Abstract
This study is a subset analysis of a retrospective multicenter study performed in Japan and its purpose was to investigate the effectiveness of stereotactic body radiotherapy (SBRT) for pulmonary oligometastases from colorectal cancer. Local control (LC), freedom from further metastases, relapse-free survival and overall survival (OS) after SBRT were retrospectively analyzed. The Kaplan–Meier method was used to estimate lifetime data and the log-rank test was performed as univariate analyses. The Cox proportional hazards model was applied in multivariate analyses. Data for 330 patients with 371 tumors were used for analyses. The median follow-up period was 25.0 months. The 3-year LC, freedom from further metastases, relapse-free survival and OS rates were 64.9, 34.9, 24.9 and 63.4%, respectively. The results of multivariate analyses showed that a higher LC rate was associated with no history of local therapy for oligometastases (P = 0.01), SBRT without concurrent chemotherapy (P < 0.01), type B calculation algorithm (P < 0.01) and higher biological effective radiation doses (≥115 Gy, P = 0.04). A longer OS was associated with no history of local therapy for oligometastases (P = 0.04), a more recent period of SBRT (2010–15, P = 0.02), tumor located in the upper or middle lobe (P < 0.01) and higher biological effective radiation doses (≥115 Gy, P = 0.01). In conclusion, OS after SBRT was good, but LC rate was relatively low. The use of high biological effective radiation doses can improve both LC and OS outcomes.
Keywords: stereotactic body radiotherapy (SBRT), pulmonary oligometastasis, colorectal cancer, colorectal metastasis
INTRODUCTION
Metastasectomy for lung metastases from colorectal cancer is a part of the standard treatment for colon cancer and rectal cancer [1, 2]. For patients who are amenable to surgical resection, stereotactic body radiotherapy (SBRT) is regarded as one of the alternative treatments. However, due to insufficient evidence regarding the efficacy and safety of SBRT, it has not become an upfront local therapy for colorectal metastases. According to the National Comprehensive Cancer Network guidelines, radiotherapy should not be used in the place of surgical resection. It has been shown that the local control (LC) rates for pulmonary oligometastases from colorectal cancer (CRC) are lower than LC rates for pulmonary oligometastases from other cancers or LC rates for early-stage non-small cell lung cancer [3, 4]. This radioresistance feature of pulumonary CRC metastases would be the same for liver metastases from CRC [5]. The results of the meta-analysis also suggested that higher LC rates would be obtained by dose escalation of SBRT, and it was shown in another study that an excellent LC rate was achieved with a maximum dose of 83–100 Gy in five fractions [6]. Another feature of CRC was that some patients with CRC showed better overall survival (OS) than patients with other malignancies, although LC rates of SBRT for CRC oligometastases were relatively low, therefore, the role of LC in survival was controversial [5, 7]. These interesting features of oligometastases from CRC motivated us to perform subset analyses using data from a nationwide multicenter retrospective study of SBRT for pulmonary oligometastases that was performed in Japan [8]. The aim of the study was to determine factors affecting LC, development of further metastases and survival after SBRT for pulmonary oligometastases from CRC.
MATERIALS AND METHODS
Eligibility criteria, data collection and definitions
This study was a subset analysis of a retrospective multicenter study performed in Japan. Methods and inclusion criteria for the whole study were as described elsewhere [8]. The whole study was conducted in 68 institutions in Japan. Rewritable compact disks (CD-RWs) containing research items (excel format) were sent from the secretariat office to each institution. Radiation oncologists at each institution filled in the research items fundamentally based on medical records, image interpretation reports, the radiological information system and treatment planning system. After confirming that there was no private information in the CD-RW, they returned the CD-RW to the secretariat office by using ‘the Letter Pack’ which provided a tracking service. The main inclusion criteria were that the number of pulmonary metastases ≤5, the primary lesion and extrathoracic metastases needed to be treated before the start of SBRT, SBRT was performed between 2004 and 2015, and a biological effective dose (BED10) ≥75 Gy or more was needed for SBRT. BED10 was calculated using the following formula: BED = n xd [1 + d/(α/β)], where n is the number of fractions, d is dose per fraction and the α/β ratio is applied for 10 Gy for the tumors. Of a total of 1378 patients in the database, there were 345 patients with pulmonary oligometastases of colorectal origin. Two cases of non-adenocarcinoma pathology (squamous cell carcinoma and endocrine cell carcinoma) were excluded from analysis. Data for 330 patients with 371 tumors from 51 institutes were analyzed in this study (Fig. 1).
Fig. 1.
