This economic evaluation assesses the cost-effectiveness of inpatient initiation of sacubitril-valsartan vs enalapril compared with no initiation or posthospitalization initiation of sacubitril-valsartan in patients who have heart failure with reduced ejection fraction.
Key Points
Question
Is sacubitril-valsartan more cost-effective than enalapril when started during hospitalization compared with 2 months after hospitalization for patients with heart failure with reduced ejection fraction?
Findings
In this economic evaluation, in a model of patients with heart failure with reduced ejection fraction, inpatient treatment with sacubitril-valsartan was associated with fewer admissions for heart failure and was cost saving in a budget analysis and from health care system and societal perspectives compared with initiation after hospitalization or no initiation.
Meaning
The findings suggest that inpatient initiation of sacubitril-valsartan for patients with heart failure with reduced ejection fraction may be associated with reduced hospitalizations, increased quality-adjusted life expectancy, and cost savings compared with no initiation or initiation after hospitalization.
Abstract
Importance
Sacubitril-valsartan use reduces mortality and hospitalizations compared with enalapril among patients with chronic heart failure with reduced ejection fraction (HFrEF); however, the cost-effectiveness of these treatments when initiated during hospitalization for HF is unknown.
Objective
To estimate the cost-effectiveness of inpatient initiation of sacubitril-valsartan vs enalapril compared with no initiation or posthospitalization initiation of sacubitril-valsartan among stabilized patients with HFrEF.
Design, Setting, and Participants
This economic evaluation included data on US patients with HFrEF who were eligible for sacubitril-valsartan treatment from December 8, 2009, to May 15, 2018.
Main Outcomes and Measures
A 5-state Markov model with all-cause mortality, HF, and non-HF hospitalization probabilities was used. Quality of life was estimated using Euro-QoL EQ-5D scores. Hospitalization, long-term care, and medication costs for sacubitril-valsartan and enalapril were modeled with a discount rate of 3%. The base-case analysis included a lifetime horizon from a health care and societal perspective.
Results
Modeled patients were a mean (SD) age of 63.8 (11.5) years. Inpatient treatment with sacubitril-valsartan ($5628 per year) was associated with 62 fewer HF-related admissions per 1000 patients compared with outpatient initiation or 116 fewer HF-related admissions compared with continuation of enalapril treatment. From a health care system perspective, initiation of sacubitril-valsartan during hospitalization saved $452 per year compared with continuing enalapril and $811 per year compared with initiation at 2 months after hospitalization and was associated with an incremental cost-effectiveness ratio of $21 532 per quality-adjusted life-year compared with continued enalapril treatment over a lifetime. From a societal perspective, inpatient initiation was estimated to save $460 per year per patient compared with no initiation of sacubitril-valsartan and $813 per year per patient compared with initiation after hospitalization. In a budget analysis, inpatient initiation of sacubitril-valsartan was estimated to save up to $449 per person for 1 year or $2550 per person over 5 years compared with continuation of enalapril.
Conclusions and Relevance
The findings suggest that, for patients with HFrEF, initiation of sacubitril-valsartan during hospitalization may be associated with reduced hospitalizations, increased quality-adjusted life expectancy, and cost savings compared with no initiation or initiation after hospitalization.
Introduction
Patients with heart failure (HF) face a challenging prognosis, with a 50% mortality rate in 5 years. In addition, HF remains a leading cause of hospitalization for adults and costs the US health care system approximately $40 billion per year.1 Over the past 3 decades, therapies that inhibit or alter neurohormonal pathways have led to improvements in morbidity and mortality among patients with HF with reduced ejection fraction (HFrEF).
In 2014, the Prospective Comparison of ARNI (angiotensin receptor–neprilysin inhibitor) with ACEI (angiotensin-converting enzyme inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial showed that sacubitril-valsartan treatment in a population with HFrEF lowered mortality and the number of hospitalizations compared with enalapril treatment.2 Subsequently, a study3 showed that sacubitril-valsartan was cost-effective compared with enalapril when initiated in a modeled outpatient population with HFrEF.3 Recently, the Comparison of Sacubitril/Valsartan Vs Enalapril on Effect on NT-proBNP (N-terminal pro b-type natriuretic peptide) in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF)4 trial showed that it was safe to administer sacubitril-valsartan to hospitalized patients with acute decompensated HFrEF and that sacubitril-valsartan reduced NT-proBNP levels significantly compared with enalapril over 8 weeks. Furthermore, in an exploratory secondary analysis, sacubitril-valsartan treatment was associated with reduced HF-related admissions compared with enalapril treatment. We examined whether administering sacubitril-valsartan to patients hospitalized with HFrEF was cost-effective compared with 2 patient groups: those who initiated sacubitril-valsartan without readmission for HF for at least 2 months after hospitalization (similar to the PARADIGM-HF cohort) and those who continued enalapril treatment indefinitely. In addition, we performed a budget analysis of widespread use of sacubitril-valsartan in patients hospitalized with HFrEF in the national health care system and evaluated societal effects, including any changes in productivity.
