Abstract
Mounting evidence suggests that the ATP-gated P2X7 receptor contributes to increased hyperexcitability in the brain. While increased expression of P2X7 in the hippocampus and cortex following status epilepticus and during epilepsy has been repeatedly demonstrated, the cell type-specific expression of P2X7 and its expression in extra-hippocampal brain structures remains incompletely explored. In this study, P2X7 expression was visualized by using a transgenic mouse model overexpressing P2X7 fused to the fluorescent protein EGFP. The results showed increased P2X7-EGFP expression after status epilepticus induced by intra-amygdala kainic acid and during epilepsy in different brain regions including the hippocampus, cortex, striatum, thalamus and cerebellum, and this was most evident in microglia and oligodendrocytes. Co-localization of P2X7-EGFP with cell type-specific markers was not detected in neurons or astrocytes. These data suggest that P2X7 activation is a common pathological hallmark across different brain structures, possibly contributing to brain inflammation and neurodegeneration following acute seizures and during epilepsy.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00573-9) contains supplementary material, which is available to authorized users.
Keywords: Purinergic signaling, P2X7, Green fluorescence protein, Status epilepticus, Epilepsy, Cell type-specific expression
Introduction
The purinergic family of receptors has garnered much attention as potential therapeutic drug targets for numerous human pathological conditions, particularly diseases of the central nervous system (CNS), such as epilepsy [1–3]. Epilepsy is a heterogeneous group of brain disorders with an incidence of 1%–2% and affects ~ 70 million people worldwide [4]. Despite the availability of > 25 seizure-suppressing drugs, drug-refractoriness remains steadfast at ~ 30%, and, even if seizure-suppressive, current pharmacological interventions are merely symptomatic without significantly altering the progression of the disease [5]. Epilepsy can be caused by genetic mutations or can be acquired, in many cases, in the aftermath of a brain injury [e.g., traumatic brain injury, infection, or following an episode of status epilepticus (SE)] [6]. Epileptogenesis, the process that lowers the seizure threshold following a precipitating insult, is an ongoing process which continues to drive the progression of the disease beyond its emergence [7]. Pathological changes that occur during epileptogenesis include delayed and ongoing neurodegeneration, changes in synaptic plasticity, neurogenesis, and chronic inflammation [6, 8]. Temporal lobe epilepsy (TLE) is the most common form of acquired epilepsy in adults and is particularly prone to drug-refractoriness [9]. Hippocampal sclerosis, characterized by a pattern of selective neuronal loss and reactive gliosis, is the most common pathological finding in the brains of TLE patients [10].
Purinergic receptors, activated by adenine and uracil nucleotides such as adenosine triphosphate (ATP), are divided into two subfamilies: the fast-acting ionotropic P2X family consisting of 7 members (P2X1–7) and the slower-acting metabotropic P2Y receptors with 8 members (P2Y1,2,4,6,11–14) [3]. Among these, the ATP-gated P2X7 receptor subtype has attracted the most attention as a possible drug target [11]. P2X7 is a non-selective, ligand-gated homotrimeric cation channel which is activated by extracellular ATP. Compared to other P2X family members, P2X7 has several unique characteristics: a long intracellular C-terminus and the ability to form a large membrane pore allowing the passage of molecules up to 900 Da. Most significantly, however, P2X7 requires much higher agonist concentrations than the low micromolar and nanomolar concentrations of extracellular ATP necessary to excite the six other P2X receptors (activation threshold: 0.3 mmol/L–0.5 mmol/L) [12–14]. This suggests that P2X7 activation occurs mainly under pathological conditions of high ATP release, for example during inflammation or increased neuronal activity (e.g., during a seizure) [15, 16]. Under normal physiological conditions, P2X7 expression in the brain seems to be mainly restricted to glial cells including microglia and oligodendrocytes [17, 18]. P2X7 has also been suggested to be expressed and to function pre-synaptically on neurons [11, 19] and astrocytes [20]; however, this remains controversial [21–23]. P2X7 has been implicated in numerous pathological processes: neurodegeneration, disruption of the blood-brain barrier, changes in neurotransmitter release, and inflammation [11, 17, 24]. P2X7 contributes to microglial activation and proliferation [25] and is a key activator of the NLRP3 inflammasome resulting in the release of the pro-inflammatory cytokine interleukin-1β (IL-1β) [17, 26]. In particular, the role of P2X7 during these inflammatory processes has sparked major interest in P2X7-targeting as a possible therapeutic approach for a plethora of diseases where underlying inflammation is one of the pathological hallmarks, including epilepsy [8].
P2X7 expression has been consistently shown to be upregulated in the hippocampus and cortex following SE and epilepsy in animal models and in the brains of patients with epilepsy [27–29]. The cell type in which P2X7 is increased following SE and during epilepsy is, however, still a matter of debate. While some studies have shown a mainly microglial upregulation of P2X7 [30], others have suggested that P2X7 is also increased on neurons. However, seizure-induced increases in the expression of P2X7 on astrocytes are absent, and have not been investigated on other brain cells, such as oligodendrocytes [27, 29, 31]. Also, there is now compelling evidence demonstrating a functional role for P2X7 during both seizure generation and epilepsy. P2X7 antagonism has been found to be anticonvulsive and neuroprotective in various models of acute seizures including the mouse model of SE induced by intra-amygdala kainic acid (KA) [27, 28, 32]. In contrast, other studies have found limited or no effect [33, 34] or even a pro-convulsant action of P2X7 antagonism during seizures [35, 36]. Less conflicting results have been obtained during chronic epilepsy. Here, drugs targeting P2X7 have been found to suppress seizure severity in one investigation [37] as well as daily seizure frequency in a second study [29].
In the present study, we used a BAC transgenic mouse model overexpressing a P2X7–EGFP fusion protein to characterize P2X7 expression changes and cell type-specific P2X7 expression in the hippocampus and non-hippocampal brain structures following unilaterally-induced SE and during the resulting chronic epilepsy [18]. Our results showed increased P2X7 expression (endogenous and P2X7-EGFP) in several structures, with transgenic P2X7-EGFP being mainly localized to microglia and oligodendrocytes.
Materials and Methods
Mouse Model of Status Epilepticus
All animal experiments were performed in accordance with the principles of the European Communities Council Directive 2010/63/EU. Procedures were reviewed and approved by the Research Ethics Committee of the Royal College of Surgeons in Ireland (REC 1322) and the Health Products Regulatory Authority (AE19127/P038; AE19127/P013) and undertaken as described previously [38] using 8–12 week-old male and female heterozygous FVB/NJ mP2X7-EGFP BAC transgenic mice [FVB/N-Tg(RP24- 114E20-P2X7/StrepHisEGFP)] that overexpress a P2X7 protein that is C-terminally fused to EGFP and is expressed under the control of the BAC-derived P2rx7 promoter [18]. Animals were housed in a controlled animal facility on a 12-h light/dark cycle at 22 °C ± 1 °C and humidity of 40%–60% with food and water ad libitum. During stereotaxic procedures, mice were anesthetized using isoflurane (5% induction, 1%–2% maintenance) and maintained normothermic by means of a feedback-controlled heat blanket (Harvard Apparatus Ltd, Edenbridge, Kent, UK). Once fully anesthetized, mice were placed in a stereotaxic frame and a midline scalp incision was made to expose the skull. A guide cannula (coordinates from bregma: AP, 0.94 mm; L, 2.85 mm) and three electrodes, one above each hippocampus and a reference electrode over the frontal cortex, were fixed in place with dental cement. SE was induced by microinjection of 0.2 µg KA [in 0.2 µL phosphate-buffered saline (PBS)] (Sigma-Aldrich, Dublin, Ireland) into the right basolateral amygdala. Vehicle-injected control animals received 0.2 µL PBS. The anticonvulsant lorazepam (6 mg/kg) (Wyetch, Taplow, UK) was delivered via intraperitoneal injection 40 min following intra-amygdala KA or vehicle to curtail seizures and reduce morbidity and mortality. The electroencephalogram (EEG) was recorded using a Xltek recording system (Optima Medical Ltd, Guildford, UK) from cortically-implanted electrodes. In a subset of P2X7-EGFP mice, spontaneous seizures were recorded using implantable telemetry devices (Model: F20-EET; Data Science International, St. Paul, MN, USA) which allows for continuous EEG recording in freely-moving mice [39]. These devices were implanted under the skin between the shoulders in the back and were connected to cortical EEG electrodes. EEG was recorded until 14 days post-SE.
Brain Dissection
To analyze endogenous P2X7 and P2X7-EGFP in the brain of naïve mice, intra-amygdala vehicle-injected control mice (Ctrl) and intra-amygdala KA-injected mice at different time-points post-SE (8 h, 24 h, 72 h, and 14 days) were killed by cervical dislocation and the brains removed. Each brain was then placed in a Petri dish on top of a cold board. Using a scalpel, the cerebellum was detached and the left and right hemispheres were separated evenly. The cortex, cerebellum, thalamus, striatum, and hippocampi were all collected for analysis. Samples were stored at − 80 °C until they were processed either for qPCR or Western blotting.
RNA Extraction and Quantitative Polymerase Chain Reaction
RNA was extracted using the TRIzol method, as previously described [39]. The quantity and quality of RNA was measured on a Nanodrop Spectrophotometer (Thermo Scientific, Rockford, IL). Samples with a 260/280 ratio between 1.8 and 2.2 were considered acceptable. 500 ng of total RNA was used to produce complementary DNA (cDNA) by reverse transcription using SuperScript III reverse transcriptase enzyme (BioScience, Dublin, Ireland) primed with 50 pmol of random hexamers (Sigma, Dublin, Ireland). qPCR was performed using the QuantiTech SYBR Green kit (Qiagen Ltd, Hilden, Germany) and LightCycler 1.5 (Roche Diagnostics, GmbH, Mannheim, Germany). Each reaction tube contained 2 μL cDNA sample, 10 μL SYBR Green Quantitect Reagent (Quiagen Ltd, Hilden, Germany), 1.25 μmol/L primer pair (Sigma) and RNAse free water (Invitrogen) to a final volume of 20 μL. Using LightCycler 1.5 software, data were analyzed and normalized to the expression of S18. The primers used (Sigma) were: P2rx7 forward: ACTGGCAGGTGTGTGTTCCATA, reverse: TTGGCAAGATGTTTCTCGTG; S18 forward: GGACCAGAGCGAAAGCATTTGCC, reverse: TCAATCTCGGGTGGCTGAACGC; Gfap forward: AGAAAACCGCATCACCATTC, reverse: TCACATCACCACGTCCTTGT; Iba-1 forward: TGGAGGGGATCAACAAGCAA, reverse: ACCCCAAGTTTCTCCAGCAT; NeuN forward: CAACCAAGTCCAAGTCCACG, reverse: GGTGGGAAAGGAAGACTGGA; Olig-2 forward: CTGCGCCTGAAGATCAACAG, reverse: CGTAGATCTCGCTCACCAGT; c-Fos forward: GGAATTAACCTGGTGCTGGA, reverse: CATTCAGACCACCTCGACAA; and Arc forward: AGCAGCAGACCTGACATCCT, reverse: GTGATGCCCTTTCCAGACAT.
