Abstract
The practical application of dual-time-point-imaging (DTPI) technique still remains controversial. One of the issues is that current parameters of DTPI quantification suffer from some deficiencies, mainly limited sampling of the diseased sites by confining measurements to specific locations. We aimed to examine the correlation between the percent change from early to delayed scans in whole-bone marrow (WBM) 18F-FDG uptake, as measured by a CT-based method of PET/CT quantification, and response to treatment in multiple myeloma (MM) patients. Pre-treatment 18F-FDG-PET/CT scans of 36 newly diagnosed MM patients were collected in a prospective study at 1 h and 3 h post tracer injection (NCT02187731). A threshold algorithm based on bone Hounsfield units on CT was applied to segment and quantify WBM 18F-FDG uptake. Patients were separated into two treatment groups: high-dose therapy with autologous stem cell transplant (HDT) and non-high dose therapy (non-HDT). The International Response Criteria for MM patients was used to determine each patient’s response to treatment. In the HDT group, WBM 18F-FDG uptake increased significantly in patients that had a poor response to treatment, from a median of 1.31 (IQR: 1.13-1.64) at 1 h to a median of 1.85 (1.45-2.10) at 3 h. The median percent change was 37.77% (IQR: 23.47-46.4), with a range of 6.10-50.73 (P = 0.003). However, no significant change in uptake was observed in patients with a complete response (P = 0.24). The same trend was observed for the non-HDT group. WBM uptake of 18F-FDG assessed with dual-time-point imaging may have a role in predicting treatment response in MM.
Keywords: Dual-time-point-imaging, positron emission tomography, 18F-FDG, multiple myeloma, whole-bone marrow, response to treatment
Introduction
Dual-time-point-imaging (DTPI), which is mostly utilized to distinguish inflammatory from malignant lesions, is a concept introduced by Zhuang et al. [1]. The technique is based on the observation that 18F-fludeoxyglucose (18F-FDG) uptake increases with time in malignant tissues until 4 hours after injection [1,2], whereas in benign and normal tissue the uptake stays the same or shows a decline after the administration of the radiotracer [1,3,4]. This phenomenon can be explained by the fact that the accumulation rate of 18F-FDG, a glucose analog, depends on the rate of transport through glucose transporter membrane proteins (GLUT) and also on hexokinase to 18F-FDG-6-phosphatase ratios, which are both higher in malignant cells [5]. Although DTPI has been shown to influence patient prognosis assessment [6-8], the practical application of this technique still remains controversial. One of the reasons is that present parameters of DTPI quantification, such as SUVmean and SUVmax, suffer from some shortcomings, including limited sampling of the diseased sites by confining measurements to specific locations. This quantitative assessment of small isolated focal lesions is sensitive to the partial volume effect, which causes significant underestimation of the true degree of metabolic activity [9-11]. Therefore, the results often show significant overlap of 18F-FDG uptake patterns between malignant and benign lesions on DTPI [1,12,13]. Another issue is that current methods of quantification suffer from high inter-observer variation, which affects the accurate and reliable differentiation of malignant from benign 18F-FDG uptake changes. Of mention, the International Myeloma Working Group (IMWG) has recently acknowledged that the high variability of PET results has limited the role of 18F-FDG PET/CT in assessing patients with multiple myeloma (MM) [14,15]. Another challenge, specific to MM disease, is that since some patients have a high number of osteolytic lesions, it is not an efficient approach to evaluate all suspicious lesions with DTPI approach. Given these issues, we think a method of quantification to evaluate the 18F-FDG uptake in the whole-bone marrow (WBM) can improve the efficiency of DTPI approach in evaluating MM patients. This region of interest (ROI) creates large volume especially in the spine where each pathological lesion often is fully included with large margins and thereby reducing the effect of partial volume correction.
