Abstract
Objectives
The vast majority of COVID-19 cases in Singapore have occurred amongst migrant workers. This paper examined trends in the hospitalised cases and tested the assumption that the low severity of disease was related to the relatively young affected population.
Methods
All patients with PCR-positive SARS-CoV-2 admitted from February to April 2020 were divided into: (i) imported cases, (ii) locally-transmitted cases outside migrant worker dormitories and (iii) migrant worker dormitory cases. They were examined for underlying comorbidities, clinical progress and outcomes.
Results
Imported cases (n = 29) peaked in mid-March 2020, followed by local cases (n = 100) in mid-April 2020; migrant worker cases (n = 425) continued to increase in late April 2020. Migrant worker cases were younger, had few medical comorbidities and less severe disease. As the migrant worker cases increased, the proportion of patients with pneumonia decreased, whilst patients presenting earlier in their illness and asymptomatic disease became more common.
Conclusion
Singapore experienced a substantial shift in the population at risk of severe COVID-19. Successful control in the community protected an aging population. Large migrant worker dormitory outbreaks occurred, but the disease incurred was less severe, resulting in Singapore having one of the lowest case fatality rates in the world.
Keywords: COVID-19, Demographics, Singapore
Introduction
Singapore was uniquely susceptible to the global Coronavirus Disease 2019 (COVID-19) pandemic, as it is one of the most densely populated countries in the world (8358/km2). It sees a high volume of travellers for both business and tourism, particularly from China (Lim et al., 2012). The public health system in Singapore was put to the test by the severe acute respiratory syndrome (SARS) outbreak in 2003, and the lessons learned prepared it well for the challenges faced by COVID-19 in 2020. Whilst Singapore acted quickly, it focused on transmission amongst those with symptomatic diseases in the community, with delayed recognition of the importance of pre-symptomatic/asymptomatic transmission, particularly in vulnerable settings like migrant worker dormitories.
The Disease Outbreak Response System Condition (DORSCON) framework, set up in 2003 to provide guidance on measures to mitigate risk of disease to the public (Ministry of Health Singapore, 2014, Goh et al., 2006), was triggered early (04 February 2020) prior to any COVID-19 cases being detected in Singapore. Singapore’s public health strategy was primarily one of surveillance and containment, effected through extensive and rapid contact tracing, the rapid roll out of diagnostic testing facilities, strict quarantine of all exposed contacts in their homes, and strict isolation of PCR-confirmed COVID-19 cases in cohort facilities (Ngiam et al., 2020, Lee et al., 2020, Ng et al., 2020). Public health messaging, which included a daily report of new cases and the COVID-19 affected areas in Singapore, was rapidly instigated in multiple languages and widely disseminated. From 14 February, returning travellers served a mandatory 14-day stay-home notice (SHN) and underwent thrice-daily temperature and symptom checks. Similarly, contacts of COVID-19 cases served an enforceable SHN. Symptomatic returning travellers and contacts of COVID-19 cases received swabs for SAS-CoV-2, with results reported within 12–24 h.
As a consequence of border closures, imported cases declined and locally transmitted cases became more common, due to importation of the virus, despite an efficient contact tracing program. This was quickly controlled with public-health measures including a strict lockdown, which included: work from home orders for all non-essential workplaces; closure of all preschools, schools and universities; no travel or movement outside of homes unless for exercise and grocery shopping; and mandatory mask-wearing in public areas for anyone aged >2 years (Lim and Wong, 2020).
Simultaneously, multiple large outbreaks were observed within migrant worker dormitories, which went on to form the majority of COVID-19 cases in Singapore to date (Koh, 2020, Bagdasarian and Fisher, 2020). Migrant workers are men–primarily from Bangladesh or India, as well as from China, Thailand and Myanmar–who work in the construction, shipping and manufacturing industries. They fill the low-wage (mean monthly income SG$828, US$590), manual labour occupations that are difficult to hire from the local labour pool (Yi et al., 2020). Housing for these workers typically consists of 20 men sharing a dormitory room with a minimum of 4.5 m2 living space per person, with communal dining and toileting facilities (Ministry of Manpower Singapore, 2020). Nearly a quarter of all registered dormitories in Singapore were gazetted as isolation zones by mid-April 2020, with workers restricted to their rooms but sharing communal toilets, making social distancing impossible (Ministry of Health Singapore, 2020). To contain these outbreaks, active case-finding first began for those with symptoms, followed by those who were asymptomatic, when asymptomatic and minimally symptomatic disease had been definitively described (Wei et al., 2020). Early in the pandemic (February to March 2020), all newly diagnosed COVID-19 cases in Singapore were transferred to tertiary hospitals for assessment and isolation (inclusive of imported cases, locally-transmitted cases and migrant worker cases). From 10 April 2020, purpose-designed community isolation facilities were set up to isolate migrant dorm workers at low risk of severe disease. Some migrant workers bypassed hospital admission and were directly transferred to these facilities for isolation. However, many low-risk, asymptomatic cases continued to be admitted to hospital in the first few months of the isolation facilities being in operation, until these facilities were subsequently sufficiently scaled-up to manage large numbers of migrant worker cases. This differed from the management of COVID-19 overseas, where hospital admissions were reserved for those with moderate-to-severe disease. This provides insight into the mild-moderate disease spectrum (Kim et al., 2020).
There was an anecdotal observation that the manifestations of COVID-19 varied amongst the different demographic groups. Severe diseases–including multi-organ system dysfunction such as myocardial injury, respiratory failure requiring intensive care and mechanical ventilation (Sia et al., 2020, Liu et al., 2020, Wang et al., 2020, Ho et al., 2020a)–were more common in the non-migrant worker population, whereas migrant worker cases were typically well and often asymptomatic/minimally symptomatic. In this retrospective study of the first 554 patients admitted to the current hospital, the clinical differences in three demographically diverse groups of COVID-19 patients early in the pandemic in Singapore were examined and quantified.