Methods
HF Disease Simulation Model Strategies
For this economic evaluation, a 5-state Markov model simulating HF was developed using both the PIONEER-HF4 and the PARADIGM-HF2 trial data from December 8, 2009, to May 15, 2018, that compared the 2 treatments: enalapril given up to 10 mg twice daily or sacubitril-valsartan given up to 97 mg/103 mg twice daily in 3 different strategies. In the first strategy, patients initiated sacubitril-valsartan treatment during hospitalization and continued treatment throughout the analysis. In the second strategy, patients received enalapril during hospitalization for HF and for the first 2 months after hospitalization; 2 months after hospitalization, they were switched to sacubitril-valsartan. In a third strategy, it was considered that patients would continue enalapril treatment indefinitely. Because no human participants were involved in this study, approval by an institutional review board was not required.
Model Structure
The model followed a standard structure of HF3,5,6,7 in which each month a patient with HF had a probability of surviving, being hospitalized, or dying. The probabilities in the different disease states were derived from previous trial results.2,4,8,9,10,11,12,13 The 5 states were (1) inpatient, (2) 1 month after hospitalization, (3) 2 months after hospitalization, (4) more than 2 months after hospitalization for HF, and (5) death. In the PIONEER-HF trial,4 there were no significant differences among any of the safety outcomes; the only feature with either health or cost implications modeled in the short term was the reduction in HF-related hospitalizations. After 2 months without a hospital admission for decompensated HF, patients had high probabilities of death and readmission to the hospital in the PARADIGM-HF trial.2 This model differed from the previous model3 in 2 fundamental ways. First, we started the model populated with a sicker population of inpatients with HFrEF similar to those in the PIONEER-HF trial, who in the 2 previous trials, had higher hospitalization and mortality rates than an outpatient population with stable health. Second, we considered patients’ health to have eventually stabilized and assumed a profile similar to a patient from the PARADIGM-HF trial if they had no additional hospital admission for at least 2 months. If a patient was readmitted, they were considered to have reverted to worse health with higher 2-month probabilities of death and readmission. Furthermore, the hazard ratio (HR) for reduction during this period in the PIONEER-HF trial using sacubitril-valsartan was greater than that in the PARADIGM-HF trial. We believed this was an improvement in the modeling reflecting the dynamic health state in patients with HFrEF, with periods of varying stability with changing risks.
Perspectives
We evaluated the 3 different strategies from 2 perspectives. The first perspective was the that of the health care sector, which accounted for costs associated with the management of HF, including medications and hospitalizations. Second, as recommended by the Second Panel on Cost-effectiveness in Health and Medicine,14,15 we evaluated the intervention from the societal perspective. This perspective included costs from the health care perspective as well as the productivity gains from a longer life and increased consumption. With nearly 25% of the population older than 65 years still working and earning approximately $40 000 per year, considering these productivity gains has been the subject of recent economic analyses.16,17 We followed previously adopted methods18 originally described by Kim et al19 that used US age-adjusted labor force participation rates and US government age-standardized estimates for productivity and consumption.
For the health care and societal perspectives, we evaluated the strategies spanning 4 different time horizons. First, we reviewed the lifetime of patients with HFrEF up to 30 years as our base case. Next, we reviewed the 2-month duration of the PIONEER-HF trial.4 Third, we modeled the effects of the strategies at 1 year. Finally, we assessed the strategies at 29 months, which was the duration of the 2 trials combined.
Budget Analysis
We evaluated annual budgetary effects for the strategies at the individual and national levels. For the individual level, we looked at a 1-year time frame. Given that enrollment in health insurance is usually done on an annual basis and the mean time in a job is 4.6 years in the US, this is a time frame important to both employers and insurers who make annual coverage decisions. In individual analyses, we reported the undiscounted costs per patient-month alive and the total cost savings for each period. For the national budget analysis, we evaluated the annual costs for any nondominated strategies and assessed the differences for hospitalizations and medications affected by the strategies. We estimated the national budgetary effects by assessing the number of current patients with HFrEF, the number of new diagnoses of HF annually, the proportion of patients with HFrEF who were admitted to the hospital, the proportion of patients who had HFrEF, and the proportion of patients who were eligible. To reflect actual reimbursements, we used Medicare reimbursement rates and the private insurance reimbursements for the proportion of patients who were eligible for each.2,20,21
Patient Population
We modeled a population similar to that in the PIONEER-HF trial, which assessed the effect of sacubitril-valsartan, an angiotensin receptor–neprilysin inhibitor, compared with enalapril on NT-proBNP; key safety events; and clinical outcomes in patients with HFrEF (EF ≤40%) who were hospitalized for acutely decompensated HFrEF. Similar criteria of rEF and elevated NT-proBNP were used in the PARADIGM-HF trial. The details of inclusion and exclusion and the study design have been reported previously.4
Statistical Analysis
Intervention Effects and Model Assumptions
For the base-case analysis, we only modeled factors that were statistically significant in the trials. Hospitalization rates during the first 2 months were based on the results of the PIONEER-HF trial for the enalapril group and the relative risk reduction in the sacubitril-valsartan group. Given that the readmission rate was a secondary outcome from the trial with a relatively small sample size, we performed a 1-way sensitivity analysis across the full range of the 95% CI of the HR and a 2-way sensitivity analysis with the wide range of estimates for the cost of HF-related hospitalizations. We assumed that sacubitril-valsartan has no association with mortality in the first 2 months. Separate HRs for all-cause mortality and HF-related hospitalizations beyond 2 months after hospitalization were calculated and are reported in Table 1.
