Summary
Background
Older adults (aged ≥70 years) are at increased risk of severe disease and death if they develop COVID-19 and are therefore a priority for immunisation should an efficacious vaccine be developed. Immunogenicity of vaccines is often worse in older adults as a result of immunosenescence. We have reported the immunogenicity of a novel chimpanzee adenovirus-vectored vaccine, ChAdOx1 nCoV-19 (AZD1222), in young adults, and now describe the safety and immunogenicity of this vaccine in a wider range of participants, including adults aged 70 years and older.
Methods
In this report of the phase 2 component of a single-blind, randomised, controlled, phase 2/3 trial (COV002), healthy adults aged 18 years and older were enrolled at two UK clinical research facilities, in an age-escalation manner, into 18–55 years, 56–69 years, and 70 years and older immunogenicity subgroups. Participants were eligible if they did not have severe or uncontrolled medical comorbidities or a high frailty score (if aged ≥65 years). First, participants were recruited to a low-dose cohort, and within each age group, participants were randomly assigned to receive either intramuscular ChAdOx1 nCoV-19 (2·2 × 1010 virus particles) or a control vaccine, MenACWY, using block randomisation and stratified by age and dose group and study site, using the following ratios: in the 18–55 years group, 1:1 to either two doses of ChAdOx1 nCoV-19 or two doses of MenACWY; in the 56–69 years group, 3:1:3:1 to one dose of ChAdOx1 nCoV-19, one dose of MenACWY, two doses of ChAdOx1 nCoV-19, or two doses of MenACWY; and in the 70 years and older, 5:1:5:1 to one dose of ChAdOx1 nCoV-19, one dose of MenACWY, two doses of ChAdOx1 nCoV-19, or two doses of MenACWY. Prime-booster regimens were given 28 days apart. Participants were then recruited to the standard-dose cohort (3·5–6·5 × 1010 virus particles of ChAdOx1 nCoV-19) and the same randomisation procedures were followed, except the 18–55 years group was assigned in a 5:1 ratio to two doses of ChAdOx1 nCoV-19 or two doses of MenACWY. Participants and investigators, but not staff administering the vaccine, were masked to vaccine allocation. The specific objectives of this report were to assess the safety and humoral and cellular immunogenicity of a single-dose and two-dose schedule in adults older than 55 years. Humoral responses at baseline and after each vaccination until 1 year after the booster were assessed using an in-house standardised ELISA, a multiplex immunoassay, and a live severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) microneutralisation assay (MNA80). Cellular responses were assessed using an ex-vivo IFN-γ enzyme-linked immunospot assay. The coprimary outcomes of the trial were efficacy, as measured by the number of cases of symptomatic, virologically confirmed COVID-19, and safety, as measured by the occurrence of serious adverse events. Analyses were by group allocation in participants who received the vaccine. Here, we report the preliminary findings on safety, reactogenicity, and cellular and humoral immune responses. This study is ongoing and is registered with ClinicalTrials.gov, NCT04400838, and ISRCTN, 15281137.
Findings
Between May 30 and Aug 8, 2020, 560 participants were enrolled: 160 aged 18–55 years (100 assigned to ChAdOx1 nCoV-19, 60 assigned to MenACWY), 160 aged 56–69 years (120 assigned to ChAdOx1 nCoV-19: 40 assigned to MenACWY), and 240 aged 70 years and older (200 assigned to ChAdOx1 nCoV-19: 40 assigned to MenACWY). Seven participants did not receive the boost dose of their assigned two-dose regimen, one participant received the incorrect vaccine, and three were excluded from immunogenicity analyses due to incorrectly labelled samples. 280 (50%) of 552 analysable participants were female. Local and systemic reactions were more common in participants given ChAdOx1 nCoV-19 than in those given the control vaccine, and similar in nature to those previously reported (injection-site pain, feeling feverish, muscle ache, headache), but were less common in older adults (aged ≥56 years) than younger adults. In those receiving two standard doses of ChAdOx1 nCoV-19, after the prime vaccination local reactions were reported in 43 (88%) of 49 participants in the 18–55 years group, 22 (73%) of 30 in the 56–69 years group, and 30 (61%) of 49 in the 70 years and older group, and systemic reactions in 42 (86%) participants in the 18–55 years group, 23 (77%) in the 56–69 years group, and 32 (65%) in the 70 years and older group. As of Oct 26, 2020, 13 serious adverse events occurred during the study period, none of which were considered to be related to either study vaccine. In participants who received two doses of vaccine, median anti-spike SARS-CoV-2 IgG responses 28 days after the boost dose were similar across the three age cohorts (standard-dose groups: 18–55 years, 20 713 arbitrary units [AU]/mL [IQR 13 898–33 550], n=39; 56–69 years, 16 170 AU/mL [10 233–40 353], n=26; and ≥70 years 17 561 AU/mL [9705–37 796], n=47; p=0·68). Neutralising antibody titres after a boost dose were similar across all age groups (median MNA80 at day 42 in the standard-dose groups: 18–55 years, 193 [IQR 113–238], n=39; 56–69 years, 144 [119–347], n=20; and ≥70 years, 161 [73–323], n=47; p=0·40). By 14 days after the boost dose, 208 (>99%) of 209 boosted participants had neutralising antibody responses. T-cell responses peaked at day 14 after a single standard dose of ChAdOx1 nCoV-19 (18–55 years: median 1187 spot-forming cells [SFCs] per million peripheral blood mononuclear cells [IQR 841–2428], n=24; 56–69 years: 797 SFCs [383–1817], n=29; and ≥70 years: 977 SFCs [458–1914], n=48).
Interpretation
ChAdOx1 nCoV-19 appears to be better tolerated in older adults than in younger adults and has similar immunogenicity across all age groups after a boost dose. Further assessment of the efficacy of this vaccine is warranted in all age groups and individuals with comorbidities.
Funding
UK Research and Innovation, National Institutes for Health Research (NIHR), Coalition for Epidemic Preparedness Innovations, NIHR Oxford Biomedical Research Centre, Thames Valley and South Midlands NIHR Clinical Research Network, and AstraZeneca.
Introduction
As of Nov 13, 2020, over 52 million people have been diagnosed with COVID-19 worldwide, with over 1·2 million confirmed deaths.1 Severe COVID-19 is more common in adults aged 70 years and older and in individuals with comorbidities such as hypertension, diabetes, cardiovascular disease, and chronic respiratory disease.2 A safe and effective vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will be an important tool in controlling the global COVID-19 pandemic. Although there are no licensed vaccines against COVID-19, 48 potential vaccine candidates based on a variety of platforms including lipid nanoparticle mRNA, DNA, adjuvanted protein, inactivated virus particles, and non-replicating viral vectors are in clinical trials (of which 11 candidates are in phase 3 trials) and a further 164 candidates are in preclinical testing.3
The WHO global target product profile of critical characteristics for prequalification of a COVID-19 vaccine requires candidates to be targeted at the most at-risk groups, including older adults; have a favourable safety profile; provide efficacy as measured by prevention of virologically confirmed disease or transmission, or both; and to provide at least 6 months of protection for individuals at ongoing risk of exposure to SARS-CoV-2.4 On Sept 25, 2020, the UK Joint Committee on Vaccination and Immunisation (JCVI) gave interim recommendations for the national prioritisation of COVID-19 vaccines.5 The following groups were provisionally prioritised: first, older adults living in residential care homes and residential care home workers; second, all adults aged 80 years or older and health-care and social-care workers; and third, all adults aged 75 years and older. However, the JCVI acknowledged that this priority ranking could change substantially if the first available vaccines were not considered safe or effective in older adults. Similar recommendations have also been made by the US Advisory Committee on Immunization Practices.6
Immunosenescence refers to the gradual deterioration and decline of the immune system brought on by ageing. Age-dependent differences in the functionality and availability of T-cell and B-cell populations are thought to have a key role in the decrease of immune response.7 There has been a drive to develop vaccines and adjuvant formulations tailored for older adults to overcome this diminished immune response after vaccination. Assessment of immune responses in older adults is therefore essential in the development of COVID-19 vaccines that could protect this susceptible population.
The spike protein of SARS-CoV-2 binds to ACE2 receptors on target cells during viral entry. Analysis of convalescent patients suggests that the spike protein is an immunodominant antigen, eliciting both antibody and T-cell responses.8 Most COVID-19 candidate vaccines have been developed to induce anti-spike protein immune responses. Clinical trials using several different vaccine platforms including mRNA,9, 10 adenoviral vectored vaccines,11, 12 inactivated virus,13, 14 and adjuvanted spike glycoprotein15 have shown neutralising antibody responses after immunisation.
Research in context.
Evidence before this study
We searched PubMed for research articles published from database inception until Nov 13, 2020, with no language restrictions, using the terms “SARS-CoV-2”, “vaccine”, AND “clinical trial”. We identified published clinical trial data on eight other vaccine candidates. Two recombinant viral vectored vaccines have been tested in clinical trials. A single dose adenovirus (Ad) 5 vector-based vaccine (CanSino Biological/Beijing Institute of Biotechnology, China) elicited neutralising antibodies and T-cell responses in a dose-dependent manner, but was less immunogenic in individuals older than 55 years. A heterologous prime-boost Ad5/Ad26-vectored vaccine schedule (Gamaleya Research Institute, Russia) generated neutralising antibody and cellular responses in adults younger than 60 years. Two nucleoside-modified mRNA vaccine candidates using a two-dose regimen were tested in adults aged 18–55 years and 65–85 years, and generated neutralising antibodies in both age groups in a dose-dependent manner, although immunogenicity decreased with age (Pfizer/BioNTech, USA). Another mRNA vaccine (Moderna, USA) was given to adults older than 56 years. The vaccine was tolerated, with neutralising antibodies induced in a dose-dependent manner, which increased after a second dose. Neutralising antibody responses with this mRNA vaccine appeared to be similar in adults older than 56 years to those aged 18–55 years who also received the vaccine. Two inactivated viral vaccines have also shown neutralising antibody responses in a dose-dependent manner in adults aged 18–59 years (Wuhan Institute Biological Products/SinoPharm, China) or adults aged 18–59 and 60 years and older (Beijing Institute Biological products/SinoPharm, China), with the second showing lower neutralising antibody titres in older adults after two doses. Finally, a clinical trial of a nanoparticle vaccine composed of adjuvanted trimeric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoproteins (Novavax, USA) reported results of a two-dose schedule given 3 weeks apart in healthy adults younger than 60 years. This vaccine was well tolerated and induced neutralisation responses that exceeded those measured in serum samples from convalescent symptomatic patients.
