Abstract
Chronic stress affects brain function, so assessing its hazards is important for mental health. To overcome the limitations of behavioral data, we combined behavioral and event-related potentials (ERPs) in an attention network task. This task allowed us to differentiate between three specific aspects of attention: alerting, orienting, and execution. Forty-one participants under chronic stress and 31 non-stressed participants were enrolled. On the performance level, the chronically stressed group showed a significantly slower task response and lower accuracy. Concerning ERP measures, smaller cue–N1, cue–N2, and larger cue–P3 amplitudes were found in the stressed group, indicating that this group was less able to assign attention to effective information, i.e., they made inefficient use of cues and had difficulty in maintaining alerting. In addition, the stressed group showed larger target–N2 amplitudes, indicating that this group needed to allocate more cognitive resources to deal with the conflict targets task. Subgroup analysis revealed lower target–P3 amplitudes in the stressed than in the non-stressed group. Group differences associated with the attention networks were found at the ERP level. In the stressed group, excessive depletion of resources led to changes in attention control. In this study, we examined the effects of chronic stress on individual executive function from a neurological perspective. The results may benefit the development of interventions to improve executive function in chronically stressed individuals.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00549-9) contains supplementary material, which is available to authorized users.
Keywords: Stress, Attention, Event-related potential, Attention control network task
Introduction
It is well known that stress has adverse effects on individual health [1]. Long-term chronic stress can have a variety of adverse effects on daily work, life, and learning [2], thus inducing corresponding changes in common behavioral patterns [3]. The American Psychological Association defines chronic stress as “stimulation lasting for a period of time, which may lead to mental and physical weakness” [4].
Like many chronic diseases, chronic stress is also considered a form of prolonged distress, which can potentially impact on individual mental health and cognitive function [5]. Individuals exposed to many stressors may also have a range of physiological and psychological responses, including distraction [6], anxiety [7], insomnia [8], muscle pain [9], hypertension, and an impaired immune system [10], and stressors can cause major diseases such as heart disease and depression [11]. Chronic stress can promote the production of dysfunctional immune cells, which further amplifies the impairment of immune function [10], thereby increasing the risk of physical threats [12]. Most of the stressors are long-term, persistent throughout life processes, or even lasting an individual’s lifetime, impairing the cognitive function of individuals [13, 14]. Prolonged stress is associated with impaired cognitive function [15, 16].
Attention control is a common concept in cognitive psychology, but its definition varies throughout the literature. Niederdeppe et al. argued that attention control includes the operation of competition planning mechanisms and the attention monitoring system (supervisory attentional system), the former being used to make decisions between conflict behaviors and the latter being used in situations where planning and decision-making face challenges [17]. Shipstead et al. believed that attention control reflects an individual’s ability to consciously activate, focus, and maintain attention to memory representations when confronted with irrelevant information interference [18]. Therefore, attention control is an important part of the executive control system, regulating goal orientation and associated with emotional information processing [19, 20].
Researchers have often used different tasks to explore the characteristics of attention control in a target population for different research purposes [21]. The attention network test [22] combines an arrowhead flanking task [23] with an attention cue paradigm [24], and can be used to explore attention control based on complex nervous system interactions. The system has three functionally and anatomically separate networks with specific and separable forms of attention, namely alarm, orientation, and executive attention. Executive attention networks are also known as collision networks [25].
The relationship between human stress and cognition was reported by Lupien et al. [26]. A previous study suggested that a highly-stressed group had significant deficits in attention and memory compared to two non-stress groups. Cohen and colleagues believed that emotions and attention are interrelated, and the influence of negative emotions on attention is crucial to human survival [27]. A previous study from our team has shown that negative emotions generally interfere with conflict monitoring and behavioral inhibition, causing a smaller P3 amplitude [28].
Morrison suggested that the attention bias of negative information interacts with life stress [29]. Excessive negative emotions lead to attention weakness. An event-related potential (ERP) study reported the effect of examination-induced academic stress on anticipation in information processing [30]; the author suggested that individuals experiencing higher academic stressors have a greater contingent negative variation, indicating that the stressed group consumed more neural resources than the control group. So it has been suggested that long-term stress may change brain function by impairing neural efficiency [31]. Moreover, individuals with post-traumatic stress disorder (PTSD) show deficits in attention allocation and emotional regulation when dealing with trauma cues, while those without PTSD are able to regulate emotions by directing attention away from threats [32].
ERPs allow the recording of neural activity in response to visual stimuli with high temporal resolution. Recent studies have demonstrated that ERPs generated during the attention network task (ANT) show differential effects of attention networks at various scalp locations, and such modulation varies between populations [33, 34]. In particular, anterior N1, P2, N2, and P3 may be involved in both alerting and orienting, while frontal–central N2 and midline P3 components seem to be involved in executive control.
N1 (attention effect) is a negative component in a time window of 100 ms–200 ms that occurs in the frontal region [35]. N1 is an early attentional electroencephalographic (EEG) indicator in the decision-making process and can characterize the early attention process. N1 likely marks the early visual processing of stimulus properties that is enhanced under conditions of heightened attention, as it tends to increase in amplitude when visual stimuli are presented to an attended-to location compared to a non-attended location [36]. The N1 effect is also reflected in the identification of conflict situations, with greater N1 volatility induced by mutually beneficial situations [37]. P2 is a positive component after N1 at ~200 ms that occurs in the central region [38]. P2 is associated with the early recognition of a target stimulus, often accompanied by N2b production at the back of the scalp.
N2 is a negative component at 200 ms after the onset of stimulation and is thought to be involved in the process of monitoring or resolving conflicts [39, 40]. When conflicts are discovered, top-down resources are recruited to improve stimulus assessment [41, 42]. Some previous studies combining the ERP with the flank conditions used in the ANT showed that the N2 of the incongruent target is significantly greater than that of the congruent target at the center of the leading edge [41, 42]. N2 mainly reflects the conflict processing before a correct response. A smaller N2 amplitude represents a decline in executive function, and the ability to monitor and resist interference is weakened.
P3 is a positive component that reaches a peak value 250 ms–500 ms after the target stimulus is presented, reflecting cognitive resource allocation in a later stage of information processing. It has mainly been used in studies of attention, memory, emotion, decision-making, and result evaluation [43]. Cue–P3 reflects the implicit attention orientation of potential targets. It precedes the reaction control process in target processing, the amount of cortical activity associated with processing incoming information, and the selection and suppression processes [44]. Target–P3 is associated with executive control. Under flank conditions, the incongruent target causes a decrease in the peak amplitude of the central and parietal P3 relative to the ipsilateral target stimulus [45, 46].
So far, few studies have combined ERPs with the ANT in individuals under chronic stress. Therefore, studying ERPs in relation to the ANT in such individuals could highlight the neuronal processes underlying the attention networks in chronic stress. Previous studies on the effect of stress on attention have mostly involved a level of laboratory manipulation of acute stress [47], and few have discussed the impact of long-term chronic stress on individuals’ attention control. Therefore, the purpose of this study was to explore ERPs associated with the ANT in individuals under chronic stress and non-stressed controls. In the current study, students who faced a postgraduate examination comprised the chronic stress group because of their prolonged chronic stress.
Based on the above discussion, we hypothesized that: (1) chronic stress group induces higher error rates and slower response times (RTs) and between-group difference with regard to RTs; In our data, we could observe a network effect in behavior. Alerting effect was as follows: double cues induced faster response time and had higher accuracy than no cues; Orienting effects: the spatial cues were faster than center cues; Executive effect was also observed in which incongruent RTs were slower than congruent targets. (2) a network effect also observe in ERPs. At the Cue-related ERPs, i.e., Cue-N1, Cue-P2, Cue-N2, Cue-P3, we could found alerting and orienting effects. At the Target-related ERPs, we could found executive effect. Moreover, we aimed to investigate between-group differences in different attention networks. Chronic stress overloads the attention system, thereby reducing the amount of attention resources available to allocate to less relevant information. This can be reflected in the N2 and P3 component. Cue-N2 was larger in the chronic stress than non-stressed group, Cue-P3 was smaller in the chronic stress than non-stressed group. So it was with Target-N2 and Target-P3.
Materials and Methods
Participants
We included 135 students from Southwest University who completed the PSS (Perceived Stress Scale), SRQ (Stress Response Questionnaire), PCL-C (PTSD checklist-civilian version), SLSI (Student-Life Stress Inventory), BDI (Beck Depression Scale), and STAI (State-trait Anxiety Inventory) surveys [48–54]. Participants whose PSS score was >28 and SLSI score was >1x+s were included in the chronic stress group [53, 54] and participants whose PSS score was <28 and SLSI score was <1x+s were included in the non-stressed group. All PCL-C scores had to be <50 and BDI scores <13 [55–57]. All STAI scores also had to be lower than the normal healthy Chinese adult score. Finally, we selected 72 healthy undergraduate students: 41 stressed candidates (21 females) from the postgraduate entrance examination group (who were due to take the 2018 postgraduate entrance examination) and 31 non-stressed candidates (15 females, who did not participate in the postgraduate entrance examination and had no other major examinations/interviews or other major events in the recent or following month). All participants reported being right-handed and having normal or corrected-to-normal vision. To prevent the influence of gender on the results, gender was controlled before the participants joined the study. In addition, we included a gender difference analysis. Before starting the experiment, all participants read the instructions and could ask questions about the experiment before giving written consent to participate. This study was approved by the Southwest University Ethics Committee. All participants completed the ANT task while EEG data were recorded.