Table 1. Model Inputs.
| Input | Value (range) | Hazard ratio (95% CI) | Source |
|---|---|---|---|
| Probability of HF hospitalization, enalapril monthly | |||
| First 2 mo after hospitalization | 0.072 | NA | PIONEER-HF4 |
| >2 mo After hospitalization | 0.022 | NA | PARADIGM-HF2 |
| Sacubitril-valsartan vs enalapril | |||
| First 2 mo after discharge | NA | 0.56 (0.37-0.84) | PIONEER-HF |
| >2 mo After discharge | NA | 0.79 (0.71-0.89) | PARADIGM-HF |
| Probability of mortality, enalapril monthly | |||
| First 2 mo after discharge | 0.017 | NA | PIONEER-HF |
| >2 mo after discharge | 0.008 (0.007-0.009) | NA | PARADIGM-HF |
| Sacubitril-valsartan vs enalapril | |||
| First 2 mo after discharge | NA | 0.66 (0.30-1.48) | PIONEER-HF |
| >2 mo After discharge | NA | 0.84 (0.76-0.93) | PARADIGM-HF |
| Costs, $ | |||
| HF hospitalization | 16 467 (14 250-29 061) | NA | Medicare fee schedule or private payers9,10,12 |
| Non-HF admission | 13 319 | NA | Medicare fee schedule |
| Enalapril, annual | 175 (48-1080) | NA | Red Book WAC Price8 |
| Sacubitril-valsartan, annual | 5628 (5600-6600) | NA | Red Book WAC Price |
| Long-term outpatient costs, annual | 4790 (4204-22 032) | NA | Dunlay11 |
| In-hospital initiation program, per patient | 200 (0-100) | NA | NA |
| Utilitiesa | |||
| Sacubitril-valsartan | 0.838 (0.0833-0.0843) | NA | PARADIGM-HF |
| Enalapril | 0.829 | NA | PARADIGM-HF |
| Discount rate | 3% | NA | Second panel on CEA13 |
Abbreviations: CEA, cost-effectiveness analysis; EQ-5D, Euro-QoL 5D scale; HF, heart failure; PARADIGM-HF, Prospective Comparison of ARNI (angiotensin receptor–neprilysin inhibitor) with ACEI (angiotensin-converting enzyme inhibitor) to Determine Impact on Global Mortality and Morbidity in Heart Failure; PIONEER-HF, Comparison of Sacubitril/Valsartan Vs Enalapril on Effect on NT-proBNP (N-terminal pro b-type natriuretic peptide) in Patients Stabilized From an Acute Heart Failure Episode; WAC, wholesale acquisition cost.
Utilities were calculated based on mixed effects model based on EQ-5D scores reported at baseline and over time during the trial. Model utilities were a function of baseline EQ-5D scores, age, time, hospitalization, and treatment status. Values reported in the table are utilities for an average-age patient in the first year of the model. Further details are available in Gaziano et al.3
In the PIONEER-HF trial, there were no significant differences in any of the adverse events. There were statistically significant differences among 4 adverse events in the PARADIGM-HF trial: symptomatic hypotension was more common in the sacubitril-valsartan arm, whereas elevated serum creatinine level, hyperkalemia (>6.0 mmol/L), and cough were more common in the enalapril group. The difference in the number of cases of angioedema was not statistically significant in either trial (the number was higher in the sacubitril-valsartan arm in the PARADIGM-HF trial and lower in the sacubitril-valsartan arm of the PIONEER-HF trial). These adverse events requiring hospitalizations were captured in the non-HF admissions included in the model.
Costs and Utilities
Medication costs were based on the annual wholesale acquisition cost for sacubitril-valsartan and enalapril listed in Table 1 and the proportion of patients who continued treatment. The monthly costs for the medications were based on wholesale acquisition costs.8 Base-case hospital costs relied on Medicare rates,12 and private insurance rates were used for budget estimates.9,10 Annual costs of long-term outpatient care for patients were used for sensitivity analyses.11 Cost of a program to initiate sacubitril-valsartan during hospitalization was estimated to range from $0 to $200 per patient, representing a range of clinicians who prescribed medications on the basis of trial results to those who would benefit from a patient identification system and reminder system managed by a nurse or pharmacist. Our base case assumed no cost to initiate the treatments. Productivity gains were estimated by multiplying age-specific labor force participation rates by mean annual earnings during each year of life from the US Department of Commerce.22 We also computed non–health care consumption costs by age for each year in the model to capture future resource use. The sources for mean annual earnings (Current Population Survey 2014), labor force participation rate (Bureau of Labor Statistics), and consumption costs (Bureau of Labor Statistics Consumer Expenditure Survey) are summarized elsewhere.19
Quality of life (QOL) or utility decrements were applied each month during which a person had HF and were based on Euro-QoL 5D scores from the PARADIGM-HF trial (Euro-QoL 5D is a standardized instrument that evaluates generic QOL). Values in Table 1 represent utilities for a person at the beginning of the simulation. Consistent with the PARADIGM-HF trial, patients continued treatment 81% of the time, which was the mean percentage of patients receiving medication in the 2 trials assuming that most of the drop-off in participation was early. In a sensitivity analysis, we tested the effects of having no additional QOL gains after the PARADIGM-HF trial.