Methods
The electronic medical records were reviewed of all patients admitted to the institution from 23 January to 30 April 2020 and who were confirmed to have COVID-19 based on a reverse transcriptase-polymerase chain reaction (RT-PCR) test from a nasopharyngeal or oropharyngeal swab on the Roche cobas® platform at the hospital clinical laboratory. The detection of ORF1ab gene target with or without the E-gene target was interpreted as a positive result. No patients were excluded from the analysis.
Data on demographic backgrounds, past medical history and presenting symptoms were collected. Day of illness was computed based on the number of days from symptom onset to the day of hospital admission. All patients had admission bloods (full blood count examination; markers of coagulation; creatinine and electrolytes; CRP, C-reactive protein; and liver function tests), baseline electrocardiography and chest X-rays. Data on patients who required intensive care, mechanical ventilation and any adverse clinical outcomes such as myocarditis/myocardial injury and death were collected. Persistent fever was defined as a fever ≥72 h. Pneumonia was defined by the presence of radiographic evidence of infiltrates on plain chest X-ray or computed tomography.
The study population was divided based on the exposure history to COVID-19: imported cases were defined as those who acquired COVID-19 overseas; locally transmitted cases as those who acquired COVID-19 within the community, outside the dormitories; and migrant worker cases were those who acquired COVID-19 as a consequence of living or working in migrant worker dormitories. The study population was analysed in two age categories: those <40 years and ≥40 years. The age of 40 was used as a cut-off because it was the age defined by Singapore’s Ministry of Health to identify the vulnerable population at elevated risk of severe COVID-19 illness. Patients aged ≥40 years were compulsorily managed in hospitals, according to a mandate by Singapore MOH, rather than in community isolation facilities.
To compare the three groups based on exposure history, one-way analysis of variance (ANOVA) was used for continuous parameters and data were presented as a mean (±standard deviation). Categorical parameters were compared by Kruskal–Wallis and Chi–squared tests for association, where appropriate, and data were presented in frequencies and percentages. Subsequent analyses by age categories were performed using t-tests and Chi–squared tests. Univariate analyses by Chi–squared tests (or Fisher’s Exact test where appropriate) were used to calculated odds ratios for parameters associated with pneumonia. Multivariable logistic regression was used to identify parameters independently associated with the outcome.
Bar graphs were used to show changes in day of illness at presentation, and proportions of patients requiring supplemental oxygen and pneumonia over time. A p-value of <0.05 was considered significant. All data analysis was performed with SPSS version 20.0 (SPSS, Inc., Chicago, Illinois). This study was approved by the hospital’s institutional review board (National Healthcare Group (NHG) Domain Specific Review Board (DSRB) 2020/00545) prior to commencing the study. Data collected was anonymised and a waiver of informed consent was obtained from the institutional review board.
Results
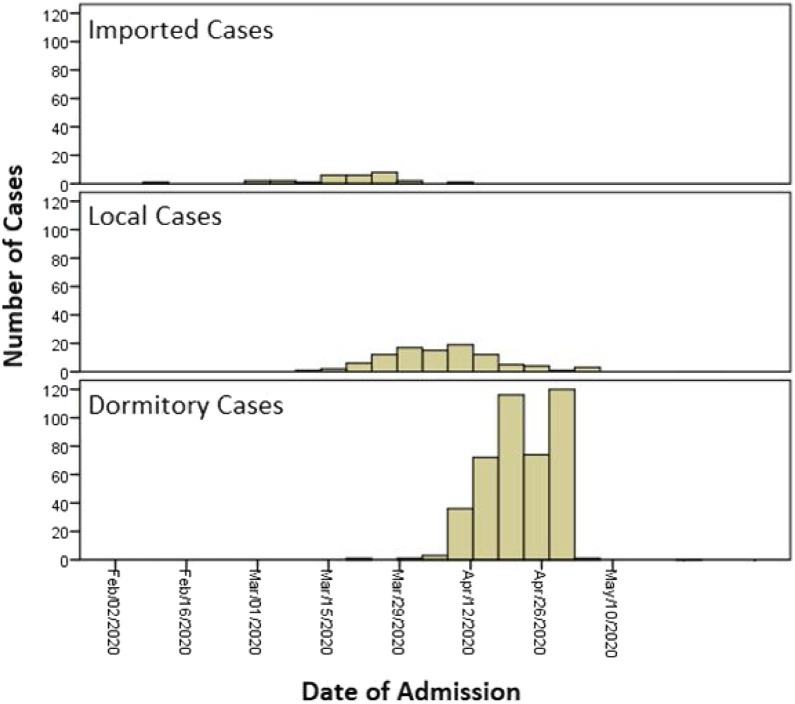
Over the 3-month period, 554 PCR-confirmed COVID-19 cases were admitted to the current centre: 477 were male (87%), with a median age of 37 years; 29 cases (5.2%) were imported; 100 were locally transmitted cases (18.1%); and 425 were migrant worker cases from dormitories (76.7%). Imported cases peaked in the second week of March, locally transmitted cases peaked in early April and migrant worker cases continued to rise in late April (Figure 1 ). No imported cases were identified after mid-April 2020 in the study population.
Figure 1.
Distribution of cases by admission date.
Local cases acquired outside the dormitories were older compared with imported or migrant worker cases (median age in years: 46 ± 16 vs 39 ± 15 vs 35 ± 9, respectively; p < 0.001). A total of 54% and 51% of imported and local cases were men, respectively, compared with 100% men in the migrant worker population. A total of 49% and 57% of local non-dormitory and imported cases were of Chinese ethnicity, respectively, and 77% of migrant workers were Indian or Bangladeshi (Table 1 ).
Table 1.
