Abstract
COVID-19 has quickly reached pandemic levels since it was first reported in December 2019. The virus responsible for the disease, named SARS-CoV-2, is enveloped positive-stranded RNA viruses. During its replication in the cytoplasm of host cells, the viral genome is transcribed into proteins, such as the structural protein spike domain S1, which is responsible for binding to the cell receptor of the host cells. Infected patients have initially flu-like symptoms, rapidly evolving to severe acute lung injury, known as acute respiratory distress syndrome (ARDS). ARDS is characterized by an acute and diffuse inflammatory damage into the alveolar-capillary barrier associated with a vascular permeability increase and reduced compliance, compromising gas exchange and causing hypoxemia. Histopathologically, this condition is known as diffuse alveolar damage which consists of permanent damage to the alveoli epithelial cells and capillary endothelial cells, with consequent hyaline membrane formation and eventually intracapillary thrombosis. All of these mechanisms associated with COVID-19 involve the phenotypic expression from different proteins transcription modulated by viral infection in specific pulmonary microenvironments. Therefore, this knowledge is fundamentally important for a better pathophysiological understanding and identification of the main molecular pathways associated with the disease evolution. Evidently, clinical findings, signs and symptoms of a patient are the phenotypic expression of these pathophysiological and molecular mechanisms of SARS-CoV-2 infection. Therefore, no findings alone, whether molecular, clinical, radiological or pathological axis are sufficient for an accurate diagnosis. However, their intersection and/or correlation are extremely critical for clinicians establish the diagnosis and new treatment perspectives.
Keywords: COVID-19, Pulmonary pathology, Molecular pathology, DAD, SARS-CoV-2
1. Introduction
The current pandemic affecting the entire world was first reported on December 31, 2019, in Wuhan province - China, as severe pneumonia in several patients epidemiologically associated with the Wuhan Seafood Market [[1], [2], [3]]. In January 2020, researchers from Shanghai and Sidney sequenced the genome of the causative agent, discovering a new coronavirus (CoV), homologous to the virus responsible for the first CoV outbreak in 2003 in Guangdong province (China) known as SARS-CoV (severe acute respiratory syndrome-coronavirus) [4,5]. Thus, this new coronavirus was named by the World Health Organization as SARS-CoV-2 and its associated disease name as 2019 Coronavirus Disease (COVID-19) [6]. COVID-19 was classified as the third outbreak caused by this virus, with SARS-CoV first and MERS-CoV (Middle East Respiratory Syndrome-Coronavirus) the second, occurred in 2012 in the Middle East [7]. SARS-CoV-2 is suspected to have been transmitted to humans via the intermediate host Pangolin, a mammal sold at the Wuhan Seafood Market, and bats as the primary host [3,8,9]. SARS-CoV-2 quickly reached pandemic levels due to its rapid proliferation and human-human infection, totaling, at the time of writing this review, 189 countries/regions with confirmed cases worldwide (John Hopkins University, Baltimore, USA) [10]. This confirmation is performed by real time RT-PCR, which detects the viral RNA extracted from patients with suspected COVID-19, after naso/oropharyngeal swab or bronchoalveolar lavage [5].
Coronavirinae, a subfamily of Coronaviridae, are enveloped positive-stranded RNA viruses of 30 kilobases, considered one of the largest RNA genomes ever identified [11,12]. They can be divided into 4 genera: Alfacoronavirus, Betacoronavirus, Gamacoronavirus and Deltacoronavirus; in which Betacoronavíruos (SARS-CoV-2 genera) is the most pathogenic subtype to humans [8,9,13]. During its replication, located in the cytoplasm of host cells, the viral genome is transcribed into: 1) structural proteins of the envelope (E), membrane (M), nucleocapsid (N) and spike (S), which is composed of domains S1 and S2, responsible for mediating the virus entry into host cells [8,14]; 2) non-structural proteins ORF1a and ORF1b; and 3) accessory proteins ORF7b, ORF8a, ORF8b and ORF9b [11,13]. After its replication, the viral RNA genome is again enveloped and merges with the plasma membrane, releasing viral replicates into the extracellular space [5,14].
Li W et al. [15] identified as the main cell receptor of SARS-CoV, the angiotensin-converting enzyme 2 (ACE2), which binds to the virus through its structural protein spike S1 domain. Due to the genetic similarity between viral strains, it is believed that the receptor is the same for SARS-CoV-2 [8,9,15]. Therefore, the new coronavirus attacks all cells that express ACE2, such as cells from gastrointestinal system, kidneys and lungs, which is the most affected organ [5].
