Summary
Human fetuses with trisomy 21 (T21) have atypical brain development that is apparent sonographically in the second trimester. We hypothesize that by analyzing and integrating dysregulated gene expression and pathways common to humans with Down syndrome (DS) and mouse models we can discover novel targets for prenatal therapy. Here, we tested the safety and efficacy of apigenin, identified with this approach, in both human amniocytes from fetuses with T21 and in the Ts1Cje mouse model. In vitro, T21 cells cultured with apigenin had significantly reduced oxidative stress and improved antioxidant defense response. In vivo, apigenin treatment mixed with chow was administered prenatally to the dams and fed to the pups over their lifetimes. There was no significant increase in birth defects or pup deaths resulting from prenatal apigenin treatment. Apigenin significantly improved several developmental milestones and spatial olfactory memory in Ts1Cje neonates. In addition, we noted sex-specific effects on exploratory behavior and long-term hippocampal memory in adult mice, and males showed significantly more improvement than females. We demonstrated that the therapeutic effects of apigenin are pleiotropic, resulting in decreased oxidative stress, activation of pro-proliferative and pro-neurogenic genes (KI67, Nestin, Sox2, and PAX6), reduction of the pro-inflammatory cytokines INFG, IL1A, and IL12P70 through the inhibition of NFκB signaling, increase of the anti-inflammatory cytokines IL10 and IL12P40, and increased expression of the angiogenic and neurotrophic factors VEGFA and IL7. These studies provide proof of principle that apigenin has multiple therapeutic targets in preclinical models of DS.
Keywords: Down syndrome, trisomy 21, prenatal treatment, transcriptome, apigenin, cytokines, inflammation
Introduction
Screening for trisomy 21 (T21) or Down syndrome (DS) (MIM: 190685) is universally offered as part of routine obstetric care in most developed countries. With the implementation of cell-free DNA sequencing of maternal plasma, the positive predictive values are on the order of 80% in the general obstetric population and ∼92% in the high-risk population.1 In continuing pregnancies, knowledge that the future child will have DS may affect the parents’ choice of where to deliver and provide opportunities for both family education and to meet with pediatric subspecialists before the child’s birth.2 Our laboratory has suggested consideration of prenatal diagnosis as a potential opportunity to treat the fetus in utero.3,4 This concept, however, has many unique challenges. Treatment cannot harm the pregnant woman or her fetus, and any therapeutic agent must cross both the placental and blood-brain barriers and improve postnatal outcomes in the baby.
Prenatal sonographic and post-mortem studies demonstrate that atypical brain growth is first detectable in second-trimester fetuses with T21, resulting in significant reduction of neurogenesis, synaptogenesis, axonal growth, and myelination.5, 6, 7, 8, 9 One study has shown that during the third trimester, fetuses with T21 have atypical patterns of habituation to a repeated auditory stimulus, suggesting that functional and sensory deficits are present prior to birth.10 We hypothesize that a safe prenatal treatment given to the pregnant woman as soon as a diagnosis of T21 is made will result in more typical fetal brain growth and development.
Until recently, almost all preclinical and clinical trials in mouse models and people with DS have been conducted in adolescents and adults because of safety concerns. As of summer 2020, 13 pharmacological interventions have been tested with little evidence of success in humans with DS.11, 12, 13, 14 Potential reasons for this failure may be related to the fact that these therapeutic interventions were carried out too late and not during the prenatal and early postnatal critical periods for brain development.14, 15, 16 To date, no prenatal treatment studies have been reported in pregnant women carrying fetuses with T21. A limited number of prenatal treatment studies using fluoxetine, maternal choline supplementation, and the neuroprotective peptides NAP and SAL have been described with the Ts65Dn mouse model of DS.17, 18, 19
In our previous studies, we integrated gene expression data from nine different cellular and tissue sources in both humans with DS and mouse models to identify common dysregulated signaling pathways and cellular processes.20,21 We demonstrated that pathway abnormalities associated with DS were the result of gene-dosage-specific effects and the consequence of a global stress response with activation of compensatory mechanisms.20 To counteract these genome-wide abnormalities, we used the Connectivity Map database22 to discover molecules that could be repurposed to rescue the transcriptome and promote more typical brain development in individuals with DS.21 One of the molecules that had the most consistent negative scores (hence, negating the dysregulated gene expression signatures in DS) across tissues and species was apigenin (4′,5,7-trihydroxyflavone). We hypothesized that prenatal treatment with apigenin would partly rescue the global gene expression dysregulation to improve neurogenesis and postnatal cognitive outcomes in DS.
Apigenin is a molecule of interest because it has no known toxicities. It is a naturally occurring compound that is present in chamomile flowers, parsley, celery, peppermint, and citrus fruits.23,24 In animal studies, apigenin has been shown to cross the blood-brain barrier. It has potent antioxidant, anti-inflammatory, and anti-apoptotic properties.24,25 In murine microglia that have been activated by interferon gamma, apigenin decreased the levels of IL-6 and TNF-alpha via its effect on phosphorylation of STAT1.26 This is notable because both humans with DS and mouse models show consistent evidence of overactivation of interferon signaling.27,28 In a double transgenic mouse model for amyloid precursor protein and presenillin 1 proteins, oral intake of apigenin for three months resulted in reduction of fibrillar amyloid deposits and improvement in learning and memory deficits.29
Here, we investigated the potential prenatal therapeutic effects of apigenin in vitro on human amniocytes and in vivo in the Ts1Cje mouse model of DS. Although we have previously described the results of an extensive comparison of three major mouse models of DS,27 the experiments reported here were initiated before the comparative study reported in Aziz et al. was completed. Whereas Ts1Cje mice are more mildly affected than Ts65Dn mice, we did not select the latter model because affected males are sterile. This requires the trisomic chromosome to be passed through an affected mother, altering the intrauterine environment in which the fetus develops. This can confound the postnatal evaluation of pup development. Furthermore, Ts65Dn mice harbor a large segmental trisomy of non-orthologous human Hsa21 genes from mouse Mmu17 for which the impact on the phenotype is still unclear.
Material and Methods
Additional description of the methods used can be found in the Supplemental Material and Methods.
In Vitro Studies on Human Amniocytes
This study was approved by the institutional review boards (IRBs) at Tufts Medical Center (protocol 5582) and Women and Infants’ Hospital (protocol 01-0028). The amniocytes were obtained after clinically indicated prenatal karyotyping. Because this was discarded material that was de-identified, patient consent was deemed unnecessary by the IRBs. Only fetal karyotype, gestational age, and sex were known. Second-trimester amniocytes were prepared as described previously.21 The initial sample set consisted of 14 flasks of amniocytes with the following metaphase karyotypes: 47, XX, +21 (n = 3); 47, XY, +21 (n = 4); 46, XX (n = 3); and 46, XY (n = 4). Gestational ages ranged from 15 3/7 to 20 2/7 weeks. Samples were matched for sex and gestational age (seven pairs were analyzed) (Table S1).
Apigenin Dose Determination
To determine the range of non-toxic doses that would be used to evaluate treatment efficacy, we either left cells untreated or treated them with five different concentrations of apigenin (1, 2, 3, 4, and 5 μM) for three consecutive days. Automatic cell counting was performed with the Scepter 2.0 Cell Counter (EMD Millipore, Billerica, MA) and CellTiter 96 cell proliferation assays (Promega, Madison, WI). We normalized cell proliferation in the untreated cells to 100% and used this as a baseline to estimate the percent of cell proliferation in apigenin-treated cells. Toxicity was defined as doses that induced significant (p < 0.05) or more than 15% (even if not statistically significant) reduction of cell proliferation in both assays.
Oxidative Stress and Antioxidant Capacity
The level of oxidative stress damage and the effects of apigenin treatment in amniocytes from fetuses with T21 and euploid fetuses was quantified with the Comet Assay kit according to the manufacturer’s instructions (Trevigen, Gaithersburg, MD). The percent of DNA in the “tail” versus “head” (nucleus) of the migrating cell, resembling a comet, was determined in 300–500 cells per cell line and compared in untreated and apigenin-treated cells.
The physiological response to oxidative stress before and after apigenin treatment was measured with the OxiSelect Total Antioxidant Capacity (TAC) kit. 5,000 μg of total protein was used to evaluate total antioxidant capacity (TAC), measured as absorbance at 490 nm and compared to a uric acid standard curve according to the manufacturer’s instructions (Cell Biolabs, San Diego, CA).
Amniocyte Gene Expression Analysis
Gene expression analysis was performed on treated and untreated cells via the GeneChip Human Transcriptome HT 2.0 array (Affymetrix, Santa Clara, CA). Only one apigenin dose (2 μM) was evaluated (see Supplemental Materials). Twenty-eight arrays were used (seven T21 and seven euploid for both controls and treated). Data were analyzed with a repeated-measures ANOVA that included genotype, treatment, and sample pairings between T21 and control samples. Probe sets for which either genotype or treatment was significant (at p < 0.001 and a Benjamini-Hochberg false discovery rate [BH-FDR] of 10%) were considered as differentially expressed (DEX) genes.30
Pathway analyses were carried out with the Database for Annotation, Visualization, and Integrated Discovery (DAVID) and Ingenuity Pathway Analysis (IPA) on the top 1% upregulated/downregulated genes (hereafter referred to as marginally expressed (MEX) genes) and gene set enrichment analysis (GSEA) on the whole transcriptome as previously described21. We used “Ingenuity Pathway Upstream Regulator Analysis” to identify potential upstream regulators that may be responsible for the gene expression changes observed in T21 amniocytes and how these genes are affected after apigenin treatment. This allows the prediction of mechanism of action of apigenin and whether identified upstream regulators are inhibited or activated on the basis of a Z score algorithm.31
In Vivo Studies on the Ts1Cje Mouse Model
The effects of apigenin treatment were analyzed at the three different stages of murine life: embryonic, neonatal, and adult.
Breeding, Prenatal Apigenin Treatment, and Genotyping
All murine experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Tufts University (protocol B2013-20). Ts1Cje males (B6 T(12;16)1Cje/CjeDnJ) were crossed with C57BL/6J females (Jackson Laboratories, Bar Harbor, ME). Breeding pairs received either purified powdered (Pow) chow F3197 (Bioserv, Flemington, NJ) or 333–400 mg/kg/day of apigenin (Apig) (2 g of apigenin in 1 kg of purified powdered chow) (SelleckChem, Houston, TX). These doses were obtained via the SD = (DD x FI)/BW formula in which SD is the single daily dose to be delivered (mg/kg/day), DD is the drug dose in the diet (2,000 mg/kg), FI is the daily food intake (5 g per mouse), and BW is the average animal weight (25–30 g).32 Treatment was given as powdered chow with apigenin starting at the time of mating and continuing throughout pregnancy and lactation until all behavioral and biochemical studies in adulthood were completed. Mice that received apigenin are referred to throughout the study with the subscript “Apig”, and untreated mice are referred to with the subscript “Pow,” indicating that they received only powdered chow. Genotyping and sex determination were performed by PCR with primers specific for the Ts1Cje mouse and Sry as described previously.33
Embryonic Forebrain Gene Expression and Pathway Analyses
For gene expression studies, total RNA was isolated from the developing forebrain via the RNA II kits following the manufacturer’s instructions (Macherey-Nagel, Bethlehem, PA). RNA was processed and hybridized on the GeneChip Mouse Gene ST 1.0 array as described previously.21 Twenty arrays were used (five wild-type (WT)Pow, five Ts1CjePow, five WTApig, and five Ts1CjeApig; three males and two females/group); each array corresponded to labeled cDNA from one sample. Analyses were performed via unpaired t tests. A gene with a p value < 0.001 and a BH-FDR of 10% was considered to be DEX. Pathway analyses were performed as in the in vitro studies.
Inflammatory, Angiogenesis, and Neurotrophic Protein Levels
Proteins were extracted from untreated and apigenin-treated Ts1Cje and WT E15.5 embryonic brain and Ts1Cje and WT adult cortex via the Cell Signaling lysis buffer supplemented with protease/phosphatase inhibitors according to the manufacturer’s instructions (Millipore-Sigma, Temecula, CA). We used 20 μg of proteins to analyze the expression of NFκB, pro-inflammatory cytokines IFNG, IL1A, IL12P70, anti-inflammatory cytokines IL10 and Il12P40, and pro-angiogenic and neurotrophic proteins VEGFA and IL7 by using the Luminex 200 system according to the manufacturer’s instructions (Millipore-Sigma, Temecula, CA). Data were acquired with the xPONENT 4.2 software (Luminex Corporation, Austin, TX). Normalization and analysis were performed with the MILLIPLEX Analyst 5.1 software (Millipore-Sigma, Temecula, CA).
Neonatal Behavior
All neonatal behavioral tests were performed blindly between postnatal (P) days P3 and P21 (weaning).34 The Fox scale is a general screening test used to evaluate body righting and coordination, strength, sensory maturation, and extinction of rotatory behavior. The criteria for successfully performing a test was under 30 s and over 2 consecutive days. The homing test was used to investigate olfactory-dependent spatial memory at postnatal day 12.35 During the testing period, pups (WTPow = 31, Ts1CjePow = 19, WTApig = 25, and Ts1CjeApig = 26) were separated from the dam and placed with nesting material in a small bowl positioned on a heating pad at 37°C. The amount of time (latency) and presence/absence of a reflex was recorded and analyzed by a single experimenter.
Adult Behavior
All adult behavioral testing paradigms were performed as previously described.35 Exploratory behavior and locomotor activity were assessed with the open field test. Exploratory behavior was tracked during a 60-min unique trial with the Ethovision 10.5 animal tracking system (Noldus, Leesburg, VA). The total distance traveled (cm) in the center versus periphery, as well as the average velocity (cm/s), were analyzed for the treated and untreated groups. Motor coordination was investigated with the rotarod test (Med Associates, Fairfax, VT) with two different protocols (fixed speed on day 1 and accelerating speed on day 2). The time to fall was recorded in seconds and analyzed for each mouse. Hippocampal-dependent memory was analyzed via the fear conditioning test. On day 1 (training session), two mild foot shocks (0.5 mA for 2 s) were administered at 180 s and 240 s. On day 2 (testing session), mice were placed in the same chambers and the extent (or percent) of freezing, used as a measure of the animal’s memory, was analyzed as time bins of 60 s via the Freeze View software (Med Associates, Fairfax, VT).
Statistical Analysis
Statistical analyses were performed with the parametric t test or two-way repeated-measure ANOVA and Tukey’s multiple comparison test for normal distributions. Non-parametric Mann-Whitney and Kruskal-Wallis tests were used if values did not follow a normal distribution. For proportions (percentages) comparison of the effects of apigenin treatment on the natural history and the homing test, chi-square and Fisher’s exact tests were used. Statistical significance was reached with a p value < 0.05. All statistical analyses were performed with GraphPad Prism 7.03 software package. Data are presented as Mean ± SEM.
