Abstract
Objectives
Limited testing capacity has characterized the ongoing coronavirus disease 2019 (COVID-19) pandemic in Spain, hampering timely control of outbreaks and opportunities to reduce the escalation of community transmission. This study investigated the potential to use sample pooling, followed by one-step retrotranscription and real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) to increase testing capacity for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).
Methods
Various pool sizes (five, 10 and 15 samples) were evaluated prior to RNA extraction followed by standard RT-qPCR for the diagnosis of COVID-19. The pool size achieving reproducible results with individual sample testing was subsequently used to assess nasopharyngeal samples in a tertiary hospital in August 2020.
Results
A pool size of five samples had higher sensitivity compared with pool sizes of 10 and 15 samples, showing a mean cycle threshold (Ct) shift of 3.5 [standard deviation (SD) 2.2] between the pooled test and positive samples in the pool. Next, a pool size of five was used to test a total of 895 pools (4475 prospective samples) using two different RT-qPCR kits. The Real Accurate Quadruplex corona-plus PCR Kit (PathoFinder) reported the lowest mean Ct shift [2.2 (SD 2.4)] between the pool and individual samples. This strategy enables detection of individual positive samples in positive pools with Ct of 16.7–39.4.
Conclusions
Grouping samples into pools of five for RT-qPCR resulted in an increase in SARS-CoV-2 testing capacity with minimal loss of sensitivity compared with testing each sample individually.
Keywords: COVID-19, SARS-CoV-2, Testing capacity, Scalability, Sample pooling
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), continues to impose a heavy burden on healthcare systems worldwide due to a shortage of consumables and demand to ‘scale up’ efficient screening approaches. In order to limit the escalation of cases and amplification of infections, there is a need to increase testing capacity and develop alternatives to the one-step real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) for regular testing of SARS-CoV-2 (Mina et al., 2020). We have previously assessed a direct heating method for nasopharyngeal (swab) samples to bypass the RNA extraction step in order to increase testing capacity (Alcoba-Florez et al., 2020a, Alcoba-Florez et al., 2020b).
In areas with a low prevalence of COVID-19, such as the Canary Islands until June 2020 (Pollán et al., 2020), testing samples in pools is another approach to increase SARS-CoV-2 testing capacity. Previous studies stated that pooling a maximum of 10 samples was desirable (Volpato et al., 2020), with six samples being the optimal pool size for the target prevalence observed in the region (Regen et al., 2020). Simulation studies show the value of testing pooled samples, with increased speed of reporting and a small impact on test sensitivity (Larremore et al., 2020). In support of this, a pooled test for SARS-CoV-2 using four samples has received Emergency Use Authorization from the USA Food and Drug Administration (FDA COVID-19 Update, 2020).
This study aimed to evaluate RT-qPCR testing on pooled swab samples in order to demonstrate its feasibility as an option to increase SARS-CoV-2 testing capacity in the region.
Materials and methods
This study was conducted in two stages at the University Hospital Nuestra Señora de Candelaria (Santa Cruz de Tenerife, Spain) in August 2020. Nasopharyngeal swab samples were collected in 2 mL of viral transmission medium (VTM) (bioMérieux, Marcy l’Etoile, France). RNA was extracted from 200 μL of pooled VTM using the MagNA Pure Compact Nucleic Acid Isolation Kit I (Roche, Basel, Switzerland) or the STARMag Viral DNA/RNA 200C Kit (Seegene, Seoul, South Korea), as described elsewhere (Alcoba-Florez et al., 2020a, Alcoba-Florez et al., 2020b). Two RT-qPCR solutions were used during the study period: the Real Accurate Quadruplex corona-plus PCR Kit (PathoFinder, Maastricht, The Netherlands) and the TaqPath COVID-19 CE-IVD RT-PCR Kit (Thermo Fisher Scientific, Waltham, MA, USA). Both kits focused on the results for the N target gene (Alcoba-Florez et al., 2020b). RT-qPCR was performed in 15-μL final volume reactions (2.5 μL of sample) using a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) following the thermal cycling specifications of each solution. Samples were considered negative when the SARS-CoV-2 target had a cycle threshold (Ct) >40. Positive and negative controls were included in all experiments, as described elsewhere (Alcoba-Florez et al., 2020a, Alcoba-Florez et al., 2020b).
In a pilot stage, the Real Accurate Quadruplex corona-plus PCR Kit was used to test 15 pools, made by combining five, 10 or 15 retrospective samples, containing equal volumes of one SARS-CoV-2-positive sample and the respective amounts of negative samples to complete the pool size. In a validation stage, the two RT-qPCR kits were used on swab samples from prospective subjects using the pool size which achieved optimal results. Subsequently, individual samples from each positive pool were subjected to RNA extraction followed by RT-qPCR using the above-mentioned methods to validate the results.