SARS-CoV-2-infected patients start experiencing flu symptoms like fever, cough, nasal congestion and fatigue [16,17]. As the viral infection progresses, patients often experience dyspnea and consistent symptoms of viral pneumonitis, such as decreased oxygen saturation and lymphopenia, as well as ground-glass opacities and alveolar exudates with intralobular involvement in chest imaging [16,17]. Thus, the outcome of these patients is a severe condition of acute lung injury, named Acute Respiratory Distress Syndrome (ARDS) [18]. Described in 1967 by Ashbaugh D et al. [19], ARDS is characterized by respiratory distress associated with hypoxemia and the presence of bilateral infiltrate on chest imaging. As there is no gold standard for its diagnosis, the definition and diagnostic criteria have been discussed since then. In 2011, at the international meeting held to review the ARDS criteria, encouraged by the European Society for Intensive Care, the Berlin Definition was created [20]. Among the established criteria, the following are briefly highlighted: 1) acute onset of respiratory symptoms; 2) presence of bilateral infiltrate on chest imaging, in which pulmonary edema cannot be fully explained by heart disease or fluid overload; and 3) hypoxemia, classified into three categories of severity [20]. Pathophysiologically, ARDS is characterized by acute and diffuse inflammatory damage into alveolar-capillary barrier associated with an increase in vascular permeability, as well as reduced compliance and the size of the aerated lung tissue, compromising gas exchange and causing hypoxemia [21]. Depending on the infection degree and lungs involvement, gas exchange becomes impossible, requiring invasive mechanical ventilation and/or extracorporeal membrane oxygenation [22].
Histopathologically, this clinical picture was named by Katzenstein et al. [23] as diffuse alveolar damage (DAD), corresponding to almost all cases of SARS-CoV-2. It consists of a permanent damage to capillary endothelial cells and alveoli epithelial cells, with consequent leakage of protein-rich fluid into the interstitial and alveolar space, ultimately culminating in hyaline membrane formation and eventually intracapillary thrombosis [24]. This scenario compromises the surfactant stabilization, providing alveolar collapse and worsening oxygenation [25].
Despite studies and advances regarding SARS-CoV-2, the pathophysiological mechanisms responsible for triggering ARDS secondary to a viral infection are still not fully understood.
2. Pathophysiology