Attention Network Test
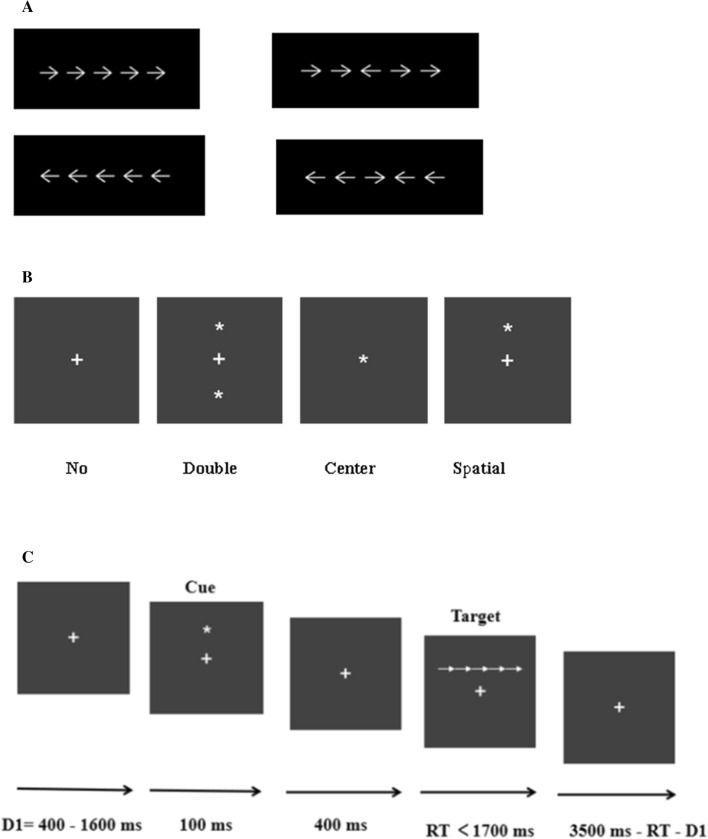
The ANT was used to investigate three network functions: attention alerting, orienting, and executive function [57]. The stimuli appeared in white against a black background, and included fixation points, cues, and target stimuli. Participants used two keys to respond to the direction of the center arrow. If the arrow pointed to the left, the participant pressed “F” with the left hand, and if the arrow pointed to the right, the participant pressed “J” with right hand. These response keys were marked with red stickers. The center arrow that required an answer appeared directly above or below the center fixation cross, with two arrows on the left and right sides, in the same (congruent) or opposite (incongruent) direction. In addition, there were four cue conditions before the target: no cue, double cue, center cue, or spatial cue. The locations of the four cue conditions are shown in Fig. 1.
Fig. 1.
Schematic of the experimental task. A The two target conditions. B The four cue conditions. C The sequence of events for the ANT used in the present study.
In the task, a fixation point appeared for 400 ms–1600 ms after which the cues were presented for 100 ms; then a fixation point was presented for 400 ms; finally, the targets were presented on the monitor until the participants responded. If participants did not respond, the targets would automatically disappear after 1700 ms. The total duration of each trial was 4000 ms. The ANT consisted of one practice block of 20 trials, followed by six test blocks of 572 trials. The averaged responses were only included in further analyses if at least 20 artefact-free segments were obtained for trials with correct responses. All channels were removed in segments containing artefacts in any channel. On average, the analyses were based on ~144 artefact-free trials per cue condition and on ~288 trials per congruent/incongruent condition. The task lasted ~30 min. Participants were instructed to remain as still as possible and to minimize their eye-blinks in order to reduce experimental artifacts during EEG data collection. The original experimental data of 72 participants were analyzed, and no response time and accuracy values were exceeded (three standard deviations). Therefore, the data from all participants were retained for statistical analysis. When examining the response of a participant’s correct response, after trials with a response time <200 ms and extreme data (>3 standard deviations) were excluded, the response times and accuracy rates were calculated for the corresponding conditions.
Procedure
Participants were asked not to smoke or drink coffee before experiments. After giving written consent, participants performed the ANT while EEG data were recorded. The procedure was approved by the Ethics Committee of the Faculty of Psychology of Southwest University.
Behavioral Analysis
A 2 (group: chronically stressed versus non-stressed) × 4 (cue: no, center, double, spatial) × 2 (target: congruent, incongruent) analysis of variance (ANOVA) was conducted for RTs and accuracy with regard to the different attention networks. Responses that occurred after the 1700 ms response deadline or were incorrect were not included in the RT analysis. To assess alertness, we compared the no and double cue situations. In terms of orienting, a comparison between center and spatial cues was conducted. Finally, the execution effects were shown by comparing the differences between congruent and incongruent targets. The targets did not include neutral targets because a preliminary analysis showed no difference in response between congruent and neutral targets [55].
EEG Data Recording and Analyses
EEG was recorded using BrainAmp equipment (Brain Products, Munich, Germany), with 64 Ag/AgCl ring electrodes mounted on an elastic cap according to the extended 10–20 system. Bipolar channels recorded vertical and horizontal electro-oculograms from above and below the midpoint of the right eye and beside the outer canthi of both eyes. Inter-electrode impedance was maintained below 5 kΩ.
The EEG data were preprocessed off-line with EEGLAB v11.0.0.0b, running in the MatLab environment (Version R2014a). Individual ERPs and grand averages were created for the ANT. We first down-sampled the data from 1000 Hz to 500 Hz [58]. EEG data were digitally filtered using a 0.1 Hz–45 Hz bandpass filter and re-referenced to the average of the two mastoids. Epochs were defined relative to the onset of the cue and target displays. Epochs were rejected on the basis of amplitude differences exceeding 100 μV. Blinks, ocular movements, and muscle artifacts were detected and removed using independent component analysis.
For analysis of the preparatory interval, the data were segmented as in previous studies [34, 59]. Analysis of cue processing was started 200 ms before presentation of the cue stimulus. Analysis of target processing was started 200 ms before target presentation. For these segments, separate averaged event-related responses were calculated based on the cue and target stimuli. Thus, we analyzed the cue amplitude in a time window of 0 ms–500 ms following presentation of the cue. Regarding target analyses, target N2 and P3 amplitudes were determined in the time window after target presentation for target trial stimuli.
For the cues, anterior N1, P2, N2, and P3 were assessed based on the topographical distribution of the grand-averaged ERP activity and previous studies. The ERP components and their time epochs were as follows: N1, 100 ms–150 ms; P2, 150 ms–190 ms; N2, 230 ms–330 ms; and P3, 330 ms–430 ms. Fz, FCz, and Cz were selected to record the anterior N1, P2, and P3. Frontal–central (Fz, FCz, and Cz) and central–parietal (Cpz and Pz) regions were selected to record N2. All electrode points were averaged. For the time window following the presentation of the cue, or in the case of the no cue situation following the time at which a cue would have otherwise occurred, a 2 (group: chronically stressed vs non-stressed) × 2 (cue: no vs double) ANOVA was conducted on the amplitudes of ERPs for alerting, with group as a between-subjects factor and cue as a within-subjects factor. A 2 (group: chronically stressed vs non-stressed) × 2 (cue: center vs spatial) ANOVA was used for orienting, with group as a between-subjects factor and cue as a within-subjects factor.
For the targets, N2 and P3 were examined following the presentation of each target stimulus. The ERP components and their time epochs were as follows: N2, 390 ms–450 ms and P3, 450 ms–600 ms. Fz, FCz, and Cz were selected to record N2. Frontal–central (Fz, FCz, and Cz) and central–parietal (Cpz and Pz) regions were selected to record P3. The peak amplitudes were evaluated by averaging the values for the electrodes at each of these sites. The ANOVAs were identical to those used for anterior cue–N1, again with separate contrasts for alerting and orienting. Since this component was considered in relation to executive function, a 2 (group: chronically stressed vs non-stressed) × 4 (cue: no, center, double, spatial) × 2 (target: congruent vs incongruent) ANOVA was used to contrast the effects of congruent and incongruent targets on target–N2 and P3 amplitudes. As in the behavioral analyses, neutral targets were not included due to the similarity of the ERPs for neutral and congruent targets [44].
All analyses were conducted with SPSS 15.0 (Chicago, IL, USA). Gaussian distribution of behavioral and ERP data was assessed with the Kolmogorov–Smirnov test. Partial eta squared was computed as an estimate of effect size. The p-values were adjusted for sphericity using the Greenhouse–Geisser method. Post-hoc t-tests used Bonferroni adjustments for multiple comparisons.
Results
Demographic Information and Self-Report Results
Demographic characteristics and group differences on several self-report questionnaires for the final sample are presented in Table 1. There was a significant difference with regard to stress between the two groups; the chronically stressed and non-stressed groups were easily distinguished, and we controlled for the level of depression and anxiety. Participants in the stressed group reported more negative emotions and higher attention impulsivity.
Table 1.
