Abstract
Immune thrombocytopenia (ITP) is characterized by decreased platelet count in the peripheral circulation. The first-line therapy is corticosteroids with 53-80% overall response rate. Eltrombopag has been used as second-line therapy in ITP for over a decade now. The long-term efficacy and safety profile have been widely reported in the western world. However, the data from the resource-constraint settings of the developing world is scarce. We aim to present the real-life experience of efficacy and safety of eltrombopag from the resource-constraint settings. This was a retrospective, single-center study conducted at a tertiary care hospital in Northern India from 2012-2019. On audit of medical records, patients of ITP receiving eltrombopag were screened for inclusion. Patients whose treatment outcomes were not available were excluded. Finally, 53 patients were analyzed using statistical packages of Python v3.7. The patients’ median age was 35 years (range 17-78), with 23 (43.4%) being female. The median time to response was 35 days (range 28-50 days) and the cumulative overall response rates (ORR) at day 30, day 60 and day 90 were 41.5%, 69.8%, and 81.1% respectively. A total of 10 patients on eltrombopag relapsed during follow up. The cumulative rate of relapse at one year, three years, and five years were 6.6%, 25.3%, and 47.7%, respectively. There was no significant difference in outcome (response rate or relapse) in any subgroups depending on age, sex, duration of disease, number of prior lines of treatment, splenectomy, or baseline platelet count. Six patients stopped eltrombopag after having a median sustained response for 796 days (range 658-1185), and after a median follow up of 624 days (range 92-1339), they continued to be in remission. Seventeen patients (17/53, 32%) reported one or more adverse events while on eltrombopag therapy. A total of 49 adverse events (n=4, grade ≥3 CTCAEv4) were noted. Anemia was the most frequent adverse event followed by hepatobiliary dysfunction as reflected by deranged AST/ALT or raised bilirubin. The use of eltrombopag among adult ITP patients in resource-constraint settings was well-tolerated and yielded excellent overall response. The benefit was found to be sustained on long-term follow up. However, events like anemia, hepatobiliary, and thrombotic complications merit closer follow up.
Keywords: Eltrombopag, ITP, second line therapy, resource constraint settings
Introduction
Immune thrombocytopenia (ITP) is characterized by decreased platelet count in peripheral circulation with or without decreased platelet production in the bone marrow [1]. Clinically, it may manifest as mucocutaneous bleeding or rarely as life-threatening bleeding, making it one of the most commonly encountered hematological emergency [2-4].
The first-line therapy is a corticosteroid (prednisolone, methylprednisolone or dexamethasone); while the Intravenous Immunoglobulin (IVIG) can be used in individual cases with or without steroids in cases where immediate platelet count elevation is desired [5]. While the overall response to corticosteroids in ITP varies from 53-80%, half of the responders finally relapse on follow-up, which leads to a waxing-waning course of the disease [6]. The reason for steroid resistance is attributable to the diverse underlying pathogenesis of ITP. The steroids act through suppression of immune-mediated destruction of platelets but have limited scope when megakaryocytic suppression plays dominant role [7-9].
In steroid-resistant ITP patients, thrombopoietin receptor agonists (TPO-RAs), rituximab and splenectomy are the standard second-line therapy options [5,10]. TPO-RAs have been used successfully in the management of ITP for over a decade now and have been found to stimulate production of platelets in both the normal as well as dysfunctional bone marrow [6,11]. Eltrombopag is one of the TPO-RA which acts through stimulation of thrombopoeitin receptors on megakaryocytes and increases the platelet production thereby overcoming the deficit due to the peripheral platelet destruction by the immune system [11].
The efficacy and safety of eltrombopag has been widely reported in the literature but predominantly from developed world [12]. In the developing countries, due to the resource constraints, the drug has poor availability restricting its use. This also results in a very limited data on efficacy and safety of eltrombopag from the developing countries. We aim to present, the real-world data on efficacy and safety of eltrombopag in ITP from resource constraint settings.
Methods
This retrospective study was conducted at a tertiary care institute in Northern India. The institutional ethics committee approved the study (AHRR IEC44/2020). The patients’ records were accessed from the outpatient department and hospital discharge and death summaries. After careful screening of the documents, data were recorded and later entered in the excel sheet. The missing data were minimized by making phone calls to the patient or the family.
Patients
Written consents were obtained from the patients. Patients with ITP who were diagnosed as per the American Society of hematology guidelines [13], and treated with eltrombopag between 2012 and 2019 were included in this study. All these patients had received at least one prior line of therapy before recieving eltrombopag. Patients who had received the additional drug with eltrombopag, and/or missing follow-up information were excluded from the study (Figure 1).
Figure 1.