Results
Effects of Apigenin Treatment on Human Amniocytes
Optimal Apigenin Dose
Separate analyses of apigenin effects on euploid and T21 amniocytes showed similar trends. High doses of apigenin (4 and 5 μM) negatively impacted cell proliferation; there was ∼10% reduction in total cell number in euploid amniocytes (U = 0 and 6, respectively, p < 0.05, Mann-Whitney test) and between 15% to 30% reduction in T21 amniocytes (p = 0.08 at 4 μM and p < 0.01 at 5 μM, respectively) (Figure S1). On the basis of these data, we selected doses between 0 and 4 μM for further evaluation of oxidative stress and antioxidant capacity, global gene expression in T21, and euploid amniocytes.
Oxidative Stress and Antioxidant Capacity
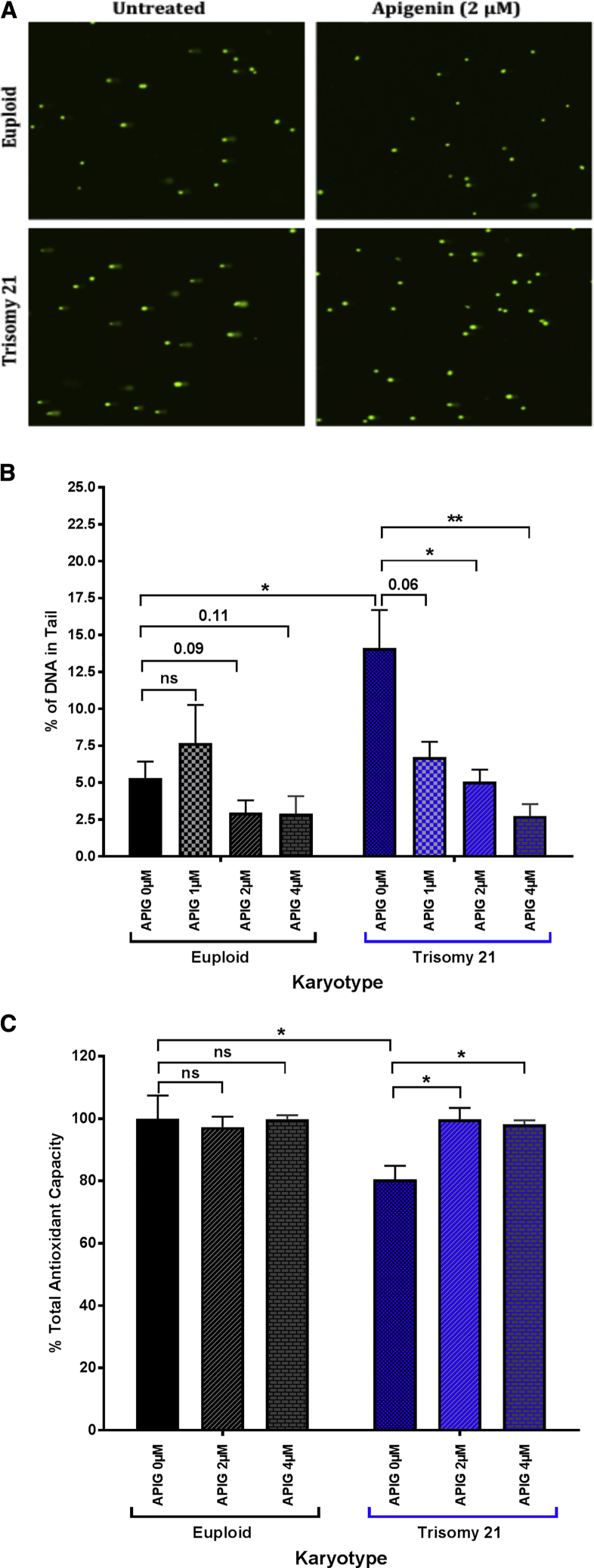
The average percent of DNA in the tail of the untreated T21 amniocytes (14.1 ± 2.6%, sum of ranks = 30, U = 0, p = 0.016, Mann-Whitney test) was significantly higher than in the euploid amniocytes (5.3 ± 1.1%, sum of ranks = 15) (Figures 1A and 1B). In treated T21 amniocytes, apigenin significantly reduced the percent of DNA in the tail in a dose-dependent manner (6.7 ± 1.0%, p = 0.06 for 1 μM of apigenin; 5.1 ± 0.8%, p = 0.03 for 2 μM; 2.7 ± 0.8%, p < 0.01 for apigenin 4 μM) (Figures 1A and 1B). Although not statistically significant, apigenin treatment also reduced the percentage of DNA in the tail of euploid amniocytes at 2 and 4 μM (2.8 ± 0.8%, p = 0.09 and 2.9 ± 1.2%, p = 0.11, respectively) (Figures 1A and 1B).
Figure 1.
Effects of Apigenin Treatment on Oxidative Stress and Antioxidant Capacity in Trisomy 21 (T21) and Euploid Amniocytes
(A) COMET assay representative images in untreated and apigenin-treated (2 μM) T21 and euploid amniocytes.
(B) Percent of DNA in tail was analyzed in T21 (n = 5) and euploid (n = 5) amniocyte cell lines untreated and treated with increasing doses of apigenin (1, 2, and 4 μM). A total of 300–500 cells were analyzed for each cell line and drug dose. Apigenin significantly reduces the percent of DNA in the tail in a dose-dependent manner in T21 amniocytes.
(C) Total antioxidant capacity (TAC) measured as absorbance at 490 nm was normalized to 100% in untreated euploid amniocytes. The percent of TAC in untreated and apigenin-treated (2 and 4 μM) T21 and euploid amniocytes was analyzed. T21 amniocytes exhibit reduced percent of TAC compared to euploid amniocytes. Apigenin treatment normalized TAC in T21 amniocytes to the level of euploid amniocytes. ∗p < 0.05, ∗∗p < 0.01.
Two-way ANOVA analysis of the percent of DNA in the tail of both euploid and T21 amniocytes highlighted a significant effect of karyotype (F = 4.7, p = 0.04), treatment (F = 7.6, p < 0.001), as well as treatment × karyotype interaction (F = 3.9, p = 0.02).
Untreated T21 amniocytes also exhibited significantly lower antioxidant capacity (80.6 ± 4.2%, p < 0.05) compared to euploid amniocytes (100.0 ± 7.4%) (Figure 1C). Treatment with 2 and 4 μM of apigenin significantly increased the total antioxidant capacity in T21 amniocytes and restored it to levels that were close to euploid amniocytes (99.8 ± 3.7% and 98.1 ± 1.3%, p > 0.05) (Figure 1C).
Amniocyte Gene Expression Analysis
DEX Genes. For gene expression studies, we selected a dose of 2 μM because it did not significantly change cell proliferation and it rescued the oxidative stress/total antioxidant capacity imbalance in T21 amniocytes. A total of 14 independent samples was used to generate seven sex- and age-matched pairs (Table S1). Using paired analysis after elimination of redundant probes, we identified over 500 genes that were DEX in T21 amniocytes compared to gestational age- and sex-matched euploid amniocytes (Table S2A). Fifty of these DEX genes mapped to chromosome 21. Chromosome 21 gene expression changes in untreated T21 versus euploid amniocytes demonstrated that only a small subset (76/506) of genes was upregulated in a gene-dosage-dependent fashion (Table S2A).
In T21 amniocytes, treatment did not have significant effects on global gene expression as demonstrated by principal-component analysis (PCA) (Figure 2A) or on the expression of the DEX genes (Tables S2B and S2C). Even though apigenin had no effect on T21 DEX genes, a closer look at the lists of MEX genes (Table S3) identified many candidate genes that have been previously reported to be direct or indirect targets of apigenin, including CCL2 (MIM: 158105), IL1A (MIM: 147760), CYP1B (MIM: 601771), MMP1 (MIM: 120353), SERPINB2 (MIM: 173390), VDR (MIM: 601769), DUSP5 (MIM: 603069), and AURKB (MIM: 604970) (Table 1), thus warranting a second pathway analysis with the MEX gene lists.
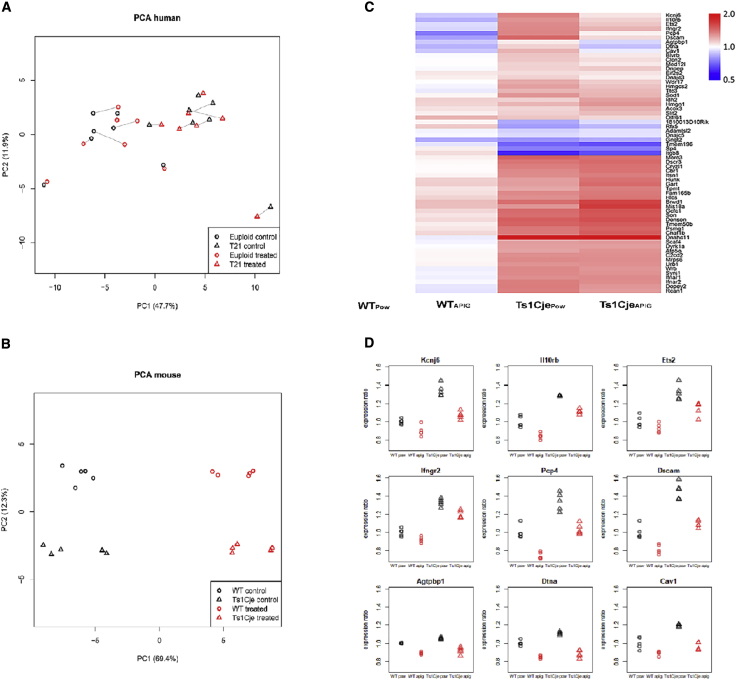
Figure 2.
Effects of Apigenin Treatment on Global Gene Expression In Vitro and In Vivo
(A) Principal-component analysis (PCA) of DEX gene expression in untreated and apigenin-treated T21 (n = 7) and sex- and age-matched euploid amniocytes (n = 7). Apigenin treatment induced subtle gene expression changes in T21 and euploid amniocytes. Solid lines link each untreated cell line to its treated counterpart.
(B) PCA of DEX gene expression in untreated and apigenin-treated Ts1Cje (n = 5) and wild-type (WT) (n = 5) E15.5 embryonic forebrain. Apigenin induced significant changes in gene expression in both Ts1Cje and WT embryos.
(C) Heatmap demonstrating the effect of apigenin on DEX genes in Ts1Cje versus WT embryos. WTPow, untreated WT; Ts1CjePow, untreated Ts1Cje; WTAPIG, apigenin-treated WT; Ts1CjeAPIG, apigenin-treated Ts1Cje.
(D) Apigenin partially rescues the expression of several Mmu16 trisomic genes, including Kcnje, Il10rb, Ets, Ifngr2, Pcp4, Dscam, Agtpbp1, Dtna, and Cav1.
Table 1.
Known Apigenin Targets that Are Dysregulated in T21 and Euploid Amniocytes After Apigenin Treatment
| Gene Symbol | T21 Untr/ Eup Untr Ratio | T21Apig/ T21 Untr Ratio | T21 Apig/ Eup Untr Ratio | Known Apigenin Effects | References |
|---|---|---|---|---|---|
| CYP1B1 | 0.88 | 0.47 | 0.38 | apigenin is a potent inhibitor of Cyp1B1; inhibition occurs at nanomolar concentrations following a mixed model; Cyp1B1 plays an important role in the regulation of cell proliferation and survival through modulation of the intracellular oxidative state and NFκB production and/or activity | Androutsopoulos et al.36 |
| MMP1 | 1.87 | 0.55 | 2.55 | apigenin suppress the expression of MMP1. MMP1 expression is induced by NFκB and activated in inflamed and cancerous tissues | Juliusson et al.37 |
| CCL2 | 1.71 | 0.62 | 0.74 | apigenin suppresses the expression of both CCL2 and IL1A through IKBKe; CCL2 and IL1A expression are increased after TNFA | Gheghiani et al.38 |
| IL1A | 1.07 | 1.05 | 0.77 | ||
| SERPINB2 | 1.41 | 0.82 | 0.77 | apigenin reduces the expression of plasminogen activator inhibitor 2 (PAI2 or SERPINB2); SERPINB2 plays an important role in the regulation of adaptive immunity, is a direct downstream target of p53, and is activated during DNA damage response; SERPINB2 is used as marker of cell senescence; SERPINB2 is activated in response to various toxic stimuli and inhibits self-renewal, migration, and differentiation potential of human stem cells | Lee et al.39 |
| VDR | 1.44 | 0.78 | 1.12 | apigenin treatment promoted cell survival and decreased LPS-induced inflammation and the production of NFκB, VDR, COX2, and iNOS in mesenchymal stem cells and human keratinocytes | Zhang et al.40 |
| DUSP5 | 0.79 | 1.53 | 2.14 | DUSP5 and DUSP6 are dual specificity phosphatases that dephosphorylates and inhibit Erk1 activity; apigenin inhibits Erk1, increases AURKB levels, and promotes the expression of DUSP5 and DUSP6 | Boeckx et al.41 |
| AURKB | 1.02 | 1.90 | 1.88 |
Amniocyte Pathway Dysregulation
In untreated amniocytes, T21 compared to euploid showed a positive enrichment of gene sets associated with immune response and JAK-STAT signaling, RNA-polymerase-I-dependent transcription, G-protein signaling, and proteolysis and G1/S mitotic cell-cycle transition. In contrast, negative enrichment of gene sets was associated with G2/M mitotic cell-cycle transition, RNA-polymerase-II-dependent transcription, translation initiation, NOTCH signaling, and response to hypoxia (Tables 2 and Table S4. Summary of DAVID-Dysregulated Pathways in Untreated and Apigenin-Treated T21 and Euploid Amniocytes, Table S5. Summary of GSEA-Dysregulated Pathways in Untreated and Apigenin-Treated T21 and Euploid Amniocytes, Table S6. Summary of IPA-Dysregulated Pathways in Untreated and Apigenin-Treated T21 and Euploid Amniocytes).
Table 2.