Differences between the pooled Ct and the positive sample Ct in the pool (Ct shift) for the two RT-qPCR kits were assessed by Mann–Whitney U-test using R v4.0.3 software. When more than one positive sample was present in a pool, the mean Ct of the positive samples was used in the calculation. Sensitivity and 95% confidence interval (CI) were assessed using MedCalc (MedCalc Software Ltd, Ostend, Belgium).
Results
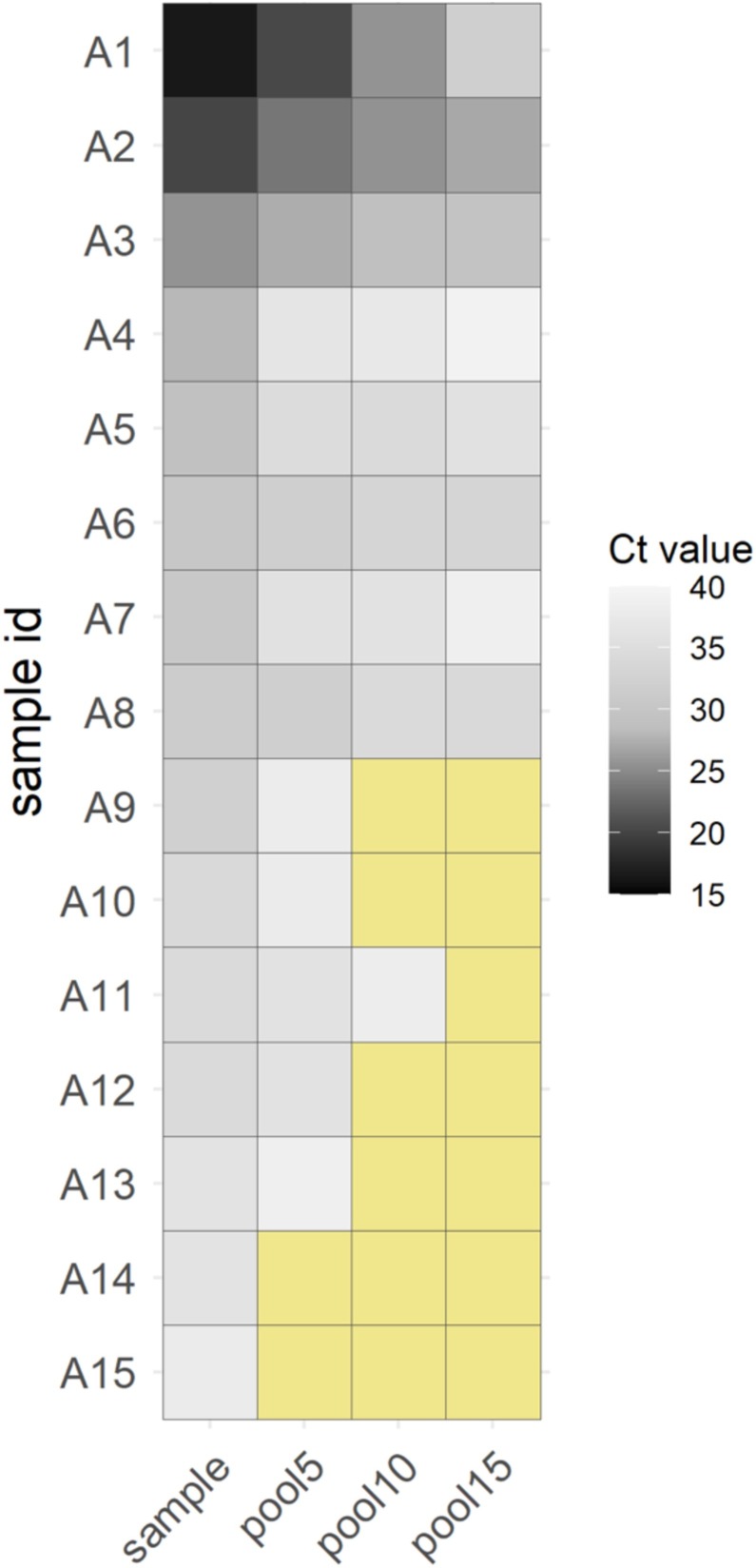
In the pilot stage, detection of SARS-CoV-2 was achieved using a pool size of five (one positive sample) in 13 of 15 independent pools (sensitivity = 86.7%, 95% CI 59.5–98.3); the detected positive samples had a maximum Ct value of 36.2 (Figure 1). A mean Ct shift of 3.5 [standard deviation (SD) 2.2] was obtained between the pool of five samples and the individual positive samples. False-negative results increased for pool sizes of 10 and 15 samples, both showing larger mean Ct shifts [5.3 (SD 2.4) and 7.2 (SD 4.4), respectively].
