To the Editor:
Increased VTE reported in patients with coronavirus disease 2019 (COVID-19)1,2 leads some to recommend routine use of therapeutic anticoagulation.3 However, the incidence of VTE in patients with COVID-19 ranges widely, depending on a number of variables. We hypothesized that ICU patients experience increased symptomatic VTE compared with ward patients, despite standard prophylactic anticoagulation.
Methods
Patients
Data on patients with COVID-19 admitted to Brigham and Women’s Hospital entered into the Research Registry for the Study of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) Infection (institutional review board approved) were retrospectively collected from March 7 to April 13, 2020; median follow-up was 7 days (interquartile range [IQR], 4-14 days). Readmissions were not included. SARS-CoV-2 positivity was determined by reverse-transcription polymerase chain reaction on nasopharyngeal samples. Subjects were classified as ICU patients if any time during admission was spent in the ICU.
Treatment protocols and ICU admission criteria for patients with COVID-19 were documented at covidprotocols.org. CBC count was measured daily; prothrombin time, activated partial thromboplastin time (aPTT), fibrinogen, lactate dehydrogenase (LDH), D-dimer, C-reactive protein (CRP), and ferritin were measured at admission and at least every 3 days. Pharmacologic VTE prophylaxis was recommended for all patients, with enoxaparin 40 mg subcutaneously daily, or unfractionated heparin 5,000 International Units subcutaneously twice or three times daily, if not contraindicated. Administered doses were confirmed in the electronic medical record. Direct oral anticoagulants were not used in admitted patients.
Outcomes
The primary outcome was symptomatic VTE caused by lower extremity DVT or pulmonary embolism (PE), determined by CT pulmonary angiography (CTPA) or compression ultrasonography (CUS). In intubated patients, symptomatic VTE was defined as imaging performed because of clinical findings suggestive of VTE. No surveillance imaging was performed. Suspected PE events in mechanically ventilated unstable patients with respiratory decompensation, right heart strain on echocardiogram, and a decision to move to therapeutic anticoagulation for suspected PE, were reviewed and confirmed by three authors. Major bleeding was defined using International Society on Thrombosis and Haemostasis4 criteria. ARDS was defined by Berlin criteria.5
Statistical Analysis
Analyses were performed in R 3.6.1 (The R Project for Statistical Computing). Variables were compared with Student t tests, analyses of variance, or rank-sum tests, as appropriate. Kaplan-Meier analyses were performed using the survival R package; we performed Tarone-Ware tests because most events occurred early in the follow-up time, and Fine and Gray6 competing risks analysis to ensure cumulative incidences were approximately similar to Kaplan-Meier models. Cox regression was used to evaluate the association of inflammatory and coagulation markers with VTE events. Proportional hazard assumptions were evaluated with Schoenfeld residual plots and tests. Association of aPTT with inflammatory markers was assessed with linear regression. Models were adjusted for age, sex, and BMI.
Results
Characteristics of the Study Population
Median follow-up time of the 210 patients (Table 1) was 7 days (IQR, 4-14 days); ICU follow-up time was significantly longer than non-ICU follow-up (12 vs 5 days, respectively). Twenty patients (9.5%) were still admitted at the end of the study. ICU patients exhibited a distinct hyperinflammatory procoagulant phenotype with prolonged aPTT and significantly higher levels of fibrinogen, D-dimer, LDH, ferritin, CRP, and lactic acid (Table 1). In linear regression, aPTT was nominally associated with CRP (β = 1.16; 95% CI, 0.09-2.2; P = .035), but not D-dimer, LDH, erythrocyte sedimentation rate, or ferritin levels.
Table 1.
