Abstract
It is clear that biofilm formation causes many serious health-care problems. Interestingly, sub minimum inhibitory concentrations (sub-MICs) of some biocides can induce biofilm formation in bacteria. We investigated whether sub-MICs of Savlon, chlorhexidine and deconex®, as biocidal products, can induce biofilm formation in clinical isolates of Pseudomonas aeruginosa. To determine MICs and biofilm formation, we performed microtitre plate assays. All three biocides induced biofilm formation at sub-MICs; Savlon was the most successful antiseptic agent to induce biofilm formation among P. aeruginosa isolates. Deconex had the best inhibition effect on planktonic cultures of P. aeruginosa isolates. We concluded that sub-MICs of Savlon and deconex could significantly induce biofilm formation.
Keywords: Biofilm formation, Pseudomonas aeruginosa, sub-MIC, biocides
Introduction
Antiseptics have been extensively applied in domestic and clinical settings as a convenient way of disinfection and protection against bacterial contamination for more than half a century [1]. Pseudomonas aeruginosa, a human opportunistic Gram-negative pathogen, is one of the most important nosocomial pathogens and is a major health problem, primarily in immunocompromised individuals. It causes a wide spectrum of infections in multiple organs, such as the respiratory, urinary and gastrointestinal tracts [[2], [3], [4]]. This organism is highly tolerant of harsh conditions and has the ability to survive in various environments, including hospital environments, on medical equipment, such as mechanical ventilators, urinary or dialysis catheters and endoscopes, and in sinks. Stability in these environments causes contamination [5]. Biofilm formation by P. aeruginosa increases morbidity and mortality through its protection against the host immune system and antibiotic treatment [6,7]. Bacterial biofilms are responsible for about 80% of all chronic human infections [8,9]. A biofilm comprises a complex aggregation of microorganisms surrounded by a matrix of extracellular polymeric substance [10], and is a mode of life that helps bacteria to resist antibiotics and survive [11]. Unfortunately, biofilm structures cause many problems through their formation on tissue and medically implanted devices [12]. Although most currently available antimicrobial agents cannot eradicate biofilm infections, some antimicrobial agents can induce their formation at sub-minimal inhibitory concentrations (sub-MICs) [13]. To eradicate the bacterial biofilm, a combination of multiple strategies to boost both activity of conventional antimicrobial agents and the host immune system is necessary [9,14]. Therefore, the aim of the study was to determine the in vitro effect of some conventional antiseptic agents, including Savlon, chlorhexidine and deconex® in biofilm formation by P. aeruginosa isolates.
Materials and methods
Ethics statement
Written informed consent was obtained from all patients and the study protocol was approved by the ethics committee of the Ilam University of Medical Sciences.
Bacterial isolates
This cross-sectional study was conducted from May 2015 to November 2016 in some of the hospitals in Ilam province, western Iran. Fifty clinical P. aeruginosa isolates (obtained from burn and urinary tract infections) were identified by standard conventional microbiological and biochemical methods [15].
Sub-MIC determination
Three biocidal agents were used. Chlorhexidine 0.2% (Iran Najo Pharmaceutical Co., Tehran, Iran) contains 0.2 g chlorhexidine per 100 mL and is recommended for use without dilution. Savlon (Behsa Pharmaceutical Co., Tehran, Iran) contains 1.5 g chlorhexidine gluconate plus 15 g cetrimide per 100 mL and is recommended for use at a 1:30 dilution. 100 g Deconex® (Borer Chemie AG, Zuchwil, Switzerland) contains 12 g ethanedial, 0.5 g pentanedial, 7.5 g didecyldimethylammonium chloride and is recommended for use at 1% and 2% dosages.