By analyzing data from a prospective myeloma trial, we aimed to assess the correlation between the percent change in WBM 18F-FDG uptake from early to delayed scans and response to treatment in MM patients. To achieve this purpose, we employed a CT-based method of PET/CT quantification. Since it has been shown that a higher percentage of bone marrow plasma cell infiltration is associated with poor outcomes [16-19], and also that 18F-FDG uptake in bone marrow is significantly correlated with the percentage of CD38/CD138 positive cells [20], which are the hallmark of MM, we hypothesized that patients with a significant increase in WBM 18F-FDG uptake would have poorer treatment responses.
Material and methods
This study was performed as a part of a prospective study (Functional Imaging in Multiple Myeloma-PET/CT and Diffusion Weighted Imaging in Multiple Myeloma (FULIMA)). The FULIMA trial approved by the Danish National Committee on Health Research Ethics, registered at ClinicalTrials.gov (NCT02187731), and conducted in accordance with the Declaration of Helsinki. The study performed between June 2013 and March 2016 at the Departments of Hematology, Odense University Hospital and Vejle Hospital, Denmark. Seventy subjects suspicious of having MM were enrolled in FULIMA trial. Exclusion criteria were history of treated MM, current or recent radiotherapy or surgery less than two weeks prior to screening, history of prior malignancy, with at least three years disease free period, known inflammatory disease or serious medical or psychiatric conditions, pregnancy or breast feeding, POEMS syndrome (Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal protein, Skin changes) [21]. Baseline FDG-PET/CT studies were performed prior to any anti-myeloma treatment including steroids or bisphosphonates. After preliminary assessment and imaging studies, the following subjects were excluded: 24 participants with other diagnosis, 3 patients who withdrew from the study following the initial consent and one patient who was younger than 50 years old (Figure 1). In addition, six MM patients were excluded since they did not have either early or delayed baseline scans or there was a technical issue with the scans (Figure 1). 18F-FDG scans of 36 MM patients (26 men, 10 women; 66.19 (range: 50-87) years) were included for further analysis (Table 1). Of these, 21 underwent high-dose therapy including induction therapy with bortezomib followed by autologous stem cell transplantation (HDT). HDT was contraindicated in the 15 remaining patients, who underwent conventional-dose chemotherapy (non-HDT). The International Response Criteria for MM patients determined treatment response. In the HDT group, 10 patients (48%) achieved a complete response, defined as either a stringent complete response (sCR) or complete response (CR). In the same group, 11 patients (52%) had a poor response to treatment, defined as either a partial response (PR) or very good partial response (VGPR). In the non-HDT group, 4 patients (27%) achieved a complete response while 11 patients (73%) had a poor response to treatment.
Figure 1.
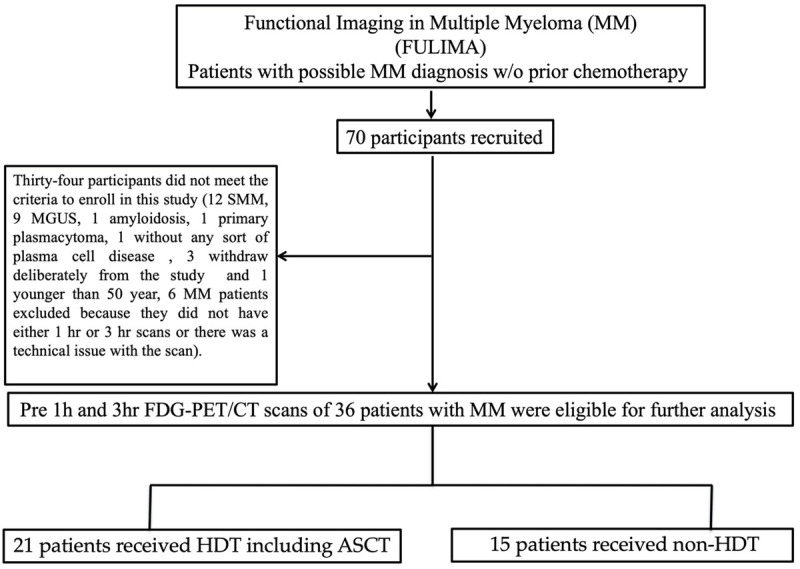
Flowchart of patient inclusion and drop-out.