Interleukin-1β ELISA
Interleukin-1β (IL-1β) levels in the hippocampus were measured using the DuoSet ELISA kits from R&D Systems as described previously (mouse IL-1β/IL-1F2, catalog #DY401-05) [40]. Briefly, the detection antibody was incubated in a 96-well ELISA plate overnight at room temperature. On the following day, 50 ng (100 μL) of the samples and standards (IL-1β: from 15.6 pg/mL to 1000 pg/mL) were added to wells and incubated for 2 h at room temperature; 100 μL of detection antibody was added to the wells and incubated for 2 h at room temperature; 100 μL of streptavidin-HRP complex was added to the wells followed by 45 min incubation in the dark. A color reaction, caused by the addition of substrate solution (100 μL) and terminated with stopping solution (50 μL), was quantified at 450 nm and 570 nm using a microplate reader. The IL-1β concentration was calculated following the manufacturer’s recommendations: the 570-nm values were subtracted from the 450-nm values. The log2 of the standard curve values were plotted, and a line of best fit was generated. The amount of IL-1β was extrapolated using the standard curve and the average calculated. The IL-1β concentration was then normalized to milligrams of total protein in tissue.
Hippocampal Cell Suspension and Fluorescence Activated Cell Sorting (FACS)
Hippocampi were dissected from adult P2X7-EGFP mouse brains. Cells were dissociated using the Worthington Papain Dissociation System (cat no LK003153) following the instructions of the manufacturer: the dissected tissue was placed in papain solution (composed by papain, 5 mL Earle’s balanced salt solution (EBSS) and 250 μL DNAseI) and incubated for 45 min at 37 °C with constant agitation. Next, mechanical trituration was performed with a sterile Pasteur pipette and the cloudy cell suspension was placed in a sterile 2 mL tube and centrifuged at 300 × g for 5 min at room temperature. The cell pellet was re-suspended in 108 mL EBSS with 12 µL reconstituted albumin-ovomucoid inhibitor solution and 6 µL DNaseI, layered on top of 200 μL albumin-inhibitor solution, then centrifuged at 70 × g for 6 min at room temperature. The cell pellet was re-suspended in 300 μL PBS in 0.4% BSA. The cell suspension was filtered using Bundle pluriStrainer® Mini 70 µm (cat no 43-10070-46). Hippocampal cells were identified by forward and side scatter, and dead cells and doublets were excluded. GFP-positive cells were sorted and acquired on a FACSAria Fusion (BD Biosciences) cell sorter, with purity > 95%. Analysis was performed using FlowJo software, confirming the gating in GFP-negative samples from a wild-type mouse hippocampus.
Western Blot Analysis
Western blots were analyzed as described previously [39]. Lysis buffer containing phosphatase and protease inhibitors was used to homogenize brain tissue and extract proteins, which were quantified using a Tecan plate reader at 560 nm. Thirty micrograms of protein per sample was loaded onto an acrylamide gel and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Following electrophoresis, proteins were transferred to a nitrocellulose membrane (GE Healthcare) and immunoblotted with extracellular P2X7 primary antibody (1:400; anti-rabbit; APR-008; Alomone Labs, Jerusalem, Israel). Membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse secondary antibodies (1:5000; Sigma-Aldrich, Dublin, Ireland). Protein bands were visualized using the Fujifilm LAS-4000 system with chemiluminescence (Immobilon western HRP substrate; Merck Millipore, Cork, Ireland) followed by analysis using Alpha-EaseFC4.0 software. The spot density option was used to evaluate the optical density of each protein band. Protein quantity was normalized to the loading control β-actin (1:1000; anti-mouse; Sigma-Aldrich).
Immunofluorescence
Immunofluorescence was carried out as before [39]. Mice were transcardially perfused with PBS followed by 4% paraformaldehyde (PFA) and post-fixed for an additional 24 h. Brains were then transferred to PBS and immersed in 4% agarose before 30-µm sagittal sections were cut on a VT1000S vibratome (Leica Biosystems, Wetzlar, Germany) and stored at − 20 °C in glycol. For immunofluorescence staining, sections were incubated with 0.1% triton/PBS and 1 mol/L glycine, followed by blocking with 1% BSA–PBS for 45 min. Sections were then incubated overnight with primary antibodies: GFP (1:400; anti-rabbit; Thermo Fisher Diagnostics, UK), Iba-1 (1:400; anti-goat; Abcam, Cambridge, UK), Olig-2 (1:400; anti-mouse; Millipore, Cork, Ireland), β-III tubulin (1:1000, anti-mouse; BioLegend, San Diego, CA, USA), syntaxin (1:400; anti-mouse; Sigma Aldrich), synaptophysin (1:400; anti-mouse; Sigma Aldrich), or GFAP (1:400; anti-mouse; Sigma Aldrich). After washing in PBS, sections were incubated with fluorescent secondary antibodies: AlexaFluor488 or AlexaFluor568 (1:400; Invitrogen, Dublin, Ireland). This was again followed by washing in PBS and brief incubation with DAPI (1:500; Sigma-Aldrich). FluorSave™ (Millipore, Cork, Ireland) was used to cover the tissue and confocal images were captured on a Zeiss 710 LSM NLO confocal microscope equipped with four laser lines (405 nm, 488 nm, 561 nm, and 653 nm) using a 40 × oil-immersion objective and ZEN 2010B SP1 software. Each image depicts a representative image from three mice. Finally, tiled images were obtained using ZEN 2010B SP1 to form a 10 × 6 mosaic of 40 × images. Cells were counted using Zen 3.0 (blue edition) by a person unaware of treatment. Cell counts were taken as the average of two images (40 ×), corresponding to an area of 0.045 mm2 per subfield. Cell counts were divided by area to determine an approximate value for cell density in each brain region. The hippocampus was further subdivided into three distinct areas: cornu ammonis 1 (CA1), CA3, and dentate gyrus (DG). Final counts are represented as cell number per square millimeter.
Fluoro-Jade B Staining
To assess SE-induced neurodegeneration, Fluoro-Jade B (FjB) staining was carried out as before [41]. Briefly, 12-μm coronal sections at the medial level of the hippocampus (bregma AP, − 1.94 mm) were cut on a cryostat. Tissue was fixed in 4% PFA, rehydrated in ethanol, and then transferred to a 0.006% potassium permanganate solution followed by incubation with 0.001% FjB (Chemicon Europe Ltd, Chandlers Ford, UK). Sections were mounted in dibutylphthalate polystyrene xylene mounting solution (Sigma Aldrich).
Statistical Analysis
GraphPad Prism 8 and StatView software (5.0.1.0) were used for statistical analysis. Data are presented as the mean ± SEM. Analysis of variance (ANOVA) with post-hoc Fisher’s protected least significant difference test was used to analyze group data of three or more. For two-group comparisons, Student’s t-test was used to determine statistical differences between groups. Significance was accepted at *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Increased P2X7-EGFP Expression in the Hippocampus Following Intra-amygdala KA-Induced Status Epilepticus
To establish P2X7 expression in the brain following seizures and during epilepsy we used a newly-developed mouse model overexpressing P2X7 protein fused to the fluorescent protein EGFP under the transcriptional control of a BAC-derived P2rx7 promoter [18] (Fig. 1A). In line with previous findings, P2rx7 mRNA levels were elevated ~ 6-fold in the hippocampus of P2X7-EGFP overexpressing reporter mice (Fig. 1B) [18]. Western blotting confirmed P2X7-EGFP to be expressed throughout the brain: the hippocampus, cortex, thalamus, striatum, and cerebellum. The highest P2X7 expression levels [transgenic (~ 95 kDa) and endogenous (~ 72 kDa)] occurred in the hippocampus, striatum, and thalamus (Fig. 1C, D). As reported previously, transgene expression was ~ 4–6 times higher than the endogenous P2X7 expression in all structures analyzed (Fig. 1D). Suggesting that P2X7-EGFP does not grossly alter normal brain hyperexcitability or inflammation, naïve P2X7-EGFP mice presented similar hippocampal mRNA levels of the neuronal activity-regulated genes c-Fos and Arc, the microglial marker Iba-1, and the astrocyte marker Gfap when compared to wild-type mice. Moreover, there was no significant difference in hippocampal baseline IL-1β levels between genotypes (Fig. S1A, B).
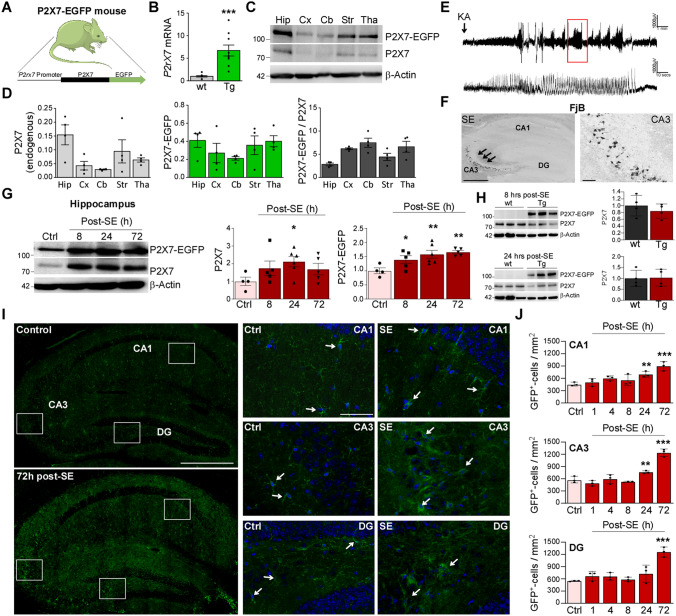
Fig. 1.
