Abstract
Background
The number of positive and death cases from coronavirus disease 2019 (COVID-19) is still increasing until now. One of the most prone individuals, even in normal situations is patients with dementia. Currently, no study provides clear evidence regarding the link between dementia and COVID-19. This study aims to analyze the relationship between dementia and poor outcomes of COVID-19 infection.
Materials and Methods
We systematically searched the PubMed and Europe PMC database using specific keywords related to our aims until October 25th, 2020. All articles published on COVID-19 and dementia were retrieved. The quality of the study was assessed using the Newcastle Ottawa Scale (NOS) tool for observational studies. Statistical analysis was done using Review Manager 5.4 software.
Results
A total of 24 studies with 46,391 dementia patients were included in this meta-analysis. This meta-analysis showed that dementia was associated with composite poor outcome [RR 2.67 (95% CI 2.06 – 3.47), p < 0.00001, I2 = 99%, random-effect modeling] and its subgroup which comprised of risk of COVID-19 infection [RR 2.76 (95% CI 1.43 – 5.33), p = 0.003, I2 = 99%, random-effect modeling], severe COVID-19 [RR 2.63 (95% CI 1.41 – 4.90), p = 0.002, I2 = 89%, random-effect modeling], and mortality from COVID-19 infection [RR 2.62 (95% CI 2.04 – 3.36), p < 0.00001, I2 = 96%, random-effect modeling].
Conclusions
Extra care and close monitoring should then be provided to patients with dementia to minimize the risk of infections, preventing the development of severe and mortality outcomes.
Keywords: Coronavirus disease 2019, Covid-19, Dementia, Memory disturbance, Neurologic disease
1. Introduction
World Health Organization (WHO) first declared coronavirus disease 2019 (COVID-19) as a pandemic in March 2020, and now, seven months after that, the number of positive and death cases are still increasing. This global pandemic has caused a significant impact on health, social, and economic aspects around the world. Thus, identification of the risk factors that contribute to the development of severe infections is important to enabling risk stratification, optimizing the hospital resources reallocation, and guiding public health recommendations and interventions. (T. I. Hariyanto & Kurniawan, 2020) Several comorbidities have been demonstrated to be associated with severe COVID-19 outcomes such as hypertension, diabetes, dyslipidemia, anemia, cardiovascular disease, thyroid disease, and pulmonary disease. (T. I. Hariyanto & Kurniawan, 2020, 2020; Huang et al., 2020; Kwenandar et al., 2020) During normal times, individuals with dementia are the most vulnerable persons as they depend on other people for their day to day survival. In a previous study, it has been shown that dementia increases the risk of morbidity and mortality among hospitalized patients, including the severity of the respiratory disease. (Liao et al., 2015) Unfortunately, until now, no study provides clear evidence regarding the link between dementia and COVID-19. This article aims to explore the potential association between dementia and poor outcomes of COVID-19 infection.
2. Materials and methods
2.1. Eligibility criteria
Studies were included in this review if met the following inclusion criteria: representation for clinical questions (P: positive/confirmed cases of COVID-19; I: a group of patients with dementia as their comorbidity; C: a group of patients without dementia; O: composite poor outcomes which comprise of risk of COVID-19 infection, severe COVID-19, and mortality), type of study was a randomized control trial, cohort, clinical trial, case-cohort, and cross-over design, and if the full-text article was available. The following types of articles were excluded: articles other than original research (e.g., review articles, letters, or commentaries); case reports; articles not in the English language; articles on research in pediatric populations (17 years of age or younger); and articles on research in pregnant women.
2.2. Search strategy and study selection
A systematic search of the literature was conducted on PubMed and Europe PMC using the keywords "dementia" OR “major cognitive impairment” OR "clinical characteristics" OR "comorbidities" OR "risk factors" AND "coronavirus disease 2019″ OR "COVID-19″, between 2019 and present time (October 25th, 2020) with language restricted to English only. The title, abstract, and full text of all articles identified that matched the search criteria were assessed, and those reporting the rate of dementia in COVID-19 patients with a clinically validated definition of "severe disease" and “mortality” were included in this meta-analysis. The references of all identified studies were also analyzed (forward and backward citation tracking) to identify other potentially eligible articles. The study was carried out per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. (Moher, Liberati, Tetzlaff & Altman, 2009)
2.3. Data extraction and quality assessment
Data extraction was performed independently by two authors, we used standardized forms that include author, year, study design, number of participants, age, gender, number of patients with dementia, and proportion of patients with each outcome of COVID-19.