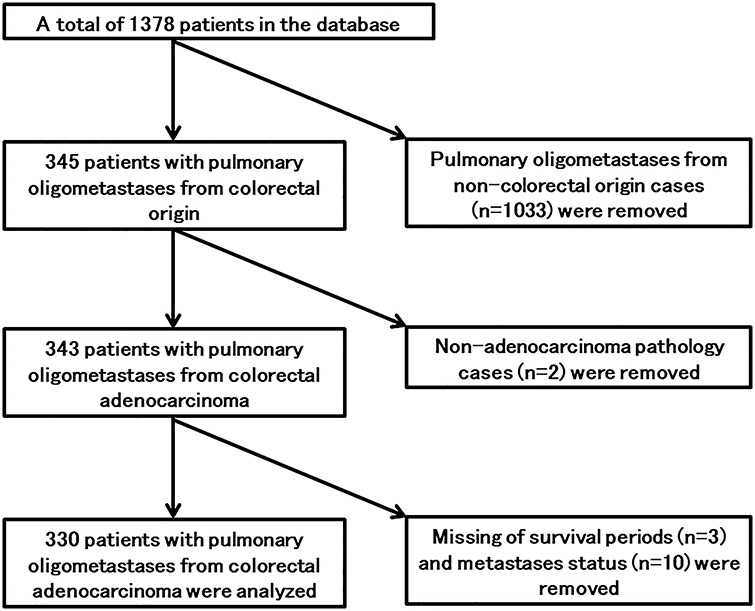
Flow chart of patients selected from the database of a retrospective multicenter study.
Patients’ characteristics are shown in Table 1, and oligometastatic tumor and SBRT characteristics are summarized in Table 2. The number of oligometastases was counted at the time of emergence of the pulmonary oligometastases targeted by SBRT. Prior local therapy or combination with another local therapy such as surgical resection was allowed. Disease-free interval (DFI) was defined as the interval between the date when the primary site was controlled and the date when the first metastasis was confirmed. Oligo-recurrences, sync-oligometastases and unclassified oligometastases were defined as DFIs of ≥6, 0 and 0–6 months, respectively. Located lobe was defined as the lung lobe in which irradiated tumor was located. When SBRT was performed metachronously, located lobe was judged based on initially irradiated tumor location. When SBRT was performed to multiple sites synchronously, at least one oligometastatic tumor located in the upper or middle lung lobe was classified into upper of middle lobe involvement. The type B dose calculation algorithm included superposition, an equivalent algorithm or a newer generation algorithm such as the Monte Carlo algorithm and type A was an older generation algorithm such as Pencil Beam Convolution.
Table 1.
Patients’ characteristics
| Patients | Total number | 330 |
|---|---|---|
| Institute | Academic | 143 (43.3%) |
| Non-academic | 187 (56.6%) | |
| Age, years | Median, range | 73, 29–93 |
| Sex | Male | 202 (61.2%) |
| Female | 128 (38.7%) | |
| ECOG performance statusa | 0 | 193 (58.4%) |
| 1 | 112 (33.9%) | |
| 2–3 | 18 (5.4%) | |
| Missing | 7 (2.1%) | |
| Primary origin | Colon | 171 (51.8%) |
| Rectum | 157 (47.5%) | |
| Missing | 2 (0.6%) | |
| DFI, months | Median, range | 17.2, 0–133.9 |
| Oligometastatic state | Oligo-recurrence | 243 (73.6%) |
| Sync-oligometastases | 35 (10.6%) | |
| Unclassified oligometastases | 27 (8.1%) | |
| Missing | 25 (7.5%) | |
| History of local therapy for metastases | Yes | 122 (36.9%) |
| No | 131 (39.6%) | |
| Missing | 77 (23.3%) | |
| Chemotherapy before SBRT | Yes | 148 (44.8%) |
| No | 179 (54.2%) | |
| Missing | 3 (0.9%) | |
| Chemotherapy concurrent with SBRT | Yes | 8 (2.4%) |
| No | 322 (97.5%) | |
| Chemotherapy after SBRT | Yes | 196 (59.3%) |
| No | 55 (16.6%) | |
| Missing | 79 (23.9%) | |
| Number of metastases | 1 | 230 (69.6%) |
| 2–5 | 97 (29.3%) | |
| Missing | 3 (0.9%) |
aECOG, Eastern Cooperative Oncology Group.
Table 2.
Characteristics of pulmonary oligometastatic tumors and SBRT
| Irradiated oligometastatic tumors | Total number | 371 |
|---|---|---|
| SBRT date | 2004–09 | 143 (38.5%) |
| 2010–15 | 228 (61.4%) | |
| Maximum tumor diameter, cm | Median, range | 1.5, 0.5–5.3 |
| Located lobe | Upper or middle lobe | 143 (38.5%) |
| Lower lobe | 143 (38.5%) | |
| Missing | 85 (22.9%) | |
| Field coplanarity | Coplanar field | 102 (27.4%) |
| Non-coplanar field | 269 (72.5%) | |
| Beam | Static | 260 (70.0%) |
| Arc | 111 (29.9%) | |
| Dose prescription | Isocenter | 256 (69.0%) |
| D95 of PTVa | 79 (21.2%) | |
| Others | 36 (9.7) | |
| Calculation algorithm | A | 143 (38.5%) |
| Bb,* | 225 (60.6%) | |
| Missing | 3 (0.8%) | |
| Energy | 6 MV only | 300 (80.8%) |
| Others | 71 (19.1%) | |
| BED10 dose of IC, Gy | Median, range | 115.3, 75.0–289.5 |
| OTTc of SBRT, days | Median, range | 7, 3–61 |
aDose covering 95% of the planning target volume.
bType B calculation algorithm included superposition, equivalent algorithm or newer generation algorithm.
cOverall treatment time.
