ABSTRACT
Background
Current dietary recommendations for cardiovascular disease (CVD) prevention focus more on dietary patterns than on single nutrients. However, randomized controlled trials using whole-diet approaches to study effects on both fasting and postprandial CVD risk markers are limited.
Objective
This randomized parallel trial compared the effects of a healthy diet (HD) with those of a typical Western diet (WD) on fasting and postprandial CVD risk markers in overweight and obese adults.
Methods
After a 2-wk run-in period, 40 men and women (50–70 y; BMI: 25–35 kg/m2) consumed the HD (high in fruit and vegetables, pulses, fibers, nuts, fatty fish, polyunsaturated fatty acids; low in salt and high-glycemic carbohydrates; n = 19) or the WD (less fruit, vegetables, and fibers; no nuts and fatty fish; and more saturated fatty acids and simple carbohydrates; n = 21) for 6 wk. Fasting and postprandial cardiometabolic risk markers were assessed as secondary outcome parameters during a 5-h mixed-meal challenge, and a per protocol analysis was performed using 1-factor ANCOVA or linear mixed models.
Results
Differences in diet-induced changes are expressed relative to the HD group. Changes in fasting plasma total cholesterol (–0.57 ± 0.12 mmol/L, P < 0.001), LDL cholesterol (–0.41 ± 0.12 mmol/L, P < 0.01), apolipoprotein B100 (–0.09 ± 0.03 g/L, P < 0.01), and apolipoprotein A1 (–0.06 ± 0.03 g/L, P = 0.05) were significantly different between the diet groups. Changes in postprandial plasma triacylglycerol (diet × time, P < 0.001) and apolipoprotein B48 (P < 0.01) differed significantly between the groups with clear improvements on the HD, although fasting triacylglycerols (–0.24 ± 0.13 mmol/L, P = 0.06) and apolipoprotein B48 (1.04 ± 0.67 mg/L, P = 0.40) did not. Significant differences between the diets were also detected in fasting systolic (–6.9 ± 3.1 mmHg, P < 0.05) and 24-h systolic (–5.0 ± 1.7 mmHg, P < 0.01) and diastolic (–3.3 ± 1.1 mmHg, P < 0.01) blood pressure.
Conclusion
A whole-diet approach targeted multiple fasting and postprandial CVD risk markers in overweight and obese adults. In fact, the postprandial measurements provided important additional information to estimate CVD risk. This trial is registered at clinicaltrials.gov as NCT02519127.
Keywords: whole-diet approach, mixed-meal challenge, postprandial metabolism, cardiovascular disease, obesity
Introduction
An increased BMI (in kg/m2), elevated serum LDL cholesterol concentrations, and a raised blood pressure are well-established risk markers for developing cardiovascular disease (CVD) (1). Therefore, in addition to physical activity and maintaining a normal body weight, a healthy diet is of utmost importance for CVD prevention. However, the relation between diet and CVD risk has mostly been investigated focusing on single nutrients, while studies using whole-diet approaches are still limited (2). In fact, the impact of nutrition on cardiovascular health can be studied at 3 different levels: nutrients, foods or food groups, and dietary patterns (2). Since dietary patterns are composed of foods, and foods deliver nutrients, these levels are inextricably linked (3). Studies focusing on isolated nutrients (e.g., dietary fibers, PUFAs, vitamins, and minerals) not only provide insight into cardiometabolic health effects but are also important for understanding the mechanisms behind the effects observed. Dietary patterns, however, better reflect the real-life situation in which eating a combination of foods delivers a wide variety of different nutrients (4). The combination of multiple nutrients and other food components may lead to different or even more advanced health effects than single nutrients only (3). For example, fruit and vegetables are important sources of fiber, vitamins, minerals, and polyphenols, displaying antioxidative properties and delivering a lower glycemic load and energy density. It has been suggested that this combination of beneficial characteristics enhances the lowering effect on CVD risk by targeting a wider spectrum of CVD risk markers (5). A well-known diet for CVD management is the Mediterranean diet, characterized by a high amount of fruit and vegetables, whole-grain products, and olive oil and less meat, dairy products, and solid fats. However, due to the many variations of this diet, results are inconsistent across randomized controlled trials, and more studies in non-Mediterranean countries are needed to demonstrate the generalization of the Mediterranean diet recommendations (6).
Not only whole diets compared with nutrients but also postprandial CVD risk markers compared with the traditional fasting measurements have been suggested to be more reflective of a real-life situation. Postprandial dyslipidemia has been identified as an important CVD risk factor, and postprandial triglycerides have been independently associated with cardiovascular events (7, 8). Considering that we spend most of our daytime in the nonfasting state, measuring postprandial profiles of CVD risk markers may be more indicative of cardiometabolic health than fasting values only (9).
The objective of this study was to assess the impact of a whole-diet approach with a healthy diet (HD) providing a combination of beneficial macro- and micronutrients (10) as compared with a more common Western diet (WD) on cardiometabolic risk markers under fasting and postprandial conditions during a 5-h mixed-meal challenge. We hypothesized that the HD compared with the WD would improve fasting CVD risk markers and postprandial responses to the mixed meal and that the postprandial measurements would provide additional information above that of fasting values.
Subjects and Methods
Subjects
Overweight and obese men and women (aged 50–70 y) with a BMI between 25 and 35 were recruited via advertisements in local newspapers. Inclusion and exclusion criteria have been described in detail previously (10). Briefly, exclusion criteria were a fasting plasma glucose concentration of ≥6.9 mmol/L, smoking, shift work, excessive alcohol consumption (>7 beverages/wk), medically prescribed or slimming diets, being vegetarian, excessive moderate to vigorous physical activity (≥3 h/wk), and medication use likely to interfere with the study measurements. All participants gave their written informed consent prior to the start of the study. The study was conducted according to the guidelines stated in the Declaration of Helsinki, and the protocol was approved by the medical ethical committee of Maastricht University Medical Centre+ and registered at www.clinicaltrials.gov as NCT02519127.