Base-Case Cost-effectiveness Analysis
We evaluated the lifetime discounted HF-associated health care costs and quality-adjusted life-years (QALYs) accrued under the 3 treatment options. Incremental cost-effectiveness ratios (ICERs) were calculated according to conventional cost-effectiveness analysis guidelines.23 Costs and QALYs were each discounted at 3%.15 We applied commonly accepted cost-effectiveness thresholds of $50 000 per QALY, $100 000 per QALY, and $150 000 per QALY to assess the optimal strategy in base-case and sensitivity analyses with $100 000 as our primary threshold.24
Sensitivity Analysis
We varied values for all variables (or groups of associated variables) through plausible ranges or used alternative values to assess the robustness of our cost-effectiveness analysis results to changes in the input parameters. Sensitivity analyses were performed on key inputs, including hospital cost, medication costs, time decays in benefit of HF hospitalization reduction, and QOL over 29 months and after 10 years. The model was run using DATA TreeAge Pro, version 2018 (TreeAge). Finally, we conducted a second-order probabilistic sensitivity analysis based on 1000 iterations using distributions appropriate to the variable25: β distributions for health probabilities bounded by 0 and 1, log normal distributions for the HRs, and γ distributions for the costs.
Model Validation
We compared the model performance with the trial results for the duration of the 2 different trials for hospitalizations and mortality. We also compared the 5-year survival rates of the enalapril arm of the model with those in community-based cohorts of patients with HF including from the Atherosclerosis Risk in Communities study, Framingham and Olmsted County (eTable 1 in the Supplement).
Results
Model Validation
Modeled patients were a mean (SD) age of 63.8 (11.5) years. At the end of the PIONEER-HF trial, with a follow-up of 8 weeks (56 days), 8.0% in the sacubitril-valsartan arm and 13.8% in the enalapril arm had been readmitted to the hospital for HF. In our model, at 2 months (61 days), we estimated that 8.1% of patients in the sacubitril-valsartan group and 14.2% in the enalapril group would be admitted to the hospital for HF. At 27 months, the model predicted a rate of 62% HF hospitalizations; the PARADIGM-HF trial reported a rate of 57% HF hospitalizations at the same follow-up time. Mortality in our model at 27 months was 21% and in the PARADIGM-HF trial was 20%. The enalapril arm in the model was within the estimated range of 5-year survival (43%) seen in the community-based cohorts (42%-54%).21,26
Cost-effectiveness Analyses
Health Care Perspective
The model predicted 44% fewer admissions in the first 2 months and 62 fewer HF-related admissions per 1000 patients compared with outpatient initiation and 116 fewer HF-related admissions compared with continuation of enalapril treatment over a lifetime. In our base-case analysis, the model predicted survival of 6.93 years (5.46 QALYs), 2.91 lifetime HF hospitalizations, and costs of $109 709 for those receiving enalapril. For those who initiated sacubitril-valsartan after hospitalization, survival was 8.1 years (6.43 QALYs), with 2.48 HF-related admissions and discounted costs of $134 409. For those who initiated sacubitril-valsartan as an inpatient, the model predicted survival of 8.44 years (6.70 QALYs), 2.40 HF-related admissions, and lifetime costs of $137 062. Compared with continuation of enalapril treatment indefinitely, initiation of sacubitril-valsartan treatment after hospitalization was associated with a cost per QALY gained of $25 705. Compared with continuation of enalapril treatment indefinitely, initiation of sacubitril-valsartan as an inpatient was associated with a cost per QALY of $21 532, and initiation of sacubitril-valsartan after hospitalization was eliminated by extended dominance when comparing all 3.