We investigated the MIC value of deconex in a total volume of 200 μL using the microtitre method from the Hengzhuang et al. procedure [16]. In the case of chlorhexidine, the dilutions used were 1:600, 1:500, 1:400, 1:200, 1:100, 1:66 and 1:50. For Savlon, the dilutions used were 1:40 000, 1:16 000, 1:8000, 1:4000, 1:2640, 1:2000 and 1:1600. Finally, 1:60 000, 1:33 300, 1:20 000, 1:10 000, 1:6600, 1:500 and 1:400 were used for deconex.
Biofilm assay
We used the microtitre plates assay for biofilm assay in Luria–Bertani medium (Merck, Darmstadt, Germany) [17]. Briefly, we added appropriately adjusted overnight bacterial cultures (0.5 McFarland, including, 1.5 × 108 CFU⁄mL) into the wells of 96-well plates (SPL, Gyeonggi-do, South Korea), followed by incubation at 37°C for 48 hours. After washing three times in phosphate-buffered saline, unattached bacterial cells were removed. Biofilm was stained with 200 μL crystal violet 0.1% (weight/volume) for 15 minutes and the wells were rewashed with phosphate-buffered saline (pH 7.2). The dye bound to the adherent cells was resolubilized with 200 μL of 95% ethanol. Optical density (OD) was measured at 492 nm using an ELISA reader (Synergy4; BioTek, Winooski, VT, USA). Each assay was performed in triplicate. Negative control wells were also included (uninoculated broth). Pseudomonas aeruginosa PAO1 (a biofilm-producing isolate) was used as positive control [18].
The adherence capabilities of the test isolates were classified into four categories; three standard deviations (SDs) above the mean OD of the negative control (broth only) was considered as the cut-off optical density (ODc). Isolates were classified as follows: if OD ≤ ODc, then the bacteria were non-adherent; if ODc < OD ≤ 2 × ODc, then the bacteria were weakly adherent; if 2 × ODc < OD ≤ 4 × ODc, then the bacteria were moderately adherent; and if 4 × ODc < OD, then the bacteria were strongly adherent.
In addition, for assaying the biofilm formation inducement, we used different concentrations of biocidal agents at sub-MICs in triplicate [19]. The biofilm value was estimated using the following formula: Biofilm value = (Test OD492 nm – Control OD492 nm).
Statistical analysis
SPSS 19.0 software was used for statistical analysis (SPSS Inc., Armonk, NY, USA). Categorical variables were compared using the chi-squared test as appropriate. Student's t-test was used to compare the different biofilm categories. Values of p < 0.05 were considered as statistically significant.
Results
Our findings showed the high ability (86%) of biofilm formation in clinical P. aeruginosa isolates (Table 1). Interestingly, the ability of biofilm formation among isolates obtained from burn skin samples was higher than isolates obtained from urine samples (p 0.012). The results of the MIC assay showed that chlorhexidine had a MIC range from 1:400 to 1:50 (mean ≈ 1:225). The MIC range was 1:33 300 to 1:100 (mean ≈ 1:16 700) for deconex and Savlon had a MIC range from 1:400 to 1:8000 (mean ≈ 1:2800).
Table 1.
The results of biofilm formation in Pseudomonas aeruginosa
| Biofilm production producer | Frequency | % |
|---|---|---|
| No biofilm producer | 7 | 14.0 |
| Weak biofilm producer | 5 | 10.0 |
| Moderate biofilm producer | 14 | 28.0 |
| Strong biofilm producer | 24 | 48.0 |
Interestingly, the burn isolates were significantly more susceptible than the urinary tract infection isolates, especially they were more susceptible to chlorhexidine (Table 2). Notably, we observed that deconex had the best inhibition effect on planktonic cultures of P. aeruginosa isolates.
Table 2.