Study design
The study design has been explained in detailed previously (10). In short, this study was a parallel-designed randomized trial with 2 intervention groups. All participants received the WD during a 2-wk run-in period. After the run-in period, all participants underwent a 5-h mixed-meal challenge to assess fasting and postprandial plasma CVD risk markers, as well as a hyperinsulinemic euglycemic clamp to assess insulin sensitivity and metabolic flexibility within the same week. All fasting and postprandial plasma markers, as well as insulin sensitivity, are considered secondary outcome parameters. The methodology and results of the clamp, as well as the main outcome parameter of this trial, metabolic flexibility (ΔRQ: the change in respiratory quotient upon insulin stimulation), have been reported previously (10). Randomization was based on a computer-generated stratified (men, women, and couples) randomization scheme, and participants were allocated to either the WD or the HD diet for 6 wk. Participants visited the test facility weekly to discuss the diets and meal plans with a research dietitian, to have their weight and waist circumference monitored, and to receive the required food products. In addition to the weekly appointments with the research dietitian, participants were asked to monitor their daily food intake by keeping a 3-d food diary during the run-in period and the final week of the intervention period. At the end of the study, the mixed-meal challenge was repeated.
Diets
For each participant, energy intake was estimated using the Harris-Benedict formula adjusted for the physical activity level (11, 12). As described previously, these outcomes were translated into individual dietary guidelines, as well as example menus and recipes, creating clear differences between the 2 diets (10). For both the WD and the HD, a standard set of food products was provided on a weekly basis, providing ∼50% of the total energy intake while taking individual food preferences into account. The rest of the products had to be bought by the participants according to the dietary guidelines. The healthy diet was high in fruit and vegetables, whole grains, pulses, fibers, nuts, and fatty fish. On a nutrient level, the diet provided a higher amount of PUFAs, less SFAs, and high-glycemic carbohydrates. To reduce dietary sodium intake and increase potassium intake, participants in the HD group were instructed to use sodium-reduced kitchen salt including potassium chloride. The HD group was also asked to consume ≥1.75 kg vegetables/wk, with 500 g rich in polyphenols (e.g., artichokes, broccoli, or tomato), 500 g rich in polyphenols and nitrate (e.g., endive, spinach, or beetroot), and 250 g rich in nitrate (e.g., rocket salat, kohlrabi, or fennel). The remaining 500 g could be chosen freely based on personal preference. The WD group was asked to avoid these vegetable groups and to choose from varieties that are relatively low in nitrate and/or polyphenols (e.g., mushrooms, carrots, or zucchini). In addition, the HD group was asked to consume ≥2 cups of green, black, or herbal tea per day, while the WD group was advised not to change their tea-drinking habits. Participants were instructed to adhere to their recommended energy intakes to prevent changes in body weight throughout the study. In case of weight loss or gain, additional counseling was provided, and energy intake was adjusted if necessary. The target nutrient compositions of both diets have been described earlier and are shown in Supplementary Table 1 (10). Example menus for both intervention groups are shown in Supplementary Table 2.
Test days and measurements
Mixed-meal challenge
Participants were asked to refrain from physical activity and from consumption of alcoholic drinks 2 d prior to the test days. On the evening before the test days, participants were instructed to consume a standardized dinner and to abstain from any other foods and drinks, except for water, after 22:00. The standardized dinner was the same for both intervention groups at the end of the run-in period and consisted of a commercially available ready-to-eat meal and dessert (761 kcal, 106 g carbohydrates, 25 g fat, and 28 g protein). The meal differed for the HD at the end of the intervention period (682 kcal, 71 g carbohydrates, 26 g fat, and 41 g protein), reflecting the targeted differences in macronutrient composition between the diets. After an overnight fast, participants came to the test facility at the Maastricht University, and a cannula was inserted in the antecubital vein for collecting a fasting blood sample. In addition, office blood pressure and endothelial function were determined. After the baseline measurements, participants were asked to consume a test meal in the form of a shake, resembling the composition of a mixed meal, within 10 min, as described previously (10) (Supplementary Table 3). The composition of the test meal was the same for all participants, regardless of their diet allocation. After consuming the shake, participants were asked not to eat or drink, except for water, for 5 h. Postprandial blood samples were taken at T15, T30, T45, T60, T90, T120, T180, T240, and T300 min after meal consumption.
Office blood pressure
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate were determined by calculating the mean of 3 consecutive measurements with an automatic blood pressure monitor (OMRON M7; OMRON Healthcare Europe BV). Before consumption of the mixed meal, a fasting measurement was performed in a seated position on the upper arm, which was not used for blood withdrawal. Mean arterial pressure (MAP) was calculated as 1/3 × SBP + 2/3 × DBP, and pulse pressure (PP) was calculated by subtracting DBP from SBP. Postprandial office blood pressure was measured hourly at T60, T120, T180, T240, and T300 after mixed-meal consumption.
Endothelial function
To measure endothelial vasodilator function, the EndoPAT 2000 (Itamar Medical Ltd) was used, as described previously (13). For this measurement, both index fingers were placed into 2 pneumatic probes to determine pulse wave amplitude in response to reactive hyperemia. A blood pressure cuff was placed on 1 arm to be occluded during the measurement. The peripheral arterial tone (1) was monitored for 5 min without inflating the cuff, followed by 5 min of blood flow occlusion by cuff inflation. Finally, the cuff was released, and the peripheral arterial tone (PAT) signal was measured for another 5 min. The reactive hyperemia index (RHI) was quantified as the post- to preocclusion PAT signal ratio in the occluded arm. RHI values were normalized to the values in the nonoccluded control arm and corrected for the baseline PAT signal. Endothelial vasodilator function was assessed before mixed-meal consumption and again at T120 after meal consumption, and the change in RHI was calculated (ΔRHI = RHIT120 – RHIT0).
24-h ambulatory blood pressure monitoring
At the end of the run-in and the intervention periods, ambulatory blood pressure was monitored for 24 h with the Mobil-O-Graph ambulatory blood pressure monitor (I.E.M. GmbH). SBP, DBP, and heart rate (HR) were measured every 15 min during daytime (07:00–23:00) and every 30 min during nighttime (23:00–07:00). MAP and PP were calculated in the same way as for the office blood pressure measurements. Mean 24-h and daytime and nighttime DBP, SBP, HR, MAP, and PP were calculated. In addition, nighttime dipping was calculated as the difference between mean daytime and mean nighttime blood pressure and expressed as a percentage of the daytime value.