Summary of Dysregulated Signaling Pathways and Cellular Processes in Untreated and Apigenin-Treated Amniocytes
| Signaling Pathway/Cellular Processes | T21 Untr/Eup Untr | T21 Apig/T21 Untr | T21 Apig/Eup Untr | Ts1Cje Untr/Eup Untr | Ts1Cje Apig/Ts1Cje Untr | Ts1Cje Apig/Eup Untr |
|---|---|---|---|---|---|---|
| Nucleosome/nucleoplasm | ↑↑ | ↑↑↑ | ↑↑↑↑ | unchanged | ↑↑↑ | ↑↑↑ |
| RNA-polymerase-I-dependent transcription | ↑↑↑ | unchanged | ↑↑↑ | unchanged | unchanged | unchanged |
| Negative regulation of transcription | ↓↓↓ | unchanged | ↑↑↑ | unchanged | ↑ | ↑ |
| RNA-polymerase-II-dependent transcription | ↓↓↓ | ↑↑↑ | unchanged | unchanged | ↑ | ↑ |
| G1/S transition of mitotic cell-cycle progression | ↑↑↑ | unchanged | ↑↑↑ | unchanged | ↑↑↑ | ↑↑↑ |
| G2/M transition of mitotic cell cycle | ↓↓ | ↑↑↑ | ↑↑↑ | ↓↓ | ↑↑↑ | ↑↑↑ |
| Mitotic role of polo-like kinase | ↓ | ↑↑↑ | ↑↑↑ | unchanged | ↑↑↑↑ | ↑↑↑↑ |
| G-protein signaling | ↑↑↑ | ↓↓↓↓ | ↓↓↓ | ↑↑↑↓↓↓ | ↓↓↓ | ↓↓↓ |
| Proteolysis | ↑↑↑ | unchanged | ↑↑↑ | ↓↓↓ | unchanged | ↓↓↓ |
| Response to hypoxia | ↓↓↓ | unchanged | ↓↓↓ | ↑↑ | unchanged | ↑↑ |
| Interferon signaling | ↑↑ | ↓↓ | unchanged | ↑↑↑↑ | ↓↓ | ↑↑ |
| Immune response | ↑↑ | ↓↓ | unchanged | unchanged | ↓ | ↓ |
| NFκB signaling pathway | unchanged | ↓ | ↓↓ | ↓↓ | unchanged | ↓↓ |
| STAT signaling pathway | ↑ | ↓↓ | unchanged | ↑↑ | unchanged | ↑↑ |
| Steroid hormone biosynthesis | unchanged | unchanged | unchanged | ↓↓↓ | unchanged | ↓↓ |
| Synaptogenesis | unchanged | unchanged | unchanged | ↑↑↑ | ↓↓↓ | unchanged |
| SLC-amino acid transport | unchanged | unchanged | unchanged | ↓↓ | unchanged | ↓↓ |
| Aryl hydrocarbon receptor signaling | unchanged | unchanged | unchanged | unchanged | ↑↑ | ↑↑ |
| Opioid signaling pathway | unchanged | unchanged | unchanged | unchanged | ↓↓↓ | ↓↓↓ |
| Dopamine DARPP32 feedback in cAMP signaling | unchanged | unchanged | unchanged | unchanged | ↓↓↓ | ↓↓↓ |
| nNOS signaling in neurons | unchanged | unchanged | unchanged | unchanged | ↓↓↓ | ↓↓↓ |
| CREB signaling in neurons | unchanged | unchanged | unchanged | unchanged | ↓↓↓ | ↓↓↓ |
Levels of upregulation and downregulation are represented by fold enrichment, normalized enrichment score (NES) in DAVID, IPA, and GSEA. Unchanged, p > 0.05; ↑, upregulation with p < 0.05; ↑↑, upregulation with p < 0.01; ↑↑↑, upregulation with p < 0.001; ↓, downregulation with p < 0.05; ↓↓, downregulation with p < 0.01; ↓↓↓, downregulation with p < 0.001. Eup Untr, untreated euploid amniocytes; T21 Untr, untreated trisomy 21 amniocytes; Eup Apig, apigenin-treated euploid amniocytes; T21 Apig, apigenin-treated trisomy 21 amniocytes.
Apigenin treatment downregulated the pro-inflammatory response and JAK-STAT signaling and upregulated RNA-polymerase-II-dependent transcription in T21 amniocytes to the level of the euploid untreated amniocytes (Table 2). Apigenin also inhibited NFκB signaling but did not affect other dysregulated pathways in untreated T21 amniocytes (Tables 2 and Table S4. Summary of DAVID-Dysregulated Pathways in Untreated and Apigenin-Treated T21 and Euploid Amniocytes, Table S5. Summary of GSEA-Dysregulated Pathways in Untreated and Apigenin-Treated T21 and Euploid Amniocytes, Table S6. Summary of IPA-Dysregulated Pathways in Untreated and Apigenin-Treated T21 and Euploid Amniocytes). Additionally, apigenin treatment induced an overexpression of gene sets associated with G2/M cell-cycle transition and positive regulation of cell proliferation, particularly through polo-like kinase pathway when compared to untreated euploid and T21 amniocytes.
Upstream Regulators in Untreated and Apigenin-Treated T21 Amniocytes
IPA upstream regulator analysis in untreated T21 amniocytes predicted the activation of interferon gamma (IFNG) (interferon signaling) and IKBKB and TLR4 (NFκB signaling) and the inhibition of VEGFA (chemokine activity and regulation of angiogenesis), APP (neurite outgrowth), TP53 (DNA damage-repair), PTGER 2 and PTGER4 (regulation of inflammatory response), and HIF1A (regulation of hypoxia) (Table S7A).
After apigenin treatment, IFNG and NFκB signaling were inhibited in T21 to a level comparable to untreated euploid amniocytes. In addition, apigenin induced an overexpression of G2/M cell transition markers through the activation of HGF and PTGER2 through E2F1 and E2F2 (transcription activators and G1/S cell-cycle transition regulators). In contrast, BNIP3L (response to hypoxia) and IRGM1 (immune response) were predicted to be significantly inhibited (Tables S7B and S7C).
Effects of Apigenin Treatment in the Ts1Cje Mouse
Natural History
When measured on embryonic days 10.5 and 15.5 (E10.5 and E15.5), WT dams fed powdered chow and powdered chow plus apigenin gained similar amounts of weight. At E15.5, they had an average of 7.2 (17 litters /123 embryos) and 7.8 (8 litters /63 embryos) embryos per litter, respectively.
At E15.5, in the untreated dams, the ratio of WT (49.6%) and Ts1Cje (50.4%) embryos followed Mendelian inheritance. In the apigenin-treated dams, 61.9% of embryos were WT, whereas only 38.1% were Ts1Cje by genotyping (Fisher’s exact test, p = 0.06) (Figure S2A). These ratios did not change postnatally (Figure S2B). Apigenin treatment did not induce any significant effects in the weights or crown-rump lengths of E15.5 embryonic mice (Figures S2C and S2D). No increase in deaths or congenital anomalies was observed after apigenin treatment in both Ts1Cje and WT pups.
A two-way ANOVA showed a statistically significant effect of the genotype on body weight throughout the pre-weaning period for both untreated and apigenin-treated pups [F(3,1718) = 378.7, p < 0.0001, η2 = 237.6]. Similar to what has previously been observed for body weight, Ts1Cje untreated and apigenin-treated pups were smaller in length compared to their WT littermates. Tukey’s multiple comparison test showed that apigenin treatment did not have a significant effect on Ts1Cje pup growth (p = 0.72) but caused a slight weight increase (p = 0.04) in WTApig versus WTPow (Figures S2E and S2F).
Embryonic Forebrain Global Gene Expression Analysis
DEX Genes. For gene expression analysis, three groups were compared: (1) the pups of untreated dams compared by genotype (Ts1CjePow versus WTPow), (2) Ts1Cje pups that were treated (Apig) versus untreated (Pow), and (3) Ts1Cje treated pups versus untreated WT. In some experiments, WTApig and WTPow were also analyzed.
Overall, apigenin treatment induced similar expression changes in both coding and non-coding genes in Ts1CjeApig (1,399 DEXs versus Ts1CjePow) and WTApig (906 DEXs versus WTPow). Even though the number of DEX genes was lower in the WTApig, PCA demonstrated that the regulation direction of genes affected by apigenin was similar between Ts1CjeApig and WTApig littermates (Figure 2B and Table S8C).
In Ts1CjePow versus WTPow embryonic forebrain, 63 protein-coding genes were differentially regulated. Forty-two of these genes mapped to the Ts1Cje aneuploid regions (38 genes on Mmu16 and 4 on Mmu12) (Table S8A). Nineteen of the DEXs mapped to chromosomes other than Hsa21 orthologs (Figure 2C).
In Ts1CjeApig versus Ts1CjePow embryonic forebrain (Figure 2C, Tables S8A–S8B) the expression of seven Mmu16 orthologous genes (Dscam, Kcnj6, Pcp4, Ets2, Il10rb, Cav1, and Dtna) was partially corrected (expression decreased after treatment by >30%) (Figure 2D) and four genes (Brwd1, Mis18a, Gart, and Hunk) were amplified (expression increased after treatment by ≥30%). These partially corrected and amplified genes were not specifically affected in Ts1CjeApig but followed similar regulation direction in the WTApig versus WTPow (Table S8C).
Importantly, apigenin-treated Ts1Cje embryonic forebrain showed a significant upregulation of genes implicated in neural stem cell proliferation (Nestin, Sox2, Sox5, Kif4, Prom1, Pax6, Mcm2, Ect2, Gli3, and Ccnd2) and proneural genes (Neurog1, Neurog2, Nhlh1, and Nhlh2) implicated in cell fate determination (Table S8C).
Embryonic Forebrain Pathway Dysregulation
Because of the low number of DEX genes in the Ts1CjePow/WTPow comparison, we examined the MEX gene lists (Table S9) for pathway analysis with the GSEA, DAVID, and IPA databases (Table S10. Summary of DAVID-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain, Table S11. Summary of GSEA-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain, Table S12. Summary of IPA-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain).
Untreated Ts1Cje embryonic forebrain exhibited a significant upregulation of immune response, interferon signaling, Jak-Stat signaling, hypoxia through Hif1a and synaptogenesis (Tables 2 and Table S10. Summary of DAVID-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain, Table S11. Summary of GSEA-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain, Table S12. Summary of IPA-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain). Dysregulation of G-protein-coupled receptor activity (mainly olfactory receptor activity) and significant downregulation of gene sets associated with cell proliferation (G2/M cell-cycle transition), SLC-mediated amino acid transport, and regulation of translation were also found.
Apigenin treatment reduced the immune response overactivation in Ts1Cje forebrain. It had a pleiotropic effect resulting in a partial improvement of some of the above-mentioned pathways, including Jak-Stat signaling, NFκB signaling, and Slc-amino acid transport (Tables 2 and Table S10. Summary of DAVID-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain, Table S11. Summary of GSEA-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain, Table S12. Summary of IPA-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain). Apigenin also induced a significant downregulation of GPCR signaling but did not have any significant effects on regulation of hypoxia through Hif1a (Table 2). Additionally, gene sets associated with cell-cycle progression (G2/M cell-cycle transition and polo-like kinase signaling), DNA-damage repair, and kinetochore organization were over-compensated by apigenin treatment. Finally, apigenin significantly inhibited the p53 signaling, opioid signaling, nNOS signaling, and Creb signaling pathways (Tables 2 and Table S10. Summary of DAVID-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain, Table S11. Summary of GSEA-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain, Table S12. Summary of IPA-Dysregulated Pathways in Untreated and Apigenin-Treated Ts1Cje and WT E15.5 Forebrain).
Embryonic Forebrain Upstream Regulators. IPA upstream regulator analysis revealed that untreated Ts1Cje embryonic brains exhibit an overactivation of Ago2 (transcription repression). Treatment with apigenin was not predicted to affect Ago2 in Ts1Cje embryos. In untreated Ts1Cje, NFκB signaling and Tp53 (DNA damage-repair) were predicted to be overactivated, which apigenin was predicted to inhibit (Table S13). Apigenin treatment was also predicted to upregulate Vegfa (angiogenesis signaling).
Apigenin also promoted the expression of G/2M markers through the activation of Ptger2 and HGF receptors and E2f1 transcription factor close to what has been observed in apigenin-treated T21 amniocytes (Table S13).
Inflammatory, Angiogenesis, and Neurotrophic Protein Levels
To validate the gene expression and pathway analyses suggesting that apigenin has potent anti-inflammatory and VEGF stimulating effects, we measured the levels of several pro-inflammatory (INFG, IL1A, IL12P70, and IL6) and anti-inflammatory (IL10 and IL12P40) cytokines as well as the angiogenic and neurotrophic factors VEGF and IL7 in apigenin-treated versus untreated WT and Ts1Cje embryonic and adult brains.
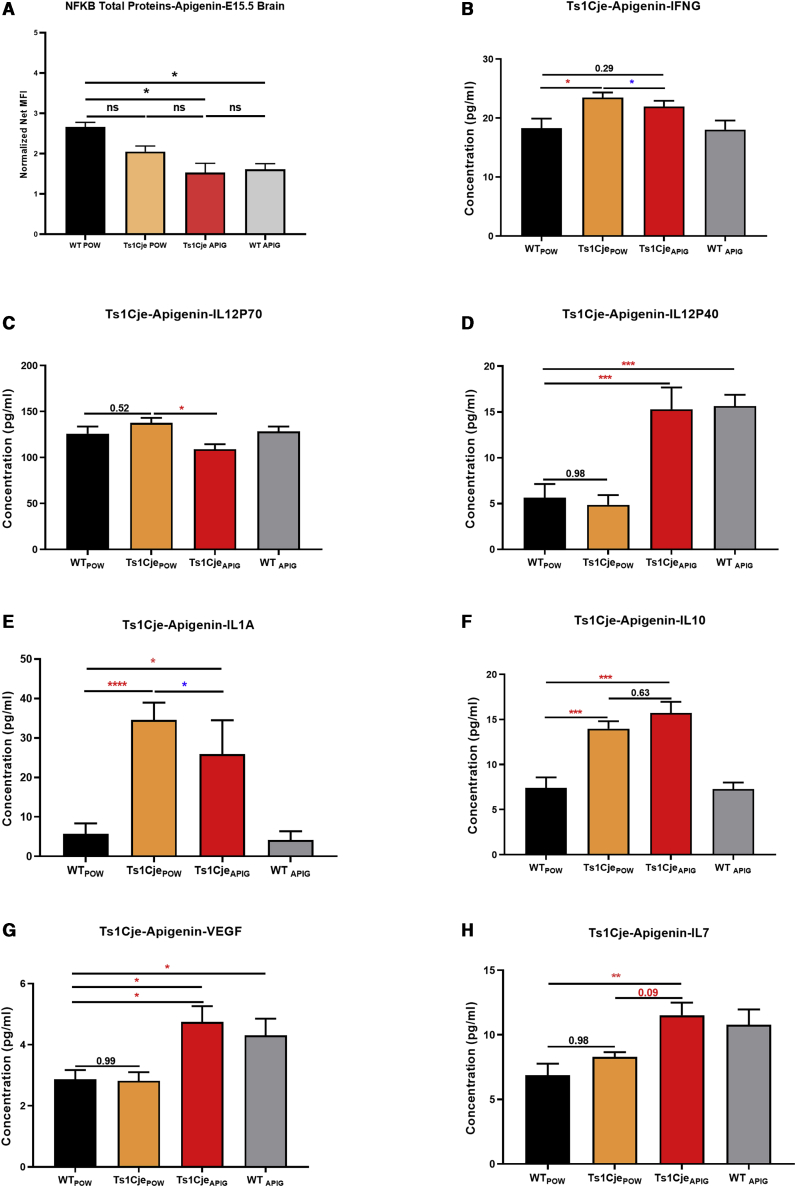
In untreated E15.5 Ts1Cje embryonic forebrains compared to those of WT littermates, Tukey’s multiple comparison test showed higher levels of INFG, IL1A, and IL10 (Figure 3). The expression of IL6, IL7, IL12P70, IL12P40, and VEGFA was unaffected.