LC was defined as freedom from local failure, and local failure was defined as enlargement of the irradiated tumor. Freedom from further metastases (FFFM) was defined as the time until emergence of any recurrence or metastasis excluding local failure. Relapse-free survival (RFS) was defined as freedom from any recurrences, any metastases, local failures or death. Toxicity that was judged to be caused by SBRT was reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 translated by the Japan Clinical Oncology Group (CTCAE-JCOG).
Ethics
The previous study was a retrospective multicenter study in Japan. The previous study and this study were approved by the ethical committee of a senior facility (Ethics Committee of Toho University Omori Medical Center, reference number: 27–148) and informed consent was waived due to the study design. All participating institutions were guaranteed the chance to opt out of participation in this study by being given the information about the study via the Internet or posters, and opt-out consent was obtained for all patients.
Data analysis
Follow-up periods and time-to-event outcomes were calculated from the first day of SBRT to the day that an event was confirmed. Cumulative LC rate, FFFM rate, RFS rate and OS rate were calculated from the Kaplan–Meier estimator, and then time-to-event outcomes were summarized using the Kaplan–Meier estimator with a log-rank test to compare stratified outcomes. Continuous covariates were divided at the median value to create stratification factors. Regarding BED10 for LC, other cut-off BED10 values of 106 and 150 Gy were used to create three groups: classic standard SBRT dose in Japan (48 Gy in 4 fractions) or less group (<106 Gy), higher than standard dose but less than ablative dose group (106–150 Gy) and ablative dose group (>150 Gy) [5]. The Kaplan–Meier curves were also described according to this group separation. In multivariate analyses (MVA), the Cox proportional hazards model was applied for factors with a log-rank P-value < 0.20 by using a stepwise backward elimination/forward addition approach with the Akaike information criterion (AIC) to construct the best MVA model. A P-value < 0.05 was defined as significant. Statistical analyses were performed using EZR version 1.37 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a modified version of R commander (R Foundation for Statistical Computing, Vienna, Austria) [9].
RESULTS
The median follow-up period for all patients was 25.0 months (range, 0.1–121.3 months). During follow-up, there were 99 local failures and 111 deaths, including death from primary cancer in 83 patients, death from unknown cause in 6 patients, death from toxicity in 2 patients and death from other causes in 20 patients. Of the 330 patients, 187 had failure of FFFM and 236 had failure of RFS. The 1- and 3-year LC rates, FFFM rates, RFS rates and OS rates were 86.1 and 64.9% [95% confidence interval (CI): 81.9–89.5 and 58.6–70.6%], 60.4 and 34.9% (95% CI: 54.7–65.7 and 28.8–41.1%), 52.6 and 24.9% (95% CI: 46.9–58.1 and 19.7–30.5%) and 94.4 and 63.4% (95% CI: 91.1–96.5 and 56.3–69.7%), respectively (Fig. 2). The median FFFM, RFS and OS periods were 19.0 months (95% CI: 15.1–24.5%), 12.6 months (95% CI: 11.2–16.0%) and 51.5 months (95% CI: 40.9–65.6%), respectively. The median time from SBRT to local failure was 12.6 months and it ranged from 4.4 months to 63.3 months. Toxicity was reported in 245 patients, and lung toxicities ≥grade 2 and ≥grade 3 occurred in 33 and 5 patients, respectively. There were two patients with grade 5 toxicity including one patient with pneumonitis and one patient with hemoptysis.
Fig. 2.
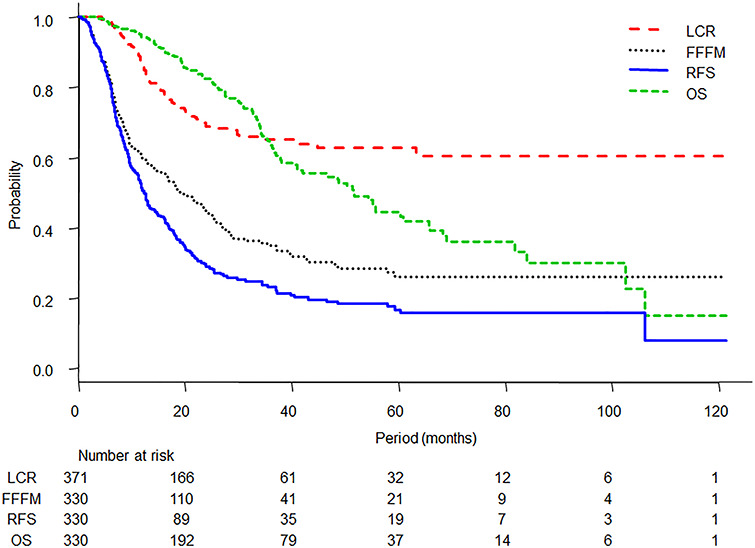
Kaplan–Meier curves of LC, FFFM, RFS and OS.