What is known about the SARS-CoV-2 pathophysiology is due to experimental studies with strains adapted from SARS-CoV [26,27] and clinical and retrospective studies in patients with this viral infection [1,17,28]. A series of pathophysiological mechanisms occur simultaneously when SARS-CoV-2 binds to ACE2 and its subsequent binding to Toll-like receptors on pneumocytes [15,18]. While the virus has its genome replicated, the host's immune system is activated, inducing the recruitment of inflammatory cells with subsequent production of pro-inflammatory cytokines and chemokines, as well as maturation of dendritic cells [5,18]. However, due to the continuous and rapid viral genome replication, the immune system is continuously activated, culminating in an uncontrolled and exacerbated response, lethal to host cells [29].
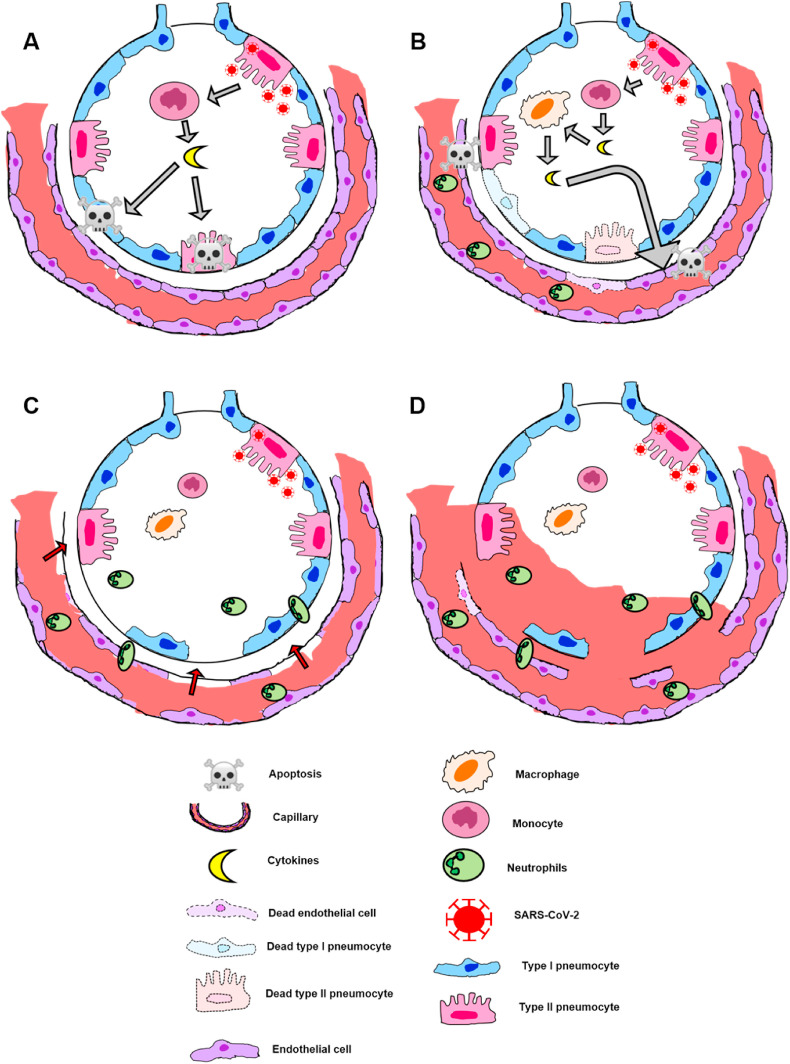
Briefly, monocytes recruited into the alveolar space secretes pro-inflammatory cytokines and induces pneumocytes apoptosis through the release of IFN (Interferon) dependent on Alpha Tumor Necrosis Factors (TNF-α), activating cell death receptors [18] (Fig. 1 A). In addition, recruited macrophages releases chemokines and others cytokines responsible for increasing capillary permeability and consequent neutrophils recruitment [30] (Fig. 1B). The excessive neutrophil degranulation causes permanent damage into pneumocytes and endothelial cells, breaking alveolar-capillary barrier [18,31] (Fig. 1C). Besides this natural mechanisms, some authors defend the formation of neutrophil extracellular traps (NETs) [30,32], that is, the release of neutrophils intracellular contents, such as DNA and proteins, in the extracellular space to capture pathogens. NETs are being recently associated with COVID-19, as shown by Zuo Y et al. [33], in which COVID-19 serum samples showed higher levels of NET markers: myeloperoxidase-DNA complexes and citrullinated histone H3.
Fig. 1.
– Scheme of SARS-COV-2 inflammatory response. The host's immune system is activated after SARS-CoV-2 binding to ACE2 receptor on type II pneumocyte surface. A - Recruited monocytes secretes pro-inflammatory cytokines, inducing pneumocytes apoptosis; B - Recruited macrophages releases other cytokines causing capillary permeability increase and consequent neutrophils recruitment; C - Neutrophils migrate into the interstitial/alveolar space and degranulate, culminating in permanent damage to pneumocytes and endothelial cells, resulting in alveolar-capillary barrier disruption; D - Interstitial and alveolar edema due to transmigration of blood proteins.
Regardless of which mechanism occurs, the end result is the transmigration of blood proteins, culminating in interstitial and alveolar edema [18] (Fig. 1D). In response to these cellular damages, there are several histopathological features that can be seen in COVID-19 patients.
3. Pathology
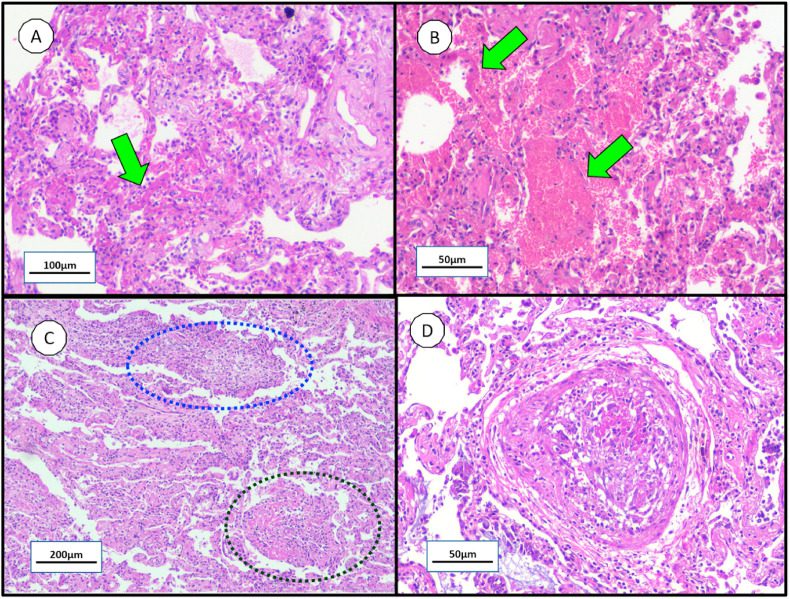
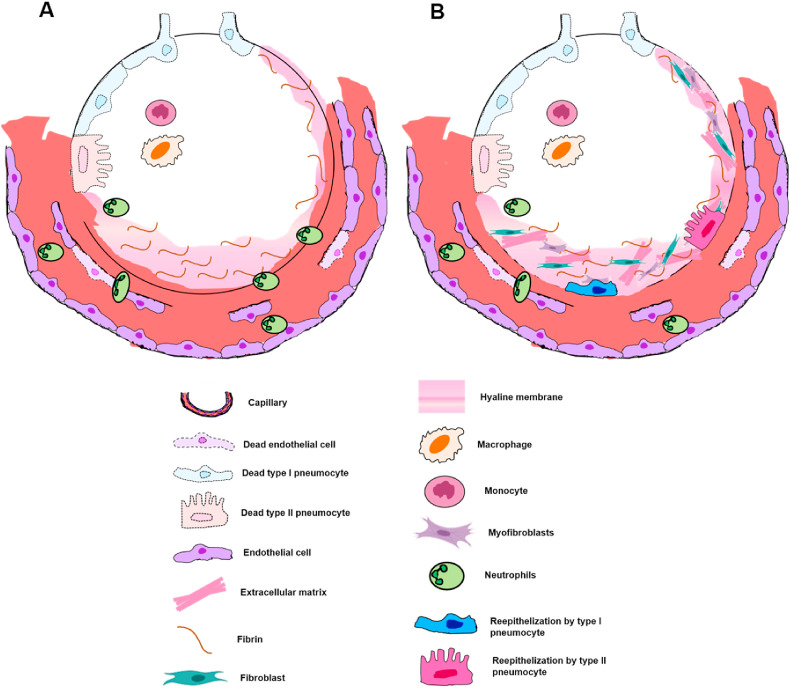
