireless, remote query ammonia sensor. Sensor. Actuat. B Chem. 2001;77:614–619. doi: 10.1016/s0925-4005(01)00766-3. [DOI] [PubMed] [Google Scholar]
- Chabukswar V.V., Pethkar S., Athawale A.A. Acrylic acid doped polyaniline as an ammonia sensor. Sensor. Actuat. B Chem. 2001;77:657–663. [Google Scholar]
- Chang S.C., Stetter J.R., Cha C.S. Amperometric gas sensors. Talanta. 1993;40:461–477. doi: 10.1016/0039-9140(93)80002-9. [DOI] [PubMed] [Google Scholar]
- Chen J.G., Crooks R.M., Seefeldt L.C., Bren K.L., Bullock R.M., Darensbourg M.Y., Holland P.L., Hoffman B., Janik M.J., Jones A.K. Beyond fossil fuel–driven nitrogen transformations. Science. 2018;360:eaar6611. doi: 10.1126/science.aar6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford P.K., Tuma D.T. Characteristics of semiconductor gas sensors I. Steady state gas response. Sensors Actuators. 1982;3:233–254. [Google Scholar]
- Cui X., Tang C., Zhang Q. A review of electrocatalytic reduction of dinitrogen to ammonia under ambient conditions. Adv. Energy Mater. 2018;8:1800369. [Google Scholar]
- Docquier N., Candel S. Combustion control and sensors: a review. Prog. Energy Combust. Sci. 2002;28:107–150. [Google Scholar]
- Faiman R., Larsson K. Assignment of the C—H stretching vibrational frequencies in the Raman spectra of lipids. J. Raman Spectrosc. 1976;4:387–394. [Google Scholar]
- Farrell M.E., Holthoff E.L., Pellegrino P.M. Surface-enhanced Raman scattering detection of ammonium nitrate samples fabricated using drop-on-demand inkjet technology. Appl. Spectrosc. 2014;68:287–296. doi: 10.1366/13-07035. [DOI] [PubMed] [Google Scholar]
- Gardiner D., Hester R., Grossman W. Ammonia in the liquid state and in solution: a Raman study. J. Raman Spectrosc. 1973;1:87–95. [Google Scholar]
- Giovannozzi A.M., Pennecchi F., Muller P., Balma Tivola P., Roncari S., Rossi A.M. An infrared spectroscopy method to detect ammonia in gastric juice. Anal. Bioanal. Chem. 2015;407:8423–8431. doi: 10.1007/s00216-015-9030-6. [DOI] [PubMed] [Google Scholar]
- Guo H., Tao S. Silver nanoparticles doped silica nanocomposites coated on an optical fiber for ammonia sensing. Sensor. Actuat. B Chem. 2007;123:578–582. [Google Scholar]
- Haber F. Salzwasser Verlag; 1905. Thermodynamik Technischer Gasreaktionen. [Google Scholar]
- Heiduschka P., Preschel M., Rösch M., Göpel W. Regeneration of an electropolymerised polypyrrole layer for the amperometric detection of ammonia. Biosens. Bioelectron. 1997;12:1227–1231. [Google Scholar]
- Hübner H.P., Drost S. Tin oxide gas sensors: an analytical comparison of gas-sensitive and non-gas-sensitive thin films. Sensor. Actuat. B Chem. 1991;4:463–466. [Google Scholar]
- Imawan C., Solzbacher F., Steffes H., Obermeier E. Gas-sensing characteristics of modified-MoO3 thin films using Ti-overlayers for NH3 gas sensors. Sensor. Actuat. B Chem. 2000;64:193–197. [Google Scholar]
- Kohl D. Function and applications of gas sensors. J. Phys. D Appl. Phys. 2001;34:R125–R149. [Google Scholar]
- Kukla A.L., Shirshov Y.M., Piletsky S.A. Ammonia sensors based on sensitive polyaniline films. Sensor. Actuat. B Chem. 1996;37:135–140. [Google Scholar]
- Lähdesmäki I., Kubiak W.W., Lewenstam A., Ivaska A. Interferences in a polypyrrole-based amperometric ammonia sensor. Talanta. 2000;52:269–275. doi: 10.1016/s0039-9140(00)00330-1. [DOI] [PubMed] [Google Scholar]
- Lähdesmäki I., Lewenstam A., Ivaska A. A polypyrrole-based amperometric ammonia sensor. Talanta. 1996;43:125–134. doi: 10.1016/0039-9140(95)01713-5. [DOI] [PubMed] [Google Scholar]
- Langseth A. Feinstruktur von Ramanbanden - II. Das ramanspektrum von Ammoniak in wässeriger lösung. Z. für Physik. 1932;77:60–71. [Google Scholar]
- Li Y., Keppler H. Nitrogen speciation in mantle and crustal fluids. Geochimica et Cosmochimica Acta. 2014;129:13–32. [Google Scholar]