Blood sampling and analyses
Blood was sampled in EDTA-containing vacutainer tubes, centrifuged, and stored as described previously (10). All risk markers were measured in plasma, and samples from the same participant were analyzed within the same analytical run at the end of the study. Fasting triacylglycerol with correction for free glycerol (TAG; Sigma Aldrich Chemie), total cholesterol, and HDL cholesterol (Roche Diagnostics GMBH), apoA1, apoB100 (Horiba ABX), and apoB48 (ELISA; Shibayagi) were all measured before and after the intervention. All fasting parameters, except for apoB48, were measured from both test days, the mixed-meal challenge, and the hyperinsulinemic euglycemic clamp (10). Fasting LDL cholesterol was calculated using the Friedewald formula (14). Since the formula is not valid with TAG concentrations exceeding 4.52 mmol/L, the LDL cholesterol concentration of 1 male participant was not calculated. Postprandial TAGs and apoB48 were measured at T30, T60, T120, T180, T240, and T300.
Statistical analyses
The primary outcome parameter of this study was the change in ΔRQ during a hyperinsulinemic euglycemic clamp. Before the start of the study, it was calculated that using a 2-sided α of 0.05, 16 participants in each diet group should complete the study to reach a power of 80% to detect a true difference of 0.04 in ΔRQ (15). To account for possible dropouts, we aimed to recruit 40 participants. Five dropouts during the run-in period were replaced, and 40 participants started and finalized the intervention period.
Results are presented as means and SEMs. The statistical analyses of fasting and postprandial parameters were performed as described earlier (10). In short, the differences between the HD and WD group in the diet-induced changes in fasting plasma markers measured before and after the intervention were assessed by a 1-factor ANCOVA with sex and baseline values of the outcome variable as covariates. For fasting concentrations of total cholesterol, LDL cholesterol, HDL cholesterol, and TAGs, the mean value of both test days (the mixed-meal challenge and the clamp days) was used for statistical analysis. For the postprandial plasma markers measured during the 5-h mixed meal before and after the intervention, a repeated-measures linear mixed model was used to examine the differences between the diet-induced overall changes (post-WD – pre-WD) compared with (post-HD – pre-HD). All models contained diet, time, and the diet × time interaction as fixed factors. Subject code was added as random factor. Sex, BMI, and age entered the model as covariates. Normality of the residuals was tested by visual inspection, as well as statistically using a Shapiro-Wilk test. The data of all outcome parameters met the normality assumptions. All measured plasma biomarkers as well as office blood pressure and endothelial function were defined as secondary end points and the 24-h blood pressure measurements as exploratory end points.
For both fasting and postprandial testing, differences were considered statistically significant at a 2-sided significance level of P < 0.05. The estimated differences and the 95% CIs of these differences are reported.
Results
Baseline characteristics and compliance
After screening, 45 participants were eligible for participation and were randomly allocated to either the HD (n = 22) or the WD (n = 23) group. The mean plasma glucose value from the screening visit was 5.27 ± 0.07 mmol/L (range: 4.4–6.2 mmol/L). As described previously, 5 participants discontinued the study due to difficulties with diet compliance (2 male and 1 female) and for non-study-related reasons (2 male). Finally, 40 participants were considered compliant and therefore included in the analysis, as shown in Supplementary Figure 1 (10). The baseline characteristics and the measured compliance markers, including urinary sodium and potassium, weight and waist circumference, and physical activity, have been reported earlier (10). The baseline characteristics are shown in Supplementary Table 4. Diet compliance was high, as shown by the analyses of the 3-d food diaries. The reported changes in nutrient intakes are shown in Figure 1 and Supplementary Table 5.
FIGURE 1.

Daily intakes of macronutrients and fatty acids obtained from 3-d food diaries at the end of the WD and HD intervention periods in overweight and obese adults. Data are mean ± SEM, n = 19 (HD) or 21 (WD). *Significantly different between the diets. CHO, carbohydrate; HD, healthy diet; WD, Western diet.
Fasting cardiometabolic risk markers
Differences in the diet-induced changes are expressed relative to the HD group. Changes in total cholesterol (–0.57 ± 0.12 mmol/L, P < 0.001), LDL cholesterol (–0.41 ± 0.12 mmol/L, P = 0.001), and apoB100 (–0.09 ± 0.03 g/L, P = 0.001) were significantly different between the diet groups (Table 1). Changes in HDL cholesterol concentrations were comparable between the 2 groups (–0.05 ± 0.03 mmol/L, P = 0.101), while apoA1 changed significantly different (–0.06 ± 0.03 g/L, P = 0.050). Plasma fasting TAG (–0.24 ± 0.13 mmol/L, P = 0.063) and apoB48 (1.04 ± 0.67 mg/L, P = 0.396) concentrations decreased on the HD, but these changes were not significantly different compared with the WD (Table 1).
TABLE 1.
Fasting plasma cardiometabolic risk markers and iAUCs at the end of the run-in period and at the end of the 6-wk interventions in overweight and obese adults and the mean differences between the healthy diet group and the Western diet group1
| WD (n = 21) | HD (n = 19) | Treatment effect2 | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Run-in | Intervention | Run-in | Intervention | Mean differences | 95% CI | P value |
| Total cholesterol, mmol/L | 5.37 ± 0.17 | 5.29 ± 0.18 | 5.54 ± 0.20 | 4.87 ± 0.18 | –0.57 ± 0.12 | –0.81, –0.33 | <0.001 |
| LDL-C, mmol/L | 3.52 ± 0.14 | 3.47 ± 0.16 | 3.57 ± 0.17 | 3.14 ± 0.14 | –0.41 ± 0.12 | –0.65, –0.18 | 0.001 |
| HDL-C, mmol/L | 1.22 ± 0.07 | 1.21 ± 0.07 | 1.24 ± 0.06 | 1.19 ± 0.05 | –0.05 ± 0.03 | –0.12, 0.01 | 0.101 |
| LDL-C/HDL-C | 3.10 ± 1.09 | 3.08 ± 1.17 | 2.93 ± 0.77 | 2.57 ± 0.62 | –0.35 ± 0.10 | –0.56, –0.14 | 0.002 |
| TAG, mmol/L | 1.40 ± 0.12 | 1.35 ± 0.09 | 1.62 ± 0.25 | 1.20 ± 0.14 | –0.24 ± 0.13 | –0.50, 0.01 | 0.064 |
| ApoB100, g/L | 1.08 ± 0.04 | 1.06 ± 0.05 | 1.09 ± 0.05 | 0.98 ± 0.04 | –0.09 ± 0.03 | –0.15, –0.04 | 0.001 |
| ApoA1, g/L | 1.36 ± 0.05 | 1.36 ± 0.05 | 1.42 ± 0.04 | 1.35 ± 0.05 | –0.06 ± 0.03 | –0.13, 0.00 | 0.050 |
| ApoB100/apoA1 | 0.82 ± 0.23 | 0.81 ± 0.25 | 0.78 ± 0.14 | 0.73 ± 0.12 | –0.04 ± 0.02 | –0.08, 0.01 | 0.129 |
| ApoB48, mg/L | 5.21 ± 0.49 | 5.30 ± 0.56 | 5.52 ± 0.87 | 4.83 ± 0.52 | –0.59 ± 0.69 | –0.31, 2.39 | 0.396 |
| iAUC TAG, mmol/L·min | 138 ± 13.3 | 188 ± 14.1 | 192 ± 19.5 | 126 ± 12.6 | –97.5 ± 19.7 | –137, –57.5 | <0.001 |
| iAUC apoB48, mg/L·min | 450 ± 51.5 | 577 ± 50.4 | 564 ± 43.7 | 425 ± 47.4 | –235 ± 73.6 | –385, –85.1 | 0.003 |
Data are expressed as means ± SEMs. HD, healthy diet; iAUC, incremental area under the curve; TAG, triacylglycerol; WD, Western diet.
