Abstract
Huntington’s (HD) and Parkinson’s diseases (PD) are neurodegenerative disorders caused by the death of GABAergic and dopaminergic neurons in the basal ganglia leading to hyperkinetic and hypokinetic symptoms, respectively. We review here the participation of purinergic receptors through intracellular Ca2+ signaling in these neurodegenerative diseases. The adenosine A2A receptor stimulates striatopallidal GABAergic neurons, resulting in inhibitory actions on GABAergic neurons of the globus pallidus. A2A and dopamine D2 receptors form functional heteromeric complexes inducing allosteric inhibition, and A2A receptor activation results in motor inhibition. Furthermore, the A2A receptor physically and functionally interacts with glutamate receptors, mainly with the mGlu5 receptor subtype. This interaction facilitates glutamate release, resulting in NMDA glutamate receptor activation and an increase of Ca2+ influx. P2X7 receptor activation also promotes glutamate release and neuronal damage. Thus, modulation of purinergic receptor activity, such as A2A and P2X7 receptors, and subsequent aberrant Ca2+ signaling, might present interesting therapeutic potential for HD and PD.
Keywords: Purinergic receptor, Central nervous system, Huntington’s disease, Parkinson’s disease, 6-hydroxydopamine
Introduction
Huntington’s and Parkinson’s Disease: Common Anatomical Basis
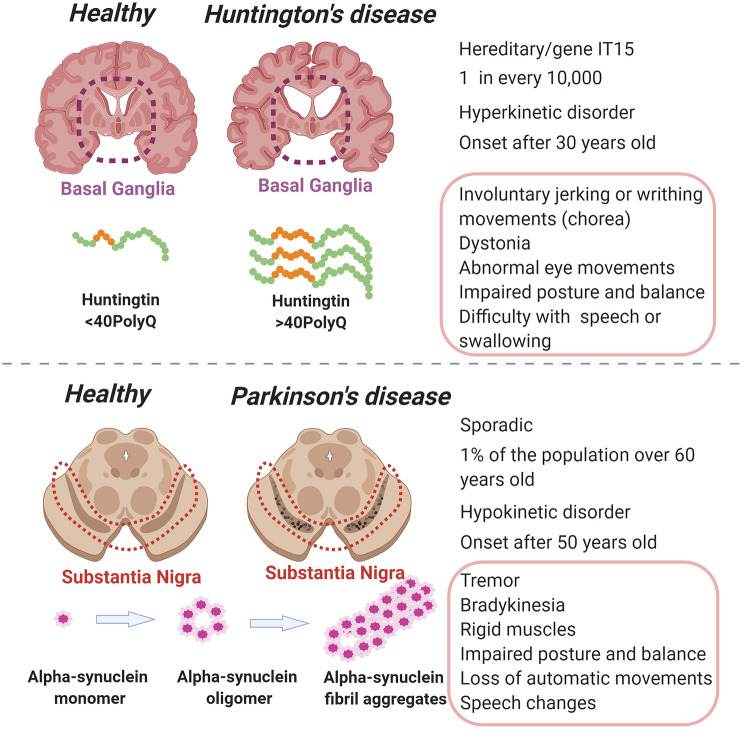
Huntington’s (HD) and Parkinson’s disease (PD) are neurodegenerative disorders that cause severe motor impairment. HD is mainly a genetic disease caused by a mutation in the huntingtin gene that was described in the ‘90s of the past century. However, no efficient therapy for HD has been developed since then. The huntingtin gene has a CAG repeat stretch that codes for a polyglutamine expansion (polyQ) in the N-terminal domain of the protein. Thus, > 36 repetitions of polyQ in this region lead to the development of the disease, resulting in massive brain degeneration marked by brain atrophy and loss of GABAergic neurons in the basal ganglia (Fig. 1) [1, 2], mainly through the accumulation of mutated protein in the cells, overwhelming the proteasome system [3] (Fig. 1)
Fig. 1.
Huntington’s and Parkinson’s disease characteristics.
PD, like HD, is associated with cell death in the basal ganglia, although the most affected cells are the dopaminergic neurons in the substantia nigra pars compacta [4]. This disease may be caused by the formation of Lewy bodies, which are intraneuronal inclusions of α-synuclein, resulting in decreased levels of dopamine secretion by the nerve terminals [5, 6] (Fig. 1)
In contrast to HD, PD is mostly a sporadic disease, and only ~ 10%–15% of cases are related to gene inheritance [7]. These genes typically encode proteins involved in autophagy and mitochondrial housekeeping, as well as endo- and exocytosis in synapses [8, 9]. Both diseases have progressive symptoms, with disease onset at ~ 30 years old in HD patients, and 60 years old in PD patients. However, there is a severe form of HD with a faster progression that occurs in children [10].
Both diseases present protein aggregates, degeneration in basal ganglia structures, and motor disabilities. However, HD is an autosomal dominant hyperkinetic disease, while PD is a mostly sporadic hypokinetic disease. HD and PD show distinct prevalence in the population and affect people in different age groups.
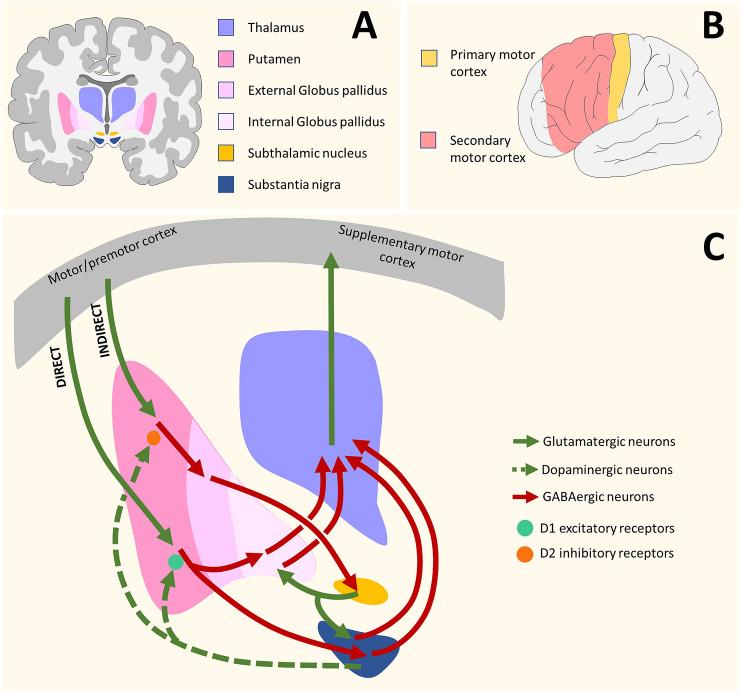
As noted above, HD and PD are basal ganglia diseases. The basal ganglia are comprised of the striatum (caudate and putamen), globus pallidus (external and internal; GPe and GPi), substantia nigra (SN), and subthalamic nucleus (STN) (Fig. 1B) [11, 12]. Glutamatergic neurons project from the thalamus to the motor cortex (divided into primary and secondary motor cortices), from the motor cortex to the putamen, and from the STN to the GP and SN (Fig. 2). GABAergic neurons project from the putamen to the GP and SN, which projects to the thalamus. Dopaminergic neurons project from the SN to the striatum, constituting the nigrostriatal pathway. Generally, thalamocortical projections must be released from inhibition for the initiation of movement [11, 12].
Fig. 2.
Basal ganglia. A Locations of primary and secondary motor cortex. B Basal ganglia structures involved in the pathophysiology of HD and PD. C Neuronal network involved in the pathophysiology of HD and PD. Cortical glutamatergic excitatory neurons connect to inhibitory GABAergic neurons in the putamen, that innervate the SN and the GP. The direct pathway is regulated in the putamen by dopaminergic afferents exerting excitatory effects through D1 receptors, and the indirect pathway with inhibitory effects through D2 receptors. These receptors are modulated by A1 and A2A receptors, respectively.
In the direct pathway (Fig. 2C), striatal GABAergic neurons expressing mainly D1 receptors, receive excitatory inputs from glutamatergic neurons of the motor cortex and project to the GPi and SN. GABAergic neurons from GPi inhibit GABAergic neurons that project to the thalamus. Thus, the disinhibition of glutamatergic neurons from the thalamus projecting to the motor cortex enables voluntary movements [11–13].
In the indirect pathway (Fig. 2C), excitatory stimuli from cortical glutamatergic neurons activate striatal GABAergic neurons expressing dopamine D2 receptors. These neurons inhibit GABAergic neurons of the GPe that project to the STN, disinhibiting this pathway. The STN has glutamatergic projections to the SN and GPi, which result in the activation of GABAergic neurons projecting to the thalamus. As expected, inhibition of thalamic neurons results in resting glutamatergic neuron projecting to the motor cortex, preventing unwanted muscle contractions would that compete with voluntary movements [11, 12].
Differential expression of excitatory D1 and inhibitory D2 receptors in both pathways leads to different effects of dopaminergic stimulation: dopamine release in the striatum activates neurons expressing D1 receptors, whereas dopamine inhibits neurons expressing D2 receptors in the indirect pathway [11, 12].
Diseases such as PD present hypokinetic symptoms. Decreased motor function by abnormally high output of the basal ganglia leads to augmented inhibition of thalamocortical projection neurons. In PD, the decrease of dopaminergic signaling in the direct pathway induces disease-characterizing symptoms: muscle rigidity, tremor at rest, and slowness to initiate and execute movements [14]. In turn, hyperkinetic disorders, such as HD, are caused by reduced basal ganglia output from the indirect pathway, leading to unwanted movements [14]. Motor symptoms such as the uncontrolled rapid and jerky movements in HD are due to the death of the GABAergic neurons that project to the GPe in the indirect pathway, resulting in disruption of the indirect pathway and thus characterizing hyperkinetic disorders [14].
Calcium Signaling as a Common Pathway in PD and HD
Besides alterations in basal ganglia neurotransmission, changes in intracellular calcium concentration ([Ca2+]i) and signaling provide another similarity between HD and PD. Ca2+ is a universal intracellular messenger, and its concentration is maintained by fine mechanisms in order to maintain homeostasis [15].