Added value of this study
This study is the fifth published clinical trial of a vaccine against SARS-CoV-2 tested in an older adult population (aged 18–55 years, 56–69 years, and ≥70 years). The vaccine was safe and well tolerated, with reduced reactogenicity in older adults. Antibody responses against the SARS-CoV-2 spike protein were induced in all age groups and were boosted and maintained at 28 days after booster vaccination, including in the 70 years and older group. Cellular immune responses were also induced in all age and dose groups, peaking at day 14 after vaccination.
Implications of all the available evidence
The populations at greatest risk of serious COVID-19 include people with coexisting health conditions and older adults. The immune correlates of protection against SARS-CoV-2 have not yet been determined, but neutralising antibodies are thought to be associated with protection, and in a COVID-19 non-human primate challenge model, neutralising antibody responses correlated with protection. These findings have led to the use of neutralisation assays to assess immune responses in recent human COVID-19 vaccine trials. Immunisation with ChAdOx1 nCoV-19 results in development of neutralising antibodies against SARS-CoV-2 in almost 100% of participants including older adults without severe comorbidities, with higher levels in boosted compared with non-boosted groups. Further assessment of the efficacy of this vaccine is warranted in all age groups and individuals with comorbidities.
Replication-deficient adenovirus vectors containing a pathogen-specific transgene have been used as novel vaccines because of their ability to induce strong humoral and cellular responses.16 However, pre-existing immunity might reduce the immunogenicity of vectors derived from human viruses; hence, use of simian adenoviruses might be preferable. ChAdOx1 nCoV-19 (AZD1222) is a replication-defective chimpanzee adenovirus-vectored vaccine expressing the full-length SARS-CoV-2 spike glycoprotein gene (GenBank accession number MN908947). Vaccination of rhesus macaques with a single dose of ChAdOx1 nCoV-19 generates humoral and cellular immune responses and protects from lower respiratory infection after subsequent challenge with SARS-CoV-2.17 Preliminary results of a phase 1/2 clinical trial of ChAdOx1 nCoV-19 in adults aged 18–55 years show that the vaccine is well tolerated and generates robust neutralising antibody and cellular immune responses against the spike glycoprotein.18 Here we present the safety and immunogenicity results of a phase 2 component of a phase 2/3 multicentre study using ChAdOx1 nCoV-19 at two different doses, in adults including those aged 56–69 years and 70 years and older, and in a one-dose or two-dose regimen.
Methods
Study design and participants
In this continuing single-blind, multicentre, randomised, controlled, phase 2/3 trial, the safety and efficacy of the ChAdOx1 nCoV-19 vaccine is being assessed, with sequential age-escalation immunogenicity substudies being done in older age groups. The study is being run at 20 centres in the UK (listed in the appendix [pp 84–87]). Here we report selected results from the phase 2 component of the trial and for which participants were enrolled at two sites in the UK: the Oxford Vaccine Centre, Centre for Clinical Vaccinology and Tropical Medicine, University of Oxford (Oxford) and the NIHR Southampton Clinical Research Facility, University Hospital Southampton NHS Foundation Trust (Southampton). Data on the participants from the phase 3 component will be published elsewhere.
We recruited participants in an age-escalation manner. We recruited adults aged 18–55 years, then adults aged 56–69 years, and then adults aged 70 years and older, without severe or uncontrolled medical comorbidities, as defined in the clinical study plan (appendix pp 48–54), through local advertisements. Participants aged 65 years and older with a Dalhousie Clinical Frailty Score of 4 or higher were excluded.19
Participants were enrolled into one of ten different groups. Recruitment was sequential with low-dose groups recruited first and standard-dose cohorts recruited after a protocol amendment was approved on June 5, 2020, that incorporated the new higher dose level. For the first stage of recruitment, participants aged 18–55 years were recruited to the low-dose group. Subsequently we recruited participants aged 56–69 years, and further extension to recruit those aged 70 years and older only occurred after safety review by the independent Data Safety Monitoring Board (DSMB). A minimum of 2 weeks of safety and immunogenicity data were reviewed by the DSMB before recruitment to each successive age cohort. The 18–55 years groups received two doses of vaccine and were randomly assigned to receive either the experimental vaccine or the control vaccine. The 56–69 years and 70 years and older groups were randomly assigned to receive either one dose or two doses of vaccine and were then randomly assigned to receive the experimental vaccine or the control vaccine. The same process was repeated with recruitment and randomisation for the standard-dose cohorts after review by the DSMB. All participants underwent a screening visit in which a full medical history, targeted examination, blood test for SARS-CoV-2 exposure, and a urinary pregnancy test in women of childbearing potential were done. Volunteers who were seropositive to SARS-CoV-2 before enrolment were excluded from participating in all groups, apart from those in the 18–55 years standard-dose cohort. Additionally, all participants included in this phase 2 component of the study, apart from those in the 18–55 years low-dose group, had additional safety tests (blood tests for HIV, hepatitis B and C serology, full blood count, and kidney and liver function tests). Full details of eligibility criteria are in the trial protocol (appendix pp 135–38).
Written informed consent was obtained from all participants, and the trial is being done in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The study was sponsored by the University of Oxford (Oxford, UK) and approved in the UK by the Medicines and Healthcare products Regulatory Agency (reference 21584/0428/001-0001) and the South-Central Berkshire Research Ethics Committee (reference 20/SC/0179). Vaccine use was authorised by Genetically Modified Organisms Safety Committees at each participating site. An independent DSMB reviewed all interim safety reports. A copy of the protocol is included in the appendix (pp 83–212).
Randomisation and masking
Participants were randomly assigned to receive either the ChAdOx1 nCoV-19 vaccine or the quadrivalent MenACWY protein-polysaccharide conjugate vaccine. MenACWY was used as a comparator vaccine rather than a saline placebo to maintain masking of participants who had local or systemic reactions. Participants aged 18–55 years were randomly assigned (1:1) in the low-dose cohort and (5:1) in the standard-dose cohort to receive either ChAdOx1 nCoV-19 or MenACWY. For both 18–55 years cohorts, participants were given two doses of study vaccine. Participants aged 56–69 years were randomly assigned (3:1:3:1) to one dose of ChAdOx1 nCoV-19, one dose of MenACWY, two doses of ChAdOx1 nCoV-19, or two doses of MenACWY. Participants aged 70 years or older were randomly assigned (5:1:5:1) to one dose of ChAdOx1 nCoV-19, one dose of MenACWY, two doses of ChAdOx1 nCoV-19, or two doses of MenACWY.
Randomisation lists, using block randomisation stratified by age and dose group and study site, were generated by the study statistician (MV). Block sizes were chosen to align with the age group and dose group sizes. Computer randomisation was done with full allocation concealment within the secure web platform used for the study electronic case report form (REDCap version 9.5.22). The trial staff administering the vaccine prepared vaccines out of sight of the participants and syringes were covered with an opaque material until ready for administration to ensure masking of participants. Participants, clinical investigators, and the laboratory team remained masked to group allocation for the duration of the study. However, trial staff administering the vaccine were unmasked.
Procedures
In the previous phase 1/2 study,18 a single standard dose of 5 × 1010 virus particles of ChAdOx1 nCoV-19 was used, based on previous experience with a ChAdOx1 Middle East respiratory syndrome (MERS) construct. In this study, we assessed a lower dose of 2·2 × 1010 virus particles and a standard dose of 3·5–6·5 × 1010 virus particles in adults of different age cohorts. Due to the need to rapidly produce large numbers of doses of vaccine manufactured using Good Manufacturing Practice to allow timely enrolment into the phase 2/3 clinical trial, two different batches of vaccine were used in this study: one manufactured and vialed by Advent (Pomezia, Italy), and one manufactured by COBRA Biologics (Keele, UK) and vialed by Symbiosis (Stirling, UK). Both were manufactured according to Good Manufacturing Practice and approved by the regulatory agency in the UK, the Medicines and Healthcare products Regulatory Agency. The 18–55 years standard-dose cohort received vaccine manufactured by COBRA Biologics for both first (ie, prime) and second (ie, boost) doses and all other cohorts received prime and boost doses, as randomised, manufactured by Advent. Analytical assessment of the batches indicates that the batches are comparable. Formal batch-to-batch comparison studies are ongoing and results will be reported when available.
ChAdOx1 nCoV-19 was administered as a single-dose or two-dose regimen (28 days apart) at either the low dose (2·2 × 1010 virus particles) or the standard dose (3·5–6·5 × 1010 virus particles). It was administered as a single intramuscular injection into the deltoid, according to specific study standard operating procedures. The MenACWY vaccine was provided by the UK Department of Health and Social Care and administered as per summary of product characteristics at the standard dose.20 Depending on the batch used for vaccination, the injection volume for the low dose of ChAdOx1 nCoV-19 was either 0·22 mL or 0·5 mL. The injection volume used for the standard dose of ChAdOx1 nCoV-19 and MenACWY was 0·5 mL.
Safety data from animal studies and our previous phase 1/2 clinical trial18 of ChAdOx1 nCoV-19 were reviewed before recruitment of participants. Volunteers were considered enrolled into the trial at the point of vaccination. Participants were observed in the clinic for a minimum of 15 min after the vaccination procedure in case of any immediate adverse events.
Participants from each group were instructed to complete a diary card to record solicited local and systemic adverse reactions for 7 days after each dose. Protocol-defined solicited local adverse events included injection-site pain, tenderness, warmth, redness, swelling, induration, and itch, and solicited systemic adverse events included malaise, muscle ache, joint pain, fatigue, nausea, headache, chills, feverishness (ie, a self-reported feeling of having a fever), and objective fever (defined as an oral temperature of 38°C or higher). All participants were given an emergency 24-h telephone number to contact the on-call study physician as required. Serious adverse events will be recorded throughout the follow-up period of 1 year after the last dose of vaccine.
Severity of adverse events was graded with the following criteria: mild (transient or mild discomfort for <48 h, no interference with activity, and no medical intervention or therapy required), moderate (mild-to-moderate limitation in activity, and no or minimal medical intervention or therapy required), severe (substantial limitation in activity and medical intervention or therapy required), or potentially life-threatening (requires assessment in emergency department or admission to hospital). All participants in the 56–69 years and 70 years and older groups and participants in the 18–55 years standard-dose group had clinical and immunogenicity assessments at 0, 7, 14, and 28 days after their prime and booster vaccinations. Participants in the 18–55 years low-dose group had clinical and immunogenicity assessments at baseline, immediately before the boost dose, and at 14 and 28 days after their booster vaccination.