The outcome of interest was the composite poor outcome that comprised of risk of COVID-19 infection, severe COVID-19, and mortality. The risk of COVID-19 infection was defined as the likelihood of someone getting contracted by COVID-19. Severe COVID-19 was defined as patients who had any of the following features at the time of, or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to a fractional concentration of oxygen inspired air (fiO2) ≤300 mmHg; or (4) critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure) or admission into ICU. Mortality outcome from COVID-19 was defined as the number of patients who were dead because of COVID-19 infection.
Two investigators independently evaluated the quality of the included cohort and case-control studies using the Newcastle–Ottawa Scale (NOS). (Margulis et al., 2014) The selection, comparability, and exposure of each study were broadly assessed and studies were assigned a score from zero to nine. Studies with scores ≥7 were considered of good quality.
2.4. Statistical analysis
A meta-analysis was performed using Review Manager 5.4 (Cochrane Collaboration) software. Dichotomous variables were calculated using the Mantel-Haenszel formula with a random-effects model regardless of heterogeneity. The effect estimate was reported as risk ratio (RR) along with its 95% confidence intervals (CIs) for dichotomous variables, respectively. P-value was two-tailed, and the statistical significance was set at ≤0.05. Subgroup analysis was performed for each component of composite poor outcomes. We performed Begg's funnel-plot analysis to qualitatively assess the risk of publication bias.
3. Results
3.1. Study selection and characteristics
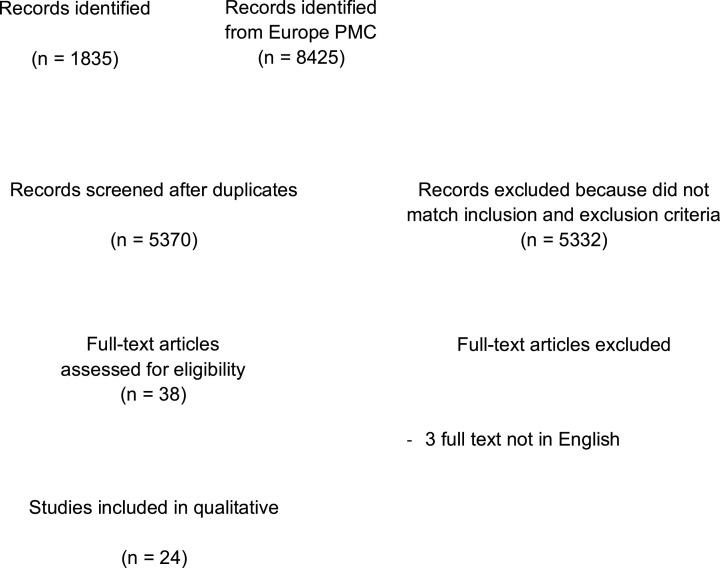
A total of 10,260 records were obtained through systematic electronic searches. After the removal of duplicates, 5370 records remained. A total of 5332 records were excluded after screening the titles/abstracts because they did not match our inclusion and exclusion criteria. After evaluating 38 full-texts for eligibility, 6 full-text articles were excluded because they do not have the control/comparison group, 5 full-text articles were excluded because they do not have the outcome of interest (risk of COVID-19 infection, severe COVID-19, and mortality), 3 full-text articles were excluded because the articles were not in English, and finally, 24 studies (Atkins et al., 2020; Bianchetti et al., 2020; Covino et al., 2020; Docherty et al., 2020; Giorgi Rossi et al., 2020; Harrison, Fazio-Eynullayeva, Lane, Underhill & Lip, 2020; Hong et al., 2020; Hwang, Kim, Park, Chang & Park, 2020; Jang et al., 2020; Ji et al., 2020; Kabarriti et al., 2020; Knights et al., 2020; Kokoszka-Bargieł, Cyprys, Rutkowska, Madowicz & Knapik, 2020; Lee et al., 2020; Miyashita et al., 2020; Mohamed et al., 2020t; Moon et al., 2020; Nystad et al., 2020; Poblador-Plou et al., 2020; Rozenfeld et al., 2020; Sapey et al., 2020; Sung et al., 2020; Wan et al., 2020; Yang et al., 2020) with a total of 4648,247 sample size and 46,391 dementia patients were included in the meta-analysis (Fig. 1 ). Of a total of 24 included studies, 21 were retrospective cohort, 2 studies were prospective cohort, while the remaining 1 study was a case-control study. The essential characteristics of the included studies are summarized in Table 1 .
