Abstract
Background
Sleep-disordered breathing (SDB) is independently associated with insulin resistance, glucose intolerance, and type 2 diabetes mellitus. However, data on whether SDB alters the metabolism of free fatty acids (FFAs) are lacking.
Research Question
The primary objective of the current study was to characterize alterations in FFA metabolism across the spectrum of SDB severity.
Study Design and Methods
The study sample included 118 participants with and without SDB who underwent full-montage polysomnography, the frequently sampled IV glucose tolerance test (FSIGTT), and body composition measurements including determination of percent body fat. Parameters of lipolysis suppression, time to FFA nadir, and FFA rebound after an IV glucose challenge were derived using a mathematical model. Multivariable regression analyses were used to characterize the independent associations between SDB severity and parameters of FFA metabolism.
Results
SDB severity, as assessed by the apnea-hypopnea index, was associated with adipocyte insulin resistance, a decrease in the glucose- and insulin-mediated suppression of lipolysis, a longer duration to reach a nadir in FFA levels during the FSIGTT, and a sluggish rebound in FFA levels after suppression. Severity of SDB-related hypoxemia was independently associated with adipocyte insulin resistance and the time to reach the FFA nadir during the FSIGTT. Finally, a higher percentage of stage N3 sleep was positively associated with greater suppression of lipolysis and a faster rebound in the FFA levels during the FSIGTT.
Interpretation
Independent of adiposity, SDB is associated with impairments in FFA metabolism, which may contribute to the development of glucose intolerance and type 2 diabetes in SDB.
Key Words: fat metabolism, free fatty acids, sleep apnea, sleep-disordered breathing
Abbreviations: adipo-IR, adipocyte insulin resistance; AHI, apnea-hypopnea index; ΔSpo2, average oxyhemoglobin desaturation; FFA, free fatty acid; FSIGTT, frequently sampled IV glucose tolerance test; Sao2, oxygen saturation; SDB, sleep-disordered breathing; TLR, Toll-like receptor
Sleep-disordered breathing (SDB) is a highly prevalent condition that commonly occurs in conjunction with obesity. Characterized by recurrent collapse of the upper airway, SDB is associated with episodes of oxyhemoglobin desaturation and arousals from sleep. There is strong evidence that SDB is independently associated with a cluster of metabolic derangements including insulin resistance and glucose intolerance.1,2 Although insulin resistance by itself increases the risk for type 2 diabetes, it often remains undetected for decades because of the associated hyperinsulinemic compensation, which maintains normal glucose homeostasis.3,4 The compensatory insulin response, which is pivotal in delaying the onset of diabetes, is attenuated in SDB.5 Although the associations between SDB and impaired insulin sensitivity and secretion have been previously described,5 little is known of the influence of SDB on free fatty acid (FFA) metabolism.
FFAs are the primary energy source in states of prolonged fasting when insulin levels are at their lowest. Rapid turnover of FFAs in the fasting state indicate that their contribution as an energy source is at least equivalent to that of glucose.6 An increase in adipose tissue lipolysis and higher circulating FFA levels have been associated with the development of insulin resistance.7 FFAs also impair secretion of insulin from the pancreatic beta cell.8 Factors that regulate adipose tissue lipolysis include the autonomic nervous system, neuroendocrine hormones (eg, growth hormone and cortisol), and various adipocytokines such as leptin, adiponectin, IL-6, and tumor necrosis factor-α.9 Because SDB is associated with an increase in sympathetic nervous system activity and alterations of several factors that regulate lipolysis (eg, adipocytokines, growth hormone), there is a good biological basis for speculating that SDB could negatively impact the regulation of FFA metabolism. Modeling FFA kinetics in a perturbed state, as during a glucose tolerance test, can provide a plethora of indexes that may offer unique insight into FFA metabolism.10,11 The aim of the current study was to assess FFA metabolism across the spectrum of SDB severity, using measures of FFA flux derived from the frequently sampled IV glucose tolerance test (FSIGTT). It was hypothesized that, independent of adiposity, FFA metabolism will be altered in SDB and be associated with the severity of underlying disease and degree of intermittent hypoxemia and sleep fragmentation.
Methods
Study Population
The study population consisted of patients with newly diagnosed SDB and a group without SDB. Recruitment of the study sample has been previously described.5 Briefly, patients undergoing polysomnography for SDB were recruited. Exclusionary criteria included a fasting glucose level greater than 125 mg/dL, prevalent type 2 diabetes, angina, myocardial infarction, coronary revascularization, congestive heart failure, and stroke. In addition, the presence of a circadian rhythm disorder, chronic insufficient sleep (< 7 h/night), obstructive lung disease, renal or hepatic dysfunction, upper airway surgery, cancer, or any chronic inflammatory condition also excluded participation. Finally, use of metformin, antiinflammatory agents (eg, steroids), supplemental oxygen, or positive airway pressure therapy was also exclusionary. After enrollment, participants were counseled to maintain at least 7 h of sleep per day and asked to consume at least 250 g of carbohydrates per day. Informed consent was obtained from all participants, and the protocol was approved by an institutional review board (WIRB 20021074).