Demographic information and self-report results.
| Participants (n = 72) | |||
|---|---|---|---|
| Measures | Stress group (n = 41; Mean± SD) | Non-stress group (n = 31; Mean ± SD) | Pa |
| Age, years | 21.2 (1.6) | 20.1 (1.8) | 0.421 |
| Age range, years | 18–25 | 18–26 | |
| PSS | 34.9 (5.6) | 20.4 (7.1) | <0.001 |
| SRQ | |||
| SR | 78.6 (24.9) | 44.4 (13.4) | <0.001 |
| ER | 33.7 (12.1) | 19.7 (7.9) | <0.001 |
| PR | 23.2 (8.1) | 13.9 (5.9) | <0.001 |
| BR | 16.3 (5.9) | 10.4 (3.4) | <0.001 |
| PCL-C | 47.8 (14.1) | 27.1 (6.5) | <0.001 |
| SLSI | 141.3 (24.4) | 105.4 (17.1) | <0.001 |
| BDI | 10.7 (3.4) | 9.1 (4.7) | 0.106 |
| STAI | |||
| S-AI | 45.1 (7.7) | 43.5 (7.0) | 0.409 |
| T-AI | 47.9 (7.6) | 45.7 (6.5) | 0.206 |
| PANAS | |||
| PA | 26.3 (4.8) | 27.9 (5.8) | 0.239 |
| NA | 25.5 (7.1) | 18.1 (5.7) | <0.001 |
| (BIS-11) | 62.5 (6.7) | 58.6 (5.9) | 0.015 |
| AI | 15.3 (1.9) | 13.6 (2.4) | 0.003 |
| MI | 19.4 (3.4) | 18.9 (2.7) | 0.494 |
| NI | 22.6 (3.5) | 21.4 (2.7) | 0.117 |
PSS, Perceived Stress Scale; SRQ, Stress Response Questionnaire; SR, Stress Response; ER, Emotional Response; PR, Physical Response; BR, Behavioral Response; PCL–C, PTSD Checklist–Civilian version; SLSI, Student–Life Stress Inventory; BDI, Beck Depression Scale; STAI, State–Trait Anxiety Inventory; PANAS, Positive and Negative Affect Schedule; PA, Positive Affect; NA, Negative Affect; BIS-11, Barratt Impulsivity Scale-11; AI, Attentional Impulse; MI, Motor Impulse; NI, Non-planning Impulse.
aSignificance level for two-tailed independent-samples t test.
Behavioral Results
Accuracy
The means and standard errors of behavioral performance are shown in Table 2. The results showed a main effect of cue [F (3,68) = 18.64, P <0.001, η2 = 0.451], with a post-hoc t-test showing the accuracy was lowest under the condition of double cue inconsistency (mean accuracy: 94.2 ± 0.7%), and highest under the condition of spatial cue congruence (98.8 ± 0.4%). Simple effect analysis showed an interaction of cue and target [F (3,68) = 2.96, P = 0.038, η2 = 0.115], and there was no Group × Cue interaction (P = 0.540).
Table 2.
Mean reaction times and accuracy for chronically stressed and non-stressed adults in each cue and target condition.
| Target | No cue | Center cue | Double cue | Spatial cue | |
|---|---|---|---|---|---|
| Mean RT (Mean ± SD) | |||||
| Stressed | Cong | 611 (8.3) | 613 (8.7) | 558 (7.7) | 534 (7.5) |
| Incong | 616 (9.7) | 612 (8.9) | 558 (7.9) | 539 (8.1) | |
| Non-stressed | Cong | 571 (9.6) | 575 (10.0) | 518 (8.9) | 490 (8.6) |
| Incong | 574 (10.4) | 578 (10.2) | 519 (9.1) | 492 (9.3) | |
| Mean accuracy (Mean ± SD) | |||||
| Stressed | Cong | 0.94 (0.009) | 0.98 (0.005) | 0.94 (0.009) | 0.98 (0.005) |
| Incong | 0.94 (0.008) | 0.98 (0.005) | 0.94 (0.010) | 0.98 (0.005) | |
| Non-stressed | Cong | 0.95 (0.010) | 0.99 (0.006) | 0.95 (0.010) | 0.99 (0.006) |
| Incong | 0.96 (0.010) | 0.99 (0.006) | 0.95 (0.011) | 0.99 (0.006) | |
For the alerting effect, we assessed the impact of the no and double cue conditions on the accuracy of analysis, and the main effect of cue [F (1,70) = 5.39, P = 0. 023, η2 = 0.072] revealed that accuracy was significantly higher following double cues relative to no cues. The difference between the two showed an alerting effect on accuracy.
To analyze the orienting effect, we conducted an accuracy analysis of the central cue and spatial cue conditions. There was no main orienting effect [F (1,70) = 1.87, P = 0.176, η2 = 0.026] or group effect [F (1,70) = 1.20, P = 0.278, η2 = 0.017] but there was a Target × Cue interaction [F (3,68) = 4.89, P = 0.030, η2 = 0.065], and simple effect analysis showed that when the target was incongruent, the accuracy of the response of the two groups to the central cues was less than that to the spatial cues [F (3,68) =5.91, P = 0.018, η2 = 0.078]. This effect was similar in both the stress and non-stressed groups, and no significant Cue × Group interaction was present [F (3,68) = 0.726, P = 0.540, η2 = 0.031].
Reaction Time
The results showed a main effect of Group [F (1,70) = 11.51, P = 0.001, η2 = 0.141], Cue, [F (3,68) = 276.23, P < 0.001, η2 = 0.924], and Target [F (1,70) = 3.97, P = 0.050, η2 = 0.054]. The response of the chronically stressed group was generally slower than that of the non-stressed group. Both groups gradually accelerated as the cues became more informative, with a decrease in RTs from the condition of no cues to central cues to double cues to spatial cues. There was a significant interaction between group and cue [F (3,68) = 2.88, P = 0.042, η2 = 0.113], and simple effect analysis showed that the RTs of the chronic stress group were significantly slower than those of the non-stressed group for all cues (all P < 0.05). In addition, the response of the stressed group to the congruent or incongruent targets was also slower than that of the non-stressed group [congruent types, F (1,70) = 11.66, P = 0.001, η2 = 0.143; incongruent types, F (1,70) = 11.18, P = 0.001, η2 = 0.138]. The difference tended to be significant in response to target type [F (1,70) = 2.99, P = 0.088, η2 = 0.041], while the non-stressed group showed no differences in trends [F (1,70) = 1.29, P = 0.260, η2 = 0.018]. Although the three-way interaction between Group, Cue, and Target was not significant [F (3,68) = 0.64, P = 0.595, η2 = 0.027], simple effect analysis showed that when the stressed group faced no cues, the difference between congruent and incongruent conditions tended to be significant [F (3,68) = 3.51, P = 0.065, η2 = 0.048], but this was not the case for other conditions.
Cue-Related ERPs
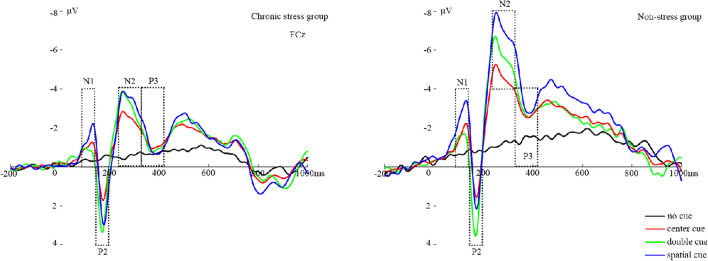
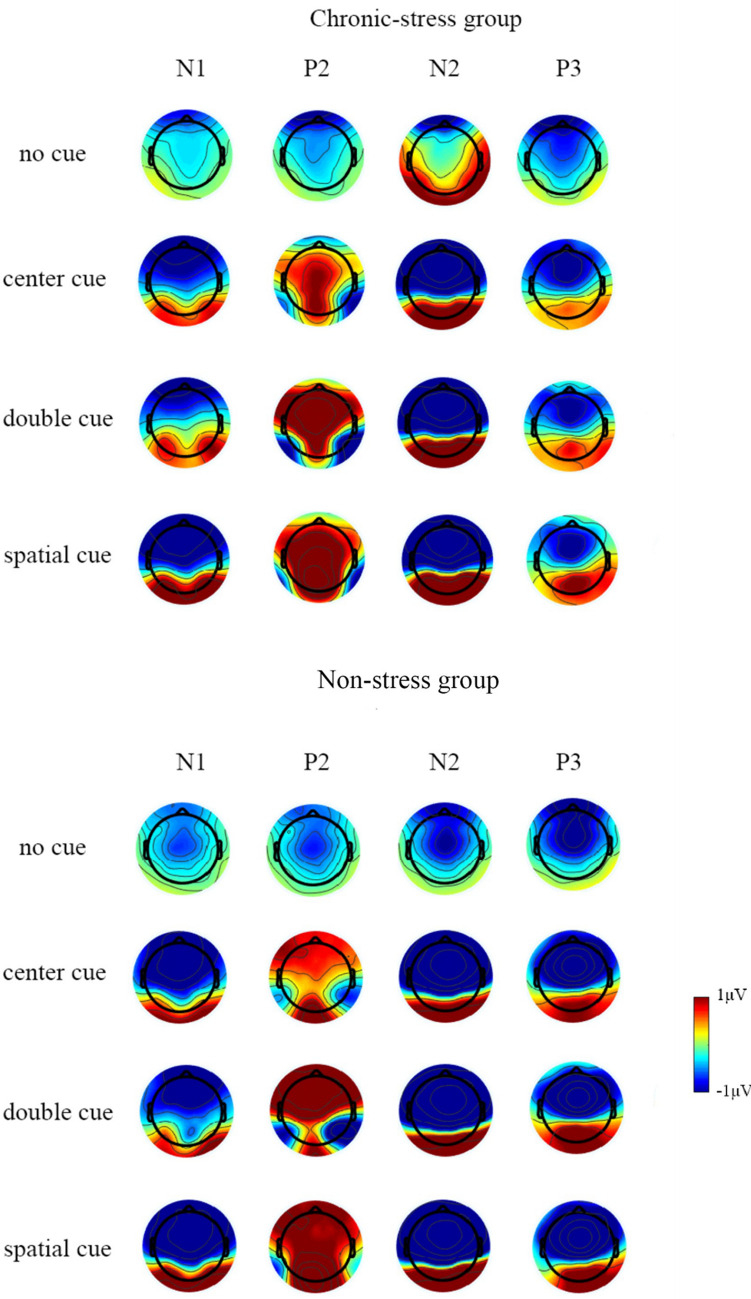
ERP waveforms of N1, P2, N2, and P3 locked to cue onset are shown in Fig. 2. Topographic plots of cue- and target-generated EEG activity for each of the cue conditions are presented in Fig. 3. The amplitude values are listed in Tables S2 and S3.