Fig. 1.
PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta-analysis.
Table 1.
Characteristics of included studies.
| Study | Sample size | Design | Outcome | Age (years) | Dementia patients n (%) | Non-dementia patients n (%) |
|---|---|---|---|---|---|---|
| Atkins et al. (2020) | 269,070 | Retrospective cohort | Risk of infection | 73.1 ± 4.4 | 888 (0.3%) | 268,182 (99.7%) |
| Bianchetti et al. (2020) | 627 | Retrospective cohort | Mortality | 70.7 ± 12.9 | 82 (13.1%) | 545 (86.9%) |
| Covino et al. (2020) | 69 | Retrospective cohort | Mortality | 85 ± 5.1 | 8 (11.6%) | 61 (88.4%) |
| Docherty et al. (2020) | 20,133 | Prospective cohort | Mortality | 70.9 ± 17.7 | 2360 (13.5%) | 15,099 (86.5%) |
| Giorgi Rossi et al. (2020) | 2653 | Prospective cohort | Mortality | 54.7 ± 18.3 | 107 (4%) | 2546 (96%) |
| Harrison et al. (2020) | 31,461 | Retrospective cohort | Mortality | 49.3 ± 20.7 | 1031 (3.3%) | 30,430 (96.7%) |
| Hong et al. (2020) | 98 | Retrospective cohort | Severity | 55.4 ± 17.1 | 3 (3.1%) | 95 (96.9%) |
| Hwang et al. (2020) | 103 | Retrospective cohort | Mortality | 67.6 ± 15.3 | 11 (10.6%) | 92 (89.4%) |
| Jang et al. (2020) | 110 | Retrospective cohort | Severity | 56.9 ± 17 | 4 (3.6%) | 106 (96.4%) |
| Ji et al. (2020) | 7341 | Case-control | Severity | 47 ± 19 | 368 (5%) | 6973 (95%) |
| Kabarriti et al. (2020) | 5902 | Retrospective cohort | Mortality | 48.3 ± 20.4 | 303 (5.1%) | 5599 (94.9%) |
| Knights et al. (2020) | 108 | Retrospective cohort | Mortality | 68.7 ± 1.5 | 16 (15%) | 92 (85%) |
| Kokoszka-Bargieł et al. (2020) | 53 | Retrospective cohort | Mortality | 62.4 ± 10.4 | 12 (22.6%) | 41 (77.4%) |
| Lee et al. (2020) | 694 | Retrospective cohort | Severity | 52.1 ± 18.2 | 10 (1.4%) | 684 (98.6%) |
| Miyashita et al. (2020) | 2071 | Retrospective cohort | Mortality | 68.5 ± 12.7 | 98 (4.7%) | 1973 (95.3%) |
| Mohamed et al. (2020t) | 144,297 | Retrospective cohort | Risk of infection | 75.8 ± 12.5 | 23,961 (16.6%) | 120,336 (83.4%) |
| Moon et al. (2020) | 352 | Retrospective cohort | Mortality | 55.6 ± 25.1 | 35 (9.9%) | 317 (90.1%) |
| Nystad et al. (2020) | 4118,831 | Retrospective cohort | Risk of infection | 53.8 ± 15.1 | 15,190 (0.4%) | 4103,641 (99.6%) |
| Poblador-Plou et al. (2020) | 4412 | Retrospective cohort | Mortality | 63.3 ± 18.1 | 468 (10.6%) | 3944 (89.4%) |
| Rozenfeld et al. (2020) | 34,503 | Retrospective cohort | Risk of infection | 61.5 ± 13.4 | 1039 (3%) | 33,464 (97%) |
| Sapey et al. (2020) | 2217 | Retrospective cohort | Mortality | 71.6 ± 19.2 | 326 (14.7%) | 1891 (85.3%) |
| Sung et al. (2020) | 3060 | Retrospective cohort | Severity | 42 ± 22.9 | 65 (2.5%) | 2621 (97.5%) |
| Wan et al. (2020) | 30 | Retrospective cohort | Mortality | 64.2 ± 14.7 | 5 (16.6%) | 25 (83.4%) |
| Yang et al. (2020) | 52 | Retrospective cohort | Mortality | 59.7 ± 13.3 | 1 (1.9%) | 51 (98.1%) |
3.2. Quality of study assessment
Studies with various study designs including cohort and case-control were included in this review and assessed accordingly with the appropriate scale or tool. Newcastle Ottawa Scales (NOS) were used to assess the cohort and case-control studies (Table 2 ). All included studies were rated ‘good’. In conclusion, all studies were seemed fit to be included in the meta-analysis.