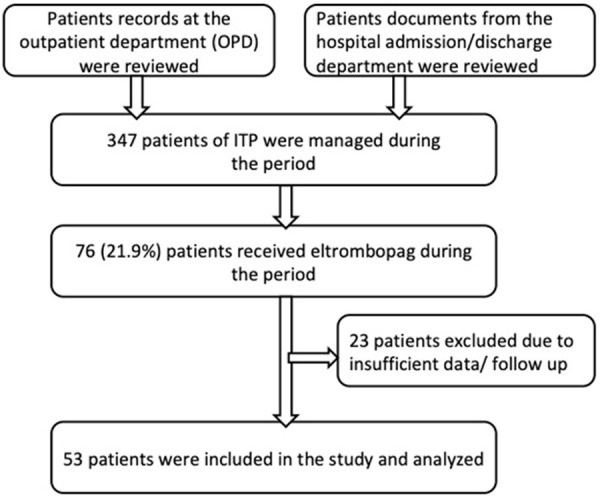
Patient screening flow diagram.
Adverse events were noted and graded as per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE)v4.0 [14]. The bleeding scores were recorded as per the World Health Organisation (WHO) bleeding scale [15].
Drug
Eltrombopag was started at 25 mg once daily dose, to be taken empty stomach in the morning. The patients were advised to avoid dairy products for four hours after intake of the drug. The patients were followed every two weeks in the clinic with complete hemogram and liver function tests. In the absence of any response, the drug dose was increased by 25 mg per week, up to maximum of 75 mg daily, if tolerated by the patients. If platelet count was increased over 2.5 lacs/μL, the dose was decreased to the previous one, or half-dose was given. For platelet count over 4.5 lacs/μL, eltrombopag was temporarily withheld till the platelet came below 2.5 lacs/μL, and the dose was decreased to the previous one or the dose was reduced by 50%.
Definitions for the study [1, 13]
Partial response (PR): A platelet count of >30,000/μL or double from the baseline, with no active bleeding manifestations.
Complete response (CR): A platelet count of >100,000/μL with no active bleeding manifestations.
Relapse: Any new-onset bleeding manifestation necessitating therapy or platelet counts <30,000/μL or patients who were started on another line of therapy or underwent splenectomy for low platelet.
Response duration: The time duration from the day of receiving eltrombopag to the day of documented relapse.
Statistical analysis
The data was stored in Microsoft Excel (R) and was analysed using statsmodels package (ver 0.11) [16] in Python 3.7 (httlps://www.python.org) and survival package [17] in R 3.5.1 [18].
Baseline variables were summarised using standard summarisation measures.
A timeline was made for each patient with entry time being the time when eltrombopag was started. Baseline clinical and laboratory characteristics were noted for each patient. Dose of eltrombopag was entered as time varying covariate.
Each patient would start in a state with disease (state 0), and then would transit into one of the following states: no disease (CR/PR) (state 1) and death (state 2). From this dataset, Kaplan Meier analysis and Cox proportional hazards regression analysis was done to assess overall response (state 0→1), relapse (state 1→0) and overall survival (0, 1→2). For the analysis of complete remission, timeline was subdivided into following states: disease present (state 0), PR (state 1), CR (state 2) and death (state 3) and same above techniques were used to analyse it. 95% confidence interval and P value <0.05 were used to assess significance of variables.
Results
Demographics and baseline laboratory parameters
A total of 347 patients of ITP were managed at our institute from 2012 to 2019. Seventy-six patients received eltrombopag during this period. After screening for inclusion and exclusion criteria, the treatment outcomes of 53 patients were analyzed. The baseline demographic characteristics and pre-eltrombopag parameters are summarised in Table 1. The median age of the study population was 35 years (range 17-78); 23/53 patients (43.4%) were females. Patients with acute, persistent, and chronic ITP who had received at least one prior line of treatment were included in the study. The median duration from diagnosis to eltrombopag was 2.06 years (32 days-14.227 year). These patients had received a median of 3 (range 1-8) prior lines of therapies; 38/53 patients (71.7%) had received ≥3 prior therapies. Eight patients (15.09%) had relapsed after splenectomy, and 16 patients (30.19%) relapsed after rituximab. The patients included in the study had a platelets counts 30000/μL or less at the time of initiation of eltrombopag. The baseline median hemoglobin, WBC, and platelets were 12.9 g/dL, 8000/μL, and 10000/μL, respectively.
Table 1.
Demographic profile and baseline clinical characteristics of the ITP patients who received eltrombopag
| Variable | Median (range) or n (%) |
|---|---|
| Evaluable pateints | 53 |
| Females | 23 (43.4%) |
| Age (years) | 35 (17-78) |
| Interval from ITP diagnosis to Eltrombopag | 2.06 year (32 days-14.227 year) |
| Number of prior therapies | 3 (1-8) |
| One line | 1 (1.89%) |
| Two lines | 12 (22.64%) |
| Three lines | 14 (26.41%) |
| Four lines | 12 (22.64%) |
| Five lines | 5 (9.43%) |
| Six lines | 2 (3.77%) |
| Seven lines | 3 (5.66%) |
| Eight lines | 2 (3.77%) |
| Different therpaies received prior to Eltrombopag | |
| Corticosteroids | 51 (96.23%) |
| Intravenous Immunoglobulin (IVIg) | 24 (45.28%) |
| Anti-RhD Immunoglobulin | 9 (16.98%) |
| Splenectomy | 8 (15.09%) |
| Rituximab | 16 (30.19%) |
| Dapsone | 44 (83.02%) |
| Danazol | 23 (43.39%) |
| Azathioprine | 18 (33.96%) |
| Vincristine | 5 (9.43%) |
| Hemoglobin (g/dL) | 12.9 (6.9-17.1) |
| WBC (/μL) | 8000 (4200-19900) |
| Platelet count (/μL) | 10000 (1000-30000) |
Response rate
The median time to response was 35 days (range 28-50 days) and the cumulative overall response rate (complete and partial response) at day 30, day 60 and day 90 were 41.5%, 69.8%, and 81.1%, respectively (Figure 2 and Table 2). Nine, 14, and 18 patients achieved complete response with eltrombopag on day 30, day 60, and day 90, respectively.