- Lundström I., Svensson C., Spetz A., Sundgren H., Winquist F. From hydrogen sensors to olfactory images - twenty years with catalytic field-effect devices. Sensor. Actuat. B. Chem. 1993;13:16–23. [Google Scholar]
- Lv D., Chen W., Shen W., Peng M., Zhang X., Wang R., Xu L., Xu W., Song W., Tan R. Enhanced flexible room temperature ammonia sensor based on PEDOT: PSS thin film with FeCl3 additives prepared by inkjet printing. Sensor. Actuat. B Chem. 2019;298:126890. [Google Scholar]
- Mahendran V., Philip J. An optical technique for fast and ultrasensitive detection of ammonia using magnetic nanofluids. Appl. Phys. Lett. 2013;102:063107. [Google Scholar]
- Max J.-J., Chapados C. Aqueous ammonia and ammonium chloride hydrates: principal infrared spectra. J. Mol. Struct. 2013;1046:124–135. [Google Scholar]
- Mount G.H., Rumburg B., Havig J., Lamb B., Westberg H., Yonge D., Johnson K., Kincaid R. Measurement of atmospheric ammonia at a dairy using differential optical absorption spectroscopy in the mid-ultraviolet. Atmos. Environ. 2002;36:1799–1810. [Google Scholar]
- Mysen B.O., Fogel M.L. Nitrogen and hydrogen isotope compositions and solubility in silicate melts in equilibrium with reduced (N+H)-bearing fluids at high pressure and temperature: effects of melt structure. Am. Mineral. 2010;95:987–999. [Google Scholar]
- Nazemi M. Georgia Institute of Technology; 2020. Investigation of (Photo) Electrocatalytic Conversion of Dinitrogen to Ammonia Using Hybrid Plasmonic Nanostructures. [Google Scholar]
- Nazemi M., Soule L., Liu M., El-Sayed M.A. Ambient ammonia electrosynthesis from nitrogen and water by incorporating palladium in bimetallic gold–silver nanocages. J. Electrochem. Soc. 2020;167:054511. [Google Scholar]
- Nicolas-Debarnot D., Poncin-Epaillard F. Polyaniline as a new sensitive layer for gas sensors. Anal. Chim. Acta. 2003;475:1–15. [Google Scholar]
- Nielander A.C., McEnaney J.M., Schwalbe J.A., Baker J.G., Blair S.J., Wang L., Pelton J.G., Andersen S.Z., Enemark-Rasmussen K., Čolić V. A versatile method for ammonia detection in a range of relevant electrolytes via direct nuclear magnetic resonance techniques. ACS Catal. 2019;9:5797–5802. [Google Scholar]
- Palmqvist E., Berggren Kriz C., Svanberg K., Khayyami M., Kriz D. DC-resistometric urea sensitive device utilizing a conducting polymer film for the gas-phase detection of ammonia. Biosens. Bioelectron. 1995;10:283–287. [Google Scholar]
- Patton C.J., Crouch S.R. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal. Chem. 1977;49:464–469. [Google Scholar]
- Qing G., Ghazfar R., Jackowski S.T., Habibzadeh F., Ashtiani M.M., Chen C.-P., Smith M.R., Hamann T.W. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 2020;120:5437–5516. doi: 10.1021/acs.chemrev.9b00659. [DOI] [PubMed] [Google Scholar]
- Riegel J., Neumann H., Wiedenmann H.M. Exhaust gas sensors for automotive emission control. Solid State Ion. 2002;152-153:783–800. [Google Scholar]
- Sanchez L.A., Lombardi J.R., Birke R.L. Surface enhanced Raman scattering of ammonia. Chem. Phys. Lett. 1984;108:45–50. [Google Scholar]
- Schmidt M.S., Hübner J., Boisen A. Large area fabrication of leaning silicon nanopillars for surface enhanced Raman spectroscopy. Adv. Mater. 2012;24:OP11–OP18. doi: 10.1002/adma.201103496. [DOI] [PubMed] [Google Scholar]
- Searle P.L. The berthelot or indophenol reaction and its use in the analytical-chemistry of nitrogen - a review. Analyst. 1984;109:549–568. [Google Scholar]
- Simonelli D., Shultz M.J. Temperature dependence for the relative Raman cross section of the ammonia/water complex. J. Mol. Spectrosc. 2001;205:221–226. doi: 10.1006/jmsp.2000.8255. [DOI] [PubMed] [Google Scholar]