Table 1.
Patient characteristics
| MM (n = 36) | |
|---|---|
| Gender, n (%) | |
| Female | 10 (28) |
| Male | 26 (72) |
| Age (y) | 66.19 |
| 50-87 | |
| B2M (mg/L) | 237 |
| 142-760 | |
| Hemoglobin (g/dl) | 7.6 |
| 3.8-9.4 | |
| Albumin (g/L) | 38 |
| 22-49 | |
| LDH (U/L) | 228.03 |
| 132-369 | |
| R-ISS, n (%) | |
| R-ISS 1 | 9 (25) |
| R-ISS 2 | 26 (72) |
| Unknown | 1 (3) |
Abbreviations: B2M: beta-2-microglobulin, LDH: lactate dehydrogenase, R-ISS: revised international staging system. Data for continuous variables is represented as median and range.
PET/CT acquisition
18F-FDG-PET/CT scans were acquired in accordance with European Association of Nuclear Medicine (EANM) guidelines, which include quality control, calibration and harmonization of the scanner and SUV calculations [22]. 18F-FDG-PET/CT scans were performed using GE Discovery (vender and type: STE, VCT, Rx, 690, 710; GE, Milwaukee, WI) and Philips Gemini TF (Philips, Amsterdam, Netherlands) PET/CT scanners. The patients received a 4 MBq/kg intravenous injection of 18F-FDG (300 MBq-520 MBq), after an overnight fast of at least 8 hours and a confirmed blood glucose concentration of below 8 mmol/L. Low-dose CT (LDCT) scans were then performed (120 kV, 30-110 mA Smart mA, collimation 16/64 * 0.625, rotation time 0.5 sec, pitch 0.984, slice thickness 3.750 mm), followed by 3-D PET scans using a whole-body acquisition protocol from skull vertex to below knee. Transaxial reconstruction was performed (standard filter, slice thickness 3.750, increment 3.270). Acquisition times were 2.5 min/field of view (FOV) for the 45-min scan. The LDCT scans were used for attenuation correction. PET images were reconstructed with iterative algorithms (Ordered Subset Expectation Maximization [OSEM], four iterations, 24 subsets).
Subjects underwent serial whole body 18F-FDG-PET/CT scans at about 1-hour and 3-hour after administration of the tracer. Between the two time-points, the patients remained in the waiting area, were ordered to fast, and received instructions to rest and walk slowly.
Image analysis
A single ROI was chosen for analyzing the skeletal disease and included most of the axial and proximal appendicular structures by adopting anatomical landmarks. This ROI included all ribs, the vertebrae, the pelvis, the proximal femur within approximately 10 cm of the lowest point of the ischium, and the proximal humerus within approximately 20 cm of the glenohumeral joint. After anatomical exclusions were manually performed, upper and lower thresholds of 1500 Hounsfield units (HU) and 150 HU, respectively, were applied to the fused PET/CT image using a growing region algorithm, segmenting the cortical and trabecular bone in the skeleton (Figure 2). A morphological closing algorithm with element radius of 20 was then applied to the ROI to include the entire medullary cavity of each bone. The entire ROI in each patient was visually confirmed to include the entire medullary cavity. WBM 18F-FDG uptake, defined as the global SUVmean (GSUVmean) obtained from this ROI, was measured.
Figure 2.
