Summary
The greenhouse gas (GHG) emissions of the marine sector were around 2.6% of world GHG emissions in 2015 and are expected to increase 50%–250% to 2050 under a “business as usual” scenario, making the decarbonization of this fossil fuel-intensive sector an urgent priority. Biofuels, which come in various forms, are one of the most promising options to replace existing marine fuels for accomplishing this in the short to medium term. Some unique challenges, however, impede biofuels penetration in the shipping sector, including the low cost of the existing fuels, the extensive present-day refueling infrastructure, and the exclusion of the sector from the Paris climate agreement. To address this, it is necessary to first identify those biofuels best suited for deployment as marine fuel. In this work, the long list of possible biofuel candidates has been narrowed down to four high-potential options—bio-methanol, bio-dimethyl ether, bio-liquefied natural gas, and bio-oil. These options are further evaluated based on six criteria—cost, potential availability, present technology status, GHG mitigation potential, infrastructure compatibility, and carbon capture and storage (CCS) compatibility—via both an extensive literature review and stakeholder discussions. These four candidates turn out to be relatively evenly matched overall, but each possesses certain strengths and shortcomings that could favor that fuel under specific circumstances, such as if compatibility with existing shipping infrastructure or with CCS deployment become pivotal requirements. Furthermore, we pay particular attention to the possibility of integrating deployment of these biofuels with CCS to further reduce marine sector emissions. It is shown that this aspect is presently not on the radar of the industry stakeholders but is likely to grow in importance as CCS acceptability increases in the broader green energy sector.
Subject Areas: Chemical Engineering, Industrial Chemistry, Biotechnology, Industrial Biotechnology, Biofuel, Energy Engineering
Graphical Abstract

Chemical Engineering; Industrial Chemistry; Biotechnology; Industrial Biotechnology; Biofuel; Energy Engineering
Introduction
Over the past decade, climate change has rapidly become the most pressing issue that is dominating global headlines (Clarke, 2019; Schiermeier, 2019; Padmanabhan et al., 2019; McGrath, 2019b), despite which both global temperatures and greenhouse gas (GHG) emissions continue to rise. However, a peak in global emissions by 2020 is a crucial element of the Paris climate agreement (McGrath, 2019a; 2018 Fourth Warmest Year in Continued Warming Trend, According to NASA, NOAA, 2019; Kennedy, 2019). The shipping sector is an important contributor to these emissions, accounting for around 2.6% of world GHG emissions in 2015 (Olmer et al., 2017). To put this into perspective, on a country basis, Japan and Germany lie fifth and sixth in the emissions table, respectively, contributing 3.3% and 2.2% of global emissions (Fleming, 2019). This is unsurprising considering that marine freight accounts for about 12% of the global transport sector energy consumption, around 300 million tons of oil equivalent (Mtoe) (IRENA, 2019). The CO2 emissions from the sector have historically risen at a rate similar to the general global increase, but over the past decade, emissions have declined from their peak and appear to be stable at the moment (Figure 1). The use of more efficient ships and the adoption of slow steaming, i.e., operating ships at significantly below the design speed to reduce fuel consumption, are the major factors that have contributed to this reduction in emissions (International Maritime Organization, 2015; Olmer et al., 2017).
Figure 1.
CO2 Emissions from the Shipping Sector as Compared with Global Emissions
(Olmer et al., 2017; International Maritime Organization, 2015; International Maritime Organization, 2009; Emissions Database for Global Atmospheric Research, 2014)
Future emissions from the sector are uncertain and depend on factors such as global trade projections, evolution in the types and number of ships, demand for commodities transport, the fuel mix assumed, the enforcement of emissions control areas, and expected improvements in overall shipping efficiency (Kirstein et al., 2018). However, most projections show that, under a Business-as-Usual (BAU) scenario, maritime CO2 emissions may be expected to rise considerably. For example, the International Transport Forum (ITF) projects a 23% GHG emissions growth by 2035 compared with 2015 under BAU (Kirstein et al., 2018), the International Maritime Organization (IMO) predicts a 0%–50% growth from 2018 to 2050 (International Maritime Organization, 2020), whereas a study by University Maritime Advisory Services for the Danish Shipowners Association projects a BAU increase of over 120% between 2010 and 2050 (Smith et al., 2016). This is exacerbated by the reduced pace of carbon intensity reduction post-2015, with the annual percentage change being just 1%–2% (International Maritime Organization, 2020). Certain market conditions can also lead to a return of higher operating speeds, potentially reversing a large portion of the reduction in carbon intensity achieved in recent years (International Maritime Organization, 2020).
The marine sector is evidently aware of the problem, and accordingly, the IMO has consistently set targets for reducing the carbon intensity of shipping. These include:
-
-
Ships delivered from 2025 must be at least 30% more CO2 efficient than those constructed before 2013 (International Chamber of Shipping, 2018)
-
-
Carbon intensity of international shipping should decrease by at least 40% by 2030, “pursuing efforts toward” 70% by 2050, compared with 2008 (International Maritime Organization, 2018)
-
-
GHG emissions of international shipping should decrease by at least 50% by 2050 compared with 2008 (International Maritime Organization, 2018)
The numerous methods proposed for emissions reduction include increasing vessel size, reducing speed, operational efficiency indexing, market-based mechanisms, etc. Unfortunately, these methods are inadequate and sometimes controversial (International Chamber of Shipping, 2018; Olmer et al., 2017). For instance, a shift to even larger ships can lead to higher emissions if not filled to capacity, whereas even with reduced emissions intensity, an increase in transport supply and operating hours can lead to an overall increase in sectoral emissions (Olmer et al., 2017). Likewise, organizations like the International Chamber of Shipping (ICS) and the Baltic and International Maritime Council (BIMCO) are often opposed to measures like mandatory operational efficiency indexing and emissions trading schemes (International Chamber of Shipping, 2018; Psaraftis, 2012). On the other hand, measures such as ship speed and size optimization, better ship design, waste heat recovery, smoother ship-port interface, and onshore power supply do offer CO2 emissions reduction potential (Kirstein et al., 2018; 't Hart, 2017) but are unlikely to be sufficient to meet the IMO targets.
A better option for reducing the CO2 footprint of shipping would be changing the propulsion technology or fuel, for which alternatives have been proposed. These include nuclear propulsion (Ondir Freire and de Andrade, 2018; Schøyen and Steger-Jensen, 2017), fuel cells (Tronstad et al., 2016; van Biert et al., 2016), batteries (DNV GL, 2016; Peralta P et al., 2019), ammonia (de Vries, 2019; Ash and Scarbrough, 2019), and various biofuels. These technologies have their advantages and disadvantages, which are briefly summarized in Table 1. It can be seen that the use of biofuels would be less disruptive and challenging than other novel propulsion options, while contributing much more to CO2 emissions reduction than measures unrelated to fuel changes (Figure 2).
Table 1.
Comparison of Novel Propulsion Options
| Technology | Pros | Cons |
|---|---|---|
| Nuclear propulsion |
|
|
| Fuel cells |
|
|
| Batteries |
|
|
| Ammonia |
|
|
| Biofuels |
|
|
Figure 2.
Comparison of Different Options for CO2 Emissions Reduction in the Shipping Sector
Adapted from Bouman et al. (2017)
The term “biofuels” refers to fuels made from biomass, i.e., organic matter such as plants. It is therefore a broad term, encompassing a range of products—methanol, ethanol, bio-oil, synthetic diesel, etc.—made via diverse processes such as fermentation, pyrolysis, and Fischer-Tropsch synthesis. Although the use of biofuels as an energy source is of pre-historic origin, in the fossil fuel era, the use of biomass as an energy source has declined, accounting for just 13% of global gross final energy consumption in 2017 (World Bioenergy Association, 2019). Most low-carbon future scenarios, however, envisage a much greater role for biofuels in the years to come. The 2DS scenario of the International Energy Agency (IEA), for example, projects a 10-fold rise in transport sector biofuels use between 2015 and 2060, with biofuels playing a particularly important role in long-haul transport like shipping and aviation (Frankl, 2018). Likewise, the REmap scenario of the International Renewable Energy Agency (IRENA) also calls for raising the contribution of biofuels in transport from 3% in 2015 to 22% in 2050 (International Renewable Energy Agency, 2018).
The vast majority of transport biofuels used today are “first-generation biofuels,” made from food crops like corn, soybean, sugarcane, and canola (Kumar et al., 2018; REN21, 2019). These fuels are unsustainable in the long run owing to their adverse impacts on food supply and land use patterns globally (Banse et al., 2011; Cai et al., 2011; Danielsen et al., 2009; Fischer et al., 2010; Havlík et al., 2011; Keeney and Hertel, 2009; Lapola et al., 2010; Pimentel et al., 2008; Ravindranath et al., 2011). “Second-generation” or “advanced” biofuels that are made from non-food feedstocks or industrial waste and residue streams promise much greater GHG reductions while minimizing competition with food crops and land use change impacts (Kumar et al., 2018; Nyström et al., 2019). These technologies are presently less mature and therefore exhibit significantly higher capital and operational costs (Padella et al., 2019; International Renewable Energy Agency, 2016; Maniatis et al., 2017). Nonetheless, owing to their lower carbon footprint, the goals outlined in the renewable energy scenarios mentioned above can only be met using advanced biofuels (Brown et al., 2020), which explains the policy and R&D interest in their development. Technologies like solar fuels can potentially reduce CO2 emissions beyond even second-generation biofuels. However, given their emergent nature, the various different processes explored all still require significant improvements and cost-competitiveness is thus unlikely before the middle of the century (Detz et al., 2018; Aro, 2016). The combination of low GHG emissions and relatively advanced technological maturity therefore make the use of second-generation biofuels an attractive option for the midterm.
Although biofuels can help reduce net GHG emissions greatly, their use, along with other renewable energy technologies, may not suffice to restrict global warming to the Intergovernmental Panel on Climate Change (IPCC) target of 1.5°C. Accordingly, the IPCC considers the use of carbon dioxide removal technologies, such as Carbon Capture and Storage (CCS), unavoidable for meeting this target (Intergovernmental Panel on Climate Change, 2018; Fuss et al., 2018). The combination of bio-energy and CCS (BECCS) can potentially lead to negative emissions and hence features heavily in Integrated Assessment Models (IAM) (Vaughan et al., 2018). In the shipping sector, the incorporation of CCS in the emissions reduction mix can potentially lead to results beyond what can be achieved using the other methods outlined above (Figure 3). However, for CCS to become a viable option, this still requires the development of more efficient sorbent materials, better CO2 storage monitoring and verification, cost reductions, a more robust legal and regulatory framework, and broader policy support (Bui et al., 2018; Global CCS Institute, 2019). As different biofuel production routes require different carbon capture technologies, the cost and ease of CCS implementation is therefore also fuel specific, and this must be taken into account while appraising fuel costs and GHG reduction potential.
Figure 3.
Shipping Sector CO2 Emissions Scenarios with Different Mitigation Options
In addition to the above, it must be noted that the use of biofuels in the shipping sector also presents some challenges not seen in other sectors like road or aviation. First, existing marine fuels like Heavy Fuel Oil (HFO) and Marine Gas Oil (MGO) are very inexpensive compared with other transport fuels, resulting in an elevated cost barrier between them and biofuel alternatives. As an illustration, IFO 380 HFO prices were in a range of 4.5–17 $/GJ between 2010 and 2019 (GL, 2020). This compares with a range of 7.5–23 $/GJ for jet fuel and 13.5–27 $/GJ for US road diesel prices in the same period (Statistics, 2020). Furthermore, the interconnected nature of international shipping means that fuel standards and distribution and storage infrastructure must be developed in a coordinated fashion. This is distinct from the road sector, where countries can and do implement independent fuel policies, such as encouraging the use of flex-fuel automobiles in Brazil (Santos et al., 2018) or promoting compressed natural gas (CNG) vehicles in countries like China, India, and Sweden (Imran Khan, 2017). It is clear, therefore, that the deployment of biofuels in the marine sector is subject to numerous hurdles and uncertainties, which tend to prevent actors in this sector from taking the initiative with respect to biofuels adoption, or other alternatives.
There have been a few works published recently reviewing the applicability of biofuels to the shipping sector. An early example examined the possibility of biodiesel, dimethyl ether (DME), straight vegetable oil (SVO), bio-liquefied natural gas (LNG), bio-ethanol, and pyrolysis bio-oil finding a foothold in the shipping industry based on the technology status as it stood in 2012 (Florentinus et al., 2012). Similarly, comprehensive analyses were later published by the IEA and by the Netherlands Maritime Knowledge Centre in 2017 (Hsieh and Felby, 2017; 't Hart, 2017). More recently, Kesieme et al. examined the prospects of SVO, biodiesel, and bio-LNG. These fuels were selected because they were deemed to be best suited from a short- to medium-term perspective, defined in terms of potential availability in 2030 (Kesieme et al., 2019). Hansson et al. used a different approach, examining the prospects of seven alternative marine fuels using a multi-criteria decision analysis and interaction with Swedish stakeholders (Hansson et al., 2019). The criteria used ranged from economic criteria like fuel price and operational cost to environmental criteria such as acidification and health impact, thus providing a holistic picture of the merits and pitfalls of the fuels.
Here, we present a study evaluating the suitability of different biofuels to the marine sector. Although we build on the work of the aforementioned authors, our approach differs in a few aspects. First, in terms of timeline, this work evaluates different biofuels options on the basis of their ability to realistically make a substantial contribution to the 2050 IMO targets. This means that very short-term (and scale-limited) options such as SVO or very long-term options like renewable hydrogen have been de-emphasized in favor of candidates that are reasonably mature while still maintaining long-term potential. Since the life span of the average ship is around 20 years (United Nations Conference on Trade and Development, 2019), this means that options that can fuel the next generation of ships in the time period of 2030–2050 have been prioritized. To reduce the subjectivity of our evaluation, a matrix rating the candidate fuels on six criteria was used and later validated and calibrated by a stakeholder assessment to arrive at a realistic estimation of the prospects of the fuels by combining the technical merits of the fuels with the needs and restrictions of the shipping sector. Lastly, the possibility of CCS use as a method of augmenting emissions reductions achieved by biofuels use is absent from most discussions on marine sector decarbonization, a breach this paper attempts to fill.