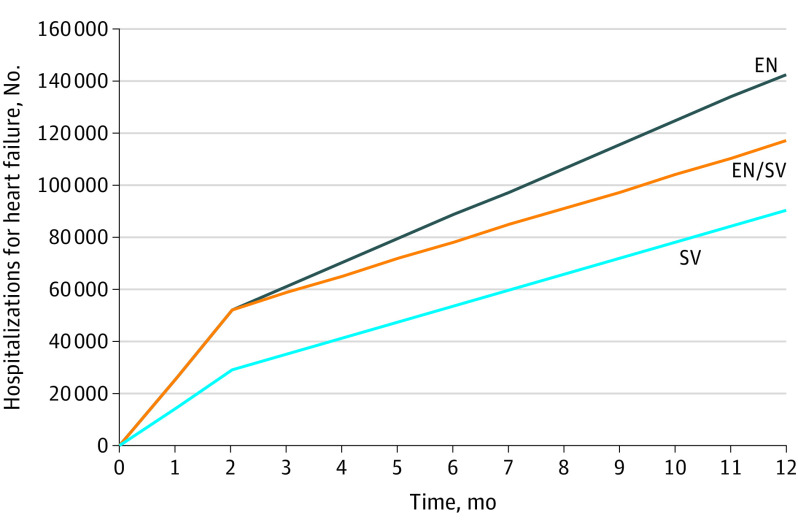
When evaluated over shorter periods of 2 months (PIONEER-HF trial duration) and 12 months, the use of sacubitril-valsartan compared with enalapril was cost saving. In 2 months, sacubitril-valsartan treatment would save the health care system more than $473 per person compared with enalapril treatment, including medications, long-term care costs, and hospitalizations. At 29 months (combined PIONEER-HF and PARADIGM-HF trials duration), when comparing initiation of sacubitril-valsartan after hospitalization with enalapril initiation at any time, the ICER was $17 040 per QALY gained; sacubitril-valsartan initiated after hospitalization was dominated with fewer QALYs and more costs than initiating as an inpatient. At a national level, 52 856 fewer HF admissions per year would be averted if all eligible patients were prescribed sacubitril-valsartan as an inpatient compared with continuing enalapril treatment indefinitely (Figure 1).
Figure 1. Comparison of Hospitalizations Over 1 Year in the US Population With Heart Failure Who Initiated Sacubitril-Valsartan During Hospitalization (S/V) vs After Hospitalization (EN/S) vs Those Who Continued Enalapril Treatment Indefinitely (EN).
Societal Perspective
From a societal perspective (Table 2), initiation of sacubitril-valsartan during hospitalization remained cost saving compared with initiation after hospitalization or continuation of enalapril treatment indefinitely. In our model, sacubitril-valsartan saved $460 per person at 2 months and up to $15 926 per person during a lifetime compared with continuation of enalapril treatment indefinitely. Sacubitril-valsartan initiated during hospitalization compared with after hospitalization saved $460 at 2 months, $813 at 1 year, and more than $7073 per person during a lifetime. Even after adjustment for possible lack of employment of up to 25% of patients with HFrEF who were in New York Heart Association functional class III or higher,2,4 sacubitril-valsartan was associated with cost savings because of increases in years of productive work-years gained.
Table 2. Lifetime Mean Costs, Effectiveness, and Cost-effectiveness Ratios.
| Strategy | Heart failure admissions | Life expectancy | Mean (95% CI) | ||
|---|---|---|---|---|---|
| Cost, $ | QALYs | ICER, $/QALY | |||
| Health system perspective | |||||
| Enalapril indefinitely | 2.91 | 6.93 | 114 778 (86 083 to 147 136) | 5.49 (5.47-5.51) | NA |
| Enalapril for 2 mo, then sacubitril-valsartan as outpatient | 2.48 | 8.10 | 139 456 (100 800 to 168 916) | 6.45 (6.43-6.47) | Extended dominancea |
| Sacubitril-valsartan, inpatient initiation | 2.40 | 8.44 | 142 097 (115 768 to 171 087) | 6.72 (6.70-6.73) | 21 532 (16 710-26 368) |
| Societal perspective | |||||
| Enalapril indefinitely | NA | NA | –85 063 (–112 065 to 50 886) | 5.49 (5.47-5.51) | Dominatedb |
| Enalapril for 2 mo, then sacubitril-valsartan as outpatient | NA | NA | –93 921 (–119 137 to 62 354) | 6.45 (6.43-6.47) | Dominatedb |
| Sacubitril-valsartan, inpatient initiation | NA | NA | –101 014 (–125 535 to –69 495) | 6.72 (6.70-6.73) | Cost savingc |
Abbreviations: ICER, incremental cost-effectiveness ratio; NA, not applicable; QALY, quality-adjusted life-years.
Extended dominance indicates that the ICER is greater than next best option.
Dominated indicates that the intervention costs more and results in fewer QALYS.
Cost saving indicates that the intervention costs less and increases QALYs.
Budget Analysis
On the basis of data from the American Heart Association21 and the trial results, approximately 367 000 patients with HF are eligible for sacubitril-valsartan treatment each year (eTable 2 in the Supplement). eTable 3 in the Supplement gives the annual budgetary effects of the different strategies at the individual and national levels. For 1 year, with sacubitril-valsartan initiated during hospitalization, there would be undiscounted savings of up to $449 or $2550 over 5 years compared with continuation of enalapril treatment indefinitely. Initiation of sacubitril-valsartan after hospitalization would be cost-effective but would cost approximately $420 annually per patient compared with continuation of enalapril treatment. Nationally, the annual savings associated with reduced HF-related hospitalizations would be approximately $1.5 billion (eTable 3 in the Supplement), and the medication costs would increase by $1.4 billion, with a net reduction in overall expenditures of $92 million per year for sacubitril-valsartan initiation during hospitalization compared with continuation of enalapril treatment indefinitely.