Polysomnography and Anthropometry
The polysomnogram included recordings of C3/M2 and C4/M1 EEGs, right and left electrooculograms, and submental and bilateral anterior tibialis electromyograms. Respiration was monitored with a nasal pressure transducer, nose and mouth thermocouples, and thoracic and abdominal strain gauges. Recording of oxyhemoglobin saturation (Sao2) was done with an oximeter (model 3700; Ohmeda). Sleep-stage scoring was performed on 30-s epochs. Apneas were identified if airflow was absent in the thermistor and nasal cannula channels for at least 10 s.12 Hypopneas were identified if there was a reduction in airflow of at least 30%, which occurred for at least 10 s, and was associated with 4% oxyhemoglobin desaturation.12 The apnea-hypopnea index (AHI) was determined as the frequency of apneas and hypopneas per hour of total sleep time. The degree of oxyhemoglobin desaturation (ΔSao2) associated with each disordered breathing event was determined, and the overall average was used as a measure of hypoxemia severity. Arousals were identified as abrupt shifts of at least 3 s in EEG frequency. Weight was measured to the nearest 0.1 kg and height was measured with a portable stadiometer to the nearest 0.5 cm. Fat mass and percent body fat were determined with a QDR-4500A DEXA (dual-energy X-ray absorptiometry) scanner (Hologic). Percent body fat was determined as the ratio of total fat mass to body weight. All body composition measurements were obtained concurrently with the FSIGTT.
Assessment of FFA Metabolism
All participants completed the insulin-modified FSIGTT after an overnight fast.13 Baseline blood samples were obtained at –15, –10, –5, and –1 min before the glucose injection. At time 0 min, a weight-adjusted dose of glucose (50% dextrose, 0.3 g/kg) was administered intravenously. Twenty minutes after the glucose injection, a weight-adjusted dose of insulin (0.03 U/kg) was administered. Blood samples were collected after the glucose injection at prespecified times over a 3-h period as previously described.5 All samples were stored at –80°C and batch processed for glucose, insulin, and FFA concentrations. Previous work has shown that analysis of FFA levels within unfractionated serum is minimally affected even with repeated freeze-thaw cycles.14 The resulting glucose and insulin values were then subjected to the minimal model analysis.15 The model for FFA metabolism has been previously described11 and is briefly summarized in e-Appendix 1 in the online article. This model was used to derive smoothed temporal profiles of plasma FFA concentrations for each participant. The resulting smoothed FFA profile was used to estimate the nadir in the FFA concentrations after the IV administration of glucose. Once the nadir was identified, a time window that included 10 min before and after the nadir was used for the estimation of lipolysis suppression slope and rebound slope, using a linear regression model. In addition to these two slope trajectories, a homeostatic index of adipocyte insulin resistance (adipo-IR) was calculated as the product of fasting FFA and insulin levels.16
Statistical Analysis
To characterize the association between SDB severity (ie, AHI) and measures of FFA flux, analysis of variance and multivariable linear regression with a robust estimation of the variance17 were used. The AHI was categorized using quartile cut-points to minimize the potential influence of outliers. Although the AHI was examined as a continuous variable, results from using the AHI as a categorical variable are reported herein for ease of exposition and interpretation. Bivariate analyses were initially conducted to examine the associations between the AHI and measures of FFA flux. For these bivariate analyses, the Spearman rank correlation coefficient (ρ) was determined. Subsequently, multivariable models were constructed to account for age, sex, race, BMI, and percent body fat derived from the DEXA scan. To assess whether severity of oxygen desaturation and sleep fragmentation influence FFA metabolism, metrics of sleep stage distribution and desaturation severity were examined. As with the AHI, the distribution of sleep stage percentages and the average oxygen desaturation were categorized to avoid excessive leverage from outliers. For sleep stage and oxygen desaturation metrics, tertiles were used given the narrow range of observed values for these variables. To examine the potentially causal impact of SDB on the disposition index, which is a product of insulin sensitivity and the acute insulin response to glucose, structural equation modeling was used. All analyses were performed with STATA 15.0 (StataCorp). Two-sided tests of hypotheses and a P value < .05 were used for statistical significance. All descriptive statistics are reported as model adjusted mean ± SEM unless otherwise stated.
Results
The study sample of 118 participants was grouped on the basis of SDB severity. Table 1 presents the clinical, anthropometric, and metabolic data for each AHI category. Not surprisingly, BMI, central obesity, and percent body fat were associated with increasing AHI. Moreover, as AHI increased there was an increase in stages N1 and N2 sleep and a decrease in stage N3 and rapid eye movement sleep. Higher AHI values were also associated with higher fasting glucose (ρ = 0.35; 95% CI, 0.21-0.50) and insulin (ρ = 0.39; 95% CI, 0.23-0.56) levels. Fasting FFA levels were not associated with the AHI (ρ = –0.05; 95% CI, –0.23 to 0.13) despite the higher BMI values in those with moderate to severe SDB.
Table 1.