Figure 1.
Heatmap representation of positive real-time quantitative reverse transcription polymerase chain reaction on a cycle threshold (Ct) scale (darker colour = lower Ct) for the pilot study with pools of five, 10 and 15 retrospective swab samples. Negative results (Ct > 40) are highlighted in yellow. The Real Accurate Quadruplex corona-plus PCR Kit (PathoFinder) was used in the experiment.
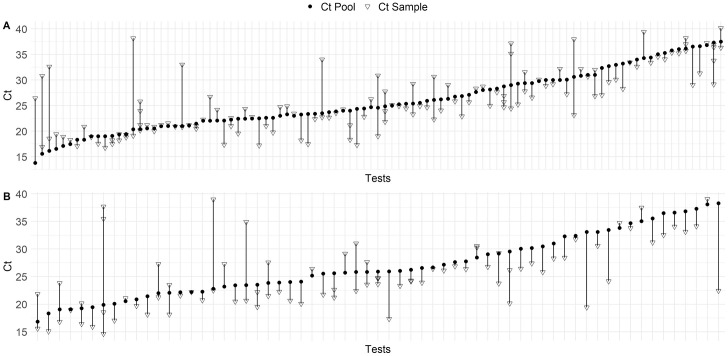
The validation stage included samples from 4475 prospective subjects between 18 and 31 August 2020. Samples were tested in 546 pools (447 negative pools) for the Real Accurate Quadruplex corona-plus PCR Kit and 349 pools (286 negative pools) for the TaqPath COVID-19 CE-IVD RT-PCR Kit. In total, 162 pools tested positive using the two kits, with 118 pools containing a single positive sample and 44 pools containing more than one positive sample (Figure 2). Compared with the individual positive samples present in the positive pools, the mean Ct shift upon pooling was 2.2 (SD 2.4) for the Real Accurate Quadruplex corona-plus PCR Kit and 3.1 (SD 2.9) for the TaqPath COVID-19 CE-IVD RT-PCR Kit (p = 0.006, Mann–Whitney U-test). Both RT-qPCR kits enabled detection of positive samples with a maximum Ct value of 39.4.
Figure 2.
Scatter plot showing cycle threshold (Ct) values and Ct shifts for all prospective cases of coronavirus disease 2019 detected by real-time quantitative reverse transcription polymerase chain reaction in pooling tests of five samples (solid circles) and individual samples (open triangles) using (A) the Real Accurate Quadruplex corona-plus PCR Kit and (B) the TaqPath COVID-19 CE-IVD RT-PCR Kit.
Discussion
This study investigated the use of RT-qPCR in pools of five swab samples, and found minimal loss of sensitivity compared with the assessment of individual samples. Consistent with these findings, using alternative RT-qPCR solutions and viral target probes, others have demonstrated that pooling up to 10 samples resulted in a slight shift in Ct (approximately 3) and therefore a decrease in sensitivity (Das et al., 2020, Volpato et al., 2020).
A major limitation of this study is that sensitivity was only evaluated in the pilot stage. However, despite the small Ct shift between the positive pools and the individual positive samples in the prospective study, a high impact of false-negative results is not expected (Cherif et al., 2020). The study data suggest that a pooling strategy of five samples is able to detect COVID-19-positive cases with Ct ≤39.4. Therefore, the data indicate that RT-qPCR testing of pooled swab samples before RNA extraction is accurate, sensitive and feasible as an option to increase SARS-CoV-2 testing capacity, and in preparedness planning to test large numbers of people in order to control community transmission of the virus and sudden uncontrolled outbreaks. Pooling with compressive sampling designs or hypercube slicing algorithms that involve repetitive tests (Mutesa et al., 2020, Shental et al., 2020), pooling of alternative less-invasive samples from the oral cavity (Watkins et al., 2020), and the use of alternative testing approximations (Peto et al., 2020) may help to further lessen the impact of the dilution factor on pooling, and continue to increase testing capacity.
Authors’ contributions
JAF and CF designed the study. JAF, HGC, DGM and ODG participated in data acquisition. JAF, LC and CF performed the analyses and data interpretation. LC, AVF, RGM and CF wrote the draft of the manuscript. All authors contributed in the critical revision and final approval of the manuscript.
Conflict of interest statement
None declared.