The results of log-rank tests for the variables are shown in Table 3 and the Kaplan–Meier LC curves stratified by BED10 of 106 Gy and 150 Gy are shown in Figure 3. By using potential factors that emerged after log-rank tests, MVA for LC, FFFM, RFS and OS revealed several related factors (Fig. 4). A history of local therapy for prior oligometastases [yes vs no, hazard ratio (HR): 1.91, 95% CI: 1.11–3.26, P = 0.01], chemotherapy concurrent with SBRT (yes vs no, HR: 4.81, 95% CI: 1.58–14.5, P < 0.01), dose calculation algorithm of SBRT (type B vs type A, HR: 0.42, 95% CI: 0.25–0.73, P < 0.01) and BED10 (≥115 Gy vs <115 Gy, HR: 0.55, 95% CI: 0.30–0.98, P = 0.04) showed significant associations with LC. MVA for FFFM showed that a history of local therapy for prior oligometastases (yes vs no, HR: 1.79, 95% CI: 1.21–2.64, P < 0.01), chemotherapy concurrent with SBRT (yes vs no, HR: 2.77, 95% CI: 1.07–7.13, P = 0.03), maximum oligometastatic tumor diameter (≥1.5 cm vs < 1.5 cm, HR: 1.52, 95% CI: 1.04–2.23, P = 0.03) and initially irradiated oligometastatic tumor-located lobe (upper or middle lobe involvement vs lower lobe, HR: 0.66, 95% CI: 0.44–0.98, P = 0.04) were significantly associated with FFFM. A history of local therapy for prior oligometastases (yes vs no, HR: 1.81, 95% CI: 1.28–2.55, P < 0.01), chemotherapy concurrent with SBRT (yes vs no, HR: 2.90, 95% CI: 1.23–6.81, P = 0.01), maximum oligometastatic tumor diameter (≥1.5 cm vs <1.5 cm, HR: 1.45, 95% CI: 1.03–2.06, P = 0.03), initially irradiated oligometastatic tumor-located lobe (upper or middle lobe involvement vs lower lobe, HR: 0.68, 95% CI: 0.48–0.95, P = 0.02), dose calculation algorithm of SBRT (type B vs type A, HR: 0.65, 95% CI: 0.43–0.96, P = 0.03) and BED10 (≥115 Gy vs <115 Gy, HR: 0.67, 95% CI: 0.47–0.97, P = 0.03) showed significant associations with RFS. In MVA for OS, a history of local therapy for prior oligometastases (yes vs no, HR: 1.70, 95% CI: 1.02–2.85, P = 0.04), SBRT treatment date (2010–15 vs 2004–09, HR: 0.54, 95% CI: 0.32–0.92, P = 0.02), initially irradiated oligometastatic tumor-located lobe (upper or middle lobe involvement vs lower lobe, HR: 0.43, 95% CI: 0.25–0.73, P < 0.01) and BED10 (≥115 Gy vs <115 Gy, HR: 0.48, 95% CI: 0.27–0.86, P = 0.01) emerged as significant prognostic factors.
Table 3.
Comparison of outcomes using the Kaplan–Meier estimator with log-rank tests for stratified outcomes
| LC | FFFM | RFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| Factorsa | 3-Year LC | P | Median time | P | Median time | P | Median time | P |
| Institute | ||||||||
| Academic | 68.3 | 17.9 | 12.7 | 42.0 | ||||
| Non-academic | 62.6 | 0.22 | 21.3 | 0.61 | 12.0 | 0.81 | 55.6 | 0.21 |
| Age, years | ||||||||
| <73 | 61.7 | 19.0 | 11.3 | 51.4 | ||||
| ≥73 | 66.4 | 0.27 | 19.7 | 0.24 | 14.3 | 0.13 | 55.6 | 0.80 |
| Sex | ||||||||
| Male | 64.8 | 21.3 | 12.0 | 55.6 | ||||
| Female | 65.1 | 0.41 | 17.9 | 0.44 | 12.4 | 0.65 | 48.8 | 0.50 |
| ECOG Performance Status | ||||||||
| 0 | 66.5 | 18.0 | 12.9 | 60.3 | ||||
| 1 | 60.9 | 23.0 | 12.0 | 40.1 | ||||
| 2–3 | 65.6 | 0.46 | 21.6 | 0.89 | 10.0 | 0.50 | 33.1 | 0.01 |
| Primary origin | ||||||||
| Colon | 64.0 | 23.5 | 14.2 | 55.2 | ||||
| Rectum | 66.5 | 0.75 | 17.8 | 0.12 | 11.7 | 0.06 | 51.7 | 0.75 |