Demographic information.
| Parameter | Overall (n = 554) | Imported cases (n = 29) | Community cases (n = 100) | Dormitory cases (n = 425) | p-value |
|---|---|---|---|---|---|
| Age (years) | 37 (±12) | 39 (±15) | 46 (±16) | 35 (±9) | <0.001 |
| Gender (male) | 477 (86.9%) | 15 (53.6%) | 51 (51.0%) | 411 (97.6%) | <0.001 |
| Ethnicity | <0.001 | ||||
| Chinese | 91 (16.5%) | 16 (57.1%) | 49 (49.0%) | 26 (6.1%) | |
| Malay | 34 (6.1%) | 1 (3.6%) | 20 (20.0%) | 13 (3.1%) | |
| Indian | 199 (36.0%) | 1 (3.6%) | 17 (17.0%) | 181 (42.6%) | |
| Bangladeshi | 175 (31.6%) | 0 (0%) | 1 (1.0%) | 174 (40.9%) | |
| Others | 55 (9.9%) | 11 (37.9%) | 13 (13.0%) | 31 (7.3%) | |
| Medical comorbidities | |||||
| Prior history of hypertension | 53 (12.3%) | 2 (12.5%) | 30 (57.7%) | 21 (5.8%) | <0.001 |
| Prior history of hyperlipidaemia | 34 (8.1%) | 6 (33.3%) | 23 (51.1%) | 5 (1.4%) | <0.001 |
| Prior history of diabetes mellitus | 21 (5.1%) | 1 (6.3%) | 15 (37.5%) | 5 (1.4%) | <0.001 |
| Prior history of asthma | 6 (1.5%) | 1 (5.9%) | 2 (5.6%) | 3 (0.8%) | 0.024 |
| No previous medical conditions | 367 (90.4%) | 11 (68.8%) | 19 (55.9%) | 337 (94.7%) | <0.001 |
| Newly-diagnosed hypertension | 12 (2.9%) | 2 (12.5%) | 1 (2.9%) | 9 (2.5%) | 0.068 |
| Newly-diagnosed diabetes mellitus | 8 (2.0%) | 0 (0.0%) | 0 (0.0%) | 8 (2.2%) | 0.560 |
| Clinical profile | |||||
| Asymptomatic illness | 66 (11.9%) | 0 (0.0%) | 3 (3.0%) | 63 (14.8%) | 0.001 |
| Day of illness at presentation | 3.5 (±5.1) | 3.8 (±2.5) | 5.8 (±5.2) | 2.9 (±5.0) | <0.001 |
| Length of days with fever | 1.2 (±2.4) | 2.0 (±2.4) | 1.6 (±2.7) | 1.1 (±2.3) | 0.025 |
| Admission temperature (oC) | 37.7 (±3.9) | 37.4 (±0.9) | 37.3 (±0.7) | 37.8 (±4.4) | 0.480 |
| Systolic blood pressure (mmHg) | 130 (±17) | 127 (±16) | 127 (±18) | 131 (±17) | 0.078 |
| Diastolic blood pressure (mmHg) | 81 (±12) | 74 (±13) | 74 (±12) | 83 (±11) | <0.001 |
| Oxygen saturation (%) | 98 (±3) | 97 (±7) | 97 (±4) | 98 (±1) | <0.001 |
| Pulse rate (/min) | 94 (±19) | 89 (±15) | 89 (±14) | 96 (±20) | 0.002 |
| Respiratory rate (/min) | 19 (±7) | 19 (±2) | 19 (±3) | 19 (±6) | 0.888 |
| Baseline laboratory investigations | |||||
| Total white cell count (×109/L) | 6.5 (±2.2) | 6.9 (±3.9) | 6.2 (±2.7) | 6.5 (±1.9) | <0.001 |
| Absolute neutrophil count (×109/L) | 4.18 (±7.9) | 4.89 (±3.91) | 3.90 (±2.37) | 4.19 (±8.88) | 0.849 |
| Absolute lymphocyte count (×109/L) | 1.90 (±2.02) | 1.33 (±0.62) | 1.59 (±1.01) | 2.00 (±2.22) | 0.062 |
| Haemoglobin (g/dL) | 14.9 (±1.6) | 14.1 (±2.1) | 13.4 (±1.6) | 15.2 (±1.4) | 0.119 |
| Platelet count (×109/L) | 228 (±60) | 204 (±48) | 230 (±69) | 229 (±59) | 0.029 |
| Haematocrit (%) | 45 (±23) | 42 (±6) | 40 (±5) | 47 (±6) | 0.007 |
| Sodium (mmol/L) | 138 (±3) | 137 (±3) | 137 (±4) | 138 (±2) | 0.679 |
| Creatinine (mmol/L) | 79 (±30) | 82 (±27) | 81 (±64) | 79 (±14) | 0.038 |
| Albumin (g/L) | 43 (±17) | 40 (±7) | 39 (±4) | 44 (±18) | <0.001 |
| AST (units/L) | 38 (±48) | 83 (±21) | 32 (±19) | 37 (±24) | 0.001 |
| ALT (units/L) | 46 (±44) | 60 (±130) | 29 (±20) | 48 (±38) | 0.009 |
| LDH (units/L) | 436 (±423) | 691 (±110) | 459 (±259) | 418 (±377) | <0.001 |
| C-reactive protein (mg/L) | 14 (±27) | 33 (±46) | 24 (±37) | 11 (±23) | <0.001 |
| Ferritin (ug/L) | 179 (±216) | 404 (±459) | 234 (±392) | 161 (±143) | <0.001 |
A total of 44.1% of local non-dormitory cases had one or more pre-existing medical conditions compared with 37.2% of imported cases and 5.3% of migrant workers. Two imported cases, one local case and nine migrant workers were newly diagnosed with hypertension requiring treatment. Eight migrant workers were newly diagnosed with diabetes mellitus.