Figure 3.
Effects of Apigenin Treatment on Inflammatory, Angiogenesis, and Neurotrophic Proteins in Ts1Cje E15.5 and WT Forebrain
(A–H) Luminex technology was used to analyze the expression of NFκB protein (A), pro-inflammatory cytokines IFNG (B) and IL12P70 (C), IL1A (E), anti-inflammatory cytokines Il12P40 (D) and IL10 (F), and pro-angiogenic and neurotrophic factors VEGF (G) and IL7 (H) in E15.5 embryonic forebrain of WTPow = 9, Ts1CjePow = 10, WTApig = 10, and Ts1CjeApig = 8.
Treatment with apigenin significantly reduced the levels of the pro-inflammatory cytokines IFNG, IL1A, and IL12P70 (Figure 3). Additionally, apigenin induced a significant increase in the levels of anti-inflammatory IL12P40 and IL10 cytokines as well as the angiogenic and neurotrophic factors IL7 and VEGFA (Figure 3). For most cytokines, the effects of apigenin in WT E15.5 forebrain followed the same expression direction as for Ts1Cje embryos.
In the untreated adult Ts1Cje cerebral cortex, there were no changes in the levels of all the inflammatory and angiogenesis markers when compared to their untreated WT littermates. However, apigenin induced a statistically significant decrease in NFκB and increase in IL10 levels in both Ts1Cje and WT mice compared to powder-fed mice (Figure S3).
Neonatal Behavior
Developmental Milestones. Ts1CjePow pups exhibited significant delays versus WTPow littermates in acquiring early developmental milestones and late coordination and sensory maturation milestones (Table 3, Figures S4 and S5). The percent of Ts1CjePow pups reaching criteria was significantly lower compared to WTPow littermates (Table 3, Figures S4 and S5).
Table 3.
Effects of Apigenin Treatment on Developmental Milestones in Ts1Cje Pups
| Milestone | Parameter Measured | WTPow (n = 31) | Ts1CjePow (n = 19) | Ts1CjeApig (n = 26) | WTApig (n = 25) |
|---|---|---|---|---|---|
| Surface righting | latency delay significant at postnatal day versus WT | P6∗, P7∗∗, P8∗∗, P9∗∗ | P7∗, P8∗∗, P9∗ | P7∗, P8∗∗, P9∗ | |
| milestone day | 7.03 ± 1.47 | 8.29 ± 1.45∗ | 7.35 ± 2.11 | 8.36 ± 2.02∗ | |
| Cliff aversion | latency delay significant at postnatal day versus WT | P5∗∗, P6∗∗, P7∗, P8∗∗, P9∗ | P5∗, P6∗, P7∗, P8∗ | P5∗, P6∗, P7∗, P8∗ | |
| milestone day | 5.71 ± 1.62 | 7.12 ± 2.20∗ | 6.46 ± 2.06 | 6.76 ± 2.83∗ | |
| Negative geotaxis | latency delay significant at postnatal day versus WT | P3∗, P4∗∗, P5∗, P6∗, P7∗∗ | P4∗, P5∗∗, P6∗, P7∗∗ | P4∗ | |
| milestone day | 6.16 ± 2.01 | 7.17 ± 2.70∗ | 7.20 ± 2.27∗ | 6.16 ± 1.54 | |
| Forelimb grasp | latency delay significant at postnatal day versus WT | P5∗, P6∗, P7∗ | P5∗, P6∗, P7∗∗∗, P8∗ | P6∗, P7∗∗, P8∗ | |
| milestone day | 7.03 ± 1.30 | 8.12 ± 1.17∗ | 8.30 ± 1.33∗∗ | 8.00 ± 1.15∗ | |
| Open field | latency delay significant at postnatal day versus WT | P14∗, P15∗∗, P16∗, P17∗, P18∗ | P14∗, P15∗∗, P16∗, P17∗, P18∗, P19∗, P20∗, P21∗ | ns | |
| milestone day | 13.54 ± 1.84 | 13.82 ± 1.42 | 14.68 ± 1.83∗ | 13.32 ± 1.40 | |
| Air righting | milestone day | 16.48 ± 1.39 | 18.88 ± 1.67∗∗∗ | 18.10 ± 2.02∗∗ | 17.00 ± 1.87 |
| Eye opening | milestone day | 14.39 ± 0.92 | 15.60 ± 2.03∗ | 14.79 ± 1.36 | 14.36 ± 0.76 |
| Ear twitch | milestone day | 16.00 ± 0.97 | 17.64 ± 1.27∗∗∗ | 17.79 ± 1.68∗∗∗ | 16.64 ± 1.25 |
| Auditory startle | milestone day | 17.12 ± 1.36 | 18.00 ± 2.00 | 17.58 ± 1.89 | 16.28 ± 1.62 |
Postnatal days at which significant delays were measured are indicated by the postnatal day (P) and the level of significance [∗ (p < 0.05), ∗∗ (p < 0.01) and ∗∗∗ (p < 0.001)] compared to WTPow. The average day at which each developmental milestone is reached (milestone day) is indicated. Significant differences are indicated with the levels of significance. ns, not significant.
Apigenin treatment partially improved several developmental milestones in Ts1Cje pups, including surface righting, cliff aversion, eye opening, and air righting. Additionally, apigenin negatively affected strength (forelimb grasp) and motor maturation (open field) but did not affect other milestones (Table 3, Figures S4 and S5).
When Ts1Cje male and female pups were analyzed separately, sex-specific differences were observed: untreated Ts1Cje females had significant deficits in surface righting, cliff aversion, and eye opening, whereas untreated Ts1Cje males were not significantly delayed in these tasks, although they followed similar trends (Figures S6 and S7). Apigenin treatment significantly improved the achievement of these milestones in Ts1Cje females and negatively affected the Ts1Cje male pups’ performance in the surface righting task.
In WT pups, apigenin negatively affected performances in early developmental milestones, including surface righting, cliff aversion, negative geotaxis, and forelimb grasp, but it did not significantly alter late milestones (Table 3, Figures S3 and S4).
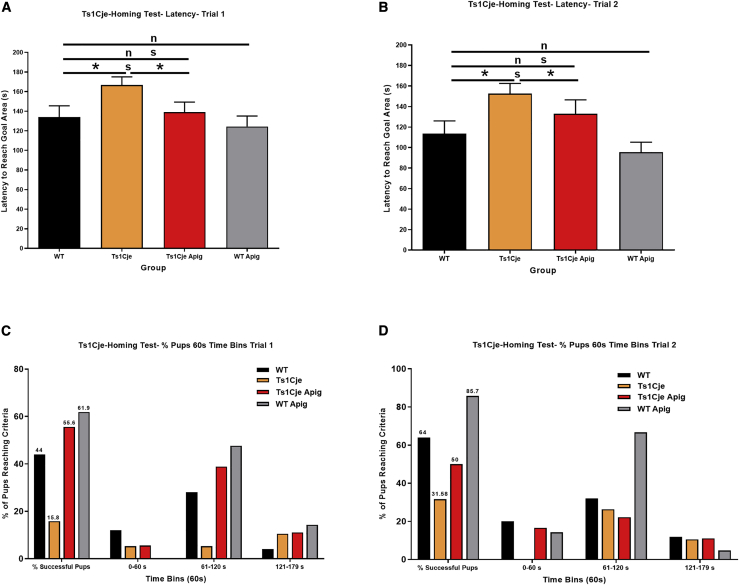
Olfactory Spatial Memory. Ts1CjePow pups displayed significant olfactory spatial memory deficits manifested by significant increases in the amount of time needed to reach the goal area in trial 1 (166.8 ± 8.2 s) compared to their WTPow littermates (134.1 ± 11.4 s, p < 0.05) (Figure 4A). Apigenin treatment significantly reduced the time required for Ts1CjeApig mice to reach the goal area (139.0 ± 10.3 s) versus Ts1CjePow mice (166.8 ± 8.2 s, p < 0.01) (Figure 4A). When compared to WTPow (134.1 ± 11.4 s), Ts1CjeApig mice exhibited similar performances (139.0 ± 10.3 s, p = 0.96) in the homing test. In trial 2, Ts1CjePow pups also took longer (152.6 ± 9.9 s) to reach the goal area compared to WTPow pups (113.8 ± 12.3 s, p = 0.02) (Figure 4B). When compared to WTPow pups (113.8 ± 12.3 s), Ts1CjeApig mice took slightly longer (132.9 ± 13.6 s, p = 0.32) to reach the goal area, but this difference was not statistically significant.
Figure 4.
Effects of Apigenin Treatment on Spatial Olfactory Memory in Untreated and Apigenin-Treated Ts1Cje and WT Neonates
The homing test was used to investigate olfactory spatial memory in untreated and apigenin-treated WT and Ts1Cje neonates (WTPow = 31, Ts1CjePow = 19, WTApig = 25, Ts1CjeApig = 26) at postnatal day 12 in two independent trials.
(A and B) Latency to reach the goal area untreated and apigenin-treated Ts1Cje and WT neonates in trials 1 and 2. Ts1Cje neonates spent significantly more time searching for the goal area compared to their WT littermates, suggesting spatial olfactory memory deficits in trisomic mice. Apigenin significantly reduced latency to reach the goal area and improved spatial olfactory memory in Ts1Cje and WT neonates.
(C and D) Percent of animals reaching the goal area during the entire testing period and 60 s time bins in trials 1 and 2. Almost 80% and over 60% of the Ts1Cje neonates and less than 50% of apigenin-treated neonates did not reach the goal area within the 180 s trial period in both trials 1 and 2. Apigenin treatment increased the percent of Ts1Cje and WT neonates reaching the goal area during the two first minutes of the trials (0–60 and 61–120 s). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Two-way ANOVA showed statistically significant effects of apigenin treatment in trials 1 [F(3,79) = 2.84, p = 0.043, η2 = 6,561] and 2 [F(3,79) = 4.34, p = 0.007, η2 = 1,2148].
Additionally, only three Ts1CjePow (15.80%) and 11 WTPow (44%, chi-square test, p < 0.0001) pups were able to reach the goal area in trial 1 (Figure 4C). On trial 2, only six Ts1CjePow pups (31.58%) reached the goal area whereas 16 WTPow (64%, chi-square test, p < 0.001) were able to successfully perform the test (Figure 4D). Apigenin treatment significantly improved performances of both WTApig and Ts1CjeApig in trial 1 (61.9% of WTApig and 55.6% of Ts1CjeApig, respectively, chi-square test, p < 0.001) and trial 2 (85.7% of WTApig and 50% of Ts1CjeApig, respectively) compared to Ts1CjePow (Figures 4C and 4D). The performance of Ts1CjeApig neonates was not statistically different from WTPow pups.
When Ts1Cje male and female pups were analyzed separately, trisomic males exhibited more significant deficits in olfactory spatial memory than trisomic females. Although apigenin treatment significantly improved olfactory spatial memory in Ts1Cje males, its effects followed the same trends in Ts1Cje females.
Adult Behavior
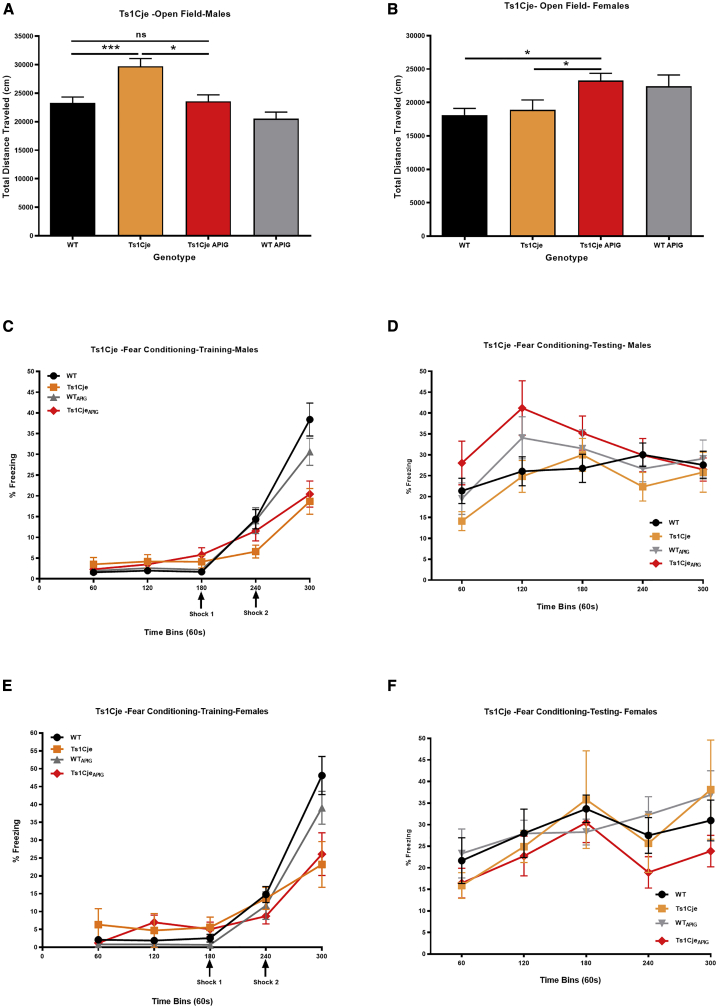
Locomotor Activity and Exploratory Behavior. Analysis of adult behavior was conducted separately in males and females. In the open field test, the total distance traveled by male Ts1CjePow mice was significantly higher (29,721 ± 1,353 cm) than their WTPow littermates (23,296 ± 1,019 cm, p < 0.001) (Figure 5A). Apigenin treatment normalized locomotor behavior in Ts1Cje male mice (distance traveled = 25,117 ± 1,443 cm) to the level of WTPow compared to Ts1CjePow animals (p < 0.05) (Figure 5A).
Figure 5.
Sex-Specific Effects of Apigenin on Exploratory Behavior and Hippocampal Memory in Untreated and Apigenin-Treated Adult Ts1Cje and WT Males and Females
Exploratory behavior and hippocampal-dependent memory were analyzed via the open field and fear conditioning tests, respectively, in untreated and apigenin-treated WT and Ts1Cje males (WTPow = 13, Ts1CjePow = 12, WTApig = 17, Ts1CjeApig = 16) and females (WTPow = 8, Ts1CjePow = 7, WTApig = 14, Ts1CjeApig = 12).
(A and B) Sex-specific effects in the open field test. Ts1Cje male but not female mice exhibit hyperactive behavior (higher distance traveled during the 60 min trial) compared to WT littermates. Apigenin treatment rescued exploratory behavior in Ts1Cje male mice but induced hyperactivity in WT and Ts1Cje female mice.