Increased P2X7-EGFP in the hippocampus after status epilepticus. A Schematic showing the transgenic approach to overexpress P2X7. Transgenic P2X7 is C-terminally fused to the fluorescent protein EGFP and expressed under the transcriptional control of the BAC-derived P2rX7 promoter. B Histogram showing a ~ 6-fold increase of P2rX7 mRNA levels in P2X7-EGFP mice when compared to wild-type (5.714 ± 1.232, P = 0.0003; n = 9 (wild-type (wt)) and P2X7-EGFP-overexpressing mice (Tg)). C Representative Western blots (n = 1 per lane) showing P2X7-EGFP expression of endogenous P2X7 (~ 72 kDa) and transgenic P2X7-EGFP-EGFP (~ 95 kDa) in the hippocampus (Hip), cortex (Cx), cerebellum (Cb), striatum (Str), and thalamus (Tha) in a P2X7-EGFP mouse. β-Actin is used as a guide to loading. D Histograms showing P2X7-EGFP is expressed ~ 4–6 times higher according to the brain structure analyzed when compared to endogenous P2X7 (n = 4 per group). E Representative EEG traces starting at the time of KA injection until treatment with anticonvulsant lorazepam 40 min later show high-amplitude high-frequency spiking during SE in P2X7-EGFP mice. F Representative FjB staining (5 × magnification) confirms typical neuropathology 24 h post-SE in P2X7-EGFP mice with damage mainly restricted to the CA3 subfield of the ipsilateral hippocampus (scale bars, 50 µm). G Representative Western blots (n = 1 per lane) and histograms showing increased P2X7-EGFP expression post-SE in the ipsilateral hippocampus [Ctrl vs 8 h, 1.000 ± 0.1035 vs 1.394 ± 0.1393, P = 0.046; Ctrl vs 24 h, 1.000 ± 0.1035 vs 1.584 ± 0.1403, P = 0.0042; Ctrl vs 72 h: 1.000 ± 0.1035 vs 1.659 ± 0.0584, P = 0.0023; n = 4 (Control), 5 (8 and 72 h post-SE) and 6 (24 h post-SE)]. Of note, the relative expression increases in P2X7-EGFP are similar to those in endogenous P2X7 (Ctrl vs 24 h, 1.000 ± 0.2359 vs 2.115 ± 0.2901, P = 0.03). β-Actin is used as a guide to loading. H Western blots (n = 1 per lane) and corresponding histograms showing no differences in the endogenous levels of P2X7 8 h and 24 h after SE between wild-type and P2X7-EGFP mice [n = 3 (wt) and 4 (P2X7-EGFP (TG)]. β-Actin is used as a guide to loading. I Left panels, representative tiled photomicrographs (40 × magnification) showing GFP-positive cells in the entire ipsilateral hippocampus in vehicle-injected control mice and mice 72 h post-SE (note increased immunoreactivity against GFP in the hippocampus of mice subjected to SE; right panels, higher magnification of each hippocampal subfield CA1, CA3, and DG (scale bars, 50 µm). J Histograms showing more GFP-positive cells in all three hippocampal subfields post-SE when compared to control-injected P2X7-EGFP mice (CA1 Ctrl vs 24 h, 459.3 ± 26.71 vs 696.3 ± 41.24, P = 0.0079, Ctrl vs 72 h, 459.3 ± 26.71 vs 896.3 ± 64.58, P < 0.0001; CA3 Ctrl vs 24 h, 577.8 ± 46.26 vs 763.0 ± 19.60, P = 0.0106, Ctrl vs 72 h, 577.8 ± 46.26 vs 1237.0 ± 53.42, P < 0.0001; DG Ctrl vs 72 h, 581.1 ± 7.407 vs 1259.0 ± 70.66, P < 0.0001; n = 3 per group). SE, status epilepticus; Ctrl, Control; *P < 0.05, **P < 0.01, ***P < 0.001.
To test whether SE leads to up-regulation of P2X7-EGFP in the brain, we used the intra-amygdala KA mouse model of status epilepticus. In this model, SE is triggered by a microinjection of KA into the basolateral nucleus of the amygdala [42]. P2X7-EGFP mice subjected to this procedure showed typical behavioral changes: Straub tail, rearing with forelimb clonus and falling, and high-amplitude high-frequency spiking on the EEG (Fig. 1E). P2X7-EGFP reporter mice also displayed typical neuropathology 72 h post-SE with cell death mainly restricted to the ipsilateral hemisphere and including the CA3 subfield of the hippocampus (Fig. 1F). We then analyzed P2X7 expression changes in the ipsilateral hippocampus of P2X7-EGFP mice via Western blot at different time-points post-SE. This revealed an increase in P2X7-EGFP protein expression at 8, 24, and 72 h following administration of the anticonvulsant lorazepam (Fig. 1G), similar to the relative expression increase found in endogenous P2X7 (Fig. 1G). Western blotting revealed similar expression levels of endogenous P2X7 in wild-type and P2X7-EGFP reporter mice at different time-points post-SE, suggesting that transgenic P2X7-EGFP had no impact on the expression of endogenous P2X7 (Fig. 1H and Fig. S1C). There was no correlation between the expression of the neuronal activity-regulated gene c-Fos and P2X7 expression (endogenous and P2X7-EGFP) (Fig. S1D), suggesting that P2X7 expression is independent of seizure severity.
We next used immunostaining against GFP to determine where in the hippocampus P2X7-EGFP is localized. Analysis of hippocampal sections using immunofluorescence against GFP at different time-points post-SE showed more GFP-positive cells in all hippocampal subfields (CA1, CA3, and DG) with GFP-positive cells presenting a mainly glial appearance (Fig. 1I, J).
Taken together, P2X7-EGFP-overexpressing reporter mice show typical pathology during intra-amygdala KA-induced SE with P2X7-EGFP expression elevated in the hippocampus post-SE.
P2X7-EGFP Expression is Mainly Confined to Microglia and Oligodendrocytes After Status Epilepticus
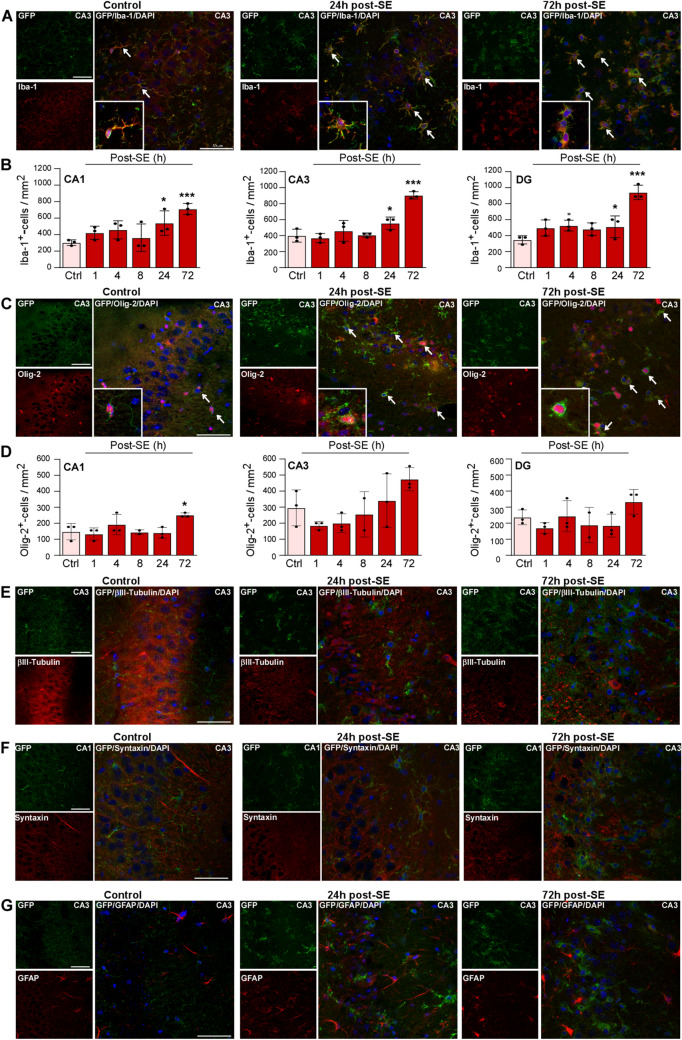
Next, to establish the cell type-specific expression of P2X7-EGFP, sections from P2X7-EGFP reporter mice were co-stained with anti-GFP antibodies and antibodies against different cell type-specific marker proteins at different time-points post-SE (Iba-1 for microglia, Olig-2 for oligodendrocytes, βIII-tubulin, synaptophysin, and syntaxin for neurons, and GFAP for astrocytes). This revealed an increase in cells positive for GFP and Iba-1 in all hippocampal subfields (CA1, CA3, and DG), which was most evident at 72 h post-SE (Fig. 2A, B and Figs S2 and 3). Increases in GFP-positive cells, which were also positive for Olig-2, were again most apparent at 72 h post-SE, although significant differences were only found in the CA1 region (Fig. 2C, D and Figs S2 and 3). The GFP signal was observed throughout the entire cell body of microglia and oligodendrocytes under physiological conditions and post-SE. Furthermore, Iba-1- and Olig-2-positive GFP cells underwent marked morphological changes following SE; during control conditions, both types of cells had a clear ramified morphology. Following SE, however, both cell types showed a strong reduction in the number of processes as well as process length which, in microglia, indicates activation [25]. This was most evident at 72 h post-SE (Fig. 2A, C). Interestingly, the relative percentage of GFP-positive cells was similar under control conditions and post-SE: ~ 70% of GFP-positive cells were also positive for Iba-1 and ~ 30% were positive for Olig-2 (Fig. S4). No co-localization of P2X7-EGFP was observed with βIII-tubulin-positive neurons, with the synaptic markers syntaxin and synaptophysin, and with GFAP-positive astrocytes at any time-point (Fig. 2E–G and Figs S2 and 5).
Fig. 2.