The MIC range of Savlon, chlorhexidine and deconex®
| Strain no.a | MIC range |
Strain no. | MIC range |
Strain no. | MIC range |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorhexidine | Deconex | Savlon | Chlorhexidine | Deconex | Savlon | Chlorhexidine | Deconex | Savlon | |||
| 1 | 1:200 | 1:33 300 | 1:4000 | 18 | 1:400 | 1:33 300 | 1:8000 | 35 | 1:100 | 1:6600 | 1:1600 |
| 2 | 1:100 | 1:33 300 | 1:2000 | 19 | 1:200 | 1:10 000 | 1:2640 | 36 | 1:50 | 1:6600 | 1:1600 |
| 3 | 1:400 | 1:10 000 | 1:2640 | 20 | 1:200 | 1:10 000 | 1:4000 | 37 | 1:50 | 1:10 000 | 1:1600 |
| 4 | 1:200 | 1:400 | 1:4000 | 21 | 1:400 | 1:6600 | 1:1600 | 38 | 1:100 | 1:10 000 | 1:1600 |
| 5 | 1:100 | 1:6600 | 1:4000 | 22 | 1:200 | 1:10 000 | 1:4000 | 39 | 1:100 | 1:10 000 | 1:1600 |
| 6 | 1:200 | 1:33 300 | 1:2640 | 23 | 1:200 | 1:6600 | 1:2640 | 40 | 1:50 | 1:6600 | 1:1600 |
| 7 | 1:66 | 1:33 300 | 1:1600 | 24 | 1:200 | 1:10 000 | 1:4000 | 41 | 1:50 | 1:6600 | 1:1600 |
| 8 | 1:50 | 1:60 000 | 1:1600 | 25 | 1:200 | 1:10 000 | 1:4000 | 42 | 1:50 | 1:10 000 | 1:1600 |
| 9 | 1:200 | 1:60 000 | 1:2000 | 26 | 1:66 | 1:33 300 | 1:4000 | 43 | 1:50 | 1:10 000 | 1:4000 |
| 10 | 1:66 | 1:60 000 | 1:1600 | 27 | 1:200 | 1:10 000 | 1:4000 | 44 | 1:50 | 1:10 000 | 1:2000 |
| 11 | 1:200 | 1:60 000 | 1:1600 | 28 | 1:400 | 1:10 000 | 1:4000 | 45 | 1:50 | 1:6600 | 1:1600 |
| 12 | 1:100 | 1:6600 | 1:4000 | 29 | 1:100 | 1:60 000 | 1:2000 | 46 | 1:50 | 1:6600 | 1:1600 |
| 13 | 1:200 | 1:6600 | 1:4000 | 30 | 1:66 | 1:60 000 | 1:1600 | 47 | 1:50 | 1:10 000 | 1:4000 |
| 14 | 1:100 | 1:6600 | 1:4000 | 31 | 1:66 | 1:10 000 | 1:4000 | 48 | 1:400 | 1:500 | 1:2640 |
| 15 | 1:200 | 1:500 | 1:4000 | 32 | 1:66 | 1:6600 | 1:1600 | 49 | 1:100 | 1:10 000 | 1:4000 |
| 16 | 1:200 | 1:6600 | 1:2640 | 33 | 1:66 | 1:10 000 | 1:1600 | 50 | 1:50 | 1:60 000 | 1:4000 |
| 17 | 1:200 | 1:10 000 | 1:2640 | 34 | 1:66 | 1:6600 | 1:1600 | ||||
Numbers 1–25, burn infection Pseudomonas aeruginosa; numbers 26–50, urinary tract infection P. aeruginosa.