Mean difference in the change between the HD and WD groups with 95% CI obtained from a 1-factor ANCOVA with sex and baseline value as covariates.
Postprandial cardiometabolic risk markers
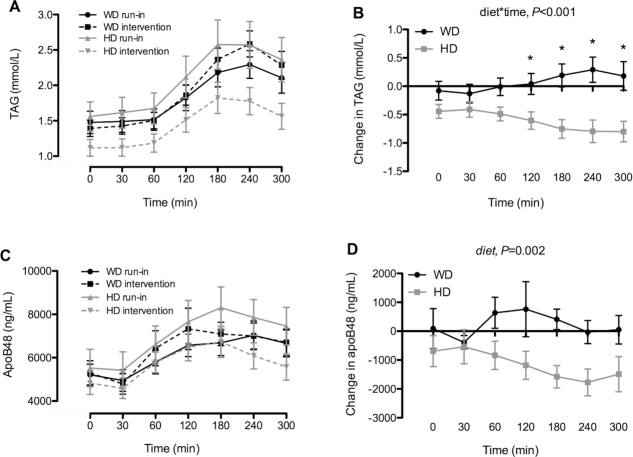
Changes in postprandial TAGs were significantly different between the diets (diet × time, P < 0.001) with decreases in the HD group compared with increases on the WD at T120 (P < 0.05), T180 (P < 0.001), T240 (P < 0.001), and T300 (P < 0.001) after mixed-meal consumption (Figure 2A, B). Significant diet effects were observed for postprandial apoB48 (P = 0.002, Figure 2C, D). Also, changes in the incremental area under the curves for TAG (–97.5 ± 19.7 mmol/L·min, P < 0.001) and apoB48 (–235 ± 73.6 mg/L·min, P = 0.003) were significantly different between the groups (Table 1).
FIGURE 2.
Postprandial plasma triacylglycerol (TAG) (A) and apoB48 (C) concentrations during the 5-h mixed-meal challenge at the end of the run-in and 6-wk WD or HD intervention periods and changes in their concentrations (B, D) in overweight and obese adults. Data are mean ± SEM, n = 19 (HD) or 21 (WD). *Significantly different between the diets. HD, healthy diet; WD, Western diet.
Office blood pressure and endothelial function
Fasting systolic blood pressure decreased more with the HD compared with the WD (–6.9 ± 3.1 mmHg, P = 0.031, Table 2). Diet-induced changes in postprandial systolic blood pressure did not differ between the diet groups (P = 0.429). Changes in fasting diastolic blood pressure were also not significantly different between the diet groups (–3.1 ± 1.7 mmHg, P = 0.069, Table 2). After baseline corrections, changes in postprandial diastolic blood pressure were comparable between the groups (P = 0.247). Changes in MAP (–4.4 ± 2.0 mmHg, P = 0.037) but not PP (–3.9 ± 2.0 mmHg, P = 0.058) were significantly different between the diet groups. No diet effects on fasting (–1.5 ± 1.2 mmHg, P = 0.230) and postprandial heart rate (P = 0.796) were observed. Endothelial function, expressed as the change in reactive hyperemia index after mixed-meal consumption (ΔRHI), was also not significantly affected by the interventions (–0.2 ± 0.3, P = 0.390).
TABLE 2.