Co-localization of P2X7-EGFP with microglia and oligodendrocytes post-status epilepticus. A Representative photomicrographs (40 × magnification) showing co-localization of GFP (green) with the microglial marker Iba-1 (red) in controls and mice subjected to SE in the CA3 subfield (note that microglia showing long processes in vehicle-injected controls change to an amoeboid appearance at 24 h and 72 h post-SE) (scale bar, 50 µm). B Histograms showing more Iba-1- and GFP-positive cells post-SE in all three hippocampal subfields (CA1 Ctrl vs 24 h, 303.7 ± 19.60 vs 540.7 ± 85.43, P = 0.0224, Ctrl vs 72 h, 303.7 ± 19.60 vs 711.1 ± 38.49, P = 0.0007; CA3 Ctrl vs 24 h, 400.0 ± 46.26 vs 555.6 ± 44, P = 0.0286, Ctrl vs 72 h, 400.0 ± 46.26 vs 903.7 ± 26.71, P < 0.0001; DG Ctrl vs 4 h, 348.1 ± 29.63 vs 525.9 ± 39.20, P = 0.034, Ctrl vs 24 h, 348.1 ± 29.63 vs 511.1 ± 78.04, P = 0.0488, Ctrl vs 72 h, 348.1 ± 29.63 vs 940.7 ± 51.85, P < 0.0001; n = 3 per group). C Representative images (40 × magnification) showing co-localization of GFP (green) with the oligodendrocyte marker Olig-2 (red) in controls and mice subjected to SE in the CA3 subfield (note that oligodendrocytes, similar to microglia, show long processes in vehicle-injected controls which change to an amoeboid appearance at 24 h and 72 h post-SE) (scale bar, 50 µm). D Histograms showing more Olig-2- and GFP-positive cells post-SE in CA1 (Ctrl vs 72 h, 148 ± 29.63 vs 251.9 ± 7.407, P = 0.0115; n = 3 per group). There are no significant differences between treatment groups for the subfields CA3 and DG (n = 3 per group). E No co-localization between the neuronal marker βIII-tubulin (red) and GFP (green) under control conditions and post-SE (scale bar, 50 µm). F No co-localization of the synaptic marker syntaxin (red) and GFP (green) under control conditions and post-SE (scale bar, 50 µm). G No co-localization between the astrocyte marker GFAP (red) and GFP (green) under control conditions and post-SE (scale bar, 50 µm). *P < 0.05, ***P < 0.001.
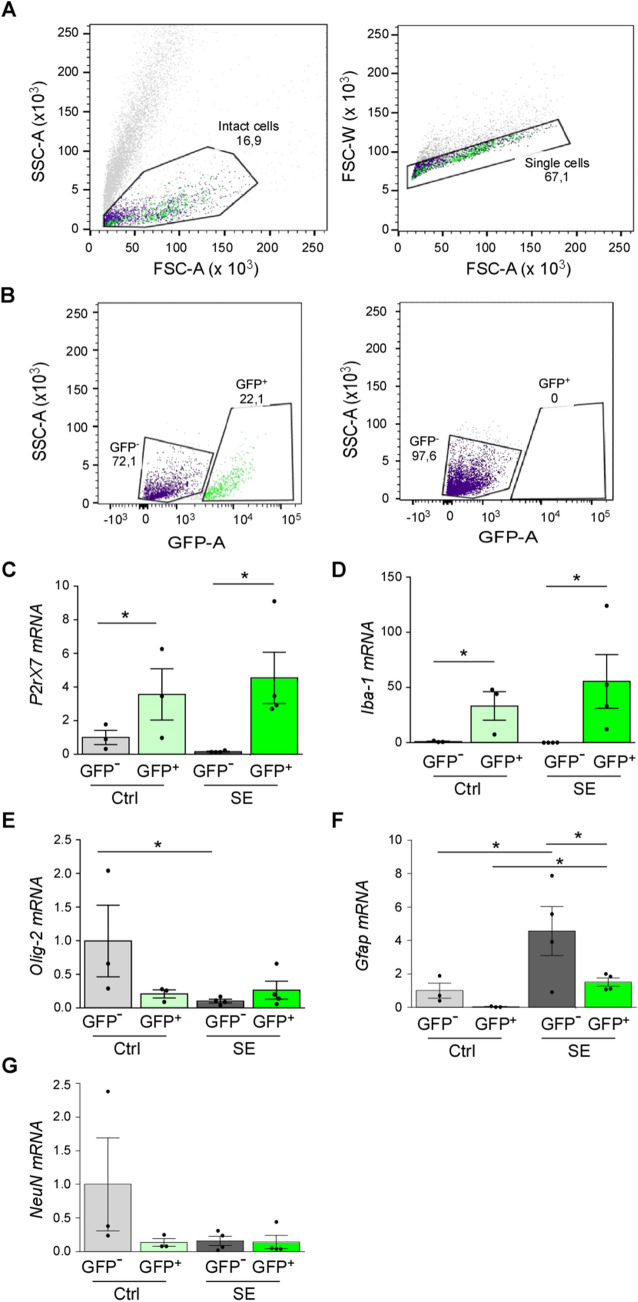
To confirm our immunofluorescence data, cell type-specific P2X7-EGFP expression was also analyzed in the ipsilateral hippocampus via FACS of GFP-positive cells (Fig. 3A, B). In line with our Western blot data, the median fluorescence intensity of the GFP signal was slightly higher 24 h post-SE than in control-injected mice (Control, n = 3 vs SE, n = 4, 600.8 ± 98.7 vs 781.8 ± 34.23; P = 0.121). The percentages of GFP-positive cells were, however, similar between conditions (Control, n = 3 vs SE, n = 4, 20.7 ± 1.64 vs 18.7 ± 0.85; P = 0.315). The absence of a GFP signal in GFP-negative samples [Ct values: 33.62 ± 0.73 (GFP+ Control) vs > 36 (GFP– Control) and 32.72 ± 0.78 (GFP+ SE) vs > 36 (GFP– SE); Ct value for negative control was > 36] and higher P2rx7 mRNA levels in GFP-positive samples than in GFP-negative samples confirmed the correct separation of cells (Fig. 3C). Interestingly, the P2rx7 mRNA levels appeared to decrease in GFP-negative cells post-SE (Fig. 3C). The Iba-1 mRNA levels were markedly elevated in GFP-positive cells, both in controls and mice subjected to SE when compared to GFP-negative cells (Fig. 3D). There were no differences in Olig-2 mRNA levels between groups (Fig. 3E). Finally, while the Gfap mRNA levels were slightly increased in the GFP-positive cell population from mice subjected to SE (Fig. 3F), no differences were found for NeuN mRNA levels between groups (Fig. 3G). This further implies, as seen using immunohistochemical approaches, that P2X7-EGFP is mainly upregulated in microglia.
Fig. 3.
Fluorescence activated cell sorting in P2X7-EGFP mice. A The forward scatter (FSC) vs the side scatter (SSC) gate was used to exclude dead cells and the FSC-A (area) vs the FSC-H (height) gate was used to identify single cells. B The GFP-A (area) vs the SSC-A (area) gate was used to discriminate GFP-positive from GFP-negative cells. Hippocampal tissue from wild-type mice was used as a negative control to set up the gate. C–G Histograms showing mRNA levels for (C) P2rx7 (Control: GFP–, 1.000 ± 0.4244 vs post-SE, GFP+, 4.548 ± 1.526, P = 0.049; post-SE: GFP–, 0.1575 ± 0.02780, GFP+, 4.548 ± 1.526, P = 0.01), (D) Iba-1 (Control: GFP–, 0.9967 ± 0.3919 vs post-SE: GFP+, 55.51 ± 24.30, P = 0.03; post-SE: GFP–, 0.1100 ± 0.01080, GFP+, 55.51 ± 24.30, P = 0.02), (E) Olig-2 (Control: GFP–, 0.9967 ± 0.5325 vs post-SE: GFP–, 0.1025 ± 0.02689, P = 0.02), (F) Gfap (Control: GFP–, 1.000 ± 0.4539 vs post-SE: GFP–, 4.573 ± 1.466, P = 0.02; Control: GFP+, 0.03667 ± 0.01667 vs post-SE: GFP–, 4.573 ± 1.466, P = 0.005; post-SE: GFP+, 1.510 ± 0.2416 vs GFP, 4.573 ± 1.466, P = 0.03), and (G) NeuN in GFP– and GFP+ FACS-separated cells from vehicle-injected controls (Ctrl) and mice 24 h post-SE (n = 3 (Control) and 4 (SE). *P < 0.05, **P < 0.01, ***P < 0.001.
Taken together, SE leads to an increase in the expression of P2X7-EGFP in the hippocampus that is mainly localized to microglia and oligodendrocytes, with increases particularly evident in Iba-1-positive microglia.