| DFI | ||||||||
| <17.2 months | 62.3 | 18.2 | 11.7 | 38.0 | ||||
| ≥17.2 months | 67.2 | 0.34 | 25.4 | 0.04 | 14.7 | 0.09 | 60.3 | 0.26 |
| Oligometastatic state | ||||||||
| Oligo-recurrence | 64.4 | 23.2 | 12.9 | 55.6 | ||||
| Sync-oligometastases | 70.9 | 9.4 | 9.4 | 35.9 | ||||
| Unclassified oligometastases | 59.5 | 0.47 | 17.9 | 0.08 | 12.6 | 0.29 | 37.6 | 0.40 |
| History of local therapy for metastases | ||||||||
| Yes | 73.3 | 14.3 | 10.6 | 42.0 | ||||
| No | 63.9 | 0.06 | 27.2 | <0.01 | 17.5 | <0.01 | 65.6 | 0.16 |
| Chemotherapy | ||||||||
| Before SBRT - yes | 69.8 | 12.7 | 11.3 | 55.6 | ||||
| Before SBRT - no | 59.5 | 0.09 | 25.0 | <0.01 | 14.7 | <0.01 | 48.8 | 0.36 |
| Concurrent SBRT - yes | N/A | 6.6 | 6.9 | 34.2 | ||||
| Concurrent SBRT - no | 65.8 | 0.01 | 21.3 | 0.02 | 12.6 | 0.01 | 54.3 | 0.16 |
| After SBRT - yes | 68.8 | 13.1 | 11.7 | 50.3 | ||||
| After SBRT - no | 68.5 | 0.92 | 23.2 | 0.08 | 12.7 | 0.41 | 51.5 | 0.66 |
| Number of metastases | ||||||||
| 1 | 60.7 | 25.0 | 12.9 | 55.8 | ||||
| 2–5 | 73.6 | 0.09 | 13.1 | <0.01 | 11.3 | 0.15 | 35.8 | 0.07 |
| SBRT date | ||||||||
| 2004–09 | 58.8 | 20.4 | 12.6 | 48.6 | ||||
| 2010–15 | 69.3 | 0.03 | 19.0 | 0.83 | 12.4 | 0.64 | 60.8 | 0.11 |
| Maximum tumor diameter | ||||||||
| <1.5 cm | 69.4 | 23.5 | 16.0 | 55.6 | ||||
| ≥1.5 cm | 56.1 | 0.07 | 16.3 | 0.03 | 11.3 | 0.02 | 48.6 | 0.01 |
| Located lobe | ||||||||
| Upper or middle lobe involvement | 70.3 | 25.0 | 16.5 | 60.8 | ||||
| Lower lobe | 67.9 | 0.75 | 14.2 | <0.01 | 11.1 | 0.02 | 36.6 | 0.02 |
| Field coplanarity | ||||||||
| Coplanar field | 60.3 | 23.0 | 13.3 | 54.3 | ||||
| Non-coplanar field | 66.7 | 0.41 | 18.5 | 0.67 | 12.4 | 0.99 | 51.5 | 0.50 |
| Beam | ||||||||
| Static | 54.5 | 21.3 | 12.7 | 55.6 | ||||
| Arc | 69.2 | 0.02 | 16.9 | 0.36 | 10.1 | 0.29 | 42.0 | 0.76 |
| Dose prescription | ||||||||
| Isocenter | 57.4 | 18.8 | 12.0 | 48.8 | ||||
| D95 of PTV | 86.3 | 25.0 | 16.3 | 68.9 | ||||
| Others | 77.7 | <0.01 | 31.3 | 0.96 | 9.2 | 0.28 | N/A | 0.62 |
| Calculation algorithm | ||||||||
| Type A | 54.5 | 18.8 | 11.6 | 51.4 | ||||
| Type B | 72.7 | <0.01 | 20.4 | 0.41 | 13.1 | 0.17 | 51.7 | 0.60 |
| Energy | ||||||||
| 6 MV only | 65.1 | 19.0 | 12.6 | 51.4 | ||||
| Others | 65.1 | 0.62 | 23.5 | 0.54 | 12.0 | 0.89 | 55.6 | 0.70 |
| BED10 dose of IC | ||||||||
| <115 Gy | 56.5 | 17.0 | 11.7 | 41.0 | ||||
| ≥115 Gy | 70.3 | 0.06 | 23.4 | 0.12 | 12.7 | 0.06 | 84.0 | 0.01 |
| OTT of SBRT | ||||||||
| <7 days | 67.1 | 18.5 | 12.7 | 50.3 | ||||
| ≥7 days | 69.2 | 0.54 | 19.7 | 0.45 | 12.4 | 0.47 | 51.5 | 0.74 |
aECOG = Eastern Cooperative Oncology Group, N/A = not applicable, D95 of PTV = dose covering 95% of the planning target volume, IC = isocenter, OTT = overall treatment time.
Fig. 3.
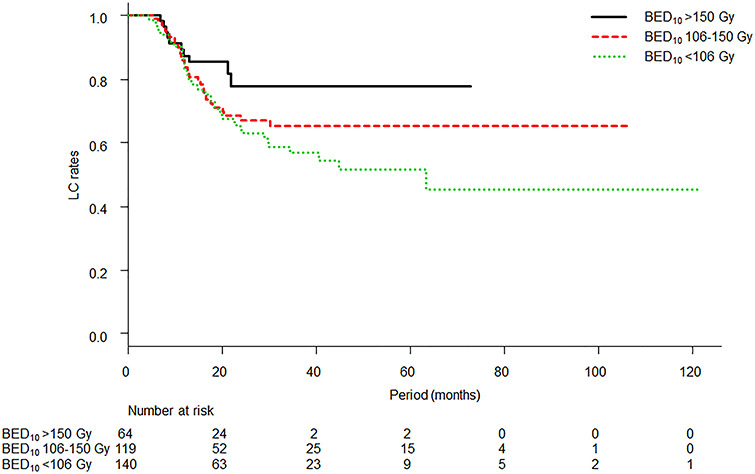
Kaplan–Meier curves of LC according to group separation: classic standard SBRT dose or less group (BED10 < 106 Gy), higher than standard dose but less than ablative dose group (BED10 106–150 Gy) and ablative dose group (BED10 > 150 Gy). The 3-year LC rates of each group were 57.0% (95% CI: 46.4–66.3%), 65.3% (95% CI: 54.1–74.4%) and 77.7% (95% CI: 61.0–88.0%), respectively. A log-rank test for the three curves was not significant (P = 0.13).