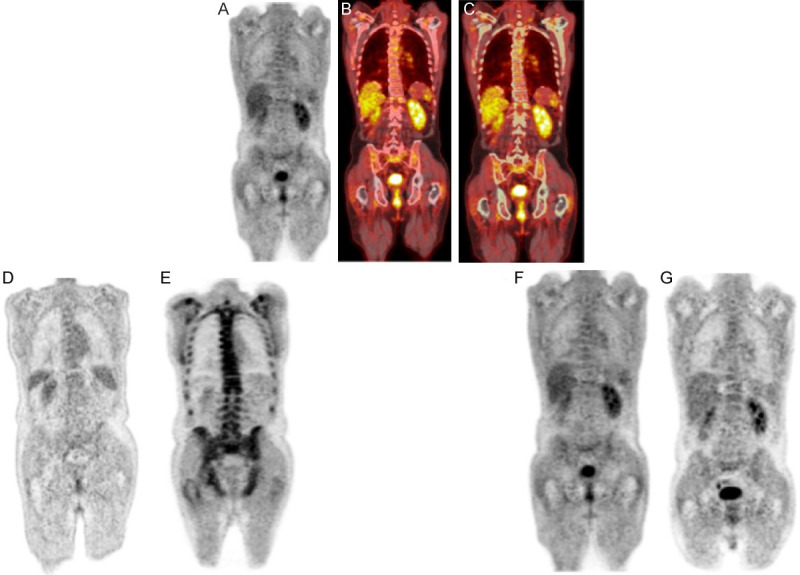
The top figures demonstrate PET (A), fused PET/CT (B), and fused PET/CT with ROI before applying morphological closing algorithm (C) in a MM patient. The left bottom figures show PET scans of a MM patient who belonged to the poor response to treatment group at 1 h (D) and 3 h (E). Whole bone-marrow FDG uptake increased from 0.89 (D) to 2.31* (E) with a percent change of 158.15%. The right bottom figures show PET scans of a MM who belonged to the complete response group at 1 h (F) and 3 h (G). There was a decrease in whole bone marrow FDG uptake from 0.83* (F) to 0.82* (G) with a percent change of -0.79%*. *The second decimal number gets rounded up.
Statistical analysis
A Wilcoxon signed-rank test was used to assess differences between 1 h and 3 h WBM FDG uptake. The median, interquartile range (IQR), and range were determined for each type of response (complete or poor) within each treatment group. Level of significance was set to 5%. Statistical analysis was performed using SPSS Statistics version 26 (IBM Corp, NY, USA).
Results
In total, WBM 18F-FDG uptake increased significantly in all patients, from 1.15 (IQR: 1.02-1.35) to 1.51 (1.11-1.84); percent change 23.560 (2.71-38.96). In the HDT group, WBM 18F-FDG uptake expressed as GSUVmean increased significantly in patients that had a poor response to treatment, from a median of 1.31 (IQR: 1.13-1.64) at 1 h to a median of 1.85 (1.45-2.10) at 3 h. The median percent change was 37.77% (IQR: 23.47-46.4), with a range of 6.10-50.73 (P = 0.003). Among patients in the HDT group with a complete response, there was a change in GSUVmean from a median of 1.12 (IQR: 0.99-1.47) at 1 h to a median of 1.27 (1.04-1.46) at 3 h. The median percent change was 2.24% (IQR: -3.40-15.21), with a range of -8.31-19.30 (P = 0.24) (Figure 3).
Figure 3.
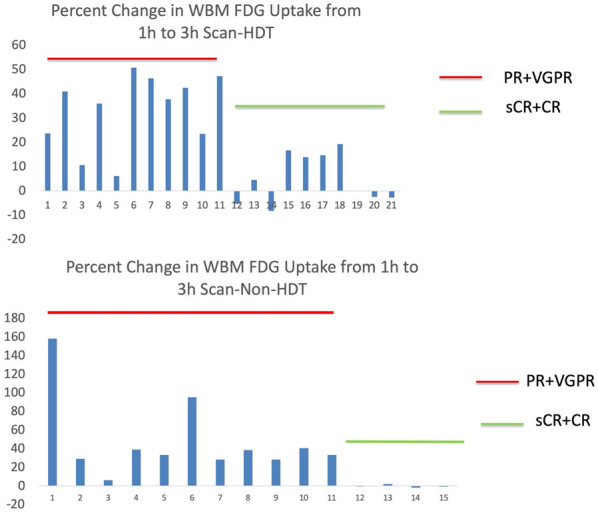
Bar charts showing percent change in WBM 18F-FDG uptake from 1 h to 3 h scans in the HDT group (top) and non-HDT group (bottom).