Increased P2X7-EGFP Expression in Extra-Hippocampal Brain Structures After Status Epilepticus
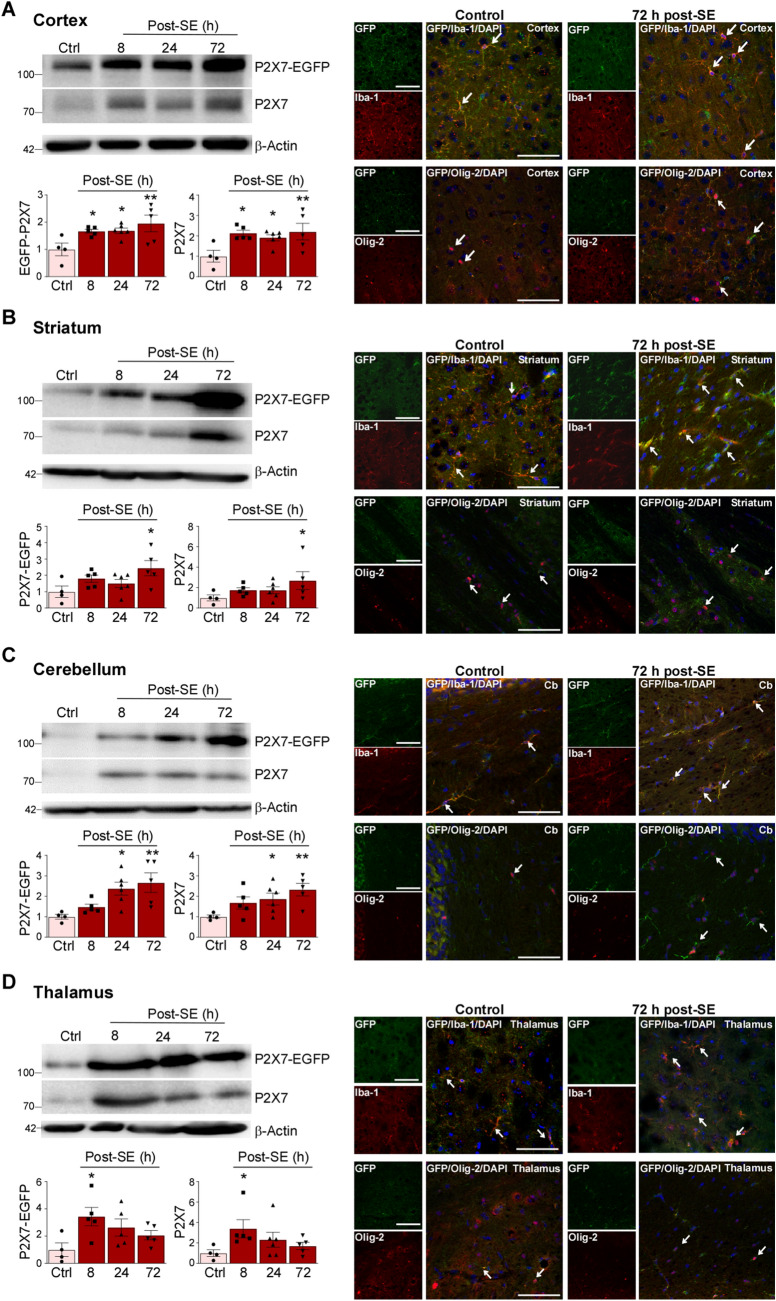
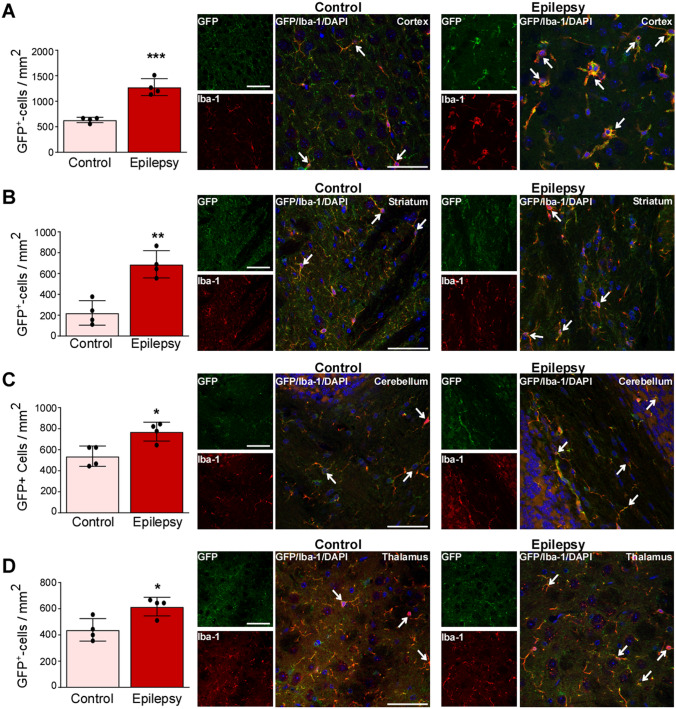
The majority of studies investigating seizure-induced changes in the expression of genes thought to be involved in the pathogenesis of epilepsy focus on the hippocampus and cortex. However, extra-hippocampal areas are also affected during epilepsy and targeting areas outside the seizure focus such as the cerebellum or thalamus has shown therapeutic potential [43, 44]. To establish whether SE leads to changes in the expression of P2X7 in extra-hippocampal areas, we analyzed four additional structures: the cortex, thalamus, striatum, and cerebellum. Analysis via Western blotting showed similar increases in P2X7 expression (P2X7-EGFP and endogenous P2X7) in all four areas (Fig. 4A–D). As shown in the hippocampus, expression changes of transgenic P2X7-EGFP were similar to those of endogenous P2X7 (Fig. 4A–D). Double immunofluorescence staining using different cell type-specific markers confirmed that microglia and oligodendrocytes are the main cell populations positive for P2X7-EGFP; both cell types displayed morphological changes post-SE similar to those observed in the hippocampus (Fig. 4A–D).
Fig. 4.
Increased P2X7 expression in extra-hippocampal areas. A–D Representative Western blots (n = 1 per lane) and histograms showing increased P2X7 expression (endogenous and P2X7-EGFP) post-SE [P2X7-EGFP, cx: Ctrl vs 8 h, 1.000 ± 0.2322 vs 1.667 ± 0.07172, P = 0.03, Ctrl vs 24 h, 1.000 ± 0.2322 vs 1.684 ± 0.09757, P = 0.03, Ctrl vs 72 h, 1.000 ± 0.2322 vs 1.953 ± 0.3028, P = 0.004; ST Ctrl vs 72 h, 1.000 ± 0.3470 vs 2.438 ± 0.4644, P = 0.01; TH Ctrl vs 8 h, 1.000 ± 0.4977 vs 3.435 ± 0.6794, P = 0.01; CB Ctrl vs 24 h, 1.000 ± 0.1172 vs 2.368 ± 0.3129, P = 0.01, Ctrl vs 72 h, 1.000 ± 0.1172 vs 2.665 ± 0.4765, P = 0.003. Endogenous P2X7 cx, Ctrl vs 8 h, 1.000 ± 0.2918 vs 2.126 ± 0.1471, P = 0.01, Ctrl vs 24 h, 1.000 ± 0.2918 vs 1.908 ± 0.1482, P = 0.03, Ctrl vs 72 h, 1.000 ± 0.2918 vs 2.205 ± 0.4071, P = 0.007; ST Ctrl vs 72 h, 1.000 ± 0.2936 vs 2.681 ± 0.8728, P = 0.04; TH Ctrl vs 8 h, 1.000 ± 0.3256 vs 3.387 ± 0.8902, P = 0.03; CB Ctrl vs 24 h, 1.000 ± 0.08098 vs 1.865 ± 0.2904, P = 0.049, Ctrl vs 72 h, 1.000 ± 0.08098 vs 2.326 ± 0.3095, P = 0.006; n = 4 (Control), 5 (8 and 72 h post-SE), and 6 (24 h post-SE) and representative images displaying P2X7-EGFP mainly localized to Iba-1-positive microglia and Olig-2-positive oligodendrocytes 72 h post- SE in (A) cortex, (B) striatum, (C) cerebellum, and (D) thalamus (n = 3 per group). β-Actin is used as a guide to loading. Scale bars, 50 µm. *P < 0.05, **P < 0.01.
In summary, increased P2X7 expression seems to be a major response to SE in the analyzed brain areas and P2X7-EGFP is mainly found on microglia and oligodendrocytes.
Increased Expression of P2X7-EGFP in the Brain During Chronic Epilepsy
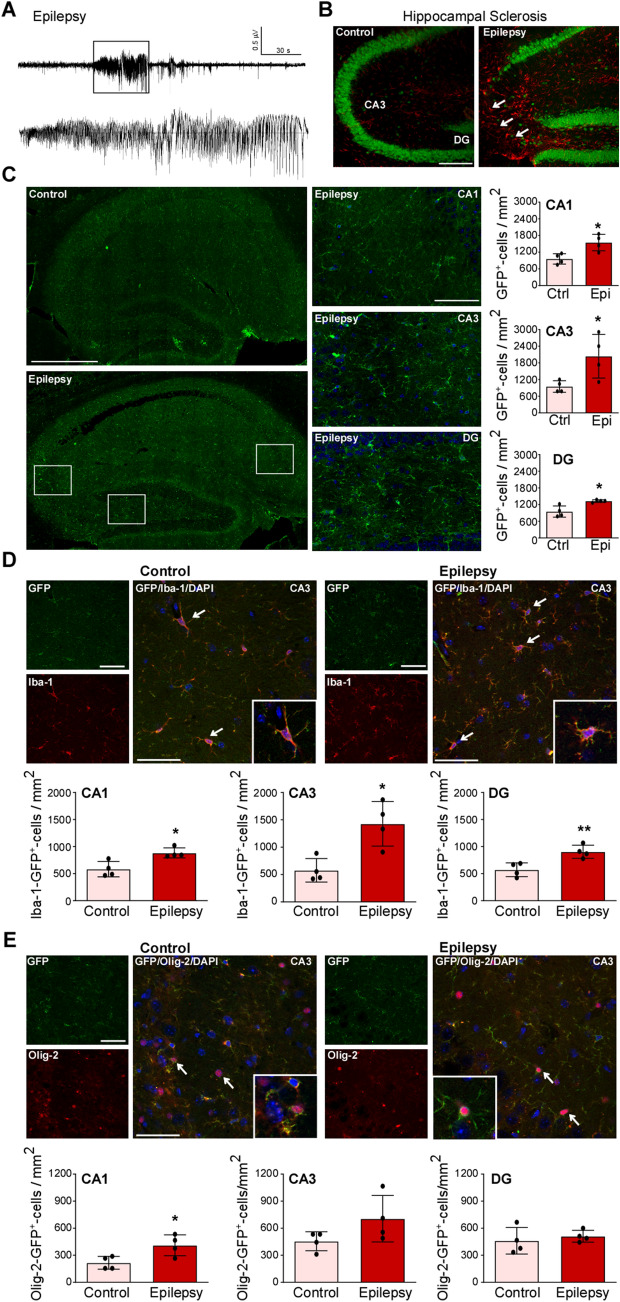
To establish whether P2X7-EGFP expression is altered during chronic epilepsy, its expression was analyzed at 14 days post-SE, a time-point when mice subjected to intra-amygdala KA are usually epileptic, experiencing unprovoked spontaneous seizures [39, 42, 45]. Long-term EEG recordings from P2X7-EGFP mice confirmed the development of epilepsy, all recorded mice (n = 3) experienced unprovoked seizures (Fig. 5A). Immunostaining in P2X7-EGFP mice at day 14 post-SE with the neuronal marker NeuN and the astrocyte marker GFAP confirmed the presence of a characteristic scar in the ipsilateral CA3 subfield of the hippocampus, mimicking the hippocampal sclerosis seen in TLE patients (Fig. 5B). Immunostaining against GFP confirmed increased GFP signals throughout the hippocampus (CA1, CA3, and DG), GFP-positive cells again displaying a mainly glial appearance (Fig. 5C). Using cell type-specific markers, we observed again, as seen before for SE, that P2X7-EGFP was mainly localized to Iba-1-positive microglia and Olig-2-positive oligodendrocytes (Fig. 5D, E). Interestingly, while the number of Iba-1- and GFP-positive cells was increased in all three hippocampal subfields (Fig. 5D), the number of GFP- and Olig-2-positive cells was only increased in CA1 (Fig. 5E). No co-localization was found between the neuronal marker βIII-tubulin and the astrocyte marker GFAP in GFP-positive cells (Fig. S6). Finally, to establish whether P2X7-EGFP is also increased in extra-hippocampal structures during epilepsy, we analyzed the same areas as before (cortex, striatum, cerebellum, and thalamus). GFP-cell counts confirmed more GFP-positive cells in all structures analyzed with P2X7-EGFP localized mainly to Iba-1-positive cells (Fig. 6A–D).