Figure 2.
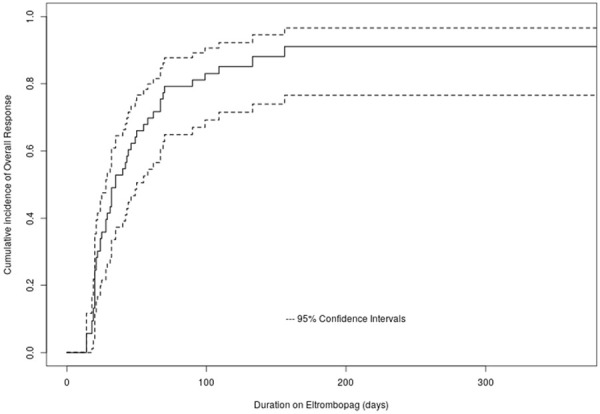
Kaplan-Meier curve showing the cumulative incidence of overall response.
Table 2.
Response to eltrombopag in ITP patients
| Variable | n (%) or median (range) | 95% CIs |
|---|---|---|
| Time to response | 35 days (28-50 days) | |
| Cumulative incidence of overall response | 48 | |
| At day-30 of Eltrombopag | 22 (41.5%) | 26.6-53.4% |
| At day-60 of Eltrombopag | 37 (69.8%) | 54.5-80% |
| At day-90 of Eltrombopag | 43 (81.1%) | 67-89.2% |
| Patients achieving complete response | ||
| At day-30 of Eltrombopag | 9 (17%) | 6.2-26.5% |
| At day-60 of Eltrombopag | 14 (26.4%) | 13.5-37.4% |
| At day-90 of Eltrombopag | 18 (34%) | 19.9-45.6 |
| Time to relapse in days | not reached | |
| Cumulative incidence of relapse | 10 | |
| At 1-year | 3 (6.6%) | 0-13.6% |
| At 2-year | 6 (15.3%) | 3.1-26% |
| At 3-year | 8 (25.3%) | 6.7-41.1% |
| At 5-year | 10 (47.7%) | 8.5-70.1% |
Factors affecting the response to eltrombopag
On univariate analysis, there was no effect of age, sex, duration of disease, prior lines of therapy received, splenectomy, and platelet count on the overall response to eltrombopag in the study population (Table 3).
Table 3.
Univariate analysis for response assessment at day+30 and day+90 after eltrombopag
| Hazard ratio | p | 30-day expected events (SE) | 90-day expected events (SE) | |
|---|---|---|---|---|
| Age (per decade) | 0.875 | 0.136 | ||
| 26-year | 0.62 (0.063) | 1.94 (0.15) | ||
| 50-year | 0.45 (0.15) | 1.41 (0.2) | ||
| Sex | 0.937 | 0.826 | ||
| Male | 0.51 (0.06) | 1.58 (0.19) | ||
| Female | 0.54 (0.09) | 1.68 (0.26) | ||
| Duration of ITP (years) | 1 (per 1 log year) | 0.999 | ||
| 1-year | 0.52 (0.05) | 1.62 (0.17) | ||
| 6-year | 0.52 (0.06) | 1.62 (0.21) | ||
| Prior therapy | 1.164 | 0.612 | ||
| ≤3 prior lines of therapy | 0.51 (0.8) | 1.57 (0.22) | ||
| >3 prior lines of therapy | 0.6 (0.08) | 1.82 (0.28) | ||
| Splenectomy | 0.846 | 0.688 | ||
| Yes | 0.54 (0.03) | 1.67 (0.18) | ||
| No | 0.45 (0.18) | 1.42 (0.58) | ||
| Hemoglobin (g/dL) | 0.955 | 0.458 | ||
| 11.7 | 0.55 (0.04) | 1.72 (0.11) | ||
| 14.9 | 0.48 (0.06) | 1.48 (0.18) | ||
| WBC (/μL) | 0.892 (per 1000 WBC/μL ) | 0.066 | ||
| 6700 | 0.64 (0.09) | 2.06 (0.23) | ||
| 10000 | 0.44 (0.05) | 1.41 (0.16) | ||
| Paltelets | 1.03 (per 1000 platelets/μL) | 0.077 | ||
| ≤5000 | 0.42 (0.09) | 1.36 (0.21) | ||
| 17000 | 0.59 (0.04) | 1.94 (0.14) | ||
| Eltrombopag dose | 1 | 0.566 | ||
| 25 mg | 0.52 (0.05) | 1.49 (0.15) | ||
| 50 mg | 0.61 (0.09) | 1.75 (0.27) |
Response evaluation at follow up
The was no significant (WHO bleeding scale ≥3) bleeding episode in the study population necessitating platelet transfusion or rescue therapy. There were only seven bleeding episodes during the follow-up, and all were WHO grade 1. A total of 10 patients on eltrombopag relapsed during follow up. The cumulative rate of relapse at one year, three years, and five years were 6.6%, 25.3%, and 47.7%, respectively. There was no significant difference in outcome (response rate or relapse) in any subgroups depending on age, sex, duration of disease, lines of prior therapies, splenectomy, or baseline platelet count (Figure 3; Table 4).