In patients who had a poor response after receiving non-HDT, GSUVmean increased significantly from a median of 1.17 (IQR: 0.89-1.20) at 1 h to a median of 1.57 (1.49-1.69) at 3 h. The median percent change was 33.39% (IQR: 28.35-40.63), with a range of 6.25-158.15 (P = 0.003). Patients with a complete response to treatment had a median GSUVmean of 1.02 (IQR: 0.87-1.05) at 1 h and a median of 1.02 (0.86-1.05) at 3 h. Median percent change was -0.62 (-1.38-0.84), with a range of -1.97-2.12 (P = 0.72) (Figures 3 and 4).
Figure 4.
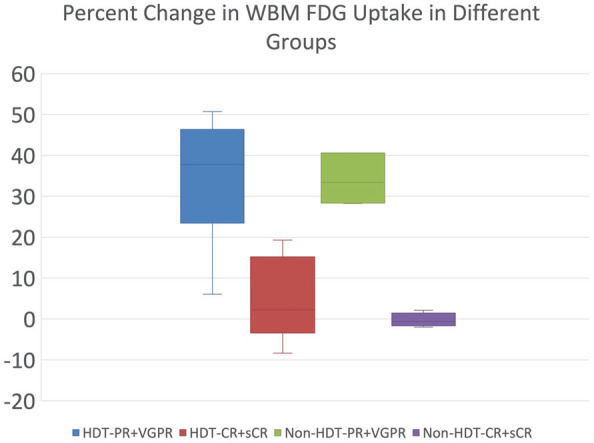
Box plots showing percent change in whole bone marrow FDG uptake from early to delayed scans. Patients were stratified by treatment group (HDT or non-HDT) and response to treatment (PR and VGPR or CR and sCR). The outliers are not shown here.
Discussion
This is the first study to the knowledge of the authors that used DTPI technique in MM patients. In particular, we employed a non-interpreter-dependent method of PET/CT quantification to evaluate the changes in 18F-FDG uptake in the WBM of MM patients from early to delayed scans. We did not observe a significant increase in WBM 18F-FDG uptake in patients who achieved a complete response to treatment (sCR and CR). However, the increase in WBM 18F-FDG uptake was significant in the patients with poor response to treatment (PR and VGPR). This result is in accordance with our hypothesis, based on the assumption that malignant cells with aggressive behavior will have increased transport rates for glucose uptake and elevated hexokinase to 18F-FDG-6-phosphatase ratios.
One limitation with current parameters of DTPI quantification is that the results are affected by the partial volume effect. Consequently, there is a significant underestimation of the true degree of disease activity of the lesions, and therefore, the results often show significant overlap of 18F-FDG uptake patterns between malignant and benign lesions on DTPI. Another issue of the conventional method of PET quantification is the high variability of results among different interpreters. According to the IMWG, the mentioned issue prevents the routine use of 18F-FDG PET imaging in MM patients [14], not only for the purpose of DTPI but also in general. Another limitation of conventional methods of PET quantification specific for MM is that it is that because of the high number of lesions it is a difficult and time consuming task to evaluate each lesions separately.
Our proposed methodology creates large volume especially in the spine where each pathological lesion often is fully included with large margins and thereby reducing the effect of partial-volume. In addition, since the methodology is based on applying a simple threshold and is therefore less susceptible to observer bias, it has the potential to ameliorate the issue of lack of reproducibility. This method of quantification is non-interpreter-dependent because it allows for segmentation of the skeleton without input from a user. This algorithm first performs a threshold of Hounsfield units in the CT image and then performs morphological closing with a set element radius. Neither the threshold algorithm nor morphological closing require manual input. As such, they are non-interpreter-dependent. The reproducibility of this methodology was confirmed by the results of several studies, including the one that was published in the Society of Nuclear Medicine and Molecular Imaging, 2019 [23]. Our approach has also shown superior speed in assessing the WBM uptake irrespective of the number of lesions (less than 2 minutes; even for patients with high number of osteolytic lesions, N ≥ 10).