Ambulatory 24-h blood pressure, office blood pressure, and endothelial function at the end of the run-in period and at the end of the 6-wk interventions in overweight and obese adults and the mean differences between the healthy diet group and the Western diet group1
| WD (n = 21) | HD (n = 19) | Treatment effect2 | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Run-in | Intervention | Run-in | Intervention | Mean differences | 95% CI | P value |
| Office blood pressure | |||||||
| SBP, mmHg | 135 ± 2.89 | 132 ± 3.09 | 133 ± 2.97 | 123 ± 3.02 | –6.9 ± 3.1 | –13.2, –0.7 | 0.031 |
| DBP, mmHg | 85.2 ± 1.52 | 83.8 ± 1.54 | 83.9 ± 2.23 | 79.9 ± 1.81 | –3.1 ± 1.7 | –6.5, 0.2 | 0.069 |
| MAP, mmHg | 101.8 ± 1.87 | 99.9 ± 1.95 | 100.2 ± 2.31 | 94.5 ± 2.07 | –4.4 ± 2.0 | –8.5, –0.3 | 0.037 |
| PP, mmHg | 49.6 ± 1.94 | 48.3 ± 2.08 | 48.8 ± 2.04 | 43.7 ± 2.06 | –3.9 ± 2.0 | –8.0, 0.1 | 0.058 |
| HR, bpm | 59.3 ± 1.67 | 59.4 ± 1.57 | 56.2 ± 1.40 | 55.6 ± 1.15 | –1.5 ± 1.2 | –4.0, 1.0 | 0.230 |
| Endothelial function | |||||||
| ΔRHI, T120–T0 | –0.43 ± 0.17 | –0.19 ± 0.17 | –0.23 ± 0.19 | –0.38 ± 0.18 | –0.2 ± 0.3 | –0.7, 0.3 | 0.390 |
| Ambulatory 24-h blood pressure | |||||||
| SBP, mmHg | 131.4 ± 2.02 | 133.7 ± 2.36 | 127.8 ± 2.29 | 125.3 ± 2.39 | –5.0 ± 1.7 | –8.5, –1.5 | 0.007 |
| DBP, mmHg | 82.6 ± 1.40 | 83.4 ± 1.30 | 80.4 ± 1.90 | 78.2 ± 1.95 | –3.3 ± 1.1 | –5.6, –1.0 | 0.006 |
| MAP, mmHg | 105.0 ± 1.53 | 106.4 ± 1.64 | 102.1 ± 1.89 | 99.9 ± 1.97 | –3.8 ± 1.3 | –6.5, –1.1 | 0.008 |
| PP, mmHg | 48.1 ± 1.11 | 50.3 ± 1.82 | 47.6 ± 1.64 | 47.2 ± 1.64 | –2.2 ± 1.0 | –4.1, –0.2 | 0.028 |
| HR, bpm | 69.9 ± 1.44 | 69.6 ± 1.65 | 67.6 ± 2.29 | 66.5 ± 1.94 | 0.0 ± 1.5 | –2.9, 2.9 | 0.986 |
| Nighttime dipping SBP, % | 11.7 ± 1.40 | 11.6 ± 1.46 | 8.71 ± 1.41 | 9.44 ± 1.35 | –1.2 ± 2.0 | –5.3, 2.8 | 0.545 |
| Nighttime dipping DBP, % | 14.8 ± 1.44 | 14.1 ± 2.07 | 11.4 ± 1.56 | 11.5 ± 1.61 | –1.0 ± 2.6 | –6.3, 4.2 | 0.696 |
| Ambulatory daytime blood pressure | |||||||
| SBP, mmHg | 133.9 ± 1.98 | 136.2 ± 2.39 | 129.8 ± 2.37 | 127.2 ± 2.54 | –5.1 ± 2.0 | –9.1, –1.1 | 0.014 |
| DBP, mmHg | 84.7 ± 1.44 | 85.5 ± 1.34 | 82.0 ± 2.00 | 79.6 ± 2.01 | –3.5 ± 1.1 | –5.8, –1.1 | 0.005 |
| MAP, mmHg | 107.3 ± 1.51 | 108.8 ± 1.66 | 103.8 ± 1.99 | 101.4 ± 2.11 | –4.2 ± 1.5 | –7.2, –1.2 | 0.007 |
| PP, mmHg | 48.5 ± 1.13 | 50.9 ± 1.87 | 47.8 ± 1.70 | 47.6 ± 1.73 | –1.9 ± 1.1 | –4.1, 0.3 | 0.084 |
| HR, bpm | 71.8 ± 1.51 | 71.3 ± 1.70 | 69.6 ± 2.42 | 68.1 ± 2.00 | –0.3 ± 1.6 | –3.6, 2.9 | 0.829 |
| Ambulatory nighttime blood pressure | |||||||
| SBP, mmHg | 118.5 ± 2.86 | 120.4 ± 2.79 | 118.2 ± 2.50 | 114.9 ± 2.24 | –5.3 ± 2.8 | –10.9, 0.3 | 0.064 |
| DBP, mmHg | 72.1 ± 1.46 | 73.4 ± 2.01 | 72.5 ± 1.97 | 70.3 ± 1.80 | –3.4 ± 2.1 | –7.7, 0.9 | 0.121 |
| MAP, mmHg | 93.5 ± 2.01 | 95.0 ± 2.31 | 93.5 ± 2.11 | 90.6 ± 1.88 | –4.4 ± 2.4 | –9.2, 0.4 | 0.070 |
| PP, mmHg | 46.2 ± 1.18 | 47.1 ± 1.38 | 45.9 ± 1.40 | 44.6 ± 1.39 | –2.1 ± 1.4 | –4.9, 0.6 | 0.127 |
| HR, bpm | 60.9 ± 1.26 | 61.3 ± 1.82 | 59.4 ± 1.76 | 58.7 ± 1.72 | –0.1 ± 1.5 | –3.1, 2.9 | 0.931 |
Data are expressed as means ± SEMs. DBP, diastolic blood pressure; HD, healthy diet; HR, heart rate; MAP, mean arterial pressure; PP, pulse pressure; RHI, reactive hyperemia index; SBP, systolic blood pressure; WD, Western diet.
Mean difference in the change between the HD and WD groups with 95% CI obtained from a 1-factor ANCOVA with sex and baseline value as covariates.
24-h ambulatory blood pressure
Changes in mean 24-h SBP (–5.0 ± 1.7 mmHg, P = 0.007), DBP (–3.3 ± 1.1 mmHg, P = 0.006), MAP (–3.8 ± 1.3 mmHg, P = 0.008), and PP (–2.2 ± 1.0 mmHg, P = 0.031) were significantly different between diets (Table 2). Ambulatory HR did not significantly differ (0.0 ± 1.5 mmHg, P = 0.986). Separate analyses of daytime measurements showed significantly different responses for SBP (P = 0.014), DBP (P = 0.005), and MAP (P = 0.007) but not PP (P = 0.084) and HR (P = 0.829), with diet-induced decreases on the HD and increases on the WD (Table 2). No statistically significant differences were detected in nighttime SBP, DBP, MAP, PP, and HR and in nighttime dipping of SBP and DBP (Table 2).
Discussion
In this randomized study with overweight and obese participants, we showed that 6 wk on a healthy diet rich in fruit and vegetables, pulses, fibers, nuts, and fatty fish and low in foods rich in SFA and high-glycemic carbohydrates significantly reduced total cholesterol, LDL cholesterol, and apoB100, as well as postprandial TAGs and apoB48 concentrations. Blood pressure also improved, but endothelial function did not.