Sixty-three of 425 (14.8%) migrant workers were asymptomatic compared with three of 100 (3.0%) of local cases and no imported cases. Migrant workers were admitted to hospital earlier in the course of illness (2.9 ± 5.0 days) compared with local and imported cases (5.8 ± 5.2 and 3.8 ± 2.5 days, respectively). Migrant workers had a higher admission heart rate (96 ± 20 beats/min; p < 0.001), higher diastolic blood pressure (83 ± 11 mmHg; p = 0.001) and a higher systolic blood pressure (131 ± 17 mmHg; p = 0.078) (Table 1).
Imported cases had the highest proportion with severe illness, as evidenced by the highest baseline inflammatory markers (CRP 33 ± 46 mg/L, ferritin 404 ± 459 ug/L, LDH 691 ± 110 ug/L; p < 0.001), the highest proportion of cases with persistent fever (n = 7, 24.1%, p < 0.001), pneumonia (n = 10, 34.5%; p < 0.001) and the highest proportion of patients who needed supplemental oxygen (n = 4, 13.8%; p < 0.001) and intensive care (5 of 29, 17.2%; p < 0.001). Those with severe illness in the imported case cohort were older (mean age 58.2 ± 3.7 years, data not shown).
A substantial number of local non-dormitory cases (23%) developed pneumonia, with 13/23 requiring ICU support. Amongst dormitory cases, 24 (5.6%) had pneumonia, 21 (5%) had persistent fever, and one dormitory worker required ICU admission and mechanical ventilation for COVID-19 pneumonia. One imported case and two local cases developed myocardial injury. There were two deaths, both in local non-dormitory cases, who were 67 and 70 years old and had hypertension and hyperlipidaemia (Table 2 ).
Table 2.
Clinical progress and outcomes.
| Parameter | Overall (n = 554) | Imported cases (n = 29) | Community cases (n = 100) | Dormitory cases (n = 4 25) | p-value |
|---|---|---|---|---|---|
| Pneumonia | 57 (10.3%) | 10 (34.5%) | 23 (23.0%) | 24 (5.6%) | <0.001 |
| Length of hospital stay (days) | 10.2 (±13.3) | 10.6 (±7.7) | 15.1 (±18.2) | 9.0 (±11.9) | <0.001 |
| Requiring supplemental oxygen | 16 (2.9%) | 4 (13.8%) | 6 (6.0%) | 6 (1.4%) | <0.001 |
| Persistent fever >72 h | 40 (7.3%) | 7 (24.1%) | 12 (12%) | 21 (5.0%) | <0.001 |
| Acute kidney injury | 45 (8.1%) | 5 (17.2%) | 15 (15.0%) | 25 (5.9%) | <0.001 |
| Required intensive care monitoring | 19 (3.4%) | 5 (17.2%) | 13 (13.0%) | 1 (0.2%) | <0.001 |
| Required mechanical ventilation | 16 (2.9%) | 3 (10.3%) | 12 (12.0%) | 1 (0.2%) | <0.001 |
| Myocarditis/myocardial injury | 3 (0.7%) | 1 (6.3%) | 2 (2%) | 0 (0.0%) | <0.001 |
| Death | 2 (0.5%) | 0 (0.0%) | 2 (2%) | 0 (0.0%) | <0.001 |
A total of 366 (60.1%) patients were aged <40 years and primarily made up of migrant workers, where 2.2% had at least one comorbidity (Table 3 ). Patients aged <40 years were more likely to be asymptomatic (p = 0.020), have a shorter duration of fever (1.0 vs 1.8 days; p < 0.001), less likely to have persistent fever (p < 0.001), have a lower admission CRP, ferritin (p < 0.001) and were less likely to have pneumonia (4.4% vs 21.8%, p < 0.001) or require intensive care (0.3% vs 9,7%, p < 0.001) when compared with those aged ≥40 years (Table 4 ).
Table 3.
Differences in clinical profile of patients by age group.
| Parameter | Overall (n = 554) | Age <40 years (n = 366) | Age ≥40 years (n = 188) | p-value |
|---|---|---|---|---|
| Age (years) | 37 (±12) | 30 (±5) | 51 (±9) | <0.001 |
| Gender (male) | 477 (86.9%) | 323 (89.2%) | 155 (82.4%) | 0.025 |
| Ethnicity | <0.001 | |||
| Chinese | 91 (16.5%) | 25 (6.8%) | 66 (35.1%) | |
| Malay | 34 (6.1%) | 19 (5.2%) | 15 (8.0%) | |
| Indian | 199 (36.0%) | 143 (39.1%) | 56 (29.8%) | |
| Bangladeshi | 175 (31.5%) | 140 (28.3%) | 35 (18.6%) | |
| Others | 55 (9.9%) | 39 (10.7%) | 16 (8.5%) | |
| Contact history | <0.001 | |||
| Imported cases | 29 (5.2%) | 16 (4.4%) | 13 (6.9%) | |