Study Sample Characteristics by Apnea-Hypopnea Index Quartile
| Characteristic | Events per Hour |
|||
|---|---|---|---|---|
| < 3.1 |
3.2-10.6 |
10.7-24.6 |
≥ 24.7 |
|
| (n = 30) | (n = 29) | (n = 30) | (n = 29) | |
| Demographics | ||||
| Sex, male | 36.7 | 55.2 | 70 | 79.3a |
| Race, white | 90 | 93.1 | 80 | 82.8 |
| Age, y | 36.4 (1.9) | 44.8 (2.4) | 49.3 (2.0) | 52.5 (1.7)b |
| Body composition | ||||
| BMI, kg/m2 | 26.3 (0.9) | 29.2 (1.3) | 29.8 (0.8) | 32.4 (1.0)b |
| Waist circumference, cm | 82.7 (2.1) | 90.2 (2.6) | 94.1 (1.9) | 105.2 (2.6)b |
| Percent body fat, % | 30.7 (2.0) | 33.0 (1.8) | 29.9 (1.7) | 34.8 (1.4) |
| Actigraphy monitoring | ||||
| Usual time in bed, h | 7.8 (0.2) | 7.6 (0.2) | 7.8 (0.1) | 7.5 (0.2) |
| Sleep duration, h | 7.2 (0.2) | 7.0 (0.2) | 7.3 (0.1) | 6.5 (0.2) |
| Polysomnography | ||||
| Total sleep time, h | 6.7 (0.3) | 6.9 (0.2) | 6.9 (0.2) | 6.4 (0.2) |
| Stage N1 sleep, % | 7.4 (0.7) | 10.1 (1.0) | 11.0 (1.1) | 15.0 (1.8)b |
| Stage N2 sleep, % | 55.7 (1.5) | 57.2 (1.5) | 62.3 (1.4) | 63.0 (2.1)b |
| Slow wave sleep (N3), % | 13.6 (1.4) | 12.7 (1.1) | 7.6 (0.9) | 5.1 (1.2)b |
| REM sleep, % | 23.3 (0.9) | 20.1 (1.1) | 19.1 (1.3) | 16.9 (1.2)b |
| Arousal index, No./h | 11.1 (0.8) | 14.2 (1.5) | 20.3 (1.3) | 45.1 (4.1)b |
| ΔSpo2, % | 3.2 (0.2) | 3.8 (0.2) | 3.7 (1.7) | 6.4 (0.8)b |
| Serum measurements | ||||
| Fasting glucose, mg/dL | 93.1 (1.3) | 98.6 (1.5) | 98.5 (1.6) | 103.0 (1.8)b |
| Fasting FFA, μM | 479.5 (37.1) | 577.5 (96.8) | 457.6 (35.4) | 482.3 (38.0) |
Values are reported are either percentages or means (SE). AHI = apnea-hypopnea index; ΔSpo2 = average oxyhemoglobin desaturation; REM = rapid eye movement.
P < .02 for comparisons across AHI groups (P values determined by analysis of variance).
P < .001 for comparisons across AHI groups (P values determined by analysis of variance).
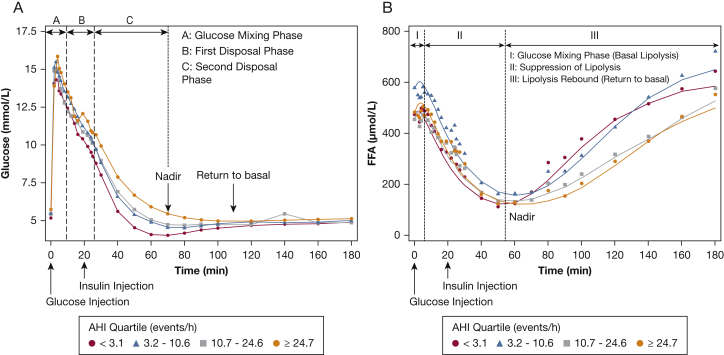
Figure 1A shows the glucose profiles during the FSIGTT for the four AHI groups. The first phase (phase A) of glucose disposal after IV infusion represents the mixing phase, during which glucose is not homogeneously distributed throughout the vascular space. Once glucose is equally distributed, the second phase (phase B) of disposal begins (approximately 8 min after the bolus), which is dominated by glucose promoting its own disposal (ie, glucose effectiveness). The third phase (phase C) of disposal begins shortly after the inflection point in the downward trajectory of glucose and is mediated predominantly by insulin action. Figure 1B displays the FFA profiles during the FSIGTT for the four corresponding AHI quartiles. As with glucose, FFA trajectories can also be segregated in distinct phases. In phase I (approximately 0-10 min after glucose bolus administration) glucose is not equally distributed through the system. Given that the cellular effects of insulin have an intrinsic delay, FFA levels remain unchanged and at basal levels. In phase II (approximately 10-60 min after glucose bolus administration), FFA levels exhibit a marked decrease as a result of glucose and insulin action. In the final phase (phase III), FFA levels rebound toward their basal levels as glucose and insulin action returns to basal levels.
Figure 1.
Glucose (A) and free fatty acid (B) profiles from the frequently sampled IV glucose tolerance test for quartiles of the apnea-hypopnea index. AHI = apnea-hypopnea index; FFA = free fatty acid.