Clinical Characteristics of All Patients Included in the Study
| Characteristics | Overall (N = 210) | Wards (n = 108) | ICU (n = 102) | P Value (ICU vs Wards) |
|---|---|---|---|---|
| Age, y | 62.21 ± 16.23 | 59.94 ± 17.19 | 64.61 ± 14.86 | .037 |
| Sex, male | 101 (48.1) | 42 (38.9) | 59 (57.8) | .009 |
| Race | .054 | |||
| Asian | 12 (5.7) | 10 (9.3) | 2 (2.0) | |
| Black | 60 (28.6) | 31 (28.7) | 29 (28.4) | |
| Hispanic | 33 (15.7) | 16 (14.8) | 17 (16.7) | |
| White | 67 (31.9) | 35 (32.4) | 32 (31.4) | |
| Other | 26 (12.4) | 14 (13.0) | 12 (11.8) | |
| Unavailable | 12 (5.7) | 2 (1.9) | 10 (9.8) | |
| Weight, kg | 84.65 ± 23.29 | 81.58 ± 19.11 | 87.91 ± 26.74 | .049 |
| BMI, kg/m2 | 29.80 ± 7.04 | 29.53 ± 6.24 | 30.08 ± 7.83 | .573 |
| Comorbidities | ||||
| Hyperlipidemia | 80 (38.1) | 37 (34.3) | 43 (42.2) | .30 |
| Hypertension | 125 (59.5) | 56 (51.9) | 69 (67.6) | .029 |
| Coronary artery disease | 18 (8.6) | 7 (6.5) | 11 (10.8) | .386 |
| Congestive heart failure | 18 (8.6) | 10 (9.3) | 8 (7.8) | .905 |
| Diabetes mellitus | 70 (33.3) | 31 (28.7) | 39 (38.2) | .188 |
| Atrial fibrillation | 17 (8.1) | 8 (7.4) | 9 (8.8) | .902 |
| Asthma | 35 (16.7) | 20 (18.5) | 15 (14.7) | .578 |
| COPD | 17 (8.1) | 7 (6.5) | 10 (9.8) | .529 |
| Idiopathic pulmonary fibrosis | 5 (2.4) | 2 (1.9) | 3 (2.9) | .676 |
| History of solid organ malignancy | 40 (19.0) | 27 (25.0) | 13 (12.7) | .037 |
| History of hematologic malignancy | 11 (5.2) | 3 (2.8) | 8 (7.8) | .126 |
| Bone marrow transplant | 4 (1.9) | 0 (0.0) | 4 (3.9) | .054 |
| Solid organ transplant | 5 (2.4) | 3 (2.8) | 2 (2.0) | > .99 |
| Tobacco history | 54 (25.7) | 29 (26.9) | 25 (24.5) | .818 |
| Prior VTE | 9 (4.3) | 5 (4.6) | 4 (3.9) | > .99 |
| Platelets, k/μL | 210.73 ± 92.82 | 203.19 ± 83.12 | 218.73 ± 101.89 | .226 |
| Fibrinogen, mg/dL | 561.32 ± 187.76 | 462.89 ± 170.01 | 605.22 ± 179.40 | < .001 |
| Prothrombin time, s | 13.85 (13.17-14.90) | 13.55 (12.90-14.60) | 13.95 (13.40-15.10) | .007 |
| Activated partial thromboplastin time, s | 34.30 (31.10-38.80) | 33.50 (29.98-35.60) | 34.80 (31.35-40.05) | .029 |
| D-dimer, ng/mL at admission | 1,064.00 (564.00-2,244.00) | 798.00 (408.00-1,446.00) | 1,456.50 (732.75-2,660.25) | < .001 |
| D-dimer, ng/mL at peak | 2,887.00 (1,122.00-4,000.00) | 1,032.50 (502.25-2,437.25) | 3,964.00 (2,499.50-4,000.00) | < .001 |
| Lactate dehydrogenase, Units/L | 330.00 (247.50-450.50) | 278.00 (230.00-346.00) | 423.00 (299.50-534.00) | < .001 |
| Ferritin, μg/L | 589.50 (269.50-1,251.75) | 407.00 (212.00-855.00) | 773.00 (359.00-1,574.00) | < .001 |