Table 2.
Newcastle-Ottawa quality assessment of observational studies.
| First author, year | Study design | Selection | Comparability | Outcome | Total score | Result |
|---|---|---|---|---|---|---|
| Atkins et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Bianchetti et al. (2020) | Cohort | ** | ** | *** | 7 | Good |
| Covino et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Docherty et al. (2020) | Cohort | **** | ** | *** | 9 | Good |
| Giorgi Rossi et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Harrison et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Hong et al. (2020) | Cohort | ** | ** | *** | 7 | Good |
| Hwang et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Jang et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Ji et al. (2020) | Case-control | *** | ** | *** | 8 | Good |
| Kabarriti et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Knights et al. (2020) | Cohort | ** | ** | *** | 7 | Good |
| Kokoszka-Bargieł et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Lee et al. (2020) | Cohort | ** | ** | *** | 7 | Good |
| Miyashita et al. (2020) | Cohort | ** | ** | *** | 7 | Good |
| Mohamed et al., 2020t) | Cohort | ** | ** | *** | 7 | Good |
| Moon et al. (2020) | Cohort | ** | ** | *** | 7 | Good |
| Nystad et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Poblador-Plou et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Rozenfeld et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Sapey et al. (2020) | Cohort | **** | ** | *** | 9 | Good |
| Sung et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Wan et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
| Yang et al. (2020) | Cohort | *** | ** | *** | 8 | Good |
3.3. Dementia and outcomes
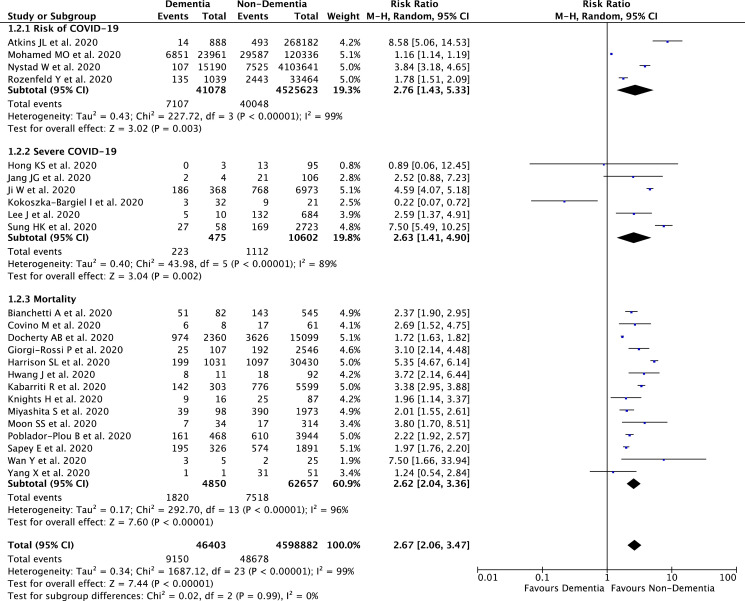
Our pooled analysis showed that dementia was associated with composite poor outcome [RR 2.67 (95% CI 2.06 – 3.47), p < 0.00001, I 2 = 99%, random-effect modeling] (Fig. 2 ). Subgroup analysis showed that dementia was associated with higher risk of COVID-19 infection [RR 2.76 (95% CI 1.43 – 5.33), p = 0.003, I 2 = 99%, random-effect modeling], severe COVID-19 [RR 2.63 (95% CI 1.41 – 4.90), p = 0.002, I 2 = 89%, random-effect modeling], and mortality from COVID-19 infection [RR 2.62 (95% CI 2.04 – 3.36), p < 0.00001, I 2 = 96%, random-effect modeling].