Humoral responses at baseline and after vaccination were assessed using Meso Scale Discovery multiplexed immunoassay against spike and receptor binding domain [RBD], a standardised total IgG ELISA against trimeric SARS-CoV-2 spike protein, and a live SARS-CoV-2 microneutralisation assay MNA80, which was done at Public Health England (Porton Down, UK), as described previously.18 Cellular responses were assessed using an ex-vivo IFN-γ enzyme-linked immunospot (ELISpot) assay to enumerate antigen-specific T cells.18 Neutralising antibodies to the ChAdOx1 vector were measured using a secreted embryonic alkaline phosphatase (SEAP)-reporter assay, which measures the reciprocal of the serum dilution required to reduce in-vitro expression of vector-expressed SEAP by 50%, 24 h after transduction.21 Due to the labour-intensive nature of neutralisation assays, we prioritised analysis of samples from the ChAdOx1 nCoV-19 groups, randomly selecting more samples from ChAdOx1 nCoV-19 participants than control samples to be sent for blinded analysis.
Outcomes
The coprimary outcomes of the trial are to assess efficacy as measured by the number of cases of symptomatic, virologically confirmed COVID-19 and safety of the vaccine as measured by the occurrence of serious adverse events. Secondary outcomes include safety, reactogenicity, and immunogenicity profiles of ChAdOx1 nCoV-19 in older adults (aged 56–69 years and ≥70 years), efficacy against severe and non-severe COVID-19, death, and seroconversion against non-spike proteins. A full list of secondary and tertiary outcomes is in the protocol (pp 118–24).
Here we report preliminary results for selected secondary endpoints, comparing local and systemic reactogenicity and cellular and humoral immunogenicity of ChAdOx1 nCoV-19 between different age groups, after one or two doses and at low or standard dose. Efficacy analyses are not included in this report.
Statistical analysis
We present safety endpoints as frequencies (%) with 95% binomial exact CIs. We present immunological endpoints as medians and IQR. Analyses were by group allocation in participants who received the vaccine.
We did comparisons across the three age groups (aged 18–55 years, aged 56–69 years, and aged ≥70 years) using Kruskal-Wallis tests within each dose level of the vaccine (low dose or standard dose) for antibody responses or unadjusted analysis of variance applied to log-transformed values for neutralisation titres. We did comparisons between low-dose and standard-dose groups using Wilcoxon rank sum tests (antibody response) or independent samples Student's t test applied to log-transformed values for neutralisation titres. We present unadjusted p values for a small number of statistical comparisons to avoid issues of multiplicity. To assess the association between responses on different assays, we used unadjusted linear regression to analyse log-transformed values after baseline.
Sample sizes were nominal for these immunogenicity subgroups and no power calculations were done.
We did all statistical analyses using SAS version 9.4 and R version 3.6.1 or later. This study is registered with ClinicalTrials.gov, NCT04400838, and with ISRCTN, 15281137.
Role of the funding source
AstraZeneca reviewed the data from the study and the final manuscript before submission, but the authors retained editorial control. All other funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
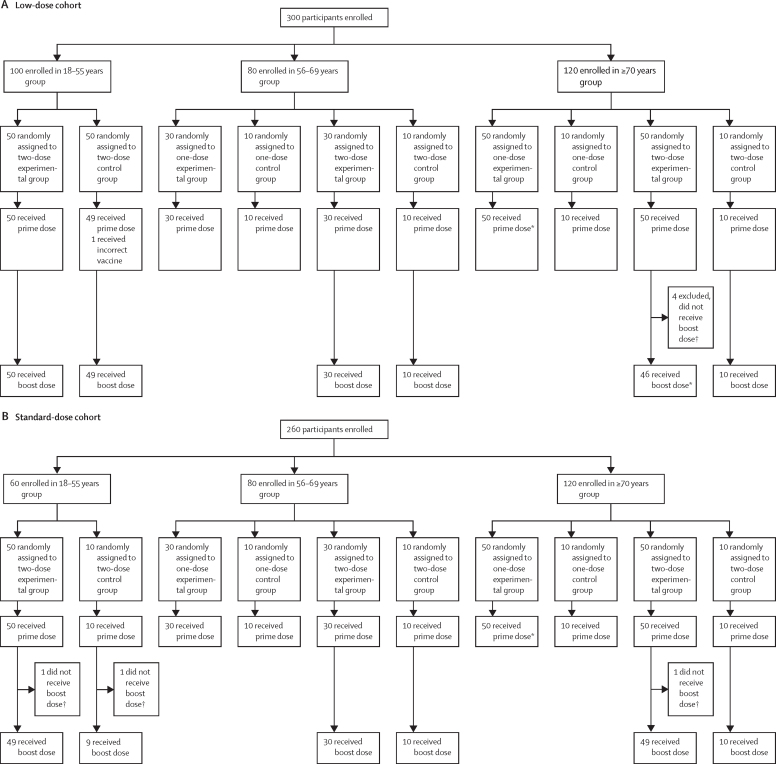
Between May 30 and Aug 8, 2020, 560 participants were enrolled in the study and randomly assigned to the experimental vaccine or control vaccine group: 160 participants aged 18–55 years (100 assigned to ChAdOx1 nCoV-19, 60 assigned to MenACWY), 160 aged 56–69 years (120 assigned to ChAdOx1 nCoV-19, 40 assigned to MenACWY), and 240 aged 70 years and older (200 assigned to ChAdOx1 nCoV-19, 40 assigned to MenACWY). Full details on randomisation are in figure 1. All participants randomly assigned to treatment were vaccinated. One participant (in the 18–55 years low-dose group) received the incorrect vaccine after randomisation and was excluded from analysis. Seven participants randomly assigned to receive two doses of vaccine chose not to continue with the boost dose and were excluded from further analyses. Three participants were excluded from immunology analyses due to incorrectly labelled samples (either incorrect participant identification numbers or incorrect timepoints noted on the label, or both; figure 1). The baseline characteristics of the participants eligible for inclusion in the analysis in each group are shown in the table. Participants 70 years and older were recruited from the NIHR Southampton Clinical Research Facility, University Hospital Southampton NHS Foundation Trust. All other participants were recruited at the Oxford Vaccine Centre, Centre for Clinical Vaccinology and Tropical Medicine, University of Oxford. Among the analysed population, 280 (50%) of 552 participants were female. 524 (95%) of 552 participants identified as white, and 540 (98%) were non-smokers. A large proportion of health-care workers who were predominantly female were enrolled in the 18–55 years and 56–69 years age groups. The median age in the 18–55 years group was 43·0 years (IQR 33·6–48·0), in the 56–69 years group was 60·0 years (57·5–63·0) and in the 70 years and older group was 73·0 years (71·0–76·0). The median age in the 70 years and older groups ranged from 73 years to 74 years across dosing groups, with the oldest participants aged 83 years.
Figure 1.
Study profile for the low-dose (A) and standard-dose (B) cohorts
*One participant excluded from immunogenicity analyses, due to mislabelling of laboratory sample. †Reasons for not receiving boost dose included that the participant moved away or was unavailable for visits, delay in receiving boost dose, or withdrawal of consent.
Table.
Baseline characteristics of prime-boost participants included in the analysis
|
Age 18–55 years |
Age 56–69 years |
Age ≥70 years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ChAdOx1 nCoV-19, two doses | MenACWY, two doses | ChAdOx1 nCoV-19, one dose | MenACWY, one dose | ChAdOx1 nCoV-19, two doses | MenACWY, two doses | ChAdOx1 nCoV-19, one dose | MenACWY, one dose | ChAdOx1 nCoV-19, two doses | MenACWY, two doses | ||
| Low dose | |||||||||||
| Number enrolled | 50 | 49 | 30 | 10 | 30 | 10 | 50 | 10 | 46 | 10 | |
| Sex | |||||||||||
| Female | 35 (70%) | 28 (57%) | 19 (63%) | 4 (40%) | 10 (33%) | 8 (80%) | 24 (48%) | 6 (60%) | 16 (35%) | 6 (60%) | |
| Male | 15 (30%) | 21 (43%) | 11 (37%) | 6 (60%) | 20 (67%) | 2 (20%) | 26 (52%) | 4 (40%) | 30 (65%) | 4 (40%) | |