As a result of the pathophysiological processes described above, the diffuse alveolar damage is a classical histopathological pattern related to ARDS which can be divided into two phases [34]. Corresponding to the first 10 days of viral infection, the first or exudative phase is mainly characterized by hyaline membrane formation from fibrin polymerization contained in the plasma liquid that leaked into the interstitial/alveolar space (Fig. 2 A – green arrow); alveolar-capillary barrier injury with red blood cell extravasation (Fig. 2B – green arrows); and intense inflammatory cells infiltration into the intra-alveolar space [18,34]. On the other hand, the second or proliferative phase is distinguished by an exacerbated fibroblast and myofibroblast proliferation which can form acute fibrinous organizing pneumonia (Fig. 2C - dark blue circle) or organizing pneumonia (Fig. 2C – dark green circle) with subsequent extracellular matrix deposition, resulting in parenchymal remodeling and pulmonary fibrosis; as well as pneumocytes squamous metaplasia and proliferation of multinucleated giant cells [[34], [35], [36], [37], [38]]. Additionally, thrombotic events in pulmonary small arteries (Fig. 2D) may occur in this phase due to NET's influence, as described by Fuchs T et al. [39] and as reported in some published cases of COVID-19 autopsies [40,41]: Wichmann et al. [38] stated the presence of deep venous thrombosis within small lung arteries in 58% of their cases (7 patients); and Fox et al. [41] reported small thrombi within small peripheral vessels in all 10 patients addressed in the study. In addition, pulmonary micro-thrombi were present in two of these patients with markedly elevated D-dimers [41]. Similarly, Ackermann M et al. [42] describes thrombi within pulmonary arteries of 1 mm–2 mm diameter in 57% of COVID-19 patients. Both phases of diffuse alveolar damage are schematically represented in Fig. 3 .
Fig. 2.
– Histopathological findings in COVID-19 lungs by minimally invasive autopsy. Note the virus-induced lung injury are temporal heterogeneity: A - alveolar hyaline membrane (green arrow); B - alveolar-capillary barrier injury with hemorrhage (green arrows); C - acute fibrinous organizing pneumonia (dark blue circle) and organizing pneumonia (dark green circle); and D - pulmonary intravascular thrombotic events. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
– Schematically representation of the two histopathological phases of DAD. A - The first or exudative phase constitutes alveolar edema, neutrophil infiltration in the intra-alveolar space and mainly by hyaline membrane formed by fibrin polymerization contained in the plasma liquid that leaked into the interstitial/alveolar space, being recognized as DAD hallmark; B - The second or proliferative phase is described essentially by an intense fibroblast/myofibroblast recruitment and proliferation, with subsequent extracellular matrix deposition. Over time and together with the fibrotic deposition, there is also the reepithelization by type I and II pneumocytes.