It is well established that water-soluble dietary fibers effectively lower plasma LDL-cholesterol concentrations (16, 17). Due to the wide variety of foods, the HD diet provided many different fibers, many of which had comparable effects on LDL cholesterol (16). It should be noted, however, that the pronounced decrease of 0.42 mmol/L in LDL cholesterol in our study was probably not solely due to the increased fiber intake but also from the replacement of SFAs by unsaturated fatty acids (18) and the decrease in cholesterol intake (19). Although HDL-cholesterol concentrations did not decrease significantly in the HD group, apoA1 concentrations—the major protein component of the HDL particle—did. This effect may be related to the exchange of saturated for unsaturated fatty acids (20). It is unlikely that the replacement of protein for carbohydrates explains the observed decreases in apoA1, as carbohydrates and proteins may have comparable effects on apoAI concentrations (21). Other dietary factors that changed with the HD, such as intakes of fiber, omega-3 [ω-3 (n–3)] fatty acids, fish, nuts, and cholesterol, do also not have significant effects on fasting apoA1 (22).
No significant effects on serum fasting TAG concentrations were observed, despite an increase in the intake of ω-3 long-chain PUFAs (LCPUFAs) from fatty fish (23), which are well known for their TAG-lowering effects. However, effects are mainly evident with supplemental amounts of 2–4 g/d (24, 25). As the HD group only consumed ∼0.7 g of EPA and DHA per day, this relatively low intake may explain the lack of a significant effect on fasting serum TAGs. In addition, TAG-lowering effects of LCPUFAs are more pronounced in hypertriglyceridemic patients (26), which might be another reason for the lack of effect in our normotriglyceridemic group. Participants in the HD group also consumed daily 3.4 g of the essential ω-3 fatty acid α-linolenic acid (ALA). However, ALA and EPA + DHA do not have comparable effects on serum TAG concentrations (27), possibly because the conversion of ALA into EPA and DHA is limited in humans (28).
In contrast to fasting TAG concentrations, the HD diet lowered postprandial TAG responses during the mixed-meal challenge. Schaefer et al. (29) also found significant decreases in postprandial but not in fasting TAG concentrations when participants consumed a diet rich in fish-derived fatty acids. However, they provided LCPUFA-enriched meals during the postprandial challenge test. Park and Harris (30) did not provide LCPUFAs on the day of the meal challenge test and also found decreased postprandial but not fasting TAG concentrations after a 4-wk intervention with a supplemental daily intake of 4 g of EPA or DHA. With regard to ALA, Finnegan et al. (31) concluded that a modest daily intake for 6 mo does not reproduce the postprandial TAG-lowering effects of EPA + DHA. Concentrations of postprandial apoB48, the characteristic protein of chylomicrons, were also significantly decreased in the HD group after meal intake. As each chylomicron contains 1 apoB48 molecule, our results suggest that less circulating chylomicron particles were present in the HD group. Therefore, a relatively low daily intake of LCPUFAs may reduce postprandial lipemia, even when not provided with the meal challenge. Unfortunately, little is known about the effect of high-protein diets on postprandial lipid responses (32), and we do not know whether the replacement of 10% carbohydrates with dietary protein has contributed to the decreased postprandial TAG concentrations in the HD group.
Our results of plasma TAGs and apoB48 show that measuring postprandial and fasting plasma markers may lead to different conclusions. In agreement, we have previously shown that fasting glucose values did not significantly differ between the HD and the WD groups (10). Surprisingly, postprandial glucose excursions during the mixed-meal challenge were significantly higher with the HD. Here, the postprandial measurements were particularly relevant since they revealed the unexpected higher glucose responses to a high glycemic load after following a healthy, low-glycemic diet (10). Therefore, nonfasting risk markers should be considered when drawing conclusions on metabolic health effects of dietary interventions.
The HD significantly reduced 24-h SBP, DBP, MAP, and PP. It has been shown that 24-h SBP may better predict cardiovascular mortality than office SBP (33). In addition, 24-h measurements allowed us to determine daytime and nighttime blood pressure separately and to calculate dipping, which is the decrease in SBP and DBP during nighttime. Impaired dipping has been defined as a risk factor for cardiovascular events and mortality (34). It is known that a higher sodium intake does not attenuate nocturnal dipping in normotensive adults (35). Prather et al. (36) observed a trend for an intervention effect of the well-known Dietary Approach to Stop Hypertension (DASH) diet on nocturnal blood pressure dipping. However, whole-diet approaches resulting in significant improvements in dipping are currently missing. In our study, only changes in daytime—and not in nighttime—SBP, DBP, and MAP were significantly different between the groups. This may be explained by the larger intraindividual variation of the nighttime measurements, possibly related to the less frequent measurements during the night. In addition, the portable blood pressure monitor might have caused discomfort and sleep disruptions in some participants. Although ambulatory blood pressure is a validated predictor for CVD risk and mortality, it was an exploratory parameter in our trial, and results should therefore be interpreted with caution.
Blood pressure can be positively affected by combined dietary approaches (37) such as the DASH diet (38) that provides comparable food products as our HD diet. Overall, this resulted in a decreased intake of sodium intake in the HD group as reflected by the significantly lower urinary sodium concentrations (10), which likely contributed to the favorable reductions in blood pressure (39). In addition, potassium intake significantly increased with the HD and may have further decreased blood pressure (40). Another approach to reduce blood pressure is to increase the intake of nitrate (NO3−) (41), which is naturally present in vegetables like beetroot, spinach, or rocket salad (42). After ingestion, NO3− is eventually converted to NO, which can act as a vasodilator and thereby reduce blood pressure (43, 44). The nitrate content of vegetables is dependent not only on the type of vegetable but also on environmental factors (45). Therefore, it is difficult to estimate the exact amount of nitrate from 750 g nitrate-rich vegetables in the HD and to predict to what extent the NO pathway has contributed to the blood pressure reduction. A number of human intervention trials have shown the antihypertensive effect of green and black tea, possibly related to the polyphenolic compounds (flavonoids) present in tea that possess antioxidative properties (46). Together with the polyphenol-rich vegetables, the regular consumption of tea in the HD group may also have contributed to a lower BP. Since we used a whole-diet approach, it can be speculated that the increased intake of fiber (47) and fish oil (48) also contributed to the reductions observed.