- Sosa C.P., Carpenter J.E., Novoa J.J. Structures and interaction energies of mixed dimers of NH3 H2O, and HF by Hartree-Fock, møller-Plesset, and density-functional methodologies. In: Brian B.L., Richard B.R., Tom Z., editors. Vol. 629. 1996. pp. 131–141. (Chemical Applications of Density-Functional Theory). [Google Scholar]
- Srivastava R.K., Lal P., Dwivedi R., Srivastava S.K. Sensing mechanism in tin oxide-based thick-film gas sensors. Sensor. Actuat. B Chem. 1994;21:213–218. [Google Scholar]
- Tang C., Qiao S.Z. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem. Soc. Rev. 2019;48:3166–3180. doi: 10.1039/c9cs00280d. [DOI] [PubMed] [Google Scholar]
- Timmer B., Olthuis W., Berg A.v. d. Ammonia sensors and their applications—a review. Sensor. Actuat. B Chem. 2005;107:666–677. [Google Scholar]
- Tzollas N.M., Zachariadis G.A., Anthemidis A.N., Stratis J.A. A new approach to indophenol blue method for determination of ammonium in geothermal waters with high mineral content. Int. J. Environ. Anal. Chem. 2010;90:115–126. [Google Scholar]
- Ujike T., Tominaga Y. Raman spectral analysis of liquid ammonia and aqueous solution of ammonia. J. Raman Spectrosc. 2002;33:485–493. [Google Scholar]
- Wang X., Miura N., Yamazoe N. Study of WO3-based sensing materials for NH3 and NO detection. Sensor. Actuat. B Chem. 2000;66:74–76. [Google Scholar]
- Xu C., Miura N., Ishida Y., Matsuda K., Yamazoe N. Selective detection of NH3 over NO in combustion exhausts by using Au and MoO3 doubly promoted WO3 element. Sensor. Actuat. B Chem. 2000;65:163–165. [Google Scholar]
- Yamazoe N. New approaches for improving semiconductor gas sensors. Sensor. Actuat. B Chem. 1991;5:7–19. [Google Scholar]
- Yampolsky S., Fishman D.A., Dey S., Hulkko E., Banik M., Potma E.O., Apkarian V.A. Seeing a single molecule vibrate through time-resolved coherent anti-Stokes Raman scattering. Nat. Photon. 2014;8:650–656. [Google Scholar]
- Yao Y., Zhu S., Wang H., Li H., Shao M. A spectroscopic study of electrochemical nitrogen and nitrate reduction on rhodium surfaces. Angew. Chem. Int. Ed. Engl. 2020;59:10479–10483. doi: 10.1002/anie.202003071. [DOI] [PubMed] [Google Scholar]
- Yao Y., Zhu S., Wang H., Li H., Shao M. A spectroscopic study on the nitrogen electrochemical reduction reaction on gold and platinum surfaces. J. Am. Chem. Soc. 2018;140:1496–1501. doi: 10.1021/jacs.7b12101. [DOI] [PubMed] [Google Scholar]
- Yeo G.A., Ford T.A. The matrix isolation infrared spectrum of the water—ammonia complex. Spectrochim. Acta A Mol. Spectrosc. 1991;47:485–492. [Google Scholar]
- Yu Y., Wang Y., Lin K., Hu N., Zhou X., Liu S. Complete Raman spectral assignment of methanol in the C–H stretching region. J. Phys. Chem. A. 2013;117:4377–4384. doi: 10.1021/jp400886y. [DOI] [PubMed] [Google Scholar]
- Zakrzewska K. Mixed oxides as gas sensors. Thin Solid Films. 2001;391:229–238. [Google Scholar]
- Zhao Y., Shi R., Bian X., Zhou C., Zhao Y., Zhang S., Wu F., Waterhouse G.I.N., Wu L.-Z., Tung C.-H., Zhang T. Ammonia detection methods in photocatalytic and electrocatalytic experiments: how to improve the reliability of NH3 production rates? Adv. Sci. 2019;6:1802109. doi: 10.1002/advs.201802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Truhlar D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008;120:215–241. [Google Scholar]
- Zilberman Y., Chen Y., Sonkusale S.R. Dissolved ammonia sensing in complex mixtures using metalloporphyrin-based optoelectronic sensor and spectroscopic detection. Sensor. Actuat.B Chem. 2014;202:976–983. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
∗OH that is not H-bonded
Data Availability Statement
No code has been used to analyze or process the data. Original data are available from the corresponding author on request.