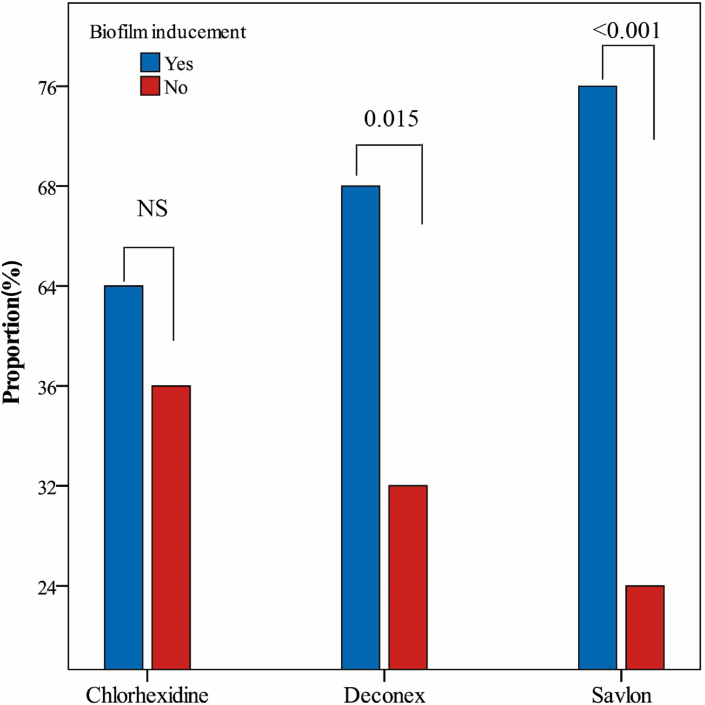
In within-group comparisons, based on the results shown in Table 3, proportions (95% CI) of biofilm inducement in chlorhexidine, deconex and Savlon were 64% (49.2%–77.1%), 68% (53.3%–80.5%) and 76% (61.8%–86.9%), respectively. The binomial test showed that proportion of biofilm inducement in chlorhexidine was not significant, but in the deconex and Savlon groups, the proportion of biofilm inducement was significant (p 0.015 and p < 0.001, respectively) (Fig. 1). Between-group comparisons, as shown in Table 3, showed that the proportions of biofilm inducement in the deconex and Savlon groups were 6% and 19% more than chlorhexidine, but these differences were not significant (p 0.673 and p 0.190, respectively).
Table 3.
Proportion of biofilm inducement in chlorhexidine, deconex and Savlon group
| Biocides | Biofilm inducement | Proportion% (95% CI) | p-value | Proportion ratio (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Chlorhexidine (n = 50) | Yes | 32 | 64% (49.2–77.1) | 0.065 | Reference group | — |
| No | 18 | 36% (22.9–50.8) | ||||
| deconex® (n = 50) | Yes | 34 | 68% (53.3–80.5) | 0.015 | 1.06 (0.80–1.40) | 0.673 |
| No | 16 | 32% (19.5–46.7) | ||||
| Savlon (n = 50) | Yes | 38 | 76% (61.8–86.9) | <0.001 | 1.19 (0.92–1.54) | 0.190 |
| No | 12 | 24% (13.1–38.2) | ||||
Fig. 1.
Proportion of biofilm inducement at sub-MICs of Savlon, chlorhexidine and deconex
Discussion
In this study the effects of the commonly used antiseptics Savlon, chlorhexidine and deconex at sub-MICs on biofilm formation were determined using clinical P. aeruginosa isolates.
Several previous studies have demonstrated that biofilm formation can be induced by sub-MICs of antibacterial agents [[20], [21], [22]]. Biofilm formation is induced when bacteria are exposed to a sub-MIC of antimicrobial agents during chemotherapy, by varying gradients of antimicrobial agents over the course of the dosing regimen, or by bacterial location depth within the biofilm structure, which causes diffusion gradients [23]. We assessed whether the sub-MIC concentrations of antiseptic agents were able to induce biofilm formation. According to MIC data, we found that deconex was the best antiseptic agent against P. aeruginosa isolates (low concentration). Ogunniyi et al. [24] reported that MIC values of Savlon were in the high dilution range (low concentration) of 1:400 and 1:350, whereas Enterobacter aerogenes needed a low dilution (1:50) for total growth inhibition.