| Community cases | 100 (18.1%) | 38 (10.4%) | 62 (33.0%) | |
| Dormitory cases | 425 (76.7%) | 312 (85.2%) | 113 (60.1%) | |
| Medical comorbidities | ||||
| Prior history of hypertension | 53 (12.3%) | 6 (2.2%) | 47 (30.5%) | <0.001 |
| Prior history of hyperlipidaemia | 34 (8.1%) | 2 (0.7%) | 32 (22.2%) | <0.001 |
| Prior history of diabetes mellitus | 21 (5.1%) | 3 (1.1%) | 18 (13.1%) | <0.001 |
| Prior history of asthma | 6 (1.5%) | 4 (1.4%) | 2 (1.5%) | 0.931 |
| No previous medical conditions | 367 (90.4%) | 269 (97.5%) | 98 (75.4%) | <0.001 |
| Clinical profile | ||||
| Asymptomatic illness | 66 (11.9%) | 52 (14.2%) | 14 (7.4%) | 0.020 |
| Day of illness at presentation | 3.5 (±5.1) | 3.1 (±5.0) | 4.2 (±5.1) | 0.012 |
| Length of days with fever | 1.2 (±2.4) | 1.0 (±2.2) | 1.8 (±2.7) | <0.001 |
| Admission temperature (oC) | 37.7 (±3.9) | 37.8 (±4.8) | 37.5 (±0.8) | 0.377 |
| Systolic blood pressure (mmHg) | 130 (±17) | 127 (±16) | 135 (±19) | <0.001 |
| Diastolic blood pressure (mmHg) | 81 (±12) | 81 (±11) | 81 (±14) | 0.771 |
| Oxygen saturation (%) | 98 (±3) | 98 (±1) | 97 (±4) | 0.001 |
| Pulse rate (/min) | 94 (±19) | 96 (±19) | 92 (±18) | 0.053 |
| Respiratory rate (/min) | 19 (±7) | 19 (±6) | 20 (±6) | 0.444 |
| Laboratory investigations | ||||
| Total white cell count (×109/L) | 6.5 (±2.2) | 6.5 (±1.9) | 6.4 (±2.7) | 0.730 |
| Absolute neutrophil count (×109/L) | 4.18 (±7.9) | 4.2 (±9.6) | 4.1 (±2.7) | 0.924 |
| Absolute lymphocyte count (×109/L) | 1.90 (±2.02) | 2.0 (±2.4) | 1.7 (±0.9) | 0.014 |
| Haemoglobin (g/dL) | 14.9 (±1.6) | 15.2 (±1.5) | 14.3 (±1.7) | <0.001 |
| Platelet count (×109/L) | 228 (±60) | 231 (±59) | 221 (±62) | 0.074 |
| Haematocrit (%) | 45 (±23) | 47 (±28) | 42 (±5) | 0.023 |
| Sodium (mmol/L) | 138 (±3) | 138 (±2) | 137 (±3) | <0.001 |
| Creatinine (mmol/L) | 79 (±30) | 77 (±15) | 83 (±47) | 0.041 |
| Albumin (g/L) | 43 (±17) | 44 (±20) | 40 (±4) | 0.010 |
| AST (units/L) | 38 (±48) | 36 (±26) | 41 (±77) | 0.428 |
| ALT (units/L) | 46 (±44) | 48 (±41) | 41 (±50) | 0.067 |
| LDH (units/L) | 436 (±423) | 413 (±409) | 485 (±448) | 0.069 |
| C-reactive protein (mg/L) | 14 (±27) | 10 (±23) | 22 (±33) | <0.001 |
| Ferritin (ug/L) | 179 (±216) | 139 (±90) | 268 (±347) | <0.001 |
Table 4.
Clinical progress and outcomes by age group.
| Parameter | Overall (n = 554) | Age < 40 years (n = 366) | Age ≥40 years (n = 188) | p-value |
|---|---|---|---|---|
| Pneumonia | 57 (10.3%) | 16 (4.4%) | 41 (21.8%) | <0.001 |
| Length of hospital stay (days) | 10.2 (±13.3) | 10.3 (±13.8) | 11.1 (±11.5) | 0.451 |
| Requiring supplemental oxygen | 16 (2.9%) | 4 (1.1%) | 12 (6.4%) | <0.001 |
| Persistent fever >72 h | 40 (7.3%) | 14 (3.8%) | 26 (14.0%) | <0.001 |
| Acute kidney injury | 45 (8.1%) | 23 (6.3%) | 22 (11.7%) | 0.027 |
| Required intensive care monitoring | 19 (3.4%) | 1 (0.3%) | 18 (9.7%) | <0.001 |
| Required mechanical ventilation | 16 (2.9%) | 0 (0.0%) | 16 (8.5%) | <0.001 |
| Myocarditis/myocardial injury | 3 (0.7%) | 0 (0.0%) | 3 (2.3%) | 0.011 |
| Death | 2 (0.5%) | 0 (0.0%) | 2 (1.5%) | 0.040 |
Patients with pneumonia (n = 57) were older, more likely to have pre-existing medical comorbidities, more likely to have elevated inflammatory markers and lymphopenia. On multivariable analyses, older age (>40 years, adjusted OR 2.39, 95% CI 1.01–5.64; p = 0.047), presence of medical comorbidities (adjusted OR 3.92, 95% CI 1.54–10.00; p = 0.004) and elevated LDH at presentation (>550 units/L, adjusted OR 4.29, 95% CI 1.71–10.75; p = 0.002) remained independently associated with the presence of COVID-19 pneumonia (Table 5 ).
Table 5.
Univariate and multivariable analyses of parameters associated with pneumonia in hospitalised patients with COVID-19.