Fig. 2.
Forest plot that demonstrates the association of dementia with composite poor outcome and its subgroup which comprises of risk of COVID-19 infection, severe COVID-19, and mortality.
3.4. Publication bias
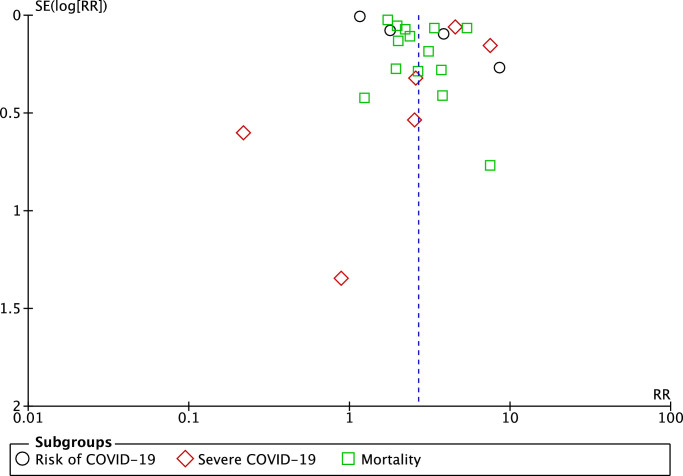
The funnel-plot analysis showed a qualitatively symmetrical inverted funnel-plot for the association between dementia and composite poor outcome (Fig. 3 ), showing no indication of publication bias.
Fig. 3.
Funnel plot analysis for the association of dementia with composite poor outcome of COVID-19.
4. Discussion
Based on our pooled analysis of available data, dementia seems to be associated with an enhanced risk of severity and mortality from COVID-19 infection. Several reasons can be proposed to explain this result. First, most of the patients with dementia were old and have other comorbid medical conditions that could increase the severity of infections. Older patients with COVID-19 infection tend to present with atypical symptoms. They may be afebrile with non-respiratory symptoms, such as delirium or isolated functional decline without any obvious physical symptoms. (D'Adamo, Yoshikawa & Ouslander, 2020) These atypical presentations of COVID-19 may impede the early recognition of the disease, increase COVID-19 spread, and mortality. Moreover, in a meta-analysis of 611,583 COVID-19 patients, age over 60 years was found to be a risk factor for disease progression. (Bonanad et al., 2020) This fact could be influenced by both the physiological aging process and, especially, the greater prevalence in older adult patients of frailty and comorbidities that contribute to a decrease in functional reserve that reduces intrinsic capacity and resilience and hinders the fight against infections. (Bonanad et al., 2020) Older people will have a condition called “immune senescence” which consist of (1) decreased neutrophil phagocytosis and oxidative burst; (2) decreased macrophages chemotaxis and phagocytosis; and (3) decreased number of peripheral naïve T and B cells which will lead to a reduced ability to respond to new antigen. (Aiello et al., 2019) Not only that, comorbidities such as cardiovascular disease, hypertension, and diabetes are highly prevalent in older adults and have been associated with worse outcomes in COVID-19. (Bonanad et al., 2020) Second, patients with dementia were associated with the ApoE e4 genotype. (Kuo, Pilling, Atkins, Kuchel & Melzer, 2020) ApoE itself has lipoprotein function and modulates the macrophage pro/anti-inflammatory phenotypes (Tudorache, Trusca & Gafencu, 2017) and is expressed by many cell types in the lung, including macrophages, type I and type II alveolar epithelial cells (Gordon et al., 2019), therefore it is possible that having one or two copies of ApoE4 can increase the risk of having severe COVID-19 infection by robust innate immune response and cytokine storm that results in acute respiratory distress syndrome (ARDS). Third, inflammation also plays an important role in the pathogenesis of dementia. In addition to amyloid protein, inflammatory molecules, including acute inflammatory reactants and inflammatory cytokines, have been found in the cerebrospinal fluid of dementia patients. (Kinney et al., 2018; Simone & Tan, 2011) These persistent