Sensitivity Analyses
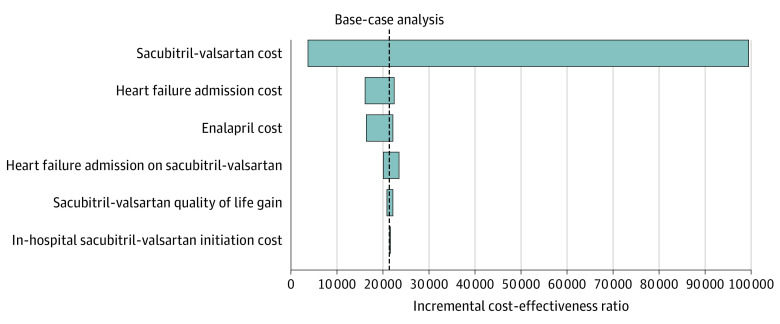
The model was most sensitive to cost of sacubitril-valsartan, with the medication being cost saving in the health system perspective at an annual cost of $1043 and less than $10 000 per QALY at $3200 per year. For the ICER to exceed $100 000 per QALY, the cost of sacubitril-valsartan would need to be $12 760 per year or higher. In the sensitivity analyses presented in the tornado diagram (Figure 2), the ICERs remained less than $100 000 per QALY in comparisons between sacubitril-valsartan and enalapril. Similar results were seen when comparing inpatient sacubitril-valsartan treatment initiation with initiation of sacubitril-valsartan after hospitalization or continuation of enalapril treatment (eFigures 1-2 in the Supplement). For the estimated cost of HF admissions, the ICER ranged from $16 186 per QALY when the cost of admission was at its highest estimate ($29 000) to $22 478 per QALY at the lowest estimate ($14 250). In a 2-way sensitivity analysis of the full range of estimates for the HR for hospital reductions (0.56; 95% CI, 0.37-0.84) and the cost of hospitalizations ($16 467; range, $14 250-$29 061), 74% of the paired results remained cost saving and more than 95% of the results remained highly cost-effective below $50 000/per QALY. At the lower range of the 95% CI (0.37-0.51) for the HR for reductions in HF hospitalizations, using sacubitril-valsartan across all estimates of the cost of hospitalizations remained cost saving. For our base-case assumptions of 0.56 HF hospitalizations with sacubitril-valsartan for each hospitalization with enalapril from the trial,4 it was no longer cost saving when the cost of hospital admission was less than $16 217. At the higher end of the HR (0.84; 95% CI, 0.76-0.93), the intervention was no longer cost saving when the cost of hospitalizations was less than $24 000 per admission. However, all strategies remained cost-effective below $60 000 per QALY.
Figure 2. Tornado Diagram of Sacubitril-Valsartan vs Enalapril Treatment.
Univariate sensitivity analyses evaluating the association of each variable’s uncertainty with the overall cost-effectiveness ratio. The dashed black line represents the base-case analysis.
When we tested the cost of enalapril from its highest ($1000) to lowest ($48) annual cost, the ICERs remained cost-effective. Finally, when we assessed the QOL benefit across its 95% CI from the trial, the ICERs remained cost-effective. When we assumed that the QOL benefit of sacubitril-valsartan dissipated after 29 months, the ICER increased by approximately $100 per QALY and up to $500 per QALY and the QOL gain was removed entirely. When we tested the effect of sacubitril-valsartan tapered after 29 months or after 10 years, the ICERs of sacubitril-valsartan vs enalapril indefinitely were $25 902 per QALY and $23 272 per QALY, respectively, and delayed sacubitril-valsartan was eliminated by extended dominance. The probabilistic sensitivity analysis revealed a mean value of $20 095 per QALY (95% CI, $16 710-$26 338 per QALY). On the cost-effectiveness acceptability curve, sacubitril-valsartan was optimal 100% of the time when the willingness-to-pay threshold was more than $27 000 per QALY.
Discussion
Our model-based analyses suggests that from a health care system perspective, initiation of sacubitril-valsartan during hospitalization in patients with recently decompensated HFrEF would be cost-effective compared with the initial use of enalapril and initiation of sacubitril-valsartan after hospitalization or the long-term use of enalapril. Initiation of sacubitril-valsartan as an outpatient was cost-effective but not cost saving compared with continued use of enalapril. In contrast, compared with no initiation of sacubitril-valsartan, inpatient initiation was cost saving at 1 year and highly cost-effective (high value) at $21 532 per QALY over a lifetime. All the results from the sensitivity analyses were considered cost saving or high value (<$50 000/QALY) to a good or intermediate value (<$150 000/QALY).27
From a societal perspective, an additional $1000 to $1500 per patient per year would be added in productivity gains. Nationally, use of sacubitril-valsartan could save eligible US patients with HFrEF more than 50 000 hospitalizations for HF each year compared with continuation of enalapril treatment. This would result in a net savings of $92.3 million per year. To place this in perspective, this represents only 0.15% to 0.30% of the estimated $30 to $60 billion spent on HF per year. The net savings would be attributable to a reduction of $1.5 billion per year in hospitalization costs per year and an increase of $1.4 billion in medication costs (eTable 3 in the Supplement). However, the net savings would be $47 million per year if only half the eligible patients initiated sacubitril-valsartan treatment during hospitalization.