| Parameter | Univariate analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| Pneumonia (n = 57) | No pneumonia (n = 497) | Odds ratio (95% CI) | p-value | Adjusted odds ratio (95% CI) | p-value | |
| Age >40 years | 41 (71.9%) | 147 (29.6%) | 6.10 (3.32–11.23) | <0.001 | 2.39 (1.01–5.64) | 0.047 |
| Asymptomatic illness | 4 (7.0%) | 62 (12.5%) | 0.53 (0.19–1.51) | 0.228 | ||
| Hypertension | 15 (26.3%) | 38 (7.6%) | 4.43 (2.20–8.94) | <0.001 | ||
| Hyperlipidaemia | 15 (26.3%) | 19 (3.8%) | 9.42 (4.35–20.40) | <0.001 | ||
| Diabetes mellitus | 7 (12.3%) | 14 (2.8%) | 4.96 (1.88–13.08) | 0.003 | ||
| Asthma | 2 (3.5%) | 4 (0.8%) | 4.68 (0.83–26.37) | 0.113 | ||
| Prior medical comorbidities | 16 (40.0%) | 23 (6.3%) | 9.94 (4.65–21.27) | <0.001 | 3.92 (1.54–10.00) | 0.004 |
| Elevated transaminases | 14 (24.6%) | 88 (17.7%) | 1.51 (0.79–2.89) | 0.206 | ||
| Elevated CRP > 60 mg/L | 11 (19.3%) | 13 (2.6%) | 8.90 (3.78–21.00) | <0.001 | 1.27 (0.32–5.10) | 0.785 |
| Elevated LDH > 550 units/L | 20 (35.1%) | 29 (5.8%) | 8.72 (4.51–16.89) | <0.001 | 4.29 (1.71–10.75) | 0.002 |
| Elevated ferritin >300 ng/L | 13 (22.8%) | 36 (7.2%) | 3.78 (1.89–7.66) | <0.001 | 2.60 (0.85–7.94) | 0.095 |
| Absolute neutrophil count >4 × 109/L | 23 (40.4%) | 172 (34.6%) | 1.28 (0.73–2.24) | 0.390 | ||
| Absolute lymphocyte count <1 × 109/L | 22 (38.6%) | 66 (13.3%) | 4.11 (2.27–7.43) | <0.001 | 2.22 (0.89–5.49) | 0.141 |
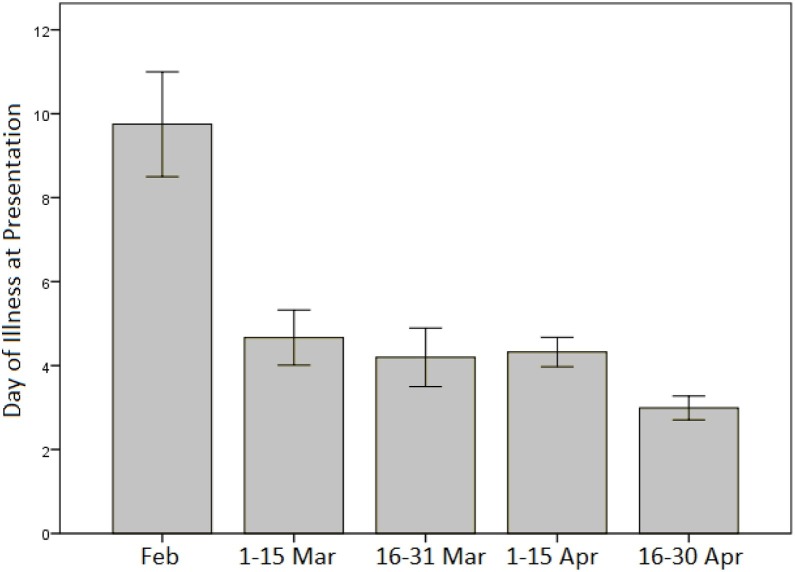
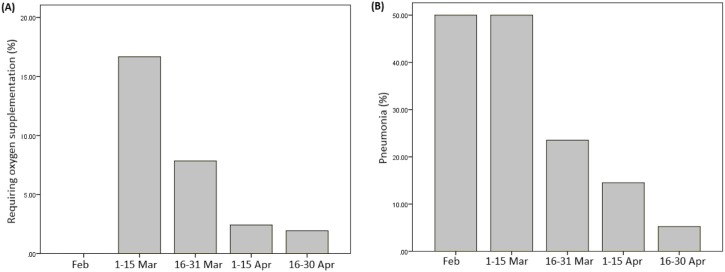
Patients had a shorter duration of symptoms at presentation later on in the disease outbreak in Singapore, with an average symptom duration of close to 10 days in February 2020, falling to a mean of 3 days by late April 2020 (Figure 2 ). A higher proportion of patients required supplemental oxygen (Chi–squared p = 0.007) and had radiographic evidence of pneumonia in March 2020 compared with April 2020 (Chi–squared p < 0.001) (Figure 3 A and B).
Figure 2.
Day of illness at presentation for COVID-19 patients from February to April 2020.
Figure 3.
Percentage of COVID-19 patients (A) requiring oxygen supplementation and with (B) radiological evidence of pneumonia from February to April 2020.
Discussion
The COVID-19 disease pandemic shifted dramatically in Singapore in the first few months, as captured by this study. Early on, the majority of patients in Singapore (late January to early March 2020) were young and healthy overseas cases. This was similar to the experience in Hong Kong, where citizens who had been working or studying abroad had returned to Singapore, thereby importing the virus (Cruz et al., 2020). Despite a third of this group having pneumonia and 10% requiring mechanical ventilation, all had good clinical outcomes, with no deaths. Of note, the patients who required intensive care this this group were all aged >40 years (mean age 58.2 ± 3.7 years). The surge in imported cases coincided with the increasing numbers of COVID-19 cases worldwide, especially in China, Europe, the United States and Canada, where these travellers had returned from (Cowling et al., 2020).
Enforcement of strict border control measures on 21 March 2020 resulted in a reduction in the number of imported cases but an increase in locally transmitted cases, with a number of large clusters particularly at a social gathering with many seniors, until the lockdown on 7 April 2020 (Figure 1) (Ministry of Health, 2020). Lockdown led to a drastic decrease in local cases from mid-April 2020, yet simultaneously, multiple large outbreaks came to light in foreign worker dormitories (Figure 1). As there was no ability to isolate and quarantine migrant workers in an efficient and effective manner, the outbreak grew exponentially (Chew et al., 2020). Active case finding within dormitories, transfer of migrant workers to hospitals and purpose-built cohort facilities were put in place in an attempt to contain the spread and the virus from the local population (Government of Singapore, 2020a, Government of Singapore, 2020b).