| Age, years, median (IQR, range) | 44·5 (39·0–51·0, 22·0–54·0) | 42·0 (32·0–48·0, 23·0–55·0) | 60·0 (58·9–62·3, 56·0–69·0) | 57·8 (56·3–60·8, 56·0–68·0) | 60·4 (57·8–66·0, 56·0–69·4) | 60·5 (58·3–63·9, 56·7–69·0) | 73·5 (71·0–76·0, 69·0–83·0) | 73·0 (70·0–74·0, 70·0–81·0) | 73·0 (71·0–75·0, 70·0–82·0) | 73·0 (71·2–74·0, 70·0–76·0) | |
| BMI, kg/m2, median (IQR, range) | 24·6 (22·9–28·9, 19·4–45·1) | 24·8 (21·6–27·7, 18·0–37·2) | 25·0 (23·2–27·3, 20·2–37·6) | 25·5 (22·5–27·3, 20·9–34·4) | 25·9 (24·0–28·8, 21·3–36·6) | 24·0 (23·2–26·0, 22·2–33·2) | 26·0 (23·8–28·0, 20·0–36·0) | 24·9 (22·3–26·9, 19·3–32·5) | 26·0 (23·4–27·7, 19·4–42·1) | 26·8 (24·3–29·5, 19·2–35·3) | |
| Smoker | 3 (6%) | 1 (2%) | 0 | 1 (10%) | 2 (7%) | 0 | 1 (2%) | 0 | 1 (2%) | 0 | |
| Alcohol drinker | 44 (88%) | 42 (86%) | 28 (93%) | 9 (90%) | 26 (87%) | 8 (80%) | 43 (86%) | 10 (100%) | 43 (94%) | 9 (90%) | |
| Health-care worker | 35 (70%) | 26 (53%) | 17 (57%) | 7 (70%) | 12 (40%) | 4 (40%) | 0 | 0 | 0 | 1 (10%) | |
| Race or ethnicity | |||||||||||
| White | 48 (96%) | 45 (92%) | 30 (100%) | 9 (90%) | 27 (90%) | 10 (100%) | 50 (100%) | 10 (100%) | 45 (98%) | 10 (100%) | |
| Black or Black British | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Asian or Asian British | 2 (4%) | 1 (2%) | 0 | 0 | 2 (7%) | 0 | 0 | 0 | 0 | 0 | |
| Mixed race or ethnicity | 0 | 3 (6%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2%) | 0 | |
| Other race or ethnicity* | 0 | 0 | 0 | 1 (10%) | 1 (3%) | 0 | 0 | 0 | 0 | 0 | |
| Comorbidities | |||||||||||
| Cardiovascular disease | 4 (8%) | 10 (20%) | 5 (17%) | 0 | 11 (37%) | 0 | 14 (28%) | 3 (30%) | 16 (35%) | 2 (20%) | |
| Respiratory disease | 12 (24%) | 9 (18%) | 7 (23%) | 0 | 7 (23%) | 0 | 6 (12%) | 2 (20%) | 6 (13%) | 1 (10%) | |
| Diabetes | 0 | 0 | 0 | 0 | 0 | 1 (10%) | 1 (2%) | 0 | 2 (4%) | 0 | |
| Standard dose | |||||||||||
| Number enrolled | 49 | 9 | 30 | 10 | 30 | 10 | 50 | 10 | 49 | 10 | |
| Sex | |||||||||||
| Female | 23 (47%) | 7 (78%) | 16 (53%) | 3 (30%) | 16 (53%) | 5 (50%) | 25 (50%) | 1 (10%) | 21 (43%) | 2 (20%) | |
| Male | 26 (53%) | 2 (22%) | 14 (47%) | 7 (70%) | 14 (47%) | 5 (50%) | 25 (50%) | 9 (90%) | 28 (57%) | 8 (80%) | |
| Age, years, median (IQR, range) | 39·0 (30·0–45·0, 19·0–55·0) | 43·0 (35·8–50·0, 32·0–54·0) | 59·0 (58·0–61·0, 56·0–69·0) | 61·5 (57·5–63·8, 57·0–66·0) | 59·5 (57·0–61·0, 56·0–67·0) | 60·5 (57·9–61·0, 56·0–64·0) | 74·0 (72·0–76·0, 70·0–80·0) | 74·0 (71·0–75·5, 70·0–78·0) | 73·0 (71·0–75·0, 70·0–83·0) | 73·5 (72·2–74·8, 71·0–81·0) | |
| BMI, kg/m2, median (IQR, range) | 26·9 (24·6–30·9, 20·2–39·7) | 24·1 (23·8–25·6, 18·6–39·0) | 26·7 (25·2–30·0, 18·6–36·8) | 28·9 (25·6–30·2, 21·7–31·9) | 24·0 (22·4–27·1, 19·9–33·5) | 26·1 (23·6–27·7, 20·5–30·2) | 25·1 (23·7–28·5, 17·5–32·6) | 26·8 (25·8–28·5, 23·0–31·7) | 27·1 (24·2–29·2, 20·3–40·2) | 25·6 (24·1–29·3, 18·9–32·5) | |
| Smoker | 1 (2%) | 0 | 0 | 0 | 0 | 1 (10%) | 1 (2%) | 0 | 0 | 0 | |
| Alcohol drinker | 45 (92%) | 6 (67%) | 29 (97%) | 10 (100%) | 29 (97%) | 10 (100%) | 39 (78%) | 9 (90%) | 42 (86%) | 9 (90·0%) | |
| Health-care worker | 13 (27%) | 5 (56%) | 10 (33%) | 2 (20%) | 12 (40%) | 5 (50%) | 2 (4%) | 0 | 0 | 0 | |
| Race or ethnicity | |||||||||||
| White | 40 (82%) | 7 (78%) | 29 (97%) | 10 (100%) | 26 (87%) | 9 (90%) | 50 (100%) | 10 (100%) | 49 (100%) | 10 (100%) | |
| Black or Black British | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Asian or Asian British | 7 (14%) | 2 (22%) | 0 | 0 | 4 (13%) | 1 (10%) | 0 | 0 | 0 | 0 | |
| Mixed race or ethnicity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other race or ethnicity* | 1 (2%) | 0 | 1 (3%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Comorbidities | |||||||||||
| Cardiovascular disease | 6 (12%) | 0 | 4 (13%) | 3 (30%) | 4 (13%) | 1 (10%) | 20 (40%) | 3 (30%) | 13 (27%) | 4 (40%) | |
| Respiratory disease | 10 (20%) | 1 (11%) | 4 (13%) | 1 (10%) | 3 (10%) | 3 (30%) | 3 (6%) | 0 | 4 (8%) | 0 | |
| Diabetes | 2 (4%) | 0 | 2 (7%) | 2 (20%) | 0 | 0 | 0 | 1 (10%) | 3 (6%) | 1 (10%) | |
Data are n (%) unless otherwise specified. BMI=body-mass index.
Included Hispanic-Columbian, Indian, Japanese, and White Irish/English.
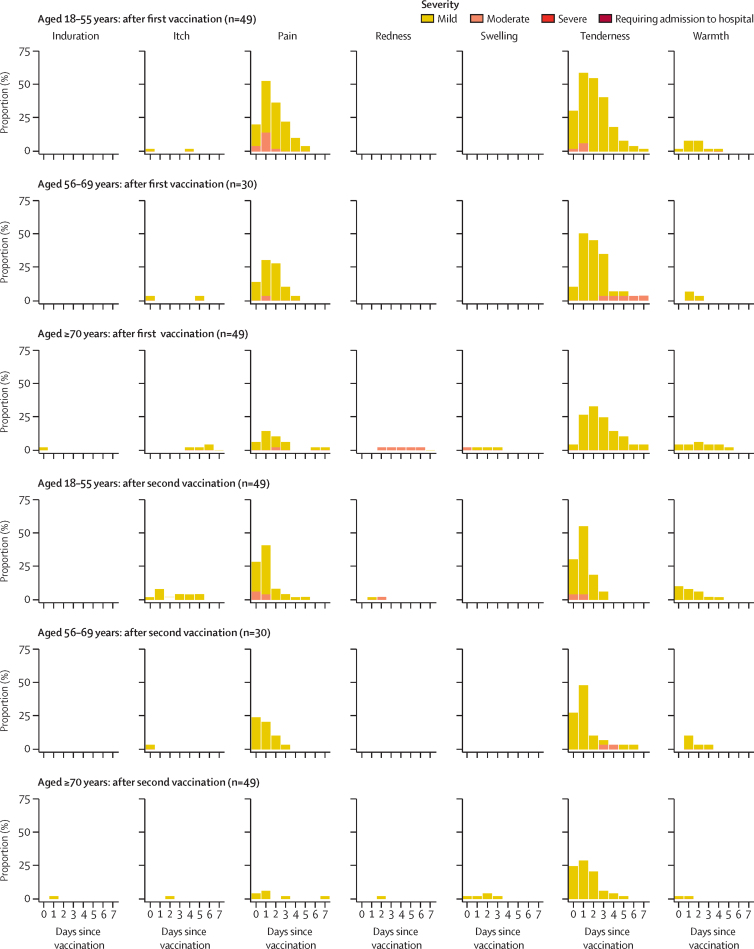
The following results for local and systemic adverse reactions are all for participants who were randomly assigned to receive two doses of vaccine. Injection-site pain and tenderness were the most common solicited local adverse reactions and occurred most frequently in the first 48 h after vaccination (data for standard-dose regimen shown in figure 2; data for the low-dose groups and control groups are shown in the appendix [pp 7, 9, 19–21]). In those aged 56 years or older, a standard dose of ChAdOx1 nCoV-19, whether the prime or boost vaccination, elicited a greater number of local or systemic reactions than did MenACWY. The difference was less clear with the low-dose vaccine in the 56–69 years and 70 years and older groups, and the number of participants in the control groups was small (appendix p 30). At least one local symptom was reported after the prime vaccination with standard-dose ChAdOx1 nCoV-19 by 43 (88%) of 49 participants in the 18–55 years group, 22 (73%) of 30 in the 56–69 years group, and 30 (61%) of 49 in the 70 years and older group (appendix p 29). Similar proportions of local symptoms were reported after the boost vaccination with the standard dose of ChAdOx1 nCoV-19, with 37 (76%) of 49 participants in the 18–55 years group, 21 (72%) of 29 in the 56–69 years group, and 27 (55%) of 49 in the 70 years and older group reporting at least one local symptom. A similar pattern was seen across the age groups in participants after their prime vaccination with low-dose ChAdOx1 nCoV-19 and after the boost vaccination with the low-dose vaccine, but with fewer total adverse reactions than in the standard-dose groups (appendix pp 7, 9, 19–21). No severe local symptoms were reported by recipients of ChAdOx1 nCoV-19. In the two-dose control groups, across both the low-dose and standard-dose cohorts, local symptoms were reported by 33 (57%) of 58 participants in the 18–55 years group, five (25%) of 20 in the 56–69 years group, and seven (35%) of 20 in the 70 years and older group after the prime vaccination with MenACWY, and by 50 (86%) of 58 in the 18–55 years group, seven (37%) of 19 in the 56–69 years group, and four (20%) of 20 in the 70 years and older group after the boost vaccination with MenACWY (appendix p 29). Data for participants randomly assigned to receive only one dose of vaccine were similar to the data after a prime dose of vaccine in the two-dose groups (data not shown).
Figure 2.
Solicited local adverse reactions in the 7 days after prime and boost doses of standard-dose vaccine, by age
Day 0 is the day of vaccination. Participants shown are those randomly assigned to receive two doses, and data are only shown for participants who received both doses of vaccine.
