Abstract
Psoriatic skin lesions are metabolically active, which makes them candidates for imaging with 18-F fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). The aim of our study was to correlate FDG-PET findings with Psoriasis Area and Severity Index (PASI) scores, the most widely-used grading system for psoriasis. Thirty-three subjects and a total of 84 FDG-PET/CT scans from a prospective clinical trial [NCT01553058] with >2 months moderate-to-severe psoriasis were included. Subjects underwent whole-body FDG-PET/CT imaging 60 min after intravenous FDG administration, prior to the start of treatment. Scans were repeated 12 weeks and 52 weeks after baseline scans were conducted and after treatment or placebo administration was initiated. Each subject and scan was graded by our “PET-PASI” scoring system, a qualitative review of multi-plane reconstructions for both attenuation-corrected (AC) and non-attenuation-corrected (NAC) PET images. PASI and PET-PASI scores were correlated using Spearman’s rho analysis. Our study demonstrated a significant positive correlation between each subject’s corresponding PET-PASI and PASI scores before and during treatment or placebo administration (r=0.53, P<0.001). We also found positive correlations between PET-PASI and PASI scores across different regions of the body (head and neck: r=0.22, upper extremities: r=0.26, trunk: r=0.48, and lower extremities: r=0.58). In conclusion, AC and NAC FDG-PET/CT images may be utilized to evaluate lesions in subjects with moderate-to-severe psoriasis. Our methodology could have future implications in the diagnosis and therapeutic management of psoriasis.
Keywords: Fluorodeoxyglucose F18, positron emission tomography computed tomography, psoriasis, PASI
Introduction
Psoriasis is a chronic autoimmune inflammatory skin disease that affects close to 125 million people worldwide. It is characterized by scaling, erythema, and pruritus [1,2]. Although it is considered to be non-life-threatening, it can lead to a substantial reduction in the quality of life, due primarily to physical discomfort and psychosocial harm [3]. In addition, a diagnosis of psoriasis has been associated with an elevated risk of diseases with higher mortality, especially cardiometabolic diseases, which may pose more severe associated health problems for patients [4].
18-F fluorodeoxyglucose (FDG), a radioactive glucose analog that enters cells via facilitative glucose transporters (GLUTs), has been widely used in diagnostic imaging as a marker to measure metabolic activity in glucose-utilizing cells [5]. The uptake of FDG in the skin depends on the number of GLUTs and activity of glucose-6-phosphate, which traps FDG and varies with the metabolic activity of the skin. As a result, FDG uptake increases in association with metabolic activity, such as inflammation seen in psoriasis. FDG-positron emission tomography/computed tomography (FDG-PET/CT) is a powerful imaging technique that has been utilized in the assessment of various pathologies [6-9]. FDG-PET/CT has shown increased FDG uptake in areas of skin lesions, psoriatic arthritis, and enthesitis [10]. Non-attenuation-corrected (NAC) FDG-PET/CT images have shown increased uptake in the skin, a cutaneously-detected finding associated with psoriasis [11]. Attenuation-corrected (AC) images are found to have reduced signals from the skin lesions and hence, NAC images should be used in addition to AC images to detect and monitor skin lesions in psoriasis [11]. Lesions such as superficial lymphoma are easier to detect on NAC compared to AC scans. This superiority could pertain to lower AC contrast resulting from inhomogeneity of data transmission, subject motion between emission and transmission, or noise from transmission measurement [12].
While treatment options for psoriasis differ widely and have variable long and short-term effects, the biological therapy agent, adalimumab, is commonly used in the management of severe psoriasis [13]. Adalimumab binds both free and membrane-bound tumor necrosis factor (TNF)-alpha, which prevents the inflammatory processes it normally propagates through its cytokine activity [14]. Narrowband ultraviolet B phototherapy (NB-UVB) is highly effective for skin lesions but unlike adalimumab, it does not lead to clinically significant changes in the systemic immune function. However, photo-therapy has been shown to produce similar improvements in psoriatic skin lesions, as well as in health-related quality of life, compared to adalimumab [15].
This study was undertaken to investigate the usefulness of FDG-PET/CT scans in detecting psoriatic skin lesions. Specifically, we analyzed AC and NAC scans of subjects with moderate-to-severe psoriasis who either received phototherapy, adalimumab, or placebo for treatment. In doing so, we assess the utility of FDG uptake as a reflection of metabolic activity in psoriatic lesions and, consequently, as a specific diagnostic indicator of psoriatic lesions.