Biofuel Alternatives
As mentioned earlier, the term “biofuels” encompasses a broad range of options, with the most prominent being fatty acid methyl esters (FAME), SVO, hydrotreated vegetable oil (HVO), Fischer-Tropsch (FT) diesel, bio-ethanol, bio-methanol, bio-DME, bio-LNG, and bio-oil (E4tech, 2018; Hsieh and Felby, 2017).
After an initial screening, several of these biofuel candidates were removed from further consideration, for the reasons listed below. Fuels that have thus been eliminated are:
Fatty Acid Methyl Esters (FAME): FAME biodiesel use is presently restricted in ISO 8217 (2017) to 7% v/v in certain distillate fuels, and 0.5% in other distillate and residual marine fuels, owing to certain technical shortcomings. These include the tendency of biodiesel to act as a solvent and degrade certain rubber and elastomer compounds; a tendency to oxidize during long-term storage (>2 months); hygroscopicity and the risk of microbial growth; and degraded low-temperature flow properties owing to gelling (Florentinus et al., 2012; Hsieh and Felby, 2017). Furthermore, feedstock supply for second-generation biodiesel production is limited. Given that biodiesel blending is already in vogue in sectors like road transport and power generation, and that FAME is currently twice as expensive as the incumbent shipping fuels (average FAME price between 2015 and 2020 around 22 €/GJ [Neste, 2020]), it is unlikely that FAME will meet a significant portion of marine fuel requirements, even in blends (E4tech, 2018; Hsieh and Felby, 2017).
Straight Vegetable Oil (SVO): SVO is not considered to be a practical fuel for large-scale or long-term use, as, owing to their high viscosity and boiling point, their use reduces the engine lifespan because of carbon build-up and damage to the engine lubricant (Hsieh and Felby, 2017). Also, although a substantial amount of vegetable oil is produced worldwide annually, almost all of this is made from food crops (corn, cottonseed, canola/rapeseed, olive, palm, peanut, safflower, sesame, soybean, and sunflower) ('t Hart, 2017; Poel-Tec, 2019). SVO is therefore mostly a first-generation biofuel, whose use as a marine fuel seems unlikely to be sustainable.
Hydrotreated Vegetable Oil (HVO): Catalytic hydrodeoxygenation (HDO) can be used to convert vegetable oil or animal fat to HVO, which is a drop-in fuel with characteristics very similar to fossil diesel (Choudhary and Phillips, 2011; Stumborg et al., 1996; Karatzos et al., 2014). A major constraint in HVO production is feedstock availability. Palm oil has historically been a major feedstock, but sustainability concerns mean that waste-based feedstocks must be used going forward. Although in theory the feedstock can be any fatty acid containing material, it must be recognized that such feedstocks are fairly limited in availability, with the global HVO potential estimated at only around 10 million t/year with currently known feedstocks (Wahl, 2018), which would amount to only around 440 PJ/year. This is a small fraction of the projected 2050 marine sector energy requirement of 11 EJ/year (DNV GL, 2018; DNV GL-Maritime, 2017). The drop-in nature of the fuel is likely to reduce its availability as a marine fuel further by eliciting a strong interest from the automotive and aviation sectors, where HVO is also at a lower price differential with existing fuels (E4tech, 2018).
Fischer-Tropsch (FT) diesel: Like HVO, FT diesel is a drop-in diesel fuel, with its production from coal and natural gas a mature technology (Ail and Dasappa, 2016; Lappas and Heracleous, 2016). In case of Biomass to Liquid (BtL), on the other hand, only a few pilot and demonstration units have been built to date, and research is currently underway on all three stages of the BtL process, namely, biomass gasification and syngas cleaning/conditioning, FT production of middle distillates, and upgrading of FT liquids to high-quality fuels (Ail and Dasappa, 2016; Dimitriou et al., 2018; Lappas and Heracleous, 2016). Virtually all operational BtL FT plants are operating at a Technology Readiness Level (TRL) of 4–7 (Molino et al., 2018), and several promising projects have been abandoned over the past decade owing to techno-economic and regulatory challenges (Lappas and Heracleous, 2016; Ravindranath et al., 2011). The large capital costs and low overall efficiencies of BtL-FT plants also result in high prices of the fuels produced (Lappas and Heracleous, 2016). BtL-FT fuels can therefore be considered to be high-price, high-quality fuels more suitable for higher-end applications. This, together with the still nascent state of the technology, precludes their consideration as a near or mid-term alternative for the marine sector.
Bioethanol: Bioethanol is the most widely used biofuel in the world but is almost exclusively made via fermentation of food-crop based sugars and starches. Advanced ethanol processes that will produce ethanol from woody biomass and waste streams are under development and may be commercially available by 2030 (E4tech, 2018). Even advanced ethanol processes, however, are likely to have a lower GHG reduction potential than alternatives like methanol and pyrolysis oil without accompanying CCS implementation (E4tech, 2018). Although the use of multifuel diesel engines may eventually open up the marine fuel market to bioethanol, their widespread installation is unlikely to occur for decades (E4tech, 2018; Hsieh and Felby, 2017). Presently, ethanol lags other biofuels in terms of evaluation as a marine fuel, as there appear to be no projects testing ethanol at present, making its on-board storage, handling, and usage unknown quantities.
Bio-methanol, bio-dimethyl ether, bio-LNG, and bio-oil are four fuels (Table 2) that scored reasonably well on all the criteria, and hence these are considered in greater detail in the following sections. The term “bio-oil” here is used to refer to a crude oil produced from biomass, most commonly by pyrolysis, hydrothermal liquefaction (HTL), or solvolysis.
Table 2.
Key Specs of the Four Biofuel Candidates
| Property | Unit | Bio-Methanol | Bio-DME | Bio-LNG | Bio-Oil |
|---|---|---|---|---|---|
| Melting point | °C | −98 | −141.5 | NA | NA |
| Boiling point | °C | 65 | −24.8 | −162 | 100–280 |
| Relative density | Water = 1 | 0.79 | 0.61 | 1.8a | 1.1–1.3 |
| Cetane number | 5 | 60 | 0 | 5–25 | |
| Octane number | 113 | 35 | 130 | NA | |
| Higher heating value (HHV) | MJ/kg | 22.9 | 31.7 | 51 | 17–34 |
| Lower heating value (LHV) | MJ/kg | 20.1 | 28.9 | 46 | 13–18 |
| Flash point (closed cup) | °C | 9.7–11 | −41 | −188 | 40–110 |
| TRL | 6 - 8 | 5 | 7 | 6–8 |
E4tech, 2018; PubChem, 2020b; Olah and Prakash, 2011; Andersson and Salazar, 2015; PubChem, 2020a; Sivebaek and Jakobsen, 2007; Chemical Book, 2020; European Biofuels Technology Platform, 2011; Groupe International des Importateurs de Gaz, 2019; Ikealumba and Wu, 2014; Hagos and Ahlgren, 2017; University of Birmingham, 2011; Linde, 2011; Bridgwater, 2012; Ben et al., 2019; Lehto et al., 2013; Wang, 2013; Kim and Lee, 2015; Oasmaa and Peacocke, 2010; Moirangthem, 2016; Nattrass et al., 2014; Perkins et al., 2018; Castello et al., 2018.
Relative density based on air = 1 for LNG.
Bio-Methanol
Production Routes
Methanol is one of the most widely produced organic chemicals, with a global production about 92 million tons per year in 2016 (Dalena et al., 2018). Currently, however, nearly all the methanol produced worldwide is derived from fossil fuel, with natural gas accounting for around two-thirds and coal the rest (Dekker, 2018).
Although methanol production from biomass can be done using enzymatic routes, this is still in the early research phase (Goeppert et al., 2014). The production of bio-methanol therefore concentrates on thermochemical routes, in which biomass is gasified in a similar manner to coal, and the syngas is then catalytically upgraded to methanol. Unlike coal, however, the single-step partial oxidation of biomass is technically challenging, and therefore biomass gasification generally follows two stages (Olah and Prakash, 2011). In the first stage, the dried biomass is pyrolyzed to pyrolysis gas and charcoal. In the second, the charcoal undergoes partial oxidation to carbon monoxide. Circulating fluidized bed gasifiers or bubbling fluidized bed gasifiers with oxygen atmosphere or indirect firing are held to be the best for large-scale syngas production for methanol synthesis (Hamelinck and Faaij, 2001; Landälv, 2017). Depending on the conversion route used, methanol can be produced from biomass with an energy efficiency of 50%–70% (Landälv, 2017). An alternative thermochemical pathway for methanol production is the biogas route. Biogas generally has a higher CO2 concentration than natural gas, and hence tri-reforming, which is a combination of steam reforming, dry reforming, and partial oxidation, may be used to obtain the required syngas composition (Bozzano et al., 2017). Bio-methanol can therefore be obtained from a range of waste biomass sources, implying a high potential availability, although the actual amount of biomass available for bio-methanol production will depend on economics and on competition from other bio-energy sources. Sustainability and practical criteria imply that the maximum global bio-methanol production capacity may be around 20 EJ/year (Hallung, 2015). The feedstock used also impacts the GHG reduction potential of bio-methanol. Using waste wood or black liquor can allow the GHG emissions over the entire bio-methanol cycle to be below 10 g CO2eq/MJ, which compares very favorably with HFO and MGO emissions of around 85 g CO2eq/MJ (DNV GL-Maritime, 2019; Chaplin, 2013). The use of crude glycerin or biogas as feedstock raises bio-methanol GHG emissions to over 30 g CO2eq/MJ, which is still a big improvement over the existing fuels (Chaplin, 2013).
Present Status of Bio-Methanol
One of the largest bio-methanol plants in operation is the Enerkem plant in Alberta, Canada, with a capacity of 38 million liters per annum from 100,000 t/year municipal solid waste (Chen, 2018). Enerkem is also the lead contractor for a 270 million liter per year methanol plant in Rotterdam, the Netherlands, a final investment decision on which is pending (Kopis, 2019). BioMCN also operates a 15,000 t/year bio-methanol plant in the Netherlands, using biogas as feedstock, with plans to augment production by using green hydrogen from a proposed 20-MW electrolyzer built by Gasunie and Nouryon (Gasunie, 2019). Bio-methanol production can be estimated to be at TRL 6–8 and is therefore more mature than most other biofuels (E4tech, 2018).
One problem in bio-methanol production is the generic challenge of biomass procurement. Although new fossil methanol plants often have a capacity of up to 10,000 t/day, this is an order of magnitude larger than bio-methanol plants can become, with 1,300–2,600 t/day being the maximum attainable in a mature future scenario (Svanberg et al., 2018). This is because for bigger plants, the low energy density of biomass means that an enormous quantity of biomass would need to be collected from vast areas and transported over long distances, which is uneconomical (Olah and Prakash, 2011). Bio-methanol, can, however, be a bridge between fossil methanol and “power-to-methanol” produced using renewable hydrogen and captured CO2, thus providing a pathway from a presently mature technology to a future large-scale renewable resource.
The production cost of bio-methanol depends on numerous factors, among which the feedstock and capital costs are the most important (Huisman et al., 2011). One study found that a feedstock cost of 5.5 €/GJ and a plant size of 20 t/h input leads to a bio-methanol cost of around 24 €/GJ, whereas a price below 10 €/GJ would be achievable if free waste feedstock was used in a plant size of 80 t/h input (Huisman et al., 2011). Other studies have reported minimum selling price (MSP) values of 18 €/GJ (feedstock cost 3 €/GJ and plant size 2,000 t/day input) (Zhu et al., 2011) and 16–25 €/GJ (feedstock cost 3–6 €/GJ and plant size 100–200 MW output) (Maniatis et al., 2017) (prices from other currencies converted to € at September 2020 rates). Despite the wide range of reported prices, it can be concluded that bio-methanol is presently somewhat more expensive than not only shipping fuels but also fossil methanol (average 2019 contract price of 16.4 €/GJ [Methanex Corporation, 2020]).
Methanol synthesis is relatively well suited for CCS. Methanol production from biomass requires adjustment of the syngas composition, as depending on the process and biomass used, bio-syngas may contain 30–45 vol% H2 and 20–25 vol% each of CO and CO2 (Rauch et al., 2014), which translates into an R-ratio (Equation 1) of 0–0.6, which is far less than the 2–2.1 required for methanol synthesis.
| (Equation 1) |
This imbalance can be redressed either by adding hydrogen or by removing CO2 (Iaquaniello et al., 2017). The latter option may be more feasible in the absence of cheap renewable hydrogen and can be accomplished by pre-combustion capture.
Methanol as a Marine Fuel
A considerable number of projects have been conducted on the use of methanol as a marine fuel. These include the Effship project, the SPIRETH project, and the PILOT methanol project (Andersson and Salazar, 2015). These have shown that methanol can be used in spark ignition and converted compression ignition engines (Grijpma, 2018). Methanol has a cetane number of 5 and an octane number of 113 (Andersson and Salazar, 2015; Bozzano and Manenti, 2016), meaning that it is intrinsically more suited to dual fuel spark ignition engines. For use as a diesel fuel, its corrosive nature and high autoignition temperature (464°C) necessitate adaptations to the engine, ignition, and fuel storage systems (Andersson and Salazar, 2015; Grijpma, 2018). The Swedish ferry Stena Germanica uses a four-stroke engine modified to use either methanol or MGO, whereas Waterfront Shipping uses dual-fuel two-stroke engines (Grijpma, 2018). It should be noted that, even in dual-fuel engines, a pilot fuel like HFO or MGO must be used (5%–-10% energy basis) for ignition (Svanberg et al., 2018). Its increasing commercial utilization has made methanol the fourth most used marine fuel, with 160,000 tons being consumed in 2018 (International Maritime Organization, 2020).