In this study, a higher percent of change in the WBM 18F-FDG uptake from early to delayed scans was associated with poor response to treatment. It was already shown that 18F-FDG uptake in the bone marrow of MM patients correlated significantly with the percentage of CD38/CD138 positive cells, which are a hallmark of MM [20]. Furthermore, the results obtained in previous studies demonstrated that MM patients with higher degree of plasma cell infiltration in the bone marrow had a poorer outcome. For instance, Rajkumar et al. in a study of seventy-five consecutive patients who underwent autologous stem cell transplantation for relapsed myeloma showed that the complete response rate was significantly lower in patients with a high bone marrow plasma cell percentage (BMPC%) [17]. In another study, Chakraborty et al. evaluated 1070 patients with newly diagnosed multiple myeloma, who completed a single line of induction therapy followed by autologous stem cell transplantation. The investigators showed that the patients with pre-transplant < 5% BMPC had a threefold likelihood of achieving sCR after transplant compared to those with BMPC ≥ 5% (45.6% vs. 16.3%; P < 0.0001) [24].
Although there is a lack of literature regarding the application of DTPI in hematological malignancies, this technique has been used in several studies in the setting of other cancers. Constantli et al. compared the sensitivity and specificity of DTPI approach with conventional single-time-point approach to differentiate malignant from benign lesions in twenty-one pediatric patients with suspected malignancy. They observed a significant improve for DTPI approach in comparison to single-time-point approach [25]. In a study of one hundred and eleven patients with newly diagnosed breast cancer, the investigators showed that ΔSUVmax% of 8% was the only significant cut-off for discrimination between invasive and non-invasive cancer [26]. These results of these studies showed that DTPI can improve the overall sensitivity and specificity of PET imaging in the setting of cancers, particularly in comparison to early imaging.
This study has some limitations. One of the limitations is the relatively small sample size that met the criteria for DTPI analysis. Despite the small number of participants, the results that we observed was in accordance with our hypothesis. Further studies with more patients may help to establish the efficacy of this proposed PET/CT quantification approach. Another limitation is that the subjects of this prospective study were collected from two different institutions. However, since the guidelines were strictly followed and the PET scanners were calibrated and harmonized, the results were not affected or minimally affected.
In conclusion, by applying a reproducible and fast method of PET/CT quantification, we assessed the correlation between the percent change from early to delayed scans in whole-bone marrow (WBM) 18F-FDG uptake and response to treatment in MM patients. We observed that WBM uptake of 18F-FDG between the two time-points (1 h and 3 h) increased significantly in patients with poor response to treatment but not in the patients that achieved complete response. Further studies are warranted to confirm these results.
Disclosure of conflict of interest
None.