| C-reactive protein, mg/L | 99.30 (43.00-169.90) | 65.70 (19.30-117.30) | 149.60 (81.60-221.50) | < .001 |
| Lactate, mmol/L | 1.50 (1.00-2.10) | 1.30 (0.93-1.70) | 1.70 (1.15-2.35) | .007 |
| Anticoagulation at start of admission | 190 (90.5) | 91 (84.3) | 99 (97.1) | .003 |
| Prophylactic anticoagulation | 169 (80.5) | 80 (74.1) | 89 (87.3) | .025 |
| Therapeutic anticoagulation | 21 (10.0) | 11 (10.2) | 10 (9.8) | > .99 |
| No. of VTE events | .006 | |||
| DVT | 7 | 0 | 7 | |
| PE | 2 | 0 | 2 | |
| Suspected PE | 2 | 0 | 2 | |
| On therapeutic anticoagulation at time of VTE event (%) | .001 | |||
| No | 8 | 0 | 8 | |
| Yes | 1 | 0 | 1 | |
| ARDS | 86 (41.0) | 0 (0.0) | 86 (84.3) | < .001 |
| Intubated | 88 (41.9) | 0 (0.0) | 88 (86.3) | < .001 |
| Bacterial pneumonia | 9 (4.3) | 0 (0.0) | 9 (8.8) | .001 |
| CVVH | 17 (8.1) | 0 (0.0) | 17 (16.7) | < .001 |
| CVVH circuit failure | 9 (4.3) | 0 (0.0) | 9 (8.8) | .001 |
| Total length of stay, d | 7.00 (4.00-15.00) | 5.00 (2.00-7.50) | 15.00 (8.00-21.50) | < .001 |
| ISTH major bleeding | 2 (1.0) | 0 (0.0) | 2 (2.0) | .235 |
| Death | 35 (16.7) | 7 (6.5) | 28 (27.5) | < .001 |
| Days followed | 7.00 (4.00-14.00) | 5.00 (2.00-8.00) | 12.00 (7.00-19.00) | < .001 |
| Discharged | 190 (90.5) | 107 (99.1) | 83 (81.4) | < .001 |
Values are mean ± SD, No. (%), median (interquartile range), or as otherwise indicated. CVVH = continuous venovenous hemofiltration; ISTH = International Society on Thrombosis and Haemostasis; PE = pulmonary embolism.
Of the 210 patients, 190 (90.5%) received anticoagulation at admission, with 169 (80.5%) with prophylactic anticoagulation. Twenty patients received no anticoagulation, for reasons including patient/family refusal (n = 5), contraindication because of bleeding risk (n = 7), transition to comfort care (n = 1), postpartum (n = 3), and unknown (n = 4). There were nine symptomatic radiographically confirmed VTE events. Seven of 17 CUSs showed proximal DVT, and two of 25 CTPA scans were positive, with both PE events involving lobar or segmental pulmonary arteries. Eight VTE events occurred in the ICU; one PE occurred after transfer to the ward after prolonged ICU admission. Eight of nine VTE events occurred while on prophylactic anticoagulation. The median time from ICU admission to VTE diagnosis was 6 days (IQR, 5-7 days), suggesting that patients were not admitted to the ICU because of VTE. An additional two ICU patients had suspected PE with increase to therapeutic anticoagulation.