Although deconex was the best antiseptic agent to remove pathogens from surfaces, it has been proved that this antiseptic agent is toxic and unstable [25]. These three antiseptic agents could induce biofilm formation at sub-MICs, and Savlon had more ability in biofilm induction. Increasing antibiotic resistance is one of the important problems related to biofilm formation [26]. Aka and Haji reported that the antibiotic concentration needed for eradicating the biofilm-forming bacteria is about 10 000 times greater than planktonic cells, and its formation can be induced by some factors or environmental conditions [22]. They investigated the effects of sub-MIC concentrations of antibiotics on the P. aeruginosa biofilm in the presence of chlorhexidine.
They also showed that P. aeruginosa isolates that are incubated in sub-inhibitory concentrations of chlorhexidine could induce stronger biofilms in the presence of sub-MICs of antibiotics. The outer membrane of P. aeruginosa is responsible for this resistance to chlorhexidine and many other antiseptics.
Lefebvre et al. [27] conducted a multistep strategy to generate a combined antibiofilm treatment (various commercial antiseptics, enzymes and EDTA) that could efficiently decrease the biomass of dense biofilms (≥6 × 107 CFU/cm2) in P. aeruginosa and Staphylococcus aureus. The combination of antiseptics, EDTA and proteases, all at low concentrations, has shown a synergistic effect leading to total eradication of dense biofilms in both P. aeruginosa and S. aureus.
Their findings showed that bacterial biofilm was enhanced by chlorhexidine culture compared with chlorhexidine-free culture [22]. Moreover, a sub-MIC concentration of antimicrobial agents has a strong effect on mutation rates and horizontal antimicrobial resistance genes transfer [28].
The current study could be useful to optimize antiseptic concentrations. It is not necessary to use a higher than MIC concentration of antimicrobial agents for eradicating the bacterial pathogens. Ebrahimi et al. showed that benzalkonium chloride at concentrations higher than the MIC have no further effects on growth and biofilm formation of planktonic cells [29]. Currently, there is limited evidence about biofilm formation and its mechanism at sub-MICs of antimicrobial agents. A global response to cell stress (by directly or indirectly inducing the SOS response) seems to play an important role. However, the concentration of antimicrobial agent and the mechanisms involved are different for each bacterial species [23,30].
Our results displayed that they might be helpful to elaborate the efficient strategies in elimination of biofilms in P. aeruginosa and favour the healing process. However, the results here may have a potential clinical impact in the area of wound healing to eliminate the biofilm formation, such as local disinfection in combination with antibiotics seems to be essential in the case of urinary or skin infections. Finally, it would be of interest to perform these tests routinely in case of persistence of these strains in case of relapse or of therapeutic failure.
Conclusions
The clinical isolates of P. aeruginosa in sub-MICs of chlorhexidine, Savlon and deconex exhibited induction of biofilm. Furthermore, deconex had a powerful inhibitory effect against P. aeruginosa isolates. However, there is little evidence about a mechanism at sub-MICs of antiseptics and the concentration of antiseptics; furthermore, the involved mechanisms differ for each bacterial species.
Conflict of interest
The authors declare that they have no competing interest.
Authors' contributions
SH and AM contributed to the conception and design of the work. ZM and NS contributed to the design of the work, and to final approval of the version to be published. EK contributed by drafting the work and revising it critically for important intellectual content. IP contributed in revising the article and final approval of the version to be published.
Funding
The work was financially supported by Ilam University of Medical Sciences for which we are very grateful.
Ethical approval
This project was approved by the Ilam University of Medical Sciences human ethics committee.
Acknowledgements
None.