Figure 3.
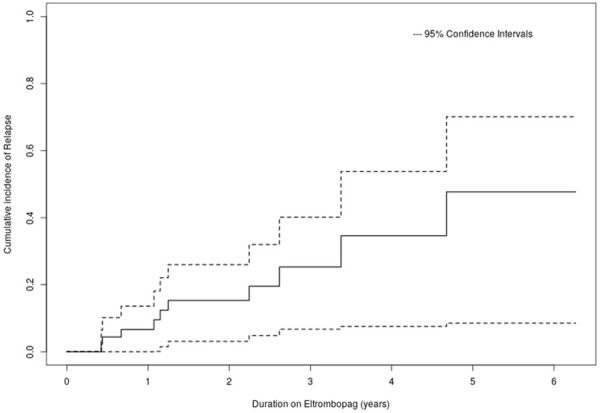
Kaplan-Meier curve showing the cumulative incidence of relapse following Eltrombopag.
Table 4.
Univariate analysis for relapse assessment at 1 year and 3 years after eltrombopag
| Hazard ratio | p | 1-year expected events (SE) | 3-year expected events (SE) | |
|---|---|---|---|---|
| Age (per decade) | 0.8 | 0.293 | ||
| 22-year | 0.08 (0.02) | 0.39 (0.04) | ||
| 46-year | 0.05 (0.01) | 0.23 (0.06) | ||
| Sex | 0.897 | 0.866 | ||
| Male | 0.06 (0.01) | 0.26 (0.08) | ||
| Female | 0.07 (0.02) | 0.29 (0.11) | ||
| Duration of ITP (years) | 0.726 (per 1 log year) | 0.198 | ||
| 1-year | 0.07 (0.007) | 0.33 (0.04) | ||
| 6-year | 0.04 (0.01) | 0.19 (0.06) | ||
| Prior therapy | 0.879 | 0.845 | ||
| ≤3 prior lines of therapy | 0.07 (0.02) | 0.3 (0.09) | ||
| >3 prior lines of therapy | 0.06 (0.02) | 0.26 (0.1) | ||
| Splenectomy | 1.375 | 0.689 | ||
| Yes | 0.06 (0.006) | 0.27 (0.02) | ||
| No | 0.08 (0.04) | 0.37 (0.18) | ||
| Hemoglobin (g/dL) | 1.243 | 0.144 | ||
| 11.7 | 0.03 (0.009) | 1.77 (0.06) | ||
| 14.9 | 0.09 (0.02) | 1.42 (0.12) | ||
| WBC (/μL) | 1.125 (per 1000 WBC/μL ) | 0.3 | ||
| 6700 | 0.05 (0.009) | 0.23 (0.04) | ||
| 10000 | 0.07 (0.009) | 0.34 (0.05) | ||
| Paltelets | 1.016 (per 1000 platelets/μL) | 0.627 | ||
| ≤5000 | 0.05 (0.02) | 0.25 (0.07) | ||
| 17000 | 0.07 (0.01) | 0.32 (0.06) |
Six patients stopped eltrombopag after having a sustained response for a median duration of 796 days (range 658-1185), and after a follow up of 624 days (92-1339), they continued to be in remission.
Side effects to eltrombopag therapy
Seventeen patients (17/53, 32%) reported one or more adverse events while on eltrombopag therapy. A total of 49 adverse events (n=4 with grade ≥3 CTCAEv4) were noted. Anemia was the most frequent adverse event followed by hepatobiliary laboratory dysfunction as measured by deranged aspartate aminotransferase (AST)/alanine aminotransferase (ALT) or raised bilirubin (Table 5).
Table 5.
Adverse events noted with eltrombopag therapy
| Total Adverse Events, n(%) | Grade >3 AEs* | Comments | |
|---|---|---|---|
| Anemia | 25 (47.1%) | 0 | |
| Thrombocytosis | 2 (3.7%) | 0 | |
| Thrombosis | 2 (3.7%) | 1 (1.9%) | Died due to CVT** |
| TMA | 1 (1.9%) | 1 (1.9%) | Died due to AKI#+MODS## |
| Increased AST/ALT@ | 7 (13.2%) | 1 (1.9%) | |
| Increased Bilirubin | 2 (3.7%) | 0 | |
| Sepsis | 2 (3.7%) | 1 (1.9%) | Died due to MODs |
| Erythromelalgia | 1 (1.9%) | 0 | |
| Headache | 2 (3.7%) | 0 | |
| Arthralgia | 1 (1.9%) | 0 | |
| Nausea/vomiting | 2 (3.7%) | 0 | |
| Diarrhoea | 1 (1.9%) | 0 | |
| Skin rash | 1 (1.9%) | 0 | |
| Total | 49 | 4 |
AEs: adverse events;
CVT: cerebral venous thrombosis;
AKI: acute kidney injury;
MODS: multi-organ dysfunction syndrome;
AST/ALT: aspartate aminotransferase/alanine aminotransferase.