Robust mechanisms fine-tune inward and outward Ca2+ currents. At rest, cells maintain [Ca2+]i levels at ~ 100 nmol/L. Increased [Ca2+]i occurs through: (a) influx from the extracellular milieu via ion channels (voltage-dependent, ligand-gated, store-operated, and other channels) and exchangers (e.g. Na+/Ca2+ exchanger); (b) efflux from the endoplasmic reticulum (ER) upon activation of inositol trisphosphate (IP3) or ryanodine receptors; and (c) release from mitochondria (mitochondrial permeability transition pore). The cellular machinery that counterbalances [Ca2+]i elevation buffers this cation by binding to proteins, pumping it into intracellular organelles like the ER and mitochondria [16–19], or extruding it from the cell through the plasma membrane.
Proteins containing an EF-hand Ca2+ binding domain, like the stromal interacting molecules, function as sensors of the ER [Ca2+] store. These proteins oligomerize and translocate to the cytosol during depletion of ER stores, leading to the opening of Orai family channels localized in the plasma membrane that replenish intracellular Ca2+ stores [20–25].
[Ca2+]i elevation triggers the activation of downstream signaling pathways comprised of kinases, phosphatases, transcription factors, and proteins related to synaptic vesicle fusion in neurons [17, 26–29]. Alterations in Ca2+ homeostasis that need stress overcompensation usually lead to cell death by excitotoxicity and apoptosis. An imbalance of Ca2+ signaling initiating cell death by excitotoxicity and apoptosis is very common in neurodegenerative diseases like HD and PD, causing massive neuronal loss.
In HD, the main cause of the dysregulation of [Ca2+]i homeostasis is the accelerated delivery of N-methyl-D-aspartate receptors (NMDARs) to the plasma membrane in GABAergic neurons [30–36]. Increased NMDAR expression augments Ca2+ influx, resulting in toxic levels of [Ca2+]i and cell death [28, 34, 35, 37].
The mutant form of huntingtin can also interact with other proteins related to Ca2+ homeostasis, such as voltage-gated Ca2+ channels and IP3 receptors [28, 38, 39]. In HD, the IP3 receptor has a higher affinity for its natural ligand IP3 and also increases the leakage of Ca2+ through ryanodine receptors and depletion of ER stores [38, 39]. As noted above, intracellular Ca2+ depletion activates stromal interacting molecules and consequently higher inflow of Ca2+ from extracellular milieu occurs [40, 41].
In PD, a new connection between Ca2+ signaling and α-synuclein has been recently described. Lautenschläger and coworkers reported that, besides the interaction of the N-terminus of α-synuclein with unilamellar vesicles, its C-terminus also binds to Ca2+, increasing its lipid-binding capacity. Moreover, the interaction with Ca2+ also controls the localization of α-synuclein at the pre-synaptic terminal and in synaptic vesicle clustering upon increased [Ca2+]i [42]. Those findings strengthen the modern consideration of PD as an ion channel disease [43].
All PD cases with hereditary characteristics are associated with mitochondrial dysfunction due to imbalanced [Ca2+]i signaling [44–46]. Dopaminergic neurons in the SN have spontaneous Ca2+ pacemaker activity triggered by L-type voltage-gated Ca2+ channels (Cav1.3), making mitochondria vulnerable to oxidative stress [47–52]. Furthermore, mutations in Orai channels result in decreased dopamine production due to reduced tyrosine hydroxylase expression [28, 53].
In addition to the voltage-gated Ca2+ channels NMDAR and Orai, other receptors like purinergic receptors also modulate [Ca2+]i signaling and have been reported to participate in PD and HD, as follows.
Purinergic Receptors
Purinergic signaling is mediated by extracellular nucleotides and nucleosides that have been implicated in a range of physiological processes. In the central nervous system (CNS), these receptors participate in neurotransmission, neuromodulation, and neuroinflammation. Studies show that the proliferation, differentiation, and apoptosis of neural cells are also mediated by purinergic receptors, indicating an important role in neurogenesis and repair [54–56].
When tissue damage occurs as a consequence of injury or pathophysiological processes, a high concentration of adenosine 5’-triphosphate (ATP) is released by dying cells into the extracellular space and is degraded by ectonucleotidases. The ectonucleotide pyrophosphatase/phosphodiesterase family together with ectonucleoside triphosphate diphosphohydrolases and 5´ectonucleotidase catalyze the hydrolysis of ATP into adenosine [57, 58].
ATP and its metabolites activate purinergic receptors on the cell membrane, divided into P1 and P2 receptors. P1 receptors, also known as adenosine receptors, are G protein-coupled and are activated by endogenous adenosine. P2 receptors are endogenously activated by the extracellular nucleotide molecules ATP, UTP, UDP, ADP and their derivatives. Based on their structural characteristics, P2 receptors can be subdivided into two categories: ligand-gated cation channels (P2X receptors) and G protein-coupled receptors (P2Y receptors) [59].
Currently, seven subtypes of the trimeric structure of the P2X receptor have been cloned, namely P2X1–7 receptors, which are mainly distributed in excitable cells such as neurons, smooth muscle cells, and glandular cells. The P2X receptor is a non-selective ATP-gated cation channel. After activation, the cell permeability to cations increases and leads to enhanced transient [Ca2+]i levels. Ca2+ influx depolarizes the cell membrane, causing a series of physiological or pathological changes such as the rapid transmission of synaptic signals in the central and peripheral nervous systems, smooth muscle contraction, platelet aggregation, macrophage activation, cell proliferation, and apoptosis. Abnormal Ca2+ influx is mainly mediated by the P2X7 receptor. Long-term or excessive activation of this receptor can open abnormal large membrane pores, leading to massive Ca2+ influx and eventually to cell death [60]. Divalent cations like Ca2+ and Mg2+ are capable of allosteric blockade of the P2X7 receptor, as proposed by Virginio and colleagues in 1997 [61].
There are eight subtypes of P2Y receptors (P2Y1, 2, 4, 6, 11, 12, 13, 14), which are widely distributed in the CNS as well as in other tissues. P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptors are coupled to Gq proteins, activate phospholipase C-β, produce IP3 and diacylglycerol, and mobilize Ca2+ from intracellular stores [62]. Subsequent protein kinase C (PKC) activation may stimulate the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase signaling pathways involved in the proliferation, differentiation and regeneration processes [63]. On the other hand, the P2Y12, P2Y13 and P2Y14 receptors are coupled to Gi proteins and, upon activation, inhibit cAMP production [62].
The P1 receptor family activated by adenosine is composed of A1, A2A, A2B and A3 subtypes. A1 and A3 receptors are coupled to Gi/Go proteins, inhibiting adenylate cyclase and reducing cAMP levels, while A2 receptors coupled to Gs protein activate adenylate cyclase and increase intracellular cAMP levels. In the CNS, ATP and adenosine receptors are associated with many neurodegenerative diseases, including PD and HD. In the following topics we focus on purinergic receptors that have been intensely investigated in PD and HD. The receptors that we do not consider (A2B, A3, P2X2–6, P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11–14) may also be involved in pathophysiology, but they are still poorly investigated in these contexts. Recently, the P2Y2 receptor has been shown to play key roles in the development of HD by controlling intracellular Ca2+ oscillations and consequently the activation of transcription factors related to GABAergic neuronal fate [64].
Shared Purinergic Receptors in HD and PD
The strict relationship between HD and PD pathophysiology extends to the involvement of purinergic receptors. Their participation in neurodegenerative diseases, including PD, is well established [55]. However, there are few studies in the literature regarding the involvement of purinergic receptors in HD. So far, two receptors have been found to be involved in both diseases: A2A and P2X7 receptors. Their function in healthy and diseased brains is summarized in Table I.
A2A Receptors
The A2A receptor is a member of the G protein-coupled receptor family that activates adenylyl cyclase to induce the synthesis of cAMP and activation of phospholipase C, consequently increasing [Ca2+]i. This receptor is strongly expressed in the caudate nucleus, putamen, nucleus accumbens, olfactory tubercles, and GPe [65]. The cortex [66], hippocampus [66, 67], cerebellum [68] and olfactory bulb [69] also express this receptor, but to a lesser extent. The A2A receptor is present in neurons, astrocytes, oligodendrocytes and microglia and may modulate dopaminergic and glutamatergic neurotransmission [70], neuroinflammation, and neurodegeneration [71, 72]. The striatopallidal pathway has already been demonstrated to play a relevant role in the pathophysiology of PD and HD. In this sense, the A2A receptor has attracted attention, since it is largely expressed in the striatum and GPe, specifically in GABAergic and glutamatergic striatopallidal medium spiny neurons [73–75].
The interaction between striatal A2A and D2 receptor subunits is well established. The A2A–D2 dimer is a functional heteroreceptor that induces allosteric inhibition. When the A2A receptor is activated by adenosine, the affinity of the D2 receptor for dopamine is reduced [76] and elicits a decrease in the G-protein coupling of the D2 receptor [77]. Under basal conditions, A2A receptor signaling is slightly activated. Consequently, the D2 receptor affinity is not impaired by A2A receptor activation, and cAMP–protein kinase A (PKA) signaling does not contribute to GABAergic neuronal activity [78]. When the A2A receptor is over-activated, adenylate cyclase stimulation occurs and induces opposite effects, commonly elicited by the D2 receptor. These stimuli lead to an increase in cAMP levels and consequent activation of PKA. The cAMP–PKA pathway activates MAPK signaling and the constitutive transcription factor CREB [78]. Further, this pathway phosphorylates AMPA receptors and DARPP-32 at Thr34 [79], decreasing its phosphorylation at Thr75 [80], and executing important signal cascade for the control of GABAergic neuronal excitability [81]. Considering the vast majority of evidence in the literature, A2A receptor activation has been strongly suggested to stimulate GABAergic neurons [74, 75, 81–88]. However, few reports have suggested that A2A receptor activation may inhibit GABAergic neuronal activity [89, 90]. A2A–D2 receptor heterodimers are present in GABAergic striatopallidal neurons and subsequent signaling may result in motor inhibition [83]. An in vivo study investigating this interaction found that mice with D2 receptor knockout exhibited motor impairment and that istradefylline, a selective A2A receptor antagonist, reverted this condition [91].
Remarkably, A2A receptors also interact with glutamate receptors, mainly NMDA and mGlu5 receptors. A2A receptors may prompt long-term potentiation by NMDA-excitatory postsynaptic currents, depending on NMDA and mGlu5 receptors due to postsynaptic Ca2+ flow [65, 92, 93]. In addition, the formation of heteromeric complexes between A2A and glutamatergic mGlu5 receptors [94, 95] may facilitate glutamate release [95]. Moreover, ample evidence indicates functional interactions between A2A, mGlu5, and D2 receptors that could contribute to the striatopallidal pathway [83, 96–99]. A study demonstrated that A2A and mGlu5 receptors have synergistic effects since their simultaneous blockade elicits a pronounced increase in locomotor activity [98]. Motor stimulation enhanced by mGlu5 antagonism depends on D2 and forebrain A2A receptors [98].