References
- 1.Houari A., Di Martino P. Effect of chlorhexidine and benzalkonium chloride on bacterial biofilm formation. Lett Appl Microbiol. 2007;45:652–656. doi: 10.1111/j.1472-765X.2007.02249.x. [DOI] [PubMed] [Google Scholar]
- 2.Neidig A., Yeung A.T., Rosay T., Tettmann B., Strempel N., Rueger M. TypA is involved in virulence, antimicrobial resistance and biofilm formation in Pseudomonas aeruginosa. BMC Microbiol. 2013;13:77. doi: 10.1186/1471-2180-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falkinham J.O., III, Hilborn E.D., Arduino M.J., Pruden A., Edwards M.A. Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ Health Persp (Online) 2015;123:749. doi: 10.1289/ehp.1408692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen B.S., Christensen N., Sorensen J., Rosenvinge F.S., Kolmos H.J., Skov M.N. Outbreak of Pseudomonas aeruginosa bacteraemia in a haematology department. Danish Med J. 2015;62:A5040. [PubMed] [Google Scholar]
- 5.Pachori P., Gothalwal R., Gandhi P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019;6:109. doi: 10.1016/j.gendis.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redelman C.V., Chakravarty S., Anderson G.G. Antibiotic treatment of Pseudomonas aeruginosa biofilms stimulates expression of the magnesium transporter gene mgtE. Microbiology. 2014;160:165–178. doi: 10.1099/mic.0.070144-0. [DOI] [PubMed] [Google Scholar]
- 7.Alhusseini L.B., Maleki A., Kouhsari E., Ghafourian S., Mahmoudi M., Al Marjani M.F. Evaluation of type II toxin-antitoxin systems, antibiotic resistance, and biofilm production in clinical MDR Pseudomonas aeruginosa isolates in Iraq. Genes Rep. 2019 Dec 1;17:100546. [Google Scholar]
- 8.Tyerman J.G., Ponciano J.M., Joyce P., Forney L.J., Harmon L.J. The evolution of antibiotic susceptibility and resistance during the formation of Escherichia coli biofilms in the absence of antibiotics. BMC Evol Biol. 2013;13:1. doi: 10.1186/1471-2148-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaïri A., Ferrières L., Latour-Lambert P., Beloin C., Tangy F., Ghigo J.-M. In vitro activities of dermaseptins K4S4 and K4K20S4 against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa planktonic growth and biofilm formation. Antimicrob Agents Chemother. 2014;58:2221–2228. doi: 10.1128/AAC.02142-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geyik A.G., Kılıç B., Çeçen F. Extracellular polymeric substances (EPS) and surface properties of activated sludges: effect of organic carbon sources. Environ Sci Poll Res. 2016;23:1653–1663. doi: 10.1007/s11356-015-5347-0. [DOI] [PubMed] [Google Scholar]
- 11.Klausen M., Heydorn A., Ragas P., Lambertsen L., Aaes-Jørgensen A., Molin S. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol. 2003;48:1511–1524. doi: 10.1046/j.1365-2958.2003.03525.x. [DOI] [PubMed] [Google Scholar]
- 12.Veerachamy S., Yarlagadda T., Manivasagam G., Yarlagadda P.K. Bacterial adherence and biofilm formation on medical implants: a review. Proc Inst Mech Eng H: J Eng Med. 2014;228:1083–1099. doi: 10.1177/0954411914556137. [DOI] [PubMed] [Google Scholar]
- 13.Mirani Z.A., Jamil N. Effect of sub-lethal doses of vancomycin and oxacillin on biofilm formation by vancomycin intermediate resistant Staphylococcus aureus. J Basic Microbiol. 2011;51:191–195. doi: 10.1002/jobm.201000221. [DOI] [PubMed] [Google Scholar]
- 14.Aparna M.S., Yadav S. Biofilms: microbes and disease. Braz J Infect Dis. 2008;12:526–530. doi: 10.1590/s1413-86702008000600016. [DOI] [PubMed] [Google Scholar]
- 15.Ningthoujam D.S., Shovarani N. Isolation and characterization of a Pseudomonas aeruginosa strain DN1 degrading p-nitrophenol. Res J Microbiol. 2008;3:345–351. [Google Scholar]