Initiation of sacubitril-valsartan treatment for patients with HFrEF while admitted for HF was cost-effective or cost saving in this modeling of long-term outcomes compared with waiting for outpatient stabilization for 2 main reasons. First, it led to reductions in readmissions for HF by 44% by the end of 2 months. Second, the absolute risk of rehospitalization in the PIONEER-HF trial for recently discharged patients with HF was 3 times higher compared with waiting until patients were stabilized, as was seen in the PARADIGM-HF trial. Initiation of therapy in this acute window was associated with greater absolute reductions in HF-related hospitalizations. When these 2 factors were taken into account and use of inpatient initiation of sacubitril-valsartan was compared with indefinite treatment with enalapril in the present analysis, the ICER decreased by approximately $23 000 per QALY compared with results from the cost-effectiveness analysis from the PARADIGM-HF trial.3 When QOL gains and drug effectiveness of sacubitril-valsartan were tested for decay over time, the results showed cost-effectiveness below $25 000 per QALY gained. This improvement in cost-effectiveness compared with initiation after hospitalization may have been associated with these patients generally having higher acuity of illness with higher near-term hospitalization and mortality risk compared with a population with stable HFrEF, such as those enrolled in the PARADIGM-HF trial.
If the HR observed in the PIONEER-HF trial applied to routine use, in-hospital initiation of sacubitril-valsartan would also be cost saving from a societal perspective. In addition to the health care savings, the results suggest that an additional $170 to $300 million per year could be gained with increases in productivity benefits per year compared with no initiation of sacubitril-valsartan or initiation after hospitalization. Even though nearly a quarter of patients with HFrEF may be unable to perform full-time work because of their age or disability, productivity gains from the remaining patients would still be associated with overall societal gains. This finding has been observed in many interventions that add QALYs; thus, this finding alone does not justify increased use but must be compared with others in the future as more economic analyses interpret productivity gains as part of their results. In a budget analysis, we found that similar gains would be made on a per–person-alive basis. For each year that a patient was receiving sacubitril-valsartan compared with receiving enalapril, a payer covering medication costs, long-term costs, and hospitalization costs would have a net savings of approximately $500 per year per individual. This excludes payments for premiums received; thus, the use of sacubitril-valsartan for all patients should be attractive to payers, in addition to those who have already approved use as an outpatient. Risk sharing may be different in different plans; thus, incentives from those holding the hospitalization risk should be given to those holding the pharmaceutical risk or the risks should be bundled to encourage the optimization of both.
Limitations
Among the limitations of our study was the short duration of the PIONEER-HF trial and its relatively small sample size. Furthermore, we based part of the analysis on an exploratory secondary outcome of HF-related hospitalizations in the PIONEER-HF trial with a relatively large 95% CI.4 Our 2-way sensitivity analysis using cost of hospitalizations and the full range of likely reductions suggested that in the first year, the inpatient initiation of sacubitril-valsartan was cost saving approximately 75% of the time and highly cost-effective 95% of the time, taking into consideration the wide 95% CIs of the estimates. After the first year, when most patients experience stabilization of their condition, use of sacubitril-valsartan remained highly cost-effective but not necessarily cost saving. When we adjusted the model to account for a more dynamic risk of hospitalizations, the ICER of inpatient initiation of sacubitril-valsartan compared with enalapril treatment only decreased considerably. We also did not model adverse events directly; however, key safety outcomes in the PIONEER-HF trial were no different in either arm, and discontinuation for safety reasons were lower in the sacubitril-valsartan arm of the PARADIGM-HF trial, which also had fewer emergency department visits. Furthermore, any differences in non-HF hospitalizations associated with adverse events were accounted for in overall hospitalizations in the model. Despite the value of in-hospital initiation of angiotensin receptor–neprilysin inhibitor therapy, prior authorization requirements and substantial out-of-pocket costs borne by patients remain barriers to greater use of angiotensin receptor–neprilysin inhibitor therapy and need to be addressed.
Conclusion
We found that for eligible patients with HFrEF, initiation of sacubitril-valsartan during hospitalization was cost saving compared with initiation 2 months after hospitalization and was cost saving or highly cost-effective compared with indefinite continuation of enalapril treatment. There may also be cost savings from a societal perspective. From a budgetary perspective, use of sacubitril-valsartan may save similar amounts for payers responsible for medication, outpatient, and inpatient costs.