Fig. 4.
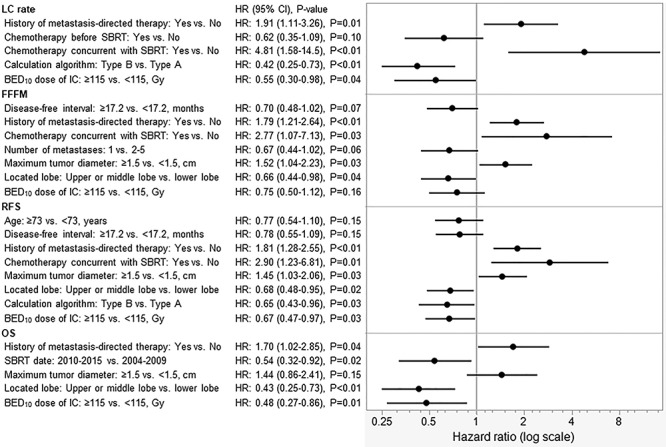
Results of multivariate Cox regression analyses for LC, FFFM, RFS and OS.
DISCUSSION
The analyses performed in this study are one of the largest-scale analyses of pulmonary oligometastases from CRC after SBRT. Although this study was a retrospective multicenter study, the results indicated possible factors related to outcomes including both pre-SBRT characteristics and treatment factors. Treatment factors would be particularly valuable because these factors such as BED10 dose and calculation algorithm can be changed from now on or in the future. The type B dose calculation algorithm of SBRT (equivalent to superposition or newer generation) and higher BED10 (≥115 Gy at the isocenter) contributed not only to a higher LC rate but also higher RFS and OS rates. The radiation dose–response relationship of pulmonary oligometastases from CRC was confirmed in the present study [3]. On the other hand, it was shown in the present study that chemotherapy concurrent with SBRT had no beneficial effect on LC or lower rate of LC.
The effect of additional chemotherapy on peri-SBRT was unclear. Chemotherapy concurrent with SBRT showed higher HRs in LC, FFFM and RFS. Although chemotherapy concurrent with SBRT was performed in only 8 patients with 11 tumors, it was shown that concurrent chemotherapy had no benefit for LC and was possibly a disadvantageous factor. On the other hand, chemotherapy prior to SBRT showed a marginally significant lower HR for LC, though the patients who received chemotherapy prior to SBRT included 8 patients with 10 tumors who also received chemotherapy concurrent with SBRT. There were opposite results that chemotherapy prior to SBRT showed higher HR for LC and systemic therapy prior to SBRT showed higher HR for OS [10, 11]. Another factors might affect these controversial results and one of possible factors, is time from diagnosis of metastases to SBRT, but this factor was not investigated in this study [11]. Chemotherapy after SBRT showed no significant relationship with outcomes. There was a report showing that chemotherapy after SBRT improved LC [12]. In multiple pulmonary oligometastases, adjuvant chemotherapy after metastasectomy might have a benefit for survival [13]. However, other reports showed that there was no association between SBRT or metastasectomy and chemotherapy [14, 15]. The role of additional chemotherapy is therefore controversial.
Interestingly, the initially irradiated oligometastatic tumor located in the lung lobe showed significant associations with FFFM, RFS and OS. A tumor located in the upper or middle lung lobe was a favorable factor. It is well known that hematogenous spread of metastases is predominant in the lower lung because of the blood distribution heterogeneity in the lung [16]. In contrast, upper lung oligometastases from CRC, which are relatively rare, might tend to be true oligometastases because there is no evidence of metastases in the more sensitive lung lobe. This advantage of location in the upper lung was found even for OS, indicating the possibility of good outcomes for patients with left upper lobe oligometastases. Metastasectomy analyses performed in a previous study showed that patients who received wedge resection had shorter survival than patients who received lobectomy [17]. However, it has been shown that lobectomy of the left upper lobe was a risk factor for cerebral infarction because of thrombosis in the pulmonary vein stump, which has been reported to occur in 13.5% of patients after left upper lobectomy [18, 19]. SBRT for CRC oligometastases located in the left upper lung lobe is possible in the place of metastasectomy.