Bio-Dimethyl Ether
Production Routes
Dimethyl ether (DME), the simplest ether, is a clean burning, high-density fuel that can be used as a direct replacement for diesel fuels (Moirangthem, 2016). It can be produced either by the catalytic dehydration of methanol or in a one-step process that permits both methanol synthesis from syngas and its dehydration in the same process unit (Olah and Prakash, 2011). The two-step process suffers from limited productivity in the methanol synthesis reactor due to equilibrium constraints, while the need for a second, separate dehydration reactor also raises costs. Direct synthesis is therefore more favorable thermodynamically and economically, but the downstream separation of DME from methanol, unreacted syngas, and other volatile compounds is complex and costly (Peng et al., 1999; Azizi et al., 2014). This makes a trade-off necessary and explains why different DME producers are currently divided between the two techniques with Haldor Topsoe, JFE Holdings, Korea Gas Corporation, Air Products, and NKK among those with technology for the direct method, whereas Toyo, MGC, Lurgi, and Uhde have indirect DME production processes (Azizi et al., 2014).
Present Status of DME
The present worldwide production of DME is 9–15 million t/year (270–450 PJ/a) ('t Hart, 2017; Frequently Asked Questions, 2020). Most of this is produced by the methanol dehydration route and is fossil fuel based. There have been a few pilot projects making DME from biomass, one such being a 4 t/day plant making DME from black liquor operated in Sweden from 2010 (Demonstration of BioDME - Dimethyl ether as an advanced biofuel at industrial scale, 2020). Presently bio-DME is at TRL 5, and a scale-up by a factor of over 30 will be required for full commercialization (TRL 8–9) (Moirangthem, 2016; Nattrass et al., 2014). That said, given that the conversion of methanol to DME is a well-developed technology, this route can readily be used for bio-DME production if needed. Therefore, from a process standpoint, the same pros and cons as mentioned for methanol largely hold for DME. This includes the ability to incorporate carbon capture as a part of the indirect synthesis process. As stated above, gas separation is harder in the direct synthesis process, and hence presently this route can be considered to be somewhat less suitable from the CCS perspective.
As direct DME production is at a lower TRL and indirect DME production has methanol as an intermediate, the production cost of DME is expected to be slightly higher than for methanol. This is borne out by cost estimates from the literature, with production costs given as 9 (Peral and Martín, 2015), 10–11 (Clausen et al., 2010), 12 (Wang et al., 2010), 19 (Zhu et al., 2011), 18–21 (Huisman et al., 2011), and 16–25 €/GJ (Maniatis et al., 2017). The similarity in the production processes of DME and methanol means that similar constraints on potential availability and GHG mitigation potential apply to DME as to methanol.
DME as a Marine Fuel
DME is a gas at ambient temperature and pressure but can be liquefied by pressurizing above 5 bar (Arcoumanis et al., 2008). Its behavior is thus similar to propane, and hence propane distribution and storage infrastructure can be used for DME distribution (Moirangthem, 2016). DME has a volumetric heating value (18.2 MJ/L) that is almost exactly half that of diesel, although its lower density (0.67 kg/L at 20°C) means that its gravimetric low heating value (28.9 MJ/kg) is closer to diesel (Bakhtyari and Rahimpour, 2018; European Biofuels Technology Platform, 2011). It is more compatible with diesel engines than methanol is, with only moderate engine modifications required (Olah and Prakash, 2011; Das and Bhatnagar, 2018). The higher cetane number of DME (55–60) and the very clean combustion obtained are advantageous as a diesel replacement, whereas the low viscosity and lubricity are drawbacks (Arcoumanis et al., 2008; Hsieh and Felby, 2017).
It must be said that ethers other than DME are also promising biofuel candidates. These include the class of oligomers called oxymethylene dimethyl ethers (OMEs), of which dimethoxymethane (DMM) is the simplest member (Sun et al., 2019). Although DMM has a cetane number of only 28, the polyoxymethylene dimethyl ethers OME3-5 exhibit physico-chemical and combustion characteristics similar to conventional diesel fuels (Hackbarth et al., 2018; Baranowski et al., 2017). OMEs can be synthesized starting from methanol or DME, but more research is needed to elucidate the reaction mechanisms and develop simple and high-selectivity reaction systems (Hackbarth et al., 2018; Baranowski et al., 2017).
Bio-LNG
Production Routes
Bio-LNG, also known as liquefied bio-methane (LBM), can essentially be produced via three different routes: anaerobic digestion, catalytic upgrading of syngas, and the “Renewable Fuel of Non-Biological Origin” (RFNBO) route (Grijpma, 2018). Of these, the RFNBO route, using CO2 from air capture and renewable hydrogen, is the least developed technologically and faces similar challenges to other RFNBO fuels. It can be said to be at TRL 5–6, or in the early demonstration stage (E4tech, 2018). The anaerobic digestion and syngas routes are more technologically developed and can therefore have more impact near-time.
Anaerobic digestion refers to the decomposition of organic matter by microorganisms in the absence of oxygen. Feedstock such as manure, sewage sludge, and energy crops can be converted to a mixture of methane and CO2 called “biogas” in an anaerobic digestor, and the raw biogas can be upgraded to bio-LNG by the removal of CO2 and trace gases followed by liquefaction (E4tech, 2018; Goeppert et al., 2014). Landfill gas is produced by a similar mechanism, although the methane concentration may be somewhat lower than in digestor gas (van den Borne, 2015; Scheutz et al., 2009). Anaerobic digestion is the most prevalent route for bio-LNG production and is practiced on a commercial scale worldwide (E4tech, 2018; Grijpma, 2018). Depending on the feedstock, biogas can contain 40%–75% methane, with the rest mostly being CO2 (van den Borne, 2015). For conversion to LNG, CO2 and contaminants like H2S and water are removed by techniques such as pressure swing adsorption (PSA), membrane separation, or cryogenic separation, and the upgraded biogas containing 99% methane can then be liquefied (van den Borne, 2015). Carbon capture is therefore an essential part of biogas to bio-LNG conversion and does not entail any added equipment or expenditure. One cubic meter of biogas can be considered to yield 0.63 m3 of natural gas equivalent (Soest et al., 2014).
The syngas route for bio-LNG production is analogous to that described for bio-methanol. Syngas is produced via biomass gasification, the H2:CO ratio is adjusted to around three by the water gas shift reaction, CO2 is removed from the syngas, and the H2 and CO are converted to methane in a methanation reactor (Birgen and Garcia, 2013). It has been calculated that around one-third of the carbon content of the feed can be removed for CCS via pre-combustion capture, with another quarter of the carbon content available for post-combustion capture applied to the gasification flue gas stream (del Álamo et al., 2015). This route can be considered to be at TRL 7, i.e., at large-scale demonstration (E4tech, 2018). Another possible thermochemical route is supercritical water gasification, in which a biomass slurry is converted under supercritical conditions into a methane-rich gas (2014). This process is related to hydrothermal liquefaction (section Hydrothermal Liquefaction) but generally operated under supercritical conditions so as to maximize gas production (Toor et al., 2011; Matsumura et al., 2005). This is a relatively novel technology and must be demonstrated beyond the prototype stage to warrant further consideration (Soest et al., 2014)).
Present Status of Bio-LNG
A major issue in bio-LNG adoption is its limited supply. In terms of feedstock availability, the anaerobic digestion and syngas routes may be viewed as being complementary rather than competitive. Wet biomass, such as manure and sewage sludge are better suited to anaerobic digestion, whereas dry biomass like lignocellulose can undergo thermochemical conversion to syngas (Soest et al., 2014). Anaerobic digestion has the advantage that its feedstocks are rarely usable for other, higher value applications (Soest et al., 2014), and hence the issue of feedstock competition that rears its head in most other cases, such as the syngas route, does not apply. Nevertheless, worldwide production of biogas was 1.33 EJ in 2017 (World Bioenergy Association, 2019), whereas bio-LNG production was only 55 million Nm3 (Tybirk et al., 2018), which in energetic terms is around 2.2 PJ. This is clearly inadequate when viewed in terms of the energy requirements of the marine sector, especially given the other competing uses. As wet biomass supply is inherently localized, a dramatic increase in bio-LNG production would appear to be a challenge. The cost of bio-LNG is also an issue. Estimated costs from the syngas route would be around 20 €/GJ (del Álamo et al., 2015), whereas the smaller scale of anaerobic digestion units mean that production costs may exceed 30 €/GJ (Verbeek and Verbeek, 2015). Not only is this far higher than current marine fuels, but it also contrasts with an average 2019 fossil LNG spot price of below 5 €/GJ (Union, 2020).
Bio-LNG as a Marine Fuel
Given the tightening maritime emission regulations, LNG has been hailed as being a potential “interim fuel” for the marine sector (Liang, 2019; Hughes, 2019; LNG positioned as greenest marine fuel, 2019). The IMO sulfur cap that came into force in January 2020 further boosted the attractiveness of LNG, as it has been calculated that the total fuel cycle SOx emissions from LNG can be as low as a quarter of that for low-sulfur (1,000 ppm) distillate marine fuel (Thomson et al., 2015). As stated in section Present Status of Bio-LNG, LNG is often available at prices below 5 €/GJ, making it extremely competitive with HFO and MGO. The increasing attractiveness of LNG as a marine fuel is reflected in the increasing number of LNG-capable vessels. This trend is expected to continue in 2020 with between 400 and 600 more such ships expected to be delivered, which, however, is still a fraction of the world fleet of over 60,000 commercial vessels (Saul and Chestney, 2018). The suitability of LNG as an interim fuel has, however, been called into question. Given that fossil fuel LNG is not suitable for long-term decarbonization targets, it is probable that a different, lower-carbon fuel will need to be utilized, perhaps from around 2030. This will mean that the expensive infrastructure being put in place for LNG supply and utilization will need to be decommissioned in favor of an alternative infrastructure, unless bio-LNG becomes a dominant fuel (Smith, 2018). The present push toward LNG is, therefore, an important driver for the prospects of bio-LNG, provided that the costs of the latter can be reduced significantly.
One concern in the use of LNG is “methane slip,” wherein a portion of the methane escapes into the atmosphere. Although biogas synthesis has a relatively low GHG emissions profile (∼20 g CO2eq/MJ) (DNV GL-Maritime, 2019), methane is a GHG with a 100-year horizon global warming potential (GWP100) of 28 (Protocol, 2016), and hence any leakage will have serious consequences for the life cycle GHG emissions. Methane can leak out in the supply chain, during transfer bunker to the ships or during its combustion in the engine. Presently, methane accounts for only 0.5% of the CO2eq GHG emissions from the shipping industry (International Maritime Organization, 2020). However, the increased use of LNG as a marine fuel and the associated rise in the use of dual-fuel engines with higher specific exhaust emissions of methane mean that methane emissions from international shipping have risen over 150% between 2012 and 2018 (International Maritime Organization, 2020), which is definitely concerning. Presently, most ships capable of using LNG as fuel use dual fuel engines, with the three most popular engines being lean-burn, spark-ignited Otto cycle engines; engines with Diesel cycle injection but lean-burn Otto cycle operation; and diesel-injected, compression-ignited Diesel cycle engines (Riviera Newsdesk, 2018). Methane slip is essentially a problem in the Otto cycle mode, not the Diesel cycle mode, but developments such as improvement in the combustion process, optimized turbocharging arrangements, and catalytic oxidation of the unburned methane are expected to reduce methane slip in the future (Riviera Newsdesk, 2018; Trakakis, 2018). The MariGreen project, for instance, has built a prototype catalytic converter for eliminating methane slip from lean-burn engines (Roosjen, 2019).
Bio-Oil
Production Routes and Present Status
Pyrolysis
Pyrolysis is a process that has been used for thousands of years for charcoal production, but over the past few decades, the development of fast pyrolysis technologies has allowed this technology to be used for liquid biofuel production. Pyrolysis refers to thermal decomposition occurring in the absence of oxygen, whereas “fast pyrolysis” refers to the use of short reaction times (∼2 s) and moderate temperatures (∼500°C) (Bridgwater, 2012). The products obtained are solid char and ash; non-condensable gases like CO, H2, and CO2; and the liquid bio-oil (Carpenter et al., 2014). The liquid fraction, which is generally a dark brown, free-flowing organic liquid, is formed by rapidly and simultaneously depolymerizing or fragmenting cellulose, hemicellulose, and lignin, followed by a rapid quenching preventing the further reaction of intermediate products (Mohan et al., 2006). Several reactor types have been used at the pilot and demonstration scales for fast pyrolysis. These include bubbling fluidized bed, circulating fluidized bed, rotary cone, auger, ablative, vacuum moving bed, and entrained flow (Bridgwater, 2012; Venderbosch and Prins, 2010). Certain researchers have examined the use of integrated catalytic pyrolysis reactors with a view of producing improved quality bio-oil and reducing the need for post-processing (Venderbosch and Prins, 2010; Bridgwater, 2012). Overall, pyrolysis is the most developed bio-oil technology, with plants by Ensyn and BTG, among others, at the commercial scale (TRL 8) (Perkins et al., 2018).
The yield, composition, and properties of bio-oil depend on the feedstock type, process conditions, and recovery techniques, and are hence difficult to generalize (Carpenter et al., 2014). Broadly speaking, though, fast pyrolysis permits the production of liquids in yields of up to 75 wt%, but about 25% of this is water that cannot be readily separated (Bridgwater, 2012). Woody biomass usually provides the highest yields, followed by energy crops and agro-residues (Roy and Dias, 2017). The bio-oil obtained is a very complex mixture of aliphatic and aromatic oxygenated hydrocarbons, with molecular weights ranging from 18 to over 10,000 g/mol, and with components including alcohols, acids, aldehydes and ketones, furans, phenolics, and alkanes (Venderbosch and Prins, 2010; Mohan et al., 2006; Marsman et al., 2007). Wood-derived bio-oil generally has an HHV of 16–19 MJ/kg (Venderbosch and Prins, 2010; Bridgwater, 2012; Mohan et al., 2006). It is highly acidic (pH ∼2.5; acid number 60–100 mg KOH/g) and has a very high oxygen content (40–50 wt%) (Bridgwater, 2012; Marsman et al., 2007; Oasmaa et al., 2010; Sundqvist et al., 2015). It is also unstable, with continuing secondary reactions such as polymerization and a loss of volatiles leading to a change in chemical composition and an increase in average molecular weight and viscosity (Diebold, 2000). This is alongside a tendency to separate into light and heavy phases at higher moisture contents and to deteriorate on storage at elevated temperatures or on exposure to oxygen (Rogers and Brammer, 2012).