Alongside the demographic shift, the clinical presentation and progress of these three groups of patients differed over time. Several factors account for this observation. First, migrant worker cases were young men with few medical comorbidities, which favoured a shorter and uncomplicated disease course and thus better COVID-19 outcomes later on in the outbreak in Singapore. This was comparable with other data, which have shown that the risk of developing pneumonia and other severe disease is related to age (Mueller et al., 2020). Singapore Ministry of Health set the age cut off as ≥40 years for determining those who are at high versus low-risk of disease, although it is acknowledged that other countries have used age cut-offs >50 years or 60 years to define the at-risk populations (Mallapaty, 2020). Second, patients presented earlier in their disease course later on in the outbreak. This was likely a consequence of greater awareness and the widening of testing. Patients diagnosed with COVID-19 were then admitted to a hospital. Similarly, all migrant workers who were quarantined within their dormitories were regularly screened for symptoms and fever. They were swabbed and transferred to a hospital if they tested positive. Additionally, widespread public-health efforts, including the establishment of designated public health preparedness clinics, greatly expanded Singapore’s testing capacity and prompted health-seeking behaviour, which likely contributed to earlier detection. Earlier detection of locally-transmitted cases within the community may have helped to limit person-to-person transmission in the non-dormitory dwelling community (Peck, 2020, Gao et al., 2020). Third, this study describes a substantial cohort of nearly 15% with asymptomatic disease, as a consequence of active case-finding in the dormitories. The likely proportion of asymptomatic disease in Singapore may in fact be substantially larger, since many migrant workers, after the construction of purpose-built facilities in Singapore, bypassed the hospital and were directly admitted to these facilities. Serology studies of this population that are currently underway may describe the true extent of asymptomatic disease in this population.
The overall case fatality rate in Singapore was low and appeared to decrease over time. This study attributed this to the changing demographics of affected patients; however, there might have been other factors that also contributed to this, namely: ecological studies have demonstrated that there is a difference in the strain that affected migrant workers in comparison with that which affected non-migrant worker populations (Young et al., 2020, GISAID, 2020). However, the impact of virulence between strains and the consequent impact on disease severity and mortality has been disputed, with a suggestion that epidemiological explanations must be first ruled out before attributing changes in risk to pathogen factors (Okell et al., 2020). The current study remained underpowered to evaluate the effects of the introduction of new treatments (e.g. remdesivir, which had been used from 13 March 2020) on mortality and outcomes in this population.
Overall, the current study population largely represented those with mild disease who were hospitalised to fulfil isolation requirements. This study showed that, despite controlling COVID-19 sufficiently well in the community, a lapse of control in migrant worker dorms led to a rapid rise in cases. The disease encountered in this population was much less severe, resulting in Singapore having one of the lowest case fatality rates in the world, approximating the age-standardised mortality rates in South Korea, Spain, China and Italy (Our World in Data, 2020).
Limitations
This was a single-centre moderately-sized cohort of patients diagnosed with COVID-19. The study only examined patients who were hospitalised and could not examine those in isolation facilities outside the hospital. Additionally, owing to the nature of active case-finding in the dormitories, there was preselection of those without symptoms or with minimal disease from the migrant worker cohort. Clinical progress and outcomes were only measured within the hospital admission, and this study was unable to longitudinally examine patients after discharge for medium-term to long-term sequelae of the disease, which has been described even amongst those with initial mild disease (Yelin et al., 2020). In the current hospital, at the time of data capture, deaths from COVID-19 were attributable to COVID-19 if the patient had PCR-confirmed disease and died from overwhelming lung infection, sepsis or acute respiratory distress syndrome. COVID-19-associated cardiac arrests or thrombotic and cardiovascular complications were insufficiently well-recognised at the time and thus the impact of these complications on the burden of COVID-19 disease and mortality rate may not have been captured (Ho et al., 2020b, Ho et al., 2020c). Similarly, pre-hospital deaths in young migrant workers from thrombo-embolic phenomena were not captured in this cohort.
Conclusions
Border closures and effective community public health measures helped to keep the spread of COVID-19 in the community outside the dormitories under control, protecting the vulnerable local aging population. However, the population residing in dormitories did not similarly benefit from these containment measures. Despite this, it was found that the majority of migrant workers in this cohort did not become severely unwell from COVID-19 respiratory disease. The degree of severity of illness in Singapore and the low mortality rate may be partly attributed to the fact that migrant workers and younger imported cases were largely protected by their young age and few medical comorbidities.
Conflict of interest
All authors have no conflicts of interest to declare.
Funding source
There was no funding for this study.
Ethical approval
This study was approved by the hospital’s institutional review board (National Healthcare Group (NHG) Domain Specific Review Board (DSRB) 2020/00545).