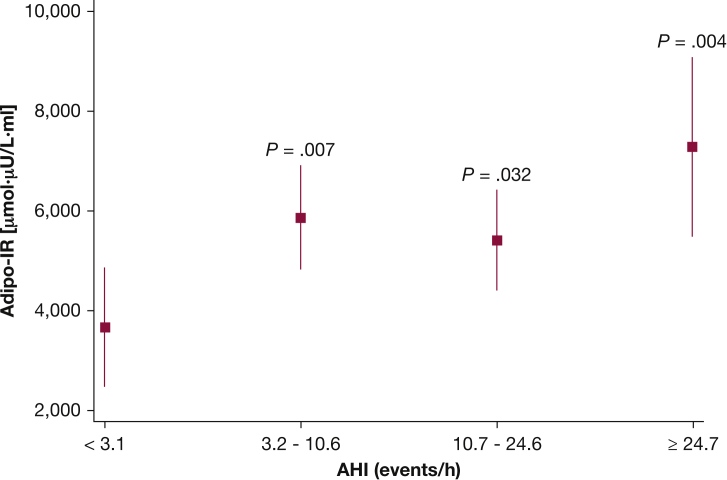
To assess the association between severity of SDB and the ability of insulin to suppress lipolysis (ie, adipose tissue insulin sensitivity), adipo-IR was examined as a function of AHI category. Adipo-IR for participants in the second and third AHI quartiles was higher compared with the reference AHI category (Fig 2), even after adjustment for age and percent body fat. Participants in the fourth AHI quartile had the highest level of adipo-IR (98% higher; P = .004) (Fig 2) compared with participants in the lowest AHI quartile despite multivariable adjustments for age, sex, and DEXA percent body fat. In these analyses, the additional role of sex and BMI as covariates was examined, and no material difference regarding the association between AHI category and adipo-IR was noted with or without these covariates.
Figure 2.
Adjusted values (mean and 95% CIs) of adipo-IR for quartiles of the AHI. adipo-IR = adipose tissue insulin resistance index. See Figure 1 legend for expansion of other abbreviation.
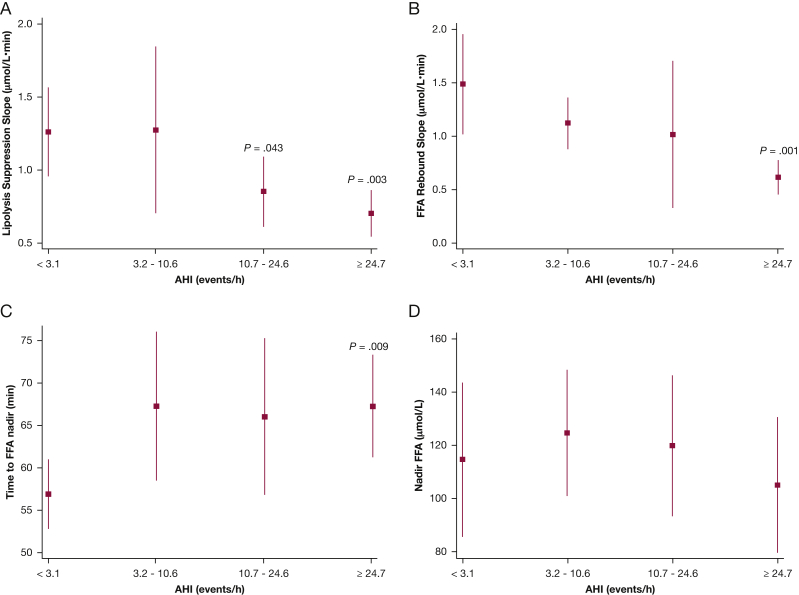
Analyses were then undertaken to examine whether SDB severity was associated with measures of FFA metabolism. Four metrics were examined, based on the FFA profiles during the FSIGTT: (1) rate of FFA suppression as assessed by the downward slope of the FFA levels; (2) rebound rate of FFA as assessed by the upward slope of the FFA; (3) time to the FFA nadir; and (4) FFA concentrations at nadir. With the exception of nadir FFA concentration, all other indexes were significantly associated with SDB severity. Increasing AHI was associated with a lower lipolysis suppression rate (Fig 3A; P < .001 for linear trend). Participants in the first AHI quartile had a lipolysis suppression rate of 1.26 μM/min (95% CI, 0.96-1.6 μM/min) (Fig 3A), whereas those in the highest AHI quartile had a rate of 0.7 μM/min (95% CI, 0.54-0.87 μM/min; P = .003). Furthermore, participants in the third quartile also exhibited a lower lipolysis suppression rate compared with the lowest AHI quartile (P = .043) (Fig 3A). Participants in the highest AHI quartile did not fare better during the plasma FFA rebound phase (Fig 3B). In contrast to the rebound rate for those without SDB (1.49 μM/min; 95% CI, 1.01-1.96 μM/min), participants with severe SDB had a slower rebound with a significantly reduced rate (0.62 μM/min; 95% CI, 0.45-0.78 μM/min; P = .001 in comparison with lowest AHI category). The mean time to reach the FFA nadir for participants in the first AHI quartile was 56.8 min (95% CI, 52.7-69.0 min). In contrast, participants with severe SDB took a significantly longer time (P = .009) to reach their FFA nadir (difference in time, severe vs normal: 10.4 min; 95% CI, 2.7-18.1 min). The times to FFA nadir for subjects in the mild and moderate SDB groups were not statistically significantly different from those without SDB (Fig 3C). Finally, FFA concentrations at the nadir time were not different across AHI groups (Fig 3D).
Figure 3.
Adjusted values (mean and 95% CIs) of lipolysis suppression rate (A), FFA rebound slope (B), time to FFA nadir (C), and nadir FFA levels (D). See Figure 1 legend for expansion of abbreviations.