Fig. 2.
Cue-locked ERP waveforms at anterior sites. ERP waveforms averaged by group (left, chronically stressed adults; right, non-stressed adults) for each cue condition. Task blocks with no, center, double, and spatial cues are depicted as black, red, green, and blue lines, respectively. Cue onset occurs at 0 ms.
Fig. 3.
Topography plots of N1, P2, N2, and P3 in non- and chronic-stress groups.
Anterior Cue–N1 Amplitude (100–150 ms)
ANOVA for anterior N1 mean amplitudes revealed a significant effect of group [F (1, 70) = 4.11, P = 0.047, η2 = 0.061] that indicated smaller cue–N1 amplitudes in chronic stress. There was a main effect of cue [F (3,68) = 22.92, P <0.001, η2 = 0.530], and post-hoc analysis revealed that the anterior cue–N1 mean amplitude was significantly more negative following spatial cues (M = –2.12 μV) than when the cue was presented in the center (M = –1.32 μV), that is, both groups of participants showed an effect of attention orienting [F (3,68) = 36.41, P <0.001, η2 = 0.366]. However, the attention alerting effect did not show up in N1, and the amplitude with double cues (M = –0.90 μV) was more negative than that of no cues (M = –0.52 μV), without a significant difference [F (3,68) = 2.43, P = 0.124, η2 = 0.037]. The Cue × Group interaction was not significant [F (3,68) = 1.03, P = 0.384, η2 = 0.048].
Anterior Cue–P2 Amplitude (150–190 ms)
In this window, ANOVA for P2 mean amplitudes showed neither group difference, nor interaction of variables. But there was a main effect of Cue [F (3,68) = 22.52, P <0.001, η2 = 0.526], that is, both groups of participants showed an effect of attention orienting [F (3,68) = 52.90, P <0.001, η2 = 0.456] such that the amplitudes were larger following spatial cues (M = 1.98 μV) than following center cues (M = 0.62 μV). There was still an alerting effect [F (3,68) = 5.72, P = 0.02, η2 = 0.083]; the amplitude of double cues (M = 0.53 μV) was more positive than that of no cues (M = –0.23 μV).
Cue–N2 Amplitude (230–330 ms)
According to the topographic map results, N2 selection involves two areas of the frontal–central and central–parietal sites. ANOVA for N2 mean amplitudes showed a main effect of sites [F (1,70) = 112.83, P <0.001, η2 = 0.642]. So N2 was larger at frontal–central sites. Not only that, the N2 amplitudes also showed a group difference [F (3,68) = 12.08, P = 0.001, η2 = 0.161], in that the mean N2 amplitude was smaller in the chronically stressed group (M = –1.16 μV) than in the non-stressed group (M = –3.15 μV). There was a main effect of Cue [F (3,68) = 12.13, P <0.001, η2 = 0.374] as well as a significant Cue × Group interaction [F (3,68) = 4.85, P = 0.004, η2 = 0.193], though this difference between groups was only significant when there were center [F (3,68) = 9.21, P = 0.003, η2 = 0.128], double [F (3,68) = 10.38, P = 0.002, η2 = 0.141], and spatial cues [F (1,72) =13.86, P <0.001, η2 = 0.180], but it was not significant when there were no cues [F (3,68) = 3.10, P = 0.083, η2 = 0.047]. As suggested by the Cue × Group interaction, the effect of alerting and orienting only showed in the non-stressed group [F (3,68) = 14.95, P <0.001, η2 = 0.424] but did not occur in the chronically stressed group [F (3,68) = 1.41, P = 0.250, η2 = 0.065].
There was a Cue × Site interaction [F (3,68) = 32.89, P <0.001, η2 = 0.618]. With respect to the effect of cue, double cues (M = –5.59 μV) led to significantly more negative cue–N2 amplitudes than did no cues (M = –1.37 μV). Coincidentally, the spatial cues (M = –6.08 μV) also led to larger cue–N2 amplitudes than center cues (M = –4.19 μV). As suggested by the Cue × Site interaction, this alerting and orienting effect was larger at a frontal–central site [M = –0.85 vs –4.12 μV; F (3,68) = 22.97, P <0.001, η2 = 0.530] than at central–parietal sites [M = –0.52 vs –1.47 μV; F (3,68) = 3.36, P = 0.024, η2 = 0.142].
Cue–P3 Amplitude (330–430 ms)
ANOVA for P3 mean amplitudes revealed a significant effect for the group [F (1,70) = 9.14, P = 0.004, η2 = 0.128], that indicated larger cue–P3 amplitudes in the chronically stressed (M = –0.95 μV) than in the non-stressed groups (M = –2.44 μV). There was a main effect of Cue [F (3,68) = 6.34, P = 0.001, η2 = 0238], though there was no Cue × Group interaction [F (3,68) = 2.10, P = 0.110, η2 = 0095], and the cue–P3 mean amplitude was significantly smaller following center cues (M = –1.83 μV) than spatial cues (M = –1.32 μV); that is, both groups showed an effect of attention orienting [F (3,68) = 7.64, P = 0.007, η2 = 0.110]. However, the attention alerting effect did not show up in P3, and the difference between double cues and no cues was not significant [F (3,68) = 3.64, P = 0.061, η2 = 0.055].
Target-Related ERPs
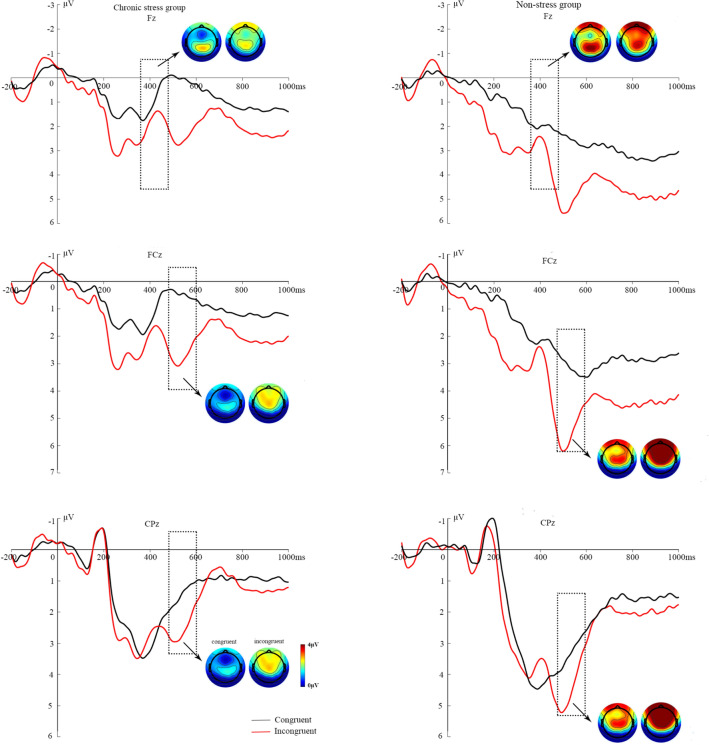
ERP waveforms at midline sites and modulation, averaged by target type, as well as topographic maps of EEG activity generated following congruent and incongruent targets are shown in Fig. 4.
Fig. 4.
Grand average ERPs during the ANT for the preparatory interval from presentation of the cue to the appearance of the target stimulus. The ERPs are depicted for groups with (left) and without stress (right). Upper: ERPs at electrode Fz. The two small vertical lines above the x-axis indicate the time window on which the Target–N2 analysis is based. Lower: ERPs at electrodes FCz and Cpz. The two small vertical lines above the x-axis indicate the time window for the Target–P3 analysis. Task blocks with congruent or incongruent types are depicted as black or red lines. Negative amplitude values are plotted upwards. Spline-interpolated maps illustrate the topography of the components (upper, Target–N2; lower, Target–P3) in the target condition (left, congruent; right, incongruent). Blue, negative amplitudes; red, positive amplitudes in the range 0 μV–4 μV.