Discussion
Eltrombopag is recommended as second-line therapy for ITP [5]. Clinical trials and real-world experiences have described its efficacy and long-term safety in the setting of ITP. However, there is a paucity of data from resource-constraint settings of the developing world. As estimated in the western world, the higher cost is a significant hurdle for its wider use in developing countries [19]. There have also been safety concerns for this drug in the Asian population due to a difference in pharmacokinetics from the western population [20]. Moreover, as we anticipate more frequent use of eltrombopag in future, there is a need to explore the data from the developing world.
The median time to response in our study was 35 days (range 28-50 days). Our study’s cumulative ORRs at day 30, day 60, and day 90 were 41.5%, 69.8%, and 81.1%, respectively. In the landmark phase 3, double-blind, placebo-controlled study (RAISE trial), the overall response rate observed was 79% [21]. Subsequently, in a retrospective study from Turkey, Ali Eser et al. reported the median time to response as 16 days (range 8-28 days) and an overall response rate of 83.9% in patients treated with eltrombopag [22]. In another study, retrospective studies from Spain, Tomas et al. reported the median time to response as 12 days (9-13 days) and an overall response rate of 88.8% in chronic ITP patients treated with eltrombopag (Table 6) [23].
Table 6.
Comparison of results from index study with the published studies on utility of eltrombopag as second-line therapy in ITP
| Study | Tomas et al. 2015 (21) | Eser et al. 2016 (20) | Tomas et al. 2017 (32) | Çekdemir et al. 2019 (31) | Present study |
|---|---|---|---|---|---|
| Study design | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective |
| Number of patients (n) | 152 | 31 | 220 | 285 | 53 |
| Median age, years(range) | 63 (45-75) | 51 (33-59) | 62 (47-75) | 43.9 ± 20.6 | 35 (17-78) |
| Females, n (%) | 109 (72%) | 16 (51.6%) | 47 (41.6%) | 187 (65.6%) | 23 (43.4%) |
| Duration of ITP, months (range) | 81 (30-192) | - | 79 (31-193) | - | 2.06 year (32 days-14.23 year) |
| Median number of prior lines of therapy, n (range) | 3 (2-4) | 4 (3-5) | 3 (2-4) | - | 3 (1-8) |
| Prior splenectomy, n (%) | 104 (68%) | 24 (77.4) | 11 (11%) | - | 8 (15.09%) |
| Prior Rituximab, n (%) | 17 (23%) | 19 (61.3) | 47 (30.7%) | - | 16 (30.19%) |
| Overall response, n (%) | 135 (88.8%) | 26 (83.9%) | 127 (90%) | 247 (86.7%) | 81.1% (on day+90) |
| Median time to response | 12 days (9-13) | 16 days (8-28) | 13 days (7-18) | 14 days (3-210) | 35 days (28-50) |
| Follow up Median | 15 months | 29 weeks (11-74) | 15 months | 18 ± 6.4 months | Not reached |
| Sustained response | 75.2% at 15 months | - | 71% | - | |
| 1-year | 93.4% | ||||
| 3-year | 74.7% | ||||
| 5-year | 52.3% | ||||
| All ADRs* | 28 (18.4%) | - | 70 (31.8%) | 62 (21.8%) | 17 (32%) |
| ADRs ≥ gd3 | 2 (1.32%) | One SCD** | 15 (6.82%) | 18 (6.3%) One SCD* | 4 (7.55%) |
ADRs: adverse drug reactions;
SCD, sudden cardiac death.
A longer median time to respond and an inferior response rate at 30 days was observed in our study compared to the clinical trials and post-marketing retrospective studies. However, the response rate slowly improved in our study and reached 69.8% & 81.1% at day 60 and day 90 of the therapy, respectively, which is comparable to the clinical trials and post-marketing real-life experiences.
In both the RAISE trial and the study by Ali Eser et al., 47% and 35.5% of patients were on concomitant anti-ITP medication in the form of corticosteroids. Both these studies also used 50 mg eltrombopag as the initial dose, and the dose was subsequently changed (increased or decreased) depending upon the response. The concomitant corticosteroid treatment may have a synergistic effect on the availability of eltrombopag [24]. Therefore, patients receiving concomitant steroids are likely to have a better response rate, as is the case in the above studies.