(C–F) Contextual fear conditioning performances of untreated and apigenin-treated male and female Ts1Cje and WT mice during the training (C and E) and testing (D and F) trials. During the testing trial, apigenin treatment significantly increased percent of freezing (improved hippocampal memory) in Ts1Cje males but not in female Ts1Cje mice during the first two minutes of testing. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Ts1CjePow females (18,871 ± 1509 cm) did not show alterations in locomotor activity compared to WTPow (distance traveled = 18,133 ± 975 cm) (Figure 5B). Apigenin treatment induced hyperactive behavior in both WTApig (distance traveled = 22,426 ± 1,695 cm, p < 0.05) and Ts1CjeApig females (distance traveled = 23,266 ± 1,105 cm, p < 0.01) (Figure 5B).
Hippocampal-Dependent Long-Term Memory. In the fear-conditioning test, during the training phase, Ts1CjePow male mice froze significantly less (18.67% ± 3.1%) after receiving the second electrical shock compared to WTPow (38.40% ± 3.98%, p < 0.001) (Figure 5C). Like their male counterparts, Ts1CjePow female mice also showed a significant decrease in percent freezing (23.21% ± 6.40%) after receiving the second electrical shock compared to WTPow (48.10% ± 5.32%, p = 0.013) (Figure 5E).
In the testing phase, sex differences were observed. A lower percent of freezing was exhibited by Ts1CjePow male mice (21.35% ± 3.03%) versus their WTPow littermates (14.13% ± 2.26%, p = 0.07) during the first 60 s, but this difference disappeared during the remaining four minutes of the trial (Figure 5D). Apigenin treatment induced a statistically significant increase in percent freezing in the Ts1CjeApig male mice (28.03% ± 5.23% in the first minute and 41.18% ± 6.54% in the second minute) versus WTPow and Ts1CjePow males during the first two minutes of testing (p < 0.05) (Figure 5D).
During the testing phase, Ts1CjePow females also exhibited a slight non-significant decrease in percent freezing (15.89% ± 2.98%) during the first minute compared to WTPow female mice (21.65% ± 5.30%) (Figure 5F). Apigenin treatment did not affect percent freezing in Ts1CjeApig females (16.44% ± 3.44%) compared to untreated Ts1CjePow females (15.89% ± 2.98%) (Figure 5F).
Motor Coordination. In Ts1Cje neonates, apigenin negatively affected strength (forelimb grasp) and motor maturation (open field) but did not affect other milestones (Table 3, Figures S4 and S5).
In adults, Ts1CjePow male mice fell significantly faster at the highest rotarod speed (86.40 ± 7.81 s at 32 RPM) than WTPow males (104.30 ± 4.68 s at 32 RPM) in the static speed trial (Figure S8A). However, Ts1CjePow females did not show any abnormalities versus their WTPow littermates (Figure S8B). After apigenin treatment, both male and female WTApig and Ts1CjeApig fell significantly faster from the rotarod at 32 RPM compared to WTPow and Ts1CjePow untreated mice (p < 0.01) (Figures S8A and S8B). No negative effects of apigenin were observed at lower speeds (16 and 24 RPM). In the accelerating speed trial, Ts1CjePow male mice also fell significantly faster (224 ± 13.5 s) than WTPow males (268.3 ± 5.46 s, p < 0.01) (Figure S8C). A similar trend, although statistically non-significant, was observed in Ts1CjePow females (234 ± 19.16 s) versus WTPow females (259.7 ± 11.8 s) (Figure S8D). Apigenin treatment did not induce any significant adverse effects in both genotypes and genders in the accelerating speed trial (Figures S8C and S8D).
Discussion
In this study, we provide proof of principle for the safety and efficacy of apigenin both in vitro (T21 amniocytes) and in vivo (Ts1Cje mouse model). Apigenin reduced oxidative stress and improved total antioxidant capacity in amniocytes derived from second-trimester fetuses with T21. Apigenin also improved some aspects of postnatal behavioral and cognitive outcomes in the Ts1Cje mouse model, and sex differences were observed. Gene expression and protein level analyses revealed that apigenin had a pleiotropic action and achieved its therapeutic effects partly through repression of pro-inflammatory responses and NFκB signaling and stimulation of anti-inflammatory, angiogenic, and neurotrophic proteins.
Effects of Apigenin Treatment on Human Amniocytes
Improvement in Oxidative Stress/Antioxidant Capacity Imbalance
There is significant transcriptomic, proteomic, and biochemical evidence that individuals with DS exhibit an imbalance between oxidative stress and antioxidant capacity, the physiological response to oxidative stress.42, 43, 44, 45, 46 This imbalance starts during fetal life, affects multiple organs, and might contribute to the atypical brain and cognitive phenotypes in DS.19,47, 48, 49 Here, we demonstrated that cultured amniocytes derived from fetuses with DS exhibit an oxidative stress (increased)/antioxidant capacity (decreased) imbalance. Apigenin treatment reduced oxidative stress and increased antioxidant capacity in T21 amniocytes, thus restoring normal redox homeostasis. Apigenin has been reported to have modulatory effects on oxidative stress and inflammation in different cell models. In cells exposed to reactive oxygen species and external stressors, apigenin has pro-proliferative, anti-inflammatory, antioxidant, and free radical scavenging effects.50, 51, 52
In healthy cells exposed to internal or external stressors, apigenin exhibits cell-specific cytoprotective and neuroprotective roles by reducing oxidative stress through its direct free radical scavenging action, upregulation of intracellular antioxidant defenses, inhibition of endoplasmic reticulum stress response, and activation of MAPK, Nrf2, and UPR (unfolded protein response) signaling cascades.53, 54, 55, 56
Suppression of Chronic Inflammation and Promotion of G2/M Cell-Cycle Progression
Untreated T21 amniocytes exhibited an upregulation of multiple genes implicated in the pro-inflammatory process and the activation of interferon and NFκB signaling pathways, including CCL2/MCP1, ICAM1 (MIM: 147840), IL18 (MIM: 600953), MMP1, TNFRSF19 (MIM: 606122), and TNFRSF21 (MIM: 605732). Although the role of NFκB in the chronic inflammatory response in DS is poorly studied, several reports have described increased plasma levels of several pro-inflammatory cytokines, including IL6 (MIM: 147620), IL10 (MIM: 124092), CCL2/MCP1, TNFA (MIM: 191160), IFNG (MIM: 147570), and MMP1, in children and both non-demented and demented adults with DS, suggesting their use as potential biomarkers for disease progression.57, 58, 59
Apigenin treatment significantly reduced the expression of several pro-inflammatory genes, including CCL2, MMP1, IL1A, NFKBIZ (MIM: 608004), INHBA (MIM: 147290), and VCAM1 (MIM: 192225), in T21 amniocytes. Multiple in vitro studies reported significant reduction of pro-inflammatory molecules (TNFA [MIM: 191160], CCL2, IL1A, IL6 [MIM: 147620], IL1B [MIM: 147720], ICAM1, and VCAM1 [MIM: 192225]) after treatment with apigenin.60, 61, 62
Both in vitro and in vivo, gene expression analyses showed that apigenin promoted the G2/M cell-cycle transition through the polo-like kinase cascade activation and through polymerase-II-dependent transcription RNA, significantly inhibited the pro-inflammatory response and NFκB signaling pathway, and downregulated G-protein signaling. In T21 amniocytes, apigenin induced a significant upregulation of multiple genes, including CDK1 (MIM: 116940), NEK2 (MIM: 604043), AURKA (MIM: 603072), AURKB (MIM: 604970), CCNB1 (MIM: 123836), CCNB2 (MIM: 602755), CDC25A (MIM: 116947), CDC25C (MIM: 157680), BIRC5 (MIM: 603352), and CDC20 (MIM: 603618), that promote entry into M phase.63,38
Effects of Apigenin Treatment in the Ts1Cje Mouse
Because of the complexity and high cost of lifespan studies in treated mice, we only used a single (high) dose of apigenin to evaluate its effects. The high dose, however, appeared to be safe because it did not increase pup loss or congenital anomalies.
Changes in Hsa21 Orthologous Genes in the Embryonic Forebrain
Apigenin treatment induced significant gene expression changes in the embryonic Ts1Cje forebrain. Several Mmu16 genes were partially compensated by apigenin, including Dscam, Kcnj6, Pcp4, Ets2, Il10rb, Cav1, and Dtna. Although the contribution of some of these genes (i.e., Il10rb, Cav1, and Dtna) to the DS phenotype is still unknown, the remaining genes (Dscam, Kcnj6, Pcp4, and Ets2) have been reported to be highly expressed in the developing brain and overexpressed in brains from individuals with DS. Their overexpression or deletion is associated with cognitive and/or motor deficits in transgenic mouse models.64, 65, 66, 67, 68, 69
Apigenin Promotes G2/M Cell-Cycle Transition and Pro-neurogenic Gene Expression
Although untreated Ts1Cje embryonic brain showed a less marked cell-cycle gene dysregulation than amniocytes, apigenin treatment targeted similar cell-cycle genes in vitro and in vivo. Indeed, apigenin treatment resulted in a significant upregulation of polo-like kinase signaling and overexpression of Ccnb1, Ccnb2, Cdk1, Racgap1, and Prc1, thus promoting G2/M cell-cycle transition in T21 amniocytes and Ts1Cje embryonic forebrains. Interestingly, apigenin treatment induced significant upregulation of pro-neurogenic genes (Nestin, Sox2, Sox5, Prom1, Pax6, Mcm2, Ect2, and Gli3) and proneural genes (Neurog1, Neurog2, Nhlh1, and Nhlh2).
Our study is the first to administer apigenin prenatally and to demonstrate that it promotes the expression of pro-neurogenic genes. A prior study investigated the pro-neurogenic effect of apigenin in the adult mouse brain.70 Daily intraperitoneal injection of apigenin for 10 consecutive days improved hippocampal-dependent memory in the Morris water maze test and significantly promoted neurogenesis in the dentate gyrus of adult mice. In a more recent study, intraperitoneal injection of luteolin, the major metabolite of apigenin, promoted hippocampal neurogenesis, significantly increased the expression of Nestin, and improved spatial memory deficits in adult Ts65Dn mice.71
Anti-inflammatory Actions of Apigenin
Similar to our results in T21 amniocytes, Ts1Cje embryonic forebrain exhibited increased neuroinflammation through overactivation of interferon signaling and its regulated genes (e.g., Ifitm3, Ifi6, and Oas1) and overproduction of the pro-inflammatory cytokine IL1A. Prenatal apigenin treatment significantly reduced this neuroinflammation through downregulation of IFNG production and NFκB protein level. In Ts1Cje mice, apigenin resulted in a significant upregulation of Chuk/Ikka (a key kinase negative regulator of NFκB) and downregulation of Irak1bp1 and Tbkbp1 (which plays a role in activating NFκB signaling) gene expression.
Consistent with our findings, the anti-inflammatory action of apigenin has been previously reported in the literature.72, 73, 74, 75 Most in vivo studies have reported inhibition of the NFκB signaling pathway and suppression of pro-inflammatory cytokines, including IFNG, TNFA, and IL6 in rodent models of different CNS insults, including Parkinson disease (PD [MIM: 168600]) and Alzheimer disease (AD [MIM: 104300]).
Overactivation of interferon signaling has emerged in the recent years as partly responsible for the chronic inflammation in individuals with DS.28,76 Analysis of gene expression changes in multiple cell types, including skin fibroblasts, lymphoblastoid cell lines, primary monocytes, and T cells have highlighted significant activation of interferon signaling and increased expression of interferon stimulated genes (ISGs), including interferon activated transcription factors or IRFs.28 When stimulated with interferons, cells from individuals with DS showed higher sensitivity and exacerbated overactivation of interferon signaling and increased expression of ISGs.28,77 Zhang et al. performed a meta-analysis of 19 studies that investigated inflammatory cytokines in 957 individuals with DS and 541 euploid individuals.78 They demonstrated that the levels of the pro-inflammatory cytokines IFNG, IL1B, and TNFA were significantly increased in individuals with DS.
In addition to the negative regulation of IFNG and NFκB, our cytokine data demonstrated that apigenin treatment normalized the ratio of IL12P70 (IL12)/IL12P40 by lowering production of the pro-inflammatory cytokine IL12P70 and increasing production of the anti-inflammatory cytokine IL12P40 in Ts1Cje embryonic brain.
Multiple studies have shown that increased activation of IL12R by IL12P70 is correlated with IFNG overproduction as a result of an inflammatory insult via lipopolysaccharide treatment, via brain infection with a scrapie agent, and in mouse models of AD.79, 80, 81 The anti-inflammatory cytokine IL12P40 acts as an antagonist of IL12P70 to inhibit IL12R signaling by binding its β subunit, thus reducing IFNG production.81,82 Several studies reported that apigenin treatment significantly suppressed IL12 signaling activation in lipopolysaccharide treated macrophages, dendritic cells, and periodontal ligament cells.83,84
Apigenin treatment also increased the production of IL10 in Ts1Cje embryonic brain above baseline. In adult Ts1Cje brains, the anti-inflammatory actions of apigenin were less pronounced than in the embryonic forebrain and were only restricted to the overproduction of the potent anti-inflammatory cytokine IL10 without affecting the expression of IFNG, NFκB, and IL1A signaling. These differences in apigenin action during the prenatal and adult stages require further investigation.
Several studies have reported increased levels of the anti-inflammatory cytokines IL10 and IL4 in blood samples obtained from children with DS compared to typically developing children, suggesting that individuals with DS develop an anti-inflammatory state at early stages as a compensatory mechanism to modulate their immune system.85, 86, 87 In adults with DS, however, the levels of IL10 are significantly lower while levels of pro-inflammatory cytokines remained significantly higher compared to euploid individuals.88,89
In Ts1Cje mice, life-long treatment with apigenin continuously promotes the production of IL10, suggesting that it may prevent long-term inflammatory-induced damage to promote brain development and cognitive outcomes. Zhang et al.90 used a mouse model of autoimmune myocarditis to show that apigenin significantly increases the production of anti-inflammatory IL10 and IL4 cytokines and represses IFNG, TNFA, and IL2 pro-inflammatory cytokines. In a second study, apigenin significantly increased the production of the anti-inflammatory cytokines IL10 and TGFβ and reduced the production of the pro-inflammatory cytokines TNFA, IL1β, and IL6 in a mouse model of sepsis.91 Dourado et al.92 reported that apigenin had neuroprotective effects in IL1β, LPS, and Aβ oligomer-treated fetal cortical neurons through the modulation of microglial activation and increased expression of IL10.
In summary, our in vitro and in vivo gene and protein expression studies suggest that apigenin has significant anti-inflammatory effects that might play a key role in improving brain development and post-natal cognitive outcome in DS. Although apigenin treatment improved similar pathways in vitro and in vivo, the genome-wide effects of the treatment were more pronounced in the Ts1Cje mouse model. One potential reason for this might be related to the high dose of apigenin that was administered in vivo to ensure that it crossed the placenta and the blood brain barrier to achieve therapeutic effects in the developing fetus. Another potential explanation might be that the in vivo metabolism of apigenin and its subsequent conversion to luteolin could have a more potent effect on gene expression and behavioral outcomes. As a cell type, amniocytes only moderately respond to apigenin in vitro. Future studies will investigate whether the effects of apigenin and its major metabolite luteolin are stronger when evaluated on neural stem cells differentiated from patient-derived induced pluripotent stem cells (iPSCs).