Fig. 5.
Increased P2X7-EGFP expression in the hippocampus during chronic epilepsy. A Representative EEG trace depicting high-frequency high-amplitude spiking during an epileptic seizure recorded from cortical electrodes in P2X7-EGFP mice at day 10 post-SE. B Representative photomicrographs (20 × magnification) showing reactive astrocytes (GFAP, red) and loss of NeuN-positive neurons (green) in the ipsilateral CA3 subfield in epileptic P2X7-EGFP mice 14 days post-SE [note decreased neuronal density in the ipsilateral CA3 subfield (arrows)]. C Left panels, representative tiled photomicrographs (40 ×) showing GFP-positive cells throughout the ipsilateral hippocampus in vehicle-injected controls and mice 14 days post-SE; right panels, higher magnification of each hippocampal subfield (CA1, CA3 and DG) from epileptic P2X7-EGFP mice and histograms showing more GFP-positive cells in each hippocampal subfield 14 days post-SE (CA1 Ctrl vs Epileptic, 959.3 ± 94.11 vs 1548 ± 148.2, P = 0.0153; CA3 Ctrl vs Epileptic, 950.0 ± 104.4 vs 2039.0 ± 392.1, P = 0.0364; DG Ctrl vs Epileptic, 950.0 ± 100.4 vs 1328.0 ± 22.91, P = 0.0105; n = 4 per group). Scale bars, 50 µm. D Representative images (40 × , CA3) and histograms showing more Iba-1-positive and GFP-positive cells in all three hippocampal subfields in epileptic P2X7-EGFP mice 14 days post-SE (CA1 Ctrl vs Epileptic, 583.3 ± 71.07 vs 883.3 ± 46.59, P = 0.0124; CA3 Ctrl vs Epileptic, 577.8 ± 107.7 vs 1428.0 ± 203.7, P = 0.0102; DG Ctrl vs Epileptic, 572.2 ± 63.75 vs 905.6 ± 60.43, P = 0.009; n = 4 per group). Scale bars, 50 µm. E Representative images (40 × , CA1) and histograms showing more Olig-2-positive and GFP-positive cells in the CA1 subfield in epileptic P2X7-EGFP mice 14 days post-SE (CA1 Ctrl vs Epileptic, 216.7 ± 35.57 vs 411.1 ± 57.74, P = 0.0285; n = 4 per group). No differences between groups were found in CA3 and DG (n = 4 per group). Scale bars, 50 µm. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 6.
Increased microglial and oligodendrocyte expression of P2X7-EGFP during chronic epilepsy. A–D Histograms and representative images (40 ×) showing more GFP-positive cells in epileptic P2X7-EGFP mice, mainly localized to Iba-1-positive microglia and Olig-2-postitive oligodendrocytes in (A) cortex (Ctrl vs Epileptic, 633.3 ± 26.45 vs 1278.0 ± 82.40, P = 0.0003; n = 4 per group), (B) striatum (Ctrl vs Epileptic, 611.1 ± 29.40 vs 844.4 ± 32.71, P = 0.0018; n = 4 per group), (C) cerebellum (Ctrl vs Epileptic, 538.9 ± 48.33 vs 755.6 ± 58.79, P = 0.0349; n = 4 per group), and (D) thalamus (Ctrl vs Epileptic, 438.9 ± 42.91 vs 616.7 ± 35.57, P = 0.0189; n = 4 per group). Scale bars, 50 µm. *P < 0.05, **P < 0.01, ***P < 0.001.
In conclusion, P2X7-EGFP expression remains increased during chronic epilepsy in several brain structures and is mainly localized to microglia and oligodendrocytes.
Discussion
In the present study, we examined the expression of P2X7 following SE and during epilepsy using a newly-generated mouse model overexpressing P2X7 fused to the fluorescent protein EGFP. This revealed that P2X7-EGFP was consistently increased in different structures of the brain where it was mainly expressed on microglia and oligodendrocytes. Thus, our data suggests that P2X7 activation is a common pathological hallmark across different brain structures, possibly contributing to extra-hippocampal brain inflammation and damage following acute seizures and during chronic epilepsy.
Increases in the expression of P2X7 following SE have been reported repeatedly in both experimental models and patients in studies focusing mainly on the hippocampus or the cortex [27–30, 46, 47]. Other structures are, however, also affected in patients with TLE and may contribute to the development of epilepsy [44, 48, 49]. We have now extended previous data demonstrating increased P2X7 in the thalamus, striatum, and cerebellum following SE and during chronic epilepsy.
The thalamus has been explored in intractable epilepsy, absence epilepsy, and seizure generation, particularly in combination with the cortex where studies exploring the cortico-thalamic pathway in epilepsy have suggested the occurrence of absence seizures in epileptic patients. Furthermore, clinical studies using EEG analysis have implicated the thalamus as a potential locus for generalized seizure onset [50, 51]. Despite being mainly investigated as a focal point of movement disorders, the role of the cerebellum in epilepsy has recently become a larger topic of interest. Ataxia and seizures, common hallmarks of epileptic patients, have been shown to be associated with cerebellar dysfunction, and further studies have also shown the detrimental effects of status epilepticus on Purkinje cells in the cerebellum. Moreover, it has been shown that Purkinje cell excitation induces a reduction in seizure frequency, further establishing an important role for the cerebellum in epilepsy [52, 53]. Finally, although the striatum has been explored extensively in neurodegenerative diseases, mostly revolving around understanding the role of dopaminergic transmission, there is evidence to suggest that dopamine may be a mediator of seizure activity. Moreover, in a study investigating the relationship between the cortex and the striatum, it was found that impairment of the cortico-striatal pathway does, in fact, trigger epilepsy in a mouse model of absence epilepsy [54–56]. Although the presence of, or the possibility of developing epilepsy, has been documented in these regions, so far, the expression of P2X7 in these structures have only been investigated in different neurodegenerative diseases. For example, thalamic expression of P2X7 was found to increase in a transgenic mouse model of Alzheimer’s disease [57], while separate studies presented evidence both in favor of and against increased striatal P2X7 in Parkinson’s disease [56, 58, 59]. Interestingly, these studies all showed increases in inflammatory markers in the areas investigated, suggesting that P2X7 may act as a common intermediary driving inflammatory processes. As inflammation has also been shown to be widespread throughout the CNS following SE and during epilepsy, increased levels of P2X7 in extra-hippocampal regions may indicate that P2X7 is one of the main contributors to inflammation in the brain during epilepsy [59–63]. Of note, while P2X7 increased gradually over time post-SE in most structures analyzed, the thalamus was an exception: P2X7 expression peaked at 8 h following lorazepam administration. While the reason for this peak remains elusive, a possible explanation may be the close proximity of the injection site (basolateral amygdala) to the thalamus with possible leakage of KA into the thalamus causing an initial, earlier upregulation of P2X7, compared with the steady increase over three days observed in the additional subfields.
As expected, P2X7-EGFP was mainly expressed on microglia following SE and during epilepsy. This is in line with its well-described role in microglial proliferation and activation [25, 64] and with the findings from animal studies in epilepsy [18, 29–31]. Morphological studies on microglia have also described changes from ameboid to reactive states following seizures and brain insults leading to neuroinflammation [65]. Here, we saw distinct changes over time, from long processes in vehicle-treated mice, to shorter, thicker processes at 24 h, as P2X7 levels increased. Finally, at 72 h, cells took on a fully active, phagocytic form. P2X7 has been described as a gatekeeper of inflammation [17], and its strong expression on microglia suggests an important role in controlling inflammatory processes during seizures and epilepsy. The release of the pro-inflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-α from activated/phagocytic microglia has been reported to be driven by P2X7 [66, 67] and, furthermore, P2X7 antagonism during SE blocks the release of the pro-convulsive cytokine IL-1β [27], while P2X7 antagonism during epilepsy reduces the amount of microgliosis and astrogliosis [29].
P2X7 is also present and functional on oligodendrocytes [68]. In our studies, we observed an increase in P2X7-EGFP-positive oligodendrocytes at 72 h post-SE and during epilepsy. Studies on the role of oligodendrocytes suggest a link between demyelination and epileptogenesis [69, 70]; therefore, discovering a link between P2X7 and oligodendrocyte proliferation and migration may have significant implications in epilepsy due to its well-known role in the maintenance and restoration of myelin sheaths in many neurodegenerative diseases [71]. Why the increase in P2X7-EGFP-positive cells was restricted to CA1 remains elusive, but it is tempting to speculate that this is due to its proximity to the corpus callosum. Similar to microglia, GFP-positive oligodendrocytes showed a reduction in processes post-SE. This may be caused by SE-induced neurodegeneration or the degeneration of oligodendrocytes themselves, as P2X7 has been shown to induce the death of oligodendrocytes during multiple sclerosis, as well as in rodent models of ischemia [72–74]. Olig-2 mRNA levels in GFP-positive cells were, however, not increased in our FACS experiments. The most likely explanation for this is that, because increases of oligodendrocytes post-SE were only found in CA1, more subtle effects may have been diluted by taking the whole hippocampus for our FACS experiments.