Frontal–Central Target–N2 Amplitude (390–450 ms)
The ANOVA for the later frontal negative N2 showed a main effect of Group [F (1,70) = 4.87, P = 0.031, η2 = 0.066] that was associated with more negative mean amplitudes in the chronically stressed group, and a significant target effect was also present [F (1,70) = 5.42, P = 0.023, η2 = 0.073], such that the fronto–central target N2 was more negative for incongruent than congruent targets in both subgroups (M = 1.65 vs 2.25 μV). The stressed and non-stressed groups were significantly different, regardless of whether there was a congruent target [F (1,70) = 5.01, P = 0.029, η2 = 0.068] or an incongruent target [F (1,70) = 3.99, P = 0.049, η2 = 0.055]. There was no Target × Group interaction [F (1,70) = 0.11, P = 0.743, η2 = 0.002]; however, the effect of Target showed a significant trend in the non-stressed group [M = 2.46 vs 3.13 μV; F (1,70) = 3.01, P = 0.085, η2 = 0.042], but not in the stressed group [M = 0.85 vs 1.36 μV; F (1,70) = 2.36, P = 0.129, η2 = 0.033].
Target–P3 Amplitude (450–600 ms)
In the frontal–central region, there was a main effect of Target on P3 [F (1,70) = 50.48, P <0.001, η2 = 0.445]; the amplitude of this component was increased for incongruent targets (M = 3.93 μV) relative to congruent targets (M = 2.02 μV). As revealed by a main effect of Group [F (1,70) = 8.71, P = 0.004, η2 = 0.121], the P3 amplitude was reduced in the chronically stressed group (M = 1.79 μV) relative to the non-stressed group (M = 4.15 μV). There was no interaction effect [F (1,70) = 0.16, P = 0.688, η2 = 0.003]. but the main effect of Target was significant in both the stressed group [M = 0.89 vs 2.70 μV; F (1,70) = 23.54, P <0.001, η2 = 0.272] and the non-stressed group [M = 3.14 vs 5.17 μV; F (1,70) = 26.95, P <0.001, η2 = 0.300].
Correspondingly, there was a similar effect in the central–parietal sites, where the mean amplitude showed significant group differences [F (1,70) = 4.61, P = 0.036, η2 = 0.068] i.e., smaller mean target–P3 amplitudes were found in the chronically stressed group (M = 1.76 vs 3.01 μV). The Target × Group interaction was not significant [F (1,70) = 0.27, P = 0.606, η2 = 0.004], but there was a main effect of target [F (1,70) = 9.66, P = 0.003, η2 = 0.133] with the P3 amplitude for the incongruent target being greater than that for the congruent target (M = 2.05 vs 2.77 μV). Post-hoc analysis revealed that the target–P3 amplitude increased for incongruent relative to congruent targets in the non-stressed group [M = 2.64 vs 3.48 μV; F (1,70) = 6.29, P = 0.015, η2 = 0.091], but there was only a significant trend in the stressed group [M = 1.46 vs 2.05 μV; F (1,70) = 3.52, P = 0.065, η2 = 0.053].
Additional analysis was performed on the topography of target–P3, and the amplitudes at the two locations were compared adding the factor of electrode site to the analysis, which resulted in a three-way mixed-model ANOVA. We found a significant group effect [F (1,70) = 7.38, P = 0.009, η2=0.105], indicating smaller mean amplitudes in the chronically stressed group than in the non-stressed group. Furthermore, an effect of site [F (1,70) = 6.51, P = 0.013, η2 = 0.094] revealed that the target–P3 mean amplitude was more positive at frontal–central sites (M = 2.97 μV) than at central–parietal sites (M = 2.41 μV) for both subgroups. There was a Site × Group interaction [F (1,70) = 5.64, P = 0.021, η2 = 0.082], which showed that there was a difference between the two sites in the non-stressed group [M = 4.12 vs 3.06 μV; F (1,70) = 11.60, P = 0.001, η2 = 0.155; but not in the stressed group [M = 1.79 vs 1.76 μV; F (1,70) = 0.02, P = 0.899, η2 <0.001]. Moreover, a Target × Site interaction occurred [F (1,70) = 69.44, P <0.001, η2 = 0.524] when the target stimulus was incongruent, and the frontal–central P3 amplitude was significantly larger than that of the central–parietal sites [M = 3.93 vs 2.77 μV; F (1,70) = 21.70, P <0.001, η2 = 0.256]. Group differences for all of the described behavioral and ERP measures of alerting, orienting, and executive function are summarized in Table 3.
Table. 3.
Summary of stress-related differences in behavioral and ERP attention network effects.
| Dependent variable | Stress-related decrease | No difference | Stress-related increase |
|---|---|---|---|
| Cue-related | |||
| Accuracy | √ | ||
| RT | √ | ||
| Cue–N1 amplitude | √ | ||
| Cue–P2 amplitude | √ | ||
| Cue–N2 amplitude | √ | ||
| Cue–P3 amplitude | √ | ||
| Target-related | |||
| Accuracy | √ | ||
| RT | √ | ||
| Target–N2 amplitude | √ | ||
| Target–P3 amplitude | √ | ||
Discussion
In this study, we assessed the effects of chronic stress on attentional control at the behavioral and neurological levels using ANT tests in chronically stressed and non-stressed groups while recording electrophysiological data. As expected, the non-stressed group exhibited typical network effects of alertness, orientation, and executive control. In all networks, between-group differences were found at both the behavioral and neurological levels. Impaired alertness, directed ability, and executive control were associated with chronic stress.
As can be seen from the behavioral results, individuals with chronic stress had a longer response and had lower accuracy than non-stressed individuals. This may reflect top-down attention control and continued attention. Participants who would take an exam experienced greater levels of stress, leading to a worse behavioral performance. This finding is consistent with previous work [60]. Navarro et al. allowed 32 participants to perform simulated penalty missions in a stress-free or a stressed environment and found that participants in the stressed situation were slower to respond and took longer to make judgments [61]. These results were repeated in another similar study [62]. In addition, Nieuwenhuys and Oudejans allowed seven police officers to conduct pistol-shooting training in low and high anxiety situations and found that in the high anxiety situations their shooting accuracy decreased and aiming time was shorter, showing that control decreased [63]. Evidence also suggests that participants are more susceptible to external environmental disturbances under stress and perform worse [64, 65].
Previously, Williams et al. reported that at the time of the cue, N1 amplitude is largest in the double cue condition, intermediate in the center and spatial cue conditions, and absent in the no cue condition [34]. They also reported that N1 amplitude is largest under spatial conditions [66]. The current study supported the latter finding for N1. We found a similar pattern in both groups. Alerting and orienting had different effects on the amplitude of the cue–N1. We found an orienting effect, but there was no alerting effect. This replicates previous research [67]. Previous studies have shown that N1 is usually associated with attention promotion [68], and may be contingent on the perceptual expectations about cue features. These results suggest that the anterior and posterior occipital N1 reflect different functions and have different neurogenic mechanisms [69]. The modulation of the anterior N1 in the present study may be linked to the voluntary control of spatial attention in the dorsal frontoparietal network and reflects top-down attentional orienting in this network [70]. Our results revealed smaller cue–N1 amplitudes in individuals under chronic stress compared to non-stressed individuals, which suggests that individuals with chronic stress may have difficulties in the perceptual processing of cue stimuli, and cannot rely on bottom-up stimulus-driven systems to react to perceived cues.
In the current study, the P2 amplitude generated by invalid cues was larger than that of valid spatial cues during the spatial orientation task, which suggested that the alerting and orienting effects on cue–P2 amplitude had the same effect. There was no inter-group difference in cue–P2. This suggests that P2 in the front of the scalp may reflect only task-related processing [71], and P2 may not be an indicator of chronic stress.
We mainly focused on frontal and central N2 amplitudes, which were more affected by chronic stress. The findings were consistent with previous results [72–74]. In addition, we found between-group differences in the cue–N2 amplitude in the attention alerting and orienting networks. Only the non-stressed group showed attentional alerting and orienting effects, and specific chronic stress-related defects were shown in these two networks. These findings suggested that the stressed group exhibited N2 amplitudes like no and center cues when faced with more efficient double and spatial cues, while the non-stressed group showed the largest amplitude when they saw the most effective cues (spatial cues). In terms of cue–N2, the amplitude in the stressed group was smaller than that in the non-stressed group, which means that they were less responsive to the effective cues of this component and had lower participation. Although the cue effect was enhanced, the alerting and orienting function in the stressed group was weakened when adjusted for the stress-related slowdown. Chen et al. argued that differences in N2 amplitude and latency may reflect the speed and efficiency of the detection of conflicting tasks [74]. Bennys et al. found that the N2 amplitude is significantly correlated with attention and execution [75], and early changes in N2 parameters may reflect attention process defects associated with frontal lobe processing, and may be related to stronger reaction inhibition [42, 76].
Concerning the ERP results relating to the executive control network, target–N2 indicates a conflict effect. Participants exhibited an effect of target incongruency on frontal–central N2. The amplitude of N2 was larger for incongruent than for congruent targets. In similar flanking missions, larger amplitudes of target–N2 under incongruent target conditions have been reported in previous studies [46, 77].
The more intriguing result was that the target–N2 was larger in the chronically stressed group, as opposed to the cue–N2. This discrepancy may be attributable to a subtle difference in target presentation in the ANT. The ANT targets are presented both above and below a central fixation point. As the target location is less predictable in the ANT, the stressed group may not have been able to process targets centrally, as previously suggested, resulting in larger N2 amplitudes than typically found. Correspondingly, regarding performance level, the stressed and non-stressed groups also had significant differences in facing different target types, and the stressed group responded more slowly. Therefore, this indicates that the conflict monitoring and solution resolution are flawed in the stressed group. As noted above, previous studies have also shown that a higher N2 amplitude in the prefrontal region represents a higher resource input in cognitive control and response inhibition [78]. Moreover, if there is an intervention for the stress, the N2 amplitude is increased, which is consistent with our study [79]. Increased N2 can make conflict monitoring more effective [80]. Participants who have been under stress for a long period pay less attention when dealing with cues and may not be able to concentrate all their attention on the upcoming cues. The alerting stimuli may be used less effectively, and the ability to locate the cue is also weaker. Moreover, we also found changes in behavioral performance in both groups, suggesting that increased alertness and orientation lead to better behavior.