In this sense, interactions among A2A, mGlu5, and D2 receptors seem to be important for the pathophysiology of PD. A literature review showed that antagonists of the mGlu5 receptor attenuate L-DOPA-induced dyskinesias in different animal models of PD [100].
Further, it has been proposed that treatment with A2A and mGlu5 receptor antagonists has antiparkinsonian effects and might enhance the antiparkinsonian activity of dopamine-based drugs [100, 101]. Similarly, synergistic effects between A2A and mGlu5 receptor antagonists stimulate locomotor actions, presenting an antiparkinsonian-like effect in the dopamine depletion model induced by reserpine, indicating that this functional interaction provides a mechanism for motor dysfunction in PD [98]. However, this mechanism needs to be deeply studied, since it has also been demonstrated a selective agonist of the mGlu5 receptor inhibits rotational behavior, an effect that is exacerbated by A2A receptor agonism and attenuated by antagonism of this receptor in the 6-hydroxydopamine (6-OHDA, a toxic dopamine analog) rat model of PD [100].
As noted above, A2A receptors modulate several mechanisms implicated in PD. In many studies, istradefylline treatment of PD patients had beneficial results when administered together with levodopa, a dopamine precursor. In fact, istradefylline is currently used as an adjunct in combination with levodopa in Japan [102]. However, the association between A2A receptors and PD is currently under investigation in order to elucidate the pathology of this neurodegenerative disease. Several findings have shown that adenosine signaling is altered in PD pathology. In the 6-OHDA animal model, a decrease in adenosine levels and an increase in A2A receptor expression has been found in the striatum [103]. These alterations were also found in the GPe and caudate–putamen of PD patients with dyskinesia, a levodopa side-effect [104, 105]. Similar to the clinical findings, 6-OHDA lesioned rats with dyskinesia exhibited increased expression of the A2A receptor in the striatum after levodopa treatment [106]. Remarkably, in the 6-OHDA rat model, basal release of GABA is augmented in the GP, and treatment with istradefylline reverses this increase [107]. These results are in agreement with the morpho-functional association between the activated striatopallidal pathway and A2A receptors in PD [107]. Dopamine depletion prompts degeneration of the spines and glutamatergic synapses of striatopallidal medium spiny neurons through increased Ca2+ influx through L-type Ca2+ channels [108]. The A2A receptor antagonist 1-(7-imino-3-propyl-2,3-dihydrothiazolo[4,5-d]pyrimidin-6(7H)-yl) urea (IDPU) counteracts the behavioral and oxidative stress impairments induced by 6-OHDA [109]. In addition, IDPU increases intracellular dopamine and decreases the glutamate and [Ca2+]i levels in 6-OHDA-lesioned rats [109]. It is hypothesized that residual A2A receptors in PD modulate GABAergic activity in the striatum [65].
In non-human primates submitted to the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD, A2A receptor expression is increased in the caudate and putamen [110]. Interestingly, when the animals were concomitantly treated with levodopa and a selective antagonist of the NR1A/2B subunits of NMDAR, A2A receptor expression was restored to control levels [110]. All this evidence indicates that the A2A receptor is extensively expressed in the striatopallidal pathway and is likely involved in the of development PD as well as in the appearance of dyskinesia.
It has been proposed that the neuronal dysfunction and toxicity mediated by exposure to α-synuclein oligomers might be due to the induction of enhanced NMDAR activity leading to increased Ca2+ influx in PD [111]. A2A receptor antagonism apparently decreases α-synuclein aggregation in the H4 cell line without affecting oligomerization [112]. In addition, in vivo analysis evidenced that A2A receptor blockade abolishes neuronal cell death, as well as the increasing expression of the NR2B subunit of NMDARs, and the impairment of long-term potentiation induced by α-synuclein [112]. Interestingly, the mouse model of α-synuclein per se revealed augmentation of A2A receptor expression accompanied by increased α-synuclein inclusions, astrocytic reactivity, cell death, and memory impairment. Genetic deletion of A2A receptors may protect against all of these disease-related effects [113]. It has been hypothesized that α-synuclein monomers bind to the A2A receptor and increases its activity [71].
In HD pathology, neuronal degeneration occurs in the striatum, in which the postsynaptic GABAergic striatopallidal neurons, the most vulnerable cell population, are localized [114]. Thus, the A2A receptor may play an important role in HD. In this sense, the rs5751876 polymorphism of the ADORA2A gene has been suggested to be a genetic modifier of the onset age of HD [115]. Unlike the findings in PD, HD patients lose A2A receptor expression [116]. During the initial phase of HD, the GPe presents an aberrant reduction of A2A receptor expression, while the decrease of A2A receptor expression in the caudate and putamen is accompanied by disease progression [116]. The same results were found in older transgenic HD model mice [117–121]. In line with these data, A2A receptor agonism delays the progressive motor impairment, reverses the reduction of brain weight, enlargement of ventricles, and stimulation of AMPK in transgenic HD mice [122].
However, simultaneous hyperactivation of D1 and A2A receptors may exacerbate cognitive impairment in the R6/1 transgenic mouse [123]. Besides, A2A receptor knockout in transgenic HD mice leads to a worsening of motor deficits and reduced life expectancy and enkephalin expression [120], suggesting impairment of GABAergic neuronal signaling. Further, some studies have shown that blockade of the A2A receptor has beneficial effects that should be considered. In R6/2 transgenic mice, the A2A receptor antagonist SCH58261 abrogates NMDA-induced toxicity, although the motor impairment is not reversed [124]. In addition, istradefylline improves working memory [125].
From these data, the roles of the A2A receptor in GABAergic and glutamatergic striatopallidal neurons are evident, besides its interaction with D2, mGlu5 and NMDA receptors. In PD pathogenesis, it has been suggested that the A2A receptor has a strong and robust association with motor impairment in striatopallidal signaling. Further functions of the A2A receptor in HD remain to be clarified, despite all the evidence pointing to an opposite mechanism of that is seen in PD.
P2X7 Receptors
P2X7 receptors are ATP-gated cation channels (for a detailed review see [126]), formerly assumed to be expressed both in neurons and glial cells, but recent results have questioned the presence of these receptors in neurons [127, 128]. This receptor was detected in cultured glial and neuronal cells by in situ hybridization, immunodetection, and electron microscopy [129–134], but the used antibodies may lack specificity [135–138]. Corroborating P2X7 receptor expression in neurons, features attributed to stimulation of these receptors in cultured neurons or synaptosomes have been demonstrated by electrophysiological recordings, [Ca2+]i measurements, and activation of intracellular signaling cascades [130, 139–143]. However, studies have suggested that glial cells could be responsible for some of these P2X7 receptor-mediated effects [144, 145]. Therefore, further studies are still required to confirm P2X7 receptor expression and function in neurons.
Regardless of the P2X7 receptor localization, its activation may lead to glutamate release, stimulation of neuroimmune responses, enhanced production of reactive oxygen species (ROS), protein misfolding, and diminished levels of brain-derived neurotrophic factor, resulting in neuroplasticity impairment and/or neuronal damage [131, 146, 147]. These key mechanisms may contribute to the pathophysiology of HD and PD.
Studies with mouse models of HD (Tet/HD94 and R6/1) have shown that treatment with Brilliant Blue G (BBG), a P2X7 receptor antagonist, ameliorates the motor coordination deficits and body weight loss, and inhibits neuronal loss [148]. Moreover, P2X7 receptor expression and P2X7 receptor-induced Ca2+ permeability are increased in these models [148]. In vitro, cultured neurons expressing mutant huntingtin show increased susceptibility to apoptosis triggered by P2X7 receptor stimulation [148].
The effects of P2X7 receptor blockade have also been investigated in the 6-OHDA rat model of PD. Injection of A-438079 (30 mg/kg, i.p.), a P2X7 receptor antagonist, before lesion establishment prevents the depletion of dopamine in the striatum with no reduction in dopaminergic neuronal cell death [149]. Moreover, chronic treatment with BBG (45 mg/kg, i.p.; every 48 h for 2 weeks) before unilateral 6-OHDA injection prevents the loss of tyrosine-hydroxylase-positive neurons and attenuates the amphetamine-induced rotational behavior and memory deficits [150]. Corroborating these data, a study by our group showed that one-week treatment with BBG (50 mg/kg, i.p.), every day after lesion establishment, reverses the dopaminergic neuronal loss in the SN and rotational behavior [151]. In a more recent study, we reported that the same treatment protocol with a higher dose of BBG (75 mg/kg) also re-establishes dopaminergic ramifications of the nigrostriatal pathway and decreases the rotation behavior in rats injured with 6-OHDA [152]. Moreover, BBG decreases Iba-1 labeling in the SN compared to the injured hemisphere of animals without treatment, which indicates a reduction of microglial activation [152].
In view of these data, in vitro studies indicated that P2X7 receptor antagonists have neuroprotective effects in PD. BV2 microglial cells pretreated with BBG (1 μmol/L) show decreased ROS production induced by α-synuclein [153]. Moreover, pretreatment of neuroblastoma SH-SY5Y cells with the P2X receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (100 μmol/L) or AZ 11645373 (10 μmol/L) prevent the abnormal Ca2+ influx induced by α-synuclein [154].
The 1513A > C (rs3751143) polymorphism that facilitates P2X7 receptor-induced pore formation and increases cell death in humans [155] has been indicated as a risk factor for sporadic PD in a Han Chinese population [156]. Taken together, these studies suggest the P2X7 receptor as a therapeutic target for the treatment of both HD and PD.
Distinctive Purinergic Receptors in HD and PD
Since purinergic receptor involvement in neurodegenerative diseases is being studied worldwide, that some receptors important for either HD or PD, may be important as well for the other disease are the focus of this review. Thus, A1, P2X1 and P2Y6 receptors are presented in this section. Table 1 summarizes functions of these receptors in healthy and diseased brains.
Table 1.