These drawbacks of raw bio-oil mean that it needs to be upgraded before use. First, physical upgrading processes like filtration, solvent addition, or emulsification may be used to reduce the ash content and viscosity of the bio-oil while enhancing its miscibility with hydrocarbon fuels (Bridgwater, 2012). This may be followed by a chemical or catalytic upgrading process. The two most commonly used catalytic methods for bio-oil upgrading are hydro-deoxygenation (HDO) and catalytic cracking. HDO is closely related to the hydrodesulfurization process used in petrochemical refining, with hydrogen being used to remove oxygen via conversion to water. HDO of bio-oil leads to the formation of at least two phases, an organic and an aqueous phase. Two organic phases, a lighter one floating on the water and the heavier one at the bottom have also been reported (Mortensen et al., 2011). Although complete deoxygenation is theoretically possible, the hydrogen consumption becomes increasingly steep with an increasing degree of deoxygenation (Venderbosch et al., 2010; Mortensen et al., 2011). In addition to the cost and carbon footprint of the hydrogen used, the high pressures required also increase the capital costs of the upgrading system (Bridgwater, 2012). Poor catalyst life due to coke deposition is another operational challenge (Bridgwater, 2012; Mortensen et al., 2011). Catalytic cracking is comparable with petrochemical fluid catalytic cracking (FCC), with zeolites commonly being used as catalysts (Mortensen et al., 2011). This route does not require hydrogen and can be carried out at atmospheric pressure, giving it a cost advantage over HDO. The oxygen elimination occurs via a mixture of dehydration, decarboxylation, and decarbonylation. The loss of a portion of the carbon as gas contributes to product yields far lower than for HDO, and the HHV of the upgraded oil (21–36 MJ/kg) is also much lower than for HDO-upgraded oil (42–45 MJ/kg) (Mortensen et al., 2011). Coking leading to catalyst deactivation is a major barrier to commercial deployment, even more so than for HDO (Mortensen et al., 2011; Butler et al., 2011).
Hydrothermal Liquefaction
Hydrothermal liquefaction (HTL) is the thermochemical conversion of biomass into liquid fuels via processing in a hot and pressurized aqueous environment (Gollakota et al., 2018; Elliott et al., 2015). Hydrothermal conditions generally refer to a temperature range of 250°C–450°C and a pressure range of 100–350 bar (Castello et al., 2018). In HTL, water acts as a solvent, a reactant, and also as a catalyst. Under HTL conditions, the dielectric constant of water is low enough to permit solubility of non-polar compounds that may not be water soluble under ambient conditions, but the ionic product is still high enough to favor ionic reactions resulting in oil products rather than radical reactions that lead to coke or gas formation (Castello et al., 2018; Dimitriadis and Bezergianni, 2017). The HTL pathway comprises three sequential steps: depolymerization, decomposition, and recombination (Gollakota et al., 2018). The process does not require a catalyst, but the use of alkali to promote aromatic oil formation has been examined (Elliott et al., 2015) as, but more rarely, has the use of heterogeneous catalysts such as Ni-containing catalysts (Toor et al., 2011). Solid-liquid separation is problematic under alkaline conditions, which can be solved by acid addition before the separation step (Tekin and Karagöz, 2012).
Most HTL processes that have been assessed operate at subcritical conditions, although supercritical conditions have also been examined (Castello et al., 2018). The optimal temperature for HTL is determined by the competition between the hydrolysis, fragmentation, and repolymerization reactions and is partially dependent on the biomass composition (Akhtar and Amin, 2011). Excessively high temperatures (>374°C) reduce liquid yields, with secondary decompositions and the Boudouard reaction leading to gas formation and free radical reactions to char formation. Temperatures below 280°C, on the other hand, lead to incomplete biomass decomposition, and therefore, temperatures between 300 and 350°C may be considered optimal (Akhtar and Amin, 2011). The role of pressure is conventionally held to be solely to maintain a single phase during liquefaction, with a negligible effect on bio-oil yields beyond this (Akhtar and Amin, 2011), but in recent years, this has been called into question, as elevated pressures can permit the creation of reaction regimes favoring liquefaction and enhanced deoxygenation (Castello et al., 2018). Other factors that affect the yield and quality of bio-oils are biomass type, particle size, heating rate, residence time, biomass-water ratio, and the reaction atmosphere (Akhtar and Amin, 2011; Xue et al., 2016).
Most types of biomass can be processed using HTL, including high-moisture-content algal biomass (Biller and Ross, 2016). Although the yields from different feedstock differ, the HHV of the produced bio-crude tends to be reasonably constant (Castello et al., 2018). If lower moisture content feedstock like woody biomass is used, then water needs to be recovered and reused for preparing the feed slurry, as this is beneficial for bio-crude generation (Biller and Ross, 2016).
HTL bio-oil is generally considered to be of higher quality than pyrolysis oil. It is more deoxygenated and viscous but less dense than pyrolysis bio-oil (Elliott et al., 2015; Haarlemmer et al., 2016). It also has a far lower moisture content (∼5%) and higher HHV (∼35 MJ/kg) (Elliott et al., 2015; Gollakota et al., 2018; Toor et al., 2011). This means that similar upgrading processes can be used for HTL bio-oil as for pyrolysis, but the procedure is expected to be less operationally challenging than for pyrolysis bio-oil (Dimitriadis and Bezergianni, 2017; Elliott, 2016). As it operates under a wet reaction environment, HTL is also suited to wet feedstock, eliminating the need for drying as in pyrolysis. However, HTL is less well developed than pyrolysis, with Licella and Steeper Energy among the most advanced with processes at a TRL of around 6 (Castello et al., 2018).
Solvolysis
Section Hydrothermal Liquefaction dealt with the use of water as a solvent for biomass liquefaction, but organic solvents like methanol, ethanol, and acetone can also be used for this purpose. As can be seen in Table 3, organic solvents generally have much lower critical temperatures and pressures than water, thereby permitting the use of milder reaction conditions, while their low dielectric constant allows facile dissolution of high-molecular-weight biomass compounds (Dimitriadis and Bezergianni, 2017).
Table 3.
Properties of Common Liquefaction Solvents
| Solvent | Critical Temperature (°C) | Critical Pressure (MPa) | Critical Density (g.cm−3) | Dielectric Constant (at 20°C) | Relative Polarity (Water = 100) |
|---|---|---|---|---|---|
| Water | 374 | 22.1 | 0.3320 | 79.7 | 100 |
| Methanol | 240 | 7.96 | 0.2720 | 32.6 | 76.2 |
| Ethanol | 243 | 6.39 | 0.2760 | 22.4 | 65.4 |
| Acetone | 235 | 4.8 | 0.2779 | 20.6 | 35.5 |
| 1,4-Dioxane | 315 | 5.21 | 0.3702 | 2.21 | 16.4 |
| Ethylene glycol | 374 | 7.7 | 0.3250 | 37.7 | 79.0 |
| Toluene | 318.8 | 4.22 | 0.2918 | 2.38 | 9.9 |
A range of organic solvents have been used on different feedstock, at different conditions, and with different desired and obtained end products (Liu and Zhang, 2008; Deng et al., 2015; Haverly et al., 2018; Durak and Aysu, 2014; Wang et al., 2013; Yip et al., 2009; Singh et al., 2015; Cheng et al., 2010; Brand and Kim, 2015; Mun and Jang, 2009). These studies have been reviewed elsewhere (Huang and Yuan, 2015; Dimitriadis and Bezergianni, 2017; Isa et al., 2018), and a detailed discussion is beyond the scope of this work. However, a few brief comments may be made here. An ideal solvent is one that gives high biocrude yield, is easily recoverable from the biocrude product, is inexpensive, and does not impact reactor cost negatively (Lange, 2018). The first of these factors is self-evidently important and generally requires empirical determination. Separation of the solvent from the bio-oil and solids is sometimes facile, for instance, removal of alcohols by drying (Isa et al., 2018). In other cases, a secondary solvent such as acetone may be used as an aid (Haverly et al., 2018). If the solvent reacts with the bio-oil, this can be considered to be an undesirable feature from both solvent recovery and product quality standpoints. On the other hand, if bio-crude or a cheap hydrocarbon solvent is used, then solvent recovery may be omitted, with the solvent going on to form a part of the product (Lange, 2018). Solvent cost is a very important factor, given that the creation of a pumpable biomass slurry may require solvent use in an amount ten times the biomass intake. Hence, if an expensive solvent is used, production costs will become prohibitive unless very complete solvent recovery and recycle is implemented (Lange, 2018). Finally, the use of corrosive solvents will increase reactor costs by requiring the use of exotic and expensive metallurgy (Lange, 2018).
The above points make it clear that there are numerous trade-offs between the different solvent options, and hence the “ideal” solvent will be rather feedstock and application specific. Nevertheless, it is also evident that the large number of organic solvents make solvolysis a promising option for bio-oil production. This technology is still in the fledgling stage, with a 50 t/day pilot plant currently operated by Vertoro being among the larger units, with plans for a 10,000 t/day demonstration unit for 2022 (Koltsova, 2018).
A downside of all the bio-oil processes is the difficulty of implementing pre-combustion carbon capture. In pyrolysis, for instance, CO2 can, depending on the feedstock and conditions, account for half or even more of the non-condensable gas (Mohabeer, 2019; Piskorz et al., 1998; Luo et al., 2004). However, as the amount of non-condensate is generally minimized so as to increase bio-oil yield and improve the carbon balance, the amount of CO2 that can be captured pre-combustion is still limited and can be complicated further by any non-condensed hydrocarbons, meaning that post-combustion capture may be a more feasible option. As a GHG mitigation option, bio-oil's efficacity is largely feedstock dependent, although production and upgrading technology also play a role, with estimates for GHG reduction compared with fossil fuels ranging from around 50% to over 90% (Chryssakis et al., 2015; Peters et al., 2015; Han et al., 2011; Geraedts, 2017). The wide range of feedstock that can be used in all the bio-oil production methods is therefore a positive point that needs to be balanced against the cost and GHG mitigation perspectives.
Most cost estimates available for bio-oil in the literature are for raw pyrolysis bio-oil. These cost estimates range from as low as 6–14 €/GJ (Brigagão et al., 2019) to as high as 19–23 €/GJ (Chryssakis et al., 2015). As with other biofuels, the assumptions made regarding the feedstock cost and plant size have a big impact on the estimated bio-oil production cost. The cost of upgraded pyrolysis bio-oil depends in addition on the chosen upgrading technique and the degree of upgrading applied, but a price range between 20 and 35 €/GJ may be realistic (Maniatis et al., 2017; Geraedts, 2017). For HTL, the lower level of technology development and expected higher plant capital costs means that HTL cost estimates tend to be higher than for pyrolysis, but the higher quality of the HTL bio-oil may partially offset this owing to lower upgrading costs (Zhu et al., 2011; Tzanetis et al., 2017; Funkenbusch et al., 2018; Zhu et al., 2014).
Bio-Oil as a Marine Fuel
The low moisture content, high calorific value and high H:C ratio of HTL bio-oil has led to it being proposed as a drop-in fuel for heavy marine engines (Hsieh and Felby, 2017). However, given the lower maturity (TRL 6) of HTL, pyrolysis bio-oil, which is a near-commercial technology, deserves attention as well. The high oxygen and moisture content, acidity, and instability of pyrolysis bio-oil mean that it must be upgraded before application as a transport fuel. The simplicity, maturity, and low cost of pyrolysis bio-oil production must therefore be balanced against the complexity and cost of its upgrading. The GHG emissions reductions must also be calculated accordingly, with poorly devised upgrading scenarios potentially raising life cycle emissions beyond that of fossil diesel (Vienescu et al., 2018).
The marine sector is conceivably more favorable in this regard than the road and aviation sectors, as marine engines are more tolerant of lower-quality fuel. For example, it has been proposed that unprocessed lignin pyrolysis oil can substitute low-sulfur marine gas oil (Obydenkova et al., 2017). However, it has also been shown that crude bio-oil cannot be blended with current marine fuels in any proportion such that the resultant mixture meets marine fuel specifications (Chong and Bridgwater, 2017). A degree of upgrading is therefore clearly necessary. Mild hydrodeoxygenation (HDO), wherein the oxygen content is reduced rather than being eliminated (Boscagli et al., 2015), might suffice for use in marine engines while also limiting the rise in costs and carbon footprint (Geraedts, 2017). Other schemes such as combining esterification with azeotropic water removal for simultaneously reducing bio-oil acidity and moisture content to a level suitable for use in slow diesel engines have also been proposed (Sundqvist et al., 2015).
Comparative Evaluation of Biofuel Alternatives
Method
The four candidate fuels profiled in the previous sections were evaluated according to six criteria:
-
a)
Present technology status: Different biofuels are currently at different stages of technological development. Given the pressing need of decarbonization, more mature biofuels will have an advantage over those unlikely to be commercially available for several years, and will therefore be awarded a higher score.
-
b)
Potential biomass feedstock availability: Biomass is a heterogeneous resource, differing widely in composition and spatial and temporal distribution. A biofuel that is more feedstock agnostic or whose feedstock is globally available perennially on a large scale without major competing uses will therefore be prioritized over one that has a more limited feedstock base.
-
c)
GHG mitigation potential: Although the principal driver for substituting fossil fuels with biofuels is reducing GHG emissions, the actual reduction achieved depends on the fuel composition, the sustainability of the feedstock used, the fuel production process, emissions arising from transporting the feedstock and/or the fuel, combustion efficiency, and other factors. Therefore, biofuels that can be assessed as having a higher GHG mitigation potential merit a higher score.
-
d)
Cost: As stated earlier, present marine fuels are very cheap, and the marine sector is highly cost-sensitive. It therefore follows that the estimated cost at which a biofuel will be available is a vital parameter.
-
e)
Infrastructure compatibility: The shipping industry has an extensive fueling infrastructure in place, and needing to replace substantial portions of this to accommodate biofuels will greatly hamper their adoption. This extends to the ships themselves, where an ideal biofuel will require minimal retrofitting of the fuel storage units and engines.
-
f)
CCS compatibility: As discussed in the Introduction, CCS is likely to play an important role in future GHG mitigation efforts. Although the storage aspect is fuel-independent, the cost of CO2 capture depends on the fuel production process and therefore needs to be kept in mind while determining the suitability of a fuel to decarbonization efforts.