Induction of Pro-angiogenic and Neurotrophic Factors by Apigenin in the Ts1Cje Embryonic Brain
Prenatal apigenin treatment significantly increased the levels of VEGFA in Ts1Cje embryonic forebrain compared to untreated WT and Ts1Cje embryos. VEGFA has neurotrophic, neuroprotective, and angiogenic properties and is expressed in endothelial cells, perivascular macrophages, neurons, and astrocytes.93,94 VEGFA is produced in neuroepithelial cells in the subventricular zone and in the deeper layers of the cortical plate at mid-gestation in humans and rodents. Its levels correlate with the onset of brain angiogenesis and corticogenesis in those two species.95, 96, 97 In mice, knockout of VEGFA or its receptor FLT1 results in defective vascular development and embryonic lethality around mid-gestation.98, 99, 100 Tetracycline-dependent overexpression of VEGF in the mouse forebrain enhanced neurogenesis and angiogenesis and improved hippocampal-dependent memory and long-term potentiation.101
Clinical studies have demonstrated that high levels of VEGF in the cerebrospinal fluid correlate with a slower age-dependent cognitive decline and a larger hippocampal volume.102,103 Compared to euploid fetuses, second-trimester fetuses with DS have low levels of VEGF and high levels of NO in amniotic fluid and mesenchymal stem cells.104,105
In rodent models of transient ischemia/reperfusion and hypoxia, gene therapy either by direct injection into the brain via adenoviral vector or transplantation of neural stem cells making human recombinant VEGF resulted in a significant increase in angiogenesis, improved neurogenesis, reduction of the infarct volume, and improvement of cognitive outcomes.106,107 In fetal growth-restricted guinea pig and sheep models, maternal therapy with the human recombinant VEGF increased fetal, brain, liver, and lung weight along with an increase in the number of newly formed microvessels producing VEGF in the subventricular zone (SVZ), periventricular, white matter, corpus callosum, and cerebral cortex.108,109
Finally, apigenin also induced a significant upregulation of IL7 in the Ts1Cje embryonic brain compared to untreated WT and Ts1Cje. IL7 is a hematopoietic cytokine that not only regulates B and T lymphocytes but also plays a neurotrophic role and is expressed in human neuronal progenitors and in the developing human brain.110 Treatment of primary hippocampal neuron cultures with increasing concentrations of IL7 significantly enhanced short-term (DIV1) and long-term (DIV7) neuronal survival in a dose-dependent manner.111 Michaelson et al.112 demonstrated that IL7 is expressed by astrocytes while IL7 receptor (IL7R) is expressed at the surface of neural progenitor cells of the subventricular zone during embryonic brain development. They also showed that treatment of embryonic cortical, hippocampal, and cerebellar neuronal cultures with IL7 enhanced neuronal survival and promoted neurite outgrowth in differentiating neurons.
Our in vitro and in vivo data suggest that apigenin might improve Ts1Cje embryonic brain development through its synergistic anti-inflammatory, pro-angiogenic, and neurotrophic actions.
Prenatal Apigenin Treatment and Behavioral Outcomes
Prenatal treatment with apigenin improved several aspects of neonatal and adult behaviors. Although the effects of apigenin on early neonatal behavior were unknown until the present study, several studies have demonstrated that apigenin and its most abundant metabolite, luteolin, improve behavioral deficits in rodent models of epilepsy and depression. Apigenin and luteolin had significant anxiolytic effects in the elevated plus maze, forced swim test, and tail suspension test.113,114 In other rodent models of neurodegenerative diseases (rotenone-induced PD and streptozotocin-induced AD models), epilepsy, and cerebral ischemia, apigenin and luteolin significantly improved hippocampal spatial memory in the Morris water maze test.29,72,115,116
Treatment with a high dose of apigenin improved exploratory behavior and hippocampal learning, yet it also had negative effects on motor coordination in both Ts1Cje males and females, as well as in some WT animals. Anusha et al.72 demonstrated beneficial effects of apigenin treatment at low doses (10 and 20 mg/kg, intraperitoneally [i.p.]) on motor coordination in a rotenone-induced rat model of PD. Treatment with high doses of apigenin or luteolin, however, inhibited motor function and induced mild sedative effects in mice and rats.114 The negative effects of high apigenin doses on motor development and coordination in neonatal and adult mice might be the result of its off-target effects and previously reported sedative effect. Further studies are needed to investigate specific dose responses. Future experiments are planned to evaluate lower doses of apigenin; we will closely monitor motor development and coordination after treatment throughout the lifespan. This applies to WT as well as the trisomic mice. In human studies, however, a euploid fetus would not be exposed to treatment.
Importantly, prenatal apigenin treatment had sex-specific effects in some adult behavioral tests, such as open field and fear conditioning. To our knowledge, no other studies have investigated the sex-specific effects of treatment with polyphenols in rodent models of CNS diseases. Further studies are needed to validate this finding. Gradoltto et al.117 studied sex- and age-specific differences in apigenin metabolism in rats after a single oral 10 mg dose. In mature males, glucuronated and sulfated derivatives of apigenin were present, but they were present in inverse proportions when compared to females and immature males and females, suggesting that sex-specific differences in behavioral outcomes after treatment might be the results of sex-specific differences in metabolism. Our studies stress the importance of evaluating the effects of treatment with polyphenols in both males and females because of their estrogenic potential.118
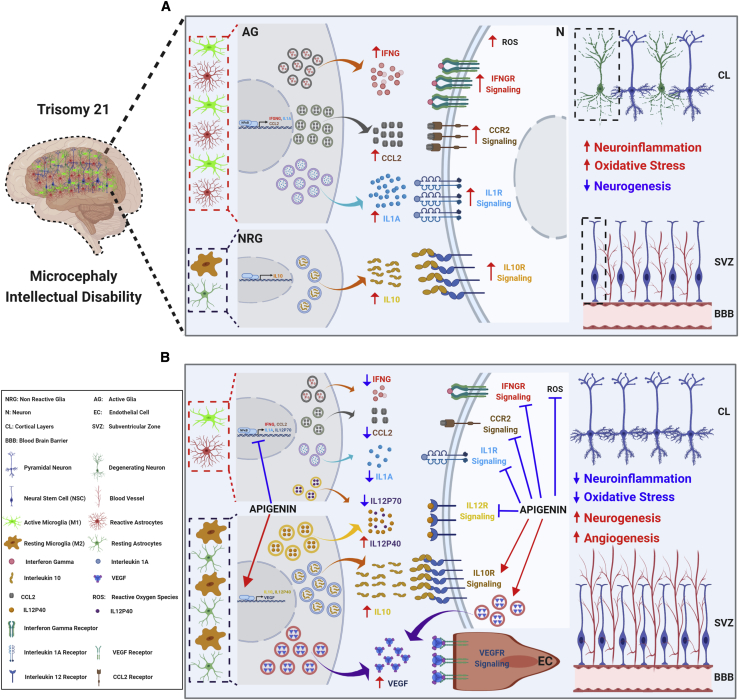
Proposed Apigenin Mechanisms of Action to Improve Brain Development and Cognition in DS
The results of the in vitro and in vivo treatment experiments described here suggest that apigenin’s mechanism of action (Figure 6) might be the result of the synergetic effects of the following qualities of apigenin.
-
(1)
ROS scavenger properties, which reduce oxidative stress and increase antioxidant responses in neural stem cells and neurons, thus reducing oxidative damage and neuronal death.
-
(2)
Increased production of the potent anti-inflammatory cytokines IL10 and IL12P40 and decreased pro-inflammatory cytokines (IL1A, IFNG, and IL12P70) and NO production through NFκB and iNOS inhibition in microglia and astrocytes. We propose that apigenin’s anti-inflammatory effects are triggered via blocking the activation of astrocytes and promoting the M2 (anti-inflammatory and neuroprotective) microglia phenotype over the M1 (pro-inflammatory and neurotoxic) phenotype.
-
(3)
Promotion of angiogenesis and neural progenitors and neuronal survival via VEGF and IL7 signaling pathways.
-
(4)
Pro-proliferative actions mediated via increased expression of many G2/M cell-cycle phase and neurogenic markers, including PRC1, PLK1, CCNB1, CCNB2, RACGAP, KI67, Nestin, SOX2, and PAX6, to cite only a few.
Figure 6.
Proposed Mechanisms of Action of Apigenin in the Brains of People with Trisomy 21
(A) Three copies of chromosome 21 are associated with increased oxidative stress, neuroinflammation, and reduced neurogenesis. Combining gene and protein expression data from human T21 amniocytes and embryonic forebrain of the Ts1Cje mouse model, we propose that the increased production of reactive oxygen species (ROSs) and pro-inflammatory cytokines (e.g., IFNG, IL1A, and CCL2) by reactive astrocytes and active microglia as well as cell-cycle delays in neural stem cells (NSCs) result in reduced neurogenesis and a lower number of mature neurons in the brain, which ultimately leads to microcephaly and intellectual disability.
(B) Apigenin treatment has pleiotropic actions by promoting anti-inflammatory IL10/IL10R signaling, reducing neuroinflammation through inhibition of NFκB, IFNG/IFNGR, CCL2/CCR2, IL1A/IL1AR, and IL12R signaling, promoting the expression of proneural genes in NSCs, and increasing angiogenesis through the activation of VEGF signaling. We propose that apigenin’s anti-inflammatory effects are triggered via blocking the activation of astrocytes and promoting the M2 (anti-inflammatory and neuroprotective) microglia phenotype over the M1 (pro-inflammatory and neurotoxic) phenotype.
In summary, our in vitro and in vivo experiments depicted a complex pleiotropic mechanism of action of apigenin on several pathways that might improve brain development and postnatal cognitive outcome in DS.
Conclusions
Combining an integrated human/murine approach and the Connectivity Map database, we identified apigenin as a candidate prenatal treatment for DS. We demonstrated that apigenin is a safe treatment that can rescue oxidative stress and total antioxidant capacity imbalance in human amniocytes from fetuses with T21. It also improved several postnatal behavioral deficits in the Ts1Cje mouse model. We also showed that apigenin achieves its therapeutic action by triggering the expression of neurogenic genes, suppressing inflammation via inhibiting NFκB, and reducing the production of pro-inflammatory cytokines while promoting the production of anti-inflammatory cytokines and angiogenic and neurotrophic factors. These in vitro and in vivo studies provide proof of principle that apigenin has therapeutic effects in preclinical models of DS.
Data and Code Availability
All gene expression data have been deposited into the Gene Expression Omnibus (GEO). The GSE identifier for the SuperSeries is GEO: GSE158385 and combines the human amniocytes series GEO: GSE158377 and the mouse embryonic forebrain series GEO: GSE158376.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
Experiments performed while the authors were at Tufts Medical Center were funded by NICHD R01 HD058880 and a sponsored research grant from Verinata Health, an Illumina company. Experiments performed after the authors moved to the National Human Genome Research Institute at the National Institutes of Health were funded by HG200399-03 and HG200399-04. The authors also wish to acknowledge the help and support of Donna Slonim and the Tufts University Neuroscience Behavior Core Facility, as well as Monica Duran Martinez, who participated in creating Figure 6.
Published: October 23, 2020
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2020.10.001.
Contributor Information
Faycal Guedj, Email: faycal.guedj@nih.gov.
Diana W. Bianchi, Email: diana.bianchi@nih.gov.