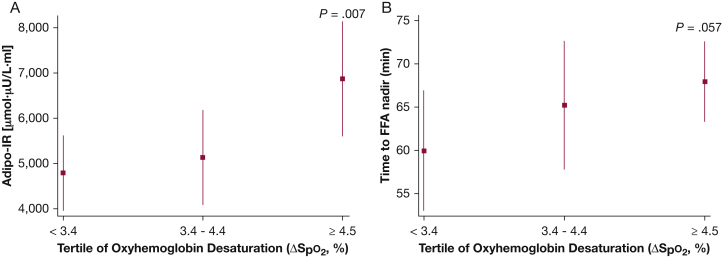
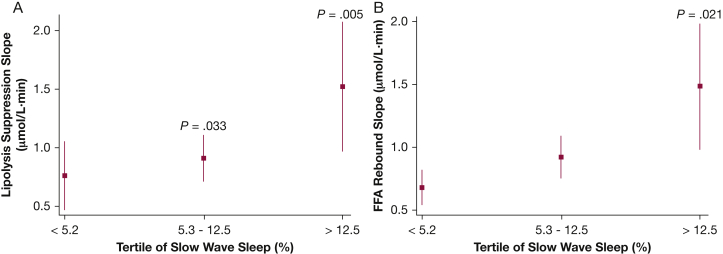
Analyses were also conducted to examine the associations between degree of nocturnal hypoxemia, sleep stage distribution, and measures of FFA metabolism. Average oxyhemoglobin desaturation (ΔSpo2) was most notably associated with the adipo-IR (ρ = 0.31; 95% CI, 0.14-0.48) and the FFA nadir (ρ = 0.32; 95% CI, 0.15-0.49). None of the other indexes of FFA metabolism were associated with ΔSpo2. Variables such as age and percent body fat were included in the multivariable linear regression model along with tertiles of ΔSpo2. Compared with the lowest ΔSpo2 tertile, the adjusted adipo-IR level was 60% higher in the highest ΔSpo2 tertile (4,791.1 vs 6,875.0 μmol⋅mU/L; P = .007) (Fig 4A). The time to FFA nadir in the highest ΔSpo2 tertile was also compared with the first ΔSpo2 tertile (P = .057) (Fig 4B). Lipolysis suppression and FFA rebound slopes were not associated with ΔSpo2. However, the lipolysis suppression rate was negatively associated (ρ = –0.39; 95% CI, –0.55 to –0.23), and the FFA rebound rate was positively associated (ρ = 0.39; 95% CI, 0.23-0.55), with percentage of stage N3 sleep. Those in the highest tertile of slow-wave sleep percentage exhibited an 84% higher lipolysis suppression rate than those in the lowest tertile (1.48 vs 0.70 μM/min; P = .005) (Fig 5A). Finally, the rate of FFA rebound was also highest in those with the greatest percentage of stage N3 sleep compared with those in the lowest tertile (P = .021) (Fig 5B). An independent association was not observed between indices of FFA metabolism, arousal frequency, and percentages of sleep stages N1, N2, or rapid eye movement sleep.
Figure 4.
Adjusted values (mean and 95% CIs) of adipo-IR (A) and time to FFA (B) nadir for tertiles of oxygen desaturation. See Figure 1 and 2 legends for expansion of abbreviations.
Figure 5.
Adjusted values (mean and 95% CIs) of lipolysis suppression rate (A) and FFA rebound slope (B) for tertiles of slow wave (N3) sleep percentage. See Figure 1 legend for expansion of abbreviation.
To examine intermediate mechanisms, a structural equation model was used to determine whether SDB increases the risk of type 2 diabetes by altering the rate of lipolysis. In this model, the specific pathway examined was whether increasing SDB severity was associated with a lower disposition index, a risk factor for type 2 diabetes, and whether that association was mediated either by lipolysis suppression or FFA rebound slopes from the FSIGTT. The structural equation model showed that severity of SDB was associated with lipolysis suppression slope and FFA rebound (see e-Appendix 1). However, only FFA rebound showed a significant association with the disposition index (P = .015). Finally, examining the association between insulin sensitivity and secretion parameters derived showed that SDB severity was associated with a lower disposition index.
Discussion
The results of this study demonstrate that SDB is independently associated with alterations in FFA metabolism. Specifically, increasing AHI was associated with adipocyte insulin resistance, a decrease in glucose- and insulin-mediated suppression of lipolysis, a longer duration to reach a nadir in FFA levels during the FSIGTT, and a sluggish rebound rate in FFA levels after suppression. Moreover, severity of oxygen desaturation was independently associated with adipocyte insulin resistance and the time to reach the FFA nadir. Finally, a higher percentage of stage N3 sleep was positively associated with greater suppression of lipolysis and a faster rebound in FFA levels. Collectively, these findings indicate that in the absence of medical comorbidities, SDB and its associated sequelae of nocturnal hypoxemia and suppressed stage N3 sleep are associated with abnormalities in FFA metabolism that may, in turn, contribute to SDB-related impairments in insulin and glucose homeostasis. The structural equation model showed that SDB may significantly decrease the disposition index by altering FFA kinetics, particularly in those with severe disease. A decrease in the disposition index increases the risk for type 2 diabetes, in part because of the effects of pancreatic beta-cell failure due to a genetic predisposition.18 The results of this study show that SDB may also have detrimental effects on the pancreatic beta cell that could be mediated by abnormalities in FFA flux.