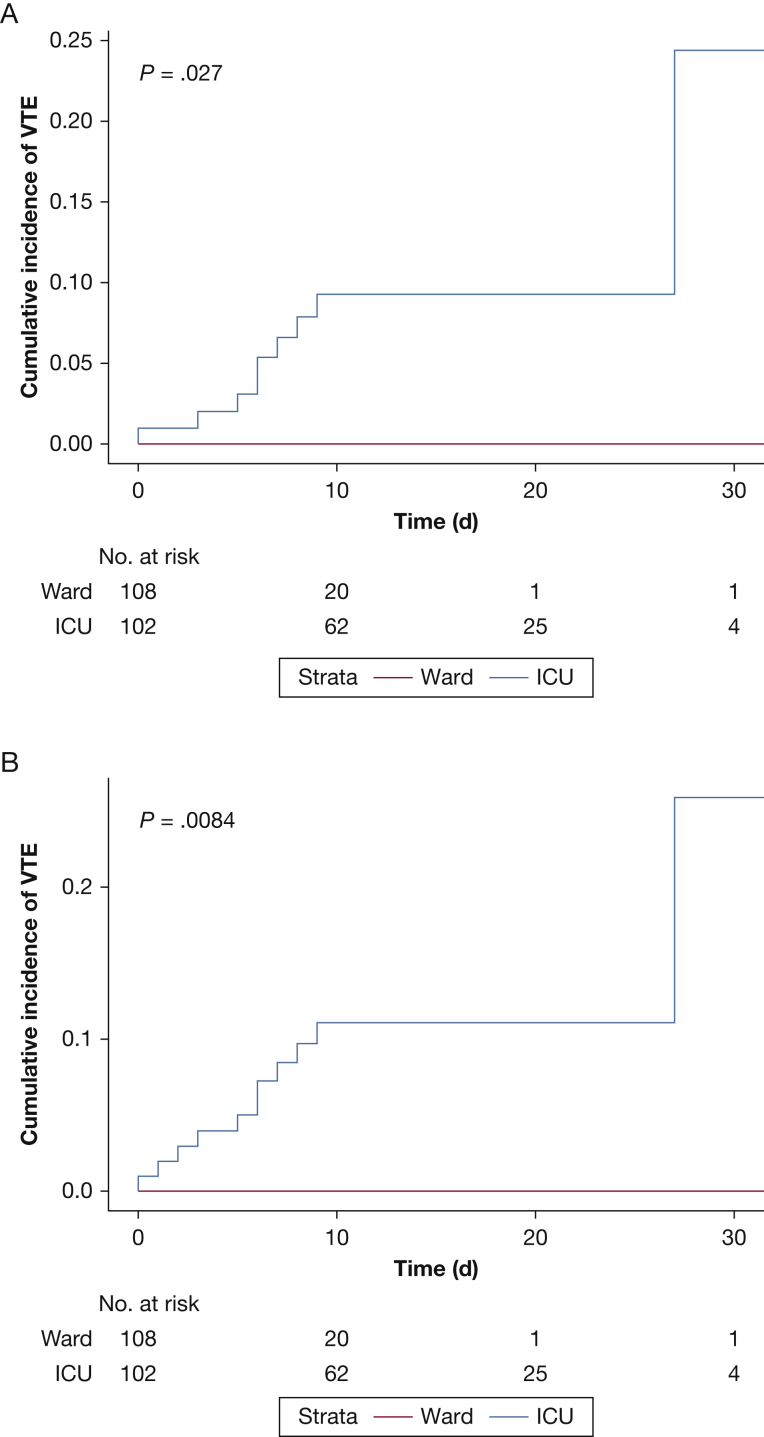
For ICU patients on anticoagulation, the 14-day cumulative incidence of radiographically confirmed VTE was 9.3% (95% CI, 4.7-17.8). No events occurred in ward patients. Including strongly suspected PEs leading to initiation of therapeutic anticoagulation, the 14-day cumulative incidence of VTE was 11.1% (95% CI, 6.1-19.7). Cumulative incidences of VTE stratified by ward and ICU patients are shown in Figure 1. Competing risk models yielded similar results. In Cox regression, LDH, CRP, ferritin, D-dimer, and aPTT were not significantly associated with an increased hazard for VTE events.
Figure 1.
A-B, Cumulative incidence plots based on Kaplan-Meier analyses, stratified by whether patients required ICU admission. Risk sets are shown below the graphs. P values are derived from Tarone-Ware tests. A, Cumulative incidences using radiographically confirmed VTE events. B, Cumulative incidences based on VTE definitions in part A and strongly suspected pulmonary embolism with right ventricular strain on echocardiography and a clinical change to therapeutic anticoagulation.