inflammatory state of dementia patients may be the cause of the increased peripheral blood white blood cells (WBC) and neutrophil counts in dementia patients with COVID-19. (Harrison et al., 2020) On the other side, a meta-analysis study has shown that a high level of leukocyte counts and the high neutrophil-lymphocyte ratio was associated with a higher risk of developing severe COVID-19 and mortality from COVID-19. (Elshazli et al., 2020) Finally, people with dementia, due to the nature of their cognitive decline, would require the care and support of carers such as dementia caregivers in order to be able to follow healthcare and preventive measures such as wearing face masks and washing their hands with water and soap or hand sanitizers. (Suzuki et al., 2020) However, during this COVID-19 pandemic, the availability of caregivers becomes limited which may cause dementia patients unable to follow healthcare and preventive measures therefore making them posed a higher risk of contracting an infection from COVID-19. (Dang et al., 2020)
The limitation of this study is that the presence of confounding factors such as patients' age, other comorbid conditions, nutritional status, and daily medication that can affect the relationship between dementia and mortality from COVID-19 should still be considered. However, with this study, we hope that dementia can further be considered as an important factor and comorbidity in COVID-19 patients.
5. Conclusions and implications
Hence, patients with dementia should be given extra care and monitoring to minimize exposure to the virus. Physicians can use the telemedicine-based practice to provide care and evaluations for dementia patients to minimize their visit to the hospitals. Physicians and caregivers should also be engaged in close monitoring of dementia patients with suspected COVID-19, for early diagnosis and treatment to avoid severe infections. The hospitals can provide Special Care Geriatric COVID-19 units for dementia and elderly patients who contract with COVID-19, which is filled with a team of mental health professionals (doctors, nurses, and psychological counselors), to give better care, more strict monitoring, and enable the healthcare providers in that units to focus only on the management of dementia patients so that the morbidity and mortality from COVID-19 can be reduced. Finally, dementia should be regarded as an important factor in future risk stratification models for COVID-19.
Funding
None.
CRediT authorship contribution statement
Timotius Ivan Hariyanto: Conceptualization, Data curation, Methodology, Investigation, Validation, Visualization, Writing - original draft, Writing - review & editing. Cynthia Putri: Conceptualization, Data curation, Methodology, Investigation, Validation, Visualization, Writing - original draft, Writing - review & editing. Jessie Arisa: Conceptualization, Data curation, Methodology, Investigation, Validation, Visualization, Writing - original draft, Writing - review & editing. Rocksy Fransisca V. Situmeang: Conceptualization, Visualization, Resources, Supervision, Writing - original draft, Writing - review & editing. Andree Kurniawan: Conceptualization, Visualization, Resources, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
None.
Acknowledgement
None.