Eltrombopag is used in the dose of 50-75 mg once daily on empty stomach. However, a lower dose has been advocated in people of Asian origin due to the higher (almost double) area under the curve in the Asian population compared to the widely reported western population [25]. With reduced initial eltrombopag doses, a lower early response rate has been reported by some studies. In a study from Japan, Tomiyama et al., reported a response rate of 22% at three weeks when 12.5 mg of eltrombopag was used. The response rate increased to 60% at six weeks, with an increase in the dose to 25 mg once daily [26]. In another study from Korea, Kim et al. reported a response rate of 72.2% when 25 mg to 50 mg of eltrombopag was used [20]. In another landmark randomized controlled trial published earlier, the use of low-dose eltrombopag (30 mg OD) was associated with an overall response rate of 28% at day 43 of therapy [27]. Therefore, the lower initial dose (25 mg) and lack of concomitant anti-ITP drugs (corticosteroids) may explain a longer time to respond as well as a lower initial cumulative response rate in our study.
The loss of follow-up had a median of 724 days (108-2288 days). After a median follow-up of 633 days (65 days-2347 days), a total of 10 patients on eltrombopag relapsed. The cumulative rate of relapse at one year, three years, and five years were 6.6%, 25.3, and 47.7%, respectively. The relapse rate in our study was comparable to a 24.8% relapse rate at a median follow up of 15 months reported by Tomas et al. in chronic ITP patients receiving eltrombopag [23].
Eltrombopag was discontinued in six patients (11.3%), after having a median duration of sustained response for 796 days (range 658-1185); after a median follow up of 624 days (range 92-1339), they continued to be in remission. Tomas et al. reported that 21% patients discontinued eltrombopag after persistent response, and 51% of evaluable patients continued to have sustained response after six months of drug discontinuation [23]. Although any direct comparison is difficult due to smaller size of our study, but our findings do suggest that eltrombopag discontinuation can be safely considered in a subgroup of patients who demonstrate sustained response despite lowering the dose.
On univariate analysis, there was no significant difference in the treatment outcome (response rate or relapse) in any subgroups depending on age, sex, duration of disease, lines of prior therapies, splenectomy, or baseline platelet count. These observations are in congruence with the findings reported earlier by Tomas et al. Similar findings, including no effect of splenectomy on the overall response rate, were also reported in the landmark RAISE trial [21,23]. However, contrary to our study, the study published by Mazza et al. suggested that splenectomy significantly reduced the response to eltrombopag therapy (P≤0.05) [28]. A smaller size may have affected the outcome of our study.
Seventeen patients (17/53, 32%) reported one or more adverse events while on eltrombopag therapy. A total of 49 adverse events were noted, including four grade 3 or more. Anemia was the commonest adverse event followed by hepatobiliary dysfunction as measured by deranged AST/ALT or raised bilirubin.
Ever since eltrombopag came into the clinical practice, hepatobiliary laboratory abnormalities (HBLAs), thrombosis, and myelofibrosis have been three significant areas of concern for long term use as also reported in the EXTEND trial [12,29].
As far as the real-life experience from the Asian continent is concerned, thrombosis and myelofibrosis have been infrequently reported. In a study from India, Tripathi et al. reported no thrombosis in a cohort of 27 patients, while 12% had asymptomatic HBLAs [30,31]. In another study from Korea, Kim et al. reported no thrombosis in a cohort of 18 patients, while 38.9% of patients had asymptomatic HBLAs, and one patient had myelofibrosis (reversed after stopping eltrombopag) [20].
Our study reported a higher incidence of anemia (47.2%) in patients treated with eltrombopag. Iron deficiency anemia in developing countries has a prevalence of up to 43%, which is much higher than in developed countries (9%) [32]. Moreover, eltrombopag has been shown to have iron chelation properties [33]. Eltrombopag has also been implicated in the development of iron deficiency in children treated for ITP [34]. The EXTEND study also reported anemia as an adverse effect of eltrombopag in up to 10% of ITP patients [12]. Iron chelating property of eltrombopag and the higher prevalence of iron deficiency anemia in developing countries can explain a higher number of anemic patients.
The subjective findings of headache, arthralgia, and nausea in our study were comparable to the reported studies so far [35,36].
Our study had the shortcoming of being a retrospective study and a small sample size. However, to the best of our knowledge, this is the largest series on eltrombopag use in ITP from India.
Conclusion
The impressive overall response and long-term sustained response with eltrombopag in ITP patients living in resource-constraint settings were comparable to the published data from clinical trials and real-world experiences from developed nations. It also has a favourable long term safety profile. However, events like anemia, hepatobiliary, and thrombotic complications need to be carefully looked for during follow up.
Disclosure of conflict of interest
None.