While evaluating different biofuels based on the above criteria, one must remember that the timeline being considered is pivotal in this regard. This is because if a short-term horizon (around 2025) is considered, then the present technology status and fuel availability become determining factors. On the other hand, if a longer horizon (around 2050) is assessed, then several candidates not presently well developed may become more prominent because of their strong showing on other fronts.
A realistic scenario is more nuanced than either of these two situations owing to the inherent inertia of the shipping sector. The lifespan of ships such as cargo ships and oil tankers can exceed 20 years (United Nations Conference on Trade and Development, 2019), and these ships are often expensive to retrofit with newer technologies, sometimes leading to premature scrapping (Jiang, 2017). A recent example is the increase in the scrapping volume of vintage units owing to the IMO sulfur cap that came into effect in January 2020 (Jiang, 2020). Allied to this is the requirement for developing new supply chains and bunker infrastructure, especially for non-drop-in fuels. Given the enormous costs and logistical complexities involved, it is clear that any fundamental decision on biofuel deployment taken today by the sector will have far-reaching consequences for decades, necessitating the incorporation of longer-term thinking into the decision making. However, this inertia in turn means that, to make a significant contribution to the IMO's GHG reduction goals for 2050, biofuels penetration needs to happen at the earliest. Thus, biofuel technologies that are more developed today are better placed to take advantage of the transition.
Although the ideal solution would be to use a fuel that scores highly in all the aforementioned criteria, and is therefore satisfactory both in the short and long haul, no fuel presently meets this demand. A fuel that presents a tangible transition pathway away from fossil fuels toward zero-emission fuels is the next best option. As an idealized illustration, the use of methanol as a marine fuel may commence with the use of fossil methanol, as this is the predominant present source. In a few years, as bio-methanol production is scaled up, it could gradually replace fossil methanol as a marine fuel without requiring any changes to the fueling infrastructure. In the farther future, RFNBO methanol made using captured CO2 and renewable hydrogen may provide an even greener and more abundant alternative (Bellotti et al., 2017; Goeppert et al., 2014), which can then seamlessly be integrated into the fueling infrastructure. This possibility of certain fuels offering the chance to combine present implementation with a longer-term transition to a more sustainable production process has therefore been given due consideration in the technology status and potential availability assessment.
The evaluation of the four biofuels was done using a two-step process (Figure 4). First, each candidate fuel was evaluated for each criterion based on the existing literature and given between 1 and 5 points depending on how promising the fuel is on that front (Table 4). The values allotted to each criterion deserve some elaboration. For the present technology status, a full score was given to a fuel if it is already produced commercially (TRL 8 or 9) and has been used as a commercial marine fuel. On the other hand, a biofuel that is still in the laboratory phase or that has never undergone any testing as a marine fuel gets the lowest score of 1. The values for potential availability and cost have been chosen so as to be comparable with existing marine fuels, with the energy consumption of international shipping expected to rise to 11–13 EJ in the time frame 2035–2050 (DNV GL, 2018), and the cost of heavy fuel oil (HFO) and marine gas oil (MGO) typically being around 10–14 €/GJ (Global 20 Ports Average: MGO, 2020). As an ideal biofuel would be practically carbon neutral, and as certain production routes have been postulated as lowering GHG emissions compared with fossil fuels by over 90% (Chaplin, 2013; Hallung, 2015), 5 points are accorded to a fuel with greater than 90% GHG mitigation potential. On the other hand, given the emphasis on potential GHG mitigation in recommending biofuels adoption, a fuel that will not decrease emissions by even 50% from present levels merits 1 point in this category. As an example of the allocation in this category, different sources ascribe a prospective GHG reduction potential of 70%–90% for bio-methanol (Chaplin, 2013; Hallung, 2015; DNV GL-Maritime, 2019), depending on the feedstock; hence, an average value of 80% was used and a score of 4 given to bio-methanol for this category.
Figure 4.
Process for Arriving at Biofuel Evaluation Scores
Table 4.
Criteria for Biofuel Evaluation
 |
The compatibility of the biofuel with the existing shipping infrastructure is also an important consideration. A drop-in fuel that will, by definition, require no changes in the infrastructure and thus avoid a costly and disruptive transition, is accorded 5 points, whereas a fuel that is incompatible with the present distribution, refueling, and shipping infrastructure 1 point. For CCS, the choice is primarily about the capture technology best suited to the process, with pre-combustion capture favored over post-combustion (or oxyfuel combustion) capture on account of the customarily lower capture costs and energy penalties (Leung et al., 2014; Vasudevan et al., 2016; Cormos et al., 2018). Within this, a certain fuel would be given a better rating based on the ease or cost of carbon capture — for instance, the removal of a large quantity of CO2 is an integral part of bio-LNG synthesis using anaerobic digestion, whereas for methanol, the amount of CO2 captured per unit of fuel would be much lower.
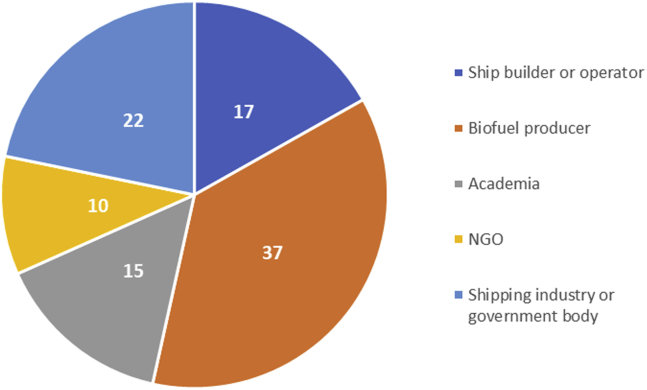
The second step involved validating these preliminary results by stakeholder consultations. A total of 41 stakeholders were consulted, with their group distribution shown in Figure 5. This was done both by one-on-one meetings, as well through a workshop where the participants were asked to anonymously rate the fuels. These ratings were used in the final calculations. Simultaneously, they were also asked to assign weights to the different criteria, since it cannot be presumed that each is equally important when it comes to determining suitability as a marine fuel candidate.
Figure 5.
Percentage Distribution of Stakeholders Consulted
Results and Interpretation
Figure 6 shows the weights allotted by the stakeholders to the different criteria. It is clear that cost is considered to be by far the most important factor (31%). This makes sense, as the cost sensitivity of the shipping sector and the low cost of present fuels are the predominant hurdles for any prospective biofuel to overcome. Potential availability and GHG mitigation potential are tied in second place (20% each), emphasizing the importance of the fuel to be available in large quantities to become more than a niche player, while GHG mitigation is the central purpose of switching to biofuels and hence fuels obviously need to fulfill that mandate. Although infrastructure compatibility is only fourth in weighting, this still means that a fuel that requires an infrastructure overhaul will only be considered if it scores highly in terms of cost, availability, and GHG reduction. The present technology status, on the other hand, received a low weight as it was held to be linked to costs and thus less important on its own. This is to say that a fuel with a low TRL level typically has a high cost and is penalized in that category. Therefore, allotting an additional low score in technology status could be considered double counting. Finally, CCS compatibility was considered to be the least important consideration, receiving a weight of only 5%.
Figure 6.
Criteria Weights as Determined by Stakeholders
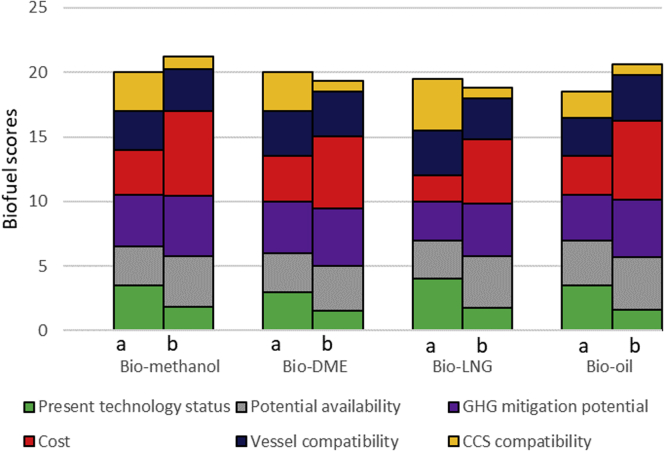
Figure 7 provides an overview of the results of the literature review and the stakeholder interactions (scores given in the Supplemental Information). It can be seen that all fuels are in fact considered fairly competitive, with methanol being marginally ahead of DME and bio-oil. Methanol scores consistently high across all criteria, and its results are the least affected by the weights given to the criteria. It is also the outright leader in terms of cost and GHG mitigation potential, which are considered to be among the most important factors. This explains why its overall score increases when one moves from the unweighted literature values to the weighted stakeholder numbers, although it is in the lead in either case. Bio-DME is neck and neck with methanol on literature evaluation alone, but the stakeholder numbers place it behind methanol. This can be attributed largely to the lower numbers given to DME in the categories of cost and potential availability. Bio-LNG gets the lowest overall stakeholder rating, which is partly caused by getting a low score on cost, the criterion with the highest weight. On most other criteria, it gets reasonably good scores. The present technological status, although lower in priority, is in particular held to be a strong suit. Surprisingly, bio-LNG also gets a low score in terms of CCS compatibility, which is at odds with literature estimates. This coupled with the low weight attached to CCS compatibility adversely affects the bio-LNG score as compared with the literature scenario, and in an emissions reduction scenario where CCS has a big part to play, this aspect will need more detailed scrutiny. Finally, bio-oil is a high scorer in all categories except for present technology status and CCS compatibility. The highest score for this fuel is in terms of infrastructure compatibility, on expectations that mildly upgraded bio-oil will be suitable for use as a drop-in fuel.
Figure 7.
Biofuel Scores, with “a” Representing Unweighted Literature Review Values and “b” Weighted Workshop Scores
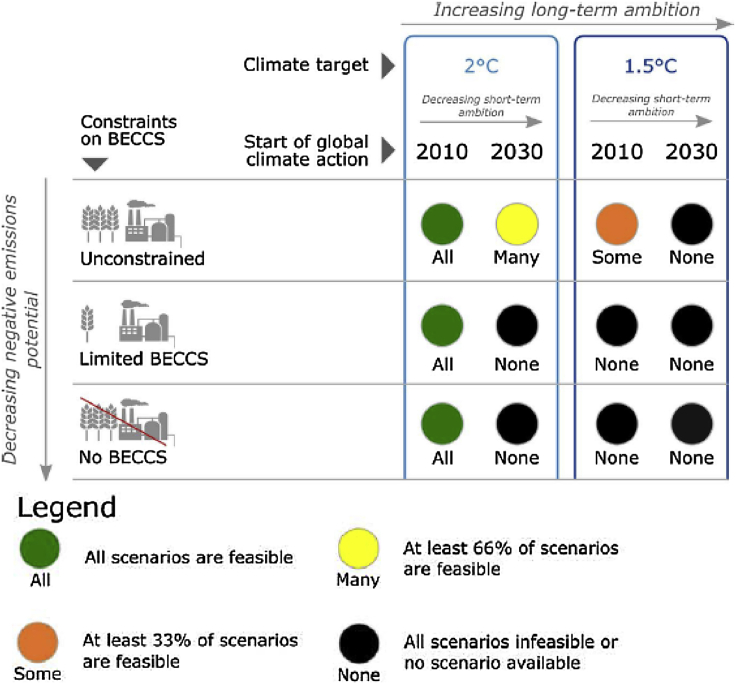
As stressed above, the actual relevance of the criteria outlined above and other exogenous developments will ultimately impact the fuel ratings. For instance, if fossil LNG use becomes widespread in the marine sector, then bio-LNG will become a more attractive alternative since the existing bunkering and refueling infrastructure and vessels can be used. The results presented here reflect the expected order of preference of the fuels under the conditions currently considered to be the most plausible by industrial stakeholders. Indeed, such stakeholders will likely have an implicit bias toward near-term scenarios, concentrating more on factors that are the most important today, and therefore preferring technologies that can be considered more developed at present. This is especially evident in the low weight given to CCS compatibility. The general consensus of the stakeholders was that, although no option should be ruled out, the techno-economics of biofuels were more important aspects that should be focused upon. CCS was considered to potentially be a distraction that could lead to the diversion of R&D and investments away from biofuels. Therefore, it should only be considered in the wake of additional cost incentives. It was also noted that CCS implementation is a broader topic beyond the remit of the IMO or the marine sector and hence needs to be discussed separately from issues specific to the shipping industry. This general apathy toward CCS contrasts with research that shows that restricting global warming to below 1.5°C or even below 2°C makes BECCS implementation almost inescapable (Fuss et al., 2018) (Figure 8). Given this reality, in combination with the expected growth of the marine transport sector, it is imperative that the shipping sector take CCS more seriously and consider how its implementation might affect future fueling choices. As an example, bio-oil, which is considered by the stakeholders as among the most promising candidates, might be penalized heavily by an emissions reduction scenario of which CCS is a big part. This indicates the need to create a greater awareness about this subject both in the shipping fraternity and in a wider setting (policy makers, biofuel producers etc.). Better quantification of the BECCS potential of the global shipping sector will be necessary to elucidate the possibilities and enable sound decision making on this front.
Figure 8.
Feasibility of Meeting Climate Targets Depending on Timeline and Extent of BECCS Implementation
Adapted from Fuss et al., 2018.
Conclusion
Decarbonization is an increasingly pressing issue for the marine sector, and the large GHG emissions reductions promised by biofuels substantiate the rising interest in their use in this sector. For this interest to translate into implementation, the promise and pitfalls of biofuels have to be rigorously scrutinized, and the most promising biofuels identified and prioritized so as to focus resources on their deployment in the shortest possible time frame. This work shows that, among the various biofuel options, bio-methanol, bio-DME, bio-LNG, and bio-oil are the most suited for the marine sector in the medium term owing to their potential for scale-up, advanced production status, and low costs. An in-depth look, combined with stakeholder engagement, provides methanol with a narrow lead over bio-oil as the most promising biofuel, whereas bio-LNG is projected to only be a leading candidate in case re-use of infrastructure installed for the use of fossil LNG becomes a priority. Although the options are close to each other in their overall suitability, it has been explained that they each have different profiles and pros and cons, which need to be considered when deciding on future investments in this area. The implementation of CCS is not presently very high on the list of priorities of the shipping industry, but this may change in line with a greater emphasis on CCS in the broader green energy sector and hence should be kept on the table as a deep decarbonization option.