Funding
This research was funded by Cabildo Insular de Tenerife (Grants CGIEU0000219140 and ‘Apuestas científicas del ITER para colaborar en la lucha contra la COVID-19’); the agreement with Instituto Tecnológico y de Energías Renovables (ITER) to strengthen scientific and technological education, training research, development and innovation in Genomics, Personalized Medicine and Biotechnology (Grant No. OA17/008); M inisterio de Ciencia e Innovación (Grant Nos. RTI2018-093747-B-100 and RTC-2017-6471-1), co-funded by the E uropean Regional Development Fund (ERDF); Lab P2+ facility (Grant No. UNLL10-3E-783), co-funded by ERDF and ‘Fundación CajaCanarias’; and the S panish HIV/AIDS Research Network (Grant No. RIS-RETIC, RD16/0025/0011), co-funded by Instituto de Salud Carlos III and ERDF. The funders had no role in the study design, collection, analysis and interpretation of data, writing the manuscript, or the decision to submit the manuscript for publication.
Ethical approval
The University Hospital Nuestra Señora de Candelaria (Santa Cruz de Tenerife, Spain) review board approved the study (CHUNSC_2020_24).
Acknowledgments
The authors wish to thank the Board of Directors and the Executive Team of University Hospital Nuestra Señora de Candelaria for their strong support and assistance in accessing the diverse resources used in this study.
References
- Alcoba-Florez J., González-Montelongo R., Íñigo-Campos A., García-Martínez de Artola D., Gil-Campesino H., The Microbiology Technical Support Team, et al. Fast SARS-CoV-2 detection by RT-qPCR in preheated nasopharyngeal swab samples. Int J Infect Dis. 2020;97:66–68. doi: 10.1016/j.ijid.2020.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcoba-Florez J., Gil-Campesino H., García-Martínez de Artola D., González-Montelongo R., Valenzuela-Fernández A., Ciuffreda L., et al. Sensitivity of different RT-qPCR solutions for SARS-CoV-2 detection. Int J Infect Dis. 2020;99:190–192. doi: 10.1016/j.ijid.2020.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherif A., Grobe N., Wang X., Kotanko P. Simulation of pool testing to identify patients with coronavirus disease 2019 under conditions of limited test availability. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA COVID-19 Update . 2020. FDA issues first emergency authorization for sample pooling in diagnostic testing.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-first-emergency-authorization-sample-pooling-diagnostic [Google Scholar]
- Das S., Lau A.F., Youn J.H., Khil P.P., Zelazny A.M., Frank K.M. Pooled testing for surveillance of SARS-CoV-2 in asymptomatic individuals. J Clin Virol. 2020;132 doi: 10.1016/j.jcv.2020.104619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larremore D.B., Wilder B., Lester E., Shehata S., Burke J.M., Hay J.A., et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 surveillance. MedRxiv. 2020 doi: 10.1101/2020.06.22.20136309. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina M.J., Parker R., Larremore D.B. Rethinking Covid-19 test sensitivity — a strategy for containment. N Engl J Med. 2020;383:e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- Mutesa L., Ndishimye P., Butera Y., Souopgui J., Uwineza A., Rutayisire R., et al. A pooled testing strategy for identifying SARS-CoV-2 at low prevalence. Nature. 2020 doi: 10.1038/s41586-020-2885-5. Preprint. [DOI] [PubMed] [Google Scholar]
- Peto L., Rodger G., Carter D.P., Osman K.L., Yavuz M., Johnson K., et al. Diagnosis of SARS-CoV-2 infection with LamPORE, a high-throughput platform combining loop-mediated isothermal amplification and nanopore sequencing. MedRxiv. 2020 doi: 10.1101/2020.09.18.20195370. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollán M., Pérez-Gómez B., Pastor-Barriuso R., Oteo J., Hernán M.A., Pérez-Olmeda M., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regen F., Eren N., Heuser I., Hellmann-Regen J. A simple approach to optimum pool size for pooled SARS-CoV-2 testing. Int J Infect Dis. 2020;100:324–326. doi: 10.1016/j.ijid.2020.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shental N., Levy S., Skorniakov S., Wuvshet V., Shemer-Avni Y., Porgador A., et al. Efficient high throughput SARS-CoV-2 testing to detect asymptomatic carriers. Sci Adv. 2020;6:eabc5961. doi: 10.1101/2020.04.14.20064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato F., Lima-Morales D., Wink P.L., Willig J., de-Paris F., Ashton-Prolla P., et al. Pooling of samples to optimize SARS-CoV-2 diagnosis by RT-qPCR: comparative analysis of two protocols. Eur J Clin Microbiol Infect Dis. 2020 doi: 10.1007/s10096-020-04071-8. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins A.E., Fenichel E.P., Weinberger D.M., Vogels C.B.F., Brackney D.E., Casanovas-Massana A., et al. Pooling saliva to increase SARS-CoV-2 testing capacity. MedRxiv. 2020 doi: 10.1101/2020.09.02.20183830. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]