References
- Aiello A., Farzaneh F., Candore G., Caruso C., Davinelli S., Gambino C.M. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Frontiers in immunology. 2019;10:2247. doi: 10.3389/fimmu.2019.02247. Sep 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J.L., Masoli J.A.H., Delgado J., Pilling L.C., Kuo C.L., Kuchel G.A. Preexisting Comorbidities Predicting COVID-19 and Mortality in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci. 2020;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetti A., Rozzini R., Guerini F., Boffelli S., Ranieri P., Minelli G. Clinical presentation of COVID19 in dementia patients. The journal of nutrition, health & aging. 2020;24(6):560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanad C., García-Blas S., Tarazona-Santabalbina F., Sanchis J., Bertomeu-González V., Fácila L. The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects. Journal of the American Medical Directors Association. 2020;21(7):915–918. doi: 10.1016/j.jamda.2020.05.045. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covino M., Matteis G.D., Santoro M., Sabia L., Simeoni B., Candelli M. Clinical characteristics and prognostic factors in COVID-19 patients aged ≥80 years. Geriatrics & gerontology international. 2020:1–5. doi: 10.1111/ggi.13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Adamo H., Yoshikawa T., Ouslander J. Coronavirus disease 2019 in geriatrics and long‐term care: The ABCDs of COVID ‐19. Journal of the American Geriatrics Society. 2020;68(5):912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- Dang S., Penney L.S., Trivedi R., Noel P.H., Pugh M.J., Finley E. Caring for Caregivers During COVID-19. Journal of the American Geriatrics Society. 2020 doi: 10.1111/jgs.16726. doi: 10.1111/jgs.16726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ (Clinical research ed.) 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshazli R.M., Toraih E.A., Elgaml A., El-Mowafy M., El-Mesery M., Amin M.N. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PloS one. 2020;15(8) doi: 10.1371/journal.pone.0238160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi Rossi P., Marino M., Formisano D., Venturelli F., Vicentini M., Grilli R., Reggio Emilia COVID-19 Working Group Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PloS one. 2020;15(8) doi: 10.1371/journal.pone.0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E., Yao X., Xu H., Karkowsky W., Kaler M., Kalchiem-Dekel O. Apolipoprotein E is a concentration-dependent pulmonary danger signal that activates the NLRP3 inflammasome and IL-1β secretion by bronchoalveolar fluid macrophages from asthmatic subjects. J Allergy Clin Immunol. 2019;144(2):426–441. doi: 10.1016/j.jaci.2019.02.027. .e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes & metabolic syndrome. 2020;14(5):1429–1430. doi: 10.1016/j.dsx.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Kurniawan A. Anemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.102926. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes & metabolic syndrome. 2020;14(5):1463–1465. doi: 10.1016/j.dsx.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., Lip G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K.S., Lee K.H., Chung J.H., Shin K.C., Choi E.Y., Jin H.J. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: A brief descriptive study. Yonsei Med J. 2020;61(5):431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes & metabolic syndrome. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.M., Kim J.H., Park J.S., Chang M.C., Park D. Neurological diseases as mortality predictive factors for patients with COVID-19: A retrospective cohort study. Neurol Sci. 2020:1–8. doi: 10.1007/s10072-020-04541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J.G., Hur J., Choi E.Y., Hong K.S., Lee W., Ahn J.H. Prognostic factors for severe coronavirus disease 2019 in Daegu, Korea. J Korean Med Sci. 2020;35(23):e209. doi: 10.3346/jkms.2020.35.e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Huh K., Kang M., Hong J., Bae G.H., Lee R. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: A nationwide case-control study. J Korean Med Sci. 2020;35(25):e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabarriti R., Brodin N.P., Maron M.I., Guha C., Kalnicki S., Garg M.K. Association of Race and Ethnicity With Comorbidities and Survival Among Patients With COVID-19 at an Urban Medical Center in New York. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s & dementia : translational research & clinical interventions. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knights H., Mayor N., Millar K., Cox M., Bunova E., Hughes M. Characteristics and outcomes of patients with COVID-19 at a district general hospital in Surrey, UK. Clin Med (Lond). 2020;20(5):e148–e153. doi: 10.7861/clinmed.2020-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszka-Bargieł I., Cyprys P., Rutkowska K., Madowicz J., Knapik P. Intensive Care Unit Admissions During the First 3 Months of the COVID-19 Pandemic in Poland: A Single-Center, Cross-Sectional Study. Med Sci Monit. 