Limitations of the Study
In this work, the scores given to the candidate biofuels were allotted based on a literature review and stakeholder consultation, and the reliability of the results accordingly depends on the limitations of both parts of the exercise. Although the authors have tried to be comprehensive in the review, it is possible that some relevant literature may have been overlooked. Additionally, interpreting and reconciling disparate literature data to assign a particular value to a fuel is inherently subjective. The results of the stakeholder consultation naturally depend on the participants, and although care was taken to include a broad range of views and areas of expertise, a different set of contributors could possibly have led to different assessments.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors would like to thank Ton Markus for help with the graphical abstract, and the UU strategic theme “Pathways to Sustainability” and the hub “Towards Industry with Negative Emissions” are acknowledged for financial support.
Author Contributions
P.B. and M.J. proposed the topic of this review, provided supervision, and edited the manuscript. A.M. conducted the review and stakeholder analysis and wrote the manuscript.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101758.
Supplemental Information
References
- 't Hart P. Maritime Knowledge Centre, TNO and TU Delft; 2017. Final Report - Framework CO2 Reduction in Shipping. [Google Scholar]
- 2018 Fourth Warmest Year in Continued Warming Trend, According to NASA, NOAA. 2019. https://www.nasa.gov/press-release/2018-fourth-warmest-year-in-continued-warming-trend-according-to-nasa-noaa
- Ail S.S., Dasappa S. Biomass to liquid transportation fuel via Fischer Tropsch synthesis – technology review and current scenario. Renew. Sustainable Energy Rev. 2016;58:267–286. [Google Scholar]
- Akhtar J., Amin N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustainable Energy Rev. 2011;15:1615–1624. [Google Scholar]
- Andersson K., Salazar C.M. FCBI Energy; 2015. Methanol as a Marine Fuel Report. [Google Scholar]
- Arcoumanis C., Bae C., Crookes R., Kinoshita E. The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: a review. Fuel. 2008;87:1014–1030. [Google Scholar]
- Aro E.M. From first generation biofuels to advanced solar biofuels. Ambio. 2016;45(Suppl 1):S24–S31. doi: 10.1007/s13280-015-0730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash N., Scarbrough T. Environmental Defense Fund Europe; 2019. Sailing on Solar. [Google Scholar]
- Azizi Z., Rezaeimanesh M., Tohidian T., Rahimpour M.R. Dimethyl ether: a review of technologies and production challenges. Chem. Eng. Process. Process Intensification. 2014;82:150–172. [Google Scholar]
- Bakhtyari A., Rahimpour M.R. Methanol. 2018. Methanol to Dimethyl Ether; pp. 281–311. [Google Scholar]
- Banse M., van Meijl H., Tabeau A., Woltjer G., Hellmann F., Verburg P.H. Impact of EU biofuel policies on world agricultural production and land use. Biomass and Bioenergy. 2011;35:2385–2390. [Google Scholar]
- Baranowski C.J., Bahmanpour A.M., Kröcher O. Catalytic synthesis of polyoxymethylene dimethyl ethers (OME): a review. Appl. Catal. B: Environ. 2017;217:407–420. [Google Scholar]
- Bellotti D., Rivarolo M., Magistri L., Massardo A.F. Feasibility study of methanol production plant from hydrogen and captured carbon dioxide. J. CO2 Utilization. 2017;21:132–138. [Google Scholar]
- Ben H., Wu F., Wu Z., Han G., Jiang W., Ragauskas A.J. A comprehensive characterization of pyrolysis oil from softwood barks. Polymers. 2019;11:1387. doi: 10.3390/polym11091387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller P., Ross A.B. Production of biofuels via hydrothermal conversion. Handbook Biofuels Prod. 2016:509–547. [Google Scholar]
- Birgen C., Garcia S. Chalmers University of Technology; 2013. Liquefied Synthetic Natural Gas from Woody Biomass. [Google Scholar]
- Boscagli C., Raffelt K., Zevaco T.A., Olbrich W., Otto T.N., Sauer J., Grunwaldt J.-D. Mild hydrotreatment of the light fraction of fast-pyrolysis oil produced from straw over nickel-based catalysts. Biomass and Bioenergy. 2015;83:525–538. [Google Scholar]
- Bouman E.A., Lindstad E., Rialland A.I., Strømman A.H. State-of-the-art technologies, measures, and potential for reducing GHG emissions from shipping – a review. Transportation Res. D: Transport Environ. 2017;52:408–421. [Google Scholar]
- Bozzano G., Manenti F. Efficient methanol synthesis: perspectives, technologies and optimization strategies. Prog. Energy Combustion Sci. 2016;56:71–105. [Google Scholar]
- Bozzano, G., Pirola, C., Italiano, C., Pelosato, R., Vita, A. and Manenti, F. (2017). Biogas: a Possible New Pathway to Methanol? 27th European Symposium on Computer Aided Process Engineering. pp. 523-528.
- Brand S., Kim J. Liquefaction of major lignocellulosic biomass constituents in supercritical ethanol. Energy. 2015;80:64–74. [Google Scholar]
- Bridgwater A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass and Bioenergy. 2012;38:68–94. [Google Scholar]
- Brigagão G.V., de Queiroz Fernandes Araújo O., de Medeiros J.L., Mikulcic H., Duic N. A techno-economic analysis of thermochemical pathways for corncob-to-energy: fast pyrolysis to bio-oil, gasification to methanol and combustion to electricity. Fuel Process. Technology. 2019;193:102–113. [Google Scholar]
- Brown A., Waldheim L., Landälv I., Saddler J., Ebadian M., McMillan J.D., Bonomi A., Klein B. IEA Bioenergy; 2020. Advanced Biofuels – Potential for Cost Reduction. [Google Scholar]
- Bui M., Adjiman C.S., Bardow A., Anthony E.J., Boston A., Brown S., Fennell P.S., Fuss S., Galindo A., Hackett L.A. Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 2018;11:1062–1176. [Google Scholar]
- Butler E., Devlin G., Meier D., McDonnell K. A review of recent laboratory research and commercial developments in fast pyrolysis and upgrading. Renew. Sustainable Energy Rev. 2011;15:4171–4186. [Google Scholar]
- Cai X., Zhang X., Wang D. Land availability for biofuel production. Environ. Sci. Technology. 2011;45:334–339. doi: 10.1021/es103338e. [DOI] [PubMed] [Google Scholar]
- Carpenter D., Westover T.L., Czernik S., Jablonski W. Biomass feedstocks for renewable fuel production: a review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green. Chem. 2014;16:384–406. [Google Scholar]
- Castello D., Pedersen T., Rosendahl L. Continuous hydrothermal liquefaction of biomass: a critical review. Energies. 2018;11:1–35. [Google Scholar]
- Chaplin A.G. Aalborg University; 2013. Renewable Methanol: An Analysis of Technological Potentials in Light of the EU Biofuels Policy Objectives of Greenhouse Gas Savings, Security of Supply and Employment. [Google Scholar]
- Chemical Book Dimethyl Ether. 2020. https://www.chemicalbook.com/ProductChemicalPropertiesCB8765901_EN.htm
- Chen A. 2018. Enerkem: A Waste-To-Biofuels Solution for China. [Google Scholar]
- Cheng S., D’cruz I., Wang M., Leitch M., Xu C. Highly efficient liquefaction of woody biomass in hot-compressed Alcohol−Water Co-solvents†. Energy & Fuels. 2010;24:4659–4667. [Google Scholar]
- Chong K.J., Bridgwater A.V. Fast pyrolysis oil fuel blend for marine vessels. Environ. Prog. Sustainable Energy. 2017;36:677–684. [Google Scholar]
- Choudhary T.V., Phillips C.B. Renewable fuels via catalytic hydrodeoxygenation. Appl. Catal. A: Gen. 2011;397:1–12. [Google Scholar]
- Chryssakis C., Brinks H., King T. DNV GL; 2015. The Fuel Trilemma: Next Generation of Marine Fuels. [Google Scholar]
- Clarke M. Australian Climate Change Policies Face Growing Criticism from Pacific Leaders. 2019. https://www.abc.net.au/news/2019-07-31/australian-climate-change-policies-criticised-pacific-leaders/11369086
- Clausen L.R., Elmegaard B., Houbak N. Technoeconomic analysis of a low CO2 emission dimethyl ether (DME) plant based on gasification of torrefied biomass. Energy. 2010;35:4831–4842. [Google Scholar]
- Cormos A.-M., Dinca C., Petrescu L., Andreea Chisalita D., Szima S., Cormos C.-C. Carbon capture and utilisation technologies applied to energy conversion systems and other energy-intensive industrial applications. Fuel. 2018;211:883–890. [Google Scholar]
- Dalena F., Senatore A., Marino A., Gordano A., Basile M., Basile A. Methanol production and applications: an overview. Methanol. 2018:3–28. [Google Scholar]
- Danielsen F., Beukema H., Burgess N.D., Parish F., Bruhl C.A., Donald P.F., Murdiyarso D., Phalan B., Reijnders L., Struebig M., Fitzherbert E.B. Biofuel plantations on forested lands: double jeopardy for biodiversity and climate. Conserv Biol. 2009;23:348–358. doi: 10.1111/j.1523-1739.2008.01096.x. [DOI] [PubMed] [Google Scholar]
- Das P., Bhatnagar A. Different feedstocks and processes for production of methanol and DME as alternate transport fuels. Prospects Altern. Transportation Fuels. 2018:131–165. [Google Scholar]
- Dekker, E. 2018. Renewable methanol and DME developments. In: Advanced Biofuels Conference, Gothenburg.
- del Álamo G., Sandquist J., Vreugdenhil B.J., Aranda Almansa G., Carbo M. IEA Bioenergy; 2015. Implementation of bio-CCS in biofuels production. [Google Scholar]
- Demonstration of BioDME - Dimethyl Ether as an Advanced Biofuel at Industrial Scale. 2020. http://www.etipbioenergy.eu/value-chains/products-end-use/products/biodme
- Deng H., Meredith W., Uguna C.N., Snape C.E. Impact of solvent type and condition on biomass liquefaction to produce heavy oils in high yield with low oxygen contents. J. Anal. Appl. Pyrolysis. 2015;113:340–348. [Google Scholar]
- Detz R.J., Reek J.N.H., van der Zwaan B.C.C. The future of solar fuels: when could they become competitive? Energy Environ. Sci. 2018;11:1653–1669. [Google Scholar]
- de Vries N. TU Delft; 2019. Safe and Effective Application of Ammonia as a Marine Fuel. [Google Scholar]
- Diebold J.P. National Renewable Energy Laboratory; 2000. A Review of the Chemical and Physical Mechanisms of the Storage Stability of Fast Pyrolysis Bio-Oils. [Google Scholar]
- Dimitriadis A., Bezergianni S. Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: a state of the art review. Renew. Sustainable Energy Rev. 2017;68:113–125. [Google Scholar]
- Dimitriou I., Goldingay H., Bridgwater A.V. Techno-economic and uncertainty analysis of Biomass to Liquid (BTL) systems for transport fuel production. Renew. Sustainable Energy Rev. 2018;88:160–175. [Google Scholar]
- DNV GL . DNV GL; 2016. DNV GL Handbook for Maritime and Offshore Battery Systems. [Google Scholar]
- DNV GL . DNV GL; 2018. Maritime Forecast to 2050. [Google Scholar]
- DNV GL-Maritime . DNV GL-Maritime; 2017. Maritime Forecast to 2050. [Google Scholar]
- DNV GL-Maritime . DNV GL-Maritime; 2019. Assessment of Selected Alternative Fuels and Technologies. [Google Scholar]
- Durak H., Aysu T. Effects of catalysts and solvents on liquefaction of Onopordum heteracanthum for production of bio-oils. Bioresour. Technol. 2014;166:309–317. doi: 10.1016/j.biortech.2014.05.051. [DOI] [PubMed] [Google Scholar]
- E4tech . E4tech; 2018. Master Plan for CO2 Reduction in the Dutch Shipping Sector - Biofuels for Shipping. [Google Scholar]
- Elliott D.C. Production of biofuels via bio-oil upgrading and refining. Handbook Biofuels Prod. 2016:595–613. [Google Scholar]
- Elliott D.C., Biller P., Ross A.B., Schmidt A.J., Jones S.B. Hydrothermal liquefaction of biomass: developments from batch to continuous process. Bioresour. Technol. 2015;178:147–156. doi: 10.1016/j.biortech.2014.09.132. [DOI] [PubMed] [Google Scholar]
- Emissions Database for Global Atmospheric Research . European Commission Joint Research Centre; 2014. Global CO2 Emissions from Fossil Fuel Use and Cement Production 1970-2013. [Google Scholar]
- European Biofuels Technology Platform Dimethyl Ether (DME) 2011. http://www.etipbioenergy.eu/images/AllBiofuelFactsheets2016.pdf
- Fischer G., Prieler S., van Velthuizen H., Berndes G., Faaij A., Londo M., de Wit M. Biofuel production potentials in Europe: sustainable use of cultivated land and pastures, Part II: land use scenarios. Biomass and Bioenergy. 2010;34:173–187. [Google Scholar]
- Fleming S. Chart of the Day: These Countries Create Most of the World’s CO2 Emissions. 2019. https://www.weforum.org/agenda/2019/06/chart-of-the-day-these-countries-create-most-of-the-world-s-co2-emissions/
- Florentinus A., Hamelinck C., van den Bos A., Winkel R., Cuijpers M. Ecofys; 2012. Potential of Biofuels for Shipping. [Google Scholar]