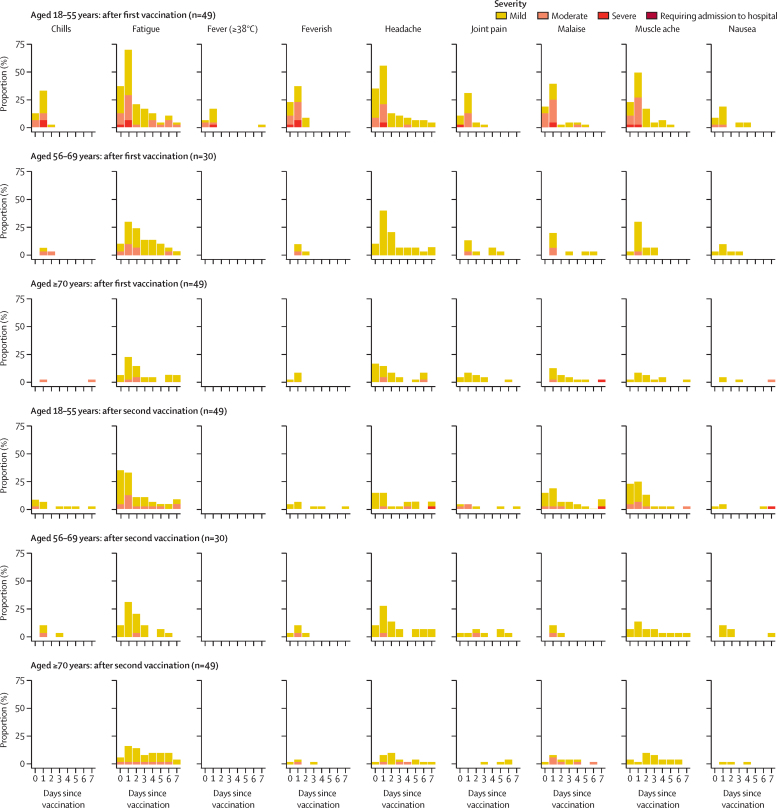
Fatigue, headache, feverishness, and myalgia were the most commonly solicited systemic adverse reactions (data for the standard-dose groups are shown in figure 3; data for the low-dose groups and control groups are shown in the appendix [pp 8, 10, 19–21]). At least one systemic symptom was reported after the prime vaccination with the standard dose of ChAdOx1 nCoV-19 by 42 (86%) of 49 participants in the 18–55 years group, 23 (77%) of 30 in the 56–69 years group, and 32 (65%) of 49 in the 70 years and older group (appendix p 29). The severity of symptoms reported in the standard-dose groups was reduced after the boost vaccination, with only one (1%) of 127 participants reporting a severe reaction compared with seven (5%) of 128 participants after the prime vaccination. At least one systemic adverse reaction after the boost vaccination of standard dose of ChAdOx1 nCoV-19 was reported by 32 (65%) of 49 participants in the 18–55 years group, 21 (72%) of 29 in the 56–69 years group, and 21 (43%) of 49 in the 70 years and older group (appendix p 29). Within 7 days after the prime vaccination with ChAdOx1 nCoV-19, the incidence of objectively measured fever was low in the 18–55 years standard-dose group (12 [24%] of 49), and no fevers were recorded in either the 56–69 years or 70 years and older standard-dose groups (appendix pp 16–18). No participants of any age who received the standard dose of ChAdOx1 nCoV-19 had objective fever after the boost vaccination. A similar pattern of decreasing reactogenicity with increasing age was seen in the low-dose groups (appendix pp 7, 8, 19–21). Similar results after the first dose were seen in those who were randomly assigned to receive only one dose of vaccine (data not shown). Data for the control groups are in the appendix (p 10)).
Figure 3.
Solicited systemic adverse reactions in the 7 days after prime and boost doses of standard-dose vaccine, by age
Day 0 is the day of vaccination. Feverish is self-reported feeling of feverishness, whereas fever is an objective fever measurement (mild: 38·0 to <38·5°C, moderate: 38·5 to <39·0°C, severe: ≥39·0°C). Participants shown are those randomly assigned to receive two doses, and data are only shown for participants who received both doses of vaccine.
As of Oct 26, 2020, 13 serious adverse events have occurred (across all age and vaccine groups), none of which are considered related to either study vaccine as assessed by the investigators (appendix p 31).
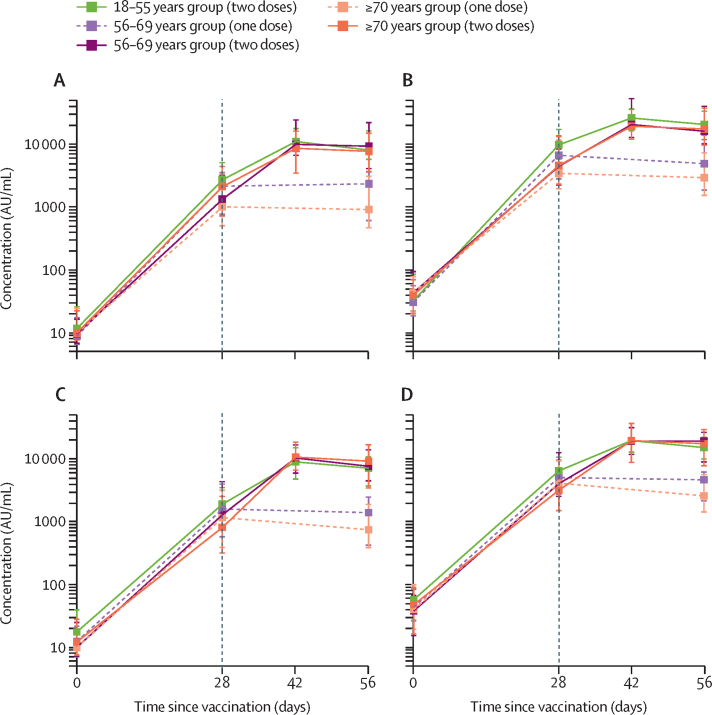
Using a multiplex immunoassay that detected total IgG against RBD and trimeric spike protein, we observed that participants who received the prime vaccination of standard-dose ChAdOx1 nCoV-19 had similar anti-spike antibody titres by day 28 after their prime vaccination as those who received a low dose (p=0·12 adjusted for age; figure 4; appendix p 12). At both dose levels, and for all dose groups combined, anti-spike IgG responses at day 28 decreased with increasing age (low-dose groups: 18–55 years, median 6439 arbitrary units [AU]/mL [IQR 4338–10 640], n=49; 56–69 years, 4553 AU/mL [2657–12 462], n=60; ≥70 years, 3565 AU/mL [1507–6345], n=93; p=0·0037; standard-dose groups: 18–55 years, median 9807 AU/mL [IQR 5847–17 220], n=43; 56–69 years, 5496 AU/mL [2548–12 061], n=55; ≥70 years, 4156 [2122–12 595], n=97; p=0·0044). By 28 days after the boost vaccination, similar antibody titres were seen across all two-dose groups, regardless of age or vaccine dose (eg, standard-dose groups: 18–55 years, median 20 713 AU/mL [IQR 13 898–33 550], n=39; 56–69 years, 16 170 AU/mL [10 233–40 353], n=26; and ≥70 years, 17 561 AU/mL [9705–37 796], n=47; p=0·68), and were higher than for those who did not receive a boost vaccination (appendix p 13). Similar results were seen with anti-RBD antibodies (figure 4; appendix p 12) and with an in-house standardised ELISA (appendix pp 12–13). Data for the control group are in the appendix (pp 12–13).
Figure 4.
SARS-CoV-2 IgG response to the receptor binding domain in the standard-dose groups (A) and low-dose groups (C) and the spike protein in the standard-dose groups (B) and the low-dose groups (D), by age
Datapoints are medians, with whiskers showing the IQRs. Solid lines show participants who were randomly assigned to and received two doses of vaccine and dashed lines indicate participants who were randomly assigned to receive one dose. The vertical black line indicates when participants who received two doses received their boost dose. Data for the control groups are shown in the appendix (p 12)). AU=arbitrary units. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
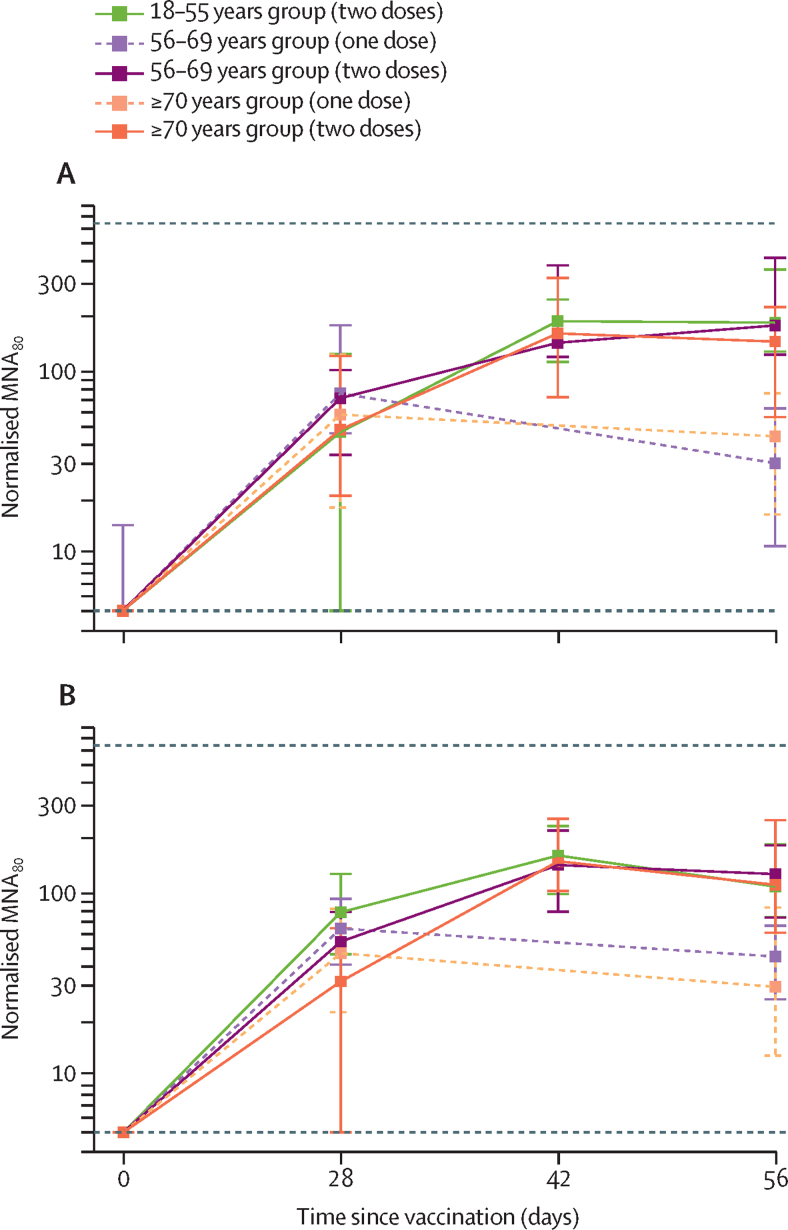
In a live SARS-CoV-2 microneutralisation assay (MNA80), median titres peaked by day 42 in most groups that received two vaccinations (figure 5). There were no significant differences in normalised titres between age groups at day 42 (low-dose groups: 18–55 years, median 161 [IQR 99–233], n=41; 56–69 years, 143 [79–220], n=28; ≥70 years, 150 [103–255], n=34; p=0·90; standard-dose groups: 18–55 years, median 193 [IQR 113–238], n=39; 56–69 years, 144 [119–347], n=20; and ≥70 years, 161 [73–323], n=47; p=0·40). Within each age group, no significant differences were seen in neutralisation titres between low-dose and standard-dose vaccine recipients at the same timepoint (18–55 years p=0·33, 56–69 years p=0·12, ≥70 years p=0·62; figure 5; appendix p 14). Neutralising titres were achieved by 14 days after the boost vaccination in 208 (>99%) of 209 recipients of a boost vaccination. The one participant with a non-neutralising level was in the 70 years and older two-dose low-dose group.