References
- Bagdasarian N., Fisher D. Heterogenous COVID-19 transmission dynamics within Singapore: a clearer picture of future national responses. BMC Med. 2020;18:166. doi: 10.1186/s12916-020-01625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew M.H., Koh F.H., Wu J.T., Ngaserin S., Ng A., Ong B.C., et al. Clinical assessment of COVID-19 outbreak among migrant workers residing in a large dormitory in Singapore. J Hosp Infect. 2020;106(1):202–203. doi: 10.1016/j.jhin.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling B.J., Ali S.T., Ng T.W.Y., Tsang T.K., Li J.C.M., Fong M.W., et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020 doi: 10.1016/S2468-2667(20)30090-6. PMID: 32311320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz C.J.P., Ganly R., Li Z., Gietel-Basten S. Exploring the young demographic profile of COVID-19 cases in Hong Kong: evidence from migration and travel history data. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0235306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Xu Y., Sun C., Wang X., Guo Y., Qiu S., et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2020;(May) doi: 10.1016/j.jmii.2020.05.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISAID . 2020. Genomic Epidemiology of hCoV-19.https://www.gisaid.org/epiflu-applications/phylodynamics/ [Accessed 10 November 2020] [Google Scholar]
- Goh K.T., Cutter J., Heng B.H., Ma S., Koh B.K.W., Kwok C., et al. Epidemiology and control of SARS in Singapore. Ann Acad Med Singapore. 2006;35:301–316. [PubMed] [Google Scholar]
- Government of Singapore . 2020. Containing COVID-19 Spread at Foreign Worker Dormitories.https://www.gov.sg/article/containing-covid-19-spread-at-foreign-worker-dormitories [Accessed August 2020] 14 April. [Google Scholar]
- Government of Singapore . 2020. Tackling Transmissions in Migrant Worker Clusters.https://www.gov.sg/article/tackling-transmissions-in-migrantworker-clusters [Accessed August 2020] 22 April. [Google Scholar]
- Ho J.S., Sia C.H., Chan M.Y., Lin W., Wong R.C. Coronavirus-induced myocarditis: a meta summary of cases. Heart Lung. 2020;49(6):681–685. doi: 10.1016/j.hrtlng.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J.S.Y., Tambyah P.A., Ho A.F.W., Chan M.Y.Y., Sia C.H. Effect of coronavirus infection on the human heart: a scoping review. Eur J Prev Cardiol. 2020;27(11):1136–1148. doi: 10.1177/2047487320925965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J.S.Y., Tambyah P.A., Sia C.H. A call for vaccines against COVID-19: implications for cardiovascular morbidity and healthcare utilization. Cardiovasc Drugs Ther. 2020;34(4):585–587. doi: 10.1007/s10557-020-06985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.U., Kim M.J., Lee J., Bae S., Jung J., Kim S.H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;26(7) doi: 10.1016/j.cmi.2020.04.040. 948.e1-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. Migrant workers and COVID-19. Occup Environ Med. 2020;77(9):634–636. doi: 10.1136/oemed-2020-106626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V.J., Chiew C.J., Khong W.X. Interrupting transmission of COVID-19: lessons from containment efforts in Singapore. J Travel Med. 2020:27. doi: 10.1093/jtm/taaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W.H., Wong W.M. COVID-19: notes from the front line, Singapore’s primary health care perspective. Ann Fam Med. 2020;18(3):259–261. doi: 10.1370/afm.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R.J., Lee T.H., Lye D.C.B. From SARS to COVID-19: the Singapore journey. Med J Australia. 2012;212(11):497–502. doi: 10.5694/mja2.50623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;(February) doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. The coronavirus is most deadly if you are older and male—new data reveal the risks. Nature. 2020;585(7823):16–17. doi: 10.1038/d41586-020-02483-2. [DOI] [PubMed] [Google Scholar]
- Ministry of Health . 2020. COVID-19 Local Situation Report Archive–Situation Report.https://www.moh.gov.sg/docs/librariesprovider5/2019-ncov/situation-report---02-may-2020.pdf [Accessed 20 September 2020] [Google Scholar]
- Ministry of Health Singapore . MOH; Singapore: 2014. MOH Pandemic Readiness and Response Plan for Influenza and Other Acute Respiratory Diseases (revised April 2014)https://www.moh.gov.sg/docs/librariesprovider5/diseasesupdates/interim-pandemic-plan-public-ver-_april-2014.pdf (viewed August 2020) [Google Scholar]
- Ministry of Health Singapore . 2020. Circuit Breaker to Minimise Further Spread of COVID-19.https://www.moh.gov.sg/news-highlights/details/circuit-breaker-to-minimise-further-spreadof-covid-19 [Accessed August 2020] 3 April. [Google Scholar]
- Ministry of Manpower Singapore . 2020. Housing for Foreign Workers.https://mom.gov.sg/passes-and-permits/work-permit-for-foreign-worker/housing [Accessed 20 September 2020] [Google Scholar]
- Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging (Albany NY) 2020;12(10):9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y., Li Z., Chua Y.X., Chaw W.L., Zhao Z., Er B., et al. Evaluation of the effectiveness of surveillance and containment measures for the first 100 patients with COVID-19 in Singapore–January 2-February 29, 2020. Morb Mortal Wkly Rep. 2020;69:307–311. doi: 10.15585/mmwr.mm6911e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngiam J.N., Tham S.M., Vasoo S., Poh K.K. COVID-19: local lessons from a global pandemic. Singapore Med J. 2020 doi: 10.11622/smedj.2020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okell L.C., Verity R., Katzourakis A., Volz E.M., Watson O.J., Misra S., et al. Host or pathogen-related factors in COVID-19 severity?—authors’ reply. Lancet. 2020;396:1397. doi: 10.1016/S0140-6736(20)32212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Our World in Data . 2020. Statistics and Research: Mortality Risk of COVID-19.https://ourworldindata.org/mortality-risk-covid?country=SGP∼USA∼GBR∼TWN#case-fatality-rate-of-covid-19-by-age . [Accessed 25 September 2020] [Google Scholar]
- Peck K.R. Early diagnosis and rapid isolation: response to COVID-19 outbreak in Korea. Clin Microbiol Infect. 2020;26(7):805–807. doi: 10.1016/j.cmi.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia C.H., Ngiam J.N., Chew N.W.S., Beh L.L.D., Poh K.K. An educational case series on electrocardiographs during the COVID-19 pandemic and their implications on therapy. Singapore Med J. 2020 doi: 10.11622/smedj.2020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;(February) doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.E., Li Z.B., Chiew C.J., Yong S.E., Toh M.P., Lee V.J. Presymptomatic transmission of SARS-CoV-2–Singapore, January 23–March 16, 2020. Morb Mort Wkly Rep. 2020;69:411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelin D., Wirtheim E., Vetter P., Kalil A.C., Bruchfeld J., Runold M., et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Ng S.T., Farwin A., Low A.P.T., Chang C.M., Lim J. Health equity considerations in COVID-19: geospatial network analysis of the COVID-19 outbreak in the migrant population in Singapore. J Travel Med. 2020 doi: 10.1093/jtm/taaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.E., Fong S.W., Chan Y.H., Mak T.M., Ang L.W., Anderson D.E., et al. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet. 2020;396:603–611. doi: 10.1016/S0140-6736(20)31757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]