As noted above, there is considerable evidence to suggest that the attention function is worse and the N200 has a lower amplitude in people who are under chronic stress [81]. However, there are also opposing findings [82]. Zhang and colleagues adopted a point detection paradigm to show that individuals under test pressure are more susceptible to exam-related threats and are more likely to consume disproportionate cognitive resources to complete a task. Attention control theory suggests that anxiety affects the efficiency of the inhibitory function and prevents attention from being directed to task-independent stimuli. Therefore, individuals with high anxiety may consume excessive cognitive resources for task-independent stimuli [83]. This may also help explain why individuals under long-term stress often have poor task performance [84]. In addition, an increase in the target–N2 amplitude in individuals with poorer functional performance has also been reported in the ANT experiment [85].
At the ERP level, our data revealed increased cue-P3 and reduced target-P3 amplitudes in the chronic stress group. As with N2, this discrepancy may also be be attributable to the different types of cue and target. Due to the unpredictability of targets, subjects in the chronic stress group are more likely to be affected, resulting in target-P3 amplitudes that behave differently from cue-P3 amplitudes. Some previous studies have also obtained this reverse results between cue-P3 and target-P3 [86]. The cue-P3 amplitude may reflect some additional features of attention in visuospatial information processing. The degree of effective information contained in the cue may be related to the cognitive resource allocation of visual spatial processing [66]. The larger cue-P3 amplitude in the chronic stress group, suggesting that they have invested more cognitive resources in the cue processing and evaluation stage, reflecting a decrease in attention control ability. Attention process appears to vary qualitatively with major defects observed during the cue process (i.e., during attention preparation). A lower the efficiency of the use of the cue, this is also consistent with the results of the behavioral data. Target-P3 is positively correlated with the ability to perform control [66].
Smaller target–P3 amplitudes reflect less attention resources are invested in the conflict, and reduced ability to adjust behavior in the chronic stress group [59, 87, 88]. The stress group show abnormal conflict management, which represents the core result of our study. In this context, the reduced amplitude of target-P3 is interpreted as impaired resource allocation [67], indicating that energy state regulation is in a sub-optimal state [70]. In line with the hypothesis of our study, the amplitude of target–P3 was affected by target consistency. When the target type was incongruent, the amplitude of target–P3 tended to increase, as previously reported [89, 90]. Furthermore, based on the results of the topographic map, we also compared the amplitudes of target–P3 in different areas. It should be noted that at fronto–central sites the non-stressed group showed an increase in P3 amplitude, which provides partial support for a more anterior distribution of executive control processes in non-stressed adults; however, we did not find such regional activation differences in the stressed group. The incongruent target also triggered greater activation of the frontal–central sites. This more strongly suggests that the frontal lobe is involved in executive control [91].
Despite our best efforts, our research has some limitations. Based on previous studies [34, 59, 67], we conducted ERP analysis, but we did not consider the ERP differences of fixation, and thus, we did not mark the fixation. In addition, the duration of the fixation before the cues was random, so it was difficult to mark fixations offline. In future, if we can show that fixation elicits similar ERPs in the two groups, this result will indicate that the cue ERP differences were specific effects of cue. What is more, there are also studies that show complex interactions among the three attention systems [92]. To reduce the impact of the interactions, future research should improve the accuracy of the ANT measurement tools to meet different application requirements. For example, the children’s version of ANT has extended the interval between cue and target, which may reduce the impact of interactions between attention networks [59, 66]. In future, we hope that this version can also be applied to the adult version of the ANT paradigm. And it is necessary to strengthen the exploration of the sub-components of attention networks. Studying how the division of labor and cooperation between the three attention networks adapt to behavior will hopefully become a common area of research in clinical, imaging, and other disciplines.
Despite these caveats, the current study contributes significantly to our understanding of the neural bases of attentional networks in chronically stressed adults. Unlike previous behavioral studies using the ANT, we have demonstrated that the difference in attention caused by chronic stress is not just a matter of discrepancies in the size of the network effect, but instead represent distinct patterns of neural activity. In establishing an understanding of typical patterns of electrophysiological activity during the ANT in chronically stressed adults, we hope that in future such an understanding will be useful in identifying pathological forms of stress.
Conclusions
Although several studies have reported the effects of negative emotions such as anxiety, PTSD, and acute combat stress on cognitive function [66, 93, 94], very few prospective studies have been conducted in people under significant long-term stress. Our findings have important implications for individuals who experience major long-term stress, such as students who face major academic stress. Prolonged exposure to stressors increases susceptibility to adverse outcomes, including psychological problems, physical illness, and cognitive decline [95]. Our results suggest that chronic stress is associated with the attention control function, providing important evidence for an interaction between these two processes. In summary, the difference in the ANT between the two groups showed that people under chronic stress may not be able to use cue stimuli efficiently and maintain alertness, and have difficulty with directional information, suggesting that the cue remains more focused in chronically stressed adults. It is important to reduce attention deficits and improve performance. In contrast, the chronically stressed group performed control with lower efficiency, and it was more difficult for them to continuously focus their attention to complete the task requirements, while the non-stressed group could maintain the information related to the target, causing concentration in attention, thereby making the best task performances. Thus, when interference is detected, the reaction mechanism is also activated to successfully resolve the conflict. Alertness and orientation are similar to the bottom-up stimulus-driven system, while executive control is similar to the top-down goal-oriented system [96]. The effect of chronic stress on attention control can be demonstrated using the ANT paradigm. Future research combining these behavioral and psychophysiological techniques with neuroimaging will help address how one process modulates another and provide understanding about whether these phenomena are manifestations of a shared neural network dysfunction. Ultimately, this may help guide treatment specifically designed to correct these processes of chronic stress.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31771237 and 81773140), the Foundation and Advanced Research Project of Chongqing Science and Technology Commission (cstc2017shmsA130007), and the Fundamental Research Funds for the Central Universities, China (SWU1709106).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Qingjin Liu and Yong Liu contributed equally to this work.
Contributor Information
Feng Xia, Email: frankfxia@163.com.
Hong Chen, Email: chenhg@swu.edu.cn.
References
- 1.Koolhaas JM, Bartolomucci A, Buwalda B, De Boer SF, Flugge G, Korte SM, et al. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Milner A, Aitken Z, Kavanagh AM, Lamontagne AD, Petrie D. Status inconsistency and mental health: A random effects and instrumental variables analysis using 14 annual waves of cohort data. Soc Sci Med. 2017;189:129–137. doi: 10.1016/j.socscimed.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association AP. Summary report of journal operations, 2012. Am Psychol. 2013;68:381–382. doi: 10.1037/a0033035. [DOI] [PubMed] [Google Scholar]
- 5.Koenen KC, Ratanatharathorn A, Ng LC, Mclaughlin KA, Bromet EJ, Stein DJ, et al. Posttraumatic stress disorder in the world mental health surveys. Psychol Med. 2017;47:2260–2274. doi: 10.1017/S0033291717000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y, Jia J, Li X, Lv Y, Sun X, Wang S, et al. Expert consensus on the care and management of patients with cognitive impairment in China. Neurosci Bull. 2020;36:307–320. doi: 10.1007/s12264-019-00444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cakir OK, Ellek N, Salehin N, Hamamci R, Keles H, Kayali DG, et al. Protective effect of low dose caffeine on psychological stress and cognitive function. Physiol Behav. 2017;168:1–10. doi: 10.1016/j.physbeh.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Jackowska M, Fuchs R, Klaperski S. The association of sleep disturbances with endocrine and perceived stress reactivity measures in male employees. Br J Psychol. 2018;109:137–155. doi: 10.1111/bjop.12250. [DOI] [PubMed] [Google Scholar]
- 9.Lindfors P, Hellstadius LF, Ostberg V. Perceived stress, recurrent pain, and aggregate salivary cortisol measures in mid-adolescent girls and boys. Scand J Psychol. 2017;58:36–42. doi: 10.1111/sjop.12347. [DOI] [PubMed] [Google Scholar]
- 10.Haggerty H G. Stress and the immune system. Encyclopedic Reference of Immunotoxicology, 2016:612–614.