Purinergic signaling in HD and PD.
| Expressed by (cell type) | Function in the CNS | Function in disease | |
|---|---|---|---|
| A2A | Neurons, astrocytes, microglia, and oligodendrocytes [70, 72, 167]. | Modulation of dopamine and glutamate neurotransmission, neuroinflammation, and synaptic plasticity [65, 71, 168]. | HD: A2A receptor expression is downregulated in HD pathogenesis and A2A receptor activation might have beneficial effects HD rodent models [116–118, 120–122], but contradictory results suggest that A2A receptor antagonism improves the motor disease [124, 125]. |
| PD: Generally, antagonists of A2A receptors promote motor and cognitive recovery [169]. A selective A2A receptor antagonist is currently used as an adjunct with levodopa [101]. Recent data show that the A2A receptor counteract the aggregation, neuronal degeneration and toxicity induced by α-synuclein [112, 113]. | |||
| P2X7 | Thought to be expressed in neurons and glia, but their presence in neurons has recently been questioned [127, 128]. | Glutamate release, stimulation of neuroimmune response, enhanced production of ROS, protein misfolding, and diminished BDNF levels, resulting in neuroplasticity impairment and/or neuronal damage [131, 146, 147]. | HD: P2X7 receptor antagonism ameliorates motor coordination deficits and body weight loss, and inhibits neuronal loss in animal models of HD (Tet/HD94 and R6/1) [148] |
|
PD: P2X7 receptor blockade before 6-OHDA lesions in rats prevents depletion of dopamine in striatum [149], hinders the loss of tyrosine-hydroxylase immunoreactivity, attenuates rotational behavior and diminishes memory deficits [150]. P2X7 receptor antagonism re-establishes the dopaminergic nigrostriatal pathway, decreases rotation behavior and reduces microglial activation in 6-OHDA lesioned rats [151, 152]. Pretreatment of BV2 microglia [153] and neuroblastoma SH-SY5Y [154] cells with P2X7 receptor antagonists decreases ROS production and prevents abnormal calcium influx induced by α-synuclein, respectively. | |||
| A1 | Neurons [170], microglia [171], astrocytes [171], oligodendrocytes [172]. | Inhibits excitatory neurotransmitter release [170] and microglial activation [171], decreases astrocyte proliferation [171], stimulates oligodendrocyte migration [172]. | A1 receptor activation protects against degeneration and decreases excitatory neurotransmission [158, 160]. |
| P2X1 | Sympathetic and sensory neurons, astrocytes, and oligodendrocytes [173]. | Noradrenaline and acetylcholine co-transmitters [173]. | PD: P2X1 receptor activation induces lysosomal dysfunction and increases α-synuclein aggregation [161]. |
| P2Y6 | mESC-derived GABAergic neurons [174] and activated microglia [175]. | Induces microglial cytokine release and phagocytic activity [164] and microglial phagoptosis [166]. | PD: P2Y6 receptor activation contributes to neurodegeneration possibly through microglial activation [152]. |
BDNF, brain-derived neurotrophic factor; HD, Huntington’s Disease; mESC, mouse embryonic stem cells; PD, Parkinson’s Disease; ROS, reactive oxygen species.
A1 Receptors
The A1 receptor, present in striatal and SN cells, has been suggested to play a significant role in neuroprotection in HD. Its main function in the CNS is to inhibit excitatory transmission, and to decrease the affinity of ligands for the D1 receptor [157]. In the rat model of HD induced by 3-nitropropionic acid, treatment with the A1 receptor agonist adenosine amine congener protects against degeneration and improves motor functions. These effects are accompanied by a decrease in the excitatory postsynaptic potential, suggesting that the neuroprotective effects of A1 receptor activation involve the modulation of aberrant [Ca2+]i flow [158]. However, different from the above-cited results, Zuchora and colleagues did not detect any neuroprotection when using the A1 receptor agonist R-PIA in the same animal model [159].
Taking into account the close relation between HD and PD, A1 receptor activity modulation could be a potential basis for PD treatment as well. In fact, a study conducted by Alfinito and colleagues showed effects of the A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine in rats injured with malonate, an inhibitor of mitochondrial function [160]. Injection of 8-cyclopentyl-1,3-dipropylxanthine into the SN exacerbated dopaminergic neuronal death, whereas striatal injection increased the GABAergic neuron loss induced by malonate [160]. Thus, in summary, A1 receptor activation protects neuronal cells against death in both PD and HD models.
P2X1 Receptors
One hallmark of the PD patient’s brain is the α-synuclein aggregation. Spread of α-synuclein clusters occurs in the neurodegenerative environment, and the ATP metabolites resulting from cellular death have been studied as key factors in this process. Since ATP stimulates α-synuclein oligomerization, P2X receptor antagonists were used to evaluate which P2X receptor subtype is implicated in this effect. NF449, a selective P2X1 receptor antagonist, reduces ATP-evoked α-synuclein aggregation in vitro [161]. P2X1 receptor activation induces lysosomal dysfunction, decreasing α-synuclein turnover and increasing its concentration [161]. Thus, ATP results in increased α-synuclein aggregation, which augments ATP production. Taking into account that lysosomal dysfunction leads to huntingtin protein accumulation in HD [162], P2X1 receptor antagonism is an interesting subject of study in this disease as well.
P2Y6 Receptors
Recently, P2Y6 receptors were also implicated in PD [152]. In the CNS, P2Y6 receptors are mainly present in neurons and microglial cells [163, 164]. Peripherally, increased expression has been found in peripheral blood mononuclear cells from PD patients [165]. Taking into account that PD induces neuroinflammation and microglia activation in vivo, rat primary microglia culture was challenged with lipopolysaccharide. These cells presented increased P2Y6 receptor expression, supporting the neuroinflammatory hypothesis regarding the role of the P2Y6 receptor in PD [165]. Moreover, microglial P2Y6 receptor activation by UDP released during the neurodegenerative process induces cytokine release and phagocytic activity [164].
Oliveira-Giacomelli and colleagues (2019) demonstrated that P2Y6 receptor antagonism prevents the death of dopaminergic neurons in SH-SY5Y cell cultures challenged with 6-OHDA, and in an animal model of PD induced by the same neurotoxin. This effect was accompanied by inhibition of microglial activation in the SN [152]. The authors suggested that this in vivo effect could be due to inhibition of the phagoptosis process, in which reactive microglial cells phagocytose viable neurons. This hypothesis is supported by a study showing that P2Y6 receptor antagonism prevents the microglial phagoptosis induced by inflammation in a mixed culture [166]. However, since the same neuroprotective effect was found in vitro in the absence of microglial cells, neuronal P2Y6 receptors could be, at least partially, responsible for the beneficial effects. In fact, P2Y6 receptor antagonism or deletion also prevents the SH-SY5Y cell death induced by 1-methyl-4-phenylpyridinium, possibly by reducing ROS production [163, 165].
Taking into account that reactive microglial cells participate in the neuronal loss in the HD brain, microglial P2Y6 receptor activation in HD development and progression seems to be a promising research subject for HD therapy.
Conclusion
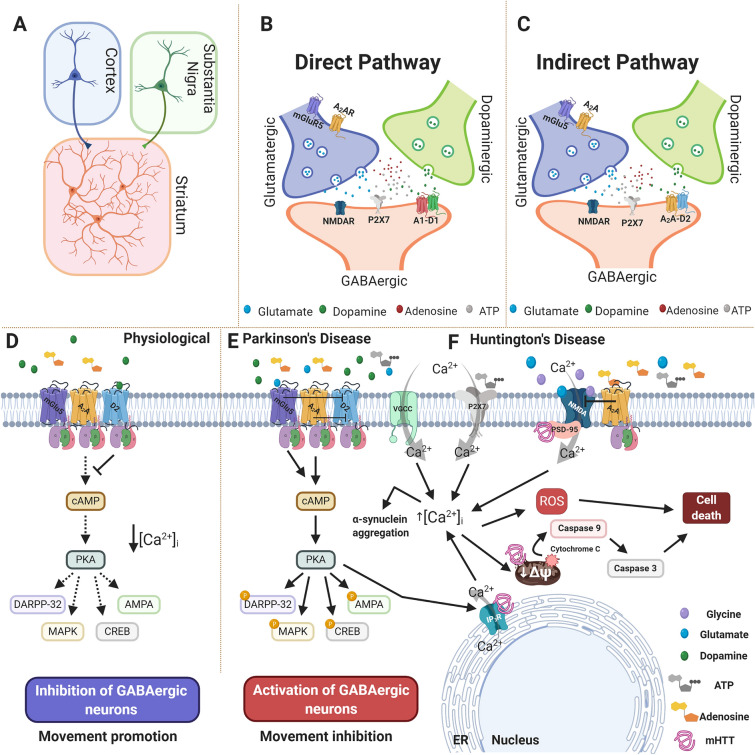
Purinergic A2A and P2X7 receptors participate in PD and HD by interfering through diverse mechanisms with intracellular Ca2+ signaling in the medium spiny neurons of the striatum. The A2A–D2 receptor dimer is a functional heteroreceptor, whose activation under physiological conditions is blocked by D2 receptor activation, resulting in movement stimulation. Under conditions of PD, the lack of dopamine maintains the heteroreceptor in the active state, triggering intracellular signaling pathways that cause movement inhibition. Thus, the same pathway, due to PKA activation, promotes neurodegeneration by facilitating glutamate release through interaction with the mGlu5 receptor, creating a disturbance of [Ca2+]i and resulting in aggregation of α-synuclein and mitochondrial oxidative stress in PD. The functions of A2A receptors in HD are controversial, although the most explored hypothesis states that A2A receptor inhibition may enhance excitotoxicity due to excessive NDMA receptor activation in some cases, or induce it in knock-out models of this glutamate receptor subtype. While P2X7 receptor activation by itself in microglial cells may lead to glutamate release and subsequent neuroplasticity impairment and/or neuronal damage, antagonism of this receptor by BBG is neuroprotective/neuroregenerative and reverses dopaminergic neuronal loss in the SN in experimental PD. Inhibition of the P2X7 receptor ameliorates the motor coordination deficits and body weight loss and inhibits the neuronal loss and apoptosis triggered by Ca2+ influx in HD (Fig. 3).
Fig. 3.