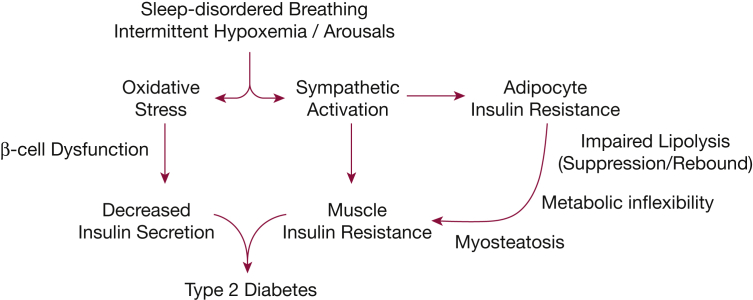
The observed alterations in FFA metabolism provide a good biological basis for the previously reported impairments in insulin sensitivity and secretion (Fig 6). Although the underlying mechanisms have not been fully elucidated, FFAs could induce insulin resistance by activating several serine kinases that inhibit insulin activity,19,20 promote serine phosphorylation of insulin receptor substrates, and thereby interfere with insulin receptor signaling.21 FFAs also activate inflammatory signaling pathways by increasing the secretion of cytokines, such as tumor necrosis factor-α and IL-6, and by interacting with members of the Toll-like receptor (TLR) family.22,23 In fact, TLR2 has been shown to mediate the initial sequence of events in FFA-induced insulin resistance,23 and inhibition of TLR2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue.22 We have previously reported that, in healthy volunteers, exposure to intermittent hypoxia decreases whole body insulin sensitivity and increases TLR2 expression in peripheral mononuclear cells.24 Thus, higher FFA flux from lipolysis could promote insulin resistance in skeletal muscle through several independent pathways. In addition to peripheral insulin resistance, FFAs decrease hepatic insulin sensitivity by attenuating insulin signaling similar to that in skeletal muscle. Finally, FFAs also stimulate gluconeogenesis and inhibit insulin- and glucose-dependent suppression of endogenous glucose production from the liver.25
Figure 6.
Possible mechanisms linking sleep-disordered breathing and the development of type 2 diabetes.
Not only can FFAs cause insulin resistance, they also impair insulin secretion. It is well known that type 2 diabetes results from a combination of insulin resistance and pancreatic beta-cell failure. The response to a decrease in insulin sensitivity is a compensatory increase in insulin secretion with a hyperbolic function linking the two.18 Thus, glucose homeostasis is maintained as the disposition index, the product of insulin sensitivity and amount of insulin secreted, remains constant. As the disposition index decreases, the hyperbolic relation between insulin secretion and sensitivity moves toward the origin, indicating an increased risk of type 2 diabetes.18 Although a decrease in the disposition index has been attributed to a genetic predisposition, the findings of this study show that SDB may also have a negative influence and that alterations in FFA flux could serve as putative mediator. Although glucose is the most important regulator of insulin secretion, other secretagogues, such as FFAs, can modulate glucose-related insulin secretion. Acute exposure to FFA increases insulin secretion and stimulates beta-cell expansion, whereas prolonged exposure leads to beta-cell apoptosis.26,27 It also appears that FFAs trigger activation of the TRL2/TLR4 signaling pathway in the beta cell, which cause it to produce inflammatory cytokines that, in turn, recruit macrophages to the islet and perpetuate the inflammatory process, eventually leading to beta-cell failure.28 Thus, abnormalities in FFA metabolism can elicit the two “hits” (ie, insulin resistance and beta-cell dysfunction) needed for the development of type 2 diabetes in SDB.