Methods
Study setting
We utilized FDG-PET/CT scans from a randomized multi-center trial [NCT01553058] involving 96 subjects across 8 centers in the United States [16]. For our current study, we included 33 subjects who underwent scanning at the Hospital of the University of Pennsylvania. One subject included in our study underwent PET/CT analysis but did not undergo randomization for the original clinical trial.
The remaining 32 subjects were included in the original clinical trial and met the following criteria: ≥18 years with a diagnosis of psoriasis for at least 6 months and that of moderate-to-severe psoriasis for at least 2 months, defined as body surface area ≥10% and psoriasis area severity index score ≥12 at baseline [17]. Excluded criteria included: UVB phototherapy within 14 days of baseline, psoralen-UVA phototherapy within 30 days of baseline, oral psoriasis treatments within 30 days of baseline, biologics within 90 days of baseline (or 180 days for ustekinumab), or investigational agents within 30 days or 5 half-lives (whichever is longer) of baseline [17]. FDG-PET/CT for these subjects was performed before the start of treatment, 12 weeks post-treatment, and 52 weeks post-treatment.
Image acquisition
After an overnight fast of at least 4 hours and a confirmed blood glucose concentration of below 150 mg/dL, FDG-PET/CT imaging was performed on a whole-body full-ring PET/CT scanner (Philips Ingenuity TF PET/CT) at the PET Center in the Department of Radiology at the University of Pennsylvania 60 minutes after 15 mCi of intravenous 18F-FDG administration. PET images were obtained from the vertex of the skull to the toes in 3D mode and then reconstructed in transverse, coronal, and sagittal views. Low-dose CT imaging was performed for attenuation correction and anatomical orientation.
Image analysis
OsiriX MD software (Pixmeo SARL, Bernex, Switzerland) was used for image analysis (Figure 1). A qualitative review of axial, sagittal, and coronal AC and NAC PET images was performed. We developed a “PET-PASI” scoring system utilizing PASI (Psoriasis Area and Severity Index score) scoring as a gold standard. Similar to the PASI scoring, we divided the body into four areas (head and neck, upper extremities, trunk (including genitals), lower extremities (including buttocks)) generating an average FDG uptake score and applying a scale from 0 to 6 depending on the percent of affected skin for each area and finally adjusting for the area involved. The score for each area was multiplied by the weight of the respective section (0.1 for head and neck, 0.2 for upper extremities, 0.3 for trunk, and 0.4 for lower extremities), yielding a PET-PASI score. The weights which were used to calculate the PET-PASI score were obtained from the PASI formula, which is an established objective calculation used in the assessment of psoriasis [18,19]. Erythema, induration, and scaling are included in the clinical PASI score, but due to the limitations of the image analysis, they could not be assessed with PET. Two readers, one of whom had more than 15 years of experience reading PET/CT scans, scored each scan. All 84 FDG-PET/CT scans were randomly provided for investigation, and the readers were blinded for patient number and clinical information including treatment, date, and time point (in the course of treatment) of the examination.
Figure 1.
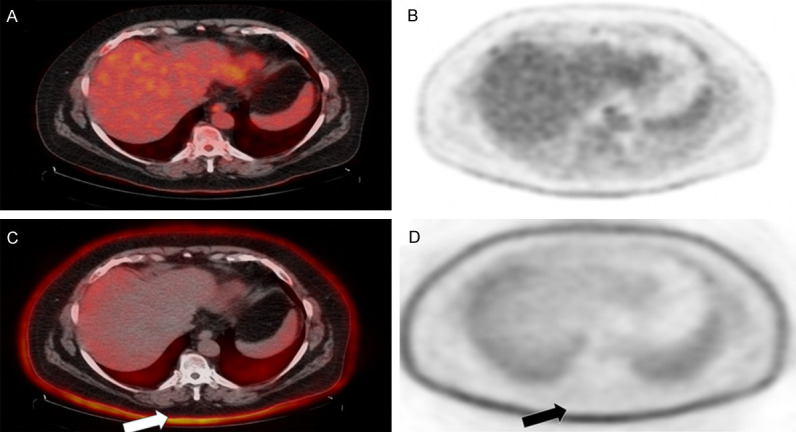
Focal areas of cutaneous radiotracer uptake on FDG-PET/CT. Non-attenuation-corrected images (A, B) demonstrate higher uptake than the attenuation-corrected images (C, D) in the corresponding areas of psoriatic skin lesions.