We did not find significant effects of the HD on endothelial function during the mixed-meal challenge (ΔRHI). In an acute study (49), a significant improvement in ΔRHI was observed 4 h after the intake of a single-dose NO3−-containing supplement, which may result in a stronger vasodilating effect than our long-term dietary intervention. In addition, the daily intake of NO3− of our participants was lower than the supplemental daily amounts used in other human trials that found an improvement in endothelial function (50). Recently, Sweazea et al. (51) also found that nitrate from fruit and vegetable supplements significantly reduced blood pressure but not endothelial function measured by flow-mediated dilation (FMD). Finally, our method (ΔRHI) might not be sensitive enough to detect NO-mediated effects on endothelial function. The PAT signal in the fingertips, reflecting small artery reactivity, is only partly mediated by NO (52), whereas NO is the main response mediator for the FMD measurement in the brachial artery (53).
In this randomized human trial, we found beneficial effects of a whole-diet approach on CVD risk markers in an overweight or obese population. One limitation of our study was the early randomization to the intervention groups. As all study dropouts occurred during the run-in period, holding the randomization until the end of the run-in period would have allowed the preferred intention-to-treat analysis. Another limitation is the possible introduction of type I errors (false positives) due to multiple comparisons. We did not correct for this bias due to the potential increase in the number of false negatives and thus reporting important physiologic effects as statistically not significant. Finally, due to the nature of the diets, blinding of participants and study team was not possible, which could have biased results.
In conclusion, a whole-diet approach targeting a wide variety of CVD risk markers was a feasible dietary strategy to improve not only fasting but also postprandial CVD risk markers. In fact, the postprandial measurements provided important additional information to estimate CVD risk beyond that of fasting values only.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Cara Op ‘t Eyndt for developing the dietary guidelines and providing dietary counseling. We also thank Esther Kornips and Maud Beckers for performing the laboratory analyses and Harry Hiemstra for supporting the statistical analyses.
The authors’ responsibilities were as follows—LB, HPFP, PS, and RPM: designed the study; EF and LB: conducted the experiments and coordinated the study; EF: performed the statistical analyses; EF, HPFP, and RPM: interpreted the data; EF: wrote the first draft of the manuscript; LB, HPFP, RPM, and PS: reviewed and edited the manuscript; EF and RPM: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript.
Notes
Sources of support: The study was funded by Unilever Food Innovation Center, Wageningen, The Netherlands.
Author disclosures: HPFP is employed by Unilever Food Innovation Center, Wageningen, The Netherlands. All other authors report no conflicts of interest.
Supplementary Figure 1 and Supplementary Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ALA, α-linolenic acid; CVD, cardiovascular disease; DASH, Dietary Approach to Stop Hypertension; DBP, diastolic blood pressure; FMD, flow-mediated dilation; HD, healthy diet; HR, heart rate; LCPUFA, long-chain PUFA; MAP, mean arterial pressure; NO3−, nitrate; PAT, peripheral arterial tone; PP, pulse pressure; RHI, reactive hyperemia index; SBP, systolic blood pressure; TAG, triacylglycerol; WD, Western diet; ΔRQ, change in respiratory quotient upon insulin stimulation.
Contributor Information
Eva Fechner, Department of Nutrition and Movement Sciences, Maastricht University Medical Center+, Maastricht, The Netherlands.
Lena Bilet, Department of Nutrition and Movement Sciences, Maastricht University Medical Center+, Maastricht, The Netherlands.
Harry P F Peters, Unilever Food Innovation Center, Wageningen, The Netherlands.
Patrick Schrauwen, Department of Nutrition and Movement Sciences, Maastricht University Medical Center+, Maastricht, The Netherlands.
Ronald P Mensink, Department of Nutrition and Movement Sciences, Maastricht University Medical Center+, Maastricht, The Netherlands.
References
- 1. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Task Force Members 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;252:207–74. [DOI] [PubMed] [Google Scholar]
- 3. Tapsell LC, Neale EP, Satija A, Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. 2016;7:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 5. Alissa EM, Ferns GA. Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr. 2017;57:1950–62. [DOI] [PubMed] [Google Scholar]
- 6. Salas-Salvado J, Becerra-Tomas N, Garcia-Gavilan JF, Bullo M, Barrubes L. Mediterranean diet and cardiovascular disease prevention: what do we know?. Prog Cardiovasc Dis. 2018;61:62–7. [DOI] [PubMed] [Google Scholar]
- 7. Higgins V, Adeli K. Postprandial dyslipidemia: pathophysiology and cardiovascular disease risk assessment. EJIFCC. 2017;28:168–84. [PMC free article] [PubMed] [Google Scholar]
- 8. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–16. [DOI] [PubMed] [Google Scholar]
- 9. Huber M KJ, Green L, van der Horst H, Jadad AR, Kromhout D. How should we define health?. BMJ. 2011;343:d4163. [DOI] [PubMed] [Google Scholar]
- 10. Fechner E, Bilet L, Peters HPF, Hiemstra H, Jacobs DM, Op‘t Eyndt C, Kornips E, Mensink RP, Schrauwen P. Effects of a whole diet approach on metabolic flexibility, insulin sensitivity and postprandial glucose responses in overweight and obese adults—a randomized controlled trial. Clin Nutr. [epub ahead of print 17 Dec 2019]. [DOI] [PubMed] [Google Scholar]
- 11. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4:370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Human energy requirements: report of a joint FAO/WHO/UNU Expert Consultation. Food Nutr Bull. 2005;26:166. [PubMed] [Google Scholar]
- 13. Axtell AL, Gomari FA, Cooke JP. Assessing endothelial vasodilator function with the Endo-PAT 2000. J Vis Exp. 2010;44:2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15. van Herpen NA, Schrauwen-Hinderling VB, Schaart G, Mensink RP, Schrauwen P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. J Clin Endocrinol Metab. 2011;96:E691–5. [DOI] [PubMed] [Google Scholar]
- 16. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30–42. [DOI] [PubMed] [Google Scholar]
- 17. Fuller S, Beck E, Salman H, Tapsell L. New horizons for the study of dietary fiber and health: a review. Plant Foods Hum Nutr. 2016;71:1–12. [DOI] [PubMed] [Google Scholar]