- 11.Mcewen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin LA, Hawker GA. Stress and the immune system: Preliminary observations in rheumatoid arthritis using an in vivo marker of immune activity. Arthritis Rheum. 1993;36:204–207. doi: 10.1002/art.1780360210. [DOI] [PubMed] [Google Scholar]
- 13.Jacques PLS, Kragel PA, Rubin DC. Neural networks supporting autobiographical memory retrieval in posttraumatic stress disorder. Cogn Affect Behav Neurosci. 2013;13:554–566. doi: 10.3758/s13415-013-0157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rooij SJH, Geuze E, Kennis M, Rademaker AR, Vink M. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology. 2015;40:667–675. doi: 10.1038/npp.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naim R, Abend R, Wald I, Eldar S, Levi O, Fruchter E, et al. Threat-Related Attention Bias Variability and Posttraumatic Stress. Am J Psychiatry. 2015;172:1242. doi: 10.1176/appi.ajp.2015.14121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naritaohtaki R, Hori H, Itoh M, Lin M, Niwa M, Ino K, et al. Cognitive function in Japanese women with posttraumatic stress disorder: Association with exercise habits. J Affect Disord. 2018;236:306–312. doi: 10.1016/j.jad.2018.02.061. [DOI] [PubMed] [Google Scholar]
- 17.Niederdeppe J, Shapiro MA, Kim HK, Bartolo D, Porticella N. Narrative Persuasion, Causality, Complex Integration, and Support for Obesity Policy. Health Commun. 2014;29:431–444. doi: 10.1080/10410236.2012.761805. [DOI] [PubMed] [Google Scholar]
- 18.Shipstead Z, Harrison TL, Engle RW. Working memory capacity and the scope and control of attention. Atten Percept Psychophys. 2015;77:1863–1880. doi: 10.3758/s13414-015-0899-0. [DOI] [PubMed] [Google Scholar]
- 19.Taylor CT, Cross K, Amir N. Attentional control moderates the relationship between social anxiety symptoms and attentional disengagement from threatening information. J Behavior Ther Exp Psychiatry. 2016;50:68–76. doi: 10.1016/j.jbtep.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelidis A, Hagenaars MA, Van Son D, Der Does WV, Putman P. Do not look away! Spontaneous frontal EEG theta/beta ratio as a marker for cognitive control over attention to mild and high threat. Biol Psychol. 2018;135:8–17. doi: 10.1016/j.biopsycho.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Finucane A, Whiteman MC, Power M. The effect of happiness and sadness on alerting, orienting, and executive attention. J Atten Disord. 2010;13:629–639. doi: 10.1177/1087054709334514. [DOI] [PubMed] [Google Scholar]
- 22.Seer C, Furkotter S, Vogts M, Lange F, Abdulla S, Dengler R, et al. Executive dysfunctions and event-related brain potentials in patients with amyotrophic lateral sclerosis. Front Aging Neurosci. 2015;7:225. doi: 10.3389/fnagi.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Attention Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 24.Posner MI. Orienting of attention: Then and now. Q J Exp Psychol. 2016;69:1864–1875. doi: 10.1080/17470218.2014.937446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White CN, Curl R. Cueing effects in the attentional network test: a spotlight diffusion model analysis. Comput Brain Behav. 2018;1:59–68. [Google Scholar]
- 26.Lupien SJ, De Leon MJ, De Santi S, Convit A, Tarshish C, Nair NPV, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 27.Cohen N, Henik A, Mor N. Can emotion modulate attention? Evidence for reciprocal links in the attentional network test. Exp Psychol. 2011;58:171–179. doi: 10.1027/1618-3169/a000083. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Zhang L, Jackson T, Wang J, Yang R, Chen H. Effects of negative mood state on event-related potentials of restrained eating subgroups during an inhibitory control task. Behav Brain Res. 2020;377:112249. doi: 10.1016/j.bbr.2019.112249. [DOI] [PubMed] [Google Scholar]
- 29.Morrison A S. Attention bias and attentional control in the development of social anxiety disorder. Dissertations & Theses - Gradworks, 2014.
- 30.Heeren A, Mcnally RJ. An integrative network approach to social anxiety disorder: The complex dynamic interplay among attentional bias for threat, attentional control, and symptoms. J Anxiety Disord. 2016;42:95–104. doi: 10.1016/j.janxdis.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Duan H, Yuan Y, Yang C, Zhang L, Zhang K, Wu J. Anticipatory processes under academic stress: An ERP study. Brain Cogn. 2015;94:60–67. doi: 10.1016/j.bandc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Adams ZW, Meinzer MC, Mandel H, Voltin J, Caughron B, Sallee FR, et al. Cue-dependent inhibition in posttraumatic stress disorder and attention-deficit/hyperactivity disorder. J Anxiety Disord. 2017;51:1–6. doi: 10.1016/j.janxdis.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Goncalves OF, Rego GG, Conde T, Leite J, Carvalho S, Lapenta OM, et al. Mind wandering and task-focused attention: ERP correlates. Sci Rep. 2018;8:7608. doi: 10.1038/s41598-018-26028-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams RS, Biel AL, Wegier P, Lapp LK, Dyson BJ, Spaniol J. Age differences in the Attention Network Test: Evidence from behavior and event-related potentials. Brain Cogn. 2016;102:65–79. doi: 10.1016/j.bandc.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton RKB, Baskinsommers AR, Newman JP. Relation of frontal N100 to psychopathy-related differences in selective attention. Biol Psychol. 2014;103:107–116. doi: 10.1016/j.biopsycho.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Wu J, Fu S, Luo Y. Orienting and focusing in voluntary and involuntary visuospatial attention conditions an event-related potential study. J Psychophysiol. 2010;24:198–209. [Google Scholar]
- 37.Boudreau C, Mccubbins MD, Coulson S. Knowing when to trust others: An ERP study of decision making after receiving information from unknown people. Soc Cogn Affect Neurosci. 2009;4:23–434. doi: 10.1093/scan/nsn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potts GF. An ERP index of task relevance evaluation of visual stimuli. Brain Cogn. 2004;56:5–13. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Johnstone SJ, Galletta D. Event-rate effects in the flanker task: ERPs and task performance in children with and without AD/HD. Int J Psychophysiol. 2013;87:340–348. doi: 10.1016/j.ijpsycho.2012.07.170. [DOI] [PubMed] [Google Scholar]
- 40.Venetacci R, Johnstone A, Kirkby KC, Matthews AJ. ERP correlates of attentional processing in spider fear: evidence of threat-specific hypervigilance. Cogn Emot. 2018;32:437–449. doi: 10.1080/02699931.2017.1310717. [DOI] [PubMed] [Google Scholar]
- 41.Larson MJ, Clayson PE, Clawson A. Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. Int J Psychophysiol. 2014;93:283–297. doi: 10.1016/j.ijpsycho.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Groom MJ, Cragg L. Differential modulation of the N2 and P3 event-related potentials by response conflict and inhibition. Brain Cogn. 2015;97:1–9. doi: 10.1016/j.bandc.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Michel R, Bolte J, Liepelt R. When a social experimenter overwrites effects of salient objects in an Individual Go/No-Go Simon Task – An ERP study. Front Psychol 2018, 9. [DOI] [PMC free article] [PubMed]
- 44.Neuhaus AH, Popescu F, Grozea C, Hahn E, Hahn C, Opgenrhein C, et al. Single-subject classification of schizophrenia by event-related potentials during selective attention. NeuroImage. 2011;55:514–521. doi: 10.1016/j.neuroimage.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 45.Larson MJ, Clayson PE. The relationship between cognitive performance and electrophysiological indices of performance monitoring. Cogn Affect Behav Neurosci. 2011;11:159–171. doi: 10.3758/s13415-010-0018-6. [DOI] [PubMed] [Google Scholar]
- 46.Purmann S, Badde S, Lunarodriguez A, Wendt M. Adaptation to frequent cConflict in the Eriksen Flanker Task. J Psychophysiol. 2011;25:50–59. [Google Scholar]
- 47.Gallagher S, Oriordan A, Mcmahon G, Creaven A. Evaluating personality as a moderator of the association between life events stress and cardiovascular reactivity to acute stress. Int J Psychophysiol. 2018;126:52–59. doi: 10.1016/j.ijpsycho.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Cohen S, Kamarck TW, Mermelstein RJ. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 49.Blanchard EB, Jonesalexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL) Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 50.Gadzella BM. Student-life stress inventory: identification of and reactions to stressors. Psychol Rep. 1994;74:395–402. doi: 10.2466/pr0.1994.74.2.395. [DOI] [PubMed] [Google Scholar]
- 51.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 52.Marteau TM, Bekker HL. The development of a six-item short-form of the state scale of the Spielberger State—Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31:301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 53.Culhane JF, Rauh V, Mccollum K, Hogan VK, Agnew K, Wadhwa PD. Maternal stress is associated with bacterial vaginosis in human pregnancy. Matern Child Health J. 2001;5:127–134. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- 54.Sabih F, Siddiqui FR, Baber MN. Assessment of stress among physiotherapy students at Riphah Centre of Rehabilitation Sciences. J Pak Med Assoc. 2013;63:346–349. [PubMed] [Google Scholar]
- 55.Evren C, Umut G, Bozkurt M, Evren B. Relationship of PTSD with impulsivity dimensions while controlling the effect of anxiety and depression in a sample of inpatients with alcohol use disorder. J Dual Diagn. 2018;14:40–49. doi: 10.1080/15504263.2017.1404665. [DOI] [PubMed] [Google Scholar]
- 56.Murphy D, Ross J, Ashwick R, Armour C, Busuttil W. Exploring optimum cut-off scores to screen for probable posttraumatic stress disorder within a sample of UK treatment-seeking veterans. Eur J Psychotraumatol. 2017;8:1398001. doi: 10.1080/20008198.2017.1398001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan J, Mccandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Zhao J, Zhang X, Gao X, Xu W, Chen H. Overweight adults are more impulsive than normal weight adults: Evidence from ERPs during a chocolate-related delayed discounting task. Neuropsychologia. 2019;133:107181. doi: 10.1016/j.neuropsychologia.2019.107181. [DOI] [PubMed] [Google Scholar]
- 59.Kratz O, Studer P, Malcherek S, Erbe K, Moll GH, Heinrich H. Attentional processes in children with ADHD: An event-related potential study using the attention network test. Int J Psychophysiol. 2011;81:82–90. doi: 10.1016/j.ijpsycho.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 60.Vine SJ, Freeman P, Moore LJ, Chandraramanan R, Wilson MR. Evaluating stress as a challenge is associated with superior attentional control and motor skill performance: Testing the predictions of the biopsychosocial model of challenge and threat. J Exp Psychol Appl. 2013;19:185–194. doi: 10.1037/a0034106. [DOI] [PubMed] [Google Scholar]
- 61.Navarro M, Miyamoto N, Der Kamp JV, Morya E, Ranvaud R, Savelsbergh GJP. The effects of high pressure on the point of no return in simulated penalty kicks. J Sport Exercise Psychol. 2012;34:83–101. doi: 10.1123/jsep.34.1.83. [DOI] [PubMed] [Google Scholar]
- 62.Navarro M, Miyamoto N, Der Kamp JV, Morya E, Savelsbergh GJP, Ranvaud R. Differential effects of task-specific practice on performance in a simulated penalty kick under high-pressure. Psychol Sport Exercise. 2013;14:612–621. [Google Scholar]
- 63.Nieuwenhuys A, Oudejans RRD. Effects of anxiety on handgun shooting behavior of police officers: a pilot study. Anxiety Stress Coping. 2010;23:225–233. doi: 10.1080/10615800902977494. [DOI] [PubMed] [Google Scholar]
- 64.Allsop J, Gray R, Bulthoff H H, Chuang L. Effects of anxiety and cognitive load on instrument scanning behavior in a flight simulation. Eye Tracking Visualization. 2016.