Figure 5.
Neutralising antibody titres measured using a live SARS-CoV-2 microneutralisation assay (MNA80) after prime and boost doses of vaccine in standard-dose groups (A) and low-dose groups (B), by age
Datapoints are medians, with whiskers showing the IQR. Solid lines show participants who were randomly assigned to and received two doses of vaccine and dashed lines indicate participants who were randomly assigned to receive one dose. Horizontal dotted lines show upper and lower limits of assay (values outside this range set to 640 beyond the upper limit and 5 beyond the lower limit). Data for the control groups are shown in the appendix (p 14)). To normalise data across assay runs, a reference sample was included in all assay runs and test samples normalised to this value by generating log10 ratios. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Anti-spike IgG levels after vaccination across all timepoints in those who received two doses of vaccine were highly correlated with neutralising titres in all age groups and for both low-dose and standard-dose vaccines (r2 from linear regression 0·42–0·75, all p<0·0001; appendix p 32).
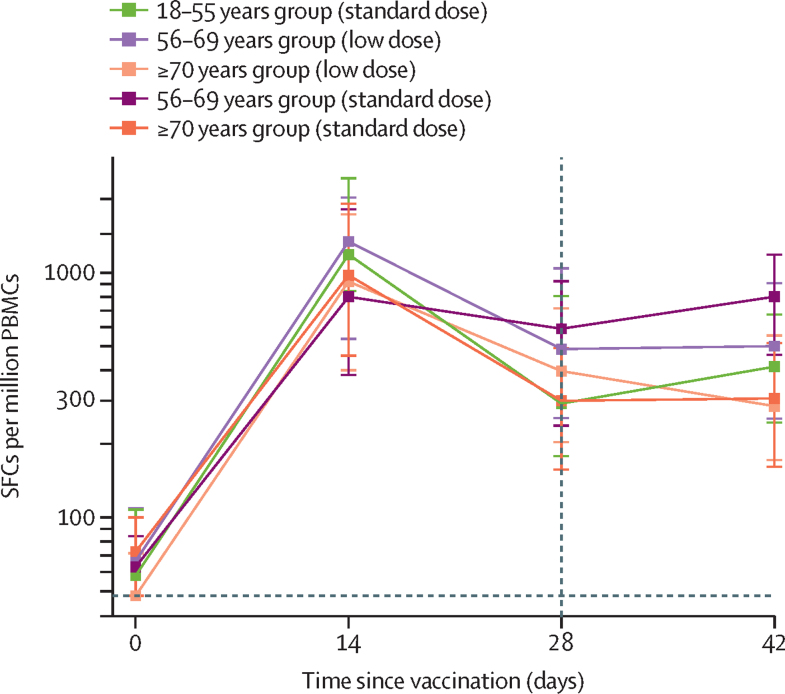
IFN-γ ELISpot responses against SARS-CoV-2 spike protein peaked 14 days after the prime vaccination (standard-dose groups: 18–55 years, median 1187 spot-forming cells [SFCs] per million peripheral blood mononuclear cells [PBMCs; IQR 841–2428], n=24; 56–69 years, 797 SFCs [383–1817], n=29; and ≥70 years, 977 SFCs [458–1914], n=48; appendix p 16) and did not increase significantly after the boost vaccination (p=0·46 from paired Student's t test of day 28 vs day 42; figure 6). ELISpot data were unavailable for the 18–55 years low-dose group because PBMCs were not collected in this group. In those who received two standard doses of vaccine, a significant difference was seen across age groups with those aged 56–69 years having higher responses at day 42 than other age groups receiving the same vaccine regimen (18–55 years, median 413 SFCs per million PBMCs [IQR 245–675], n=23; 56–69 years, 798 SFCs [462–1186], n=28; and ≥70 years, 307 SFCs [161–516], n=47; p<0·0001; appendix p 15).
Figure 6.
IFN-γ ELISpot response to peptides spanning the SARS-CoV-2 spike insert after prime and boost doses of vaccine for all participants who were given two doses of vaccine, by age group and vaccine dose
ELISpot data were unavailable for the 18–55 years low-dose group because PBMCs were not collected in this group. Datapoints are medians, with whiskers showing the IQR. The lower limit of detection is 48 SFCs per million PBMCs (horizontal dotted line). Day 42 samples are from participants who received the boost dose at day 28 (vertical dotted line). Data for both one-dose and two-dose groups, with numbers analysed at each timepoint, are in the appendix (p 15)). ELISpot=enzyme-linked immunospot. PBMC=peripheral blood mononuclear cells. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. SFC=spot-forming cells.
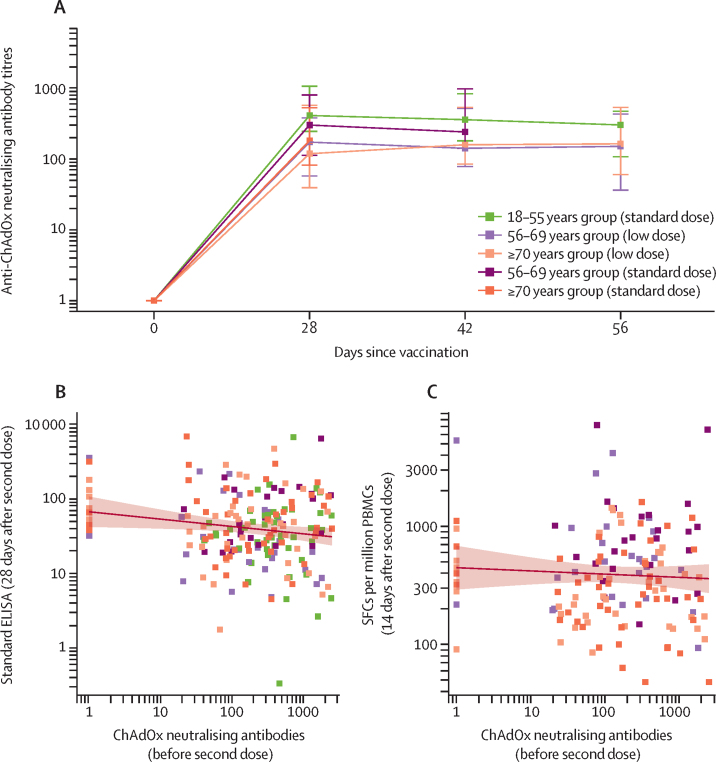
Anti-ChAdOx1 neutralising antibody titres across different age and dose groups are shown in figure 7. Titres increased with the prime vaccination with ChAdOx1 nCoV-19 in all groups to similar levels, but were not increased further after a boost dose of vaccine at day 28. This observation was in contrast with the anti-SARS-CoV-2 spike protein antibody levels, which were increased 28 days after the boost vaccination (figure 4). Anti-ChAdOx1 neutralising titres immediately before the boost vaccination were negatively correlated with standardised ELISA values 28 days after the boost vaccination (p=0·037; figure 7), but no significant correlation was seen between anti-ChAdOx1 neutralising titres immediately before the boost vaccination and ELISpot responses 14 days after the boost vaccination (p=0·22; figure 7).
Figure 7.
Anti-ChAdOx1 vector neutralising titres after prime and boost doses of vaccine, by age and vaccine dose, and the correlation between pre-boost dose anti-ChAdOx1 neutralising antibodies and 28 days after boost dose antibody and T-cell responses
(A) Anti-ChAdOx1 neutralising antibody titres in participants who received ChAdOx1 nCoV-19 vaccine by age and dose: datapoints are medians, with whiskers showing the IQR. Values below the limit of detection were assigned a value of 1. (B) Anti-ChAdOx1 neutralising antibody titre immediately before boost dose of vaccine versus standardised IgG ELISA against SARS-CoV-2 spike 28 days after the boost dose of vaccine with linear regression of logged values (p=0·037). (C) Anti-ChAdOx1 neutralising antibody titres immediately before boost dose of vaccine versus SARS-CoV-2 spike specific T cells measured by IFN-γ ELISpot on day 14 after the boost dose of vaccine with linear regression of logged values (p=0·22). In B and C, each datapoint is one participant and the solid line shows the linear regression, with the shaded area showing the 95% CI from an unadjusted linear regression of anti-vector neutralisation titres against logged ELISA (in B) or ELISpot (in C) response. Data were unavailable at day 56 for the 56–69 years standard-dose group. ELISpot=enzyme-linked immunospot. PBMC=peripheral blood mononuclear cells. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. SFC=spot-forming cells.
Discussion
Our findings show that the ChAdOx1 nCoV-19 vaccine was safe and well tolerated with a lower reactogenicity profile in older adults than in younger adults. Immunogenicity was similar across age groups after a boost vaccination. If these responses correlate with protection in humans, these findings are encouraging because older individuals are at disproportionate risk of severe COVID-19 and so any vaccine adopted for use against SARS-CoV-2 must be effective in older adults.
Most of the reported local and systemic adverse events were mild to moderate in severity, in line with our previous phase 1 study of the ChAdOx1 nCoV-19 vaccine18 and previously reported studies of ChAdOx1-vectored vaccines.22, 23, 24 Fewer adverse events were reported after the boost vaccination than after the prime vaccination and reactogenicity reduced with increasing age. The lower dose of vaccine was less reactogenic than the standard dose of vaccine across all age groups.
The serious adverse events observed during the trial in these study groups were judged to be unrelated to the study vaccines and occurred at frequencies expected for these conditions in the general population. None of the participants included in this report had any suspected unexpected serious adverse reactions. In the phase 3 component of the trial, suspected unexpected serious adverse reactions occurred in other groups, and will be reported in detail in a subsequent publication. We carefully monitored suspected unexpected serious adverse reactions and other adverse events to ensure that no pattern of unexplained illnesses emerged that could indicate a safety concern. Independent assessments have led to the recommendation that the trial is safe to continue.
The ChAdOx1 nCoV-19 vaccine induced a specific antibody response to the SARS-CoV-2 spike glycoprotein and RBD at 28 days after a single dose across all age groups, including adults aged 70 years and older. A clear effect of a boost vaccination on antibody titres at day 56 was seen that was unrelated to dose regimen or age group. Similar patterns were observed with neutralising antibody responses, with no difference in the magnitude of the response at day 28 after the prime vaccine regardless of age or vaccine dose, but a booster effect was observed in individuals who received a second dose of vaccine.