Web Resources
Connectivity Map database, https://www.broadinstitute.org/CMap
OMIM, https://www.omim.org/
Supplemental Information
References
- 1.Taylor-Phillips S., Freeman K., Geppert J., Agbebiyi A., Uthman O.A., Madan J., Clarke A., Quenby S., Clarke A. Accuracy of non-invasive prenatal testing using cell-free DNA for detection of Down, Edwards and Patau syndromes: a systematic review and meta-analysis. BMJ Open. 2016;6:e010002. doi: 10.1136/bmjopen-2015-010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ralston S.J., Wertz D., Chelmow D., Craigo S.D., Bianchi D.W. Pregnancy outcomes after prenatal diagnosis of aneuploidy. Obstet. Gynecol. 2001;97:729–733. doi: 10.1016/s0029-7844(01)01129-2. [DOI] [PubMed] [Google Scholar]
- 3.Guedj F., Bianchi D.W. Noninvasive prenatal testing creates an opportunity for antenatal treatment of Down syndrome. Prenat. Diagn. 2013;33:614–618. doi: 10.1002/pd.4134. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi D.W. From prenatal genomic diagnosis to fetal personalized medicine: progress and challenges. Nat. Med. 2012;18:1041–1051. doi: 10.1038/nm.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt-Sidor B., Wisniewski K.E., Shepard T.H., Sersen E.A. Brain growth in Down syndrome subjects 15 to 22 weeks of gestational age and birth to 60 months. Clin. Neuropathol. 1990;9:181–190. [PubMed] [Google Scholar]
- 6.Guidi S., Bonasoni P., Ceccarelli C., Santini D., Gualtieri F., Ciani E., Bartesaghi R. Neurogenesis impairment and increased cell death reduce total neuron number in the hippocampal region of fetuses with Down syndrome. Brain Pathol. 2008;18:180–197. doi: 10.1111/j.1750-3639.2007.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidi S., Ciani E., Bonasoni P., Santini D., Bartesaghi R. Widespread proliferation impairment and hypocellularity in the cerebellum of fetuses with down syndrome. Brain Pathol. 2011;21:361–373. doi: 10.1111/j.1750-3639.2010.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisniewski K.E., Schmidt-Sidor B. Postnatal delay of myelin formation in brains from Down syndrome infants and children. Clin. Neuropathol. 1989;8:55–62. [PubMed] [Google Scholar]
- 9.Ábrahám H., Vincze A., Veszprémi B., Kravják A., Gömöri É., Kovács G.G., Seress L. Impaired myelination of the human hippocampal formation in Down syndrome. Int. J. Dev. Neurosci. 2012;30:147–158. doi: 10.1016/j.ijdevneu.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Hepper P.G., Shahidullah S. Habituation in normal and Down’s syndrome fetuses. Q. J. Exp. Psychol. B. 1992;44:305–317. doi: 10.1080/02724999208250617. [DOI] [PubMed] [Google Scholar]
- 11.Guedj F., Bianchi D.W., Delabar J.M. Prenatal treatment of Down syndrome: a reality? Curr. Opin. Obstet. Gynecol. 2014;26:92–103. doi: 10.1097/GCO.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 12.Stagni F., Giacomini A., Guidi S., Ciani E., Bartesaghi R. Timing of therapies for Down syndrome: the sooner, the better. Front. Behav. Neurosci. 2015;9:265. doi: 10.3389/fnbeh.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardiner K.J. Pharmacological approaches to improving cognitive function in Down syndrome: current status and considerations. Drug Des. Devel. Ther. 2014;9:103–125. doi: 10.2147/DDDT.S51476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S.E., Duran-Martinez M., Khantsis S., Bianchi D.W., Guedj F. Challenges and Opportunities for Translation of Therapies to Improve Cognition in Down Syndrome. Trends Mol. Med. 2020;26:150–169. doi: 10.1016/j.molmed.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice D., Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meredith R.M. Sensitive and critical periods during neurotypical and aberrant neurodevelopment: a framework for neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2015;50:180–188. doi: 10.1016/j.neubiorev.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Guidi S., Stagni F., Bianchi P., Ciani E., Giacomini A., De Franceschi M., Moldrich R., Kurniawan N., Mardon K., Giuliani A. Prenatal pharmacotherapy rescues brain development in a Down’s syndrome mouse model. Brain. 2014;137:380–401. doi: 10.1093/brain/awt340. [DOI] [PubMed] [Google Scholar]
- 18.Incerti M., Horowitz K., Roberson R., Abebe D., Toso L., Caballero M., Spong C.Y. Prenatal treatment prevents learning deficit in Down syndrome model. PLoS ONE. 2012;7:e50724. doi: 10.1371/journal.pone.0050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ash J.A., Velazquez R., Kelley C.M., Powers B.E., Ginsberg S.D., Mufson E.J., Strupp B.J. Maternal choline supplementation improves spatial mapping and increases basal forebrain cholinergic neuron number and size in aged Ts65Dn mice. Neurobiol. Dis. 2014;70:32–42. doi: 10.1016/j.nbd.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slonim D.K., Koide K., Johnson K.L., Tantravahi U., Cowan J.M., Jarrah Z., Bianchi D.W. Functional genomic analysis of amniotic fluid cell-free mRNA suggests that oxidative stress is significant in Down syndrome fetuses. Proc. Natl. Acad. Sci. USA. 2009;106:9425–9429. doi: 10.1073/pnas.0903909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guedj F., Pennings J.L., Massingham L.J., Wick H.C., Siegel A.E., Tantravahi U., Bianchi D.W. An integrated human/murine transcriptome and pathway approach to identify prenatal treatments for Down syndrome. Sci. Rep. 2016;6:32353. doi: 10.1038/srep32353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb J., Crawford E.D., Peck D., Modell J.W., Blat I.C., Wrobel M.J., Lerner J., Brunet J.P., Subramanian A., Ross K.N. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 23.Venigalla M., Gyengesi E., Münch G. Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural Regen. Res. 2015;10:1181–1185. doi: 10.4103/1673-5374.162686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venigalla M., Sonego S., Gyengesi E., Sharman M.J., Münch G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2016;95:63–74. doi: 10.1016/j.neuint.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Kalivarathan J., Chandrasekaran S.P., Kalaivanan K., Ramachandran V., Carani Venkatraman A. Apigenin attenuates hippocampal oxidative events, inflammation and pathological alterations in rats fed high fat, fructose diet. Biomed. Pharmacother. 2017;89:323–331. doi: 10.1016/j.biopha.2017.01.162. [DOI] [PubMed] [Google Scholar]
- 26.Rezai-Zadeh K., Ehrhart J., Bai Y., Sanberg P.R., Bickford P., Tan J., Shytle R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflammation. 2008;5:41. doi: 10.1186/1742-2094-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aziz N.M., Guedj F., Pennings J.L.A., Olmos-Serrano J.L., Siegel A., Haydar T.F., Bianchi D.W. Lifespan analysis of brain development, gene expression and behavioral phenotypes in the Ts1Cje, Ts65Dn and Dp(16)1/Yey mouse models of Down syndrome. Dis. Model Mech. 2018;11:dmm031013. doi: 10.1242/dmm.031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan K.D., Lewis H.C., Hill A.A., Pandey A., Jackson L.P., Cabral J.M., Smith K.P., Liggett L.A., Gomez E.B., Galbraith M.D., DeGregori J., Espinosa J.M. Trisomy 21 consistently activates the interferon response. Elife. 2016;5:e16220. doi: 10.7554/eLife.16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao L., Wang J.L., Liu R., Li X.X., Li J.F., Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules. 2013;18:9949–9965. doi: 10.3390/molecules18089949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- 31.Krämer A., Green J., Pollard J., Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30:523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricci M. Dosing animal via diet: This can be a simple and efficient method for delivering compounds to lab animals when done correctly. ALN World. 2012;5:1–4. [Google Scholar]
- 33.Ferrés M.A., Bianchi D.W., Siegel A.E., Bronson R.T., Huggins G.S., Guedj F. Perinatal Natural History of the Ts1Cje Mouse Model of Down Syndrome: Growth Restriction, Early Mortality, Heart Defects, and Delayed Development. PLoS ONE. 2016;11:e0168009. doi: 10.1371/journal.pone.0168009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill J.M., Lim M.A., Stone M.M. Developmental Milestones in the Newborn Mouse. Neuromethods. 2008;39:131–149. [Google Scholar]
- 35.Guedj F., Pennings J.L., Ferres M.A., Graham L.C., Wick H.C., Miczek K.A., Slonim D.K., Bianchi D.W. The fetal brain transcriptome and neonatal behavioral phenotype in the Ts1Cje mouse model of Down syndrome. Am. J. Med. Genet. A. 2015;167A:1993–2008. doi: 10.1002/ajmg.a.37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Androutsopoulos V.P., Papakyriakou A., Vourloumis D., Spandidos D.A. Comparative CYP1A1 and CYP1B1 substrate and inhibitor profile of dietary flavonoids. Bioorg. Med. Chem. 2011;19:2842–2849. doi: 10.1016/j.bmc.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 37.Juliusson S.J., Nielsen J.K., Runarsdottir V., Hansdottir I., Sigurdardottir R., Björnsson E.S. Lifetime alcohol intake and pattern of alcohol consumption in patients with alcohol-induced pancreatitis in comparison with patients with alcohol use disorder. Scand. J. Gastroenterol. 2018;53:748–754. doi: 10.1080/00365521.2018.1455893. [DOI] [PubMed] [Google Scholar]
- 38.Gheghiani L., Loew D., Lombard B., Mansfeld J., Gavet O. PLK1 Activation in Late G2 Sets Up Commitment to Mitosis. Cell Rep. 2017;19:2060–2073. doi: 10.1016/j.celrep.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 39.Lee N.H., Cho A., Park S.R., Lee J.W., Sung Taek P., Park C.H., Choi Y.H., Lim S., Baek M.K., Kim D.Y. SERPINB2 is a novel indicator of stem cell toxicity. Cell Death Dis. 2018;9:724. doi: 10.1038/s41419-018-0748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H.T., Zha Z.G., Cao J.H., Liang Z.J., Wu H., He M.T., Zang X., Yao P., Zhang J.Q. Apigenin accelerates lipopolysaccharide induced apoptosis in mesenchymal stem cells through suppressing vitamin D receptor expression. Chin. Med. J. (Engl.) 2011;124:3537–3545. [PubMed] [Google Scholar]
- 41.Boeckx C., Op de Beeck K., Wouters A., Deschoolmeester V., Limame R., Zwaenepoel K., Specenier P., Pauwels P., Vermorken J.B., Peeters M. Overcoming cetuximab resistance in HNSCC: the role of AURKB and DUSP proteins. Cancer Lett. 2014;354:365–377. doi: 10.1016/j.canlet.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 42.Barone E., Arena A., Head E., Butterfield D.A., Perluigi M. Disturbance of redox homeostasis in Down Syndrome: Role of iron dysmetabolism. Free Radic. Biol. Med. 2018;114:84–93. doi: 10.1016/j.freeradbiomed.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muchová J., Žitňanová I., Ďuračková Z. Oxidative stress and Down syndrome. Do antioxidants play a role in therapy? Physiol. Res. 2014;63:535–542. doi: 10.33549/physiolres.932722. [DOI] [PubMed] [Google Scholar]
- 44.Pagano G., Castello G. Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv. Exp. Med. Biol. 2012;724:291–299. doi: 10.1007/978-1-4614-0653-2_22. [DOI] [PubMed] [Google Scholar]
- 45.Lott I.T. Antioxidants in Down syndrome. Biochim. Biophys. Acta. 2012;1822:657–663. doi: 10.1016/j.bbadis.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iannello R.C., Crack P.J., de Haan J.B., Kola I. Oxidative stress and neural dysfunction in Down syndrome. J. Neural Transm. Suppl. 1999;57:257–267. doi: 10.1007/978-3-7091-6380-1_17. [DOI] [PubMed] [Google Scholar]
- 47.Gimeno A., García-Giménez J.L., Audí L., Toran N., Andaluz P., Dasí F., Viña J., Pallardó F.V. Decreased cell proliferation and higher oxidative stress in fibroblasts from Down Syndrome fetuses. Preliminary study. Biochim. Biophys. Acta. 2014;1842:116–125. doi: 10.1016/j.bbadis.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Perluigi M., di Domenico F., Fiorini A., Cocciolo A., Giorgi A., Foppoli C., Butterfield D.A., Giorlandino M., Giorlandino C., Schininà M.E., Coccia R. Oxidative stress occurs early in Down syndrome pregnancy: A redox proteomics analysis of amniotic fluid. Proteomics Clin. Appl. 2011;5:167–178. doi: 10.1002/prca.201000121. [DOI] [PubMed] [Google Scholar]
- 49.de Haan J.B., Susil B., Pritchard M., Kola I. An altered antioxidant balance occurs in Down syndrome fetal organs: implications for the “gene dosage effect” hypothesis. J. Neural Transm. Suppl. 2003;67:67–83. doi: 10.1007/978-3-7091-6721-2_6. [DOI] [PubMed] [Google Scholar]
- 50.Sharma H., Kanwal R., Bhaskaran N., Gupta S. Plant flavone apigenin binds to nucleic acid bases and reduces oxidative DNA damage in prostate epithelial cells. PLoS ONE. 2014;9:e91588. doi: 10.1371/journal.pone.0091588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang S.S., Lee J.Y., Choi Y.K., Kim G.S., Han B.H. Neuroprotective effects of flavones on hydrogen peroxide-induced apoptosis in SH-SY5Y neuroblostoma cells. Bioorg. Med. Chem. Lett. 2004;14:2261–2264. doi: 10.1016/j.bmcl.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 52.An F., Cao X., Qu H., Wang S. Attenuation of oxidative stress of erythrocytes by the plant-derived flavonoids vitexin and apigenin. Pharmazie. 2015;70:724–732. [PubMed] [Google Scholar]
- 53.Nabavi S.F., Khan H., D’onofrio G., Šamec D., Shirooie S., Dehpour A.R., Argüelles S., Habtemariam S., Sobarzo-Sanchez E. Apigenin as neuroprotective agent: Of mice and men. Pharmacol. Res. 2018;128:359–365. doi: 10.1016/j.phrs.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Jeon B.J., Yang H.M., Lyu Y.S., Pae H.O., Ju S.M., Jeon B.H. Apigenin inhibits indoxyl sulfate-induced endoplasmic reticulum stress and anti-proliferative pathways, CHOP and IL-6/p21, in human renal proximal tubular cells. Eur. Rev. Med. Pharmacol. Sci. 2015;19:2303–2310. [PubMed] [Google Scholar]
- 55.Wu P.S., Yen J.H., Kou M.C., Wu M.J. Luteolin and Apigenin Attenuate 4-Hydroxy-2-Nonenal-Mediated Cell Death through Modulation of UPR, Nrf2-ARE and MAPK Pathways in PC12 Cells. PLoS ONE. 2015;10:e0130599. doi: 10.1371/journal.pone.0130599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McKay D.L., Blumberg J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) Phytother. Res. 2006;20:519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- 57.Corsi M.M., Dogliotti G., Pedroni F., Palazzi E., Magni P., Chiappelli M., Licastro F. Plasma nerve growth factor (NGF) and inflammatory cytokines (IL-6 and MCP-1) in young and adult subjects with Down syndrome: an interesting pathway. Neuroendocrinol. Lett. 2006;27:773–778. [PubMed] [Google Scholar]
- 58.Licastro F., Chiappelli M., Porcellini E., Trabucchi M., Marocchi A., Corsi M.M. Altered vessel signalling molecules in subjects with Downs syndrome. Int. J. Immunopathol. Pharmacol. 2006;19:181–185. [PubMed] [Google Scholar]
- 59.Iulita M.F., Ower A., Barone C., Pentz R., Gubert P., Romano C., Cantarella R.A., Elia F., Buono S., Recupero M. An inflammatory and trophic disconnect biomarker profile revealed in Down syndrome plasma: Relation to cognitive decline and longitudinal evaluation. Alzheimers Dement. 2016;12:1132–1148. doi: 10.1016/j.jalz.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X., Wang G., Gurley E.C., Zhou H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE. 2014;9:e107072. doi: 10.1371/journal.pone.0107072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong J., Fristiohady A., Nguyen C.H., Milovanovic D., Huttary N., Krieger S., Hong J., Geleff S., Birner P., Jäger W. Apigenin and Luteolin Attenuate the Breaching of MDA-MB231 Breast Cancer Spheroids Through the Lymph Endothelial Barrier in Vitro. Front. Pharmacol. 2018;9:220. doi: 10.3389/fphar.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bauer D., Redmon N., Mazzio E., Soliman K.F. Apigenin inhibits TNFα/IL-1α-induced CCL2 release through IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells. PLoS ONE. 2017;12:e0175558. doi: 10.1371/journal.pone.0175558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qi F., Chen Q., Chen H., Yan H., Chen B., Xiang X., Liang C., Yi Q., Zhang M., Cheng H. WAC Promotes Polo-like Kinase 1 Activation for Timely Mitotic Entry. Cell Rep. 2018;24:546–556. doi: 10.1016/j.celrep.2018.06.087. [DOI] [PubMed] [Google Scholar]