Purinergic receptors and Ca2+ signaling as common mechanisms in PD and HD. A Schematic presentation of the corticostriatal and nigrostriatal pathways. The striatum is mainly composed of GABAergic medium spiny neurons that project to direct or indirect movement pathways. B, C Glutamate, dopamine, adenosine and ATP control the direct and indirect signaling pathways, depending on the formation of adenosinergic–dopaminergic receptor dimers. The direct pathway stimulates GABAergic medium spiny neurons expressing D1 excitatory receptors, that inhibit motor function when dimerized with the A1 receptor. C, D The indirect pathway stimulates GABAergic medium spiny neurons expressing D2 inhibitory receptors. When D2 receptors form complexes with A2A and mGlu5 receptors, these neurons are inhibited with consequent movement promotion. E In PD, the dopamine levels in synapses are diminished, favoring A2A receptor activity, which leads to PKA activation and the inhibition of movements, while increasing [Ca2+]i and the aggregation of α-synuclein. F In HD, P2X7 receptors mediate increased Ca2+ inflow, moving the cell towards apoptosis pathways through depolarization of mitochondrial membrane potential, the release of cytochrome c and the activation of caspases. Moreover, NMDA receptors interact with mHtt, presenting abnormal Ca2+ influx and leading to the same cell death pathways, including the formation of ROS. Abbreviations: NMDA, N-methyl-D-aspartate; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; DARPP-32, dopamine- and cAMP-regulated neuronal phosphoprotein; MAPK, mitogen-activated protein kinase/extracellular signal-regulated kinase; CREB, cAMP response element-binding protein; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ΔΨ, change in membrane potential; ROS, reactive oxygen species; IP3R, inositol trisphosphate receptor; ER, endoplasmic reticulum; mHtt, mutant huntingtin protein.
Altogether, the above data indicate that the negative effects of [Ca2+]i signaling in PD and HD may be controlled or counterbalanced by the pharmacological modulation of purinergic receptor activity, such as by A2A and P2X7 receptors and possibly other purinergic receptors. Without any doubt, more studies are need to deepen the understanding of the mechanisms of these receptors, mainly in HD, in which their actions are yet controversial.
Acknowledgements
This review was supported by the São Paulo Research Foundation (FAPESP; 2018/07366-4) and a Fellowship from the National Council for Scientific and Technological Development (CNPq; 306392/2017-8). TG, AOG, and DER are grateful for postdoctoral fellowships from FAPESP (2015/13345-1, 2019/26852-0, and 2018/17504-5). RA is grateful for a doctoral fellowship from FAPESP (2019/24553-5). LBM is thankful for a master fellowship from CNPq (133396/2019-3). QY is grateful for the fellowship from National Key R&D Program of China (2019YFC1709101), The Project First-Class Disciplines Development (CZYHW1901) of Chengdu University of TCM and Sichuan Science and Technology Program (2019YFH0108, 2018SZ0257). AS work was supported by Russian Science Foundation grant 20-14-00241. Figures were created with BioRender.com.
Conflict of interest
The authors claim that there are no conflict of interest.
Footnotes
Talita Glaser and Roberta Andrejew contributed equally to this review.
References
- 1.MacDonald Gillian P.Buckler, Alan J.Altherr, MichaelTagle, DaniloSnell, Russell et al. MEB. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 1993, 72:971–983. [DOI] [PubMed]
- 2.Rikani AA, Choudhry Z, Choudhry AM, Rizvi N, Ikram H, Mobassarah NJ, et al. The mechanism of degeneration of striatal neuronal subtypes in Huntington disease. Ann Neurosci. 2014;21:112–114. doi: 10.5214/ans.0972.7531.210308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W, Xie J, Qiang Q, Li L, Lin X, Ren Y, et al. Gedunin degrades aggregates of mutant huntingtin protein and intranuclear inclusions via the proteasomal pathway in neurons and fibroblasts from patients with Huntington’s disease. Neurosci Bull. 2019;35:1024–1034. doi: 10.1007/s12264-019-00421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lees AJ, Hardy J, Revesz T, Lila R. Parkinson ’ s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 5.Jellinger KA. Significance of brain lesions in Parkinson disease dementia and Lewy body dementia. Front Neurol Neurosci. 2009;24:114–125. doi: 10.1159/000197890. [DOI] [PubMed] [Google Scholar]
- 6.Mor DE, Ischiropoulos H. The convergence of dopamine and alpha-synuclein: implications for Parkinson’s disease. J Exp Neurosci. 2018;12:1179069518761360. doi: 10.1177/1179069518761360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming SM. Mechanisms of gene-environment interactions in Parkinson’s disease. Curr Env Heal Rep. 2017;4:192–199. doi: 10.1007/s40572-017-0143-2. [DOI] [PubMed] [Google Scholar]
- 8.Kumar KR, Djarmati-Westenberger A, Grunewald A. Genetics of Parkinson’s disease. Semin Neurol. 2011;31:433–440. doi: 10.1055/s-0031-1299782. [DOI] [PubMed] [Google Scholar]
- 9.Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat Rev Neurol. 2013;9:445–454. doi: 10.1038/nrneurol.2013.132. [DOI] [PubMed] [Google Scholar]
- 10.Quarrell OW, Nance MA, Nopoulos P, Paulsen JS, Smith JA, Squitieri F. Managing juvenile Huntington’s disease. Neurodegener Dis Manag 2014, 3. [DOI] [PMC free article] [PubMed]
- 11.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 12.DeLong MR. Functional and pathophysiological models of the basal ganglia: therapeutic implications. Rinsho Shinkeigaku. 2000;40:1184. [PubMed] [Google Scholar]
- 13.Curtis AR, Fey C, Morris CM, Bindoff LA, Ince PG, Chinnery PF, et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet. 2001;28:350–354. doi: 10.1038/ng571. [DOI] [PubMed] [Google Scholar]
- 14.Nambu A. Seven problems on the basal ganglia. Curr Opin Neurobiol. 2008;18:595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 16.Capiod T. Extracellular calcium has multiple targets to control cell proliferation. Adv Exp Med Biol. 2016;898:133–156. doi: 10.1007/978-3-319-26974-0_7. [DOI] [PubMed] [Google Scholar]
- 17.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 18.La Rovere RM, Roest G, Bultynck G, Parys JB. Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium. 2016;60:74–87. doi: 10.1016/j.ceca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Toth AB, Shum AK, Prakriya M. Regulation of neurogenesis by calcium signaling. Cell Calcium. 2016;59:124–134. doi: 10.1016/j.ceca.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraft R. STIM and ORAI proteins in the nervous system. Channels (Austin) 2015;9:245–252. doi: 10.1080/19336950.2015.1071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 22.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, et al. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brini M, Calì T, Ottolini D, Carafoli E. Neuronal calcium signaling: Function and dysfunction. Cell Mol Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popugaeva E, Pchitskaya E, Bezprozvanny I. Dysregulation of neuronal calcium homeostasis in Alzheimer’s disease - A therapeutic opportunity? Biochem Biophys Res Commun. 2017;483:998–1004. doi: 10.1016/j.bbrc.2016.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pchitskaya E, Popugaeva E, Bezprozvanny I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium. 2018;70:87–94. doi: 10.1016/j.ceca.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glaser T, Arnaud Sampaio VF, Lameu C, Ulrich H. Calcium signalling: A common target in neurological disorders and neurogenesis. Semin Cell Dev Biol. 2019;95:25–33. doi: 10.1016/j.semcdb.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Chen N, Luo T, Wellington C, Metzler M, McCutcheon K, Hayden MR, et al. Subtype-specific enhancement of NMDA receptor currents by mutant huntingtin. J Neurochem. 1999;72:1890–1898. doi: 10.1046/j.1471-4159.1999.0721890.x. [DOI] [PubMed] [Google Scholar]
- 31.Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s disease. Prog Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Zeron MM, Hansson O, Chen N, Wellington CL, Leavitt BR, Brundin P, et al. Increased sensitivity to N-methyl-D-aspartate in a mouse model of Huntington’s disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 33.Levine MS, Cepeda C, André VM. Location, location, location: Contrasting roles of synaptic and extrasynaptic NMDA receptors in Huntington’s disease. Neuron. 2010;65:145–147. doi: 10.1016/j.neuron.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milnerwood AJ, Gladding CM, Pouladi MA, Kaufman AM, Hines RM, Boyd JD, et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Plotkin JL, Surmeier DJ. Corticostriatal synaptic adaptations in Huntington’s disease. Curr Opin Neurobiol. 2015;33:53–62. doi: 10.1016/j.conb.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raymond LA. Striatal synaptic dysfunction and altered calcium regulation in Huntington disease. Biochem Biophys Res Commun. 2017;483:1051–1062. doi: 10.1016/j.bbrc.2016.07.058. [DOI] [PubMed] [Google Scholar]
- 37.Barbin G, Pollard H, Gaiarsa JL, Ben-Ari Y, Barria A, Muller D, et al. Spatial and temporal requirements for huntingtin (Htt) in neuronal migration and survival during brain development. J Neurosci. 2001;81:1807–1817. doi: 10.1523/JNEUROSCI.2774-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, et al. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, et al. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang TS, Guo C, Wang H, Chen X, Bezprozvanny I. Neuroprotective effects of inositol 1,4,5-trisphosphate receptor C-terminal fragment in a Huntington’s disease mouse model. J Neurosci. 2009;29:1257–1266. doi: 10.1523/JNEUROSCI.4411-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinas R, et al. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc Natl Acad Sci U S A. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lautenschläger J, Stephens AD, Fusco G, Ströhl F, Curry N, Zacharopoulou M, et al. C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction. Nat Commun. 2018;9:712–725. doi: 10.1038/s41467-018-03111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Xue B, Wang J, Liu H, Shi L, Xie J. Potassium channels: A potential therapeutic target for Parkinson’s disease. Neurosci Bull. 2018;34:341–348. doi: 10.1007/s12264-017-0177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cieri D, Brini M, Cali T. Emerging (and converging) pathways in Parkinson’s disease: keeping mitochondrial wellness. Biochem Biophys Res Commun. 2016;483:1020–1030. doi: 10.1016/j.bbrc.2016.08.153. [DOI] [PubMed] [Google Scholar]