A history of local therapy for prior oligometastases, also known as metastasis-directed therapy, showed significant associations with LC, FFFM, RFS and OS, while the SBRT treatment period was significantly associated only with OS. Although prior oligometastatic sites were not investigated in this study, a history of liver metastases has been reported to be a negative prognostic factor for survival after surgery [17]. Potential micrometastases might gradually acquire resistance to radiotherapy and chemotherapy through local therapy with or without chemotherapy for tangible oligometastases. As for the SBRT treatment periods, improvement in OS over time seemed to be mainly due to developments in systemic therapy and partly due to advances in SBRT and progress in surgery.
The LC rate of this study was relatively low, as expected from previous findings [3, 4]. Higher BED10 tended to achieve higher LC but was not significant because of the effects of other factors such as the dose calculation algorithm (Fig. 3). In MVA, higher BED10 (≥115 Gy at the isocenter) was significantly related not only to a higher LC rate but also better RFS and OS. Sharma et al. have recently reported that a BED10 ≥100 Gy contributed significant improvements to both LC and OS [10]. The current study shows that a higher cut-off value of SBRT dose (the median dose of this study) contributes the same improvements and this result dispels concerns over dose escalation because toxicity will increase as the SBRT dose increases. SBRT has mostly been performed for patients who were unfit for surgery. It is important in such cases to achieve a higher LC rate because metastasectomy as a salvage treatment after local failure would be difficult. Although there were very limited data because of the lack of questions about salvage therapy in this survey, salvage metastasectomy was performed for at least 4 tumors out of 29 in ≥3-year survivors with local failure according to comments from collaborators. LC might be beneficial especially in long survivors. Therefore, dose escalation to achieve higher LC is justified around the range of BED10 in this survey (range 75.0–289.5 Gy and interquartile range 105.6–134.4 Gy at the isocenter). If a sufficient SBRT dose is delivered to the tumor, the edge dose, such as the dose covering 95% of planning target volume, might have significance. The results of survival inferiority of wedge resection (vs lobectomy) or positive surgical margins from an individual data meta-analysis for lung metastasectomy suggested that radiation dose at the edge of the target volume might be important [17]. In fact, it was indicated that marginal tumor doses are important as in the case of SBRT for early-stage non-small cell lung cancer [20].
There are some limitations in the present study. Some possible relevant factors such as carcinoembryonic antigen levels, comorbidities in the patients, time from diagnosis of metastases to SBRT and tumor-located lobe which was treated by another local therapy were not investigated in this survey. This study was a retrospective multicenter study and there were therefore missing data for very short-term follow-up and various treatment protocols at the institutions. Some confounding and bias would not have been controlled because of the retrospective nature of this study.
In conclusion, although the LC rate after SBRT is relatively low, the use of high BED10 (≥115 Gy) and the use of a type B or newer generation dose calculation algorithm for SBRT can improve LC. Patients who received high BED10 also showed prolonged RFS and OS. The role of peri-SBRT chemotherapy remains unclear, but, from the current results, chemotherapy concurrent with SBRT should be avoided. Oligometastases located in the upper or middle lung lobe showed higher FFFM, RFS and OS, therefore, they are good candidates for local therapy and possibly better candidates for SBRT than left upper lobectomy if oligometastases are located in the left upper lung lobe.
ACKNOWLEDGEMENTS
We acknowledge the collaboration of many radiation oncologists in Japan. We thank Drs Hideya Yamazaki, Hidekazu Tanaka, Hideomi Yamashita, Hisao Kakuhara, Ichiro Ogino, Kazunari Yamada, Katsuyuki Shirai, Kenji Nagata, Kosuke Amano, Koutaro Terashima, Kuniaki Katsui, Masayoshi Yamada, Michiko Imai, Mitsuru Kobayashi, Nanae Yamaguchi, Nobuhiko Yoshikawa, Nobuhisa Mabuchi, Osamu Suzuki, Saeko Hirota, Satoshi Itasaka, Seiji Kubota, Shigetoshi Shimamoto, Shinichi Ogawa, Shizuko Ohashi, Takanori Fukuda, Takashi Sakamoto, Takashi Kawanaka, Takuya Yamazaki, Tetsuo Nonaka, Tetsuya Inoue, Toshiki Kawamura, Toshinori Soejima, Toru Sakayauchi, Tsunehiko Kan, Yasuhiro Dekura, Yoshiaki Okamoto, Yoshisuke Matsuoka, Yo Ushijima and Yu Takada.
Contributor Information
Takaya Yamamoto, Department of Radiation Oncology, Graduate School of Medicine, Tohoku University, Sendai, Japan.
Yuzuru Niibe, Department of Radiology, Toho University Omori Medical Center, Tokyo, Japan; Department of Public Health, Kurume University School of Medicine, Kurume, Japan.
Yasuo Matsumoto, Department of Radiation Oncology, Niigata Cancer Center, Niigata, Japan.
Hiroshi Onishi, Department of Radiology, Yamanashi University, Chuo, Japan.
Masahiko Aoki, Department of Radiation Oncology, Hirosaki University, Hirosaki, Japan.
Atsushi Nishikawa, Department of Radiation Oncology, Shikoku Cancer Center, Matsuyama, Japan.
Ryoong-Jin Oh, Department of Radiology, Miyakojima IGRT Clinic, Osaka, Japan.