Statistical analysis
STATA software (Stata/IC Version 10.1, StataCorp, College Station, TX) was used to analyze our PET-PASI results from the visual assessment of FDG-PET/CT with the PASI score. Spearman’s rho analysis, which is a non-parametric measure of rank correlation, was used to assess the correlation between PET-PASI and PASI scores.
Results
Subjects characteristics are listed in Table 1. Our study found a significant overall positive correlation between the PET-PASI and PASI scores before and during treatment or placebo administration (r=0.53, P<0.001). The linear regression of the data obtained from the entire body surface area can be observed in Figure 2. We also found positive correlations between PET-PASI and PASI scores for each of the four regions of the body (head and neck: r=0.22, upper extremities: r=0.26, trunk: r=0.48, and lower extremities: r=0.58). Linear regressions representing PET-PASI and PASI score correlations for each of the four anatomical sections can be found in Figure 3. These correlations suggest that PET-PASI can successfully identify psoriatic disease to at least the same extent as PASI alone. These results demonstrate the significant association between the degree of psoriatic disease measured by both PASI and PET-PASI, which indicates that both methods may be useful diagnostically. Coupled with the power of FDG as a marker for glucose uptake in metabolically active cells, the strong positive correlations between PET-PASI and PASI scores indicate that PET-PASI is a reliable alternative to PASI in the assessment of psoriasis, and it may have the potential to determine disease extent with more precision.
Table 1.
Subject characteristics
| Variable | Total (N=33) |
|---|---|
| Mean Age in years (SD) | 43.6 ± 15.6 |
| Sex | Female, 9 (27.3%) |
| Male, 24 (72.3%) | |
| Race | Asian: 3 (9.1%) |
| Black/African-American: 7 (21.2%) | |
| White: 19 (57.6%) | |
| Other: 4 (12.1%) | |
| Ethnicity | Hispanic/Latino: 3 (9.1%) |
| Not Hispanic/Latino: 29 (87.9%) | |
| Missing: 1 (3%) | |
| BMI (SD) | 31.4 ± 8.73 |
| History of Diabetes Mellitus | 0 (0%) |
| History of Hypertension | 6 (18.2%) |
| History of Hypertension Medication | 6 (18.2%) |
| History of Hyperlipidemia | 7 (21.2%) |
| History of Statin Use | 3 (9.1%) |
| History of Additional Lipid Medication | 0 (0%) |
BMI - body mass index, SD - standard deviation.
Figure 2.

Correlation between PET-PASI and PASI scores for the entire body surface area (r=0.53, P<0.001).
Figure 3.

Correlation between PET-PASI and PASI scores in the (A) head and neck (r=0.22), (B) upper extremities (r=0.26), (C) trunk (r=0.48), and (D) lower extremities (r=0.58).
Discussion
To our knowledge, this is the first study to compare visual findings seen on both AC and NAC PET images with clinical findings in subjects with moderate-to-severe psoriasis. In the present study, we demonstrate that both AC and NAC FDG-PET/CT can be used to assess psoriatic lesions in patients. Specifically, we have elucidated a significant correlation between clinical PASI scores and our PET-PASI analysis of FDG-PET/CT imaging. This finding suggests that PET-PASI analysis has the potential to function similarly to PASI in evaluating the severity of psoriatic disease. This conclusion may be applied to expand our methodology to additional cutaneous lesions, which may offer new diagnostic techniques used in dermatologic pathologies.
Psoriasis is a disease characterized by chronic inflammation. A number of studies have previously used FDG-PET/CT in psoriasis patients. Bruna-Muraille et al. first noted skin uptake of FDG in a psoriasis patient who received incidental FDG-PET/CT scanning for recurrent papillary thyroid carcinoma [20]. Mehta et al. then demonstrated that FDG-PET/CT may be used to identify skin, liver, joints, tendons, and aorta inflammatory lesions in subjects with moderate to severe psoriasis [21]. More recently, Chaudhari et al. utilized high-resolution FDG-PET/CT to evaluate joint and bone involvement in psoriatic arthritis patients [10]. Taken together, these studies demonstrate the feasibility of FDG-PET/CT in the evaluation of psoriasis patients. Both Mehta et al. or Chaudhari et al. utilized mean standardized uptake value to quantify lesion involvement; moreover, neither compared global uptake to disease severity, as measured by PASI or other clinical scales. In our study, we combine a visual PET/CT assessment with the surface area of involvement as assessed clinically to generate our PET-PASI score. Moreover, we correlate our findings to subjects’ corresponding PASI scores, thus suggesting a clinical use for FDG-PET/CT in psoriasis patients.