- 45.Cali T, Ottolini D, Brini M. Calcium signaling in Parkinson’s disease. Cell Tissue Res. 2014;357:439–454. doi: 10.1007/s00441-014-1866-0. [DOI] [PubMed] [Google Scholar]
- 46.Zaichick SV, McGrath KM, Caraveo G. The role of Ca2+ signaling in Parkinson’s disease. Dis Model Mech. 2017;10:519–535. doi: 10.1242/dmm.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 48.Guzman JN, Sanchez-Padilla J, Chan CS, Surmeier DJ. Robust pacemaking in substantia nigra dopaminergic neurons. J Neurosci. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puopolo M, Raviola E, Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J Neurosci. 2007;27:645–656. doi: 10.1523/JNEUROSCI.4341-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pchitskaya E, Popugaeva E, Bezprozvanny I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium. 2017;70:87–94. doi: 10.1016/j.ceca.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surmeier DJ, Guzman JN, Sanchez J, Schumacker PT. Physiological phenotype and vulnerability in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009290. doi: 10.1101/cshperspect.a009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Surmeier DJ, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E. Calcium and Parkinson’s disease. Biochem Biophys Res Commun. 2016;483:1013–1019. doi: 10.1016/j.bbrc.2016.08.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pathak T, Agrawal T, Richhariya S, Sadaf S, Hasan G. Store-operated calcium entry through orai is required for transcriptional maturation of the flight circuit in Drosophila. J Neurosci. 2015;35:13784–13799. doi: 10.1523/JNEUROSCI.1680-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burnstock G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology. 2016;104:4–17. doi: 10.1016/j.neuropharm.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 55.Oliveira-Giacomelli ágatha, Naaldijk Y, Sardá-Arroyo L, Gonçalves MCB, Corrêa-Velloso J, Pillat MM, et al. Purinergic receptors in neurological diseases with motor symptoms: Targets for therapy. Front Pharmacol 2018, 9: 325. 10.3389/fphar.2018.00325. [DOI] [PMC free article] [PubMed]
- 56.Ribeiro DE, Glaser T, Oliveira-Giacomelli, Ulrich H. Purinergic receptors in neurogenic processes. Brain Res Bull 2019, 151: 3–11. [DOI] [PubMed]
- 57.Ghiringhelli F, Bruchard M, Chalmin F, Rebe C. Production of adenosine by ectonucleotidases: a key factor in tumor immunoescape. J Biomed Biotechnol. 2012;2012:473712. doi: 10.1155/2012/473712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmermann H. Ectonucleotidases in the Nervous System. Purinergic Signalling in Neuron-Glia Interactions. Wiley. 2008;7:423–436. [Google Scholar]
- 59.Burnstock G. Introductory overview of purinergic signalling. Front Biosci (Elite Ed) 2011;3:896–900. doi: 10.2741/e298. [DOI] [PubMed] [Google Scholar]
- 60.Jimenez-Mateos EM, Smith J, Nicke A, Engel T. Regulation of P2X7 receptor expression and function in the brain. Brain Res Bull. 2019;151:153–163. doi: 10.1016/j.brainresbull.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 62.von Kügelgen I. Pharmacology of P2Y receptors. Brain Res Bull. 2019;151:12–24. doi: 10.1016/j.brainresbull.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Neary JT. Trophic actions of extracellular ATP: gene expression profiling by DNA array analysis. J Auton Nerv Syst. 2000;81:200–204. doi: 10.1016/s0165-1838(00)00154-5. [DOI] [PubMed] [Google Scholar]
- 64.Glaser T, Shimojo H, Ribeiro DE, Martins PPL, Beco RP, Kosinski M, et al. ATP and spontaneous calcium oscillations control neural stem cell fate determination in Huntington’s disease: a novel approach for cell clock research. Mol Psychiatry. 2020 doi: 10.1038/s41380-020-0717-5. [DOI] [PubMed] [Google Scholar]
- 65.Mori A. Mode of action of adenosine A2A receptor antagonists as symptomatic treatment for Parkinson’s disease. Int Rev Neurobiol. 2014;119:87–116. doi: 10.1016/B978-0-12-801022-8.00004-0. [DOI] [PubMed] [Google Scholar]
- 66.Lee YC, Chien CL, Sun CN, Huang CL, Huang NK, Chiang MC, et al. Characterization of the rat A2A adenosine receptor gene: A 4.8-kb promoter-proximal DNA fragment confers selective expression in the central nervous system. Eur J Neurosci 2003, 18:1786–1796. [DOI] [PubMed]
- 67.Cunha RA, Johansson B, Ploeg I, Sebastião AM, Ribeiro JA, Fredholm BB. Evidence for functionally important adenosine A2A receptors in the rat hippocampus. Brain Res. 1994;649:208–216. doi: 10.1016/0006-8993(94)91066-9. [DOI] [PubMed] [Google Scholar]
- 68.Svenningsson P, Hall H, Sedvall G, Fredholm BB. Distribution of adenosine receptors in the postmortem human brain: An extended autoradiographic study. Synapse. 1997;27:322–335. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 69.Kaelin-Lang A, Lauterburg T, Burgunder J-M. Expression of adenosine A2A receptors gene in the olfactory bulb and spinal cord of rat and mouse. Neurosci Lett. 1999;261:189–191. doi: 10.1016/s0304-3940(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 70.Cervetto C, Venturini A, Passalacqua M, Guidolin D, Genedani S, Fuxe K, et al. A2A-D2 receptor–receptor interaction modulates gliotransmitter release from striatal astrocyte processes. J Neurochem. 2017;140:268–279. doi: 10.1111/jnc.13885. [DOI] [PubMed] [Google Scholar]
- 71.Borroto-Escuela DO, Hinz S, Navarro G, Franco R, Müller CE, Fuxe K. Understanding the role of adenosine A2AR heteroreceptor complexes in neurodegeneration and neuroinflammation. Front Neurosci. 2018;12:1–11. doi: 10.3389/fnins.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vuorimaa A, Rissanen E, Airas L. In vivo PET imaging of adenosine 2A receptors in neuroinflammatory and neurodegenerative disease. Contrast Media Mol Imaging. 2017;2017:6975841. doi: 10.1155/2017/6975841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shindou T, Mori A, Kase H, Ichimura M. Adenosine A 2A receptor enhances GABA A-mediated IPSCs in the rat globus pallidus. J Physiol. 2001;532:423–434. doi: 10.1111/j.1469-7793.2001.0423f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shindou T, Nonaka H, Richardson PJ, Mori A, Kase H, Ichimura M. Presynaptic adenosine A2A receptors enhance GABAergic synaptic transmission via a cyclic AMP dependent mechanism in the rat globus pallidus. Br J Pharmacol. 2002;136:296–302. doi: 10.1038/sj.bjp.0704702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shindou T, Richardson PJ, Mori A, Kase H, Ichimura M. Adenosine modulates the striatal GABAergic inputs to the globus pallidus via adenosine A2A receptors in rats. Neurosci Lett. 2003;352:167–170. doi: 10.1016/j.neulet.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 76.Ferre S, Von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A. 1991;88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferré S, Snaprud P, Fuxe K. Opposing actions of an adenosine A2 receptor agonist and a GTP analogue on the regulation of dopamine D2 receptors in rat neostriatal membranes. Eur J Pharmacol. 1993;244:311–315. doi: 10.1016/0922-4106(93)90157-5. [DOI] [PubMed] [Google Scholar]
- 78.Ferré S, Ciruela F, Quiroz C, Luján R, Popoli P, Cunha RA, et al. Adenosine receptor heteromers and their integrative role in striatal function. ScientificWorldJournal. 2007;7:74–85. doi: 10.1100/tsw.2007.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm BB, et al. Regulation of the phosphorylation of the dopamine-and cAMP-regulated phosphoprotein of 32 kDa in vivo by dopamine D1, dopamine D2, and adenosine A2A receptors. Proc Natl Acad Sci U S A. 2000;97:1856–1860. doi: 10.1073/pnas.97.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindskog M, Svenningsson P, Pozzi L, Kim Y, Flenberg AA, Bibb JA, et al. Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature. 2002;418:774–778. doi: 10.1038/nature00817. [DOI] [PubMed] [Google Scholar]
- 81.Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shindou T, Mori A, Kase H, Ichimura M. Adenosine A2A receptor enhances GABAA-mediated IPSCs in the rat globus pallidus. J Physiol. 2001;532:423–434. doi: 10.1111/j.1469-7793.2001.0423f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beggiato S, Tomasini MC, Borelli AC, Borroto-Escuela DO, Fuxe K, Antonelli T, et al. Functional role of striatal A2A, D2, and mGlu5 receptor interactions in regulating striatopallidal GABA neuronal transmission. J Neurochem. 2016;19:254–264. doi: 10.1111/jnc.13652. [DOI] [PubMed] [Google Scholar]
- 84.Beggiato S, Antonelli T, Tomasini M, Borelli A, Agnati L, Tanganelli S, et al. Adenosine A2A-D2 receptor-receptor interactions in putative heteromers in the regulation of the striato-pallidal GABA pathway: Possible relevance for Parkinson’s disease and its treatment. Curr Protein Pept Sci. 2014;15:673–680. doi: 10.2174/1389203715666140901103205. [DOI] [PubMed] [Google Scholar]