It is worth mentioning that these stages do not occur sequentially, often occurring simultaneously through the lung tissue: while the immune system tries to contain the microorganism (exudative phase) in one histopathological region, another lung tissue area begins to organize itself in order to repair the affected areas (proliferative phase).
The association between ARDS, DAD and SARS-CoV (2003) is well known and several studies report it, as revealed by Nicholls J et al. [35], Ding Y et al. [28] and Pei F et al. [36] (Table 1 ) [1,17,43]. In these studies, the histopathological parameters commonly present in patients with SARS-CoV (2003) are: 1) alveolar edema; 2) desquamation of alveolar epithelial cells; 3) alveolar and interstitial inflammatory cells infiltration, like macrophages, monocyte neutrophils and lymphocytes; and 4) hyaline membrane formation. Additionally, patients autopsies reported by Nicholls J et al. [35] and Pei F et al. [36], share the histopathological findings of: 1) hemophagocytosis; and 2) pneumocytes squamous metaplasia. Moreover, other important features of DAD were also recorded by Pei F et al. [36]: 1) alveolar hemorrhage; 2) pneumocytic hyperplasia; 3) thickening of the pulmonary septa and 4) intra-alveolar hemorrhage, also shared by Ding Y et al. [28].
Table 1.
Summary of the main histopathological attributes secondary to SARS-CoV infection described in the literature.
| HISTOPATHOLOGICAL CHARACTERISTICS | Li G et al.(1) | Gu J et al.(17) | Ding Y et al.(28) | Nicholls JM et al.(35) | Pei F et al.(36) | Xu Z el at(37) | Wichmann D et al.(38) | Barton L et al.(40) | Fox S et al.(41) | Ackermann M et al.42) | Franks T J et al.(43) | Tian S et al.(44) | Lacy J et al.(45( | Konopka K et al.(46) | Zhang H et al.47) | Tian S et al.48) | Bradley B T et al.49) | Grillo F et al.50) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alveolar hemorrhage | X | X | X | X | X | X | X | |||||||||||
| Alveolar septa thickening | X | X | X | X | ||||||||||||||
| Fibrin deposition | X | X | X | X | X | X | X | X | X | X | ||||||||
| Fibroblastic proliferation | X | X | X | X | ||||||||||||||
| Focal necrosis | X | X | X | |||||||||||||||
| Haemophagocytosis | X | X | X | |||||||||||||||
| Hyaline membrane | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Inflammatory cells | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Intra-alveolar edema | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Micro-thrombi | X | X | X | X | ||||||||||||||
| Pneumocyte desquamation | X | X | X | X | X | X | X | X | X | X | ||||||||
| Pneumocytes squamous metaplasia | X | X | X | X | X | |||||||||||||
| Pneumocyte hyperplasia | X | X | X | X | X | X | X | X | X | X | ||||||||
| Viral cytopathic effecta | X | X | X |
Structural changes in host cells caused by viral invasion, such as: large nuclei, prominent nucleoli and amphophilic granular cytoplasm.
As mentioned earlier, CoV infection and its consequences are not restricted to the lungs only. Findings from patients autopsies with SARS-CoV indicate cell damage (necrosis and monocytes and lymphocytes infiltration) in the digestive tract, kidneys, liver, spleen and brain [17,28].
Regarding the new SARS-CoV-2, several pathological attributes similar to those seen in SARS-CoV were found in recent autopsies, establishing its association with ARDS and DAD (Table 1). The main damages shared by both SARS-CoV-2 and SARS-CoV are: 1) alveolar hyaline membrane; 2) desquamation of alveolar epithelial cells; 3) alveolar edema and 4) alveolar and interstitial inflammatory cells infiltration, such as macrophages, neutrophil monocytes and lymphocytes [37,38,40,41,[43], [44], [45], [46]]. In addition to these histopathological features, Xu Z et al. [37] reported a lung autopsy of SARS-CoV-2 consistent with the exudative phase of DAD: 1) cellular fibromyxoid exudates; 2) desquamation of alveolar epithelial cells; and 3) viral cytopathic-like changes on pneumocytes in the intra-alveolar spaces (large nuclei, prominent nucleoli and amphophilic granular cytoplasm), also noted by Fox et al. [41]. Similarly, Konopka K et al. [46] also described histopathological findings of DAD phase one: 1) interstitial edema; 2) focal pneumocyte hyperplasia; 3) hyaline membranes; and 3) fibrinous airspace exudate with neutrophils. Likewise, in the case report presented by Barton et al. [40]: 1) hyaline membranes without interstitial organization; 2) T-lymphocytes infiltration; and 3) alveolar/interstitial edema focally; Lacy et al. [45] reported a case of an unexpected death due to COVID-19 in which the authors identified: 1) hyaline membranes; 2) mild mononuclear infiltrates; 3) desquamating pneumocyte hyperplasia with focal multinucleated cells; 4) acute alveolar hemorrhage focally present; 5) alveolar fibrin; but without any evidence of viral inclusions, specific cytopathic changes, granulomas or fibroblast proliferation.