The PASI score is based on 16 variables: average redness, thickness, and scaliness of the lesions across 4 areas of involvement (head and neck, upper extremities, trunk, and lower extremities) [22]. While this score represents the most comprehensive assessment of psoriasis disease severity for clinical trials, it may be too unwieldy for clinical practice. Louden et al. developed the Simplified Psoriasis Area Severity Index (which takes into account redness, thickness, scaliness of lesions, and total body surface area involved) as a 97% sensitive alternative to the PASI [23]. Moreover, while a high PASI score is generally indicative of more severe disease, the clinical implications of any single score are inconsistent among patients, and it has poor sensitivity to small areas of involvement [24,25]. By assessing FDG uptake in areas that may not physically appear to be clinically diseased, as opposed to simply using average redness, thickness, and scaliness of lesions as indicators of disease severity, the PET-PASI approach may be able to more accurately detect and quantify systemic disease burden in each anatomic region.
FDG-PET/CT is a powerful modality and could have future implications in the diagnostic and therapeutic interventions for the psoriasis patient population. Among the limitations of our current study is the lack of an established and validated qualitative method to grade FDG activity of the psoriatic lesions. Erythema, induration, and scaling, important parameters to quantify disease severity, are difficult to measure by FDG-PET/CT. However, high metabolic uptake in a background of normal activity is indicative of inflammatory processes, which is indirect evidence of psoriasis. In addition, it is nearly impossible to confirm whether the high activity seen on an FDG-PET/CT scan is due to a psoriatic lesion or another skin lesion. Finally, because there were only 33 participants included in our retrospective analysis, larger prospective studies are indicated to strengthen the power and generalizability of this data. However, one of the major strengths of this study was the fact that both scan readers were blinded towards treatment type, baseline and follow-up status of the scans, and patient identity. Coupled with the statistically significant correlation between PET-PASI and PASI scores, these results have the potential to expand the diagnostic repertoire in the field of dermatopathology, which will improve the development of patient-tailored treatment plans.
Conclusion
This study has demonstrated the feasibility of FDG-PET/CT in assessing the metabolic activity of psoriatic skin lesions in subjects with moderate-to-severe psoriasis as diagnosed with PASI score. We observed a significant positive correlation between PET-PASI and PASI scores in both general body surface area and individual anatomic regions. Future prospective studies with a larger number of subjects are warranted to assess the correlation between clinical severity and metabolic activity as assessed on FDG-PET/CT imaging in patients with psoriasis.
Acknowledgements
We thank the staff of the VIP research study and the participants for their contributions.
Disclosure of conflict of interest
None.
References
- 1.Szepietowski JC, Reich A. Pruritus in psoriasis: an update. Eur J Pain Lond Engl. 2016;20:41–6. doi: 10.1002/ejp.768. [DOI] [PubMed] [Google Scholar]
- 2.Chong HT, Kopecki Z, Cowin AJ. Lifting the silver flakes: the pathogenesis and management of chronic plaque psoriasis. BioMed Res Int. 2013;2013:168321. doi: 10.1155/2013/168321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891–5. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278–85. [PMC free article] [PubMed] [Google Scholar]
- 5.Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med. 2011;31:3–13. doi: 10.4103/0256-4947.75771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borja AJ, Hancin EC, Zhang V, Revheim ME, Alavi A. Potential of PET/CT in assessing dementias with emphasis on cerebrovascular disorders. Eur J Nucl Med Mol Imaging. 2020;47:2493–2498. doi: 10.1007/s00259-020-04697-y. [DOI] [PubMed] [Google Scholar]