References
- 1.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, Godeau B, Lechner K, Mazzucconi MG, McMillan R, Sanz MA, Imbach P, Blanchette V, Kühne T, Ruggeri M, George JN. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 2.Mishra K, Jandial A, Malhotra P, Varma N. Wet purpura: a sinister sign in thrombocytopenia. Case Reports. 2017;2017:1–2. doi: 10.1136/bcr-2017-222008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nampoothiri RV, Singh C, Mishra K, Jandial A, Lad D, Prakash G, Khadwal A, Malhotra P, Varma N, S V. Immune thrombocytopenia is the commonest diagnosis on consultative hematology - a single centre experience. Indian J Hematol Blood Transfus. 2017;33:S104. doi: 10.1007/s12288-018-1045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra K, Malhotra P, Jandial A, Kumar LDP, Varma N, Dhiman R, Yanamandra U, Khadwal A, Prakash G, Ahluwalia J. Bleeding risk assessment by sonoclot in severe immune thrombocytopenia. Blood. 2017;130:2320–2320. [Google Scholar]
- 5.Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, Cuker A, Despotovic JM, George JN, Grace RF, Kühne T, Kuter DJ, Lim W, McCrae KR, Pruitt B, Shimanek H, Vesely SK. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829–3866. doi: 10.1182/bloodadvances.2019000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stasi R, Provan D. Management of immune thrombocytopenic purpura in adults. Mayo Clin Proc. 2004;79:504–522. doi: 10.4065/79.4.504. [DOI] [PubMed] [Google Scholar]
- 7.McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44:S3–S11. doi: 10.1053/j.seminhematol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Houwerzijl EJ, Blom NR, van der Want JJ, Esselink MT, Koornstra JJ, Smit JW, Louwes H, Vellenga E, de Wolf JT. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood. 2004;103:500–506. doi: 10.1182/blood-2003-01-0275. [DOI] [PubMed] [Google Scholar]
- 9.Mishra K, Jandial A, Sandal R, Porchezhian P, Charan S, Kumar LDP, Prakash G, Khadwal A, Dhiman R, Varma N. Poor platelet function on sonoclot signature is associated with high incidence of bleeding in severe immune thrombocytopenia. Blood. 2018;132:4991–4991. [Google Scholar]
- 10.Khera S, Pramanik SK, Yanamandra U, Mishra K, Kapoor R, Das S. Dapsone: an old but effective therapy in pediatric refractory immune thrombocytopenia. Indian J Hematol Blood Transfus. 2020;36:690–694. doi: 10.1007/s12288-020-01286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson-Miller CL, Delorme E, Tian SS, Hopson CB, Landis AJ, Valoret EI, Sellers TS, Rosen J, Miller SG, Luengo JI, Duffy KJ, Jenkins JM. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27:424–430. doi: 10.1634/stemcells.2008-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong RSM, Saleh MN, Khelif A, Salama A, Portella MSO, Burgess P, Bussel JB. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130:2527–2536. doi: 10.1182/blood-2017-04-748707. [DOI] [PubMed] [Google Scholar]
- 13.Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson TM, Ryan SJ, Bennett AV, Stover AM, Saracino RM, Rogak LJ, Jewell ST, Matsoukas K, Li Y, Basch E. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24:3669–3676. doi: 10.1007/s00520-016-3297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Seabold S, Perktold J. statsmodels: Econometric and statistical modeling with python. 2010 [Google Scholar]
- 17.Terry M. Modeling Survival Data: Extending the {C}ox Model. New York: Springer; 2000. Therneau PMG. [Google Scholar]
- 18.Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 19.Tremblay G, Dolph M, Roy AN, Said Q, Forsythe A. The cost-effectiveness of eltrombopag for the treatment of immune thrombocytopenia in the United States. Clin Ther. 2020;42:860–872. e868. doi: 10.1016/j.clinthera.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Kim YK, Lee SS, Jeong SH, Ahn JS, Yang DH, Lee JJ, Kim HJ. Efficacy and safety of eltrombopag in adult refractory immune thrombocytopenia. Blood Res. 2015;50:19–25. doi: 10.5045/br.2015.50.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, Arning M, Stone NL, Bussel JB. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377:393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 22.Eser A, Toptas T, Kara O, Sezgin A, Noyan-Atalay F, Yilmaz G, Ozgumus T, Pepedil-Tanrikulu F, Kaygusuz-Atagunduz I, Firatli-Tuglular T. Efficacy and safety of eltrombopag in treatment-refractory primary immune thrombocytopenia: a retrospective study. Blood Coagul Fibrinolysis. 2016;27:47–52. doi: 10.1097/MBC.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 23.González-López TJ, Alvarez-Román MT, Pascual C, Sánchez-González B, Fernández-Fuentes F, Jarque I, Pérez-Rus G, Pérez-Crespo S, Bernat S, Hernández-Rivas JA, Andrade MM, Cortés M, Gómez-Nuñez M, Olivera P, Martínez-Robles V, Fernández-Rodríguez A, Fuertes-Palacio MA, Fernández-Miñano C, de Cabo E, Fisac R, Aguilar C, Bárez A, Peñarrubia MJ, García-Frade LJ, González-Porras JR. Eltrombopag safety and efficacy for primary chronic immune thrombocytopenia in clinical practice. Eur J Haematol. 2016;97:297–302. doi: 10.1111/ejh.12725. [DOI] [PubMed] [Google Scholar]