- 64.Saito Y., Oka A., Mizuguchi M., Motonaga K., Mori Y., Becker L.E., Arima K., Miyauchi J., Takashima S. The developmental and aging changes of Down’s syndrome cell adhesion molecule expression in normal and Down’s syndrome brains. Acta Neuropathol. 2000;100:654–664. doi: 10.1007/s004010000230. [DOI] [PubMed] [Google Scholar]
- 65.Barlow G.M., Micales B., Lyons G.E., Korenberg J.R. Down syndrome cell adhesion molecule is conserved in mouse and highly expressed in the adult mouse brain. Cytogenet. Cell Genet. 2001;94:155–162. doi: 10.1159/000048808. [DOI] [PubMed] [Google Scholar]
- 66.Cooper A., Grigoryan G., Guy-David L., Tsoory M.M., Chen A., Reuveny E. Trisomy of the G protein-coupled K+ channel gene, Kcnj6, affects reward mechanisms, cognitive functions, and synaptic plasticity in mice. Proc. Natl. Acad. Sci. USA. 2012;109:2642–2647. doi: 10.1073/pnas.1109099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang X., Liu C., Yu T., Zhang L., Meng K., Xing Z., Belichenko P.V., Kleschevnikov A.M., Pao A., Peresie J. Genetic dissection of the Down syndrome critical region. Hum. Mol. Genet. 2015;24:6540–6551. doi: 10.1093/hmg/ddv364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raveau M., Nakahari T., Asada S., Ishihara K., Amano K., Shimohata A., Sago H., Yamakawa K. Brain ventriculomegaly in Down syndrome mice is caused by Pcp4 dose-dependent cilia dysfunction. Hum. Mol. Genet. 2017;26:923–931. doi: 10.1093/hmg/ddx007. [DOI] [PubMed] [Google Scholar]
- 69.Mouton-Liger F., Sahún I., Collin T., Lopes Pereira P., Masini D., Thomas S., Paly E., Luilier S., Même S., Jouhault Q. Developmental molecular and functional cerebellar alterations induced by PCP4/PEP19 overexpression: implications for Down syndrome. Neurobiol. Dis. 2014;63:92–106. doi: 10.1016/j.nbd.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Taupin P. Apigenin and related compounds stimulate adult neurogenesis. Mars, Inc., the Salk Institute for Biological Studies: WO2008147483. Expert Opin. Ther. Pat. 2009;19:523–527. doi: 10.1517/13543770902721279. [DOI] [PubMed] [Google Scholar]
- 71.Zhou W.B., Miao Z.N., Zhang B., Long W., Zheng F.X., Kong J., Yu B. Luteolin induces hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neural Regen. Res. 2019;14:613–620. doi: 10.4103/1673-5374.248519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anusha C., Sumathi T., Joseph L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017;269:67–79. doi: 10.1016/j.cbi.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Smolinski A.T., Pestka J.J. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew) Food Chem. Toxicol. 2003;41:1381–1390. doi: 10.1016/s0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 74.Patil S.P., Jain P.D., Sancheti J.S., Ghumatkar P.J., Tambe R., Sathaye S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology. 2014;86:192–202. doi: 10.1016/j.neuropharm.2021.108876. [DOI] [PubMed] [Google Scholar]
- 75.Chen L., Xie W., Xie W., Zhuang W., Jiang C., Liu N. Apigenin attenuates isoflurane-induced cognitive dysfunction via epigenetic regulation and neuroinflammation in aged rats. Arch. Gerontol. Geriatr. 2017;73:29–36. doi: 10.1016/j.archger.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Kirsammer G., Crispino J.D. Signaling a link between interferon and the traits of Down syndrome. Elife. 2016;5:e20196. doi: 10.7554/eLife.20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan Y.H., Schneider E.L., Tischfield J., Epstein C.J., Ruddle F.H. Human chromosome 21 dosage: effect on the expression of the interferon induced antiviral state. Science. 1974;186:61–63. doi: 10.1126/science.186.4158.61. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Che M., Yuan J., Yu Y., Cao C., Qin X.Y., Cheng Y. Aberrations in circulating inflammatory cytokine levels in patients with Down syndrome: a meta-analysis. Oncotarget. 2017;8:84489–84496. doi: 10.18632/oncotarget.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Häusler K.G., Prinz M., Nolte C., Weber J.R., Schumann R.R., Kettenmann H., Hanisch U.K. Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages. Eur. J. Neurosci. 2002;16:2113–2122. doi: 10.1046/j.1460-9568.2002.02287.x. [DOI] [PubMed] [Google Scholar]
- 80.Abbas N., Bednar I., Mix E., Marie S., Paterson D., Ljungberg A., Morris C., Winblad B., Nordberg A., Zhu J. Up-regulation of the inflammatory cytokines IFN-gamma and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP(SWE) transgenic mice. J. Neuroimmunol. 2002;126:50–57. doi: 10.1016/s0165-5728(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 81.Tribouillard-Tanvier D., Striebel J.F., Peterson K.E., Chesebro B. Analysis of protein levels of 24 cytokines in scrapie agent-infected brain and glial cell cultures from mice differing in prion protein expression levels. J. Virol. 2009;83:11244–11253. doi: 10.1128/JVI.01413-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.D’Andrea A., Aste-Amezaga M., Valiante N.M., Ma X., Kubin M., Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karunarathne W.A.H.M., Lee K.T., Choi Y.H., Jin C.Y., Kim G.Y. Anthocyanins isolated from Hibiscus syriacus L. attenuate lipopolysaccharide-induced inflammation and endotoxic shock by inhibiting the TLR4/MD2-mediated NF-κB signaling pathway. Phytomedicine. 2020;76:153237. doi: 10.1016/j.phymed.2020.153237. [DOI] [PubMed] [Google Scholar]
- 84.Jeong G.S., Lee S.H., Jeong S.N., Kim Y.C., Kim E.C. Anti-inflammatory effects of apigenin on nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. Int. Immunopharmacol. 2009;9:1374–1380. doi: 10.1016/j.intimp.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 85.Cetiner S., Demirhan O., Inal T.C., Tastemir D., Sertdemir Y. Analysis of peripheral blood T-cell subsets, natural killer cells and serum levels of cytokines in children with Down syndrome. Int. J. Immunogenet. 2010;37:233–237. doi: 10.1111/j.1744-313X.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- 86.Nateghi Rostami M., Douraghi M., Miramin Mohammadi A., Nikmanesh B. Altered serum pro-inflammatory cytokines in children with Down’s syndrome. Eur. Cytokine Netw. 2012;23:64–67. doi: 10.1684/ecn.2012.0307. [DOI] [PubMed] [Google Scholar]
- 87.Huggard D., Kelly L., Ryan E., McGrane F., Lagan N., Roche E., Balfe J., Leahy T.R., Franklin O., Doherty D.G., Molloy E.J. Increased systemic inflammation in children with Down syndrome. Cytokine. 2020;127:154938. doi: 10.1016/j.cyto.2019.154938. [DOI] [PubMed] [Google Scholar]
- 88.Cavalcante L.B., Tanaka M.H., Pires J.R., Apponi L.H., Aparecida Giro E.M., Valentini S.R., Palomari Spolidório D.M., Capela M.V., Rossa C., Jr., Scarel-Caminaga R.M. Expression of the interleukin-10 signaling pathway genes in individuals with Down syndrome and periodontitis. J. Periodontol. 2012;83:926–935. doi: 10.1902/jop.2011.110056. [DOI] [PubMed] [Google Scholar]
- 89.Rodrigues R., Debom G., Soares F., Machado C., Pureza J., Peres W., de Lima Garcias G., Duarte M.F., Schetinger M.R., Stefanello F. Alterations of ectonucleotidases and acetylcholinesterase activities in lymphocytes of Down syndrome subjects: relation with inflammatory parameters. Clin. Chim. Acta. 2014;433:105–110. doi: 10.1016/j.cca.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 90.Zhang S., Liu X., Sun C., Yang J., Wang L., Liu J., Gong L., Jing Y. Apigenin Attenuates Experimental Autoimmune Myocarditis by Modulating Th1/Th2 Cytokine Balance in Mice. Inflammation. 2016;39:678–686. doi: 10.1007/s10753-015-0294-y. [DOI] [PubMed] [Google Scholar]
- 91.Karamese M., Erol H.S., Albayrak M., Findik Guvendi G., Aydin E., Aksak Karamese S. Anti-oxidant and anti-inflammatory effects of apigenin in a rat model of sepsis: an immunological, biochemical, and histopathological study. Immunopharmacol. Immunotoxicol. 2016;38:228–237. doi: 10.3109/08923973.2016.1173058. [DOI] [PubMed] [Google Scholar]
- 92.Dourado N.S., Souza C.D.S., de Almeida M.M.A., Bispo da Silva A., Dos Santos B.L., Silva V.D.A., De Assis A.M., da Silva J.S., Souza D.O., Costa M.F.D. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated With Alzheimer’s Disease. Front. Aging Neurosci. 2020;12:119. doi: 10.3389/fnagi.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greene J., Banasr M., Lee B., Warner-Schmidt J., Duman R.S. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34:2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jais A., Solas M., Backes H., Chaurasia B., Kleinridders A., Theurich S., Mauer J., Steculorum S.M., Hampel B., Goldau J. Myeloid-Cell-Derived VEGF Maintains Brain Glucose Uptake and Limits Cognitive Impairment in Obesity. Cell. 2016;165:882–895. doi: 10.1016/j.cell.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 95.Norman M.G., O’Kusky J.R. The growth and development of microvasculature in human cerebral cortex. J. Neuropathol. Exp. Neurol. 1986;45:222–232. [PubMed] [Google Scholar]
- 96.Virgintino D., Errede M., Robertson D., Girolamo F., Masciandaro A., Bertossi M. VEGF expression is developmentally regulated during human brain angiogenesis. Histochem. Cell Biol. 2003;119:227–232. doi: 10.1007/s00418-003-0510-y. [DOI] [PubMed] [Google Scholar]
- 97.Breier G., Clauss M., Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev. Dyn. 1995;204:228–239. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- 98.Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O’Shea K.S., Powell-Braxton L., Hillan K.J., Moore M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 99.Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., Fahrig M., Vandenhoeck A., Harpal K., Eberhardt C. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 100.Shalaby F., Rossant J., Yamaguchi T.P., Gertsenstein M., Wu X.F., Breitman M.L., Schuh A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 101.Licht T., Goshen I., Avital A., Kreisel T., Zubedat S., Eavri R., Segal M., Yirmiya R., Keshet E. Reversible modulations of neuronal plasticity by VEGF. Proc. Natl. Acad. Sci. USA. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hohman T.J., Bell S.P., Jefferson A.L., Alzheimer’s Disease Neuroimaging Initiative The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 2015;72:520–529. doi: 10.1001/jamaneurol.2014.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paterson R.W., Bartlett J.W., Blennow K., Fox N.C., Shaw L.M., Trojanowski J.Q., Zetterberg H., Schott J.M., Alzheimer’s Disease Neuroimaging Initiative Cerebrospinal fluid markers including trefoil factor 3 are associated with neurodegeneration in amyloid-positive individuals. Transl. Psychiatry. 2014;4:e419. doi: 10.1038/tp.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salvolini E., Orciani M., Lucarini G., Vignini A., Tranquilli A.L., Di Primio R. VEGF and nitric oxide synthase immunoexpression in Down’s syndrome amniotic fluid stem cells. Eur. J. Clin. Invest. 2011;41:23–29. doi: 10.1111/j.1365-2362.2010.02370.x. [DOI] [PubMed] [Google Scholar]
- 105.Tranquilli A.L., Bezzeccheri V., Scagnoli C., Mazzanti L., Garzetti G.G. Amniotic levels of vascular endothelial growth factor and nitric oxide at the second trimester in Down’s syndrome. J. Matern. Fetal Neonatal Med. 2003;13:28–31. doi: 10.1080/jmf.13.1.28.31. [DOI] [PubMed] [Google Scholar]
- 106.Zhu W., Mao Y., Zhou L.F. Reduction of neural and vascular damage by transplantation of VEGF-secreting neural stem cells after cerebral ischemia. Acta Neurochir. Suppl. (Wien) 2005;95:393–397. doi: 10.1007/3-211-32318-x_80. [DOI] [PubMed] [Google Scholar]
- 107.Zhu W., Mao Y., Zhao Y., Zhou L.F., Wang Y., Zhu J.H., Zhu Y., Yang G.Y. Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia. Neurosurgery. 2005;57:325–333, discussion 325–333. doi: 10.1227/01.neu.0000166682.50272.bc. [DOI] [PubMed] [Google Scholar]
- 108.Baburamani A.A., Castillo-Melendez M., Walker D.W. VEGF expression and microvascular responses to severe transient hypoxia in the fetal sheep brain. Pediatr. Res. 2013;73:310–316. doi: 10.1038/pr.2012.191. [DOI] [PubMed] [Google Scholar]
- 109.Swanson A.M., Rossi C.A., Ofir K., Mehta V., Boyd M., Barker H., Ledwozyw A., Vaughan O., Martin J., Zachary I. Maternal Therapy with Ad.VEGF-A165 Increases Fetal Weight at Term in a Guinea-Pig Model of Fetal Growth Restriction. Hum. Gene Ther. 2016;27:997–1007. doi: 10.1089/hum.2016.046. [DOI] [PubMed] [Google Scholar]
- 110.Moors M., Vudattu N.K., Abel J., Krämer U., Rane L., Ulfig N., Ceccatelli S., Seyfert-Margolies V., Fritsche E., Maeurer M.J. Interleukin-7 (IL-7) and IL-7 splice variants affect differentiation of human neural progenitor cells. Genes Immun. 2010;11:11–20. doi: 10.1038/gene.2009.77. [DOI] [PubMed] [Google Scholar]
- 111.Araujo D.M., Cotman C.W. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- 112.Michaelson M.D., Mehler M.F., Xu H., Gross R.E., Kessler J.A. Interleukin-7 is trophic for embryonic neurons and is expressed in developing brain. Dev. Biol. 1996;179:251–263. doi: 10.1006/dbio.1996.0255. [DOI] [PubMed] [Google Scholar]
- 113.Sharma P., Sharma S., Damanpreet S. Apigenin reverses behavioral impairments and cognitive decline in kindled mice via CREB-BDNF upregulation in the hippocampus. Nutr. Neurosci. 2020;23:118–127. doi: 10.1080/1028415X.2018.1478653. [DOI] [PubMed] [Google Scholar]
- 114.Crupi R., Paterniti I., Ahmad A., Campolo M., Esposito E., Cuzzocrea S. Effects of palmitoylethanolamide and luteolin in an animal model of anxiety/depression. CNS Neurol. Disord. Drug Targets. 2013;12:989–1001. doi: 10.2174/18715273113129990084. [DOI] [PubMed] [Google Scholar]
- 115.Tsai F.S., Cheng H.Y., Hsieh M.T., Wu C.R., Lin Y.C., Peng W.H. The ameliorating effects of luteolin on beta-amyloid-induced impairment of water maze performance and passive avoidance in rats. Am. J. Chin. Med. 2010;38:279–291. doi: 10.1142/S0192415X10007841. [DOI] [PubMed] [Google Scholar]
- 116.Tu F., Pang Q., Huang T., Zhao Y., Liu M., Chen X. Apigenin Ameliorates Post-Stroke Cognitive Deficits in Rats Through Histone Acetylation-Mediated Neurochemical Alterations. Med. Sci. Monit. 2017;23:4004–4013. doi: 10.12659/MSM.902770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gradolatto A., Basly J.P., Berges R., Teyssier C., Chagnon M.C., Siess M.H., Canivenc-Lavier M.C. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab. Dispos. 2005;33:49–54. doi: 10.1124/dmd.104.000893. [DOI] [PubMed] [Google Scholar]
- 118.Lóránd T., Vigh E., Garai J. Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: phytoestrogens and xenoestrogens. Curr. Med. Chem. 2010;17:3542–3574. doi: 10.2174/092986710792927813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All gene expression data have been deposited into the Gene Expression Omnibus (GEO). The GSE identifier for the SuperSeries is GEO: GSE158385 and combines the human amniocytes series GEO: GSE158377 and the mouse embryonic forebrain series GEO: GSE158376.