Takashi Shintani, Department of Radiation Oncology and Image-Applied Therapy, Graduate School of Medicine, Kyoto University, Kyoto, Japan.
Katsuya Yahara, Department of Radiology, University of Occupational and Environmental Health, Fukuoka, Japan.
Masatoki Ozaki, Department of Radiation Oncology, Shizuoka City Shimizu Hospital, Shizuoka, Japan.
Yoshihiko Manabe, Department of Radiology, Nagoya City University, Nagoya, Japan.
Keiichi Jingu, Department of Radiation Oncology, Graduate School of Medicine, Tohoku University, Sendai, Japan.
CONFLICT OF INTEREST
Y.N. has received lecturer fees from Janssen Pharmaceutical K.K. All other authors have nothing to disclose.
FUNDING
This work was supported by Grant-in-aid for research on radiation oncology of JASTRO 2015–2016.
References
- 1. National Comprehensive Cancer Network . Clinical practice guidelines in oncology. Colon Cancer, Version 4. 2019. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (December 13, 2019, date last accessed). [Google Scholar]
- 2. National Comprehensive Cancer Network . Clinical practice guidelines in oncology. Rectal Cancer, Version 3. 2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (December 13, 2019, date last accessed). [Google Scholar]
- 3. Jingu K, Matsushita H, Yamamoto T et al. Stereotactic radiotherapy for pulmonary Oligometastases from colorectal cancer: A systematic review and meta-analysis. Technol Cancer Res Treat 2018;17:1533033818794936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang X, Zamdborg L, Ye H et al. A matched-pair analysis of stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer versus early stage non-small cell lung cancer. BMC Cancer 2018;18:962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andratschke N, Alheid H, Allgäuer M et al. The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): Patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases. BMC Cancer 2018;18:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takeda A, Sanuki N, Tsurugai Y et al. Stereotactic body radiotherapy for patients with oligometastases from colorectal cancer: Risk-adapted dose prescription with a maximum dose of 83-100 Gy in five fractions. J Radiat Res 2016;57:400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klement RJ, Abbasi-Senger N, Adebahr S et al. The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: A combined analysis of 388 patients with 500 metastases. BMC Cancer 2019;19:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niibe Y, Yamamoto T, Onishi H et al. Pulmonary Oligometastases Treated by Stereotactic, Body Radiation Therapy: A Nationwide Survey of 1,378 Patients. Anticancer Res 2020;40:393–9. [DOI] [PubMed] [Google Scholar]
- 9. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma A, Baker S, Duijm M et al. Prognostic factors for local control and survival for inoperable pulmonary colorectal oligometastases treated with stereotactic body radiotherapy. Radiother Oncol 2020;144:23–9. [DOI] [PubMed] [Google Scholar]
- 11. Franzese C, Comito T, Toska E et al. Predictive factors for survival of oligometastatic colorectal cancer treated with stereotactic body radiation therapy. Radiother Oncol 2019;133:220–6. [DOI] [PubMed] [Google Scholar]
- 12. Jingu K, Matsuo Y, Onishi H et al. Dose escalation improves outcome in stereotactic body radiotherapy for pulmonary Oligometastases from colorectal cancer. Anticancer Res 2017;37:2709–13. [DOI] [PubMed] [Google Scholar]
- 13. Guerrera F, Mossetti C, Ceccarelli M et al. Surgery of colorectal cancer lung metastases: Analysis of survival, recurrence and re-surgery. J Thorac Dis 2016;8:1764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agolli L, Bracci S, Nicosia L et al. Lung metastases treated with stereotactic ablative radiation therapy in Oligometastatic colorectal cancer patients: Outcomes and prognostic factors after long-term follow-up. Clin Colorectal Cancer 2017;16:58–64. [DOI] [PubMed] [Google Scholar]
- 15. Salah S, Watanabe K, Welter S et al. Colorectal cancer pulmonary oligometastases: Pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann Oncol 2012;23:2649–55. [DOI] [PubMed] [Google Scholar]
- 16. Nemec SF, Bankier AA, Eisenberg RL. Lower lobe-predominant diseases of the lung. AJR Am J Roentgenol 2013;200:712–28. [DOI] [PubMed] [Google Scholar]
- 17. Zabaleta J, Iida T, Falcoz PE et al. Individual data meta-analysis for the study of survival after pulmonary metastasectomy in colorectal cancer patients: A history of resected liver metastases worsens the prognosis. Eur J Surg Oncol 2018;44:1006–12. [DOI] [PubMed] [Google Scholar]
- 18. Ohtaka K, Hida Y, Kaga K et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg 2013;95:1924–8. [DOI] [PubMed] [Google Scholar]
- 19. Ohtaka K, Hida Y, Kaga K et al. Left upper lobectomy can be a risk factor for thrombosis in the pulmonary vein stump. J Cardiothorac Surg 2014;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao L, Zhou S, Balter P et al. Planning target volume D95 and mean dose should be considered for optimal local control for stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys 2016 15;95:1226–35. [DOI] [PubMed] [Google Scholar]