- Frankl P. 2018. IEA Technology Roadmap: The Need to Scale up Sustainable Bioenergy. [Google Scholar]
- Frequently Asked Questions. 2020. https://aboutdme.org/FAQ
- Funkenbusch L.T., Mullins M.E., Vamling L., Belkhieri T., Srettiwat N., Winjobi O., Shonnard D.R., Rogers T.N. Vol. 8. Wiley Interdisciplinary Reviews: Energy and Environment; 2018. Technoeconomic assessment of hydrothermal liquefaction oil from lignin with catalytic upgrading for renewable fuel and chemical production. [Google Scholar]
- Fuss S., Lamb W.F., Callaghan M.W., Hilaire J., Creutzig F., Amann T., Beringer T., de Oliveira Garcia W., Hartmann J., Khanna T. Negative emissions—Part 2: costs, potentials and side effects. Environ. Res. Lett. 2018;13:1–47. [Google Scholar]
- Gasunie BioMCN to Produce Renewable Methanol with Green Hydrogen. 2019. https://www.gasunie.nl/en/news/biomcn-to-produce-renewable-methanol-with-green-hydrogen
- Geraedts S. GoodFuels; 2017. Potential for pyrolysis in the marine market. [Google Scholar]
- GL D Current Price Development Oil and Gas. 2020. https://www.dnvgl.com/maritime/lng/current-price-development-oil-and-gas.html
- Global 20 Ports Average: MGO. 2020. https://shipandbunker.com/prices/av/global/av-g20-global-20-ports-average#MGO
- Global CCS Institute . Global CCS Institute; 2019. Global Status of CCS 2019. [Google Scholar]
- Goeppert A., Czaun M., Jones J.-P., Surya Prakash G.K., Olah G.A. Recycling of carbon dioxide to methanol and derived products – closing the loop. Chem. Soc. Rev. 2014;43:7995–8048. doi: 10.1039/c4cs00122b. [DOI] [PubMed] [Google Scholar]
- Gollakota A.R.K., Kishore N., Gu S. A review on hydrothermal liquefaction of biomass. Renew. Sustainable Energy Rev. 2018;81:1378–1392. [Google Scholar]
- Grijpma P. Utrecht University; 2018. Sustainable Marine Biofuel for the Dutch Bunker Sector. [Google Scholar]
- Groupe International des Importateurs de Gaz . Groupe International des Importateurs de Gaz; 2019. LNG Information Paper #1. [Google Scholar]
- Haarlemmer G., Guizani C., Anouti S., Déniel M., Roubaud A., Valin S. Analysis and comparison of bio-oils obtained by hydrothermal liquefaction and fast pyrolysis of beech wood. Fuel. 2016;174:180–188. [Google Scholar]
- Hackbarth K., Haltenort P., Arnold U., Sauer J. Recent progress in the production, application and evaluation of oxymethylene ethers. Chem. Ingenieur Technik. 2018;90:1520–1528. [Google Scholar]
- Hagos D.A., Ahlgren E. Chalmers University of Technology; 2017. A State-Of-The Art Review on the Development of CNG/LNG Infrastructure and Natural Gas Vehicles (NGVs) [Google Scholar]
- Hallung C. Chalmers University Of Technology; 2015. Evaluation of Alternative Fuels from a Heavy-Duty Vehicle Perspective. [Google Scholar]
- Hamelinck C., Faaij A. Utrecht University; 2001. Future Prospects for Production of Methanol and Hydrogen from Biomass. [Google Scholar]
- Han J., Elgowainy A., Palou-Rivera I., Dunn J.B., Wang M.Q. Argonne National Laboratory; 2011. Well-to-Wheels Analysis of Fast Pyrolysis Pathways with GREET. [Google Scholar]
- Hansson J., Månsson S., Brynolf S., Grahn M. Alternative marine fuels: prospects based on multi-criteria decision analysis involving Swedish stakeholders. Biomass and Bioenergy. 2019;126:159–173. [Google Scholar]
- Haverly M.R., Schulz T.C., Whitmer L.E., Friend A.J., Funkhouser J.M., Smith R.G., Young M.K., Brown R.C. Continuous solvent liquefaction of biomass in a hydrocarbon solvent. Fuel. 2018;211:291–300. [Google Scholar]
- Havlík P., Schneider U.A., Schmid E., Böttcher H., Fritz S., Skalský R., Aoki K., Cara S.D., Kindermann G., Kraxner F. Global land-use implications of first and second generation biofuel targets. Energy Policy. 2011;39:5690–5702. [Google Scholar]
- Hsieh C.-w.C., Felby C. IEA Bioenergy; 2017. Biofuels for the Marine Shipping Sector. [Google Scholar]
- Huang H.-j., Yuan X.-z. Recent progress in the direct liquefaction of typical biomass. Prog. Energy Combustion Sci. 2015;49:59–80. [Google Scholar]
- Hughes D. LNG, a Fuel for the Future? Not Everyone Agrees. 2019. https://www.businesstimes.com.sg/transport/lng-a-fuel-for-the-future-not-everyone-agrees
- Huisman G.H., Van Rens G.L.M.A., De Lathouder H., Cornelissen R.L. Cost estimation of biomass-to-fuel plants producing methanol, dimethylether or hydrogen. Biomass and Bioenergy. 2011;35:S155–S166. [Google Scholar]
- Iaquaniello G., Centi G., Salladini A., Palo E., Perathoner S., Spadaccini L. Waste-to-methanol: process and economics assessment. Bioresour. Technol. 2017;243:611–619. doi: 10.1016/j.biortech.2017.06.172. [DOI] [PubMed] [Google Scholar]
- Ikealumba W.C., Wu H. Some recent advances in liquefied natural gas (LNG) production, spill, dispersion, and safety. Energy & Fuels. 2014;28:3556–3586. [Google Scholar]
- Imran Khan M. Policy options for the sustainable development of natural gas as transportation fuel. Energy Policy. 2017;110:126–136. [Google Scholar]
- Intergovernmental Panel on Climate Change . Intergovernmental Panel on Climate Change; 2018. Global Warming of 1.5°C: Summary for Policymakers. [Google Scholar]
- International Chamber of Shipping . International Chamber of Shipping; 2018. Reducing CO2 Emissions to Zero: The ‘Paris Agreement for Shipping’. [Google Scholar]
- International Maritime Organization . International Maritime Organization; 2009. Second IMO GHG Study 2009. [Google Scholar]
- International Maritime Organization . International Maritime Organization; 2015. Third IMO Greenhouse Gas Study 2014. [Google Scholar]
- International Maritime Organization . International Maritime Organization; 2018. Resolution MEPC.304(72) [Google Scholar]
- International Maritime Organization . International Maritime Organization; 2020. Fourth IMO GHG Study 2020. [Google Scholar]
- International Renewable Energy Agency . International Renewable Energy Agency; 2016. Innovation Outlook: Advanced Liquid Biofuels. [Google Scholar]
- International Renewable Energy Agency . International Renewable Energy Agency; 2018. Global Energy Transformation: A Roadmap to 2050. [Google Scholar]
- IRENA . IRENA; 2019. Navigating the way to a renewable future: solutions to decarbonise shipping. [Google Scholar]
- Isa K.M., Abdullah T.A.T., Ali U.F.M. Hydrogen donor solvents in liquefaction of biomass: a review. Renew. Sustainable Energy Rev. 2018;81:1259–1268. [Google Scholar]
- Jiang J. Are Ships Set for Shorter Lifespans? 2017. https://splash247.com/ships-set-shorter-lifespans/
- Jiang J. IMO 2020 and Demolition Volumes. 2020. https://splash247.com/imo-2020-and-demolition-volumes/
- Karatzos S., McMillan J.D., Saddler J. IEA Bioenergy; 2014. The Potential and Challenges of Drop-In Biofuels. [Google Scholar]
- Keeney R., Hertel T.W. The indirect land use impacts of United States biofuel policies: the importance of acreage, yield, and bilateral trade responses. Am. J. Agric. Econ. 2009;91:895–909. [Google Scholar]
- Kennedy R. 'Reaching End Game': New Paper on Climate Change Raises Alarm. 2019. https://www.aljazeera.com/news/2019/06/game-paper-climate-change-raises-alarm-190608101953511.html
- Kesieme U., Pazouki K., Murphy A., Chrysanthou A. Biofuel as an alternative shipping fuel: technological, environmental and economic assessment. Sustainable Energy Fuels. 2019;3:899–909. [Google Scholar]
- Kim T.Y., Lee S.H. Combustion and emission characteristics of wood pyrolysis oil-butanol blended fuels in a DI diesel engine. Int. J. Automotive Technology. 2015;16:903–912. [Google Scholar]
- Kirstein L., Halim R., Merk O. International Transport Forum; 2018. Decarbonising maritime transport: pathways to zero-carbon shipping by 2035. [Google Scholar]
- Koltsova O. Making Crude Oil from Paper Pulp? Vertoro Creates This ‘green Gold’. 2018. https://innovationorigins.com/making-crude-oil-from-paper-pulp-vertoro-makes-this-green-gold/
- Kopis M. 2019. W2C Rotterdam Project Welcomes Shell as Partner. [Google Scholar]
- Kumar A., Ogita S., Yau Y.-Y. Springer; 2018. Biofuels: Greenhouse Gas Mitigation and Global Warming. [Google Scholar]
- Landälv I. Luleå University of Technology; 2017. Methanol as a Renewable Fuel – A Knowledge Synthesis. [Google Scholar]
- Lange J.P. Lignocellulose liquefaction to biocrude: a tutorial review. ChemSusChem. 2018;11:997–1014. doi: 10.1002/cssc.201702362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapola D.M., Schaldach R., Alcamo J., Bondeau A., Koch J., Koelking C., Priess J.A. Indirect land-use changes can overcome carbon savings from biofuels in Brazil. Proc. Natl. Acad. Sci. U S A. 2010;107:3388–3393. doi: 10.1073/pnas.0907318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas A., Heracleous E. Handbook of Biofuels Production; 2016. Production of biofuels via Fischer–Tropsch synthesis; pp. 549–593. [Google Scholar]
- Lehto J., Oasmaa A., Solantausta Y., Kytö M., Chiaramonti D. VTT Technical Research Centre of Finland; 2013. Fuel Oil Quality and Combustion of Fast Pyrolysis Bio-Oils. [Google Scholar]
- Leung D.Y.C., Caramanna G., Maroto-Valer M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustainable Energy Rev. 2014;39:426–443. [Google Scholar]
- Liang L.H. LNG as Fuel Garners Interest but Still a Long Way to Go. 2019. https://www.seatrade-maritime.com/asia/lng-fuel-garners-interest-still-long-way-go
- Linde . Linde; 2011. Liquefied natural gas material safety data sheet. [Google Scholar]
- Liu Z., Zhang F.-S. Effects of various solvents on the liquefaction of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2008;49:3498–3504. [Google Scholar]
- LNG positioned as greenest marine fuel. 2019. https://www.rina.org.uk/LNG_positioned_as_greenest_marine_fuel.html
- Luo Z., Wang S., Liao Y., Zhou J., Gu Y., Cen K. Research on biomass fast pyrolysis for liquid fuel. Biomass and Bioenergy. 2004;26:455–462. [Google Scholar]
- Maniatis K., Landälv I., Waldheim L., Heuvel E.v.d., Kalligeros S. European Commission Sustainable Transport Forum Sub Group on Advanced Biofuels; 2017. Final Report: Building up the Future. [Google Scholar]
- Marsman J.H., Wildschut J., Mahfud F., Heeres H.J. Identification of components in fast pyrolysis oil and upgraded products by comprehensive two-dimensional gas chromatography and flame ionisation detection. J. Chromatogr. A. 2007;1150:21–27. doi: 10.1016/j.chroma.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Minowa T., Potic B., Kersten S., Prins W., Vanswaaij W., Vandebeld B., Elliott D., Neuenschwander G., Kruse A. Biomass gasification in near- and super-critical water: status and prospects. Biomass and Bioenergy. 2005;29:269–292. [Google Scholar]
- Mazaheri H., Lee K.T., Bhatia S., Mohamed A.R. Sub/supercritical liquefaction of oil palm fruit press fiber for the production of bio-oil: effect of solvents. Bioresour. Technol. 2010;101:7641–7647. doi: 10.1016/j.biortech.2010.04.072. [DOI] [PubMed] [Google Scholar]
- McGrath M. Climate Change: 12 Years to Save the Planet? Make that 18 Months. 2019. https://www.bbc.com/news/science-environment-48964736
- McGrath M. Climate Change: UK's 10 Warmest Years All Occurred since 2002. 2019. https://www.bbc.com/news/science-environment-49167797
- Methanex Corporation . Methanex Corporation; 2020. Methanex Monthly Average Regional Posted Contract Price History. [Google Scholar]
- Mohabeer C.C. Ph.D. thesis, L’Institut National des Sciences Appliquées de Rouen Normandie; 2019. Bio-oil production by pyrolysis of biomass coupled with a catalytic de-oxygenation treatment. [Google Scholar]
- Mohan D., Pittman C.U., Steele P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy & Fuels. 2006;20:848–889. [Google Scholar]
- Moirangthem K. Joint Research Centre, European Commission; 2016. Alternative Fuels for Marine and Inland Waterways. [Google Scholar]
- Molino A., Larocca V., Chianese S., Musmarra D. Biofuels production by biomass gasification: a review. Energies. 2018;11:1–31. [Google Scholar]
- Mortensen P.M., Grunwaldt J.D., Jensen P.A., Knudsen K.G., Jensen A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A: Gen. 2011;407:1–19. [Google Scholar]
- Mun S.P., Jang J.P. Liquefaction of cellulose in the presence of phenol using p-toluene sulfonic acid as a catalyst. J. Ind. Eng. Chem. 2009;15:743–747. [Google Scholar]
- Nattrass L., Ripken R., Taylor R., Bauen A., Twisse F., Groves L., Bates J., O’Brien S. Arup URS Consortium; 2014. Advanced biofuel demonstration competition: feasibility study. [Google Scholar]