In contrast to other studies [1, 27, 31], we did not detect P2X7-EGFP on neurons. The presence of P2X7 on neurons remains, however, a matter of debate in which functional P2X7 on neurons is repeatedly questioned [22]. There are several possible explanations for the discrepancies between studies. In the present study, we used a mouse model with P2X7 fused to the reporter protein EGFP [18]. Previous attempts to localize P2X7 have either used antibodies or a different P2X7 reporter mouse where the GFP is inserted downstream of the P2rx7 promoter [27], so the GFP signal is rather a readout of P2rx7 transcription than a readout of translation into protein. Our previous work has shown that P2X7 is targeted post-transcriptionally via microRNA-22 following SE, blocking its translation into protein [75]. Therefore, a potential explanation for the lack of P2X7 protein in neurons is that while P2rx7 mRNA is transcribed in neurons, this is not translated into functional P2X7 protein. Another possible explanation for the apparent absence of P2X7 from both neurons and astrocytes is P2X7 is simply not observed as it is below the detection threshold in these cell types in both immunohistochemical and FACS analysis. Thus, future studies using, for example, patch clamp or Ca2+ imaging can be used to determine whether P2X7 is functional in these cell types during and after seizures. Finally, here we analyzed transgenic P2X7 fused to EGFP. Endogenous P2X7 may be subject to different regulatory mechanisms allowing its expression in different cells including neurons. In line with this, our FACS experiments showed that, while P2X7-EGFP seemed to increase post-SE, endogenous P2X7 decreased following SE. However, this may also be an artefact of our FACS approach as discussed below.
In agreement with previous studies, P2X7-EGFP did not co-localize with the astrocyte marker GFAP following SE and during epilepsy [27, 29, 31]. Nevertheless, using FACS analysis we did find that GFP-positive cells expressing GFAP increased at 24 h after SE. While GFP-expressing astrocytes were not observed, GFAP may also be used as a marker for progenitor cells which subsequently give rise to both neurons and oligodendrocytes [76, 77]. Lastly, whereas P2X7 expression increased as soon as 8 h post-SE, P2X7-EGFP cell counts increased only 24 h–72 h following SE. This suggests that early increases are most likely the result of increased P2X7 levels within the cell, while increased cell proliferation probably contributes to the overall increases in P2X7 expression at later stages, possibly via driving proliferation of microglia [25].
There are several possible limitations of our study which should be taken into account. Although P2X7-EGFP appeared to have no major impact on hyperexcitability and brain inflammation during naïve conditions, P2X7-EGFP overexpression may have an impact on seizure generation, seizure-induced pathology, and the epileptic phenotype and thereby also on P2X7 expression itself (endogenous and transgenic). While outside the scope of the present manuscript, future studies are therefore needed to investigate whether P2X7-EGFP overexpression impacts on seizures and the development of epilepsy. In this context, it is noteworthy that fold-changes in the actual protein expression of transgenic P2X7 were similar to those of endogenous P2X7, and endogenous P2X7 expression seemed not to be altered by transgenic P2X7-EGFP expression, suggesting that our genetic model reflects the expression changes in the brains of wild-type mice. While the FACS experiments confirmed our cell counting analysis showing Iba-1-positive cells were the main cell population expressing P2X7-EGFP, this technique has several limitations which should be kept in mind when interpreting our results. During tissue digestion, cellular branched extensions such as dendrites, axons, and microglial processes may be lost and our subsequent mRNA analysis may therefore be biased towards somatic mRNA [78]. Moreover, while our qPCR analysis suggested that all major cell types were present, we do not know whether our cell suspension had the same cell composition as the intact brain.
In conclusion, our data demonstrate that P2X7 expression is increased in several brain areas following acute seizures and during chronic epilepsy, suggesting that P2X7 may be a common pathological factor in the brain possibly contributing to the widespread inflammation and damage seen in TLE. Our study therefore reinforces the potential for P2X7-based therapeutic strategies for seizure control and disease-modification in epilepsy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by funding from the Health Research Board (HRA-POR-2015-1243), the Science Foundation Ireland (17/CDA/4708 and co-funded under the European Regional Development Fund and by FutureNeuro industry partners 16/RC/3948), H2020 Marie Skłodowksa-Curie Actions Individual Fellowships (753527, 796600 and 844956), the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement (766124), and the Deutsche Forschungsgemeinschaft (German Research Foundation; Project-ID 335447717 - SFB 1328).
Conflict of interest
All authors claim that there are no conflicts of interest.
References
- 1.Engel T, Alves M, Sheedy C, Henshall DC. ATPergic signalling during seizures and epilepsy. Neuropharmacology. 2016;104:140–153. doi: 10.1016/j.neuropharm.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Purinergic signalling: Therapeutic developments. Front Pharmacol. 2017;8:661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G. Introduction to purinergic signalling in the brain. Adv Exp Med Biol. 2020;1202:1–12. doi: 10.1007/978-3-030-30651-9_1. [DOI] [PubMed] [Google Scholar]
- 4.Moshe SL, Perucca E, Ryvlin P. Tomson T. Epilepsy: new advances. Lancet. 2015;385:884–898. doi: 10.1016/S0140-6736(14)60456-6. [DOI] [PubMed] [Google Scholar]
- 5.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 6.Pitkanen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 7.Pitkanen A, Lukasiuk K, Dudek FE, Staley KJ. Epileptogenesis. Cold Spring Harb Perspect Med 2015, 5. [DOI] [PMC free article] [PubMed]
- 8.Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:459–472. doi: 10.1038/s41582-019-0217-x. [DOI] [PubMed] [Google Scholar]
- 9.Engel J., Jr Approaches to refractory epilepsy. Ann Indian Acad Neurol. 2014;17:S12–17. doi: 10.4103/0972-2327.128644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 11.Sperlagh B, Illes P. P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci. 2014;35:537–547. doi: 10.1016/j.tips.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Mateos EM, Smith J, Nicke A, Engel T. Regulation of P2X7 receptor expression and function in the brain. Brain Res Bull. 2019;151:153–163. doi: 10.1016/j.brainresbull.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Kopp R, Krautloher A, Ramirez-Fernandez A, Nicke A. P2X7 Interactions and signaling - making head or tail of it. Front Mol Neurosci. 2019;12:183. doi: 10.3389/fnmol.2019.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beamer E, Conte G, Engel T. ATP release during seizures - A critical evaluation of the evidence. Brain Res Bull. 2019;151:65–73. doi: 10.1016/j.brainresbull.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Kaczmarek-Hajek K, Zhang J, Kopp R, Grosche A, Rissiek B, Saul A, et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. Elife 2018, 7. [DOI] [PMC free article] [PubMed]
- 19.Armstrong JN, Brust TB, Lewis RG, MacVicar BA. Activation of presynaptic P2X7-like receptors depresses mossy fiber-CA3 synaptic transmission through p38 mitogen-activated protein kinase. J Neurosci. 2002;22:5938–5945. doi: 10.1523/JNEUROSCI.22-14-05938.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan MT, Deussing J, Tang Y, Illes P. Astrocytic rather than neuronal P2X7 receptors modulate the function of the tri-synaptic network in the rodent hippocampus. Brain Res Bull. 2019;151:164–173. doi: 10.1016/j.brainresbull.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Jabs R, Matthias K, Grote A, Grauer M, Seifert G, Steinhauser C. Lack of P2X receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia. 2007;55:1648–1655. doi: 10.1002/glia.20580. [DOI] [PubMed] [Google Scholar]
- 22.Illes P, Khan TM, Rubini P. Neuronal P2X7 receptors revisited: Do They Really Exist? J Neurosci. 2017;37:7049–7062. doi: 10.1523/JNEUROSCI.3103-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miras-Portugal MT, Sebastian-Serrano A, de Diego Garcia L, Diaz-Hernandez M. Neuronal P2X7 receptor: Involvement in neuronal physiology and pathology. J Neurosci. 2017;37:7063–7072. doi: 10.1523/JNEUROSCI.3104-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miras-Portugal MT, Queipo MJ, Gil-Redondo JC, Ortega F, Gomez-Villafuertes R, Gualix J, et al. P2 receptor interaction and signalling cascades in neuroprotection. Brain Res Bull. 2019;151:74–83. doi: 10.1016/j.brainresbull.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Chen Y. Inflammation: A network in the pathogenesis of status epilepticus. Front Mol Neurosci. 2018;11:341. doi: 10.3389/fnmol.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel T, Gomez-Villafuertes R, Tanaka K, Mesuret G, Sanz-Rodriguez A, Garcia-Huerta P, et al. Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J. 2012;26:1616–1628. doi: 10.1096/fj.11-196089. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Pacheco A, Mesuret G, Sanz-Rodriguez A, Tanaka K, Mooney C, Conroy R, et al. Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia. 2013;54:1551–1561. doi: 10.1111/epi.12257. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez-Pacheco A, Diaz-Hernandez M, Arribas-Blazquez M, Sanz-Rodriguez A, Olivos-Ore LA, Artalejo AR, et al. Transient P2X7 receptor antagonism produces lasting reductions in spontaneous seizures and gliosis in experimental temporal lobe epilepsy. J Neurosci. 2016;36:5920–5932. doi: 10.1523/JNEUROSCI.4009-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rappold PM, Lynd-Balta E, Joseph SA. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089:171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 31.Dona F, Ulrich H, Persike DS, Conceicao IM, Blini JP, Cavalheiro EA, et al. Alteration of purinergic P2X4 and P2X7 receptor expression in rats with temporal-lobe epilepsy induced by pilocarpine. Epilepsy Res. 2009;83:157–167. doi: 10.1016/j.eplepsyres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Huang C, Chi XS, Li R, Hu X, Xu HX, Li JM, et al. Inhibition of P2X7 receptor ameliorates nuclear factor-Kappa B mediated neuroinflammation induced by status epilepticus in rat hippocampus. J Mol Neurosci. 2017;63:173–184. doi: 10.1007/s12031-017-0968-z. [DOI] [PubMed] [Google Scholar]