References
- 1.Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, Mozley PD, Rossman MD, Albelda SM, Alavi A. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42:1412–1417. [PubMed] [Google Scholar]
- 2.Basu S, Kung J, Houseni M, Zhuang H, Tidmarsh GF, Alavi A. Temporal profile of fluorodeoxyglucose uptake in malignant lesions and normal organs over extended time periods in patients with lung carcinoma: implications for its utilization in assessing malignant lesions. Q J Nucl Med Mol Imaging. 2009;53:9–19. [PubMed] [Google Scholar]
- 3.Ziai P, Hayeri MR, Salei A, Salavati A, Houshmand S, Alavi A, Teytelboym OM. Role of optimal quantification of FDG PET imaging in the clinical practice of radiology. Radiographics. 2016;36:481–496. doi: 10.1148/rg.2016150102. [DOI] [PubMed] [Google Scholar]
- 4.Mavi A, Basu S, Cermik TF, Urhan M, Bathaii M, Thiruvenkatasamy D, Houseni M, Dadparvar S, Alavi A. Potential of dual time point FDG-PET imaging in differentiating malignant from benign pleural disease. Mol Imaging Biol. 2009;11:369–378. doi: 10.1007/s11307-009-0212-5. [DOI] [PubMed] [Google Scholar]
- 5.Luna A, Vilanova JC, da Cruz LCH Jr, Rossi SE. Functional imaging in oncology: biophysical basis and technical approaches-volume 1. Springer Science & Business Media. 2013 [Google Scholar]
- 6.Houshmand S, Salavati A, Segtnan EA, Grupe P, Hoilund-Carlsen PF, Alavi A. Dual-time-point imaging and delayed-time-point fluorodeoxyglucose-PET/computed tomography imaging in various clinical settings. PET Clin. 2016;11:65–84. doi: 10.1016/j.cpet.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Cheng G, Torigian DA, Zhuang H, Alavi A. When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET? Eur J Nucl Med Mol Imaging. 2013;40:779–787. doi: 10.1007/s00259-013-2343-9. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Loving VA, Chauhan A, Zhuang H, Mitchell S, Alavi A. Potential of dual-time-point imaging to improve breast cancer diagnosis with (18)F-FDG PET. J Nucl Med. 2005;46:1819–1824. [PubMed] [Google Scholar]
- 9.Sadıç M, Houshmand S, Alavi A. Quantitative global disease assessment of FDG-PET/CT Yeni Bir Teknik: FDG-PET/CT ile quantitative global hastalık degerlendirme. J Clin Anal Med. 2015;6 [Google Scholar]
- 10.Taghvaei R, Zadeh MZ, Sirous R, Shamchi SP, Raynor WY, Seraj SM, Moghbel M, Wang S, Werner TJ, Zhuang H. Pre-treatment partial-volume-corrected TLG is the best predictor of overall survival in patients with relapsing/refractory non-hodgkin lymphoma following radioimmunotherapy. Am J Nucl Med Mol Imaging. 2018;8:407. [PMC free article] [PubMed] [Google Scholar]
- 11.Hess S, Blomberg BA, Rakheja R, Friedman K, Kwee TC, Høilund-Carlsen PF, Alavi A. A brief overview of novel approaches to FDG PET imaging and quantification. Clin Transl Imaging. 2014;2:187–198. [Google Scholar]
- 12.Kim SJ, Kim YK, Kim IJ, Kim YD, Lee MK. Limited predictive value of dual-time-point F-18 FDG PET/CT for evaluation of pathologic N1 status in NSCLC patients. Clin Nucl Med. 2011;36:434–439. doi: 10.1097/RLU.0b013e31820adef8. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Kim BH, Jeon YK, Kim SS, Kim IJ. Limited diagnostic and predictive values of dual-time-point 18 F FDG PET/CT for differentiation of incidentally detected thyroid nodules. Ann Nucl Med. 2011;25:347–353. doi: 10.1007/s12149-011-0468-0. [DOI] [PubMed] [Google Scholar]
- 14.Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S, Hillengass J, Engelhardt M, Usmani SZ, Vesole DH, San-Miguel J, Kumar SK, Richardson PG, Mikhael JR, da Costa FL, Dimopoulos MA, Zingaretti C, Abildgaard N, Goldschmidt H, Orlowski RZ, Chng WJ, Einsele H, Lonial S, Barlogie B, Anderson KC, Rajkumar SV, Durie BGM, Zamagni E. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the international myeloma working group. Lancet Oncol. 2017;18:e206–e217. doi: 10.1016/S1470-2045(17)30189-4. [DOI] [PubMed] [Google Scholar]