- 7.Kothekar E, Yellanki D, Borja AJ, Al-Zaghal A, Werner TJ, Revheim ME, Gerke O, Saboury B, Gholamrezanezad A, Høilund-Carlsen PF, Alavi A. 18F-FDG-PET/CT in measuring volume and global metabolic activity of thigh muscles: a novel CT-based tissue segmentation methodology. Nucl Med Commun. 2020;41:162–8. doi: 10.1097/MNM.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 8.Kothekar E, Borja AJ, Gerke O, Werner TJ, Alavi A, Revheim ME. Assessing respitatory muscle activity with 18F-FDG-PET/CT in patients with COPD. Am J Nucl Med Mol Imaging. 2019;9:309–15. [PMC free article] [PubMed] [Google Scholar]
- 9.Borja A, Hancin E, Dreyfuss A, Rojulpote C, Zhang V, Gonuguntla K, Lin A, Feigenberg SJ, Swisher-McClure S, Alavi A, Revheim ME. FDG-PET/CT in the quantification of photon radiation therapy-induced vasculitis. J Nucl Med. 2020;61:1360–1360. [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhari AJ, Ferrero A, Godinez F, Yang K, Shelton DK, Hunter JC, Naguwa SM, Boone JM, Raychaudhuri SP, Badawi RD. High-resolution 18F-FDG PET/CT for assessing disease activity in rheumatoid and psoriatic arthritis: findings of a prospective pilot study. Br J Radiol. 2016;89:20160138. doi: 10.1259/bjr.20160138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakshi A, Gholami S, Alavi A, Gelfand JM, Takeshita J. Assessing cutaneous psoriasis activity using FDG-PET. Clin Nucl Med. 2015;40:727–9. doi: 10.1097/RLU.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houseni M, Chamroonrat W, Basu S, Bural G, Mavi A, Kumar R, Alavi A. Usefulness of non attenuation corrected 18F-FDG-PET images for optimal assessment of disease activity in patients with lymphoma. Hell J Nucl Med. 2009;12:5–9. [PubMed] [Google Scholar]
- 13.Gisondi P, Del Giglio M, Girolomoni G. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci. 2017;18:2427. doi: 10.3390/ijms18112427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yost J, Gudjonsson JE. The role of TNF inhibitors in psoriasis therapy: new implications for associated comorbidities. F1000 Med Rep. 2009;1:30. doi: 10.3410/M1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noe MH, Wan MT, Shin DB, Armstrong AW, Duffin KC, Chiesa Fuxench ZC, Kalb RE, Menter A, Simpson EL, Takeshita J, Tyring SK, Van Voorhees AS, Mehta NN, Gelfand JM. Patient-reported outcomes of adalimumab, phototherapy, and placebo in the vascular inflammation in psoriasis trial: a randomized controlled study. J Am Acad Dermatol. 2019;81:923–30. doi: 10.1016/j.jaad.2019.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vascular Inflammation in Psoriasis Trial (The VIP Trial)-Full Text View-ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/ NCT01553058 (accessed December 6, 2019)
- 17.Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of two psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394. doi: 10.1161/CIRCIMAGING.117.007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Information NC for B, Pike USNL of M 8600 R, MD B, Usa 20894. Validity of outcome measures. Canadian Agency for Drugs and Technologies in Health; 2018. [Google Scholar]
- 19.Feldman SR, Menter A, Koo JY. Improved health-related quality of life following a randomized controlled trial of alefacept treatment in patients with chronic plaque psoriasis. Br J Dermatol. 2004;150:317–26. doi: 10.1111/j.1365-2133.2004.05697.x. [DOI] [PubMed] [Google Scholar]
- 20.Bruna-Muraille C, Pochart JM, Papathanassiou D, Guedec-Ghelfi R, Cuif-Job A, Liehn JC. Incidental finding of F-18 FDG skin uptake in a patient with psoriasis during the evaluation of a recurrent papillary thyroid carcinoma. Clin Nucl Med. 2011;36:34–5. doi: 10.1097/RLU.0b013e3181feedff. [DOI] [PubMed] [Google Scholar]
- 21.Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A, Baer A, Antigua J, Van Voorhees AS, Torigian DA, Alavi A, Gelfand JM. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F] -fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031–9. doi: 10.1001/archdermatol.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredriksson T, Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica. 1978;157:238–44. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 23.Louden BA, Pearce DJ, Lang W, Feldman SR. A Simplified Psoriasis Area Severity Index (SPASI) for rating psoriasis severity in clinic patients. Dermatol Online J. 2004;10:7. [PubMed] [Google Scholar]
- 24.Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J Am Acad Dermatol. 2004;51:563–9. doi: 10.1016/j.jaad.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64:ii65–8. doi: 10.1136/ard.2004.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]