- 33.Fischer W, Franke H, Krugel U, Muller H, Dinkel K, Lord B, et al. Critical evaluation of P2X7 receptor antagonists in selected seizure models. PLoS One. 2016;11:e0156468. doi: 10.1371/journal.pone.0156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieoczym D, Socala K, Wlaz P. Evaluation of the anticonvulsant effect of brilliant blue G, a selective P2X7 receptor antagonist, in the iv PTZ-, maximal electroshock-, and 6 Hz-induced seizure tests in mice. Neurochem Res. 2017;42:3114–3124. doi: 10.1007/s11064-017-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JE, Ryu HJ, Kang TC. P2X7 receptor activation ameliorates CA3 neuronal damage via a tumor necrosis factor-alpha-mediated pathway in the rat hippocampus following status epilepticus. J Neuroinflammation. 2011;8:62. doi: 10.1186/1742-2094-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozmer K, Gao P, Araujo MGL, Khan MT, Liu J, Rong W, et al. Pilocarpine-induced status epilepticus increases the sensitivity of P2X7 and P2Y1 receptors to nucleotides at neural progenitor cells of the Juvenile Rodent hippocampus. Cereb Cortex. 2017;27:3568–3585. doi: 10.1093/cercor/bhw178. [DOI] [PubMed] [Google Scholar]
- 37.Amhaoul H, Ali I, Mola M, Van Eetveldt A, Szewczyk K, Missault S, et al. P2X7 receptor antagonism reduces the severity of spontaneous seizures in a chronic model of temporal lobe epilepsy. Neuropharmacology. 2016;105:175–185. doi: 10.1016/j.neuropharm.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Moran C, Sanz-Rodriguez A, Jimenez-Pacheco A, Martinez-Villareal J, McKiernan RC, Jimenez-Mateos EM, et al. Bmf upregulation through the AMP-activated protein kinase pathway may protect the brain from seizure-induced cell death. Cell Death Dis. 2013;4:e606. doi: 10.1038/cddis.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engel T, Sanz-Rodgriguez A, Jimenez-Mateos EM, Concannon CG, Jimenez-Pacheco A, Moran C, et al. CHOP regulates the p53-MDM2 axis and is required for neuronal survival after seizures. Brain. 2013;136:577–592. doi: 10.1093/brain/aws337. [DOI] [PubMed] [Google Scholar]
- 40.Alves M, De Diego Garcia L, Conte G, Jimenez-Mateos EM, D’Orsi B, Sanz-Rodriguez A, et al. Context-specific switch from Aanti- to pro-epileptogenic function of the P2Y1 receptor in experimental epilepsy. J Neurosci. 2019;39:5377–5392. doi: 10.1523/JNEUROSCI.0089-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engel T, Gomez-Sintes R, Alves M, Jimenez-Mateos EM, Fernandez-Nogales M, Sanz-Rodriguez A, et al. Bi-directional genetic modulation of GSK-3beta exacerbates hippocampal neuropathology in experimental status epilepticus. Cell Death Dis. 2018;9:969. doi: 10.1038/s41419-018-0963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouri G, Jimenez-Mateos E, Engel T, Dunleavy M, Hatazaki S, Paucard A, et al. Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res. 2008;1213:140–151. doi: 10.1016/j.brainres.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 43.Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–1025. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Streng ML, Krook-Magnuson E. The cerebellum and epilepsy. Epilepsy Behav 2020: 106909. [DOI] [PMC free article] [PubMed]
- 45.Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G, et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med. 2012;18:1087–1094. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vianna EP, Ferreira AT, Naffah-Mazzacoratti MG, Sanabria ER, Funke M, Cavalheiro EA, et al. Evidence that ATP participates in the pathophysiology of pilocarpine-induced temporal lobe epilepsy: fluorimetric, immunohistochemical, and Western blot studies. Epilepsia. 2002;43(Suppl 5):227–229. doi: 10.1046/j.1528-1157.43.s.5.26.x. [DOI] [PubMed] [Google Scholar]
- 47.Beamer E, Fischer W, Engel T. The ATP-gated P2X7 receptor as a target for the treatment of drug-resistant epilepsy. Front Neurosci. 2017;11:21. doi: 10.3389/fnins.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeCarli C, Hatta J, Fazilat S, Fazilat S, Gaillard WD, Theodore WH. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol. 1998;43:41–45. doi: 10.1002/ana.410430110. [DOI] [PubMed] [Google Scholar]
- 49.Moran NF, Lemieux L, Kitchen ND, Fish DR, Shorvon SD. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain. 2001;124:167–175. doi: 10.1093/brain/124.1.167. [DOI] [PubMed] [Google Scholar]
- 50.Blumenfeld H. The thalamus and seizures. Arch Neurol. 2002;59:135–137. doi: 10.1001/archneur.59.1.135. [DOI] [PubMed] [Google Scholar]
- 51.Tschampa HJ, Greschus S, Sassen R, Bien CG, Urbach H. Thalamus lesions in chronic and acute seizure disorders. Neuroradiology. 2011;53:245–254. doi: 10.1007/s00234-010-0734-1. [DOI] [PubMed] [Google Scholar]
- 52.Liu RS, Lemieux L, Bell GS, Sisodiya SM, Bartlett PA, Shorvon SD, et al. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia. 2005;46:1482–1494. doi: 10.1111/j.1528-1167.2005.51603.x. [DOI] [PubMed] [Google Scholar]
- 53.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro 2014, 1. [DOI] [PMC free article] [PubMed]
- 54.Kucker S, Tollner K, Piechotta M, Gernert M. Kindling as a model of temporal lobe epilepsy induces bilateral changes in spontaneous striatal activity. Neurobiol Dis. 2010;37:661–672. doi: 10.1016/j.nbd.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Miyamoto H, Tatsukawa T, Shimohata A, Yamagata T, Suzuki T, Amano K, et al. Impaired cortico-striatal excitatory transmission triggers epilepsy. Nat Commun. 1917;2019:10. doi: 10.1038/s41467-019-09954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deransart C, Riban V, Le B, Marescaux C, Depaulis A. Dopamine in the striatum modulates seizures in a genetic model of absence epilepsy in the rat. Neuroscience. 2000;100:335–344. doi: 10.1016/s0306-4522(00)00266-9. [DOI] [PubMed] [Google Scholar]
- 57.Martin E, Amar M, Dalle C, Youssef I, Boucher C, Le Duigou C, et al. New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol Psychiatry. 2019;24:108–125. doi: 10.1038/s41380-018-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bozzi Y, Borrelli E. The role of dopamine signaling in epileptogenesis. Front Cell Neurosci. 2013;7:157. doi: 10.3389/fncel.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crabbe M, Van der Perren A, Bollaerts I, Kounelis S, Baekelandt V, Bormans G, et al. Increased P2X7 receptor binding is associated with neuroinflammation in acute but not chronic rodent models for Parkinson’s disease. Front Neurosci. 2019;13:799. doi: 10.3389/fnins.2019.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei H, Zou H, Sheikh AM, Malik M, Dobkin C, Brown WT, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vezzani A, Friedman A. Brain inflammation as a biomarker in epilepsy. Biomark Med. 2011;5:607–614. doi: 10.2217/bmm.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen YC, Zhu GY, Wang X, Shi L, Du TT, Liu DF, et al. Anterior thalamic nuclei deep brain stimulation reduces disruption of the blood-brain barrier, albumin extravasation, inflammation and apoptosis in kainic acid-induced epileptic rats. Neurol Res. 2017;39:1103–1113. doi: 10.1080/01616412.2017.1379241. [DOI] [PubMed] [Google Scholar]
- 63.Savio LEB, de Andrade Mello P, da Silva CG, Coutinho-Silva R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front Pharmacol. 2018;9:52. doi: 10.3389/fphar.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia. 2016;64:1772–1787. doi: 10.1002/glia.23001. [DOI] [PubMed] [Google Scholar]
- 65.Domercq M, Vazquez-Villoldo N, Matute C. Neurotransmitter signaling in the pathophysiology of microglia. Front Cell Neurosci. 2013;7:49. doi: 10.3389/fncel.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci U S A. 2008;105:17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends Neurosci. 2014;37:55–65. doi: 10.1016/j.tins.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matute C. P2X7 receptors in oligodendrocytes: a novel target for neuroprotection. Mol Neurobiol. 2008;38:123–128. doi: 10.1007/s12035-008-8028-x. [DOI] [PubMed] [Google Scholar]
- 69.Luo Y, Hu Q, Zhang Q, Hong S, Tang X, Cheng L, et al. Alterations in hippocampal myelin and oligodendrocyte precursor cells during epileptogenesis. Brain Res. 2015;1627:154–164. doi: 10.1016/j.brainres.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 70.Hu X, Wang JY, Gu R, Qu H, Li M, Chen L, et al. The relationship between the occurrence of intractable epilepsy with glial cells and myelin sheath - an experimental study. Eur Rev Med Pharmacol Sci. 2016;20:4516–4524. [PubMed] [Google Scholar]
- 71.Amadio S, Parisi C, Piras E, Fabbrizio P, Apolloni S, Montilli C, et al. Modulation of P2X7 Receptor during Inflammation in Multiple Sclerosis. Front Immunol. 2017;8:1529. doi: 10.3389/fimmu.2017.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matute C, Torre I, Perez-Cerda F, Perez-Samartin A, Alberdi E, Etxebarria E, et al. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci. 2007;27:9525–9533. doi: 10.1523/JNEUROSCI.0579-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, et al. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res. 2008;1194:45–55. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 74.Feng JF, Gao XF, Pu YY, Burnstock G, Xiang Z, He C. P2X7 receptors and Fyn kinase mediate ATP-induced oligodendrocyte progenitor cell migration. Purinergic Signal. 2015;11:361–369. doi: 10.1007/s11302-015-9458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jimenez-Mateos EM, Arribas-Blazquez M, Sanz-Rodriguez A, Concannon C, Olivos-Ore LA, Reschke CR, et al. microRNA targeting of the P2X7 purinoceptor opposes a contralateral epileptogenic focus in the hippocampus. Sci Rep. 2015;5:17486. doi: 10.1038/srep17486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed AI, Shtaya AB, Zaben MJ, Owens EV, Kiecker C, Gray WP. Endogenous GFAP-positive neural stem/progenitor cells in the postnatal mouse cortex are activated following traumatic brain injury. J Neurotrauma. 2012;29:828–842. doi: 10.1089/neu.2011.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sahoo PK, Smith DS, Perrone-Bizzozero N, Twiss JL. Axonal mRNA transport and translation at a glance. J Cell Sci 2018, 131. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.