eTable 1. Comparison of Model Results With Trial and Community-based Estimates
eTable 2. Derivation of Number of Persons per Year Eligible for Heart Failure Treatment
eTable 3. Annual Budget Impact at the Individual and National Levels
eFigure 1. One-Way Sensitivity Analyses Comparing Sacubitril/Valsartan Inpatient Vs Enalapril Inpatient Then Sacubitril/Valsartan as an Outpatient
eFigure 2. One-Way Sensitivity Analyses Comparing Enalapril Throughout Vs Enalapril Inpatient Then Sacubitril/Valsartan as an Outpatient
eReferences
References
- 1.Voigt J, John MS, Taylor A, Krucoff M, Reynolds MR, Gibson CM. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol. 2014;37(5):312-321. doi: 10.1002/clc.22260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMurray JJ, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 3.Gaziano TA, Fonarow GC, Claggett B, et al. . Cost-effectiveness analysis of sacubitril/valsartan vs enalapril in patients with heart failure and reduced ejection fraction. JAMA Cardiol. 2016;1(6):666-672. doi: 10.1001/jamacardio.2016.1747 [DOI] [PubMed] [Google Scholar]
- 4.Velazquez EJ, Morrow DA, DeVore AD, et al. ; PIONEER-HF Investigators . Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539-548. doi: 10.1056/NEJMoa1812851 [DOI] [PubMed] [Google Scholar]
- 5.Chan DC, Heidenreich PA, Weinstein MC, Fonarow GC. Heart failure disease management programs: a cost-effectiveness analysis. Am Heart J. 2008;155(2):332-338. doi: 10.1016/j.ahj.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 6.Delea TE, Vera-Llonch M, Richner RE, Fowler MB, Oster G. Cost effectiveness of carvedilol for heart failure. Am J Cardiol. 1999;83(6):890-896. doi: 10.1016/S0002-9149(98)01066-2 [DOI] [PubMed] [Google Scholar]
- 7.Gaziano TA. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112(23):3547-3553. doi: 10.1161/CIRCULATIONAHA.105.591792 [DOI] [PubMed] [Google Scholar]
- 8.Red Book. 2019. Accessed December 31, 2019. https://www.ibm.com/products/micromedex-red-book
- 9.O’Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29(8):693-704. doi: 10.2165/11584620-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 10.Optum. DRG Expert 2014. OPTUMInsight; 2013.
- 11.Dunlay SM, Shah ND, Shi Q, et al. . Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4(1):68-75. doi: 10.1161/CIRCOUTCOMES.110.957225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. doi: 10.2147/RMHP.S130341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, eds. Cost-effectiveness in health and medicine. 2nd ed. Oxford University Press; 2016. doi: 10.1093/acprof:oso/9780190492939.001.0001 [DOI] [Google Scholar]
- 14.Jönsson B. Ten arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ. 2009;10(4):357-359. doi: 10.1007/s10198-009-0173-2 [DOI] [PubMed] [Google Scholar]
- 15.Sanders GD, Neumann PJ, Basu A, et al. . Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 16.Lee Y, Mozaffarian D, Sy S, et al. . Health Impact and Cost-Effectiveness of Volume, Tiered, and Absolute Sugar Content SugarSweetened Beverage Tax Policies in the United States: A Microsimulation Study. Circulation. 2020;142:00-00. doi: 10.1161/CIRCULATIONAHA.119.042956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Mozaffarian D, Sy S, et al. ; FOOD-PRICE (Policy Review and Intervention Cost-Effectiveness) Project . Health and Economic Impacts of the National Menu Calorie Labeling Law in the United States: A Microsimulation Study. Circ Cardiovasc Qual Outcomes. 2020;13(6):e006313. doi: 10.1161/CIRCOUTCOMES.119.006313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaziano TA, Pandya A, Sy S, et al. . Modeling the cost effectiveness and budgetary impact of Polypills for secondary prevention of cardiovascular disease in the United States. Am Heart J. 2019;214:77-87. doi: 10.1016/j.ahj.2019.04.020 [DOI] [PubMed] [Google Scholar]
- 19.Kim DD, Basu A, Duffy SQ, Zarkin GA. Appendix A: the cost-effectiveness of treatments for individuals with alcohol use disorders: a reference case analysis In: Neumann P, Sanders GD, Russell LB, Siegel JE, Ganiats TG, eds. Cost-effectiveness in Health and Medicine. 2nd ed Oxford University Press; 2016. [Google Scholar]
- 20.Heidenreich PA, Albert NM, Allen LA, et al. ; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council . Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 22.United States Census Bureau. Current population survey tables for personal income. Accessed in March 2018. https://www.census.gov/data/tables/time-series/demo/income-poverty/cps-pinc.html
- 23.Hunink MGM, Weinstein MC, Wittenberg E, et al. . Decision Making in Health and Medicine: Integrating Evidence and Values. 2nd ed Cambridge University Press; 2014. doi: 10.1017/CBO9781139506779 [DOI] [Google Scholar]
- 24.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 25.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479-500. doi: 10.2165/00019053-200017050-00006 [DOI] [PubMed] [Google Scholar]
- 26.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646-659. doi: 10.1161/CIRCRESAHA.113.300268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson JL, Heidenreich PA, Barnett PG, et al. ; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines . ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345. doi: 10.1161/CIR.0000000000000042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Comparison of Model Results With Trial and Community-based Estimates
eTable 2. Derivation of Number of Persons per Year Eligible for Heart Failure Treatment
eTable 3. Annual Budget Impact at the Individual and National Levels
eFigure 1. One-Way Sensitivity Analyses Comparing Sacubitril/Valsartan Inpatient Vs Enalapril Inpatient Then Sacubitril/Valsartan as an Outpatient
eFigure 2. One-Way Sensitivity Analyses Comparing Enalapril Throughout Vs Enalapril Inpatient Then Sacubitril/Valsartan as an Outpatient
eReferences