- 85.Dayne Mayfield R, Larson G, Orona RA, Zahniser NR. Opposing actions of adenosine A2a and dopamine D2 receptor activation on GABA release in the basal ganglia: evidence for an A2a/D2 receptor interaction in globus pallidus. Synapse. 1996;22:132–138. doi: 10.1002/(SICI)1098-2396(199602)22:2<132::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 86.Mayfield RD, Suzuki F, Zahniser NR. Adenosine A2a receptor modulation of electrically evoked endogenous GABA release from slices of rat globus pallidus. J Neurochem. 1993;60:2334–2337. doi: 10.1111/j.1471-4159.1993.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 87.Nam HW, Bruner RC, Choi DS. Adenosine signaling in striatal circuits and alcohol use disorders. Mol Cells. 2013;36:195–202. doi: 10.1007/s10059-013-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 89.Chergui K, Bouron A, Normand E, Mulle C. Functional GluR6 kainate receptors in the striatum: Indirect downregulation of synaptic transmission. J Neurosci. 2000;20:2175–2182. doi: 10.1523/JNEUROSCI.20-06-02175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mori A, Shindou T, Ichimura M, Nonaka H, Kase H. The role of adenosine A2A receptors in regulating GABAergic synaptic transmission in striatal medium spiny neurons. J Neurosci. 1996;76:605–611. doi: 10.1523/JNEUROSCI.16-02-00605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aoyama S, Kase H, Borrelli E. Rescue of locomotor impairment in dopamine D2 receptor-deficient mice by an Adenosine A2A receptor antagonist. J Neurosci. 2000;20:5848–5852. doi: 10.1523/JNEUROSCI.20-15-05848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A(2A) receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- 93.Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: Morphological substrates for integration of striatal function. Neurology. 2003;61(11 Suppl 6):S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- 94.Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueño J, Gutiérrez MA, et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: Implications for striatal neuronal function. Proc Natl Acad Sci U S A. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA. Co-localization and functional interaction between adenosine A2A and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem. 2005;92:433–441. doi: 10.1111/j.1471-4159.2004.02887.x. [DOI] [PubMed] [Google Scholar]
- 96.Díaz-Cabiale Z, Vivó M, Del Arco A, O’Connor WT, Harte MK, Müller CE, et al. Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A2A and dopamine D2 receptors. Neurosci Lett 2002, 324:154–158. [DOI] [PubMed]
- 97.Ferré S, Popoli P, Rimondini R, Reggio R, Kehr J, Fuxe K. Adenosine A(2A) and group I metabotropic glutamate receptors synergistically modulate the binding characteristics of dopamine D2 receptors in the rat striatum. Neuropharmacology. 1999;38:129–140. doi: 10.1016/s0028-3908(98)00154-3. [DOI] [PubMed] [Google Scholar]
- 98.Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25:10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Popoli P, Pèzzola A, Torvinen M, Reggio R, Pintor A, Scarchilli L, et al. The selective mGlu5 receptor agonist CHPG inhibits quinpirole-induced turning in 6-hydroxydopamine-lesioned rats and modulates the binding characteristics of dopamine D2 receptors in the rat striatum: Interactions with adenosine A2a receptors. Neuropsychopharmacology. 2001;25:505–513. doi: 10.1016/S0893-133X(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 100.Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 101.Zhu C, Wang G, Li J, Chen L, Wang C, Wang Y, et al. Adenosine A2A receptor antagonist istradefylline 20 versus 40 mg/day as augmentation for Parkinson’s disease: a meta-analysis. Neurol Res. 2014;36:1028–1034. doi: 10.1179/1743132814Y.0000000375. [DOI] [PubMed] [Google Scholar]
- 102.Pinna A, Corsi C, Carta AR, Valentini V, Pedata F, Morelli M. Modification of adenosine extracellular levels and adenosine A2A receptor mRNA by dopamine denervation. Eur J Pharmacol. 2002;446:75–82. doi: 10.1016/s0014-2999(02)01818-6. [DOI] [PubMed] [Google Scholar]
- 103.Calon F, Dridi M, Hornykiewicz O, Bédard PJ, Rajput AH, Di Paolo T. Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain. 2004;127:1075–1084. doi: 10.1093/brain/awh128. [DOI] [PubMed] [Google Scholar]
- 104.Mishina M, Ishiwata K, Naganawa M, Kimura Y, Kitamura S, Suzuki M, et al. Adenosine A2A receptors measured with [11C]TMSX pet in the striata of parkinson’s disease patients. PLoS One. 2011;6:e17338. doi: 10.1371/journal.pone.0017338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramlackhansingh AF, Bose SK, Ahmed I, Turkheimer FE, Pavese N, Brooks DJ. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology. 2011;76:1811–1816. doi: 10.1212/WNL.0b013e31821ccce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tomiyama M, Kimura T, Maeda T, Tanaka H, Kannari K, Baba MA. Upregulation of striatal adenosine A2A receptor mRNA in 6-Hydroxydopamine-lesioned rats intermittently treated with L-DOPA. Synapse. 2004;52:218–222. doi: 10.1002/syn.20011. [DOI] [PubMed] [Google Scholar]
- 107.Ochi M, Koga K, Kurokawa M, Kase H, Nakamura J, Kuwana Y. Systemic administration of adenosine A(2A) receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: A microdialysis study. Neuroscience. 2000;100:53–62. doi: 10.1016/s0306-4522(00)00250-5. [DOI] [PubMed] [Google Scholar]
- 108.Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 109.Kumari N, Agrawal S, Kumari R, Sharma D, Luthra P. Neuroprotective effect of IDPU (1-(7-imino-3-propyl-2,3-dihydrothiazolo[4,5-d]pyrimidin-6(7H)-yl)urea) in 6-OHDA induced rodent model of hemiparkinson’s disease. Neurosci Lett. 2018;675:74–82. doi: 10.1016/j.neulet.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 110.Morissette M, Dridi M, Calon F, Hadjtahar A, Meltzer L, Bédard PJ, et al. Prevention of dyskinesia by an NMDA receptor antagonist in MPTP monkeys: Effect on adenosine A2A receptors. Synapse. 2006;60:239–250. doi: 10.1002/syn.20295. [DOI] [PubMed] [Google Scholar]
- 111.Diógenes MJ, Dias RB, Rombo DM, Vicente Miranda H, Maiolino F, Guerreiro P, et al. Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J Neurosci. 2012;32:11750–11762. doi: 10.1523/JNEUROSCI.0234-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferreira DG, Batalha VL, Miranda HV, Coelho JE, Gomes R, Gonçalves FQ, et al. Adenosine A2A receptors modulate α-synuclein aggregation and toxicity. Cereb Cortex. 2017;27:718–730. doi: 10.1093/cercor/bhv268. [DOI] [PubMed] [Google Scholar]
- 113.Hu Q, Ren X, Liu Y, Li Z, Zhang L, Chen X, et al. Aberrant adenosine A2A receptor signaling contributes to neurodegeneration and cognitive impairments in a mouse model of synucleinopathy. Exp Neurol. 2016;283:213–223. doi: 10.1016/j.expneurol.2016.05.040. [DOI] [PubMed] [Google Scholar]
- 114.Reiner A, Albin RL, Anderson KD, D’Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci U S A. 1988;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dhaenens CM, Burnouf S, Simonin C, Van Brussel E, Duhamel A, Defebvre L, et al. A genetic variation in the ADORA2A gene modifies age at onset in Huntington’s disease. Neurobiol Dis. 2009;35:474–476. doi: 10.1016/j.nbd.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 116.Glass M, Dragunow M, Faull RLM. The pattern of neurodegeneration in Huntington’s disease: A comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000;97:505–519. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 117.Bauer A, Zilles K, Matusch A, Holzmann C, Riess O, Von Hörsten S. Regional and subtype selective changes of neurotransmitter receptor density in a rat transgenic for the Huntington’s disease mutation. J Neurochem. 2005;94:639–650. doi: 10.1111/j.1471-4159.2005.03169.x. [DOI] [PubMed] [Google Scholar]
- 118.Cha JHJ, Frey AS, Alsdorf SA, Kerner JA, Kosinski CM, Mangiarini L, et al. Altered neurotransmitter receptor expression in transgenic mouse models of Huntington’s disease. Philos Trans R Soc B Biol Sci. 1999;354:981–989. doi: 10.1098/rstb.1999.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiang MC, Lee YC, Huang CL, Chern Y. cAMP-response element-binding protein contributes to suppression of the A2A adenosine receptor promoter by mutant huntingtin with expanded polyglutamine residues. J Biol Chem. 2005;280:14331–14340. doi: 10.1074/jbc.M413279200. [DOI] [PubMed] [Google Scholar]
- 120.Mievis S, Blum D, Ledent C. A2A receptor knockout worsens survival and motor behaviour in a transgenic mouse model of Huntington’s disease. Neurobiol Dis. 2011;41:570–576. doi: 10.1016/j.nbd.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 121.Villar-Menéndez I, Blanch M, Tyebji S, Pereira-Veiga T, Albasanz JL, Martín M, et al. Increased 5-methylcytosine and decreased 5-hydroxymethylcytosine levels are associated with reduced striatal A2AR levels in Huntington’s disease. Neuromolecular Med. 2013;15:295–309. doi: 10.1007/s12017-013-8219-0. [DOI] [PubMed] [Google Scholar]
- 122.Chou SY, Lee YC, Chen HM, Chiang MC, Lai HL, Chang HH, et al. CGS21680 attenuates symptoms of Huntington’s disease in a transgenic mouse model. J Neurochem. 2005;93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- 123.Tyebji S, Saavedra A, Canas PM, Pliassova A, Delgado-García JM, Alberch J, et al. Hyperactivation of D1 and A2A receptors contributes to cognitive dysfunction in Huntington’s disease. Neurobiol Dis. 2015;74:41–57. doi: 10.1016/j.nbd.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 124.Domenici MR, Scattoni ML, Martire A, Lastoria G, Potenza RL, Borioni A, et al. Behavioral and electrophysiological effects of the adenosine A2A receptor antagonist SCH 58261 in R6/2 Huntington’s disease mice. Neurobiol Dis. 2007;28:197–205. doi: 10.1016/j.nbd.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 125.Li W, Silva HB, Real J, Wang YM, Rial D, Li P, et al. Inactivation of adenosine A2A receptors reverses working memory deficits at early stages of Huntington’s disease models. Neurobiol Dis. 2015;79:70–80. doi: 10.1016/j.nbd.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 126.Sluyter R. The P2X7 receptor. Advances in experimental medicine and biology. 2017;1051:17–53. doi: 10.1007/5584_2017_59. [DOI] [PubMed] [Google Scholar]
- 127.Illes P, Khan TM, Rubini P. Neuronal P2X7 receptors revisited: Do they really exist? J Neurosci. 2017;37:7049–7062. doi: 10.1523/JNEUROSCI.3103-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miras-Portugal MT, Sebastián-Serrano Á, de Diego García L, Díaz-Hernández M. Neuronal P2X7 receptor: Involvement in neuronal physiology and pathology. J Neurosci. 2017;37:7063–7072. doi: 10.1523/JNEUROSCI.3104-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sim JA. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24:6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, et al. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sperlágh B, Köfalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, et al. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem. 2002;81:1196–1211. doi: 10.1046/j.1471-4159.2002.00920.x. [DOI] [PubMed] [Google Scholar]
- 132.Wirkner K, Köfalvi A, Fischer W, Günther A, Franke H, Gröger-Arndt H, et al. Supersensitivity of P2X7 receptors in cerebrocortical cell cultures after in vitro ischemia. J Neurochem. 2005;95:1421–1437. doi: 10.1111/j.1471-4159.2005.03465.x. [DOI] [PubMed] [Google Scholar]