2020;26 doi: 10.12659/MSM.926974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Pilling L.C., Atkins J.L., Kuchel G.A., Melzer D. ApoE e2 and aging-related outcomes in 379,000 UK Biobank participants. Aging. 2020;12:12222–12233. doi: 10.18632/aging.103405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwenandar F., Japar K.V., Damay V., Hariyanto T.I., Tanaka M., Lugito N.P.H. Coronavirus disease 2019 and cardiovascular system: A narrative review. International journal of cardiology. Heart & vasculature. 2020;29 doi: 10.1016/j.ijcha.2020.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Hong S.W., Hyun M., Park J.S., Lee J.H., Suh Y.S. Epidemiological and clinical characteristics of coronavirus disease 2019 in Daegu, South Korea. Int J Infect Dis. 2020;98:462–466. doi: 10.1016/j.ijid.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K.M., Lin T.C., Li C.Y., Yang Y.H. Dementia increases severe sepsis and mortality in hospitalized patients with chronic obstructive pulmonary disease. Medicine. 2015;94(23):e967. doi: 10.1097/MD.0000000000000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis A.V., Pladevall M., Riera-Guardia N., Varas-Lorenzo C., Hazell L., Berkman N.D. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: The Newcastle-Ottawa Scale and the RTI item bank. Clinical epidemiology. 2014;6:359–368. doi: 10.2147/CLEP.S66677. https://doi/org/10.2147/CLEP.S66677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita S., Yamada T., Mikami T., Miyashita H., Chopra N., Rizk D. Impact of dementia on clinical outcomes in elderly patients with coronavirus 2019 (COVID-19): An experience in New York. Geriatrics & gerontology international. 2020;20(7):732–734. doi: 10.1111/ggi.13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.O., Gale C.P., Kontopantelis E., Doran T., de Belder M., Asaria M. Sex Differences in Mortality Rates and Underlying Conditions for COVID-19 Deaths in England and Wales. Mayo Clin Proc. 2020;95(10):2110–2124. doi: 10.1016/j.mayocp.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S.S., Lee K., Park J., Yun S., Lee Y.S., Lee D.S. Clinical Characteristics and Mortality Predictors of COVID-19 Patients Hospitalized at Nationally-Designated Treatment Hospitals. J Korean Med Sci. 2020;35(36):e328. doi: 10.3346/jkms.2020.35.e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystad W., Hjellvik V., Larsen I.K., Ariansen I., Helland E., Johansen K.I. Underlying conditions in adults with COVID-19. Tidsskr Nor Laegeforen. 2020;140(13) doi: 10.4045/tidsskr.20.0512. [DOI] [PubMed] [Google Scholar]
- Poblador-Plou B., Carmona-Pírez J., Ioakeim-Skoufa I., Poncel-Falcó A., Bliek-Bueno K., Cano-Del Pozo M. Baseline Chronic Comorbidity and Mortality in Laboratory-Confirmed COVID-19 Cases: Results from the PRECOVID Study in Spain. International journal of environmental research and public health. 2020;17(14):5171. doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld Y., Beam J., Maier H., Haggerson W., Boudreau K., Carlson J. A model of disparities: Risk factors associated with COVID-19 infection. International journal for equity in health. 2020;19(1):126. doi: 10.1186/s12939-020-01242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapey E., Gallier S., Mainey C., Nightingale P., McNulty D., Crothers H. All clinicians and students at University Hospitals Birmingham NHS Foundation Trust. Ethnicity and risk of death in patients hospitalised for COVID-19 infection in the UK: An observational cohort study in an urban catchment area. BMJ open respiratory research. 2020;7(1) doi: 10.1136/bmjresp-2020-000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone M.J., Tan Z.S. The role of inflammation in the pathogenesis of delirium and dementia in older adults: A review. CNS neuroscience & therapeutics. 2011;17(5):506–513. doi: 10.1111/j.1755-5949.2010.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H.K., Kim J.Y., Heo J., Seo H., Jang Y.S., Kim H. Korea National Committee for Clinical Management of COVID-19. Clinical Course and Outcomes of 3,060 Patients with Coronavirus Disease 2019 in Korea, January-May 2020. J Korean Med Sci. 2020;35(30):e280. doi: 10.3346/jkms.2020.35.e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Hotta M., Nagase A., Yamamoto Y., Hirakawa N., Satake Y. The behavioral pattern of patients with frontotemporal dementia during the COVID-19 pandemic. International psychogeriatrics / IPA. 2020:1–4. doi: 10.1017/S104161022000109X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudorache I., Trusca V., Gafencu A. Apolipoprotein E - A multifunctional protein with implications in various pathologies as a result of its structural features. Computational and structural biotechnology journal. 2017;15:359–365. doi: 10.1016/j.csbj.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Wu J., Ni L., Luo Q., Yuan C., Fan F. Prognosis analysis of patients with mental disorders with COVID-19: A single-center retrospective study. Aging. 2020;12(12):11238–11244. doi: 10.18632/aging.103371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet. Respiratory medicine. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]