- 24.Gibiansky E, Zhang J, Williams D, Wang Z, Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011;51:842–856. doi: 10.1177/0091270010375427. [DOI] [PubMed] [Google Scholar]
- 25.Shida Y, Takahashi N, Nohda S, Hirama T. Pharmacokinetics and pharmacodynamics of eltrombopag in healthy japanese males. Rinsho yakuri/Japanese Journal of Clinical Pharmacology and Therapeutics. 2011;42:11–20. [Google Scholar]
- 26.Tomiyama Y, Miyakawa Y, Okamoto S, Katsutani S, Kimura A, Okoshi Y, Ninomiya H, Kosugi H, Nomura S, Ozaki K, Ikeda Y, Hattori T, Katsura K, Kanakura Y. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012;10:799–806. doi: 10.1111/j.1538-7836.2012.04695.x. [DOI] [PubMed] [Google Scholar]
- 27.Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, Arning M, Provan D, Jenkins JM. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357:2237–2247. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 28.Mazza P, Minoia C, Melpignano A, Polimeno G, Cascavilla N, Di Renzo N, Specchia G. The use of thrombopoietin-receptor agonists (TPO-RAs) in immune thrombocytopenia (ITP): a “real life” retrospective multicenter experience of the Rete Ematologica Pugliese (REP) Ann Hematol. 2016;95:239–244. doi: 10.1007/s00277-015-2556-z. [DOI] [PubMed] [Google Scholar]
- 29.Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, Brainsky A. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121:537–545. doi: 10.1182/blood-2012-04-425512. [DOI] [PubMed] [Google Scholar]
- 30.Tripathi AK, Shukla A, Mishra S, Yadav YS, Yadav DK. Eltrombopag therapy in newly diagnosed steroid non-responsive ITP patients. Int J Hematol. 2014;99:413–417. doi: 10.1007/s12185-014-1533-y. [DOI] [PubMed] [Google Scholar]
- 31.Sahu KK, Varma SC. Cortical vein thrombosis in a case of idiopathic thrombocytopenic purpura. Platelets. 2015;26:374–375. doi: 10.3109/09537104.2014.898180. [DOI] [PubMed] [Google Scholar]
- 32.Al-Alimi AA, Bashanfer S, Morish MA. Prevalence of Iron Deficiency Anemia among University Students in Hodeida Province, Yemen. Anemia. 2018;2018:4157876. doi: 10.1155/2018/4157876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlachodimitropoulou E, Chen YL, Garbowski M, Koonyosying P, Psaila B, Sola-Visner M, Cooper N, Hider R, Porter J. Eltrombopag: a powerful chelator of cellular or extracellular iron(III) alone or combined with a second chelator. Blood. 2017;130:1923–1933. doi: 10.1182/blood-2016-10-740241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambert MP, Witmer CM, Kwiatkowski JL. Therapy induced iron deficiency in children treated with eltrombopag for immune thrombocytopenia. Am J Hematol. 2017;92:E88–E91. doi: 10.1002/ajh.24705. [DOI] [PubMed] [Google Scholar]
- 35.Çekdemir D, Güvenç S, Özdemirkıran F, Eser A, Toptaş T, Özkocaman V, Haydaroğlu Şahin H, Ermiş Turak E, Esen R, Cömert M, Sadri S, Aslaner M, Uncu Ulu B, Karakuş A, Selim Bapur D, Alacacıoğlu İ, Aydın D, Tekinalp A, Namdaroğlu S, Ceran F, Tarkun P, Kiper D, Çetiner M, Yenerel M, Demir AM, Yılmaz G, Terzi H, Atilla E, Malkan Ü Y, Acar K, Öztürk E, Tombak A, Sunu C, Salim O, Alayvaz N, Sayan Ö, Ozan Ü, Ayer M, Gökgöz Z, Andıç N, Kızılkılıç E, Noyan F, Özen M, Pepedil Tanrıkulu F, Alanoğlu G, Özkan HA, Aslan V, Çetin G, Akyol Erikçi A, Deveci B, Ersoy Dursun F, Dermenci H, Aytan P, Gündüz M, Karakuş V, Özlü C, Demircioğlu S, Akay Yanar OM, Özatlı D, Ündar L, Tiftik EN, Türköz Sucak AG, Haznedaroğlu İ, Özcan M, Şencan M, Tombuloğlu M, Özet G, Bilgir O, Turgut B, Özcan MA, Payzın KB, Sönmez M, Ayyıldız O, Dal MS, Ertop Ş, Turgut M, Soysal T, Kaya E, Ünal A, Pehlivan M, Atagündüz I, Tuğlular Fıratlı T, Saydam G, Diz Küçükkaya R. A multi-center study on the efficacy of eltrombopag in management of refractory chronic immune thrombocytopenia: a real-life experience. Turk J Haematol. 2019;36:230–237. doi: 10.4274/tjh.galenos.2018.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.González-López TJ, Fernández-Fuertes F, Hernández-Rivas JA, Sánchez-González B, Martínez-Robles V, Alvarez-Román MT, Pérez-Rus G, Pascual C, Bernat S, Arrieta-Cerdán E, Aguilar C, Bárez A, Peñarrubia MJ, Olivera P, Fernández-Rodríguez A, de Cabo E, García-Frade LJ, González-Porras JR. Efficacy and safety of eltrombopag in persistent and newly diagnosed ITP in clinical practice. Int J Hematol. 2017;106:508–516. doi: 10.1007/s12185-017-2275-4. [DOI] [PubMed] [Google Scholar]