Although the exudative phase is highly present in COVID-19 cases, since it represents the first inflammatory response against virus, the proliferative phase is also noticed in several autopsies. In the 12 autopsies described by Wichmann et al. [38], 67% (8 cases) were consistent with exudative phase of DAD, while the other cases had typical proliferative phase attributes: 1) squamous metaplasia; and 2) extensive granulocytic infiltration of the alveoli and bronchi. Additionally, lungs autopsies from African Americans reported by Fox et al. [41] also revealed not only the exudative phase, but also the proliferative, as well as features consistent with the transition between both phases. Similarly, loose interstitial fibrosis, intra alveolar loose fibrous plugs of organizing pneumonia and intra-alveolar organizing fibrin was also described in the histopathological analysis by Zhang H et al. [47]. Tian S et al. [44] described focal fibril clusters as well as ongoing reparative process with severe pneumocyte hyperplasia and interstitial/alveolar thickening. In another work also from Tian S et al. [48], organization with fibroblasts in the intralveolar space was reported. Additionally, Bradley B T et al. [49], reported fibroblastic proliferation in 92% of pulmonary autopsies from COVID-19 patients and Grillo F et al. [50], fibrous parenchymal remodeling with fibroblast proliferation. Therefore, it can be inferred that there is a miscellaneous in cases of DAD secondary to COVID-19, since both exudative and proliferative phases are present in the cases, and sometimes even in the same patient, denoting a heterogeneity of lung damage caused by the virus. Furthermore, although COVID-19 is still recent, it is possible to infer that interstitial fibrosing could be an important future sequel in recovered patients. As revealed by Schwensen H F [51], destruction of lung parenchyma with fibrous organization and beginning of immature fibroblasts proliferation, was reported in an autopsy performed after clinical cure of COVID-19, indicating interstitial fibrosing as an important histomorphological sequel of this new disease.
Regarding the presence of SARS-CoV-2 infection and cell damage in other organs, Xu Z et al. [37] reported few infiltration of interstitial mononuclear inflammatory cells in cardiac tissue and microvesicular steatosis in liver biopsy, which may be due to COVID-19 or induced by drug. Interestingly, viral myocarditis was not present in some autopsy's records [40,45]. Although Fox et al. [41] described rare areas of lymphocytes adjacent to degenerating myocytes, the authors could not infer if that could represent an early manifestation of myocarditis.
All these histo-pathophysiological mechanisms associated with COVID-19 involve the phenotypic expression from different proteins transcription modulated by viral infection in specific pulmonary microenvironments. Therefore, understanding the molecular biology/pathways associated with the disease evolution is of fundamental importance.
4. Molecular biology
While there are thousands of works regarding SARS-CoV and MERS-CoV, little is known about the genetic mechanisms involved in SARS-CoV-2 replication. However, some recent papers published this year address the molecular biology of this virus, enabling its better understanding.
Wu F et al. [9] and Lu R et al. [8] compared SARS-CoV-2 with SARS-CoV at molecular and phylogenetic level, showing interesting results. Phylogenetic analyzes of both studies revealed that there are 5 subgenera belonging to Betacoronarivus, with Sarbecovirus the subgenera of SARS-CoV-2 [8,9]. Wu F et al. [9] performed deep meta-transcriptomic sequencing from the bronchoalveolar lavage fluid of a 41-year-old patient admitted to a hospital in Wuham. The phylogenetical analysis of 4 subgenera of Sarbecovirus with the new SARS-CoV-2 extracted from the patient revealed that the topological position of these groups changed as the analysis was performed, that is, according to the criteria chosen for the phylogenetic analysis, implying that recombination has occurred in the past [9]. The criteria used for these analyzes were: the nucleotide sequence of the entire genome, the non-structural protein genes ORF1a and ORF1b and the structural proteins encoded by the S, E, M and N genes. In this manner, SARS-CoV-2 appeared as a sister group of bat-SL-CoVZC45 and bat-SL-CoVZXC21 (bat-infecting viral genomes) when the criteria chosen was the gene encoding the non-structural protein ORF1a [9].