- Neste Biodiesel prices (SME & FAME) 2020. https://www.neste.com/investors/market-data/biodiesel-prices-sme-fame
- Riviera Newsdesk LNG Fuel and the Ship Emissions Debate. 2018. https://www.rivieramm.com/opinion/opinion/lng-fuel-and-the-ship-emissions-debate-23849
- Nyström I., Bokinge P., Franck P.-Å. CIT Industriell Energi AB; 2019. Production of liquid advanced biofuels - global status. [Google Scholar]
- Oasmaa A., Peacocke C. Vol. 731. VTT Publications: Finland; 2010. Properties and fuel use of biomass-derived fast pyrolysis liquids; p. p79. [Google Scholar]
- Oasmaa A., Elliott D.C., Korhonen J. Acidity of biomass fast pyrolysis bio-oils. Energy & Fuels. 2010;24:6548–6554. [Google Scholar]
- Obydenkova S.V., Kouris P.D., Hensen E.J.M., Heeres H.J., Boot M.D. Environmental economics of lignin derived transport fuels. Bioresour. Technol. 2017;243:589–599. doi: 10.1016/j.biortech.2017.06.157. [DOI] [PubMed] [Google Scholar]
- Olah A.G.A., Prakash G.S. Wiley-VCH; 2011. Beyond Oil and Gas: The Methanol Economy, 2nd, Updated and Enlarged Edition. [Google Scholar]
- Olmer N., Comer B., Roy B., Mao X., Rutherford D. International Council on Clean Transportation; 2017. Greenhouse Gas Emissions from Global Shipping, 2013–2015. [Google Scholar]
- Ondir Freire L., de Andrade D.A. Economically feasible mobile nuclear power plant for merchant ships and remote clients. Nucl. Technology. 2018;205:766–780. [Google Scholar]
- Padella M., O’Connell A., Prussi M. What is still limiting the deployment of cellulosic ethanol? Analysis of the current status of the sector. Appl. Sci. 2019;9:1–13. [Google Scholar]
- Padmanabhan V., Alexander S., Srivastava P. The Growing Threat of Climate Change in India. 2019. https://www.livemint.com/news/india/the-growing-threat-of-climate-change-in-india-1563716968468.html
- Peng X.D., Wang A.W., Toseland B.A., Tijm P.J.A. Single-step syngas-to-dimethyl ether processes for optimal productivity, minimal emissions, and natural gas-derived syngas. Ind. Eng. Chem. Res. 1999;38:4381–4388. [Google Scholar]
- Peral E., Martín M. Optimal production of dimethyl ether from switchgrass-based syngas via direct synthesis. Ind. Eng. Chem. Res. 2015;54:7465–7475. [Google Scholar]
- Peralta P C., Vieira G., Meunier S., Vale R., Salles M., Carmo B. Evaluation of the CO2 emissions reduction potential of Li-ion batteries in ship power systems. Energies. 2019;12:1–19. [Google Scholar]
- Perkins G., Bhaskar T., Konarova M. Process development status of fast pyrolysis technologies for the manufacture of renewable transport fuels from biomass. Renew. Sustainable Energy Rev. 2018;90:292–315. [Google Scholar]
- Peters J.F., Iribarren D., Dufour J. Simulation and life cycle assessment of biofuel production via fast pyrolysis and hydroupgrading. Fuel. 2015;139:441–456. [Google Scholar]
- Pimentel D., Marklein A., Toth M., Karpoff M., Paul G., McCormack R., Kyriazis J., Krueger T. Biofuel impacts on world food supply: use of fossil fuel, land and water resources. Energies. 2008;1:41–78. [Google Scholar]
- Piskorz J., Majerski P., Radlein D., Scott D.S., Bridgwater A.V. Fast pyrolysis of sweet sorghum and sweet sorghum bagasse. J. Anal. Appl. Pyrolysis. 1998;46:15–29. [Google Scholar]
- Poel-Tec Straight Vegetable Oil SVO as Diesel Replacement Fuel. 2019. https://www.poel-tec.com/english/straight_vegetable_oil.php
- Protocol G.G. Global Warming Potential Values. 2016. https://www.ghgprotocol.org/sites/default/files/ghgp/Global-Warming-Potential-Values%20%28Feb%2016%202016%29_1.pdf
- Psaraftis H.N. Market-based measures for greenhouse gas emissions from ships: a review. WMU J. Maritime Aff. 2012;11:211–232. [Google Scholar]
- PubChem Dimethyl Ether. 2020. https://pubchem.ncbi.nlm.nih.gov/compound/Dimethyl-ether
- PubChem Methanol. 2020. https://pubchem.ncbi.nlm.nih.gov/compound/Methanol
- Rauch R., Hrbek J., Hofbauer H. Vol. 3. Wiley Interdisciplinary Reviews: Energy and Environment; 2014. Biomass gasification for synthesis gas production and applications of the syngas; pp. 343–362. [Google Scholar]
- Ravindranath N.H., Sita Lakshmi C., Manuvie R., Balachandra P. Biofuel production and implications for land use, food production and environment in India. Energy Policy. 2011;39:5737–5745. [Google Scholar]
- REN21 . REN21; 2019. Renewables 2019 Global Status Report. [Google Scholar]
- Rogers J.G., Brammer J.G. Vol. 36. Biomass and Bioenergy; 2012. Estimation of the production cost of fast pyrolysis bio-oil; pp. 208–217. [Google Scholar]
- Roosjen S. Methane Catalyst for LNG Engines. 2019. http://marigreen.eu/wordpress_marigreen/wp-content/uploads/2019/07/4.-Factsheet-A5-Methane-Catalyst.pdf
- Roy P., Dias G. Prospects for pyrolysis technologies in the bioenergy sector: a review. Renew. Sustainable Energy Rev. 2017;77:59–69. [Google Scholar]
- Santos A.S., Gilio L., Halmenschlager V., Diniz T.B., Almeida A.N. Flexible-fuel automobiles and CO 2 emissions in Brazil: parametric and semiparametric analysis using panel data. Habitat Int. 2018;71:147–155. [Google Scholar]
- Saul J., Chestney N. New Fuel Rules Push Shipowners to Go Green with LNG. 2018. https://www.reuters.com/article/us-shipping-fuel-lng-analysis/new-fuel-rules-push-shipowners-to-go-greenwith-lng-idUSKBN1L01I8
- Scheutz C., Kjeldsen P., Bogner J.E., De Visscher A., Gebert J., Hilger H.A., Huber-Humer M., Spokas K. Microbial methane oxidation processes and technologies for mitigation of landfill gas emissions. Waste Manag. Res. 2009;27:409–455. doi: 10.1177/0734242X09339325. [DOI] [PubMed] [Google Scholar]
- Schiermeier Q. Climate Change Made Europe’s Mega-Heatwave Five Times More Likely. 2019. https://www.nature.com/articles/d41586-019-02071-z [DOI] [PubMed]
- Schøyen H., Steger-Jensen K. Nuclear propulsion in ocean merchant shipping: the role of historical experiments to gain insight into possible future applications. J. Clean. Prod. 2017;169:152–160. [Google Scholar]
- Singh R., Bhaskar T., Balagurumurthy B. Vol. 93. Process Safety and Environmental Protection; 2015. Effect of solvent on the hydrothermal liquefaction of macro algae Ulva fasciata; pp. 154–160. [Google Scholar]
- Sivebaek I.M., Jakobsen J. The viscosity of dimethyl ether. Tribology Int. 2007;40:652–658. [Google Scholar]
- Smallwood I. Butterworth-Heinemann; 2012. Handbook of Organic Solvent Properties. [Google Scholar]
- Smith T. Why LNG as the Ship Fuel of the Future Is a Massive Red Herring. 2018. https://splash247.com/lng-ship-fuel-future-massive-red-herring/
- Smith T., Raucci C., Hosseinloo S.H., Rojon I., Calleya J., Suarez De La Fuente S., Wu P., Palmer K. 2016. CO2 Emissions from International Shipping. [Google Scholar]
- Soest J.P.v., Wiltink H., Soest H.v., Luken E., Londo M., Mozaffarian H., Himbergen H.v., Dorp R.v., Mechelen X.v., Dumont M., Wismeijer R. De Gemeynt, ECN, Groen Gas Nederland, RVO.nl; 2014. Green Gas Roadmap. [Google Scholar]
- Statistics B o T Diesel and Jet Fuel Prices. 2020. https://www.bts.gov/diesel-and-jet-fuel-prices
- Stumborg M., Wong A., Hogan E. Hydroprocessed vegetable oils for diesel fuel improvement. Bioresour. Technology. 1996;56:13–18. [Google Scholar]
- Sun R., Delidovich I., Palkovits R. Dimethoxymethane as a cleaner synthetic fuel: synthetic methods, catalysts, and reaction mechanism. ACS Catal. 2019;9:1298–1318. [Google Scholar]
- Sundqvist T., Oasmaa A., Koskinen A. Upgrading fast pyrolysis bio-oil quality by esterification and azeotropic water removal. Energy & Fuels. 2015;29:2527–2534. [Google Scholar]
- Svanberg M., Ellis J., Lundgren J., Landälv I. Renewable methanol as a fuel for the shipping industry. Renew. Sustainable Energy Rev. 2018;94:1217–1228. [Google Scholar]
- Tekin K., Karagöz S. Non-catalytic and catalytic hydrothermal liquefaction of biomass. Res. Chem. Intermediates. 2012;39:485–498. [Google Scholar]
- Thomson H., Corbett J.J., Winebrake J.J. Natural gas as a marine fuel. Energy Policy. 2015;87:153–167. [Google Scholar]
- Toor S.S., Rosendahl L., Rudolf A. Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy. 2011;36:2328–2342. [Google Scholar]
- Trakakis A. Methane Slip from LNG Fueled Engines. 2018. https://safety4sea.com/methane-slip-from-lng-fueled-engines/
- Tronstad T., Åstrand H.H., Haugom G.P., Langfeldt L. DNV GL; 2016. Study on the Use of Fuel Cells in Shipping. [Google Scholar]
- Tybirk K., Solberg F.E., Wennerberg P., Wiese F., Danielsen C.G. 2018. Biogas Liquefaction and Use of Liquid Biomethane. [Google Scholar]
- Tzanetis K.F., Posada J.A., Ramirez A. Analysis of biomass hydrothermal liquefaction and biocrude-oil upgrading for renewable jet fuel production: the impact of reaction conditions on production costs and GHG emissions performance. Renew. Energy. 2017;113:1388–1398. [Google Scholar]
- Union I.G. International Gas Union; 2020. 2020 world LNG report. [Google Scholar]
- United Nations Conference on Trade and Development . United Nations Conference on Trade and Development; 2019. Review of Maritime Transport 2019. [Google Scholar]
- University of Birmingham . The Energy and Fuel Data Sheet. University of Birmingham; 2011. [Google Scholar]
- van Biert L., Godjevac M., Visser K., Aravind P.V. A review of fuel cell systems for maritime applications. J. Power Sourc. 2016;327:345–364. [Google Scholar]
- van den Borne B. TU Delft; 2015. Exploring Policy Options to Stimulate Bio LNG in the Netherlands. [Google Scholar]
- Vasudevan S., Farooq S., Karimi I.A., Saeys M., Quah M.C.G., Agrawal R. Energy penalty estimates for CO2 capture: comparison between fuel types and capture-combustion modes. Energy. 2016;103:709–714. [Google Scholar]
- Venderbosch R.H., Prins W. Fast pyrolysis technology development. Biofuels, Bioproducts and Biorefining. 2010;4:178–208. [Google Scholar]
- Vaughan N.E., Gough C., Mander S., Littleton E.W., Welfle A., Gernaat D.E.H.J., van Vuuren D.P. Evaluating the use of biomass energy with carbon capture and storage in low emission scenarios. Environ. Res. Lett. 2018;13:1–11. [Google Scholar]
- Venderbosch R.H., Ardiyanti A.R., Wildschut J., Oasmaa A., Heeres H.J. Stabilization of biomass-derived pyrolysis oils. J. Chem. Technology Biotechnol. 2010;85:674–686. [Google Scholar]
- Verbeek R., Verbeek M. TNO; 2015. LNG for Trucks and Ships: Fact Analysis. [Google Scholar]
- Vienescu D.N., Wang J., Le Gresley A., Nixon J.D. A life cycle assessment of options for producing synthetic fuel via pyrolysis. Bioresour. Technol. 2018;249:626–634. doi: 10.1016/j.biortech.2017.10.069. [DOI] [PubMed] [Google Scholar]
- Wahl H. CO2 Reduction Obligation – a Way to Combine Advanced Biofuels and Global Afforestation. 2018. https://www.svebio.se/app/uploads/2016/10/ABC-2018-programme-FINAL.pdf
- Wang S. Biomass Now - Sustainable Growth and Use; 2013. High-Efficiency Separation of Bio-Oil. [Google Scholar]
- Wang T., Li Y., Ma L., Wu C. Frontiers of Energy and Power Engineering in China. 2010. Biomass to dimethyl ether by gasification/synthesis technology-an alternative biofuel production route. [Google Scholar]
- Wang Y., Wang H., Lin H., Zheng Y., Zhao J., Pelletier A., Li K. Effects of solvents and catalysts in liquefaction of pinewood sawdust for the production of bio-oils. Biomass and Bioenergy. 2013;59:158–167. [Google Scholar]
- World Bioenergy Association . World Bioenergy Association; 2019. Global Bioenergy Statistics 2019. [Google Scholar]
- Xue Y., Chen H., Zhao W., Yang C., Ma P., Han S. A review on the operating conditions of producing bio-oil from hydrothermal liquefaction of biomass. Int. J. Energy Res. 2016;40:865–877. [Google Scholar]
- Yaws C.L., Yaws C. McGraw-Hill Education; 1999. Chemical Properties Handbook: Physical, Thermodynamics, Environmental Transport, Safety & Health Related Properties for Organic &. [Google Scholar]
- Yip J., Chen M., Szeto Y.S., Yan S. Comparative study of liquefaction process and liquefied products from bamboo using different organic solvents. Bioresour. Technology. 2009;100:6674–6678. doi: 10.1016/j.biortech.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Rahardjo S.T., Valkenburg C., Snowden-Swan L., Jones S., Machinal M. U.S. Department of Energy; 2011. Techno-economic Analysis for the Thermochemical Conversion of Biomass to Liquid Fuels. [Google Scholar]
- Zhu Y., Biddy M.J., Jones S.B., Elliott D.C., Schmidt A.J. Techno-economic analysis of liquid fuel production from woody biomass via hydrothermal liquefaction (HTL) and upgrading. Appl. Energy. 2014;129:384–394. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.