Other clinical trials have also assessed safety, tolerability, and immunogenicity of SARS-CoV-2 vaccines in older adults. An adenovirus 5 vector-based vaccine also had reduced reactogenicity in adults aged 55 years and older compared with adults aged 18–54 years after a single dose of vaccine, although immunogenicity was concurrently reduced in this older age group.11 A two-dose mRNA vaccine has also been shown to be immunogenic in adults older than 56 years with dose-dependent immune responses and similar neutralising antibody titres and cellular immune responses to younger adults.9 Another two-dose mRNA vaccine has shown immunogenicity in older adults, but absolute neutralising antibody responses in adults aged 65–85 years were lower than in those aged 18–55 years.10 By contrast with our observations, in both these studies, reactogenicity was more common after the second dose of an mRNA vaccine. A two-dose inactivated virus vaccine has also shown lower absolute neutralising antibody titres in adults aged 60 years and older than in adults aged 18–59 years, but reactogenicity was not formally compared between the first and second doses in this study.13
T-cell responses are important in controlling disease in natural infection8 and therefore generation of a robust cellular immune response is a desirable attribute for a vaccine against SARS-CoV-2. Here, we found that spike-specific T-cell responses measured with ELISpot peaked at 14 days after the prime vaccination, consistent with previous studies of simian adenovirus-vectored vaccines,25 and were similar in all groups regardless of age and vaccine dose. Spike protein T-cell responses measured with ELISpot have also been reported in studies with other adenovirus-vectored vaccines against SARS-CoV-2,12 including in adults older than 55 years.11 Theoretical concerns about vaccine-enhanced disease have led to a view that a type 1 T-helper (Th1)-biased CD4 response is a preferred coronavirus vaccine characteristic.26 An adjuvanted nanoparticle vaccine has been shown to induce spike-specific CD4 T-cell cytokine responses with a predominantly Th1 profile,15 as has an mRNA vaccine in small numbers of adults aged 56–70 years and 71 years and older.9 More detailed investigations of antigen-specific T-cell responses in our study participants are ongoing.
The robust humoral and cellular immune responses obtained in our older adult population were encouraging given that a number of studies have shown that decreasing immune function with age leads to decreased immune responses to vaccines. This fact holds true for vaccines such as for influenza, for which pre-existing immune memory exists,27 and vaccines that induce primary immune responses, such as hepatitis B.28 Other adenovirus-vector platforms against SARS-CoV-2 have either shown reduced immunogenicity in an older age group11 (although this study was of a single-dose regimen and so not directly comparable with our prime-boost regimen) or have not yet been tested in an older population.12
However, our results are consistent with previous studies of adenovirus-vector-based vaccines against respiratory pathogens that evoke humoral and T-cell responses in older adults, including a human adenovirus-vectored respiratory syncytial virus (RSV) vaccine29 and a simian adenovirus-vectored RSV vaccine.30 Our results with ChAdOx1 nCoV-19 are also consistent with those of a ChAdOx1-vectored vaccine against influenza that showed good immunogenicity in adults older than 50 years.22
Notably, the anti-spike antibody responses in our study increased after a boost vaccination at an interval of 1 month but the neutralising anti-vector antibody responses did not. There was also no difference in anti-vector immunity by age. We observed a small negative correlation between anti-vector antibody titres and anti-spike total IgG, but not T-cell ELISpot responses. Further work is needed to investigate if homologous boosting with adenovirus-vectored vaccines can be done without loss of immunogenicity to the pathogen-specific transgene.
In the absence of a clear serological correlate of protection against SARS-CoV-2, clinical studies have focused on measuring neutralising antibodies because these have been shown to confer protection from challenge in animal models.9, 10, 11, 12, 13, 14, 15 Live virus neutralisation assays are labour intensive and can only be done in specialist laboratories under category 3 biological safety conditions. We found here that anti-spike IgG levels correlate with neutralising antibody titres for all age groups. This finding suggests that, should neutralising antibodies be shown to be protective in humans, routine serological assays could be used for the standardised evaluation of functional antibody by vaccine candidates in clinical trials.
A limitation of this study is its single-blind design. However, all laboratory analyses and clinical assessments reported in this manuscript were done in a blinded fashion. A further limitation is possible variation of severity of local reactions due to the difference in injection volumes between different batches of vaccine in the low-dose group. Ongoing studies in larger groups will investigate the reactogenicity of a booster dose in more detail. Finally, the selection of participants aged 70 years and older, with a median age of 73–74 years between dose groups and with few comorbidities, might not be representative of the general older population, including those living in residential care settings or older than 80 years. Early phase studies in older adults require healthy volunteers to be enrolled for safety assessments, and recruitment to the study occurred during a period of national lockdown when more susceptible individuals were advised by Public Health England to self-isolate. Therefore, we excluded volunteers with substantial comorbidities or clinical frailty. Larger studies are now underway to assess immunogenicity, safety, and efficacy in older adults with a wider range of comorbidities.
Ultimately, licensure of a vaccine relies on the demonstration of efficacy in preventing COVID-19 and safety. Phase 3 studies with ChAdOx1 nCoV-19 are ongoing in the UK, Brazil, and the USA to assess vaccine efficacy and safety. Here we found similar safety and immunogenicity of ChAdOx1 nCoV-19 in older adults compared with younger adults, which could support the use of this vaccine in this older age group, if it is shown to be protective in phase 3 trials.
This online publication has been corrected. The corrected version first appeared at thelancet.com on December 17, 2020
Data sharing
The study protocol and clinical study plan are provided in the appendix (pp 45–212). Anonymised participant data will be made available when the trial is complete, upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement. All data will be made available for a minimum of 5 years from the end of the trial.
Acknowledgments
Acknowledgments
This Article reports independent research funded by UK Research and Innovation (MC_PC_19055), Engineering and Physical Sciences Research Council (EP/R013756/1), Coalition for Epidemic Preparedness Innovations, and the National Institute for Health Research (NIHR). We acknowledge support from Thames Valley and South Midland's NIHR Clinical Research Network and the staff and resources of NIHR Southampton Clinical Research Facility, and the NIHR Oxford Health Biomedical Research Centre. PMF received funding from the Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior, Brazil (finance code 001). ALF was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Science, China (grant number: 2018-I2M-2-002). KJE is an NIHR Biomedical Research Centre Senior Research Fellow. AJP and SNF are NIHR senior investigators. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care. We thank the senior management at AstraZeneca for facilitating and funding the Mesoscale antibody assay included in this manuscript. We also thank the volunteers who participated in this study.
Contributors
AJP and SCG conceived and designed the trial and AJP is the chief investigator. AJP, AMM, HR, MNR, MV, and PMF contributed to the protocol and design of the study. AVSH and SNF were the study site principal investigators. ALF, CD, EAC, KJE, RM, and TL were responsible for laboratory testing and assay development. MV and NGM did the statistical analysis. SCG and TL were responsible for vaccine development. ADD, CG, and RT were responsible for vaccine manufacture. AJP, AMM, MNR, MV, NGM, and TL contributed to the preparation of the report. AMM, DRO, HR, KJE, MNR, PKA, and PMF contributed to the implementation of the study. All other authors contributed to the implementation of the study and data collection. All authors critically reviewed and approved the final version.
Declaration of interests
Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19 (AZD1222). AstraZeneca reviewed the data from the study and the final manuscript before submission, but the authors retained editorial control. SCG is cofounder of Vaccitech (a collaborator in the early development of this vaccine candidate) and named as an inventor on a patent covering use of ChAdOx1-vectored vaccines (PCT/GB2012/000467) and a patent application covering this SARS-CoV-2 vaccine. TL is named as an inventor on a patent application covering this SARS-CoV-2 vaccine and was consultant to Vaccitech. PMF is a consultant to Vaccitech. AJP is Chair of the UK Department of Health and Social Care's JCVI, but does not participate in policy advice on coronavirus vaccines, and is a member of the WHO Strategic Advisory Group of Experts (SAGE). AVSH is a cofounder of and consultant to Vaccitech and is named as an inventor on a patent covering design and use of ChAdOx1-vectored vaccines (PCT/GB2012/000467). MDS reports grants from Janssen, GlaxoSmithKline, MedImmune, Novavax, and MCM Vaccine and grants and non-financial support from Pfizer outside of the submitted work. CG reports personal fees from the Duke Human Vaccine Institute outside of the submitted work. ADD reports grants and personal fees from AstraZeneca outside of the submitted work. All other authors declare no competing interests.
Contributor Information
Maheshi N Ramasamy, Email: maheshi.ramasamy@paediatrics.ox.ac.uk.