- 18. Jacobson TA, Maki KC, Orringer CE, Jones PH, Kris-Etherton P, Sikand G, La Forge R, Daniels SR, Wilson DP, Morris PB et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9:S1–S122. [DOI] [PubMed] [Google Scholar]
- 19. Griffin JD, Lichtenstein AH. Dietary cholesterol and plasma lipoprotein profiles: randomized-controlled trials. Curr Nutr Rep. 2013;2:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins: a meta-analysis of 27 trials. Arterioscler Thromb. 1992;12:911–9. [DOI] [PubMed] [Google Scholar]
- 21. Roussell MA, Hill AM, Gaugler TL, West SG, Heuvel JP, Alaupovic P, Gillies PJ, Kris-Etherton PM. Beef in an Optimal Lean Diet study: effects on lipids, lipoproteins, and apolipoproteins. Am J Clin Nutr. 2012;95:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smolders L, Plat J, Mensink RP. Dietary strategies and novel pharmaceutical approaches targeting serum apoA-I metabolism: a systematic overview. J Nutr Metab. 2017;2017:5415921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alhassan A, Young J, Lean MEJ, Lara J. Consumption of fish and vascular risk factors: a systematic review and meta-analysis of intervention studies. Atherosclerosis. 2017;266:87–94. [DOI] [PubMed] [Google Scholar]
- 24. Bradberry JC, Hilleman DE. Overview of omega-3 fatty acid therapies. P T. 2013;38:681–91. [PMC free article] [PubMed] [Google Scholar]
- 25. Skulas-Ray AC, West SG, Davidson MH, Kris-Etherton PM. Omega-3 fatty acid concentrates in the treatment of moderate hypertriglyceridemia. Expert Opin Pharmacother. 2008;9:1237–48. [DOI] [PubMed] [Google Scholar]
- 26. Harris WS. n-3 fatty acids and lipoproteins: comparison of results from human and animal studies. Lipids. 1996;31:243–52. [DOI] [PubMed] [Google Scholar]
- 27. Goyens PLL, Mensink RP. Effects of alpha-linolenic acid versus those of EPA/DHA on cardiovascular risk markers in healthy elderly subjects. Eur J Clin Nutr. 2006;60:978–84. [DOI] [PubMed] [Google Scholar]
- 28. Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care. 2002;5:127–32. [DOI] [PubMed] [Google Scholar]
- 29. Schaefer EJ, Lichtenstein AH, Lamon-Fava S, Contois JH, Li Z, Goldin BR, Rasmussen H, McNamara JR, Ordovas JM. Effects of National Cholesterol Education Program Step 2 diets relatively high or relatively low in fish-derived fatty acids on plasma lipoproteins in middle-aged and elderly subjects. Am J Clin Nutr. 1996;63:234–41. [DOI] [PubMed] [Google Scholar]
- 30. Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res. 2003;44:455–63. [DOI] [PubMed] [Google Scholar]
- 31. Finnegan YE, Minihane AM, Leigh-Firbank EC, Kew K, Meijer GW, Muggli R, Calder PC, Williams CM. Plant and marine-derived n-3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. Am J Clin Nutr. 2003;77:783–95. [DOI] [PubMed] [Google Scholar]
- 32. Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr. 2007;98:458–73. [DOI] [PubMed] [Google Scholar]
- 33. Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O'Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–61. [DOI] [PubMed] [Google Scholar]
- 34. Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertens Res. 2012;35:695–701. [DOI] [PubMed] [Google Scholar]
- 35. Brian MS, Dalpiaz A, Matthews EL, Lennon-Edwards S, Edwards DG, Farquhar WB. Dietary sodium and nocturnal blood pressure dipping in normotensive men and women. J Hum Hypertens. 2017;31:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prather AA, Blumenthal JA, Hinderliter AL, Sherwood A. Ethnic differences in the effects of the DASH diet on nocturnal blood pressure dipping in individuals with high blood pressure. Am J Hypertens. 2011;24:1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM, American Heart Association . Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
- 38. Kucharska A, Gajewska D, Kiedrowski M, Sinska B, Juszczyk G, Czerw A, Augustynowicz A, Bobinski K, Deptala A, Niegowska J. The impact of individualised nutritional therapy according to DASH diet on blood pressure, body mass, and selected biochemical parameters in overweight/obese patients with primary arterial hypertension: a prospective randomised study. Kardiol Pol. 2018;76:158–65. [DOI] [PubMed] [Google Scholar]
- 39. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER III, Simons-Morton DG et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 40. Appel LJ. The effects of dietary factors on blood pressure. Cardiol Clin. 2017;35:197–212. [DOI] [PubMed] [Google Scholar]
- 41. Jonvik KL, Nyakayiru J, Pinckaers PJ, Senden JM, van Loon LJ, Verdijk LB. Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults. J Nutr. 2016;146:986–93. [DOI] [PubMed] [Google Scholar]
- 42. Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. [DOI] [PubMed] [Google Scholar]
- 43. Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525–32. [DOI] [PubMed] [Google Scholar]
- 44. Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–3. [DOI] [PubMed] [Google Scholar]
- 45. Ashworth A, Mitchell K, Blackwell JR, Vanhatalo A, Jones AM. High-nitrate vegetable diet increases plasma nitrate and nitrite concentrations and reduces blood pressure in healthy women. Public Health Nutr. 2015;18:2669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li DX, Wang RR, Huang JB, Cai QS, Yang CS, Wan XC, Xie ZW. Effects and mechanisms of tea regulating blood pressure: evidences and promises. Nutrients. 2019;11:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Streppel MT, Arends LR, van't Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med. 2005;165:150–6. [DOI] [PubMed] [Google Scholar]
- 48. Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–9. [DOI] [PubMed] [Google Scholar]
- 49. Houston M, Hays L. Acute effects of an oral nitric oxide supplement on blood pressure, endothelial function, and vascular compliance in hypertensive patients. J Clin Hypertens. 2014;16:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lara J, Ashor AW, Oggioni C, Ahluwalia A, Mathers JC, Siervo M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: a systematic review and meta-analysis. Eur J Nutr. 2016;55:451–9. [DOI] [PubMed] [Google Scholar]
- 51. Sweazea KL, Johnston CS, Miller B, Gumpricht E. Nitrate-rich fruit and vegetable supplement reduces blood pressure in normotensive healthy young males without significantly altering flow-mediated vasodilation: a randomized, double-blinded, controlled trial. J Nutr Metab. 2018;2018:1729653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–8. [DOI] [PubMed] [Google Scholar]
- 53. Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol Heart C. 1996;270:H1435–H40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.