- 65.Herzog S, D’Andrea W, Depierro J, Khedari V. When stress becomes the new normal: Alterations in attention and autonomic reactivity in repeated traumatization. J Trauma Dissociation. 2018;19:362–381. doi: 10.1080/15299732.2018.1441356. [DOI] [PubMed] [Google Scholar]
- 66.Abramov DM, Pontes M, Pontes AT, Mouraojunior CA, Vieira JV, Cunha CQ, et al. Visuospatial information processing load and the ratio between parietal cue and target P3 amplitudes in the Attentional Network Test. Neurosci Lett. 2017;647:91–96. doi: 10.1016/j.neulet.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 67.Galvaocarmona A, Gonzalezrosa JJ, Hidalgomunoz AR, Paramo D, Benitez ML, Izquierdo G, et al. Disentangling the attention network test: behavioral, event related potentials, and neural source analyses. Front Hum Neurosci. 2014;8:813. doi: 10.3389/fnhum.2014.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends Cogn Sci. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- 69.Chan LKH, Hayward WG. Dissociating goal-directed and stimulus-driven determinants in attentional capture. I-perception. 2011;2:323. [Google Scholar]
- 70.Marzecova A, Schettino A, Widmann A, Sanmiguel I, Kotz SA, Schroger E. Attentional gain is modulated by probabilistic feature expectations in a spatial cueing task: ERP evidence. Sci Rep. 2018;8:54. doi: 10.1038/s41598-017-18347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Potts GF, Martin LE, Burton PC, Montague PR. When things are better or worse than expected: the medial frontal cortex and the allocation of processing resources. J Cogn Neurosci. 2006;18:1112–1119. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- 72.Cole M, Repovs G, Anticevic A. The frontoparietal control system a central role in mental health. Neuroscientist. 2014;20:652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dixon ML, La Vega AD, Mills C, Andrewshanna JR, Spreng RN, Cole M, et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Nat Acad Sci U S A 2018, 115. [DOI] [PMC free article] [PubMed]
- 74.Chen C, Zhang JX, Li L, Wang R. Bilingual memory representations in less fluent Chinese-English bilinguals: An event-related potential study. Psychol Rep. 2015;116:230–241. doi: 10.2466/28.PR0.116k13w7. [DOI] [PubMed] [Google Scholar]
- 75.Bennys K, Rondouin G, Benattar E, Gabelle A, Touchon J. Can event-related potential predict the progression of mild cognitive impairment? J Clin Neurophysiol. 2011;28:625–632. doi: 10.1097/WNP.0b013e31823cc2d3. [DOI] [PubMed] [Google Scholar]
- 76.Melynyte S, Ruksenas O, Griskovabulanova I. Sex differences in equiprobable auditory Go/NoGo task: effects on N2 and P3. Exp Brain Res. 2017;235:1565–1574. doi: 10.1007/s00221-017-4911-x. [DOI] [PubMed] [Google Scholar]
- 77.Grutzmann R, Riesel A, Klawohn J, Kathmann N, Endrass T. Complementary modulation of N2 and CRN by conflict frequency. Psychophysiol. 2014;51:761–772. doi: 10.1111/psyp.12222. [DOI] [PubMed] [Google Scholar]
- 78.Yin J, Yuan K, Feng D, Cheng J, Li Y, Cai C, et al. Inhibition control impairments in adolescent smokers: electrophysiological evidence from a Go/NoGo study. Brain Imaging Behav. 2016;10:497–505. doi: 10.1007/s11682-015-9418-0. [DOI] [PubMed] [Google Scholar]
- 79.Chamine I, Oken B. Expectancy of stress-reducing aromatherapy effect and performance on a stress-sensitive cognitive task. Evid Based Complement Alternat Med. 2015;2015:419812. doi: 10.1155/2015/419812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olfers KJF, Band GPH. Game-based training of flexibility and attention improves task-switch performance: near and far transfer of cognitive training in an EEG study. Psychol Res. 2018;82:186–202. doi: 10.1007/s00426-017-0933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Felmingham KL, Bryant RA, Kendall C, Gordon E. Event-related potential dysfunction in posttraumatic stress disorder: the role of numbing. Psychiatry Res. 2002;109:171–179. doi: 10.1016/s0165-1781(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 82.Zhang X, Dong Y, Zhou R. Examination stress results in attentional bias and altered neural reactivity in test-anxious individuals. Neural Plast. 2018;2018:1–7. doi: 10.1155/2018/3281040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eysenck MW, Derakshan N. New perspectives in attentional control theory. Pers Individ Differ. 2011;50:955–960. [Google Scholar]
- 84.Shi Z, Gao X, Zhou R. Emotional working memory capacity in test anxiety. Learn Individ Differ. 2014;32:178–183. [Google Scholar]
- 85.Johnstone SJ, Barry RJ, Markovska V, Dimoska A, Clarke AR. Response inhibition and interference control in children with AD/HD: a visual ERP investigation. International Journal of Psychophysiology. 2009;72:145–153. doi: 10.1016/j.ijpsycho.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 86.Finke M, Escera C, Barcelo F. The effects of foreknowledge and task-set shifting as mirrored in cue- and target-locked event-related potentials. PLoS One 2012, 7. [DOI] [PMC free article] [PubMed]
- 87.Brouwer A, Van Schaik MG, Van Erp JBF, Korteling H. Neuroticism, Extraversion and Stress: Physiological Correlates. Affective Computing & Intelligent Interaction 2013: 429–434. 10.1109/ACII.2013.77.
- 88.Sumner JA, Hagan KA, Grodstein F, Roberts AL, Harel B, Koenen KC. Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depress Anxiety. 2017;34:356–366. doi: 10.1002/da.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang R, Hu Z, Debi R, Zhang L, Li H, Liu Q. Neural processes underlying the “same”-“different” judgment of two simultaneously presented objects- An EEG study. PLoS One 2013, 8. [DOI] [PMC free article] [PubMed]
- 90.Rusnakova S, Daniel P, Chladek J, Jurak P, Rektor I. The executive functions in frontal and temporal lobes: a flanker task intracerebral recording study. J Clin Neurophysiol. 2011;28:30–35. doi: 10.1097/WNP.0b013e31820512d4. [DOI] [PubMed] [Google Scholar]
- 91.Zhu Y, Jiang X, Ji W. The Mechanism of cortico-striato-thalamo-cortical neurocircuitry in response inhibition and emotional responding in attention deficit hyperactivity disorder with comorbid disruptive behavior disorder. Neurosci Bull. 2018;34:566–572. doi: 10.1007/s12264-018-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Cui Q, Liu F, Huo Y, Lu F, Chen H, et al. A new method for computing attention network scores and relationships between attention networks. PLoS One 2014, 9. [DOI] [PMC free article] [PubMed]
- 93.Paltell K, Bingcanar H, Ranney RM, Tran JK, Berenz EC, Vujanovic AA. Anxiety Sensitivity Moderates the Effect of Posttraumatic Stress Disorder Symptoms on Emotion Dysregulation among Trauma-Exposed Firefighters. J Psychopathol Behav Assess. 2019;41:524–535. [Google Scholar]
- 94.Paulus DJ, Gallagher MW, Bartlett BA, Tran J, Vujanovic AA. The unique and interactive effects of anxiety sensitivity and emotion dysregulation in relation to posttraumatic stress, depressive, and anxiety symptoms among trauma-exposed firefighters. Compr Psychiatry. 2018;84:54–61. doi: 10.1016/j.comppsych.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 95.Alshargie F, Kiguchi M, Badruddin N, Dass SC, Hani AFM, Tang TB. Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomed Opt Express. 2016;7:3882–3898. doi: 10.1364/BOE.7.003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pachecounguetti AP, Acosta A, Callejas A, Lupianez J. Attention and anxiety different attentional functioning under state and trait anxiety. Psychol Sci. 2010;21:298–304. doi: 10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.