A novel finding of the current study is that SDB severity was associated with whole-body adipose tissue insulin resistance as quantified by the adipo-IR index even after accounting for percent body fat. The inability of insulin to quickly suppress adipose tissue lipolysis resulted in a delay in reaching the FFA nadir after a glucose and insulin challenge. Given that increasing SDB was also associated with a sluggish rebound in FFA levels, it appears that SDB alters adipose tissue behavior, resulting in decreasing flexibility in response to a metabolic challenge. Interestingly, hypoxemia severity and suppression of stage N3 sleep seem to explicate the observed alterations in FFA metabolism in SDB. The SDB-related diminished flexibility in adipose tissue response most likely leads to altered FFA signaling at the pancreatic beta cell, resulting in impaired insulin secretion. Metabolic inflexibility (ie, diminished insulin-mediated suppression of lipolysis and sluggish recovery) may also contribute to intramyocellular lipid accumulation and insulin resistance.29,30
The observations that fasting FFA levels were comparable across AHI groups and that the final FFA levels in severe SDB are lower than in those without SDB are unexpected (Fig 1). Because percent body fat is a key determinant of lipolysis rate, fasting FFA levels would be expected to increase with percent body fat. Given that the difference in percent body fat between the lowest and highest AHI quartile in the current study was only 12%, it would seem reasonable that fasting FFA levels would not be significantly different. The finding that, despite increasing severity of SDB, fasting FFA levels are comparable in the current study is not consistent with previous work,31 which has demonstrated higher fasting FFA levels as a function of AHI. The inconsistency between the current and previous work is likely the result of residual confounding due to percent body fat and suggests statistical adjustments for BMI, as made in previous studies,31 may not be sufficient. Lastly, the lower levels of final FFA levels in severe SDB in the current study can be explained by the decrease in lipolysis rate, delay in reaching a nadir, and slow rebound. These alterations in FFA metabolism could be due to sympathetic overactivation, which is known to lead to beta-cell and adipocyte “exhaustion.”32,33 It is also important to note that factors beyond basal levels of circulating FFA can increase the risk for the development of type 2 diabetes. For example, inadequate insulin-mediated suppression of lipolysis as noted in moderate and severe SDB has been associated with impairments in skeletal muscle insulin action.34
There are several limitations in the current study that merit discussion. First, the cross-sectional nature of this study does not allow for inferences regarding causal effects. Second, modeling of FFA kinetics was not based on isotope-labeled tracer-based techniques (radioactive or stable isotope) to quantify adipose tissue lipolysis and FFA appearance in plasma. Although such techniques can yield unique insights into lipid metabolism, the choice of tracer, the analytical methods used, the need for simultaneous arteriovenous balance measurement, and the participant burden represent major drawbacks. The approach to modeling FFA profiles after a glucose challenge offers a simpler alterative that is less cumbersome. Third, although percent body fat was considered a confounder, the lack of data on visceral fat mass is a limitation. Regional fat depots vary considerably regarding fatty acid metabolism.35 Postprandial FFA release, for example, is greater with visceral obesity compared with lower body obesity.36, 37, 38 Thus, there is a possibility of residual confounding because visceral fat mass was not accounted for in the analyses. Finally, dietary intake of carbohydrates and fat before the FSIGTT was not assessed. Dietary content and amount of carbohydrates and fat are important determinants of FFA metabolism.39,40 Despite these limitations, there are several notable strengths. These include a relatively large sample size with full-montage polysomnography, exclusion of prevalent medical conditions, assessment of adiposity, and use of a dynamic model to characterize FFA metabolism. Certainly, the findings presented herein motivate further research interrogating pathways through which SDB may alter FFA metabolism. It also remains to be determined whether treatment of SDB with positive pressure therapy improves FFA kinetics. Finally, the usefulness of pharmacologic agents that can modulate FFA metabolism (eg, nicotinic acid and fibrates) also needs to be explored as a means to attenuate SDB-related metabolic risk. In conclusion, the results of this study show that there is an independent association between severity of SDB and aberrant lipid metabolism, which may be responsible for the lack of hyperinsulinemic compensation in SDB. These findings are particularly relevant given that altered FFA metabolism results in increased lipid deposition and contributes to the development of type 2 diabetes and associated cardiovascular complications.
Acknowledgments
Author contributions: N. M. P. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. D. S., R. C. B., and N. M. P. contributed substantially to the data analysis and interpretation, and the writing of the manuscript. N. M. P. is the guarantor of the content of this manuscript including study design, data collection, and analysis.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Esra Tasali, MD (University of Chicago) for input regarding the content of this manuscript.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Supported by the following grants from the National Heart, Lung, and Blood Institute: HL11716 and HL146709.
Supplementary Data
References
- 1.Kent B.D., McNicholas W.T., Ryan S. Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J Thorac Dis. 2015;7(8):1343–1357. doi: 10.3978/j.issn.2072-1439.2015.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reutrakul S., Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leahy J.L. Natural history of beta-cell dysfunction in NIDDM. Diabetes Care. 1990;13(9):992–1010. doi: 10.2337/diacare.13.9.992. [DOI] [PubMed] [Google Scholar]
- 4.Tripathy D., Chavez A.O. Defects in insulin secretion and action in the pathogenesis of type 2 diabetes mellitus. Curr Diab Rep. 2010;10(3):184–191. doi: 10.1007/s11892-010-0115-5. [DOI] [PubMed] [Google Scholar]