Two major bleeding events occurred: one hemodynamically significant bleed with a drop in hemoglobin ≥ 2 g/dL in < 24 h, and one intracranial hemorrhage. Both patients were receiving therapeutic anticoagulation at the time: one patient with DVT, and one admitted on therapeutic anticoagulation for prior VTE.
Discussion
Patients in the ICU had a higher incidence of symptomatic VTE events compared with ward patients, with a 14-day cumulative incidence of 9.3% despite the use of at least standard dose VTE prophylaxis in all patients experiencing an event. Our findings suggest that standard dose VTE prophylaxis is effective for ward patients, but may be insufficient to prevent VTE in ICU patients with COVID-19.
The cumulative incidence of 9.3% for VTE in ICU patients with COVID-19 is somewhat lower than recently reported cumulative incidences of VTE in ICU patients with COVID-19 ranging from 11% to 70%.1,2,7,8 In two studies,1,2 the dose of prophylactic anticoagulation was 25% lower, possibly explaining their higher VTE rates. One study using screening CUS found DVTs in 100% of those on prophylactic and 56% of those on therapeutic anticoagulation8; however, our study focuses on symptomatic VTE events leading to a change in anticoagulation. The threshold to obtain CTPA or CUS in patients with COVID-19 at our institution is extremely high given infection control concerns and difficulty moving mechanically ventilated patients; VTE rates are likely underestimated. Patients in the ICU had significantly higher fibrinogen, LDH, CRP, and D-dimer levels. Because of recent reports of the presence of lupus anticoagulants resulting in elevated aPTT, we found that although the aPTT was associated with CRP levels, elevated aPTT levels did not predict VTE events.9
In summary, ICU patients exhibited a distinct phenotype characterized by elevated inflammatory markers, ARDS, and a significant increase in the cumulative incidence of symptomatic VTE compared with those not requiring ICU care, suggesting that standard VTE prophylaxis is insufficient in these patients. Heparins were used for VTE prophylaxis, not direct oral anticoagulants; therefore, failure was not caused by drug-drug interactions. Although individual inflammatory markers did not predict VTE events, it is likely that the development of a thromboinflammatory phenotype is a harbinger of critical illness, need for ICU care, and risk of VTE. Most VTE events occurred early in hospital admission, with a second set of events later in the course for ICU patients. These findings suggest that patients requiring ICU care experience events early in the setting of profound host inflammatory responses to SARS-CoV-2, but later VTE risk may be caused by factors associated with prolonged ICU care. Further investigation into the appropriate dose of prophylactic anticoagulation and associated risks and benefits in ICU patients with COVID-19 is urgently needed.
Acknowledgments
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank the Research Registry for the Study of SARS-CoV-2 Infection (COVID-19) at Brigham and Women’s Hospital.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES: The authors have reported to CHEST the following: J. M. C. received personal fees from Bristol-Myer Squibb, Abbott, Portola, and Pfizer; and research funding for the institution from CSL Behring. R. M. B. serves on the advisory board for Merck. M. H. C. received personal fees from AstraZeneca and Illumina, and research funding for the institution from GSK and Bayer. R. L. Z. is a consultant and stockholder for Amagma Therapeutics. None declared (M. M., K. W. S., E. C. C., V. C., N. T. C., L. E. F., A. E. W.).
FUNDING/SUPPORT: M. M. is supported by the National Heart, Lung, and Blood Institute, NIH [Grants T32HL007427, U01 HL089897, U01 HL089856]. M. H. C. is supported by the NIH [Grants R01HL137927, R01HL135142].
References
- 1.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulman S., Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 5.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Fine J.P., GR A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94:496–509. [Google Scholar]
- 7.Poissy J., Goutay J., Caplan M. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 8.Llitjos J.-F., Leclerc M., Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]