- 16.Hengzhuang W., Ciofu O., Yang L., Wu H., Song Z., Oliver A. High β-lactamase levels change the pharmacodynamics of β-lactam antibiotics in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2013;57:196–204. doi: 10.1128/AAC.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badmasti F., Siadat S.D., Bouzari S., Ajdary S., Shahcheraghi F. Molecular detection of genes related to biofilm formation in multidrug-resistant Acinetobacter baumannii isolated from clinical settings. J Med Microbiol. 2015;64:559–564. doi: 10.1099/jmm.0.000058. [DOI] [PubMed] [Google Scholar]
- 18.Perez L.R.R., Costa M., Freitas AL, Barth A.L.J. Evaluation of biofilm production by Pseudomonas aeruginosa isolates recovered from cystic fibrosis and non-cystic fibrosis patients. Braz J Microbiol. 2011 Jun;42:476–479. doi: 10.1590/S1517-838220110002000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saeed Hemati N.S., Ghafurian S., Maleki F., Mahdavi Z., Hassan A., Valadbeigi H. The association of biofilm formation and sub-minimal inhibitory concentrations of antimicrobial agents. J Bas Res Med Sci. 2016;3:26–30. [Google Scholar]
- 20.Tsai S.-H., Lai H.-C., Hu S.-T. Subinhibitory doses of aminoglycoside antibiotics induce changes in the phenotype of Mycobacterium abscessus. Antimicrob Agents Chemother. 2015;59:6161–6169. doi: 10.1128/AAC.01132-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan J.B., Izano E.A., Gopal P., Karwacki M.T., Kim S., Bose J.L. Low levels of β-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. MBio. 2012;3(4) doi: 10.1128/mBio.00198-12. e00198–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aka S.T., Haji S.H. Sub-MIC of antibiotics induced biofilm formation of Pseudomonas aeruginosa in the presence of chlorhexidine. Braz J Microbiol. 2015;46:149–154. doi: 10.1590/S1517-838246120140218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan J.B. Antibiotic-induced biofilm formation. Int J Artif Organs. 2011;34:737–751. doi: 10.5301/ijao.5000027. [DOI] [PubMed] [Google Scholar]
- 24.Ogunniyi T.A., Oni P.O., Juba A., Asaolu S.O., Kolawole D.O. Disinfectants/antiseptics in the management of Guinea worm ulcers in the rural areas. Acta Trop. 2000;74:33–38. doi: 10.1016/s0001-706x(99)00057-1. [DOI] [PubMed] [Google Scholar]
- 25.Ghaneian M.T., Ehrampoush M.H., Jebali A., Hekmatimoghaddam S., Mahmoudi M. Antimicrobial activity, toxicity and stability of phytol as a novel surface disinfectant. Environ Health Eng Manage J. 2015;2:13–16. [Google Scholar]
- 26.Greene C., Vadlamudi G., Newton D., Foxman B., Xi C. The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am J Infect Contr. 2016;44:e65–e71. doi: 10.1016/j.ajic.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefebvre E., Vighetto C., Di Martino P., Garde V.L., Seyer D. Synergistic antibiofilm efficacy of various commercial antiseptics, enzymes and EDTA: a study of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Int J Antimicrob Agents. 2016;48:181–188. doi: 10.1016/j.ijantimicag.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Laureti L., Matic I., Gutierrez A. Bacterial responses and genome instability induced by subinhibitory concentrations of antibiotics. Antibiotics. 2013;2:100–114. doi: 10.3390/antibiotics2010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebrahimi A., Hemati M., Shabanpour Z., Dehkordi S.H., Bahadoran S., Lotfalian S. Effects of benzalkonium chloride on planktonic growth and biofilm formation by animal bacterial pathogens. Jundishapur J Microbiol. 2015;8(2) doi: 10.5812/jjm.16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jolivet-Gougeon A., Bonnaure-Mallet M. Biofilms as a mechanism of bacterial resistance. Drug Discov Today:Technol. 2014;11:49–56. doi: 10.1016/j.ddtec.2014.02.003. [DOI] [PubMed] [Google Scholar]