- 15.Zadeh MZ, Raynor WY, Seraj SM, Ayubcha C, Kothekar E, Werner T, Alavi A. Evolving roles of fluorodeoxyglucose and sodium fluoride in assessment of multiple myeloma patients: introducing a novel method of PET quantification to overcome shortcomings of the existing approaches. PET Clin. 2019;14:341–352. doi: 10.1016/j.cpet.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Cavo M, Baccarani M, Gobbi M, Lipizer A, Tura S. Prognostic value of bone marrow plasma cell infiltration in stage I multiple myeloma. Br J Haematol. 1983;55:683–690. doi: 10.1111/j.1365-2141.1983.tb02851.x. [DOI] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, Therneau TM, Kyle RA, Greipp PR, Gertz MA. Methods for estimation of bone marrow plasma cell involvement in myeloma: predictive value for response and survival in patients undergoing autologous stem cell transplantation. Am J Hematol. 2001;68:269–275. doi: 10.1002/ajh.10003. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian R, Basu D, Dutta T. Prognostic significance of bone marrow histology in multiple myeloma. Indian J Cancer. 2009;46:40. doi: 10.4103/0019-509x.48594. [DOI] [PubMed] [Google Scholar]
- 19.Terpstra WE, Lokhorst HM, Blomjous F, Meuwissen OJ, Dekker AW. Comparison of plasma cell infiltration in bone marrow biopsies and aspirates in patients with multiple myeloma. Br J Haematol. 1992;82:46–49. doi: 10.1111/j.1365-2141.1992.tb04592.x. [DOI] [PubMed] [Google Scholar]
- 20.Ak İ, Gulbas Z. F-18 FDG uptake of bone marrow on PET/CT scan: it’s correlation with CD38/CD138 expressing myeloma cells in bone marrow of patients with multiple myeloma. Ann Hematol. 2011;90:81–87. doi: 10.1007/s00277-010-1037-7. [DOI] [PubMed] [Google Scholar]
- 21.Pourhassan Shamchi S, Zirakchian Zadeh M, Østergaard B, Kim J, Raynor WY, Khosravi M, Taghvaei R, Nielsen AL, Gerke O, Werner TJ, Holdgaard P, Abildgaard N, Revheim ME, Høilund-Carlsen PF, Alavi A. Brain glucose metabolism in patients with newly diagnosed multiple myeloma significantly decreases after high-dose chemotherapy followed by autologous stem cell transplantation. Nucl Med Commun. 2020;41:288–293. doi: 10.1097/MNM.0000000000001144. [DOI] [PubMed] [Google Scholar]
- 22.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zadeh MZ, Ostergaard B, Raynor W, Ayubcha C, Acosta-Montenegro O, Yellanki D, Taghvaei R, Gerke O, Constantinescu C, Werner T. Assessment of uptake of FDG in the whole bone marrow of multiple myeloma and smoldering multiple myeloma patients through a novel method of PET/CT quantification: comparison with a control group. J Nucl Med. 2019;60:143–143. [Google Scholar]
- 24.Chakraborty R, Muchtar E, Kumar SK, Buadi FK, Dingli D, Dispenzieri A, Hayman SR, Hogan WJ, Kapoor P, Lacy MQ. Impact of pre-transplant bone marrow plasma cell percentage on post-transplant response and survival in newly diagnosed multiple myeloma. J Leuk. 2017;58:308–315. doi: 10.1080/10428194.2016.1201572. [DOI] [PubMed] [Google Scholar]
- 25.Costantini DL, Vali R, Chan J, McQuattie S, Charron M. Dual-time-point FDG PET/CT for the evaluation of pediatric tumors. Am J Roentgenol. 2013;200:408–413. doi: 10.2214/AJR.12.8930. [DOI] [PubMed] [Google Scholar]
- 26.Zytoon AA, Murakami K, El-Kholy MR, El-Shorbagy E. Dual time point FDG-PET/CT imaging… Potential tool for diagnosis of breast cancer. Clin Radiol. 2008;63:1213–1227. doi: 10.1016/j.crad.2008.03.014. [DOI] [PubMed] [Google Scholar]