Similarly, Lu R et al. [8] found the same result. The authors performed next generation sequencing of samples from bronchoalveolar lavage from 9 patients in China. When comparing the SARS-CoV-2 viral genome with gene sequences available on the GenBank (Blast), they found that the new coronavirus is a sister group of the bat-SL-CoVs (bat-SL-CoVZC45 and bat-SL-CoVZXC21), with 88% amino acid identity [8]. In this way, these analyzes reveal the surprising finding that, contrary to what was thought, the new SARS-CoV-2 may be phylogenetically distant from SARS-CoV. However, depending on the phylogenetic criteria, this perspective can differ.
When the criteria chosen was the gene encoding structural protein spike S1 domain, Wu F et al. [9] and Lu R et al. [8] noticed a high phylogenetic proximity between SARS-CoV-2 and SARS-CoV, with 75% amino acid identity. Thus, the new coronavirus and covoravirus from 2003, share the same host cell receptor, the ACE2 [8,9,15].
These results are of extreme relevance in the therapeutic scope. By blocking the viral S1 domain or the ACE2 receptor and/or the proteins from the renin-angiotensin system (RAS), of which ACE2 belongs, the virus is unable to infect host cells. Some authors already address this subject, such as the therapy proposed by Phadke M and Saunik S in February 2020 [52]. The authors propose to use known angiotensin 1 receptor (AT1R) blockers (E.g.: losartan and telmisartan), as an attempt to treat patients with COVID-19. This proposal is based on the fact that SARS-CoV-2 when bound to ACE2, decreases the expression of this protein, consequently increasing its alternative pathway in RAS: the angiotensin-converting enzyme (ACE) [53,54]. ACE, in turn, converts angiotensin I into angiotensin II, which by binding to its receptor (AT1R), promotes an exaggerated constriction vessel, increasing blood pressure [53,54]. In addition, AT1R increases pulmonary vasculature permeability, favoring alveolar edema and SARS, contributing to the lung injury caused by the virus [53,54].
In this way, the advantageous therapy suggested by Phadke M and Saunik S [52] aims to block AT1R combined with increased expression of ACE2, preventing the increase in vascular permeability and supplying the decrease in the expression of ACE2 caused by its binding to SARS-CoV-2. More details regarding the ACE and ACE2 pathways and their role with SARS-CoV-2 are detailed in Gurwitz D's review [55].
Recently, much has been said about the use of chloroquine/hydroxychloroquine for treatment of COVID-19 patients. Used for more than 70 years, chloroquine/hydroxychloriquein is originally indicated for malaria and rheumatic diseases due to its anti-inflammatory effects [56]. This substance reduces the inflammatory cytokines secretion (TNFα, INFα, IL-6 and IL-1) and decreases the expression of TNFα receptors in monocytes, reducing their activation and consequent leukocyte extravasation [57].
With this in mind, Savarino et al. [57] hypothesized that this drug could have beneficial effects in the treatment of patients infected with SARS-CoV. By reducing the secretion of pro-inflammatory cytokines, the drug would reduce the consequent epithelial and endothelial damage caused by the cytokines excess, thus preventing an increase in vascular permeability [57]. Therefore, the use of chloroquine/hydroxychloriquein reduces the DAD caused by SARS-CoV-2.
In addition, in the in vitro trial published this year by Wang M et al. [58], the researchers infected Vero E6 cells with SARS-CoV-2 and subsequently treated it with low concentration chloroquine. The authors identified that this drug has an antiviral effect by reducing the effectiveness of virus replication [58]. By increasing the cellular pH, the steps of viral replication dependent on an alkaline pH, such as membrane fusion and encapsulation, become impaired [56,58]. Although the effects of chloroquine/hydroxychloriquein appear to be beneficial in the treatment of patients with COVID-19, its use is still controversial.
5. Concluding remarks
Taking together all the information described in this review, it is evident that the clinical findings, signs and symptoms of a patient are the phenotypic expression of the pathophysiological and molecular mechanisms of SARS-CoV-2 infection. Thus, no findings alone, whether molecular, clinical, radiological or pathological axis are sufficient for an accurate diagnosis. However, their intersection and/or correlation are extremely valuable and critical for a proper diagnosis. All things considered, the clinical-radio-patho-molecular correlation is extremely fundamental for pulmonologists establish the diagnosis and new treatment perspectives.
Funding
Nothing to declare.
Authors contributions
SSB, ATF: Performed literature search, design, and wrote the manuscript. ATF: Supervised and critically reviewed the manuscript.
Declaration of competing interest
Nothing to declare.
Acknowledgements
Nothing to declare.