Oxford COVID Vaccine Trial Group:
Jeremy Aboagye, Kelly Adams, Aabidah Ali, Elizabeth R. Allen, Lauren Allen, Jennifer L. Allison, Foteini Andritsou, Rachel Anslow, Edward H. Arbe-Barnes, Megan Baker, Natalie Baker, Philip Baker, Ioana Baleanu, Debbie Barker, Eleanor Barnes, Jordan R. Barrett, Kelly Barrett, Louise Bates, Alexander Batten, Kirsten Beadon, Rebecca Beckley, Duncan Bellamy, Adam Berg, Laura Bermejo, Eleanor Berrie, Amy Beveridge, Kevin Bewley, Else M. Bijker, Geeta Birch, Luke Blackwell, Heather Bletchly, Caitlin L. Blundell, Susannah R. Blundell, Emma Bolam, Elena Boland, Daan Bormans, Nicola Borthwick, Konstantinos Boukas, Thomas Bower, Francesca Bowring, Amy Boyd, Tanja Brenner, Phillip Brown, Charlie Brown-O'Sullivan, Scott Bruce, Emily Brunt, Jamie Burbage, Joshua Burgoyne, Karen R. Buttigieg, Nicholas Byard, Ingrid Cabera Puig, Susana Camara, Michelangelo Cao, Federica Cappuccini, Melanie Carr, Miles W. Carroll, Paul Cashen, Ana Cavey, Jim Chadwick, Ruth Challis, David Chapman, David Charles, Irina Chelysheva, Jee-Sun Cho, Liliana Cifuentes, Elizabeth Clark, Sarah Collins, Christopher P. Conlon, Naomi S. Coombes, Rachel Cooper, Cushla Cooper, Wendy E.M. Crocker, Sarah Crosbie, Dan Cullen, Christina Cunningham, Fiona Cuthbertson, Brad E. Datoo, Lynne Dando, Mehreen S. Datoo, Chandrabali Datta, Hannah Davies, Sarah Davies, Elizabeth J. Davis, Judith Davis, David Dearlove, Tesfaye Demissie, Stefania Di Marco, Claudio Di Maso, Danielle DiTirro, Claire Docksey, Tao Dong, Francesca R. Donnellan, Naomi Douglas, Charlotte Downing, Jonathan Drake, Rachael Drake-Brockman, Ruth E. Drury, Susanna J. Dunachie, Christopher J. Edwards, Nick J. Edwards, Omar El Muhanna, Sean C. Elias, Ryan S. Elliott, Michael J. Elmore, Marcus Rex English, Sally Felle, Shuo Feng, Carla Ferreira Da Silva, Samantha Field, Richard Fisher, Carine Fixmer, Karen J. Ford, Jamie Fowler, Emma Francis, John Frater, Julie Furze, Pablo Galian-Rubio, Celine Galloway, Harriet Garlant, Madita Gavrila, Felicity Gibbons, Karyna Gibbons, Ciaran Gilbride, Hardeep Gill, Kerry Godwin, Katherine Gordon-Quayle, Giacomo Gorini, Lyndsey Goulston, Caroline Grabau, Lara Gracie, Nichola Graham, Nicola Greenwood, Oliver Griffiths, Gaurav Gupta, Elizabeth Hamilton, Brama Hanumunthadu, Stephanie A. Harris, Tara Harris, Daisy Harrison, Thomas C. Hart, Birgit Hartnell, Louise Haskell, Sophia Hawkins, John Aaron Henry, Macarena Hermosin Herrera, David Hill, Jennifer Hill, Gina Hodges, Susanne H.C. Hodgson, Katie L. Horton, Elizabeth Howe, Nicola Howell, Jessica Howes, Ben Huang, Jonathan Humphreys, Holly E. Humphries, Poppy Iveson, Frederic Jackson, Susan Jackson, Sam Jauregui, Helen Jeffers, Bryony Jones, Christine E. Jones, Elizabeth Jones, Kathryn Jones, Amar Joshi, Reshma Kailath, Jade Keen, Dearbhla M. Kelly, Sarah Kelly, Debbie Kelly, David Kerr, Liaquat Khan, Baktash Khozoee, Annabel Killen, Jasmin Kinch, Lloyd D.W. King, Thomas B. King, Lucy Kingham, Paul Klenerman, Julian C. Knight, Daniel Knott, Stanislava Koleva, Gail Lang, Colin W. Larkworthy, Jessica P.J. Larwood, Rebecca Law, Arlene Lee, Kim Y.N. Lee, Emily A. Lees, Stephanie Leung, Yuanyuan Li, Amelia M. Lias, Aline Linder, Samuel Lipworth, Shuchang Liu, Xinxue Liu, Stephanie Lloyd, Lisa Loew, Raquel Lopez Ramon, Meera Madhavan, David O. Mainwaring, Garry Mallett, Kushal Mansatta, Spyridoula Marinou, Phedra Marius, Emma Marlow, Paula Marriott, Julia L. Marshall, Jane Martin, Shauna Masters, Joanne McEwan, Joanna L. McGlashan, Lorna McInroy, Nicky McRobert, Clare Megson, Alexander J. Mentzer, Neginsadat Mirtorabi, Celia Mitton, Maria Moore, Marni Moran, Ella Morey, Róisín Morgans, Susan J. Morris, Hazel Morrison Morrison, Gertraud Morshead, Richard Morter, Nathifa A. Moya, Ekta Mukhopadhyay, Jilly Muller, Claire Munro, Sarah Murphy, Philomena Mweu, Andrés Noé, Fay L. Nugent, Katie O'Brien, Daniel O'Connor, Blanché Oguti, Victoria Olchawski, Catarina Oliveira, Peter John O'Reilly, Piper Osborne, Lydia Owen, Nelly Owino, Panagiotis Papageorgiou, Helena Parracho, Karen Parsons, Bhumika Patel, Maia Patrick-Smith, Yanchun Peng, Elizabeth J. Penn, Marco Polo Peralta-Alvarez, James Perring, Christos Petropoulos, Daniel J. Phillips, Dimitra Pipini, Samuel Pollard, Ian Poulton, Danny Pratt, Laura Presland, Pamela C. Proud, Samuel Provstgaard-Morys, Sophie Pueschel, David Pulido, Ria Rabara, Kajal Radia, Durga Rajapaska, Fernando Ramos Lopez, Helen Ratcliffe, Sara Rayhan, Byron Rees, Emilia Reyes Pabon, Hannah Roberts, Isla Robertson, Sophie Roche, Christine S. Rollier, Rossana Romani, Zoe Rose, Indra Rudiansyah, Sabeha Sabheha, Stephannie Salvador, Helen Sanders, Katherine Sanders, Iman Satti, Chloe Sayce, Annina B. Schmid, Ella Schofield, Gavin Screaton, Cynthia Sedik, Samiullah Seddiqi, Rameswara R. Segireddy, Beatrice Selby, Imam Shaik, Hannah R. Sharpe, Robert Shaw, Adam Shea, Sarah Silk, Laura Silva-Reyes, Donal T. Skelly, David J. Smith, Daniel C. Smith, Nicholas Smith, Alexandra J. Spencer, Louise Spoors, Elizabeth Stafford, Imogen Stamford, Lisa Stockdale, David Stockley, Lisa V. Stockwell, Matthew Stokes, Louise H. Strickland, Arabella Stuart, Sulaiman Sulaiman, Eloise Summerton, Zoe Swash, Anna Szigeti, Abdessamad Tahiri-Alaoui, Rachel Tanner, Iona Taylor, Keja Taylor, Ursula Taylor, Rebecca te Water Naude, Andreas Themistocleous, Merin Thomas, Tonia M. Thomas, Amber Thompson, Kevin Thompson, Viv Thornton-Jones, Lan Tinh, Adriana Tomic, Susan Tonks, James Towner, Nguyen Tran, Julian A. Tree, Adam Truby, Cheryl Turner, Richard Turner, Marta Ulaszewska, Rachel Varughese, Dennis Verbart, Marije K. Verheul, Iason Vichos, Laura Walker, Matthew E. Wand, Bridget Watkins, Jessica Welch, Alison J. West, Caroline White, Rachel White, Paul Williams, Mark Woodyer, Andrew T. Worth, Daniel Wright, Terri Wrin, Xin Li Yao, Diana-Andreea Zbarcea, and Dalila Zizi
Supplementary Material
References
- 1.WHO . World Health Organization; Geneva: Nov 13, 2020. Coronavirus disease (COVID-19) dashboard.https://covid19.who.int [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; Geneva: Nov 12, 2020. Draft landscape of COVID-19 vaccine candidates.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [Google Scholar]
- 4.WHO . World Health Organization; Geneva: April 29, 2020. WHO target product profiles for COVID-19 vaccines: version 3.https://www.who.int/docs/default-source/blue-print/who-target-product-profiles-for-covid-19-vaccines.pdf?sfvrsn=1d5da7ca_5 [Google Scholar]
- 5.Joint Committee on Vaccination and Immunisation Priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI, 25 September 2020. UK Government. Sept 25, 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-25-september-2020
- 6.US Centers for Disease Control and Prevention The COVID-19 vaccination program interim operational guidance for jurisdictions playbook. Nov 5, 2020. https://www.cdc.gov/vaccines/covid-19/covid19-vaccination-guidance.html
- 7.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y, Mentzer AJ, Liu G. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson EJ, Rouphael NG, Widge AT. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020 doi: 10.1056/NEJMoa2028436. published online Sept 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh EE, Frenck RW, Jr, Falsey AR. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020 doi: 10.1056/NEJMoa2027906. published online Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu FC, Guan XH, Li YH. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logunov DY, Dolzhikova IV, Zubkova OV. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia S, Zhang Y, Wang Y. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30831-8. published online Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia S, Duan K, Zhang Y. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keech C, Albert G, Cho I. Phase 1-2 Trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020 doi: 10.1056/NEJMoa2026920. published online Sept 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milligan ID, Gibani MM, Sewell R. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA. 2016;315:1610–1623. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 17.van Doremalen N, Lambe T, Spencer A. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folegatti PM, Ewer KJ, Aley PK. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockwood K, Song X, MacKnight C. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nimenrix: summary of medicinal product characteristics. Oct 28, 2020. https://www.medicines.org.uk/emc/medicine/26514#gref
- 21.Dicks MD, Spencer AJ, Edwards NJ. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coughlan L, Sridhar S, Payne R. Heterologous two-dose vaccination with simian adenovirus and poxvirus vectors elicits long-lasting cellular immunity to influenza virus A in healthy adults. EBioMedicine. 2018;29:146–154. doi: 10.1016/j.ebiom.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapia MD, Sow SO, Mbaye KD. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: a randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2020;20:719–730. doi: 10.1016/S1473-3099(20)30019-0. [DOI] [PubMed] [Google Scholar]
- 24.Wilkie M, Satti I, Minhinnick A. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime - MVA85A boost in healthy UK adults. Vaccine. 2020;38:779–789. doi: 10.1016/j.vaccine.2019.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewer K, Rampling T, Venkatraman N. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. N Engl J Med. 2016;374:1635–1646. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert PH, Ambrosino DM, Andersen SR. Consensus summary report for CEPI/BC March 12-13, 2020 meeting: assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine. 2020;38:4783–4791. doi: 10.1016/j.vaccine.2020.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 28.Denis F, Mounier M, Hessel L. Hepatitis-B vaccination in the elderly. J Infect Dis. 1984;149 doi: 10.1093/infdis/149.6.1019. [DOI] [PubMed] [Google Scholar]
- 29.Williams K, Bastian AR, Feldman RA. Phase 1 safety and immunogenicity study of a respiratory syncytial virus vaccine with an adenovirus 26 vector encoding prefusion F (Ad26.RSV.preF) in adults aged ≥60 years. J Infect Dis. 2020;222:979–988. doi: 10.1093/infdis/jiaa193. [DOI] [PubMed] [Google Scholar]
- 30.Green CA, Sande CJ, Scarselli E. Novel genetically-modified chimpanzee adenovirus and MVA-vectored respiratory syncytial virus vaccine safely boosts humoral and cellular immunity in healthy older adults. J Infect. 2019;78:382–392. doi: 10.1016/j.jinf.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol and clinical study plan are provided in the appendix (pp 45–212). Anonymised participant data will be made available when the trial is complete, upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement. All data will be made available for a minimum of 5 years from the end of the trial.