- 133.Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, et al. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation. 2017;14:102. doi: 10.1186/s12974-017-0865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, et al. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res. 2008;1194:45–55. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 135.Metzger MW, Walser SM, Aprile-Garcia F, Dedic N, Chen A, Holsboer F, et al. Genetically dissecting P2rx7 expression within the central nervous system using conditional humanized mice. Purinergic Signal. 2017;13:153–170. doi: 10.1007/s11302-016-9546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Masin M, Young C, Lim K, Barnes SJ, Xu XJ, Marschall V, et al. Expression, assembly and function of novel C-terminal truncated variants of the mouse P2X7 receptor: Re-evaluation of P2X7 knockouts. Br J Pharmacol. 2012;165:978–993. doi: 10.1111/j.1476-5381.2011.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sánchez-Nogueiro J, Marín-García P, Miras-Portugal MT. Characterization of a functional P2X7-like receptor in cerebellar granule neurons from P2X7 knockout mice. FEBS Lett. 2005;17:3783–3788. doi: 10.1016/j.febslet.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 138.Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24:6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anderson CM, Nedergaard M. Emerging challenges of assigning P2X7 receptor function and immunoreactivity in neurons. Trends Neurosci. 2006;29:257–262. doi: 10.1016/j.tins.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 140.Carrasquero LMG, Delicado EG, Bustillo D, Gutiérrez-Martín Y, Artalejo AR, Miras-Portugal MT. P2X7 and P2Y13 purinergic receptors mediate intracellular calcium responses to BzATP in rat cerebellar astrocytes. J Neurochem. 2009;110:879–889. doi: 10.1111/j.1471-4159.2009.06179.x. [DOI] [PubMed] [Google Scholar]
- 141.Díaz-Hernandez M, del Puerto A, Díaz-Hernandez JI, Diez-Zaera M, Lucas JJ, Garrido JJ, et al. Inhibition of the ATP-gated P2X7 receptor promotes axonal growth and branching in cultured hippocampal neurons. J Cell Sci. 2008;121:3717–3728. doi: 10.1242/jcs.034082. [DOI] [PubMed] [Google Scholar]
- 142.Hervás C, Pérez-Sen R, Miras-Portugal MT. Coexpression of functional P2X and P2Y nucleotide receptors in single cerebellar granule cells. J Neurosci Res. 2003;73:384–399. doi: 10.1002/jnr.10676. [DOI] [PubMed] [Google Scholar]
- 143.Ortega F, Pérez-Sen R, Delicado EG, Miras-Portugal MT. P2X7 Nucleotide Receptor is Coupled to GSK-3 Inhibition and Neuroprotection in Cerebellar Granule Neurons. Neurotox Res. 2009;15:193–204. doi: 10.1007/s12640-009-9020-6. [DOI] [PubMed] [Google Scholar]
- 144.Rubini P, Pagel G, Mehri S, Marquardt P, Riedel T, Illes P. Functional P2X7 receptors at cultured hippocampal astrocytes but not neurons. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:943–954. doi: 10.1007/s00210-014-1005-1. [DOI] [PubMed] [Google Scholar]
- 145.Khan MT, Deussing J, Tang Y, Illes P. Astrocytic rather than neuronal P2X7 receptors modulate the function of the tri-synaptic network in the rodent hippocampus. Brain Res Bull. 2018;151:164–173. doi: 10.1016/j.brainresbull.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 146.Sperlágh B, Illes P. P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci. 2014;35:537–547. doi: 10.1016/j.tips.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 147.Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. The P2X7 receptor: A main player in inflammation. Biochem Pharmacol. 2018;151:234–244. doi: 10.1016/j.bcp.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 148.Díaz-Hernández M, Díez-Zaera M, Sánchez-Nogueiro J, Gómez-Villafuertes R, Canals JM, Alberch J, et al. Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23:1893–1906. doi: 10.1096/fj.08-122275. [DOI] [PubMed] [Google Scholar]
- 149.Marcellino D, Suárez-Boomgaard D, Sánchez-Reina MD, Aguirre JA, Yoshitake T, Yoshitake S, et al. On the role of P2X7 receptors in dopamine nerve cell degeneration in a rat model of Parkinson’s disease: studies with the P2X7 receptor antagonist A-438079. J Neural Transm. 2010;117:681–687. doi: 10.1007/s00702-010-0400-0. [DOI] [PubMed] [Google Scholar]
- 150.Carmo MRS, Menezes APF, Nunes ACL, Pliássova A, Rolo AP, Palmeira CM, et al. The P2X7 receptor antagonist Brilliant Blue G attenuates contralateral rotations in a rat model of Parkinsonism through a combined control of synaptotoxicity, neurotoxicity and gliosis. Neuropharmacology. 2014;81:142–152. doi: 10.1016/j.neuropharm.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 151.Ferrazoli EG, de Souza HDN, Nascimento IC, Oliveira-Giacomelli Á, Schwindt TT, Britto LR, et al. Brilliant blue-G but not fenofibrate treatment reverts hemiparkinsonian behavior and restores dopamine levels in an animal model of Parkinson’s disease. Cell Transplant. 2017;26:669–677. doi: 10.3727/096368917X695227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oliveira-Giacomelli Á, M. Albino C, de Souza HDN, Corrêa-Velloso J, de Jesus Santos AP, Baranova J, et al. P2Y6 and P2X7 receptor antagonism exerts neuroprotective/ neuroregenerative effects in an animal model of Parkinson’s disease. Front Cell Neurosci 2019, 13: 476–89. [DOI] [PMC free article] [PubMed]
- 153.Jiang T, Hoekstra J, Heng X, Kang W, Ding J, Liu J, et al. P2X7 receptor is critical in α-synuclein-mediated microglial NADPH oxidase activation. Neurobiol Aging. 2015;36:2304–2318. doi: 10.1016/j.neurobiolaging.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 154.Wilkaniec A, Gąssowska M, Czapski GA, Cieślik M, Sulkowski G, Adamczyk A. P2X7 receptor-pannexin 1 interaction mediates extracellular alpha-synuclein-induced ATP release in neuroblastoma SH-SY5Y cells. Purinergic Signal. 2017;13:347–361. doi: 10.1007/s11302-017-9567-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, et al. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001;276:11135–11142. doi: 10.1074/jbc.M010353200. [DOI] [PubMed] [Google Scholar]
- 156.Liu H, Han X, Li Y, Zou H, Xie A. Association of P2X7 receptor gene polymorphisms with sporadic Parkinson’s disease in a Han Chinese population. Neurosci Lett. 2013;546:42–45. doi: 10.1016/j.neulet.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 157.Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 158.Blum D, Gall D, Galas MC, D’Alcantara P, Bantubungi K, Schiffmann SN. The adenosine A1 receptor agonist adenosine amine congener exerts a neuroprotective effect against the development of striatal lesions and motor impairments in the 3-nitropropionic acid model of neurotoxicity. J Neurosci. 2002;22:9122–9133. doi: 10.1523/JNEUROSCI.22-20-09122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zuchora B, Urbańska EM. Effect of adenosine receptor agonists on neurodegenerative and convulsive activity of mitochondrial toxin, 3-nitropropionic acid. Pol J Pharmacol. 2001;53:69–71. [PubMed] [Google Scholar]
- 160.Alfinito PD, Wang SP, Manzino L, Rijhsinghani S, Zeevalk GD, Sonsalla PK. Adenosinergic protection of dopaminergic and GABAergic neurons against mitochondrial inhibition through receptors located in the substantia nigra and striatum, respectively. J Neurosci. 2003;23:10982–10987. doi: 10.1523/JNEUROSCI.23-34-10982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gan M, Moussaud S, Jiang P, McLean PJ. Extracellular ATP induces intracellular alpha-synuclein accumulation via P2X1 receptor-mediated lysosomal dysfunction. Neurobiol Aging. 2015;36:1209–1220. doi: 10.1016/j.neurobiolaging.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Koh JY, Kim HN, Hwang JJ, Kim YH, Park SE. Lysosomal dysfunction in proteinopathic neurodegenerative disorders: Possible therapeutic roles of cAMP and zinc. Mol Brain. 2019;12:18. doi: 10.1186/s13041-019-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qian Y, Xu S, Yang X, Xiao Q. Purinergic receptor P2Y6 contributes to 1-methyl-4-phenylpyridinium-induced oxidative stress and cell death in neuronal SH-SY5Y cells. J Neurosci Res. 2018;96:253–264. doi: 10.1002/jnr.24119. [DOI] [PubMed] [Google Scholar]
- 164.Kim B, Jeong HK, Kim JH, Lee SY, Jou I, Joe EH. Uridine 5’-Diphosphate induces chemokine expression in microglia and astrocytes through activation of the P2Y6 receptor. J Immunol. 2011;186:3701–3709. doi: 10.4049/jimmunol.1000212. [DOI] [PubMed] [Google Scholar]
- 165.Yang X, Lou Y, Liu G, Wang X, Qian Y, Ding J, et al. Microglia P2Y6 receptor is related to Parkinson’s disease through neuroinflammatory process. J Neuroinflammation. 2017;14:38. doi: 10.1186/s12974-017-0795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neher JJ, Neniskyte U, Hornik T, Brown GC. Inhibition of UDP/P2Y 6purinergic signaling prevents phagocytosis of viable neurons by activated microglia in vitro and in vivo. Glia. 2014;62:1463–1475. doi: 10.1002/glia.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res, 1992, 14:186–195 [DOI] [PubMed]
- 168.Chen J-F. Adenosine receptor control of cognition in normal and disease. International Review of Neurobiology, vol. 119. Elsevier; 2014:257–307. [DOI] [PubMed]
- 169.Morelli M, Di Paolo T, Wardas J, Calon F, Xiao D, Schwarzschild MA. Role of adenosine A2A receptors in parkinsonian motor impairment and l-DOPAinduced motor complications. Prog Neurobiol, 2007, 83:293–309. [DOI] [PubMed]
- 170.Haas HL, Selbach O. Functions of neuronal adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol, 2000, 362:375–381. [DOI] [PubMed]
- 171.Haskó G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci, 2005, 26:511–516. [DOI] [PMC free article] [PubMed]
- 172.Othman T, Yan H, Rivkees SA. Oligodendrocytes Express Functional A1 Adenosine Receptors That Stimulate Cellular Migration. Glia, 2003, 44:166–172. [DOI] [PubMed]
- 173.Burnstock G, Kennedy C. P2X Receptors in Health and Disease. Advances in Pharmacology, vol. 61. Academic Press Inc.; 2011:333–372. [DOI] [PubMed]
- 174.Khaira SK, Pouton CW, Haynes JM. P2X2, P2X4 and P2Y1 receptors elevate intracellular Ca 2+ in mouse embryonic stem cell-derived GABAergic neurons. Br J Pharmacol, 2009, 158:1922–1931. [DOI] [PMC free article] [PubMed]
- 175.Burnstock G. Introduction: ATP and Its Metabolites as Potent Extracellular Agents. Curr Top Membr, 2003:1–27.