References
- 1.Li G., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babiker A., et al. SARS-CoV-2 testing. Am. J. Clin. Pathol. 2020;153(6):706–708. doi: 10.1093/ajcp/aqaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashour H.M., et al. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9(3) doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes E.C., Zhang Y.Z. Novel 2019 coronavirus genome. 2020. http://virological.org/t/novel-2019-coronavirus-genome/319 Available from:
- 5.Yi Y., et al. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo J.R., et al. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect. Dis. 2020;20(6):678–688. doi: 10.1016/S1473-3099(20)30162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Hazmi A. Challenges presented by MERS corona virus, and SARS corona virus to global health. Saudi J. Biol. Sci. 2016;23(4):507–511. doi: 10.1016/j.sjbs.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu F., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins Universidade John, Baltimore E. Coronavirus COVID-19 global cases by the center for systems science and engineering (CSSE) 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available from:
- 11.Marra M.A., et al. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300(5624):1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 12.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo P.C., et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86(7):3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wilde A.H., et al. Host factors in coronavirus replication. Curr. Top. Microbiol. Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop. Med. Int. Health. 2020;25(3):278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu J., et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthay M.A., et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashbaugh D.G., et al. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 20.Force A.D.T., et al. Acute respiratory distress syndrome: the Berlin Definition. J. Am. Med. Assoc. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 21.Bellani G., et al. Epidemiology, patterns of Care, and mortality for patients with acute respiratory distress syndrome in intensive Care units in 50 countries. J. Am. Med. Assoc. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 22.Meyer N.J., Calfee C.S. Novel translational approaches to the search for precision therapies for acute respiratory distress syndrome. Lancet Respir Med. 2017;5(6):512–523. doi: 10.1016/S2213-2600(17)30187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzenstein A.L., Bloor C.M., Leibow A.A. Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. Am. J. Pathol. 1976;85(1):209–228. [PMC free article] [PubMed] [Google Scholar]
- 24.Brigham K.L., Staub N.C. Pulmonary edema and acute lung injury research. Am. J. Respir. Crit. Care Med. 1998;157(4 Pt 2):S109–S113. doi: 10.1164/ajrccm.157.4.nhlbi-8. [DOI] [PubMed] [Google Scholar]
- 25.Pham T., Rubenfeld G.D. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am. J. Respir. Crit. Care Med. 2017;195(7):860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 26.Nagata N., et al. Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am. J. Pathol. 2008;172(6):1625–1637. doi: 10.2353/ajpath.2008.071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts A., et al. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J. Virol. 2005;79(9):5833–5838. doi: 10.1128/JVI.79.9.5833-5838.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y., et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes B.J., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J. Clin. Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinkmann V., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 33.Zuo Y., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholls J., et al. SARS: clinical virology and pathogenesis. Respirology. 2003;8(Suppl):S6–S8. doi: 10.1046/j.1440-1843.2003.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholls J.M., et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361(9371):1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei F., et al. [Lung pathology and pathogenesis of severe acute respiratory syndrome: a report of six full autopsies] Zhonghua Bing Li Xue Za Zhi. 2005;34(10):656–660. [PubMed] [Google Scholar]
- 37.Xu Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wichmann D., et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020;4(268):173–277. doi: 10.7326/L20-1206. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs T.A., Brill A., Wagner D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012;32(8):1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barton L.M., et al. COVID-19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox S.E., et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):P681–P686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ackermann M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franks T.J., et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum. Pathol. 2003;34(8):743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian S., et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacy J.M., et al. Covid-19: postmortem diagnostic and biosafety considerations. Am. J. Forensic Med. Pathol. 2020;41(3):143–151. doi: 10.1097/PAF.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konopka K.E., Wilson A., Myers J.L. Postmortem lung findings in a patient with asthma and coronavirus disease 2019. Chest. 2020;158(3):e99–e101. doi: 10.1016/j.chest.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H., et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian S., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradley B.T., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grillo F., et al. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect. Dis. 2020;S1473-3099(20)30582-X doi: 10.1016/S1473-3099(20)30582-X. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwensen H.F., et al. Fatal pulmonary fibrosis: a post-COVID-19 autopsy case. J. Clin. Pathol. 2020;jclinpath-2020-206879 doi: 10.1136/jclinpath-2020-206879. In press. [DOI] [PubMed] [Google Scholar]
- 52.S P.M.S. Rapid response: use of angiotensin receptor blockers such as telmisartan, losartsan in nCoV wuhan corona virus infections – novel mode of treatment. 2020. https://www.bmj.com/content/368/bmj.m406/rr-2 Available from.
- 53.Kuba K., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imai Y., et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020;81(5):537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 57.Savarino A., et al. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]