- 5.Punjabi N.M., Beamer B.A. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179(3):235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raz I., Eldor R., Cernea S., Shafrir E. Diabetes: insulin resistance and derangements in lipid metabolism: cure through intervention in fat transport and storage. Diabetes Metab Res Rev. 2005;21(1):3–14. doi: 10.1002/dmrr.493. [DOI] [PubMed] [Google Scholar]
- 7.Morigny P., Houssier M., Mouisel E., Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. doi: 10.1016/j.biochi.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Grill V., Qvigstad E. Fatty acids and insulin secretion. Br J Nutr. 2000;83(suppl 1):S79–S84. doi: 10.1017/s0007114500000994. [DOI] [PubMed] [Google Scholar]
- 9.Fruhbeck G., Mendez-Gimenez L., Fernandez-Formoso J.A., Fernandez S., Rodriguez A. Regulation of adipocyte lipolysis. Nutr Res Rev. 2014;27(1):63–93. doi: 10.1017/S095442241400002X. [DOI] [PubMed] [Google Scholar]
- 10.Sumner A.E., Bergman R.N., Vega G.L. The multiphasic profile of free fatty acids during the intravenous glucose tolerance test is unresponsive to exogenous insulin. Metabolism. 2004;53(9):1202–1207. doi: 10.1016/j.metabol.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Boston R.C., Moate P.J. A novel minimal model to describe NEFA kinetics following an intravenous glucose challenge. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1140–R1147. doi: 10.1152/ajpregu.00749.2007. [DOI] [PubMed] [Google Scholar]
- 12.Berry R.B., Budhiraja R., Gottlieb D.J., American Academy of Sleep Medicine Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergman R.N., Ider Y.Z., Bowden C.R., Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236(6):E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 14.Zivkovic A.M., Wiest M.M., Nguyen U.T., Davis R., Watkins S.M., German J.B. Effects of sample handling and storage on quantitative lipid analysis in human serum. Metabolomics. 2009;5(4):507–516. doi: 10.1007/s11306-009-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boston R.C., Stefanovski D., Moate P.J., Sumner A.E., Watanabe R.M., Bergman R.N. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5(6):1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 16.Søndergaard E., Espinosa De Ycaza A.E., Morgan-Bathke M., Jensen M.D. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2017;102(4):1193–1199. doi: 10.1210/jc.2017-00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert J.H., Kott P.S. Robust variance estimation in linear regression. J Appl Stat. 1988;15(3):341–345. [Google Scholar]
- 18.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 19.Itani S.I., Ruderman N.B., Schmieder F., Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 20.Savage D.B., Petersen K.F., Shulman G.I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87(2):507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin M.E., Marcucci M.J., Cline G.W. Free fatty acid-induced insulin resistance is associated with activation of protein kinase Cθ and alterations in the insulin signaling cascade. Diabetes. 1999;48(6):1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 22.Caricilli A.M., Nascimento P.H., Pauli J.R. Inhibition of Toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol. 2008;199(3):399–406. doi: 10.1677/JOE-08-0354. [DOI] [PubMed] [Google Scholar]
- 23.Senn J.J. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281(37):26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 24.Polotsky V.Y., Bevans-Fonti S., Grigoryev D.N., Punjabi N.M. Intermittent hypoxia alters gene expression in peripheral blood mononuclear cells of healthy volunteers. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0144725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boden G., Cheung P., Stein T.P., Kresge K., Mozzoli M. FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am J Physiol Endocrinol Metab. 2002;283(1):E12–E19. doi: 10.1152/ajpendo.00429.2001. [DOI] [PubMed] [Google Scholar]
- 26.Haber E.P., Ximenes H.M., Procopio J., Carvalho C.R., Curi R., Carpinelli A.R. Pleiotropic effects of fatty acids on pancreatic beta-cells. J Cell Physiol. 2003;194(1):1–12. doi: 10.1002/jcp.10187. [DOI] [PubMed] [Google Scholar]
- 27.Sharma R.B., Alonso L.C. Lipotoxicity in the pancreatic beta cell: not just survival and function, but proliferation as well? Curr Diab Rep. 2014;14(6):492. doi: 10.1007/s11892-014-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin J., Peng Y., Wu J., Wang Y., Yao L. Toll-like receptor 2/4 links to free fatty acid-induced inflammation and beta-cell dysfunction. J Leukoc Biol. 2014;95(1):47–52. doi: 10.1189/jlb.0313143. [DOI] [PubMed] [Google Scholar]
- 29.Galgani J.E., Moro C., Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295(5):E1009–E1017. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodpaster B.H., Sparks L.M. Metabolic flexibility in health and disease. Cell Metab. 2017;25(5):1027–1036. doi: 10.1016/j.cmet.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barcelo A., Pierola J., de la Pena M. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur Respir J. 2011;37(6):1418–1423. doi: 10.1183/09031936.00050410. [DOI] [PubMed] [Google Scholar]
- 32.Poitout V., Robertson R.P. An integrated view of beta-cell dysfunction in type-II diabetes. Annu Rev Med. 1996;47:69–83. doi: 10.1146/annurev.med.47.1.69. [DOI] [PubMed] [Google Scholar]
- 33.Mori S., Nojiri H., Yoshizuka N., Takema Y. Rapid desensitization of lipolysis in the visceral and subcutaneous adipocytes of rats. Lipids. 2007;42(4):307–314. doi: 10.1007/s11745-007-3034-8. [DOI] [PubMed] [Google Scholar]
- 34.Abdul-Ghani M.A., Molina-Carrion M., Jani R., Jenkinson C., Defronzo R.A. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol. 2008;45(3):147–150. doi: 10.1007/s00592-008-0033-z. [DOI] [PubMed] [Google Scholar]
- 35.Jensen M.D. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen M.D., Haymond M.W., Rizza R.A., Cryer P.E., Miles J.M. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989;83(4):1168–1173. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roust L.R., Jensen M.D. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes. 1993;42(11):1567–1573. doi: 10.2337/diab.42.11.1567. [DOI] [PubMed] [Google Scholar]
- 38.Guo Z., Hensrud D.D., Johnson C.M., Jensen M.D. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48(8):1586–1592. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- 39.Wolever T.M., Bentum-Williams A., Jenkins D.J. Physiological modulation of plasma free fatty acid concentrations by diet: metabolic implications in nondiabetic subjects. Diabetes Care. 1995;18(7):962–970. doi: 10.2337/diacare.18.7.962. [DOI] [PubMed] [Google Scholar]
- 40.Terra L.F., Lobba A.R. High-fat diet and fish oil affect adipocyte metabolism in a depot-specific manner. J Physiol. 2017;595(6):1859–1860. doi: 10.1113/JP273828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.